Abstract
Objective
To evaluate longitudinal changes in grey matter (GM) volume and cognitive performance among individuals exposed to neurotropic herpes simplex virus subtype 1 (HSV1). There is a replicable association of HSV1 exposure with smaller prefrontal volumes (1, 2) and cognitive impairments (3, 4) in schizophrenia (SZ).
Method
We concurrently examined the whole-brain longitudinal trajectory of GM volumes and executive functions measured using the Wisconsin Card Sorting Test (WCST) among first-episode antipsychotic-naive SZ subjects and healthy subjects (HS) over 1 year. Age, gender, socioeconomic status (SES) and exposure to cytomegalovirus - another virus of herpes family associated with cognitive impairments in our previous study (4), were the covariates.
Results
Significant GM loss in the posterior cingulate gyrus (PCG) was noted among HSV1 seropositive SZ subjects over 1 year compared to baseline but not among other groups. Prefrontal GM volumes did not show longitudinal changes. Binomial mixed-effects models indicated that a significantly higher number of HSV1 seropositive SZ subjects completed fewer categories and committed proportionately more errors compared to HSV1 seronegative SZ and HS regardless of the serological status; significant 3-way diagnosis-by-HSV1 status-by-time interactions predicted both categories completed (p=0.0004) and perseverative errors (p=0.042) on WCST. Higher number of perseverative errors at year 1 compared to baseline but not number of categories completed correlated with longitudinal PCG volume loss.
Conclusions
These observations suggest that HSV1 exposure may be associated with longitudinal GM loss in the PCG and decline in executive functions among SZ subjects in contrast to the comparison groups.
Keywords: Schizophrenia, Cognition, Executive functions, Neuroanatomy, Prognosis, Herpes Simplex virus, Neurotropic viruses
Background
Cognitive impairments are considered to be a core deficit (5) of schizophrenia (SZ). SZ subjects show impairments in several cognitive domains compared to matched healthy subjects (HS), e.g. memory (6) and executive functions (7). There is an urgent need for systematic research in this area because such deficits: (a) profoundly affect the long-term outcome (8), (b) do not remit between the episodes adding enormous burden on the families (9), (c) have little correlation with positive symptoms (10), and (d) current medications minimally impact cognitive impairments (11).
Genetic and environmental factors may contribute to cognitive impairments. Among the environmental factors, emerging evidence supports the role of neurotropic viruses, e.g. herpes viruses. These agents can cause lifelong infection in the brain, with latency and reactivation cycles associated with neuronal damage or dysfunction (12, 13). Elevated prevalence of HSV1 seropositivity in SZ is inconsistent (14, 15) and whether such agents confer risk for SZ is uncertain. Nonetheless, there are replicable associations of herpes simplex virus, 1 (HSV1) exposure with cognitive impairments (3, 4) and decreased prefrontal (PFC) volume (1, 2). Besides, cross-sectional PFC GM reduction was correlated with impaired performance on the trails tests among HSV1 exposed SZ subjects (2). These associations have medium to large effect sizes and appear to be more prominent in SZ than in controls. Separately, we have noted a different pattern of association of cognitive impairments with cytomegalovirus (CMV) exposure, another virus within the herpes family (4). However, these associations do not necessarily prove etiological links. Natural history of lifelong periodic reactivations leading to longitudinal decline in cognitive function and related neurobiological measures could further support the role of HSV1 exposure in cognitive impairments.
In this study, our goals were to test the hypothesis that HSV1 exposure would be associated with longitudinal changes in executive functions and longitudinal grey matter (GM) loss in regions implicated in the regulation of executive functions. We examined executive functions performance and GM changes over one year among first-episode SZ subjects who were antipsychotic-naïve at baseline in comparison to HS. We included CMV exposure to investigate the relative specificity of the associations with HSV1 exposure.
Methods
Subjects (SZ and HS) were recruited through an ongoing study. All participants were interviewed on the Structured Clinical Interview for DSM-IV (SCID) and followed up for ≥6 months, when data were synthesized and consensus DSM-IV diagnoses derived by senior clinicians. Cases with SZ/schizoaffective disorder were included. HS were recruited from the same geographic region as cases through advertisements and referrals from other studies. None of the HS met DSM-IV criteria for mental retardation, substance dependence (past 6 months) or abuse (past month). Subjects with medical or neurological disorders including head injury, encephalitis and epilepsy were excluded. Physical examination and full medical history was obtained at study entry on all subjects. Demographic data including the socioeconomic status (SES) using the Hollingshead scale were obtained. For the cognitive study, we selected subjects who were followed up at 1 year and had available serum. We allowed up to 30% attrition at year 1 for Wisconsin Card Sorting Test (WCST) data. For the imaging study, participants with both baseline and follow-up structural MRI were included with no attrition (SZ=18; HS=24). This cohort was incidentally a subset of a larger cohort that we examined at baseline only in our previous cross-sectional study (1) (Table 1). After fully explaining the experimental procedures, written informed consents were obtained from all subjects. The Institutional Review Board of the University of Pittsburgh approved the study.
Table 1.
Distribution of first episode SZ and HS across the follow up period and their serological status at baseline
| Neuropsychological Study1 | Neuroimaging study2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Baseline | 26 Wks | 52 Wks | Baseline | 52 Wks | ||||||
|
| ||||||||||
| HSV1+ | HSV1− | HSV1+ | HSV1− | HSV1+ | HSV1− | HSV1+ | HSV1− | HSV1+ | HSV1− | |
| HS | 14 | 24 | 10 | 21 | 12 | 17 | 7 | 17 | 7 | 17 |
| SZ | 12 | 12 | 11 | 10 | 9 | 5 | 12 | 6 | 12 | 6 |
Note: HS: Healthy Subjects; SZ: Schizophrenia/Schizoaffective disorder
Serological status of SZ and HS included in the neuropsychological study at baseline (χ2=2.63, df 3, p=0.45), 6 months (χ2=3.23, df 3, p=0.36) and at 1 year (χ2=1.70, df 3, p=0.64) was not different.
Serological status of subjects within the neuroimaging study were not significantly different between the groups (χ2=6.17, df 3, p=0.1).
Neuroimaging methods
Both baseline and follow-up scans were acquired on GE 1.5T whole-body scanner using the same protocol: three-dimension spoiled gradient recalled (SPGR), steady-state pulse sequence (124 coronal slices, 1.5 mm thickness, TE=5 msec, TR=25 msec, acquisition matrix=256×192, FOV=24 cm, flip angle=40°). None of the images in this cohort had motion artifacts.
Cross-sectional voxel-based morphometry was implemented to compare HSV1 seropositive and seronegative subjects within each diagnostic group using the Statistical Parametric Mapping, 2 (SPM2) (16) to further examine our previous findings on an expanded sample (1). The regions-of-interest (ROI) were specified a priori based on our previous observations on Brodmann areas (BA) 8, 9 and 32 using the Wake Forest University PickAtlas toolbox for SPM2 (17) with regional definitions (18). Using AlphaSim (19), we conducted 10,000 Monte Carlo simulations using our imaging parameters and ROI masks to estimate combined voxel-extent and α threshold that would provide corrected experiment-wise error rate at p<0.05 from >64,500 voxels across both hemispheres. This approach is more powerful than intensity α threshold only because the combined α threshold and voxel extent threshold is less likely to be false positive according to the random field theory. The simulations indicated that a combined voxel extent threshold of 78 voxels and an uncorrected α threshold/voxel of p=0.005 was sufficient to keep the corrected experiment-wise rate at p<0.05. Volumes were extracted from above-mentioned regions by creating masks.
Longitudinal image processing poses challenges because of global morphological changes over time. We implemented deformation fields analysis (DFA) on SPM2 to examine longitudinal GM changes (20). For longitudinal studies, DFA is superior to ROI measurement at two time-points because the method offers sensitivity to overall minimal changes and specificity to ROI volume change in the context of other brain region changes. Besides, ROI measurements are labor-intensive and error-prone owing to differences in local anatomical features and rater-drift. DFA reduce errors by applying uniform criteria across all subjects, examining the entire brain simultaneously and are sensitive to changes in volumes up to 0.5% (21). Unique subject-specific morphology and complexity of brain structure are used to achieve accurate matching and estimation of deformation fields. Therefore, instead of measuring ROI volumes at baseline and follow up, we used a sensitive previously validated computational approach (20). Age at baseline and year 1, sex and SES were the covariates. Baseline and follow-up scans from each group (SZ HSV1+/−; HS HSV1+/−) were evaluated in separate paired t-test designs. All results were corrected for multiple comparisons using false discovery rate (FDR) approach. Briefly, customized priors were created followed by whole-brain templates using scans from both baseline and follow-up. Customized GM templates were then, created by normalizing each original image onto the whole-brain template followed by segmenting, averaging and smoothing. Next, GM loss was estimated subject-by-subject by transforming the follow-up scan so that it is identical to the first one. The estimation is based on individual high-dimensional warping of the follow-up image onto the baseline image. The Jacobian determinants of the resulting deformation field contain an estimate of the change in volume between the two images. The estimated baseline data multiplied by the Jacobian determinant is an estimate of the follow-up volumetric data. We conducted post hoc multiple regression analyses by including age, sex and SES as covariates, to examine the correlation between change in WCST scores and the change in GM volumes over 1 year using the Jacobian images that contain the deformation data on all subjects first and then within each group.
Neuropsychological evaluations
The executive functions were evaluated at baseline, 6 months and 1 year using WCST (Table 1). We selected the number of categories completed and perseverative errors as indices of executive functions.
We conducted stratified analysis to examine changes in the number of categories completed and perseverative errors over the follow-up period using binomial mixed-effects growth models. Mixed-effects models were used instead of repeated measures ANOVA because they allow for more flexible and realistic longitudinal structure of longitudinal data (22), and provide a more accurate estimation of missing data than last observation carried forward (23). This method uses subject’s own data to generate robust maximum-likelihood parameter estimates in the presence of missing data, thus avoiding potentially biased imputations. Our choice of the statistical method was further motivated by its acceptability to the FDA (23). Binomial models were used due to the non-normal and highly skewed distribution of WCST scores that could not be handled by non-linear transformation as is common on neuropsychological tests. Therefore, data were dichotomized by median split (>9 perseverative errors and >5 categories completed) and are presented as the percentage of subjects exceeding the median. We accepted missing neuropsychological data points of up to 30%. Decreasing the missing data rate to <30% led to having fewer n in each cell with loss of power. We included CMV exposure, age, sex and SES as covariates.
Immunological assays
To test for viral exposure, IgG antibodies to HSV1 and CMV were assayed with solid-phase enzyme immunoassay directed at the HSV1/CMV specific gG1 glycoprotein (3, 4). Assays using different microtiter plates were standardized by using reference samples on each assay plate. Antibody titers were quantified as signal/cutoff ratios. Subjects with antibody ratios ≥1.3 were considered HSV1 seropositive and those with <1.3 were considered HSV1 seronegative.
Results
Demographic and clinical characteristics
At baseline, the sample comprised of 26 subjects with SZ (64% male; mean age 23.59±7.82 years; illness duration -the duration between the onset of first psychotic symptom to the time of evaluation: 2.12±2.26 years) and 38 HS (55% male; mean age 23.05±4.85 years). The majority of subjects were available for follow-up (Table 1). SZ subjects differed from HS with regard to SES (SZ, 36.06±12.93; HS, 44.80±10.44, p=0.004) but not on age, sex or serostatus at baseline, 6 months and 1 year. The positive (28.62±10.77 vs. 24.00±11.05) and negative (40.85±8.07 vs. 46.85±40.85) symptom severity between HSV1 seropositive and seronegative subjects at baseline (all p>0.11) and at follow-up (positive symptoms 7.60±6.79 vs. 9.80±7.12; negative symptoms, 39.00±11.40 vs. 33.60±9.42) (all p>0.37) were not different. Age, sex, illness duration, or BPRS/SANS/SAPS scores did not differ between individuals followed up for 1 year or not (all p>0.31). However, individuals not available at 1-year did have lower SES (p=0.028).
MRI studies were completed by 18 SZ subjects and 24 HS. There were no significant differences in the mean age (SZ, 24.0±6.82 years; HS, 23.75±6.09 years), sex or SES between subjects in the MRI study. Age, SES, sex, illness duration, and BPRS/SANS/SAPS scores did not differ between participants included or excluded from the imaging analyses (all p=0.4).
Structural imaging
Cross-sectional morphometric studies
In the entire sample (cases + controls), we did not observe a diagnosis-by-HSV1 status interaction on GM differences at baseline or follow-up. When SZ and HS were analyzed separately, SZ subjects seropositive for HSV1 showed decreased BA 9 and 32 GM volumes compared to seronegative counterparts at baseline (HSV1− 4.92±0.20 cc, HSV1+ 4.50±0.14 cc; t=3.23, p=0.005) and at follow-up (HSV1− 4.67±0.28 cc, HSV1± 4.26+0.24 cc; t=5.18, p=0.00009) but not HS (both p>0.16). The GM reductions across HSV1 status were not different between SZ and HS.
Longitudinal morphometric studies
The DFA revealed that SZ subjects as a group showed reduction in the bilateral posterior cingulate gyri (PCG) (left, T=11.43, FDR p=0.002; right, T=8.49, p=0.005) over 1 year. The estimated PCG GM volume reduction at 1 year compared to baseline was 0.82 cc on the left and 0.18 cc on the right PCG. HS did not show significant GM changes in any region during the same period.
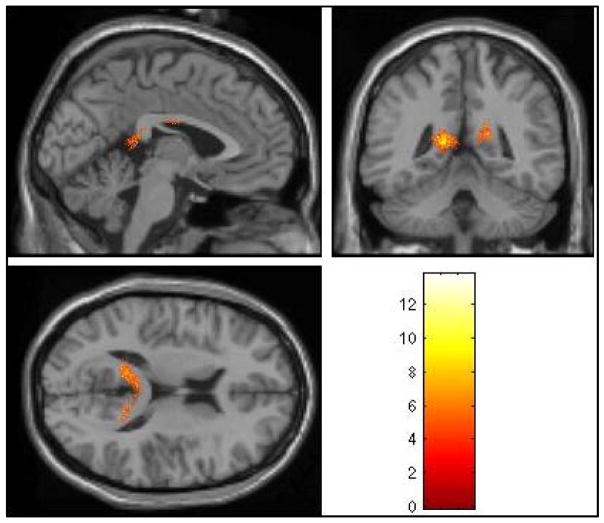
Within HSV1 seropositive SZ subjects, the DFA revealed significant left PCG GM volume reductions at 1 year compared to baseline (T=13.77, FDR p=0.034) and a trend for the right PCG (T=9.93, FDR p=0.07) (Fig 1). The estimated GM volume reduction was 1.6 cc on the left PCG and 0.66 cc on the right over 1 year relative to the baseline. Seronegative SZ subjects and HS regardless of the HSV1 status did not show significant GM loss during the same period.
Fig. 1.
Longitudinal changes in grey matter volume among HSV1 seropositive SZ subjects. In the whole brain analysis, the left posterior cingulate gyrus showed significant grey matter volume loss over 1 year after correcting for multiple comparisons using the false discovery rate (FDR) approach (T=13.77, FDR p=0.034). The grey matter volume in the right posterior cingulate gyrus showed a trend toward significance after correcting for multiple comparisons (T=9.93, FDR p=0.07).
Executive functions
WCST Categories completed
Using the mixed effects model, we evaluated the main effects of diagnosis, follow-up duration, and HSV1 and CMV status separately. Next, diagnosis-by-HSV1, diagnosis-by-time interactions, and three-way diagnosis-by-serostatus-by-time interactions were examined separately for HSV1 and CMV (Table 2).
Table 2.
Mixed model analysis of cognitive variables in relation to serological status
| Categories completed | Perseverative Errors | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| B | SE | P | B | SE | p | |
| Intercept | 0.84 | 2.21 | 0.706 | −4.01 | 3.31 | 0.229 |
| Age | 0.02 | 0.05 | 0.741 | 0.05 | 0.07 | 0.481 |
| Sex | −1.00 | 0.60 | 0.099 | 1.55 | 0.89 | 0.088 |
| SES | 0.06 | 0.02 | 0.011 | −0.02 | 0.04 | 0.618 |
| Diagnosis | −2.20 | 0.96 | 0.026 | 4.74 | 1.71 | 0.007 |
| Follow up duration | 1.75 | 0.74 | 0.020 | −1.99 | 0.82 | 0.017 |
| CMV | 0.44 | 0.83 | 0.597 | 1.38 | 1.17 | 0.244 |
| HSV1 | −1.56 | 0.83 | 0.067 | 0.85 | 1.18 | 0.476 |
| Diagnosis x Follow up duration | −0.99 | 1.04 | 0.345 | −0.74 | 1.99 | 0.710 |
| Diagnosis x CMV | −0.39 | 1.19 | 0.745 | −1.38 | 1.81 | 0.448 |
| CMV x Follow up duration | −3.97 | 1.10 | 0.0005 | −0.78 | 1.80 | 0.666 |
| Diagnosis x HSV1 | 2.09 | 1.24 | 0.097 | −3.63 | 1.99 | 0.073 |
| HSV1 x Follow up duration | 4.05 | 1.35 | 0.004 | −3.62 | 2.11 | 0.089 |
| Diagnosis x Follow up duration x CMV | 4.40 | 1.36 | 0.002 | 1.16 | 2.29 | 0.614 |
| Diagnosis x Follow up duration x HSV1 | −5.91 | 1.59 | 0.0004 | 5.57 | 2.79 | 0.042 |
In the entire sample, a main effect of diagnosis and time suggested that SZ subjects completed fewer categories than HS and that the subjects (cases +controls) completed more categories over 1 year. A trend for HSV1 serostatus (p=0.067) suggested that the HSV1 exposed subjects (cases + controls) completed fewer categories compared to unexposed subjects. A significant time-by-HSV1 status and time-by-CMV status interactions suggested that the subjects (cases + controls) completed fewer categories when exposed to either HSV1 or CMV compared to those unexposed.
Within the diagnostic groups, the number of HSV1 negative SZ subjects who could complete the median number of categories for the sample (>5) increased by 57% over 1 year. In contrast, the number of seropositive SZ subjects who completed >5 categories fell by 40% at 1 year. HS did not show such changes possibly because of their near-ceiling performance at baseline (Fig 2). CMV seropositive subjects showed a similar pattern during the follow-up (data not shown). The 3-way interaction (time*diagnosis*CMV status) was significant (p=0.002). After including the CMV association in the model, the 3-way interaction (time*diagnosis*HSV1 status) remained highly significant (p=0.0004) (Fig 2). These models accounted for baseline differences as well as variance contributed by CMV status.
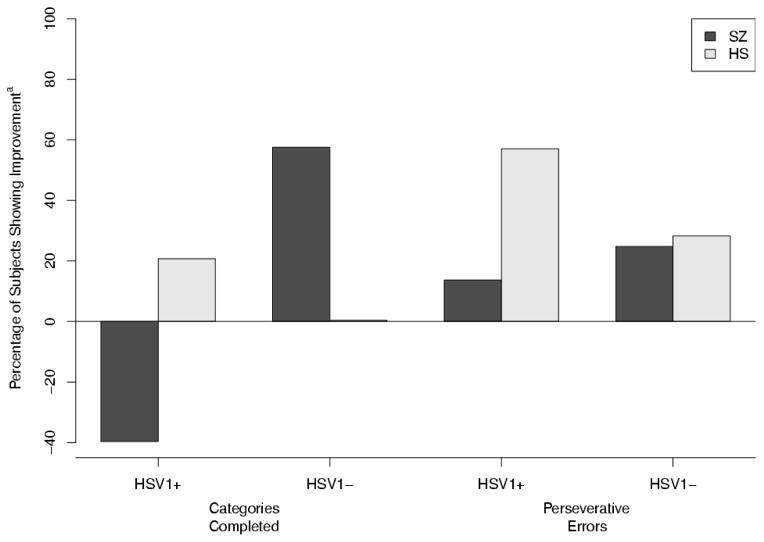
Fig. 2.
Longitudinal changes in executive function measures.
Baseline represents the median split for number of categories completed (>5) and the number of perseverative errors (>9). Bars for SZ (black) and HS (grey) represent the percentage of subjects performing above or below the median split at 1 year compared to baseline. Over 1 year, the percentage of HSV1 negative SZ subjects who could complete >5 categories increased by 57% whereas the percentage of seropositive SZ subjects who completed >5 categories fell by 40%. Similarly, the percentage of seronegative SZ subjects who committed <9 perseverative errors increased by 24.81% compared with 13.70% in seropositive SZ subjects at 1 year compared to baseline.
aImprovement was defined as movement from below-median performance at baseline to above-median performance at 1-year follow-up. Negative values indicate movement from above-median performance at baseline to below-median performance at year 1. Perseverative errors were transformed so that higher scores indicated improvement.
WCST Perseverative errors
A separate mixed model examined the main effects and interactions in the same manner as the categories completed (Table 2). In the combined sample (cases + controls), we observed main effects of diagnosis and time. SZ subjects committed more errors compared to HS, and over time all subjects committed fewer errors. We did not observe main effect of CMV (p=0.2) or HSV1 status (p=0.5). There was a trend for HSV1 status-by-diagnosis (p=0.07) and HSV1 status-by-time (p=0.089) but not diagnosis-by-CMV status interactions. Individuals (cases + controls) exposed to HSV1 committed more errors compared to those unexposed.
Within the diagnostic groups, over 1 year, the number of HSV1 seronegative SZ patients who committed less than the median number of errors for the sample (<9) increased by 24.81% compared with 13.70% in HSV1 seropositive SZ patients (Fig 2). CMV seropositive subjects did not show such associations. The final model that accounted for baseline differences and CMV exposure showed a significant 3-way interaction (time*diagnosis*HSV1 status; p=0.042) indicating greater differential reduction in perseverative errors among HSV1 seronegative patients compared to seropositive patients.
Correlation of Change in Executive Function with Change in GM volumes
Post hoc multiple regressions on the combined sample (cases + controls) showed significant correlation of change in perseverative errors over 1 year with change in PCG volume (Pearson r=0.604, p=0.0002). These observations were most prominent among HSV1 seropositive SZ subjects (Pearson r=0.73, p=0.007; Fig 3). We did not observe significant correlations with the number of categories completed.
Fig. 3.
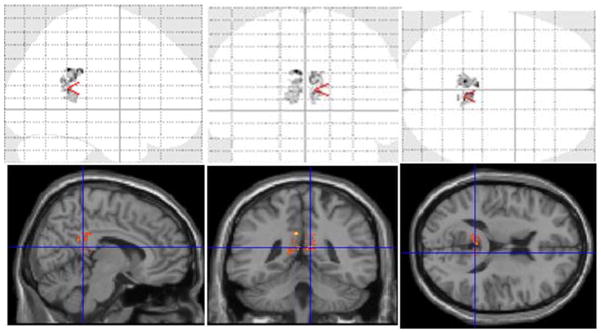
Correlation of change in posterior cingulate cortex volume with change in perseverative error scores over 1 year among HSV1 positive SZ patients (Pearson r=0.73, p=0.007).
Discussion
We observed significant PCG GM loss over one year among HSV1 seropositive SZ subjects. Consistent with the volume loss, more HSV1 seropositive SZ subjects showed poorer performance on executive functions over 1-year compared to HSV1 seronegative SZ subjects. Specifically, more HSV1 seropositive SZ subjects completed fewer WCST categories and committed proportionately more perseverative errors compared with HSV1 seronegative cases. Although CMV exposure was associated with significant reduction in the number of categories completed (not perseverative errors), it did not account for the HSV1-associated changes. The neuropsychological and the structural MRI findings appear to be convergent. The PCG is thought to be involved in the regulation of executive control and working memory (24). However, structure-function relationship can be complex (25). Previous follow-up studies on HSV1 encephalitis survivors indicate continued GM loss and cognitive impairments (26). Our pilot study shows for the first time that HSV1 exposure is associated with longitudinal GM loss and executive functions decline without history of acute encephalitis. SZ subjects may be particularly susceptible to such progression.
There are several plausible explanations for the observed changes. In the rodent/rabbit models of central nervous system HSV1 exposure, latent infection and reactivation directly affected function through neuronal death/dysfunction. Neuronal death resulted from apoptosis (27). Neuronal dysfunction during reactivation and latency resulted from modulation of apoptosis (12) and autophagy (13), host cell translational shut-off (28), oxidative damage (29), and/or neurotransmitter alterations (30). Even with peripheral infections, HSV1 could alter neurotransmission through release of cytokines, especially chemokines (31) that may be elevated in HSV1-exposed individuals (31). Human studies support some of these observations (32). These processes occur throughout the life of an infected subject.
The precise reasons for PCG GM loss are unknown. The PCG GM loss in longitudinal SZ studies is inconsistent (33, 34), possibly because sensitive computational approaches were not used. Using DFA, we note longitudinal GM loss in SZ regardless of serostatus. Our previous cross-sectional study did not observe PCG volume changes at baseline for SZ and HS across HSV1 serostatus (1). Another study reported baseline PCG volume reductions among HSV1 positive SZ subjects that correlated with trails test performance (2). In this study, both DFA and extracted volumes show GM loss in HSV1 seropositive SZ but volume loss observed on extracted volumes should be viewed with caution because they are based on rule-based boundaries assigned by the Talairach database and may not precisely represent the absolute anatomy of the regions (17). Longitudinal changes are noted in animals and in successfully treated HSV1 encephalitis patients. In animals, long-term HSV1 exposure followed by acyclovir treatment but not short-term exposure was associated with cingulate cortex neuronal loss (35). Similarly, recovering acyclovir-treated HSV1-encephalitis patients continue to show PCG GM loss (26). HSV1 infection may also activate proinflammatory cytokines (36) that are associated with decreased PCG volume (37). We did not observe longitudinal PFC changes possibly because our relatively small sample of structural scans might have shown significant changes for a region with the largest effect size. For regions with smaller effects (Cohen’s d≥0.2), we need to double the sample size.
The sensitive and specific nature of the serum antibody assays used here reliably indicates prior exposure to the neurotropic agents providing a non-invasive and sensitive proxy for brain infection. The presence of HSV1 DNA in the trigeminal ganglia in human postmortem studies significantly correlates with peripheral evidence of HSV1 infection (38). However, there is no reliable method for directly demonstrating current brain infection; PCR assays detect viral DNA in the cerebrospinal fluid for only a proportion of acute encephalitis patients (39). Further, serological assays cannot precisely indicate the timing or duration of the infection, e.g. prenatal/postnatal exposure. There are no systematic human studies on exposure at different developmental periods and specific differential effects on brain structure/function and cognitive performance. Animal studies suggest that prenatal exposure (particularly within the first few weeks) may be more important than postnatal exposure for SZ risk (40). However, it is unclear whether the same holds for cognitive impairments. It is possible that the HSV1 positive individuals in this study were infected in the prenatal/perinatal period, with the current assays indicating past exposure/recrudescences. Thus, seropositive individuals may reflect the impact of acute and/or chronic exposure, as well as alterations in a number of neurodevelopmental processes. Since several cellular and molecular changes have been demonstrated following HSV1 exposure, a person exposed for longer time can be expected to show more abnormalities.
It is unclear why the HSV1 positive SZ subjects, but not the HSV1 positive HS showed significant longitudinal changes. In contrast to SZ subjects, HS performed optimally at baseline and thus may have greater compensatory reserve. The SZ group may have had more prolonged exposure than the controls. Alternatively, variations in the host immune response or exposure to other infectious agents could explain case-control differences.
Based on existing evidence from cross-sectional studies that showed impaired working memory and executive functions, we hypothesized longitudinal changes in executive functions. Our analyses support this prediction, but there are several limitations. Practice effects might explain part of the variance. Since all subjects took the tests at both time points, it is unlikely that the practice effects solely explain these observations. Specifically, practice effects may explain improved performance for HSV1 negative SZ subjects (Figure 2), but they cannot explain the decline in function observed in the HSV1 exposed subjects. Differences in medications between the HSV1 exposed and unexposed patients may explain some of the results, though the severity of positive and negative symptoms was not different between the HSV1 seropositive groups at baseline and follow up. All subjects evaluated at baseline were not available for follow-up evaluations, but the proportions of seropositive SZ or HS did not change significantly. Further, clinical and demographic characteristics did not differ between those who followed-up and those who dropped out except for SES; all results were controlled for SES differences. Thus, differential attrition may not significantly account for the observed differences.
In conclusion, we report longitudinal changes in PCG GM volume and executive functions among SZ subjects exposed to HSV1, after accounting for variance due to CMV exposure and other potential confounds. Significant changes were not observed for the imaging or cognitive measures among HSV1 seronegative SZ subjects or among the HS. These results are important because of consistent and substantial correlation between cognitive impairments and long-term outcome in SZ. Cohort-based studies can clarify whether these associations reflect an etiological effect of HSV1 exposure. Such studies are justifiable, as effective medications to treat HSV1 infection are available.
Acknowledgments
Grant Support: National Institute of Mental Health (NIMH) MH 72995, NARSAD Young Investigators Award (KMP), NIMH MH 63480 and 07R-1712 from the Stanley Medical Research Institute (VLN).
We thank Drs. Cameron S. Carter MD (CSC), Gretchen L. Haas PhD (GLH), Nina R. Schooler PhD (NRS), the clinical core staff and the Director of the Conte Center for the Neuroscience of Mental Disorders (Dr. David A. Lewis, MD: MH45156) for their assistance in diagnostic and psychopathological assessments.
Footnotes
This paper was presented at the 65th Annual Meeting of the Society of Biological Psychiatry, May 20–22, 2010, New Orleans, LA
Disclosures: None of the authors discloses any conflicts of interests.
Conflict of Interest
None of the authors have any conflict of interests to declare that would impinge on the design, execution, analysis or reporting of this study.
Bibliography
- 1.Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12(1):105–113. doi: 10.1038/sj.mp.4001915. [DOI] [PubMed] [Google Scholar]
- 2.Schretlen DJ, Vannorsdal TD, Winicki J, Mushtaq Y, Hikida T, Sawa A, Yolken RH, Dickerson FB, Cascella NG. Neuroanatomic and Cognitive Abnormalities Realted to Herpes Simplex Virus Type 1 in Schizophrenia. Schizophr Res. doi: 10.1016/j.schres.2010.01.008. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60(5):466–72. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- 4.Shirts BH, Prasad KM, Pogue-Geile MF, Dickerson F, Yolken RH, Nimgaonkar VL. Antibodies to cytomegalovirus and Herpes Simplex Virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2–3):268–74. doi: 10.1016/j.schres.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14(1):1–21. [PubMed] [Google Scholar]
- 6.Aleman A, Hijman R, de Haan EHF, Kahn RS. Memory Impairment in Schizophrenia: A Meta-Analysis. Am J Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 7.Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57(9):907–13. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 8.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Bora E, Gokcen S, Kayahan B, Veznedaroglu B. Deficits of social-cognitive and social-perceptual aspects of theory of mind in remitted patients with schizophrenia: effect of residual symptoms. J Nerv Ment Dis. 2008;196(2):95–9. doi: 10.1097/NMD.0b013e318162a9e1. [DOI] [PubMed] [Google Scholar]
- 10.Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain Res Cogn Brain Res. 1996;5(1–2):175–81. doi: 10.1016/s0926-6410(96)00054-7. [DOI] [PubMed] [Google Scholar]
- 11.Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25(2):233–55. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- 12.Aubert M, O’Toole J, Blaho JA. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J Virol. 1999;73(12):10359–70. doi: 10.1128/jvi.73.12.10359-10370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orvedahl A, Levine B. Autophagy and viral neurovirulence. Cellular Microbiology. 2008;10(9):1747–1756. doi: 10.1111/j.1462-5822.2008.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delisi LE, Smith SB, Hamovit JR, Maxwell ME, Goldin LR, Dingman CW, Gershon ES. Herpes simplex virus, cytomegalovirus and Epstein-Barr virus antibody titres in sera from schizophrenic patients. Psychol Med. 1986;16(4):757–63. doi: 10.1017/s0033291700011764. [DOI] [PubMed] [Google Scholar]
- 15.Niebuhr DW, Millikan AM, Yolken R, Li Y, Weber NS. Results From a Hypothesis Generating Case-Control Study: Herpes Family Viruses and Schizophrenia Among Military Personnel. Schizophr Bull. 2007 doi: 10.1093/schbul/sbm139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 17.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 18.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 19.Ward BD. In: Simultaneous Inference for fMRI data. Milwaukee WI, editor. 2000. [Google Scholar]
- 20.Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27(4):934–46. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Csernansky JG, Davatzikos C, Fox NC, Frisoni GB, Thompson PM. Computer-assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurol. 2003;2(2):79–88. doi: 10.1016/s1474-4422(03)00304-1. [DOI] [PubMed] [Google Scholar]
- 22.Raudenbush DSW, Bryk DAS. Hierarchical LInear Models: Applications and data analysis methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 23.Siddiqui O, Ali MW. A comparison of the random-effects pattern mixture model with last-observation-carried-forward (LOCF) analysis in longitudinal clinical trials with dropouts. J Biopharm Stat. 1998;8(4):545–63. doi: 10.1080/10543409808835259. [DOI] [PubMed] [Google Scholar]
- 24.Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Ford JM, Gollub RL, White T, Wible C, Belger A, Bockholt HJ, Clark VP, Lauriello J, O’Leary D, Mueller BA, Lim KO, Andreasen N, Potkin SG, Calhoun VD. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Human Brain Mapping. 2009;9999(9999):NA. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demakis GJ. Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, stroop test, and trail-making test. J Clin Exp Neuropsychol. 2004;26(3):441–50. doi: 10.1080/13803390490510149. [DOI] [PubMed] [Google Scholar]
- 26.Markoula S, Giannopoulos S, Pelidou SH, Argyropoulou M, Lagos G, Kyritsis AP. MRI deterioration in herpes simplex encephalitis despite clinical recovery. Neurologist. 2009;15(4):223–6. doi: 10.1097/NRL.0b013e3181921abc. [DOI] [PubMed] [Google Scholar]
- 27.Kraft RM, Nguyen ML, Yang X-H, Thor AD, Blaho JA. Caspase 3 activation during herpes simplex virus 1 infection. Virus Research. 2006;120(1–2):163–175. doi: 10.1016/j.virusres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Feng P, Everly DN, Jr, Read GS. mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J Virol. 2005;79(15):9651–64. doi: 10.1128/JVI.79.15.9651-9664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valyi-Nagy T, Dermody TS. Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histol Histopathol. 2005;20(3):957–67. doi: 10.14670/HH-20.957. [DOI] [PubMed] [Google Scholar]
- 30.Paivarinta MA, Marttila RJ, Lonnberg P, Rinne UK. Decreased raphe serotonin in rabbits with experimental herpes simplex encephalitis. Neurosci Lett. 1993;156(1–2):1–4. doi: 10.1016/0304-3940(93)90424-j. [DOI] [PubMed] [Google Scholar]
- 31.Wuest TR, Carr DJ. The role of chemokines during herpes simplex virus-1 infection. Front Biosci. 2008;13:4862–72. doi: 10.2741/3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill JM, Zhao Y, Clement C, Neumann DM, Lukiw WJ. HSV-1 infection of human brain cells induces miRNA-146a and Alzheimer-type inflammatory signaling. Neuroreport. 2009;20(16):1500–5. doi: 10.1097/WNR.0b013e3283329c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry. 2008;65(7):746–60. doi: 10.1001/archpsyc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58(9):713–23. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 35.McLean JH, Shipley MT, Bernstein DI, Corbett D. Selective lesions of neural pathways following viral inoculation of the olfactory bulb. Exp Neurol. 1993;122(2):209–22. doi: 10.1006/exnr.1993.1121. [DOI] [PubMed] [Google Scholar]
- 36.Oakes JE, Monteiro CA, Cubitt CL, Lausch RN. Induction of interleukin-8 gene expression is associated with herpes simplex virus infection of human corneal keratocytes but not human corneal epithelial cells. J Virol. 1993;67(8):4777–84. doi: 10.1128/jvi.67.8.4777-4784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Kern DM, Tsai WY, Schaefer CA, Brown AS. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121(1–3):46–54. doi: 10.1016/j.schres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill JM, Ball MJ, Neumann DM, Azcuy AM, Bhattacharjee PS, Bouhanik S, Clement C, Lukiw WJ, Foster TP, Kumar M, Kaufman HE, Thompson HW. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82(16):8230–4. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauerbrei A, Eichhorn U, Hottenrott G, Wutzler P. Virological diagnosis of herpes simplex encephalitis. J Clin Virol. 2000;17(1):31–6. doi: 10.1016/s1386-6532(00)00069-x. [DOI] [PubMed] [Google Scholar]
- 40.Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13(3):241–56. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]