Abstract
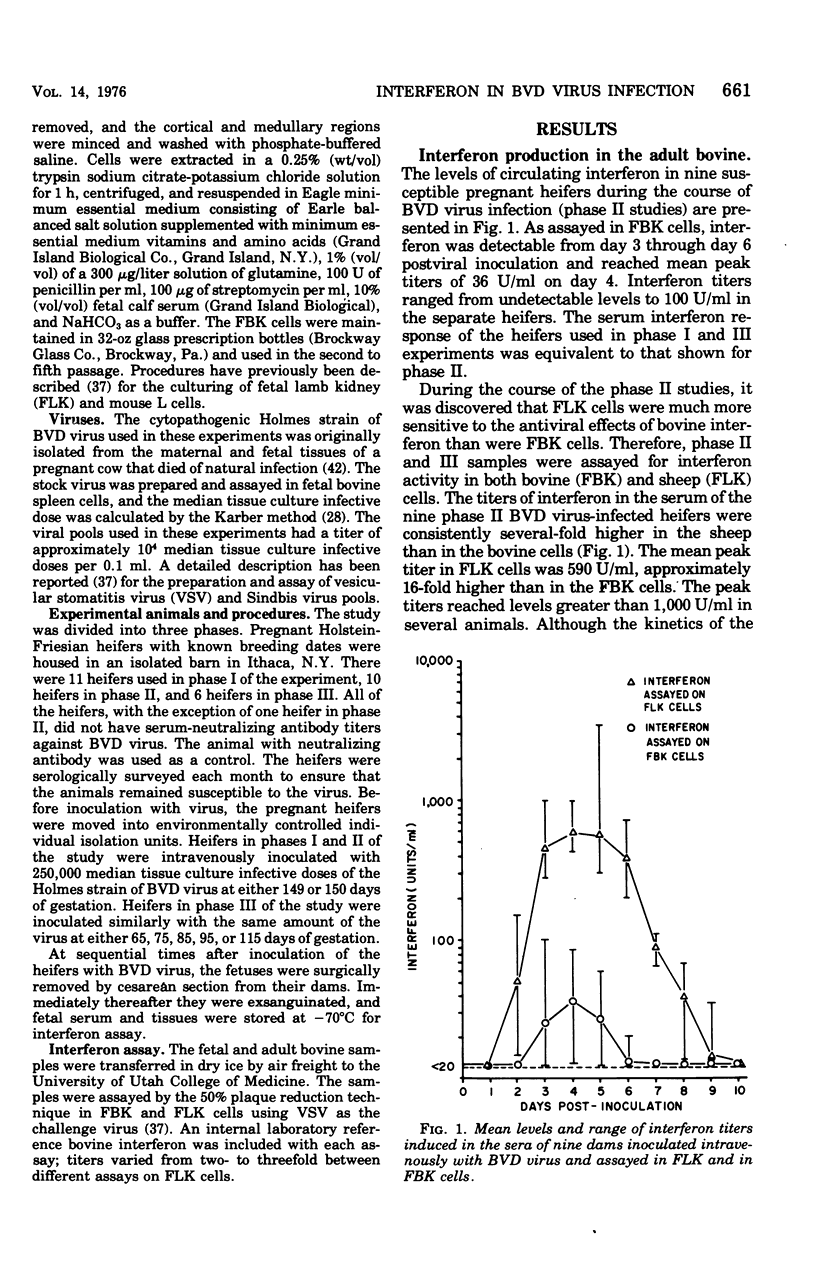
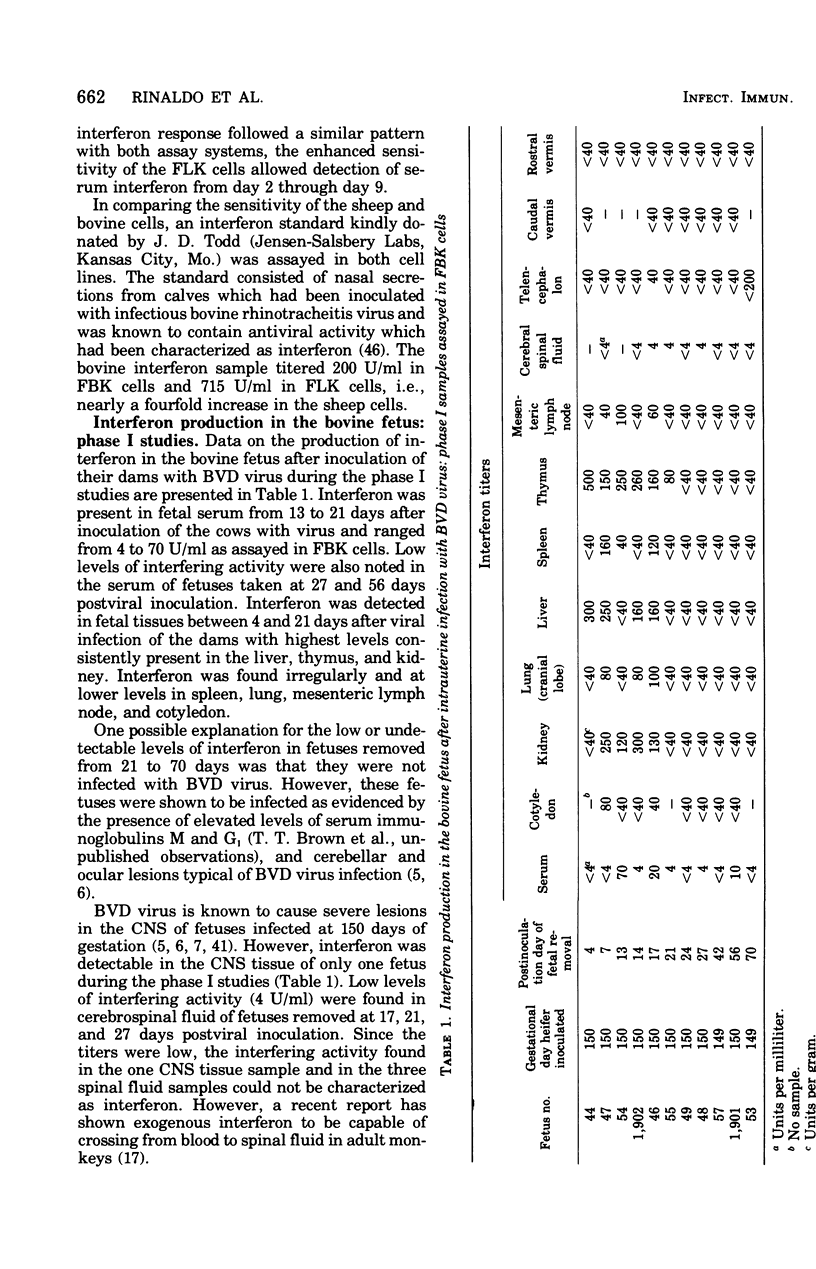
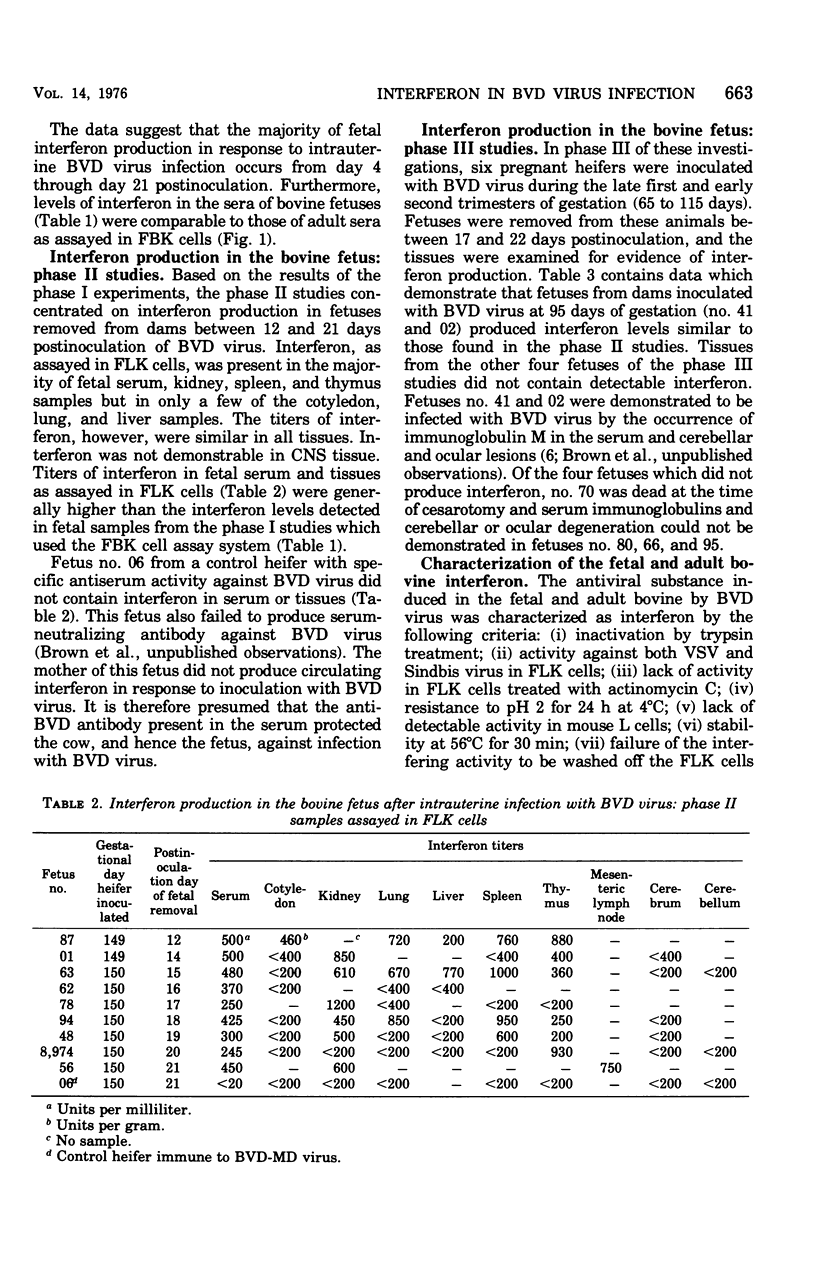
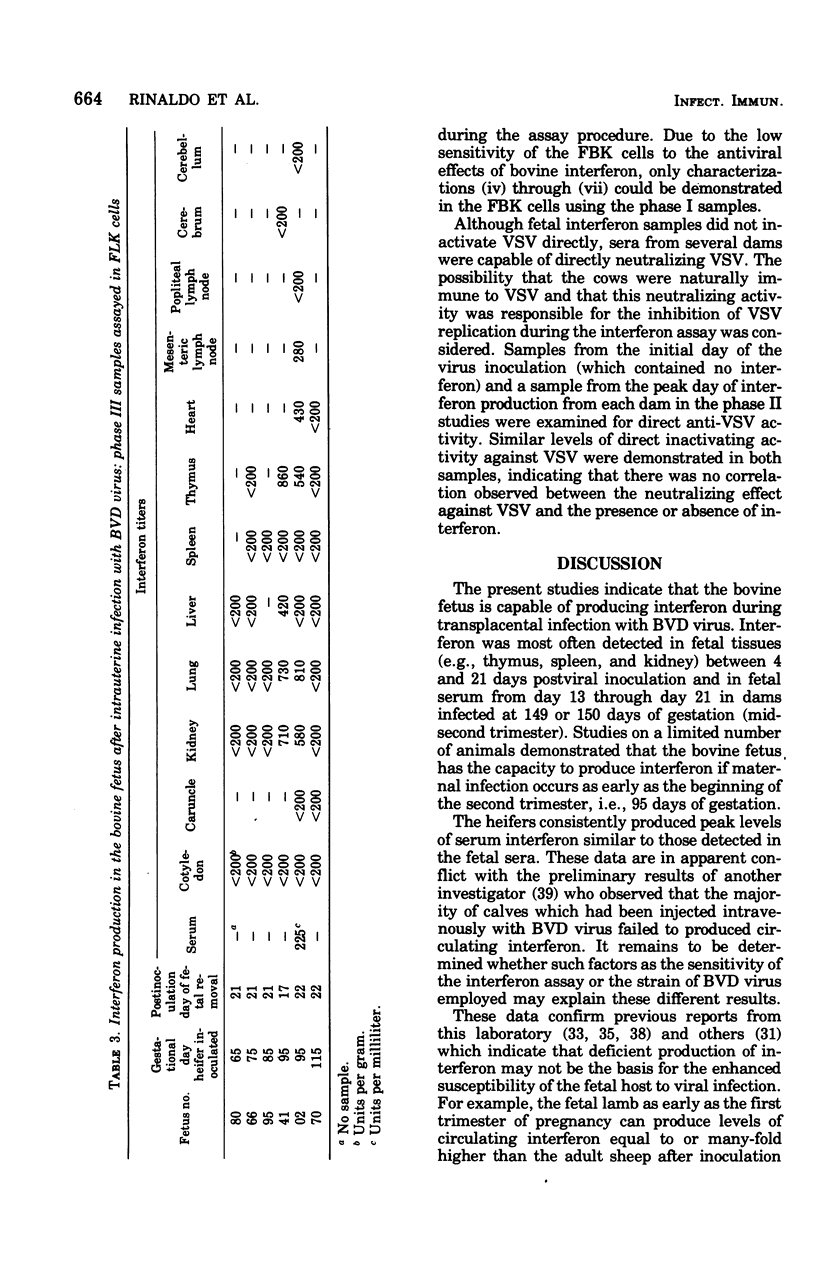
Levels of interferon in adult bovine serum and in fetal bovine serum and tissues were examined during the course of transplacental bovine viral diarrhea virus infection. The cows produced circulating interferon between 2 and 9 days after viral inoculation, with mean peak levels in the serum on day 4. Interferon could be routinely detected in fetal tissues (e.g., thymus, spleen, and kidney) between days 4 and 21 after viral inoculation of the cows at 149 to 150 days of gestation (mid-second trimester) and in fetal serum from day 13 through day 21. Interferon was also detectable in the serum and tissues of fetuses from dams infected at day 95 of gestation (the beginning of the second trimester). In general, no differences were found between the ability of the adult and fetus to produce interferon. Fetal lamb kidney cells were more sensitive to the antiviral effects of bovine interferon than were fetal bovine kidney cells. The antiviral substance from the fetal and adult animals was characterized as interferon by standard criteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARON S., ISAACS A. Mechanism of recovery from viral infection in the chick embryo. Nature. 1961 Jul 1;191:97–98. doi: 10.1038/191097a0. [DOI] [PubMed] [Google Scholar]
- Barbosa L. H., London W. T., Hamilton R., Buckler C. Interferon response of the fetal Rhesus monkey after viral infection. Proc Soc Exp Biol Med. 1974 Jun;146(2):398–400. doi: 10.3181/00379727-146-38113. [DOI] [PubMed] [Google Scholar]
- Blattner R. J., Williamson A. P., Heys F. M. Role of viruses in the etiology of congenital malformations. Prog Med Virol. 1973;15:1–41. [PubMed] [Google Scholar]
- Braun R. K., Osburn B. I., Kendrick J. W. Immunologic response of bovine fetus to bovine viral diarrhea virus. Am J Vet Res. 1973 Sep;34(9):1127–1132. [PubMed] [Google Scholar]
- Brown T. T., Bistner S. I., de Lahunta A., Scott F. W., McEntee K. Pathogenetic studies of infection of the bovine fetus with bovine viral diarrhea virus. II. Ocular lesions. Vet Pathol. 1975;12(5-6):394–404. doi: 10.1177/0300985875012005-00606. [DOI] [PubMed] [Google Scholar]
- Brown T. T., De Lahunte A., Scott F. W., Kahrs R. F., McEntee K., Gillespie J. H. Virus induced congenital anomalies of the bovine fetus. II. Histopathology of cerebellar degeneration (hypoplasia) induced by the virus of bovine viral diarrhea-mucosal disease. Cornell Vet. 1973 Oct;63(4):561–578. [PubMed] [Google Scholar]
- Brown T. T., DeLahunta A., Bistner S. I., Scott F. W., McEntee K. Pathogenetic studies of infection of the bovine fetus with bovine viral diarrhea virus. I. Cerebellar atrophy. Vet Pathol. 1974;11(6):486–505. doi: 10.1177/030098587401100604. [DOI] [PubMed] [Google Scholar]
- Casaro A. P., Kendrick J. W., Kennedy P. C. Response of the bovine fetus to bovine viral diarrhea-mucosal disease virus. Am J Vet Res. 1971 Oct;32(10):1543–1562. [PubMed] [Google Scholar]
- Catalano L. W., Jr, Sever J. L. The role of viruses as causes of congenital diseases. Annu Rev Microbiol. 1971;25:255–282. doi: 10.1146/annurev.mi.25.100171.001351. [DOI] [PubMed] [Google Scholar]
- Cole G. A., Wisseman C. L., Jr Pathogenesis of type 1 dengue virus infection in suckling, weanling and adult mice. 1. The relation of virus replication to interferon and antibody formation. Am J Epidemiol. 1969 Jun;89(6):669–680. doi: 10.1093/oxfordjournals.aje.a120981. [DOI] [PubMed] [Google Scholar]
- Desmyter J., Rawls W. E., Melnick J. L. A human interferon that crosses the species line. Proc Natl Acad Sci U S A. 1968 Jan;59(1):69–76. doi: 10.1073/pnas.59.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmyter J., Rawls W. E., Melnick J. L., Yow M. D., Barrett F. F. Interferon in congenital rubella: response to live attenuated measles vaccine. J Immunol. 1967 Oct;99(4):771–777. [PubMed] [Google Scholar]
- Dudgeon J. A. Congenital rubella. Pathogenesis and immunology. Am J Dis Child. 1969 Jul;118(1):35–44. doi: 10.1001/archpedi.1969.02100040037007. [DOI] [PubMed] [Google Scholar]
- Dunne H. W., Clark C. D. Embryonic death, fetal mummification, stillbirth, and neonatal death in pigs of gilts vaccinated with attenuated live-virus hog cholera vaccine. Am J Vet Res. 1968 Apr;29(4):787–796. [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-boye D., Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974 Oct 11;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- HEINEBERG H., GOLD E., ROBBINS F. C. DIFFERENCES IN INTERFERON CONTENT IN TISSUES OF MICE OF VARIOUS AGES INFECTED WITH COXSACKIE B1 VIRUS. Proc Soc Exp Biol Med. 1964 Apr;115:947–953. doi: 10.3181/00379727-115-29086. [DOI] [PubMed] [Google Scholar]
- Habif D. V., Lipton R., Cantell K. Interferon crosses blood-cerebrospinal fluid barrier in monkeys. Proc Soc Exp Biol Med. 1975 May;149(1):287–289. doi: 10.3181/00379727-149-38790. [DOI] [PubMed] [Google Scholar]
- Hanshaw J. B. Congenital cytomegalovirus infection: a fifteen year perspective. J Infect Dis. 1971 May;123(5):555–561. doi: 10.1093/infdis/123.5.555. [DOI] [PubMed] [Google Scholar]
- Howell P. G., Verwoerd D. W. Bluetongue virus. Virol Monogr. 1971;9:35–74. doi: 10.1007/978-3-7091-3987-5_2. [DOI] [PubMed] [Google Scholar]
- ISAACS A., BARON S. Antiviral action of interferon in embryonic cells. Lancet. 1960 Oct 29;2(7157):946–947. doi: 10.1016/s0140-6736(60)92022-5. [DOI] [PubMed] [Google Scholar]
- Kendrick J. W. Bovine viral diarrhea-mucosal disease virus infection in pregnant cows. Am J Vet Res. 1971 Apr;32(4):533–544. [PubMed] [Google Scholar]
- Kibrick S., Loria R. M. Rubella and cytomegalovirus. Current concepts of congenital and acquired infection. Pediatr Clin North Am. 1974 May;21(2):513–526. [PubMed] [Google Scholar]
- Korsantiya B. M., Smorodintsev A. A. Transplacental transmission of endogenous interferon in pregnant mice inoculated with influenza or Newcastle disease viruses. Nature. 1971 Aug 20;232(5312):560–561. doi: 10.1038/232560b0. [DOI] [PubMed] [Google Scholar]
- Lavelle G. C., Starr T. J. Interferon response and age-related resistance of germfree mice to mouse hepatitis virus. J Reticuloendothel Soc. 1968 Oct;5(5):422–435. [PubMed] [Google Scholar]
- Mendelson J., Kapusta R., Dick V. Maternal and fetal interferon production in the rat. Am J Obstet Gynecol. 1970 Jul 15;107(6):902–907. doi: 10.1016/s0002-9378(16)34044-3. [DOI] [PubMed] [Google Scholar]
- Mims C. A. Pathogenesis of viral infections of the fetus. Prog Med Virol. 1968;10:194–237. [PubMed] [Google Scholar]
- Morahan P. S., Grossberg S. E. Age-related cellular resistance of the chicken embryo to viral infections. I. Interferon and natural resistance to myxoviruses and vesicular stomatitis virus. J Infect Dis. 1970 Jun;121(6):615–623. doi: 10.1093/infdis/121.6.615. [DOI] [PubMed] [Google Scholar]
- Overall J. C., Jr, Glasgow L. A. Fetal response to viral infection: interferon production in sheep. Science. 1970 Feb 20;167(3921):1139–1141. doi: 10.1126/science.167.3921.1139. [DOI] [PubMed] [Google Scholar]
- Overall J. C., Jr, Glasgow L. A. Virus infections of the fetus and newborn infant. J Pediatr. 1970 Aug;77(2):315–333. doi: 10.1016/s0022-3476(70)80346-8. [DOI] [PubMed] [Google Scholar]
- Reinarz A. B., Broome M. G., Sagik B. P. Age-dependent resistance of mice to sindbis virus infection: viral replication as a function of host age. Infect Immun. 1971 Feb;3(2):268–273. doi: 10.1128/iai.3.2.268-273.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Overall J. C., Jr, Cole B. C., Glasgow L. A. Mycoplasma-associated induction of interferon in ovine leukocytes. Infect Immun. 1973 Nov;8(5):796–803. doi: 10.1128/iai.8.5.796-803.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Overall J. C., Jr, Glasgow L. A. Viral replication and interferon production in fetal and adult ovine leukocytes and spleen cells. Infect Immun. 1975 Nov;12(5):1070–1077. doi: 10.1128/iai.12.5.1070-1077.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWICKI L. Influence of age of mice on the recovery from experimental Sendal virus infection. Nature. 1961 Dec 30;192:1258–1259. doi: 10.1038/1921258a0. [DOI] [PubMed] [Google Scholar]
- SELLERS R. F., FITZPATRICK M. An assay of interferon produced in rhesus monkey and calf kidney tissue cultures using bovine enterovirus M6 as challenge. Br J Exp Pathol. 1962 Dec;43:674–683. [PMC free article] [PubMed] [Google Scholar]
- Scott F. W., Kahrs R. F., De Lahunte A., Brown T. T., McEntee K., Gillespie J. H. Virus induced congenital anomalies of the bovine fetus. I. Cerebellar degeneration (hypoplasia), ocular lesions and fetal mummification following experimental infection with bovine viral diarrhea-mucosal disease virus. Cornell Vet. 1973 Oct;63(4):536–560. [PubMed] [Google Scholar]
- Scott F. W., Kahrs R. F., Parsonson I. M. A cytopathogenic strain of bovine viral diarrhea-mucosal disease virus isolated from a bovine fetus. Cornell Vet. 1972 Jan;62(1):74–84. [PubMed] [Google Scholar]
- Subrahmanyan T. P. A study of the possible basis of age-dependent resistance of mice to poxvirus diseases. Aust J Exp Biol Med Sci. 1968 Jun;46(3):251–265. doi: 10.1038/icb.1968.20. [DOI] [PubMed] [Google Scholar]
- TYRRELL D. A. Interferon produced by cultures of calf kidney cells. Nature. 1959 Aug 8;184(Suppl 7):452–453. doi: 10.1038/184452a0. [DOI] [PubMed] [Google Scholar]
- Todd J. D., Volenec F. J., Paton I. M. Interferon in nasal secretions and sera of calves after intranasal administration of avirulent infectious bovine rhinotracheitis virus: association of interferon in nasal secretions with early resistance to challenge with virulent virus. Infect Immun. 1972 May;5(5):699–706. doi: 10.1128/iai.5.5.699-706.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILCEK J. PRODUCTION OF INTERFERON BY NEWBORN AND ADULT MICE INFECTED WITH SINDBIS VIRUS. Virology. 1964 Apr;22:651–652. doi: 10.1016/0042-6822(64)90091-1. [DOI] [PubMed] [Google Scholar]
- Ward G. M., Roberts S. J., McEntee K., Gillespie J. H. A study of experimentally induced bovine viral diarrhea-mucosal disease in pregnant cows and their progeny. Cornell Vet. 1969 Oct;59(4):525–538. [PubMed] [Google Scholar]


