Abstract
The new the International Association for the Study of Lung Cancer (IASLC)/the American Thoracic Society (ATS)/the European Respiratory Society (ERS) pathologic classification of lung cancer has markedly changed the pathologic diagnosis of lung adenocarcinoma. This classification deals with many aspects that directly affect clinical practice, and opens new gateways for future research. By means of a multidisciplinary approach, it differs significantly from the former 2004 the World Health Organization (WHO) classification, which was mainly written by pathologist. The present review, in line with the consensus article, is divided in two components: the diagnosis and classification of lung adenocarcinoma in resection specimens and the diagnosis of lung cancer in small biopsies and cytology. Resection specimens are currently classified according to the predominant histologic pattern after comprehensive subtyping in 5% increments. This approach has led to the addition of new pathologic subtypes [adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA) and micropapillary predominant adenocarcinoma)] and to the discontinuation of some heterogeneous entities included in the former 2004 WHO classification (mixed subtype adenocarcinoma and bronchioloalveolar carcinoma). Overall, these changes have resulted in a better stratification of lung adenocarcinoma tumors in more homogeneous morphologic, clinical and biological subgroups. Pathologic subtyping has demonstrated prognostic utility in resected stage I-III patients, and recent data support their predictive role for the benefit of adjuvant chemotherapy. Moreover, comprehensive pathologic subtyping may potentially affect TNM staging and surgical management or early-stage tumors. On the other hand, for the first time, the novel pathologic classification provides standardized terminology and diagnostic criteria of small biopsies and cytology. Criteria are proposed not only for adenocarcinoma but also for other histologies, but special emphasis was put on the distinction between adenocarcinoma and squamous-cell carcinoma due to its major clinical implications. This review outlines the main issues of the new lung adenocarcinoma classification from a clinical perspective. We describe the different pathologic subtypes in resection specimens, with their most relevant clinical implications. Further on, we address the new terminology and diagnostic criteria for lung adenocarcinomas in small specimens, oriented to their importance for the management and treatment of metastatic lung cancer patients. Finally, we discuss some unanswered questions and relevant issues for the near future.
Keywords: Non-small cell lung cancer (NSCLC), adenocarcinoma, the International Association for the Study of Lung Cancer (IASLC)/the American Thoracic Society (ATS)/the European Respiratory Society (ERS) pathologic classification, clinical relevance
Introduction
Lung cancer is the most common cause of cancer-related mortality worldwide. Up to 85% of all cases are classified as non-small cell lung cancer (NSCLC) and adenocarcinoma subtype accounts for more than 50% of these cases in our setting. A better understanding of NSCLC biology has markedly changed treatment paradigms in the past few years, which have led to major improvements in prognosis especially for advanced-stage disease. Lung cancer pathology has played a pivotal role in achieving this success. Histology guided therapies based on pemetrexed and bevacizumab could overcome the therapeutic plateau of 1-year overall survival (OS) in advanced non-squamous tumors (1,2). On the other hand, genotype-based targeted therapies predominately for lung adenocarcinoma spawned a new horizon towards personalized therapy in lung cancer (3). With this background and due to the diverse pathologic spectrum of pulmonary adenocarcinoma, a new histologic classification of lung adenocarcinoma was published in 2011 (4). The new treatment strategies and a better understanding of lung cancer biology led to major changes compared to the previous pathologic classification by the World Health Organization (WHO), last updated in 2004 (5). First, this project recognized the need for a multidisciplinary approach integrating pathologist but also clinicians, molecular biologist, radiologist and surgeons, under the sponsorship of the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS) and the European Respiratory Society (ERS). Second, the new review addressed the issue of management, diagnosis and classification of small biopsies and cytology, which account for nearly 70% of lung cancer specimens in daily clinical practice (4).
Here, we review the new pathologic classification of lung adenocarcinoma with special focus on its relevance for routine clinical practice. As in the original consensus article (4), this review is divided in two components: the classification and description of pathologic subtypes in resection specimens and the diagnosis, management and terminology of small biopsies and cytology specially for the advanced disease setting.
Diagnosis and pathologic classification of resection specimens
Changes to the previous 2004 WHO classification
The high histologic and clinical heterogeneity inherent to lung adenocarcinoma has been one of the major challenges for its pathologic classification. The new approach to subtyping lung adenocarcinoma provides a significant advance over previous classifications, as it permits to stratify the tumors in more homogenous subgroups with morphologically, clinically and biologically meaningful correlations. The most significant change of the new classification was the discontinuation of the term bronchiolo-alveolar carcinoma (BAC). This concept was exclusively based on a distinctive histo-morphologic pattern, but encompassed at least five different entities with disparate clinical and biological characteristics (6), as we will see below. Apart from this conceptual change, other major changes include the following: first, one of the main limitations of previous classifications was the fact that up to 90% of the invasive adenocarcinoma cases fell into the mixed subtype category, which provided little information and had limited clinical applicability. In order to solve this problem, the discontinuation of the term “mixed subtype adenocarcinoma” was proposed. Instead, invasive adenocarcinomas are now classified according to the predominant subtype after comprehensive histologic subtyping, which implies a semiquantitative estimation of the percentage of different subtypes in 5% increments (4). Second, two new entities are introduced for small solitary adenocarcinomas (3 cm) with a lepidic (alveolar/in situ) pattern that either lack of invasion (adenocarcinoma in situ, AIS), or have an invasive component of [5 mm, minimally invasive adenocarcinoma (MIA)]. This means the recognition of AIS as the second preinvasive lesion of lung adenocarcinomas, together with adenomatous hyperplasia (7). Third, micropapillary predominant adenocarcinoma is recognized as an independent subtype. This inclusion was justified due to the poor prognosis shown in previous reports even in resected stage I patients (4). Forth, the use of the term “lepidic predominant adenocarcinoma” for invasive adenocarcinomas with a predominant lepidic growth but with at least one focus of invasion (non-lepidic) measuring >5 mm. Fifth, mucinous BAC tumors are no longer in the same category as non-mucinous BAC. Instead, they are classified as mucinous AIS or MIA and a new term is introduced, invasive mucinous adenocarcinoma (IMA, former mucinous BAC), with distinctive molecular, radiological and clinical behaviors compared to the bulk invasive adenocarcinomas. Hence, the five different entities previously lumped together as BAC include: AIS, MIA, lepidic predominant adenocarcinoma, IMA and advanced-stage adenocarcinomas with a lepidic component (6). Finally, signet ring and clear cell adenocarcinomas are excluded from this classification. These patterns are now recognized as cytologic changes that can be seen in virtually all the histologic subtypes. The current approach is to record any percentage of these particular features instead of requiring any amount to consider them independent histologic subtypes (7). Table 1 summarizes the most important modifications to the 2004 WHO classification that apply to resection specimens and the current pathologic classification of lung adenocarcinoma.
Table 1. Pathology of lung adenocarcinomas according to previous 2004 WHO and current IASLC/ATS/ERS classifications.
| 2004 WHO classification |
| Mixed subtype |
| Acinar |
| Papillary |
| BAC |
| Non mucinous |
| Mucinous |
| Mixed |
| Solid adenocarcinoma |
| Colloid |
| Fetal |
| Mucinous cystadenocarcinoma |
| Signet-ring |
| Clear-cell |
| Major changes in the new IASLC/ATS/ERS classification |
| Discontinuation of the term BAC |
| Discontinuation of the mixed subtype |
| Comprehensive pathologic subtyping in 5% increments and classification of adenocarcinomas according to the predominant subtype |
| Introduction of AIS and MIA as new entities |
| Introduction of micropapillary adenocarcinoma as a predominant subtype |
| Introduction of lepidic predominant adenocarcinoma and lepidic growth as new terminologies |
| Exclusion of signet-ring and clear cell adenocarcinomas |
| IASLC/ATS/ERS classification |
| Pre-invasive lesions |
| Atypical adenomatous hyperplasia |
| AIS |
| Non-mucinous |
| Mucinous |
| Mixed |
| MIA |
| Non-mucinous |
| Mucinous |
| Mixed |
| Invasive adenocarcinomas |
| Lepidic predominant |
| Acinar predominant |
| Papillary predominant |
| Micropapillary predominant |
| Solid predominant with mucin production |
| Variants of invasive adenocarcinomas |
| IMA |
| Colloid |
| Fetal |
| Enteric |
WHO, World Health Organization; IASLC, International Association for the Study of Lung Cancer; ATS, American Thoracic Society; ERS, European Respiratory Society; BAC, bronchioloalveolar carcinoma; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IMA, invasive mucinous adenocarcinoma.
Description of the different pathologic subtypes
Preinvasive or minimally invasive adenocarcinomas
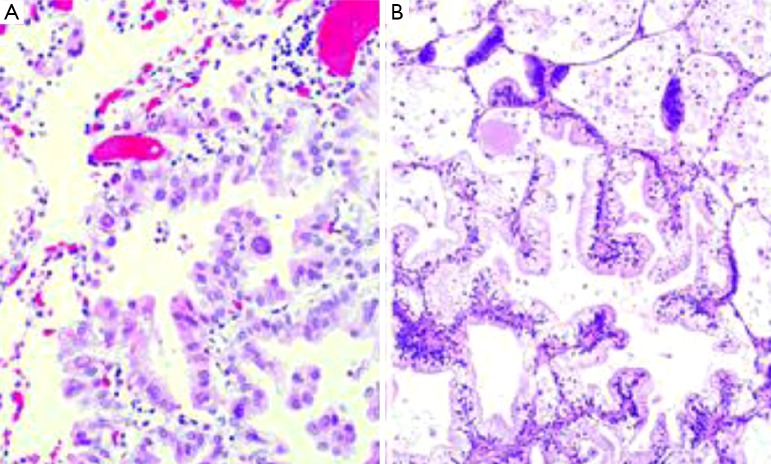
AIS is defined as a small tumor (3 cm) which neoplastic growth is restricted along pre-existing alveolar structure, so called lepidic growth. It lacks stromal, vascular or pleural invasion, and neither papillary or micropapillary patterns nor intra-alveolar tumor cells must contain (Figure 1A). Virtually all AIS are non-mucinous, but rarely may be mucinous (Figure 1B). The distinction between atypical adenomatous hyperplasia (the other preinvasive lesion among pulmonary adenocarcinomas) and AIS can be difficult in resected specimens. Multiple characteristics including size, architectural and cytologic features are needed for a correct diagnosis, but usually atypical adenomatous hyperplasias tend to be small (<5 mm), more cellular, frequently multiple and cytologically atypical (7). From an epidemiological perspective, AIS is the less frequent of lung adenocarcinoma subtypes, accounting for 1-5% of the cases in Caucasian populations (8,9) and 5-8% in Asiatic series (10-15). Although previously lumped together with BAC tumors, it should be noted that the majority of cases diagnosed as BAC were invasive tumors (6). MIA is also a solitary 3 cm tumor with a predominant lepidic growth pattern but containing a small (5 mm) invasive focus. This invasive component shows no lepidic growth but other histologic patterns (acinar, papillary, micropapillary and/or solid), or is defined as tumor cells infiltrating a myofibroblastic estroma. MIAs must lack of lymphatic, blood vessels or pleural invasion, and tumor necrosis must be equally absent. Like AIS, almost all MIA are non-mucinous. Similar prevalence as to AIS has been reported among different series (<8-10% overall), but can vary across different populations (8-15).
Figure 1.
Non-mucinous AIS (A) and mucinous AIS (B) (Hematoxylin-Eosin stain; Magnification is 20×). AIS, adenocarcinoma in situ.
It must be emphasized that complete tumor sampling is mandatory for the diagnosis of AIS or MIA, and thus resected specimens are required for both diagnostics. Sometimes the review of preoperative CT-scans is helpful in order to assess if the lesion has been completely resected or sampled (7). Lepidic growth is usually correlated as ground glass opacity in the CT-scans, while the invasive components are radiologically solid (16).
Invasive adenocarcinomas
Invasive tumors account for 70-90% of resected pulmonary adenocarcinomas, and often consist of multiple heterogeneous mixtures of pathologic patterns. Thus, as previously stated, most adenocarcinomas fell into de mixed subtype in the former 2004 WHO classification (5). With the current comprehensive pathologic subtyping, invasive adenocarcinomas are now classified according to the predominant pathologic subtype. Expert recommendations suggest that the percentage of the non-predominant patterns should also be described in pathologic reports (7).
Lepidic predominant adenocarcinoma shares morphology similarities with AIS and MIA with regard to lepidic growth, but must additionally contain at least one of the following: one focus >5 mm of invasive tumor; gross tumor size >3 cm; invasion of lymphatics, blood vessels or pleura; tumor necrosis. Deserves mentioning that a lepidic growth can be seen in any subtype of invasive adenocarcinoma, invasive mucinous adenocarcinoma and also in metastatic tumors. However, the term lepidic adenocarcinoma is defined as a non-mucinous tumor whose predominant pathologic pattern is a lepidic growth. As in previous classifications there was no quantitative assessment of the lepidic growth (former BAC pattern), some lepidic predominant adenocarcinomas were interspersed within the heterogeneous mixed subtype (6,7).
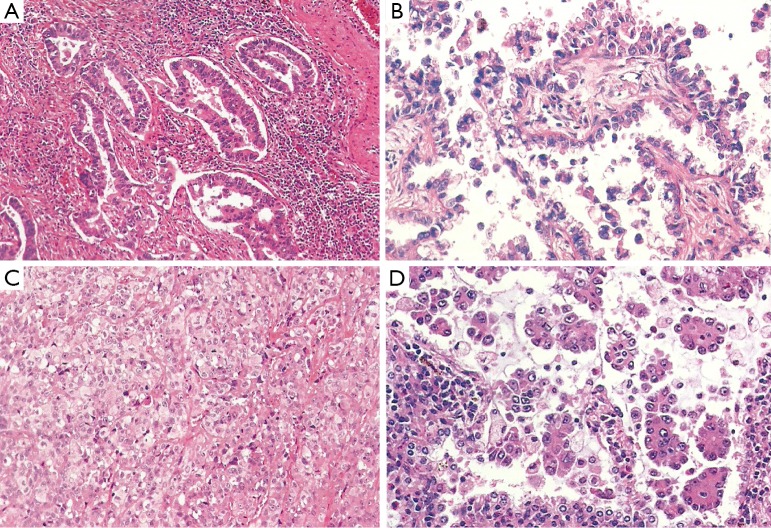
Acinar predominant adenocarcinoma was already contemplated as an independent subtype in former classifications. Morphologically is composed of round glands with the lumen surrounded by tumors cells (Figure 2A). It is probably the most prevalent subtype of pulmonary adenocarcinoma, accounting up to 40% of all invasive adenocarcinoma cases in some series (8). This certainly represents a limitation, since acinar tumors are clinically heterogeneous. In this regard, the presence of a significant amount cribriform pattern has been identified as a subset of acinar tumors with poor prognosis in patients with resected stage I lung adenocarcinoma. Indeed, cribriform predominant tumors (10%) have been proposed as a new pathologic subtype of lung adenocarcinoma (17). Further validation is required before this can be translated into routine pathologic diagnosis and clinical practice.
Figure 2.
Most frequent subtypes of invasive adenocarcinomas. (A) Acinar predominant adenocarcinoma; (B) papillary predominant adenocarcinoma; (C) solid predominant adenocarcinoma; (D) micropapillary predominant adenocarcinoma. (Hematoxylin-Eosin stain; Magnification is 20×).
Papilar predominant adenocarcinoma was included in the previous 2004 WHO classification (5). Morphologically shows glandular cells growing along fibrovascular cores (Figure 2B).
Solid predominant adenocarcinoma shows polygonal tumor cells that form sheets, but no other specific patterns (lepidic, acinar, papillary or micropapillary) are apparent (Figure 2C). This subtype was equally recognized in the former pathologic classification (5). Of note, clear cell and signet ring features are found strongly associated to solid predominant tumors, while they may be apparent in any subtype (7).
Micropapillary predominant pattern was mentioned in the 2004 WHO classification (5), but at that time there were not sufficient data to consider it an independent pathologic subtype (5). Since then, many studies highlighted the poor prognosis and clinical relevance of this tumor, which came with the inclusion as a formal entity in the current classification (4). Morphologically, these neoplasms show tumor cells in papillary tufts lacking fibrovascular cores (Figure 2D).
Variants of invasive adenocarcinomas (VIA)
IMAs are, among VIA, the clinically more relevant tumors. Formerly included as mucinous BAC, they are known to have invasive components in the majority of the cases. They contain tumor cells with globet morphology and abundant cytoplasmic mucin. As previously stated, if they meet the criteria of AIS/MIA, they are regarded as mucinous AIS/MIA. IMAs have distinct clinical, radiological and biological properties. They usually appear as multicentric and multilobar lung tumors, with frequent bilateral lung involvement (usually linked to poor prognosis) (6). Other characteristic CT-scan findings include consolidations and air bronchograms (16). At a molecular level, they consistently show a high prevalence of k-RAS mutations and lack of EGFR mutations (6). Their prevalence is relatively low, not exceeding 5% in the majority of the series (8-10,13-15,17).
The rest of the entities included as VIA are very rare. Colloid and fetal adenocarcinomas are maintained to the previous WHO classifications. Former mucinous cystadenocarcinoma is removed and included within colloid adenocarcinoma. Enteric adenocarcinoma is added as a new variant. It must be distinguished from colorectal carcinoma, with whom shares morphologic and immunohistochemistry features (7).
Reproducibility of comprehensive pathologic subtyping
Warth et al. evaluated a total of 100 consecutive cases of resected lung adenocarcinomas by five pulmonary pathologists and two residents. They found high agreement for pulmonary pathologist [κ= (range, 0.44-0.72)], but lower concordance for inexperienced pathologist [κ= (range, 0.38-0.47)]. Inter-observer variability was significantly higher in cases with more slide numbers available (P=0.028), but was considerably reduced after training. The most difficult patterns to evaluate were papillary and micropapillary patterns (18). Another international inter-observer study reached similar conclusions. The identification of the predominant pattern as well as the distinction of invasive from lepidic growth patterns showed high reproducibility. However, as authors concluded, a better training is required to improve recognition of purely in situ adenocarcinomas (19). Overall, these data indicate that the novel adenocarcinoma classification is reproducible and generally applicable for routine pathologic diagnosis, but should be performed by specifically trained pathologist.
Clinical implications of comprehensive pathologic subtyping in resection specimens for early and locally advanced disease
There are many ways that subtyping lung adenocarcinoma according to predominant pathologic patterns could change the current clinical practice. The first and more obvious is the potential impact this could have on TNM staging. First, measuring invasive tumor size to determine T factor rather than total tumor size (adjusting for lepidic growth) has shown to be an independent predictor of survival in resected patients, and even a better predictor than gross tumor size in several reports (8,11,13,15,20). In line with this, AIS and MIA may be separated from the bulk T1 tumors and regarded as Tis (“in situ”) and Tmi (“microinvasive”) in the next TNM edition (15). And finally, the use of comprehensive pathologic subtyping of multiple lung adenocarcinoma nodules in the same patient may help to determine whether they are intrapulmonary metastases or primary tumors (6).
Probably the most relevant clinical implication is the prognostic and potential predictive role of the predominant pathologic patterns in completely resected lung adenocarcinoma patients. The first study highlighting the prognostic impact of lung adenocarcinoma subtyping was published in 2011. After comprehensive histologic subtyping of 514 surgically resected stage I tumors, three overall prognostic groups/grades were identified according to 5-year disease-free survival (DFS) rates: AIS and MIA were classified in the low-grade group with 100% DFS; intermediate grade comprised non-mucinous lepidic predominant, papillary predominant and acinar predominant, with 90%, 83% and 84% DFS respectively; and high-grade group including solid predominant (70%), micropapillary predominant (67%), IMA (76%) and colloid predominant tumors (71%). Significant differences in DFS were found for each group separately, with 100%, 84% and 71% for high, intermediate and low groups respectively (P<0.001). Multivariate analysis showed that pathologic grading remained as an independent prognostic factor (HR =1.68, P=0.038) for high risk group compared to intermediate/low risk groups). No statistically significant differences were found in OS (P=0.06) (8). Subsequently several reports including resected stage I-III patients have validated these results. Consistently, these studies show that AIS/MIA have survival rates close to 100%. Among invasive adenocarcinomas, lepidic predominant subtype shows the better DFS survival rates, ranging from 75-85% at 5 years. Acinar and papillary subtypes have intermediate prognosis, with some variability in survival rates among different reports (5-year DFS 50-70%). Finally, solid and micropapillary predominant tumors clearly have the worst prognosis, with 5-year survival rates of 30-40% (9-14,17,20-24). In addition, some of these series have validated its prognostic utility also for OS (9,13,14,20,25).
Importantly, a potential predictive role of pathologic subtypes for the benefit of adjuvant chemotherapy has been recently reported in the World Conference of Lung Cancer (WCLC, Sydney 2013). A statistically significant interaction was observed between adjuvant chemotherapy and pathologic subtypes (P=0.007), and patients in the micropapillary and solid subgroup obtained significant DFS benefit from adjuvant chemotherapy (HR =0.58, 95% CI: 0.43-0.80, P<0.001) compared to the patients in the acinar and papillary subgroup (HR =1.12, 95% CI: 0.79-1.59, P=0.53) (25).
The new lung adenocarcinoma classification may potentially impact on the surgical management of pulmonary tumors. It is well known that lobectomy with systematic nodal dissection or lymph node sampling remains the cornerstone of treatment of early-stage NSCLC. However, more limited resections (segmentectomy or wedge resections) may be a treatment alternative for small (2 cm) and peripheral tumors in some cases, but no prospective data are yet available confirming this possibility (26). The prognostic relevance of pathologic subtyping in resected stage I adenocarcinomas raises the hypothesis of whether not only tumor size but also histologic subtypes could guide surgical management of these patients. Nitadori et al. have recently shown that the application of the IASLC/ATS/ERS classification identifies the presence of 5% of micropapillary component as independently associated with the risk of recurrence in patients with small tumors (2 cm) treated with limited resections (HR =3.11, P=0.003) (22). Undoubtedly, these data clearly affect the surgical planning for those patients selected for elective sublobar resections. In routine clinical practice, the sole presence of a solid or partly solid nodule in a CT scan should take aware of elected sublobar resections, since the presence of significant amount of micropapillary component cannot be excluded (27).
Diagnosis in small biopsies and cytology
Changes to the previous 2004 WHO classification
Previous 2004 WHO classification focused exclusively on lung cancer diagnosis in resection specimens (5). Up to 70% of lung cancer patients present with advanced-stage disease at diagnosis, so the primary method for diagnosis in routine clinical practice is obtaining small specimens. Therefore, the definition of homogeneous terminology and criteria for small biopsies and cytology was one of the most important aspects in the new classification. The IASLC/ATS/ERS committee had to make recommendations for all histologic types including other than lung adenocarcinoma, because there were no specific recommendations for the management and diagnosis of small biopsies or cytology. Immunohistochemistry was given high importance for this purpose (4). As the current review is focused primarily on lung adenocarcinoma, we will provide only a few comments on the diagnostic methods and terminology criteria to distinguish adenocarcinoma from squamous-cell carcinoma, with its corresponding clinical implications.
Another important issue highlighted in the new classification was the necessity of a multidisciplinary strategy to optimize the obtaining of the tissue sample, as well as the importance of adequate tissue management for molecular diagnosis. No specific recommendations on these issues were provided because of the high variability in infrastructure and expertise among different centers. However, it was emphasized that each institution should develop a strategy for the correct diagnosis and management of small specimens (4). To provide recommendations on the tissue management or guidelines for molecular testing is not the objective of this review, and have been reviewed elsewhere (28,29).
Diagnostic methods and terminology for small biopsies/cytology
When standard morphologic criteria for either adenocarcinoma (glandular differentiation or the presence of mucin) or squamous-cell carcinoma (keratinization, pearls or intercellular bridges) are present, the diagnosis can be firmly established and no other tools are required. In these situations the WHO diagnostic terms (“adenocarcinoma” and “squamous-cell carcinoma”) can be used. For the particular case of adenocarcinoma, the description of identifiable patterns (lepidic, acinar, papilar, micropapilar, solid, mucinous) when present is recommended. Contrary, when a tumor does not reflect classic morphologic criteria of glandular or squamous features, a limited stain workup must be performed in order to classify them further (4). Current guidelines recommend a judicious use of immunohistochemistry stains to preserve as much tissue as possible for further molecular testing. The use of only one marker for adenocarcinoma and another squamous marker is suggested (28). TTF-1 is probably the most widely used and single best marker for adenocarcinoma. In addition, Napsin A is probably the most specific marker for pulmonary adenocarcinomas. From a practical perspective, probably the most accepted antibody pair is that formed by TTF-1 for adenocarcinoma and P63 for squamous tumors. P40 (the most specific) or CK 5/6 are other accepted markers for squamous tumors (30). Tumors staining for adenocarcinoma markers are termed as NSCLC-favor adenocarcinoma, while tumors positive for squamous markers are classified as NSCLC-favor squamous-cell carcinoma. Apart from immunohistochemistry, cytology combined with biopsy specimens is a powerful tool to classify NSCLC. Tumors that cannot be further classified in spite of these methods remain diagnosed as NSCLC-not otherwise specified (NOS) (4). Ideally, no more than 5% of the cases should be diagnosed as NSCLC-NOS and this term should be used as little as possible. On the other hand, the term large-cell carcinoma should be avoided in this setting. This concept is restricted to resection specimens, where the tumor is completely sampled excluding a differentiated pattern. Finally it can occur that some samples may show morphologic features or immunohistochemical expression of both squamous and adenocarcinoma markers. P63 can be positive in up to 1/3 of adenocarcinomas. Indeed, virtually all tumors showing co-expression of P63 and TTF-1 are adenocarcinomas. The possibility of adenosquamous tumor cannot be completely excluded in certain cases, but this diagnosis can only be established in resection specimens (28). Table 2 summarizes the terminology criteria and diagnostic recommendations for small specimens.
Table 2. Diagnosis, terminology and recommendations in small specimens.
| Pathologic characteristics | 2004 WHO terminology | IASLC/ATS/ERS terminology |
|---|---|---|
| Clear morphologic features present | ||
| Presence of glands or mucin | Adenocarcinoma. Patterns are described if clearly present | Adenocarcinoma. Describe patterns if clearly present. Considerations: AIS/MIA cannot be diagnosed in small biopsies; if pure lepidic pattern/growth seen, add comment on invasive adenocarcinoma cannot be excluded nor assumed |
| Presence of keratinization, pearls or intercellular bridges | Squamous-cell carcinoma | Squamous-cell carcinoma |
| No morphologic features but distinctive positive immunostaining | ||
| TTF-1 (or napsin A) | No specific terminology. Usually diagnosed as solid adenocarcinomas | NSCLC-favor adenocarcinoma |
| p63 (or p40 or CK 5/6) | No specific terminology | NSCLC-favor squamous-cell carcinoma |
| Conflicting results of morphology and IHC (Mixed) | No clear recommendations | Individualize. Considerations: adenosquamous tumors can only be diagnosed in resection specimens with >10% of each component present; almost all TTF-1 and p63 positive are adenocarcinomas |
| No differentiation by morphology or IHC (both negative) | Large-cell carcinoma | NSCLC-NOS. Considerations: avoid the use of the term large-cell carcinoma in small specimens (restricted to resection specimens); this term should be used as little as possible |
WHO, World Health Organization; IASLC, International Association for the Study of Lung Cancer; ATS, American Thoracic Society; ERS, European Respiratory Society; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; NSCLC, non-small-cell lung cancer; NOS, not otherwise specified; IHC, immunohistochemistry.
Clinical implications for the management of advanced-stage patients
Currently, the terminology and tissue processing criteria proposed by the IASLC/ATS/ERS committee are essential for the adequate treatment of patients with advanced stage NSCLC. Histology, and in particular the distinction between adenocarcinoma and squamous-cell carcinoma, guides treatment strategies in metastatic NSCLC patients. Molecular profiling is the first step before choosing a specific therapy for these patients. Patients with somatic EGFR activating mutations and ALK rearrangements have shown dramatic responses with EGFR TKI inhibitors (gefitinib, erlotinib, afatinib) and ALK inhibitors (crizotinib) respectively (31,32). These drugs have changed the natural history of lung cancer patients harboring these genetic abnormalities, and are currently the treatment of choice above chemotherapy in the first-line setting. Currently, such targetable genetic alterations with drugs approved for clinical use are mostly restricted to adenocarcinoma histology. Other somatic mutations linked to adenocarcinoma histology such as k-RAS may have clinical prognostic relevance, but lack of targetable drugs so they are not widely performed in all institutions for daily clinical practice (33). Therefore, according to published guidelines, EGFR and ALK mutation testing is recommended for tumors diagnosed as adenocarcinoma, NSCLC-favor adenocarcinoma and NSCLC-NOS, while squamous tumors are usually excluded from molecular testing (as long as patients are not never smokers) (29). This selectivity has important implications: first, histologic diagnosis is crucial to guide molecular testing; second, reasonable use of classificatory immunostains must be made in order to preserve enough tissue for further genetic testing; and third, in the setting of a tumor with negative adenocarcinoma markers and weak or uncertain presence of squamous markers, is better to classify it as NSCLC-NOS instead of NSCLC-favor squamous-cell carcinoma, in order to give the patient the opportunity for molecular testing (28).
Another implication for clinical practice is whether predominant pathologic subtypes of lung adenocarcinomas can further predict genetic background. The strongest association between predominant patterns and genetic mutations has been shown with IMAs, since a high percentage of these tumors (range, 80-100%) have k-RAS mutations and lack of EGFR mutations (7). Other weaker associations include: negative correlation between the solid and mucinous predominant tumors for EGFR mutations (13,15,34-38); negative correlation between the presence of lepidic components and k-RAS mutations (13); positive correlation between ALK rearranged tumors and the presence of solid signet-ring cell pattern or mucinous cribriform morphology (39,40). However, several reports on this issue have shown that virtually all adenocarcinoma subtypes can harbor these somatic abnormalities (13,15,34-36,38,41). Therefore, predominant pathologic subtypes should not be used to guide molecular testing in daily clinical practice.
Histology also plays a pivotal role in the treatment algorithm of patients lacking of targetable somatic mutations. As it is well known, pemetrexed for efficacy reasons (42) and bevacizumab for safety concerns (bleeding events in squamous histology) (1) are restricted for patients with non-squamous tumors, and this directly affects first and second-line treatment paradigms in these patients. All these issues clearly reflect the pivotal role pathologist play for the correct management of advanced NSCLC patients.
Conclusions and future prospects
The new IASLC/ATS/ERS classification of lung adenocarcinoma has markedly changed the pathologic diagnosis of lung cancer. This classification deals with many aspects that directly affect clinical practice, and opens new gateways for future research. The most relevant finding is that the predominant pathologic subtype within a tumor after comprehensive pathologic subtyping clearly determines prognosis of resected patients, and might even be predictive for the benefit of adjuvant therapies in high-risk patients. The significant interaction observed with regard to the benefit of adjuvant chemotherapy between high-grade tumors (micropapillary and solid predominant) and intermediate/low grade tumors should be further replicated in other ideally prospective series before drawing definitive conclusions. If confirmed, this may have two major implications in the near future: future adjuvant trials should be stratified according to predominant patterns of lung adenocarcinoma; patients with micropapillary or solid predominant tumors may be selected for adjuvant chemotherapy even in stage I. On the other hand, there are several ways the new classification could affect the 8th TNM edition. However, many of the issues regarding TNM classification require future validation before it can be considered by the International Union Against Cancer and American Joint Committees. A wider implementation of CT-scan as a screening method for lung cancer diagnosis will surely increment the diagnosis of small solitary tumors. Prospective data of randomized controlled trials on the ideal surgical method for these patients (lobectomy vs. LR) are awaited. In addition to tumor size, a higher local recurrence observed for tumors with micropapillary pattern raises the need to considerer pathologic subtypes together with tumor size to define the best surgical strategies for these tumors. Radiology will definitely play an important role in this setting. And finally, several druggable molecular alterations are being recently discovered linked predominately to squamous tumors, such as FGFR1 amplifications and PIK3CA and DDR2 mutations (43). This would emphasize even more the need of an accurate distinction between adenocarcinoma and squamous-cell carcinoma to guide genetic testing. Contrary, given the huge expansion and probable wider implementation of the next-generation sequencing technologies for routine practice in the near future, molecular profiling will possibly be implemented for all patients irrespective of histology. Ultimately, in one way or another, the impact of this new adenocarcinoma classification and other upcoming future prospects highlight the importance of a close collaboration between oncologist, pathologist, molecular biologist, radiologist and surgeons in a multidisciplinary approach, in order to offer the best treatment opportunities to our lung cancer patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [DOI] [PubMed] [Google Scholar]
- 2.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [DOI] [PubMed] [Google Scholar]
- 3.Pikor LA, Ramnarine VR, Lam S, et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013;82:179-89. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Müller-Hermelink HK, et al. eds. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004. [Google Scholar]
- 6.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013;31:992-1001. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung adenocarcinoma in resected specimens: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:685-705. [DOI] [PubMed] [Google Scholar]
- 8.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [DOI] [PubMed] [Google Scholar]
- 9.Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [DOI] [PubMed] [Google Scholar]
- 10.Woo T, Okudela K, Mitsui H, et al. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int 2012;62:785-91. [DOI] [PubMed] [Google Scholar]
- 11.Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Wu J, Tan Q, et al. Why do pathological stage IA lung adenocarcinomas vary from prognosis?: a clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol 2013;8:1196-202. [DOI] [PubMed] [Google Scholar]
- 13.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [DOI] [PubMed] [Google Scholar]
- 14.Gu J, Lu C, Guo J, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol 2013;107:474-80. [DOI] [PubMed] [Google Scholar]
- 15.Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [DOI] [PubMed] [Google Scholar]
- 16.Austin JH, Garg K, Aberle D, et al. Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology 2013;266:62-71. [DOI] [PubMed] [Google Scholar]
- 17.Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol 2014;27:690-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warth A, Stenzinger A, von Brünneck AC, et al. Interobserver variability in the application of the novel IASLC/ATS/ERS classification for pulmonary adenocarcinomas. Eur Respir J 2012;40:1221-7. [DOI] [PubMed] [Google Scholar]
- 19.Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [DOI] [PubMed] [Google Scholar]
- 21.Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013;258:1079-86. [DOI] [PubMed] [Google Scholar]
- 22.Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Z, Zhu H, Guo Z, et al. Prognostic value of the IASLC/ATS/ERS classification in stage I lung adenocarcinoma patients--based on a hospital study in China. Eur J Surg Oncol 2013;39:1262-8. [DOI] [PubMed] [Google Scholar]
- 24.Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [DOI] [PubMed] [Google Scholar]
- 25.Brambilla E, Pignon J, Le Chevalier T, et al. Prognostic and predictive value of a new IASLC/ATS/ERS lung adenocarcinoma classification in a pooled analysis of four adjuvant chemotherapy trials: a LACE-Bio study. Oral abstract presented at: WCLC 2013. Proceedings of the 15th World Conference Lung Cancer; 2013 Oct 27-31; Sidney, Australia. (O17.01). [Google Scholar]
- 26.Baltayiannis N, Chandrinos M, Anagnostopoulos D, et al. Lung cancer surgery: an up to date. J Thorac Dis 2013;5:S425-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pass HI. Lung cancer, histologic stratification, and resection extent: something for surgeons to think about. J Natl Cancer Inst 2013;105:1168-9. [DOI] [PubMed] [Google Scholar]
- 28.Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:668-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conde E, Angulo B, Izquierdo E, et al. Lung adenocarcinoma in the era of targeted therapies: histological classification, sample prioritization, and predictive biomarkers. Clin Transl Oncol 2013;15:503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line--is there a difference? J Clin Oncol 2013;31:1081-8. [DOI] [PubMed] [Google Scholar]
- 32.Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol 2013;31:1105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts PJ, Stinchcombe TE. KRAS mutation: should we test for it, and does it matter? J Clin Oncol 2013;31:1112-21. [DOI] [PubMed] [Google Scholar]
- 34.Shim HS, Lee da H, Park EJ, et al. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 2011;135:1329-34. [DOI] [PubMed] [Google Scholar]
- 35.Russell PA, Barnett SA, Walkiewicz M, et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol 2013;8:461-8. [DOI] [PubMed] [Google Scholar]
- 36.Song Z, Zhu H, Guo Z, et al. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol 2013;30:645. [DOI] [PubMed] [Google Scholar]
- 37.Sun PL, Seol H, Lee HJ, et al. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol 2012;7:323-30. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011;35:1226-34. [DOI] [PubMed] [Google Scholar]
- 40.Nishino M, Klepeis VE, Yeap BY, et al. Histologic and cytomorphologic features of ALK-rearranged lung adenocarcinomas. Mod Pathol 2012;25:1462-72. [DOI] [PubMed] [Google Scholar]
- 41.Motoi N, Ishikawa Y, Sato S, et al. Morphological and Mucin profile of lung adenocarcinoma harboring driver mutation. Oral abstract presented at: WCLC 2013. Proceedings of the 15th World Conference Lung Cancer; 2013 Oct 27-31; Sydney, Australia. (O17.03). [Google Scholar]
- 42.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist 2009;14:253-63. [DOI] [PubMed] [Google Scholar]
- 43.Drilon A, Rekhtman N, Ladanyi M, et al. Squamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol 2012;13:e418-26. [DOI] [PubMed] [Google Scholar]