Abstract
Adenocarcinoma has become the most common histologic type of lung cancers. Ground glass nodules (GGN), most of them early stage noninvasive or minimally invasive adenocarcinomas (MIA), have been encountered more frequently with the application of computed tomography (CT) screening. The International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) histologic lung adenocarcinoma classification combines radiologic, histologic, clinic, and molecular features to form a diagnostic approach for different subgroups of diseases. One of the major focuses of this new classification is the introduction of adenocarcinoma in situ (AIS) and MIA, to replace the old term of bronchioloalveolar carcinoma (BAC). Not all GGNs are malignant lesions that should be surgically resected upon first presentation. A management approach different to solid nodules has been suggested based on the understanding that these lesions tend to have a more indolent nature. Hasty intervention should be avoided and potential surgical risks, radiation exposure, patient psychology, and socio-economical burden must be balanced comprehensively before surgery is decided upon. In the mean time, surgical issues concerning extent of resection and lymphadenectomy should also be carefully contemplated once intervention is deemed necessary. Extremely good prognosis with a near 100% disease-free survival could be expected when a pure GGN is completely resected. This has led to re-evaluation of sublobar resections, including both segmentectomy and big wedge resection, for small (≤2 cm) less invasive histology (AIS or MIA) appearing as GGN on CT scan. Evidences are accumulating that these limited resections are oncologically equivalent to standard lobectomy. And extensive lymph node dissection may not have additional staging or prognostic benefit. These would add new meaning to the contemporary definition of minimally invasive surgery for lung cancers. Overall, joint effort from a multiple disciplinary team is imperative, and decision making should be based on both anatomical and biological nature of the disease.
Keywords: Lung cancer, adenocarcinoma, histology, surgery
In the past decades, adenocarcinoma has become the most common histologic type of lung cancer. The tendency is similar in western populations as well as in China (1). In the meantime, management for adenocarcinomas of lung has gained significant improvement both in diagnostic and therapeutic approaches. With the application of computed tomography (CT) screening, ground glass nodules (GGN) often corresponding to early stage noninvasive or minimally invasive adenocarcinomas (MIA), which would have been difficult or even impossible to detect on routine chest X-ray, have been encountered more and more frequently in daily practice. On the other hand, only 20-30% of non-small cell lung cancer (NSCLC) patients is diagnosed with stage I-IIIa disease and thus may be amenable to surgical resection. Even for completely resected stage Ia NSCLC, 5-year survival is 73% only, with 27% of patients eventually dying from recurrent disease (2). And currently there is no accurate way to identify patients at high risk of recurrence, and thus would potentially benefit from adjuvant therapies.
With the intention to direct clinical practice as well as future research, the International Association for the Study of Lung Cancer (IASLC), together with American Thoracic Society (ATS) and European Respiratory Society (ERS) recently proposed a new histologic classification system for lung adenocarcinomas (3). Through a multidisciplinary effort, this new classification combines radiologic, histologic, clinic, and particularly molecular features to form a diagnostic approach for different subgroups of lung adenocarcinoma. Highlighted by the introduction of adenocarcinoma in situ (AIS, small adenocarcinomas less than 3 cm in diameter with pure lepidic growth) and MIA (MIA, small solid adenocarcinomas showing predominant lepidic growth with ≤5 mm invasion), these new concepts are now used to replace the ill-defined terminology of bronchioloalveolar carcinoma (BAC), and to define a subgroup of patients who would have near 100% disease-specific survival after complete resection (4). Specific issues have thus been raised concerning surgical indications, approaches, extent of resection and lymph node dissection for early stage NSCLC. Answers to these questions will have to be depended not only on the traditionally anatomical TNM staging, but also on a comprehensive understanding of other prognostic factors including histology, radiologic characteristics, and genetic information of the disease.
Follow-up strategies and timing for intervention
As mentioned above, the new classification represents a joint effort of the IASLC/ATS/ERS working force to define clinical, histologic, radiologic, and molecular subtypes of lung adenocarcinoma. One of the major focuses is on the original term of BAC, a long existing discrepancy between clinical and pathologic identities. On imaging study, BAC most often appears to be a GGN. This refers to a hazy opacity that does not obscure the underlying bronchial structures or pulmonary vessels on CT scan (5). Pure GGN is a lesion with no solid components, whereas part-solid GGN contains both GGN and a solid component. In 1995 Noguchi et al. reviewed 236 surgically resected small (≤2 cm) peripheral adenocarcinomas and proposed a histologic classification of six types based on tumor growth patterns (6). According to this classification, GGN can be found in type A, B and C tumors that show a replacement growth pattern along the alveolar lining cells. The IASLC multidisciplinary force focused on the lepidic subtype defined as a proliferation of atypical cells (type II pneumocytes and Clara cells) along the alveolar walls, without invasion and therefore without alveolar collapse. A good correlation between the ground glass appearance within the tumor and the lepidic component has been established because of persistent air spaces within the alveoli leading to relatively low CT density. According to the new IASLC/ATS/ERS proposal, atypical adenomatous hyperplasia (AAH) and AIS are mostly pure GGN, corresponding to Noguchi types A and B (Figure 1). MIA often appears as part solid GGN, roughly coincides with type C in Noguchi’s classification, as do most lepidic predominant invasive adenocarcinomas (7).
Figure 1.
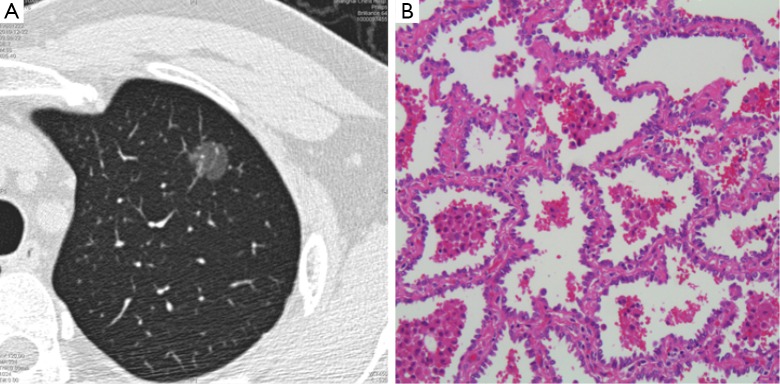
CT image (A) and histologic slide (B) of a 1.4 cm pure GGN resected via VATS lobectomy. Upon histologic examination, the lesion was an AIS purely consisted of atypical cells along the alveolar walls, without invasion or without alveolar collapse (B, magnification is 100×). CT, computed tomography; AIS, adenocarcinoma in situ; GGN, ground glass nodule.
It has not been well defined if all GGNs should be surgically resected upon first presentation, or if under follow-up, when would be the most suitable timing for surgical intervention. It should be reminded that not all GGNs are malignant lesions. They actually include a wide variety of clinical features, including benign conditions, such as focal interstitial fibrosis, inflammation, and hemorrhage (8). Therefore, it is absolutely necessary to understand the natural history of GGNs before embarking on decision making strategies. In the multicentric Italian lung detection (MILD) trial, Silva et al. (9) followed 56 consecutive participants with 76 GGNs among 1,866 individuals identified by CT screening for a mean time of 50.26 months. Fifteen of 48 pure GGNs (31.3%) resolved, 4 of 48 (8.3%) decreased in size, 21 of 48 (43.8%) remained stable, and only 8 of 48 (16.7%) progressed. Among part-solid GGNs with a solid component smaller than 5 mm, 3 of 26 (11.5%) resolved, 11 of 26 (42.3%) remained stable, and 12 of 26 (46.2%) progressed. One of the two part-solid GGNs with a solid component larger than 5 mm remained stable, and the other decreased in size. Only four lung cancers were detected among these GGN subjects, with only one arose from a part-solid GGN which turned out to be in stage Ia. They concluded that progression rate of the GGNs toward clinically relevant disease was extremely low and supported an active surveillance attitude. Kobayashi et al. (10) also followed 108 lesions in 61 asymptomatic individuals for a median observation period of 4.2 years. Their inclusion criteria were: (I) tumor diameter of 3 cm or less; (II) ground-glass opacity proportion of 50% or more; and (III) observation without treatment for 6 months or more. Most of the findings remained stable with observation up to 12 years. Only 29 lesions (27%) increased in size and/or in solid component. All lesions showing growth did so within the first 3 years. Twenty-one patients went on to surgical resection because of growing lesions. Almost half the lesions turned out to be AAH or AIS, with most of the remaining lesions being MIA.
In general, lung adenocarcinomas are thought to follow a linear multistep progression, whereby AAH progresses to AIS and is followed by invasive adenocarcinoma. However, Yatabe et al. suggested a novel scenario for the progression of lung adenocarcinoma, which differs to this linear progression schema (11). Molecular biomarker studies also suggest the possibility, for KRAS-mutated AAH rarely progresses to more advanced tumors, as KRAS mutation is seldom detected in well-differentiated adenocarcinomas (12). This means that some GGNs may remain unchanged (Figure 2). And given the very indolent nature of these abnormalities, some of the malignant lesions might be ‘over diagnosis’ of lung cancer. In Kobayashi’s study (10), 12 resections were for these premalignant lesions, although the authors correctly suggested that GGNs should be observed for at least 3 years before deciding that they would remain stable. Currently there is no worldwide consensus on management strategies for pure GGN. But almost all societies (13-16) propose an approach different to solid nodules based on the understanding that these lesions have a more indolent nature (Tables 1,2). Evidences currently available suggest that surgical intervention for pure GGNs less than 10-15 mm and remain unchanged during follow-up is at least imprudent, if not overtreatment. A comprehensive balance of potential surgical risks, radiation exposure, patient psychology, and socio-economical burden is necessary before surgery is decided.
Figure 2.
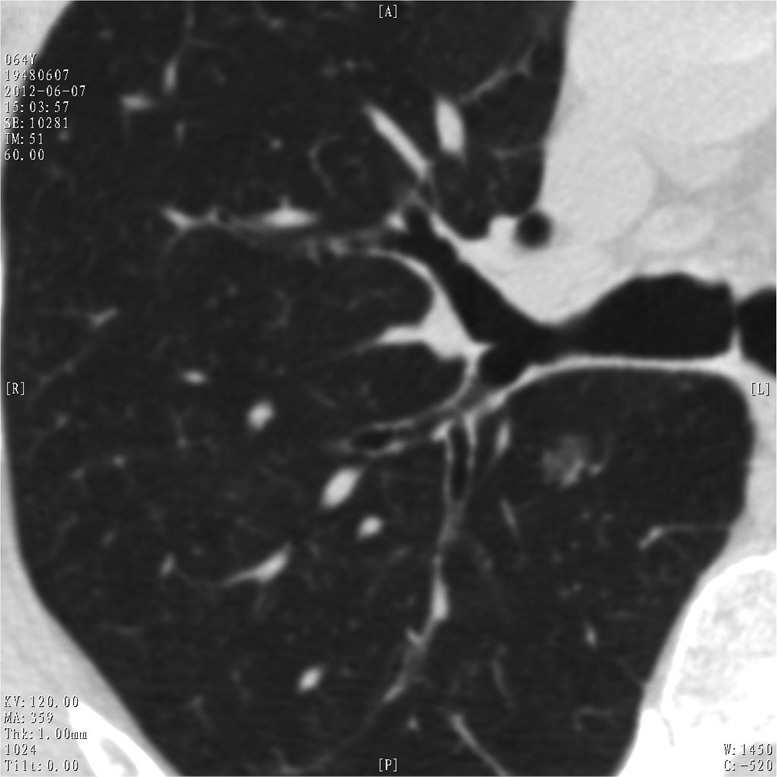
Pure ground glass nodule detected by HRCT on routine physical check-up in a 64-year-old male patient. The lesion showed no change after 6 years follow-up.
Table 1. Recommendations for solitary pure GGN.
| ≤5 mm |
5-10 mm |
10-15 mm |
15-30 mm |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Further evaluation | CT follow-up | Further evaluation | CT follow-up | Further evaluation | CT follow-up | Further evaluation | CT follow-up | ||||
| Fleischner Society (13) | contiguous 1-mm-thick sections to confirm pure GGN | Not required | PET not recommended (limited value and potentially misleading) | 3 months to confirm persistence, then annual surveillance for a minimum of 3 years | Same as 5-10 mm | ||||||
| American Association for Thoracic Surgery (15) | Not mentioned | Annual screen until 79 yr | Not mentioned | 6 months, then annual screen until 79 yr | Not mentioned | 3-6 months, then 6-12 months or intervention | Same as 10-15 mm | ||||
| Japanese Society of CT Screening (16) | Not mentioned | 3, 12, 24 months, then continue | Same as ≤5 mm | Workup | Not mentioned | ||||||
CT, computed tomography; GGN, ground glass nodules.
Table 2. Recommendations for solitary part-solid GGN.
| ≤5 mm |
5-10 mm |
10-15 mm |
15-30 mm |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT follow-up | Further workup | CT follow-up | Further workup | CT follow-up | Further workup | CT follow-up | Further workup | ||||
| Fleischner Society (14) | N/A | At 3 months. If persistent and solid component <5 mm, then yearly for a minimum of 3 years | If persistent and solid component ≥5 mm, then biopsy or surgical resection | Same as 5-10 mm | Consider PET | Same as -10 mm | Consider PET | ||||
| American Association for Thoracic Surgery (15) | Annual screen until 79 yr | Not mentioned | At 6 months. If stable then annual screen until 79 yr | Suspicious change in size or appearance then surgical excision | At 3-6 months. If stable then at 6-12 months, then annually | Biopsy or surgery. Suspicious change in size or appearance then surgical excision | Same as 10-15 mm | ||||
| Japanese Society of CT Screening (16) | N/A | At 3, 6, 12, 18, 24 months if solid component ≤5 mm | Workup if solid component >5 mm | Same as 10-15 mm | May choose follow-up if solid component ≤5 mm | Workup | |||||
CT, computed tomography; GGN, ground glass nodules.
On the other hand, part-solid nodules should follow a more stringent strategy, as solid components on CT scan often represent the invasive histological component (Figure 3). The cut-off value for malignant versus benign pulmonary nodules is often set to be a doubling time of 400 days (17,18). Takashima et al. (19) found the volume doubling time to be for 122 days for squamous cell carcinoma, 384 days for invasive adenocarcinoma, 567 days for AIS, and 988 days for AAH. The same authors also reported a mean doubling time of 149 days for solid nodules, 457 days for part-solid nodules and 813 days for pure GGNs in a 3-year follow-up of 82 malignant lesions (20). Based on these observations, it can be deducted that pure GGNs require a longer follow-up to rule out the possibility of malignancy, whereas part-solid nodules (mixed GGN) should be followed more closely. The IASLC/ATS/ERS work force has suggested measuring the solid component within mixed GGNs in addition to tumor size, taking into account both volume and density of the two components (3).
Figure 3.

CT image of a 2.5 cm mixed ground glass nodule in a 60-year-old female patient. On the third year of follow-up, multiple metastatic nodules in bilateral lungs were detected. CT, computed tomography.
Similar principles should be endorsed when considering the timing of intervention for asymptomatic GGNs. Changes of at least 25% in volume, or over 2 mm in maximal diameter, are deemed significant. If a pure GGN over 10-15 mm does not go away upon follow-up, or even increases in size, surgery is warranted for the purpose of histologic diagnosis and eradication of the lesion. One reason for preference of surgical resection over fine needle biopsy is that the latter has been shown to be quite unsatisfactory in case of GGN. The overall diagnostic yield was merely 51% for GGN dominant lesions (GGN ratio >50%) and only 35% for GGN dominant lesions smaller than 10 mm (21). The other reason favoring surgery rather than biopsy is that extremely good prognosis with a near 100% disease-free survival could be expected when a pure GGN is completely resected (4,6,22). Presence or a newly developed solid component is often another indication for further work-up including biopsy or surgical resection. Listed in Table 3 are the guidelines for intervention of GGNs in different societies (13-16,23). Clearly difference in surgical indication for pure or mixed GGNs reflect the understanding that solid component often represents invasive adenocarcinomas with metastatic potential and thus, should be managed more actively. Although well researched and justified by retrospective data currently available, these guidelines are yet to be verified prospectively by outcome-oriented studies.
Table 3. Comparison of indications for intervention.
| Pure GGNs | Mixed GGNs | |
|---|---|---|
| Japanese Society of CT Screening (16) | Size >15 mm. Increase in size, or newly developed solid component upon follow-up | Surgery when detected |
| American Association for Thoracic Surgery (15) | 5-10 mm: suspicious change in size or appearance upon follow-up; >10 mm: stable upon follow-up | Same as pure GGNs |
| Fleischner Society (13,14) | Size >10 mm and persistent | Persistent and solid component ≥5 mm |
| National Comprehensive Cancer Network (23) | 5-10 mm: increase in size and/or become solid/part-solid upon follow-up; >10 mm: stable, or increase in size and/or become solid/part-solid upon follow-up | 4-8 mm: increase in size upon follow-up; >8 mm: increase in size, or suspicious on PET |
CT, computed tomography; GGNs, ground glass nodules.
Surgical approach and extent of resection
It is based on the understanding of the histologic sub-typing and corresponding oncologic behavior of lung adenocarcinomas that IASLC now offers a comprehensive recommendation for their management. This should be incorporated into surgical treatment of early stage NSCLC, especially in the case of low-grade tumors including AIS, MIA, and invasive adenocarcinomas with a predominant lepidic growth pattern. It is high time to reconsider the current indication and selection of approach or resection extent, which traditionally has been established on anatomical base (TNM staging) only. In 1995, the Lung Cancer Study Group reported a randomized trial in stage Ia (T1N0M0) NSCLC, comparing limited resection in 122 patients (82 segmentectomies and 40 wedge resections) with lobectomy in 125 patients (24). The results showed that compared with lobectomy, limited resection was associated with 75% increase in recurrence (P=0.02), tripling of local recurrence (P=0.008), 30% increase in overall death (P=0.08), and 50% increase in cancer death (P=0.09). Thereafter, lobectomy was established as the standard procedure for stage I NSCLC, while sublobar resection is reserved only for those who could not tolerate lobectomy due to marginal lung function and/or significant comorbidities. Other evidences supporting lobectomy over limited resection were all retrospective, like the one from Warren et al. (25). They found that comparing to lobectomy in 105 stage I (T1-2N0) NSCLC, segmentectomy in 68 patients with similar disease was associated with more frequent locoregional recurrence (22.7% vs. 4.9%). Lobectomy also carried a significant survival advantage over segmentectomy. Looking back at these two studies, it should be noticed that both came from the time when only TNM staging were considered for surgical strategy, and neither mentioned histologic subtypes of their patients. The launch of the new IASLC/ATS/ERS classification signaled a new era that for the first time tumor histology and related biomarkers are introduced into lung cancer prognostic grouping and hence therapeutic decision making. Size of the lesion under investigation should also be taken into consideration, given that in the 7th edition Union for International Cancer Control (UICC) staging system for NSCLC, T1 disease was subdivided into T1a (≤2 cm) and T1b (>2 cm). The inclusion criterion for the Lung Cancer Study Group trial was T1N0M0. And it did not stratify the results between T1a and T1b. In the study by Warren et al., where both T1 and T2 patients were included, the survival advantage for lobectomy over segmentectomy was retained only in the subgroup of T2a tumors >3 cm (P=0.006). Neither in tumors ≤2 cm (P=0.24) nor in tumors 2-3 cm (P=0.58) was any survival difference detected.
Okada et al. (26) did a more detailed study involving 1,272 stage I NSCLC patients. They found that 5-year cancer-specific survivals were similar after lobectomy (92.4%) or segmentectomy (96.7%) when the tumor size was ≤20 mm. Survival after wedge resection was a little lower (85.7%), but the difference was not statistically significant either. For tumors sized between 21-30 mm, survival after wedge resection became significantly lower (39.4%), while there was still no difference between lobectomy (87.4%) and segmentectomy (84.6%). And only in lesions sized >30 mm was survival significantly different among the three procedures (81.3% for lobectomy, 62.9% for segmentectomy but none after wedge resection). Their results suggest that segmentectomy should be distinguished from wedge resection, and limited resections may be acceptable for stage I tumor ≤20 mm. The same authors once carried out a nonrandomized study at three Japanese institutions (27). Two-hundred and sixty-two lobectomies were compared with 305 limited resections, including 230 segmentectomies, for peripheral cT1N0M0 NSCLC sized ≤2 cm. Morbidity (7.3% vs. 6.6%), survival (89.1% vs. 89.6%), overall recurrence rate (17.2% vs. 14.1%), local recurrence (6.9% vs. 4.9%) were all similar between the two groups. They suggested sublobar resection should be considered as an alternative for stage Ia NSCLC. In recent years, several retrospective studies have supported the above observation in the context of minimal invasive surgery (Table 4). VATS segmentectomy for small-sized (diameter ≤2.0 cm) stage Ia NSCLC appeared to be at least comparable to VATS lobectomy regarding surgical outcomes as well as prognosis and local recurrence (28-30).
Table 4. Retrospective studies comparing VATS segmentectomy with VATS lobectomy for stage I NSCLC.
| Author | Procedure | No. | Surgical outcome |
Recurrence |
Survival |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOH (d) | Morbidity (%) | Overall (%) | Local (%) | Overall (%) | Cancer death (%) | |||||
| Shapiro (28) | Lobe | 113 | 4 | / | 17.2 | 3.5 | / | / | ||
| Segment | 31 | 4 | / | 20.4 | 3.6 | / | / | |||
| Yamashita (29) | Lobe | 71 | / | / | / | 5.6 | 84 | 2.6 | ||
| Segment | 38 | / | / | / | 7.9 | 87 | 4.6 | |||
| Zhong (30) | Lobe | 81 | 6.9 | 12.3 | 7.4 | 0 | 83.4 | / | ||
| Segment | 39 | 6.4 | 10.3 | 7.7 | 0 | 88.5 | / | |||
NSCLC, non-small cell lung cancer.
Not only does size but radiologic feature and corresponding histology also matters. Compared with segmentectomy, non-anatomical wedge resection has been reported to be an inferior oncologic approach (31). Yet, clinical results of wedge resection for small-sized (diameter ≤2.0 cm) stage Ia NSCLC is now under reevaluation (26,27). Nakayama et al. (32) studied sublobar resections (49 wedge resections, 14-segmentectomy) for 63 cT1N0M0 adenocarcinomas ≤2 cm in size. Overall survival was 95% for GGN and 69% for solid lesions, while recurrence-free survival was 100% in the former versus 57% in the latter. They suggested that for small air-containing adenocarcinomas on preoperative CT scan, sublobar resection might be acceptable.
We have also investigated the impact of extent of resections on prognosis of early stage NSCLC. This was a retrospective study at three institutions in Japan and China including 173 segmentectomy patients and 181 patients with wedge resection. The results showed that postoperative morbidity was significantly higher after segmentectomy than after wedge resection (11.0% vs. 1.7%, respectively). Overall, lung cancer-specific survivals, as well as local recurrence rates (4.5% vs. 4.4%) were similar between the two groups. No patient with GGN had local recurrence. And lung cancer-specific survival rate at 10 years was significantly better in patients with GGN than in those with solid tumors (98.2% vs. 85.1%, P<0.0001). In multivariate Cox regression analysis, GGN was identified as an independent prognostic factor, while extent of resection had no impact on survival (personal data). Our results support the notion that sublobar resection might be an acceptable procedure for early stage adenocarcinomas that are small and without lymph node involvement. They also suggest that wedge resection may not be inferior to segmentectomy for GGN type tumors.
The necessity of lymph node dissection
Along with the extent of resection is the issue of lymph node clearance. While systemic mediastinal dissection has been accepted as a standard practice for NSCLC surgery, it is not known whether it is truly necessary for early stage adenocarcinomas, especially in those less invasive histologic subtypes. Recently published results from ACSOG Z0030 trial (33) revealed that after mediastinal lymph node dissection, occult N2 disease was found in only less than 4% (21 of 525) clinically N0-1 patients. And there were no significant differences in 5-year disease-free survival (69% vs. 68%), or in local, regional, or distant recurrence after systemic dissection or sampling. We also carried out a randomized controlled trial in 202 patients who were assigned to undergo either skeletonized complete mediastinal lymph node dissection or minimal mediastinal lymph node dissection. Significantly more stations of lymph nodes were harvested through complete dissection (8.9 vs. 6.2, P<0.001). Yet, the pN2 rates (27.1% vs. 24.2%), skip-mediastinal metastasis (9.3% vs. 7.4%), and multi-stational mediastinal involvement (15.0% vs. 16.8%) were all similar between the two groups. And the survival benefit of complete dissection was mainly seen in stage II-III or low-differentiation carcinoma (34).
Accuracy for predicting lymphatic involvement increases significantly when radiologic features are incorporated with tumor size. In the multicenter prospective study to predict pathological noninvasiveness, Suzuki et al. reported that the specificities for pathological invasiveness were 96.4% for adenocarcinomas ≤3 cm with solid component ratio ≤50%, and 98.7% for adenocarcinomas ≤2 cm with solid component ratio ≤50% (35). Cho et al. (36) studied risk of nodal involvement in 770 clinically stage I NSCLC patients. They found that rates of N1 and N2 were merely 5.0% and 4.3% for tumors ≤2 cm, and both were 1.4% for par-solid lesions. What was more, none of the pure GGNs had intrapulmonary or mediastinal lymphatic involvement. Both GGN and tumor size were indentified as independent predictive factors for nodal disease. Nagamoto et al. (37) examined hilar and mediastinal nodal status in 84 patients who underwent systemic dissection. Nodal involvement was found in 6 of 25 tumors sized over 20 mm, but none was detected in 59 tumors <20 mm. All 11 AIS and 4 MIA showed no lymph node metastasis. The authors thus suggested that no lymph node dissection is necessary for AIS or MIA less than 20 mm in size.
Currently there are two ongoing trials assessing the efficacy of limited resection for small, peripheral lung cancers, with the intention to compare extent of surgery for stage Ia NSCLC (JCOG0802 and CALGB140503). However, the inclusion criteria of both of these two trials are still anatomical (stage Ia and ≤2.0 cm). They do not include histology or even radiologic features such as GGN or solid types for their study. Neither is extent of lymph node clearance considered. Therefore, prospective trials based on histologic subtyping are definitely in need to clarify the issue. The importance of such studies lies not in the reviving of procedures less used before, but in adding new meaning to ‘minimally invasive’ lung cancer surgery, in addition to the advantage already brought about by VATS approach.
All in all, diagnosis and management strategies for lung adenocarcinomas have changed greatly in the past decade. The IASLC/ATS/ERS histologic classification depicted a new scenario of the disease and hence suggested a multidisciplinary approach to different subgroups of patients. Specifically for GGN lesions and corresponding histology, joint effort among surgeons, medical oncologists, radiologists, and pathologists is imperative for decision making in follow-up strategy and timing of intervention. Procedure planning based on both anatomical factors and tumor biology would help make future lung cancer surgery truly minimal invasive.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Curado MP, Edwards B, Shin HR, et al. eds. Cancer incidence in five continents, Vol. IX. Lyon: IARC Scientific Publications, 2007. [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [DOI] [PubMed] [Google Scholar]
- 5.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [DOI] [PubMed] [Google Scholar]
- 7.Lederlin M, Revel MP, Khalil A, et al. Management strategy of pulmonary nodule in 2013. Diagn Interv Imaging 2013;94:1081-94. [DOI] [PubMed] [Google Scholar]
- 8.Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics 2007;27:391-408. [DOI] [PubMed] [Google Scholar]
- 9.Silva M, Sverzellati N, Manna C, et al. Long-term surveillance of ground-glass nodules: evidence from the MILD trial. J Thorac Oncol 2012;7:1541-6. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y, Fukui T, Ito S, et al. How long should small lung lesions of ground-glass opacity be followed? J Thorac Oncol 2013;8:309-14. [DOI] [PubMed] [Google Scholar]
- 11.Yatabe Y, Borczuk AC, Powell CA. Do all lung adenocarcinomas follow a stepwise progression? Lung Cancer 2011;74:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto H, Shimizu J, Horio Y, et al. Disproportionate representation of KRAS gene mutation in atypical adenomatous hyperplasia, but even distribution of EGFR gene mutation from preinvasive to invasive adenocarcinomas. J Pathol 2007;212:287-94. [DOI] [PubMed] [Google Scholar]
- 13.Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [DOI] [PubMed] [Google Scholar]
- 14.MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson FL, Austin JH, Field JK, et al. Development of The American Association for Thoracic Surgery guidelines for low-dose computed tomography scans to screen for lung cancer in North America: recommendations of The American Association for Thoracic Surgery Task Force for Lung Cancer Screening and Surveillance. J Thorac Cardiovasc Surg 2012;144:25-32. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines for the Management of Pulmonary Nodules Detected by Low-dose CT Lung Cancer Screening. Available online: http://www.jscts.org/pdf/guideline/ gls3rd_english120719.pdf
- 17.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [DOI] [PubMed] [Google Scholar]
- 18.Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177-84. [DOI] [PubMed] [Google Scholar]
- 19.Takashima S, Sone S, Li F, et al. Indeterminate solitary pulmonary nodules revealed at population-based CT screening of the lung: using first follow-up diagnostic CT to differentiate benign and malignant lesions. AJR Am J Roentgenol 2003;180:1255-63. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [DOI] [PubMed] [Google Scholar]
- 23.Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012;10:240-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [DOI] [PubMed] [Google Scholar]
- 25.Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087-93; discussion 1093-4. [PubMed] [Google Scholar]
- 26.Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [DOI] [PubMed] [Google Scholar]
- 27.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro M, Weiser TS, Wisnivesky JP, et al. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg 2009;137:1388-93. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita S, Chujo M, Kawano Y, et al. Clinical impact of segmentectomy compared with lobectomy under complete video-assisted thoracic surgery in the treatment of stage I non-small cell lung cancer. J Surg Res 2011;166:46-51. [DOI] [PubMed] [Google Scholar]
- 30.Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. [DOI] [PubMed] [Google Scholar]
- 31.Sienel W, Dango S, Kirschbaum A, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728-34. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama H, Yamada K, Saito H, et al. Sublobar resection for patients with peripheral small adenocarcinomas of the lung: surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg 2007;84:1675-9. [DOI] [PubMed] [Google Scholar]
- 33.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Mao T, Gu Z, et al. Comparison of complete and minimal mediastinal lymph node dissection for non-small cell lung cancer: Results of a prospective randomized trial. Thoracic Cancer 2013;4:416-21 [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [DOI] [PubMed] [Google Scholar]
- 36.Cho S, Song IH, Yang HC, et al. Predictive factors for node metastasis in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg 2013;96:239-45. [DOI] [PubMed] [Google Scholar]
- 37.Nagamoto N, Saito Y, Ohta S, et al. Relationship of lymph node metastasis to primary tumor size and microscopic appearance of roentgenographically occult lung cancer. Am J Surg Pathol 1989;13:1009-13. [DOI] [PubMed] [Google Scholar]


