Abstract
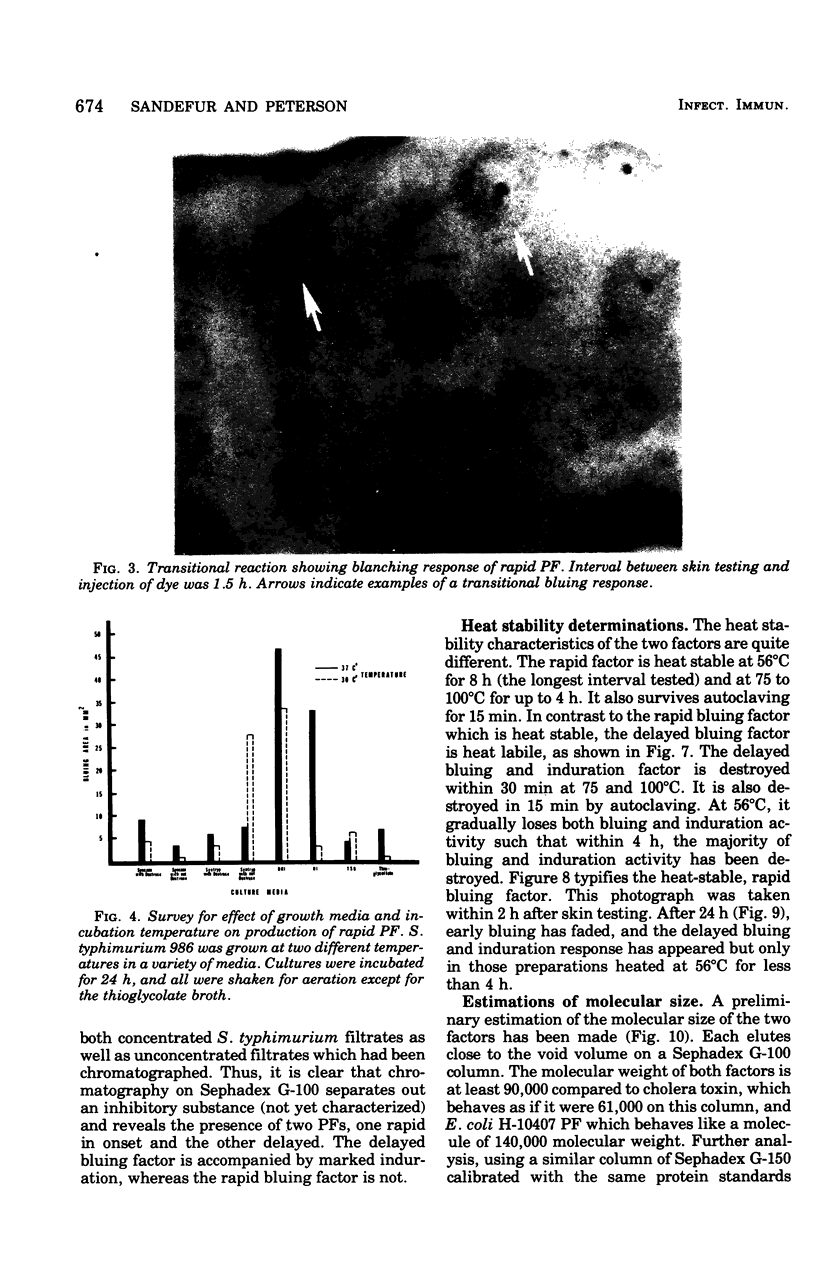
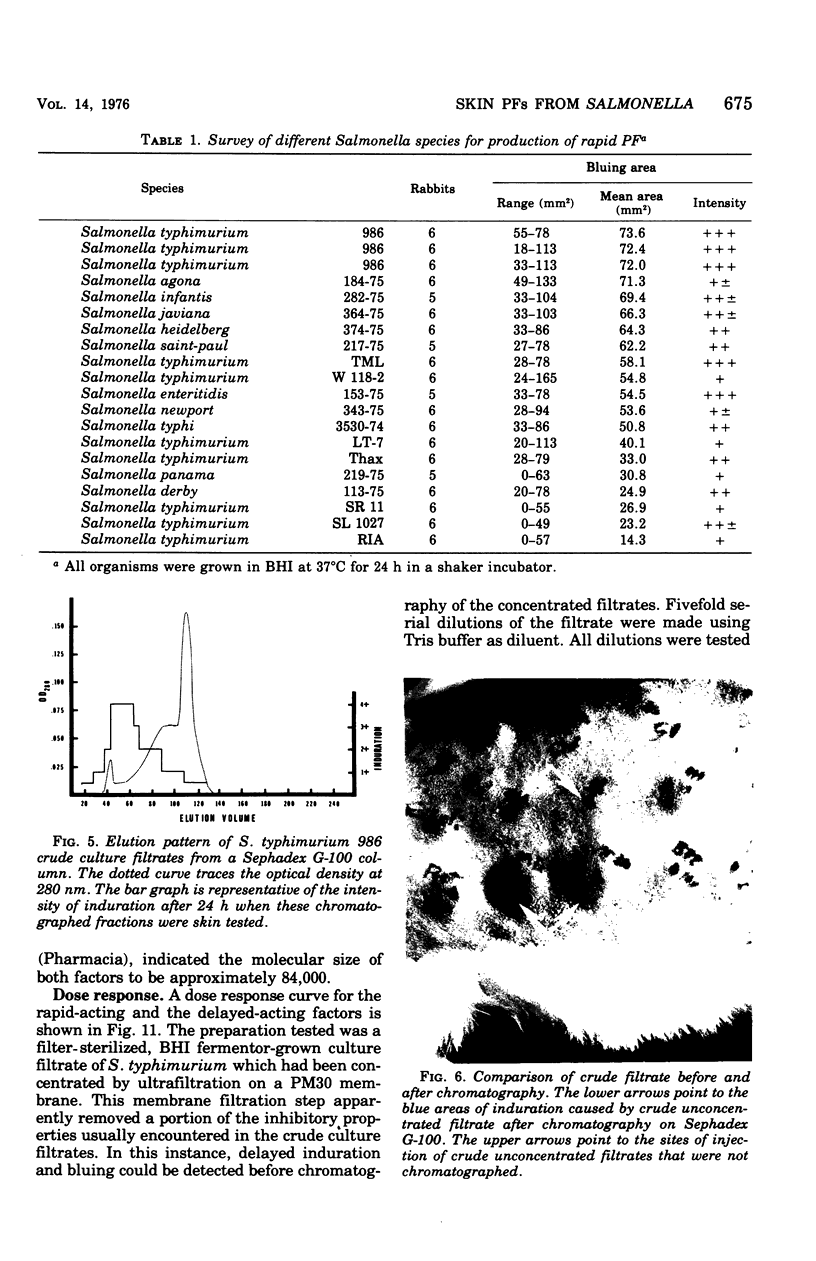
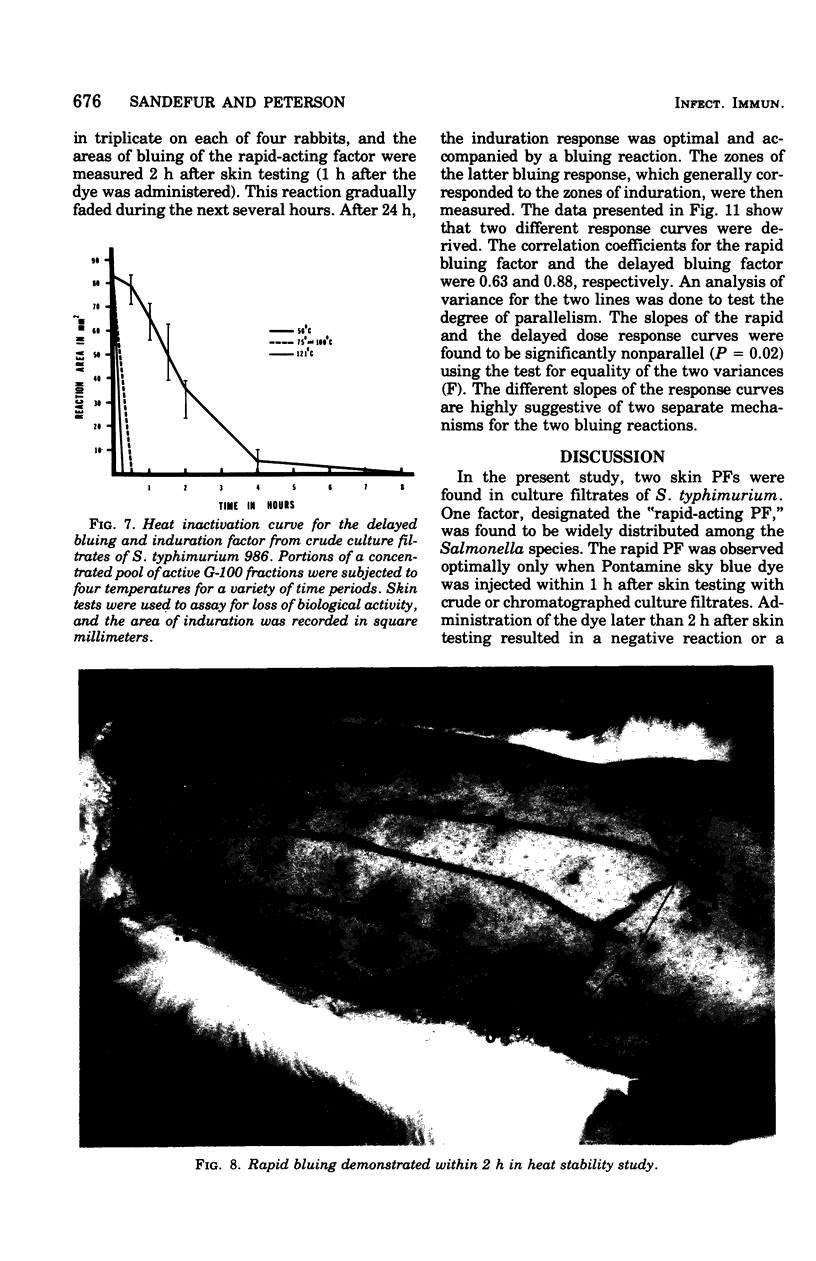
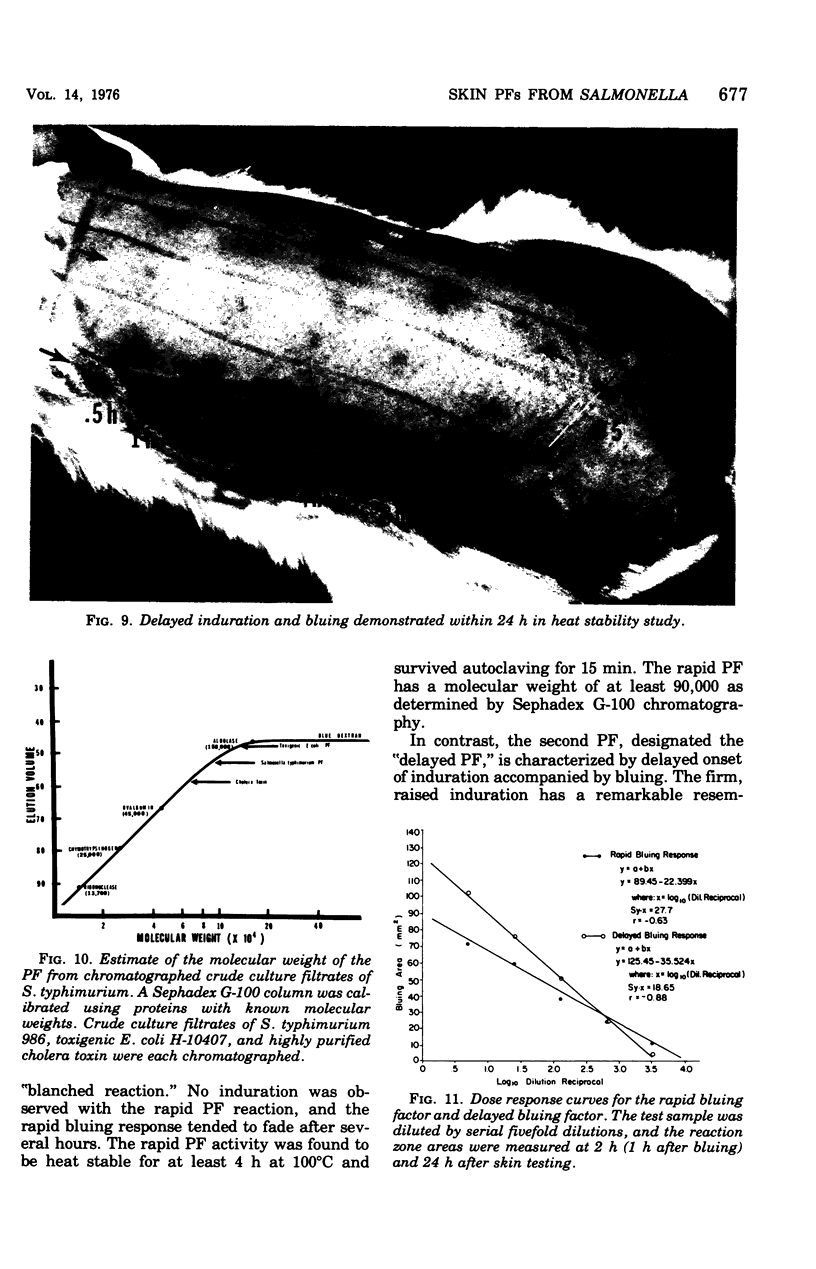
Engerotoxins isolated from Vibrio cholerae and toxigenic Escherichia coli cause permeability alterations in rabbit skin. Firm induration and erythema are observed within 18 to 24 h, and visualization of the reaction may be enhanced by intravenous injection of Pontamine sky blue dye. Two skin permeability factors (PF) have been found in culture filtrates of Salmonella typhimurium. A rapid acting factor, produced optimally in brain heart infusion broth at 37 degrees C by numerous Salmonella species, has a critical bluing time of 1 h after completion of skin testing. This rapid PF is heat stable at 100 degrees C for at least 4 h and has no associated induration. The delayed factor is heat labile, being completly destroyed within 30 min at 75 degrees C and causes marked duration of the rabbit skin within 18 h that is indistinguishable from the permeability reactions of V. cholerae and E. coli enterotoxins. Induration produced by the delayed PF is observed only after chromatography of the culture filtrate on a Sephadex G-100 column. Thus, the effects of the delayed PF appear to be masked or blocked by an inhibitor-like substance present in crude culture filtrates. Both early and delayed factors are estimated to have a molecular weight of at least 90,000. It is postulated that one or both of these factors may participate in the pathogenesis of Salmonella infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axon A. T., Poole D. Salmonellosis presenting with cholera-like diarrhoea. Lancet. 1973 Apr 7;1(7806):745–746. doi: 10.1016/s0140-6736(73)92129-6. [DOI] [PubMed] [Google Scholar]
- Banwell J. G., Sherr H. Effect of bacterial enterotoxins on the gastrointestinal tract. Gastroenterology. 1973 Sep;65(3):467–497. [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Craig J. P., Eichner E. R., Hornick R. B. Cutaneous responses to cholera skin toxin in man. I. Responses in unimmunized American males. J Infect Dis. 1972 Mar;125(3):203–215. doi: 10.1093/infdis/125.3.203. [DOI] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Ileal loop fluid accumulation and production of diarrhea in rabbits by cell-free products of Clostridium perfringens. J Bacteriol. 1969 Oct;100(1):86–94. doi: 10.1128/jb.100.1.86-94.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Gorbach S. L. Identification of enterotoxigenic Escherichia coli and serum antitoxin activity by the vascular permeability factor assay. Infect Immun. 1973 Nov;8(5):731–735. doi: 10.1128/iai.8.5.731-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Formal S. B., Dammin G. J., Collins H. Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J Clin Invest. 1973 Feb;52(2):441–453. doi: 10.1172/JCI107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Grady G. F., Mata L. J., McIver J. The pathogenesis of Shigella diarrhea. I. Enterotoxin production by Shigella dysenteriae I. J Clin Invest. 1972 May;51(5):1212–1218. doi: 10.1172/JCI106915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupal L. R., Deibel R. H. Assay, characterization, and localization of an enterotoxin produced by Salmonella. Infect Immun. 1975 Jan;11(1):14–22. doi: 10.1128/iai.11.1.14-22.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Liu P. V. An enterotoxin of Pseudomonas aeruginosa. J Infect Dis. 1971 Jan;123(1):97–98. doi: 10.1093/infdis/123.1.97. [DOI] [PubMed] [Google Scholar]
- Sakazaki R., Tamura K., Nakamura A., Kurata T. Enteropathogenic and enterotoxigenic activities on ligated gut loops in rabbits of Salmonella and some other enterobacteria isolated from human patients with diarrhea. Jpn J Med Sci Biol. 1974 Feb;27(1):45–48. doi: 10.7883/yoken1952.27.45. [DOI] [PubMed] [Google Scholar]
- Smith N. W., Sack R. B. Immunologic cross-reactions of enterotoxins from Escherichia coli and Vibrio cholerae. J Infect Dis. 1973 Feb;127(2):164–170. doi: 10.1093/infdis/127.2.164. [DOI] [PubMed] [Google Scholar]
- Zinnaka Y., Carpenter C. C., Jr An enterotoxin produced by noncholera vibrios. Johns Hopkins Med J. 1972 Dec;131(6):403–411. [PubMed] [Google Scholar]