Abstract
Two embryonic cell populations, the neural crest and cranial ectodermal placodes, between them give rise to many of the unique characters of vertebrates. Neurogenic placode derivatives are vital for sensing both external and internal stimuli. In this speculative review, we discuss potential developmental and evolutionary relationships between two placode series that are usually considered to be entirely independent: lateral line placodes, which form the mechanosensory and electroreceptive hair cells of the anamniote lateral line system as well as their afferent neurons, and epibranchial placodes (geniculate, petrosal and nodose), which form Phox2b+ visceral sensory neurons with input from both the external and internal environment. We illustrate their development using molecular data we recently obtained in shark embryos, and we describe their derivatives, including the possible geniculate placode origin of a mechanosensory sense organ associated with the first pharyngeal pouch/cleft (the anamniote spiracular organ/amniote paratympanic organ). We discuss how both lateral line and epibranchial placodes can be related in different ways to the otic placode (which forms the inner ear and its afferent neurons), and how both are important for protective somatic reflexes. Finally, we put forward a highly speculative proposal about the original function of the cells whose evolutionary descendants today include the derivatives of the lateral line, otic and epibranchial placodes, namely that they produced sensory receptors and neurons for Phox2b-dependent protective reflex circuits. We hope this review will stimulate both debate and a fresh look at possible developmental and evolutionary relationships between these seemingly disparate and independent placodes.
Keywords: neurogenic placodes, lateral line, otic, epibranchial, spiracular/paratympanic organ, Scyliorhinus canicula, Eya4, Tbx3, Pax2, Phox2b
The importance of cranial placodes for vertebrate development and evolution
Vertebrate evolution is inextricably bound up with the evolution of two transient embryonic cell populations: the neural crest and cranial placodes. Neural crest cells are neuroepithelial cells that undergo an epithelial-mesenchymal transition and actively migrate into the periphery to form many different cell types, including teeth, cranial cartilage and bone, pigment cells, various endocrine cells, and most of the peripheral nervous system (reviewed in Le Douarin and Kalcheim, 1999). Cranial placodes are transient, bilateral, thickened patches of cranial embryonic ectoderm whose derivatives are vital for sensing both external and internal stimuli. Placodes form the sensory receptors, afferent innervation and support cells of the paired peripheral sense organs (nose, inner ear, lateral line system); the lenses of the paired eyes; cranial somatosensory (trigeminal) neurons; visceral sensory (epibranchial) neurons that transmit a wide variety of information from the heart, gut and other visceral organs, as well as taste buds; and the endocrine adenohypophysis (reviewed in Baker and Bronner-Fraser, 2001; Schlosser, 2006). Different placodes express different genes, are induced by different tissues and molecules, and form different cell types, but they are united by their common origin from a “pre-placodal region” of ectoderm at the cranial neural plate border that expresses Six and Eya genes (reviewed in Baker and Bronner-Fraser, 2001; Bailey and Streit, 2006; Schlosser, 2006). Induction of “pre-placodal” markers may confer competence to respond to subsequent specific placode-inducing signals (Martin and Groves, 2006).
In two seminal papers, Northcutt and Gans proposed (1) that most of the unique characters of vertebrates derive from the neural crest and cranial placodes, in association with a muscularised hypomere, and (2) that these evolved in the vertebrate ancestor in association with a shift from filter-feeding to active predation (Gans and Northcutt, 1983; Northcutt and Gans, 1983; also see Northcutt, 2005a). Thus, the extraordinary variety of neural crest and placode derivatives could theoretically be unified as adaptations to a predatory lifestyle. The most primitive extant craniates, hagfishes and lampreys, have essentially the entire suite of neural crest and placodes, while the presence in the invertebrate chordates of bona fide neural crest and placodes, or even identifiable evolutionary precursors of these cells, is controversial and under active investigation (see Baker and Bronner-Fraser, 1997; Baker and Schlosser, 2005).
In this speculative review, we will discuss potential developmental and evolutionary relationships between two series of placodes that are usually considered to be entirely independent: the lateral line placodes, which form the sensory receptors and afferent neurons of the mechanosensory and electoreceptive lateral line system in anamniotes (input exclusively from the external environment), and the epibranchial placodes, which form visceral sensory neurons (input from both the external and internal environment) and, possibly, mechanosensory receptors in the paratympanic organ.
Lateral line placodes and derivatives
Lateral line placodes, which have been lost in amniotes, are part of a “dorsolateral” series of placodes, primitively three pre-otic pairs (anterodorsal, anteroventral, and otic lateral line placodes) and three post-otic pairs (middle, supratemporal and posterior lateral line placodes) (Fig. 1A; see Northcutt, 1997; Schlosser, 2002b; Gibbs, 2004). The posterior lateral line placode actively migrates along the trunk, depositing mechanosensory neuromasts at intervals; the control of this process has been the subject of much recent investigation in zebrafish (reviewed in Ghysen and Dambly-Chaudière, 2004, 2005). The other lateral line placodes elongate to form sensory ridges in characteristic lines over the head (Fig. 1B). Neuromasts (Fig. 1C) differentiate in the central zones of the sensory ridges, while electroreceptive ampullary organs (Fig. 1E) differentiate in the lateral zones of the sensory ridges (reviewed in Northcutt, 2005b). Neuromasts are displacement detectors of water flow around the animal; they are used in obstacle avoidance, schooling behaviour, and prey detection. They contain axon-less mechanosensory hair cells very similar to those found in the inner ear, each with a kinocilium and a polarised bundle of linked microvilli (stereocilia), whose height decreases with increasing distance from the kinocilium (Fig. 1C,D; reviewed in Fritzsch et al., 2006). They may be superficial or buried in subepidermal canals, which are connected to the surface by pores (Fig. 1C). Ampullary organs comprise collections of axon-less electroreceptor cells (modified hair cells, usually with an apical kinocilium surrounded by microvilli; see Jørgensen, 1989; Gibbs, 2004; Fritzsch et al., 2006) and support cells; they are found either at the surface, or recessed in pores filled with a mucous jelly of very low electrical resistance (Fig. 1E) (reviewed in Gibbs, 2004; Jørgensen, 2005; Bodznick and Montgomery, 2005). The detection of weak electric fields by ampullary organs is used for locating prey, for orientation and (in some species) for communication (reviewed in Bullock, 1982; Bullock et al., 2005). The lateral line placode origin of electroreceptive ampullary organs has only been tested experimentally in axolotls, where grafting and ablation experiments showed that individual lateral line placodes form both neuromasts and ampullary organs (Northcutt et al., 1995). Different embryological origins for electroreceptors have also been proposed. General surface ectoderm was suggested to form catfish (teleost) electroreceptors, based on experiments suggesting that electroreceptor development in this species requires the presence of, or is induced locally by, afferent lateral line axons, while neuromasts develop autonomously from lateral line placodes in the absence of afferent innervation (Roth, 2003). Neural crest cells were proposed to give rise to shark electroreceptors, based on electroreceptor expression of the transcription factor Sox8 and cross-reaction with the HNK1 antibody (Freitas et al., 2006); however, neither marker is specific for neural crest cells. We have recently obtained some descriptive molecular evidence in support of a lateral line placode origin for shark electroreceptors, by showing that in the lesser-spotted dogfish, Scyliorhinus canicula, the transcription co-factor Eya4 is expressed in the lateral line system throughout its development, including in sensory ridges, and also in the ampullary organs forming on either side of the sensory ridges (Fig. 2A-G; O’Neill et al., 2007).
Fig. 1.
Development of the lateral line system. (A) Schematic showing position of lateral line placodes (black) in a 17-day skate (elasmobranch) embryo (modified after Northcutt, 1997). Epibranchial placodes (light grey ovals) are shown dorsocaudal to each pharyngeal (branchial) cleft. (B) Schematic showing distribution of lateral line neuromasts (black ovals), both at the surface and buried in subepidermal lateral line canals, on the head of a juvenile channel catfish (teleost). Modified after Northcutt et al., (2000). (C) Schematic section through a mechanosensory neuromast: the kinocilia and stereocilia of each hair cell project into a gelatinous cupula, secreted by support cells. Redrawn from Baker (2005). (D) Schematic showing a mechanosensory lateral line hair cell bearing a kinocilium and stereocilia, innervated by lateral line neurites, with an adjacent support cell. Redrawn from Baker (2005). (E) Basic structure of an elasmobranch electroreceptive ampullary organ, with electroreceptor cells and support cells recessed in a pore canal filled with a mucous jelly. Redrawn from Cernuda-Cernuda and García-Fernández (1996). (F) Scanning electron micrograph of a lateral line neuromast on the trunk of a stage 49 (12-day) Xenopus tadpole, showing the bundle of long kinocilia with smaller stereocilia at its base. Scale bar: 1μm. Xenopus staging after Nieuwkoop and Faber (1967). (G) Higher-power view of the base of the kinocilia from panel F. Scale bar: 1μm. Abbreviations: ad, anterodorsal lateral line placode; av, anteroventral lateral line placode; io, infraorbital lateral line canal; m, middle lateral line placode; o, otic lateral line placode; ot, otic lateral line canal; ov, otic vesicle; po, posterior lateral line placode; prm, preoperculomandibular lateral line canal; so, supraorbital lateral line canal; sp, spiracle (first pharyngeal cleft); st, supratemporal lateral line placode; t, temporal lateral line canal.
Fig. 2.
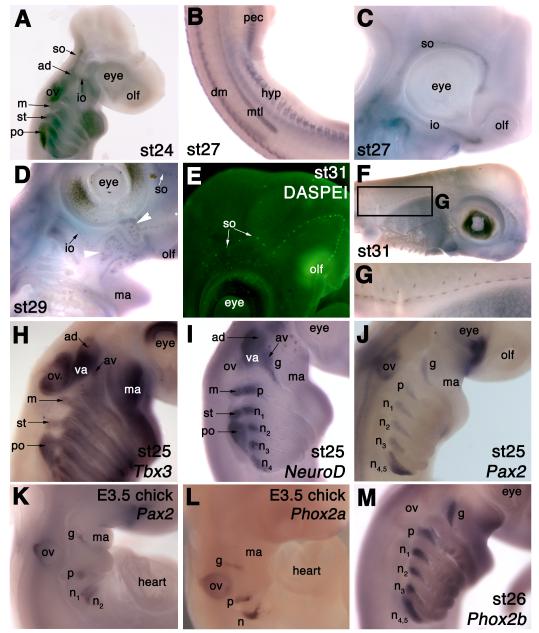
Molecular analysis of lateral line and epibranchial placode development in the shark, S. canicula. (A) At stage 24 (30-31.5 days), Eya4 is expressed in the anterodorsal lateral line placode (ad) and in the supraorbital (so) and infraorbital (io) ridges beginning to extend from it, as well as in the middle (m), supratemporal (st) and posterior (po) lateral line placodes, and in the otic vesicle (ov). (B) By stage 27 (42-46 days), the migrating Eya4+ main trunk line primordium (mtl) that originated from the posterior lateral line placode has reached a point midway between the pectoral (pec) and pelvic fin buds. dm, dermomyotome; hyp, hypaxial muscle primordia. (C) Also at stage 27 (42-46 days), Eya4 is expressed in the supraorbital (so) and infraorbital (io) ridges extending from the anterodorsal lateral line placode (ad). (D) At stage 29 (49-53 days), Eya4 is expressed in lateral line neuromasts of the supraorbital (so) and infraorbital (io) lateral lines, and in individual ampullary organs in the ampullary fields dorsal and ventral to these lines (white arrowheads). (E) DASPEI-stained embryo at stage 31 (60-80 days), showing neuromasts in the supraorbital (so) lateral line. (F,G) Eya4 expression in lateral line neuromasts at stage 31 (60-80 days). (H) At stage 25 (31-38 days), Tbx3 is expressed in lateral line and vestibuloacoustic ganglia and in the branchial arches, most strongly in the mandibular arch (ma). (I) NeuroD expression at stage 25 for comparison with panels H and J, also showing expression in the epibranchial placode-derived ganglia (g, geniculate; p, petrosal; n, nodose). (J) Pax2 expression at stage 25 in the epibranchial placodes (g, geniculate; p, petrosal; n, nodose), as well as in the dorsomedial otic vesicle (ov) and the optic fissure. (K) A 3.5-day chick embryo, showing Pax2 expression in the epibranchial placodes (g, geniculate; p, petrosal; n, nodose), dorsomedial otic vesicle (ov) and optic fissure. (L) A 3.5-day chick embryo, showing Phox2a expression in the epibranchial placode-derived ganglia (g, geniculate; p, petrosal; n, nodose). The staining in the otic vesicle (ov) is trapping. (M) At stage 26 (37-42 days), Phox2b expression in S. canicula is likewise seen specifically in the epibranchial placode-derived ganglia (g, geniculate; p, petrosal; n, nodose). Abbreviations: ad, anterodorsal lateral line placode/ganglion; av, anteroventral lateral line placode/ganglion; dm, dorsal dermomyotome; g, geniculate placode/ganglion; hyp, hypaxial muscle primordia; io, infraorbital sensory ridge; m, middle lateral line placode/ganglion; ma, mandibular arch; mtl, main trunk line primordium; n, nodose placode/ganglion; olf, olfactory pits; ov, otic vesicle; p, petrosal placode/ganglion; pec, pectoral fin bud; pel, pelvic fin bud; po, posterior lateral line placode/ganglion; so, supraorbital sensory ridge; st, supratemporal lateral line placode/ganglion; va, vestibuloacoustic ganglion.
Before migrating or elongating, lateral line placodes give rise to the sensory neurons in cranial lateral line ganglia that provide afferent innervation to both neuromasts and (where present) ampullary organs (reviewed in Baker and Bronner-Fraser, 2001; Schlosser, 2006; Ghysen and Dambly-Chaudière, 2004, 2005). Lateral line ganglia are often fused or closely associated with epibranchial placode-derived ganglia, however we have recently found that the transcription factor Tbx3 is a useful specific marker for lateral line (as opposed to epibranchial) ganglia in the shark, S. canicula (Fig. 2H; O’Neill et al., 2007). The growth cones of lateral line axons closely track, indeed seem to be “towed” by, the migrating lateral line primordium (Harrison, 1904; Gilmour et al., 2004), allowing coordination of sense organ formation and afferent innervation. The hair cells also receive efferent innervation, from two closely apposed efferent nuclei in the hindbrain, near the facial motor nucleus (reviewed in Fritzsch, 1989; Roberts and Meredith, 1989; also see Sapède et al., 2005). Lateral line efferents provide inhibitory input to the neuromast hair cells and suppress self-stimulation from the animal’s own movements (see Bodznick, 1989) and vocalisations (see Weeg et al., 2005). In zebrafish, efferent axons track the migrating posterior lateral line primordium but are not “towed” by it, as are the lateral line afferents; instead, they migrate along the lateral line afferent nerve bundle (Sapède et al., 2005).
The lateral line system is as old as the earliest craniates. Eptatretid hagfish embryos have three lateral line placodes (Wicht and Northcutt, 1995) and the adults have a simple lateral line system composed of three groups of shallow grooves, lined with flask-shaped hair cells that lack a cupula (Braun and Northcutt, 1997). The grooves are innervated by lateral line nerves (Kishida et al., 1987; Braun and Northcutt, 1997). Myxinid hagfish lack a lateral line system altogether, probably through secondary loss, and the simplified nature of the eptatretid hagfish lateral line system may also be a secondary reduction (Braun and Northcutt, 1997). Lampreys have a well-developed lateral line system similar to gnathostome fish, with neuromasts (though lacking cupulae) and electroreceptors (reviewed in Braun and Northcutt, 1997). Surprisingly, a third modality of the lateral line system is found uniquely in lampreys, namely photoreception, via solitary multivillous receptor cells found throughout the skin; these are similar to electroreceptors but contain pigment, and are innervated by lateral line nerves (reviewed in Braun and Northcutt, 1997). Electroreceptors are found in living agnathans (lampreys, though not hagfish), chondrichthyans (sharks, rays, chimeras), sarcopterygians (coelacanths and lungfish; urodele and caecilian amphibians) and basal actinopterygians (polypterids, paddlefish and sturgeons). They were lost in various lineages, including neopterygian ray-finned fish (gars, bowfins and teleosts), anuran amphibians, and amniotes. However, they seem to have independently “re-evolved” at least twice in two different teleost groups, including the weakly electric teleosts (mormyrids, gymnotids and gymnarchids), and siluriform catfish. The main teleost developmental model, the zebrafish, lacks electroreceptors.
Relationship with the otic placode
The dorsolateral placode series also includes the otic placode, which produces the entire inner ear, including its mechanosensory hair cells (very similar to lateral line hair cells) and the afferent neurons of the vestibulocochlear ganglion that innervate the hair cells (reviewed in (Baker and Bronner-Fraser, 2001; Schlosser, 2006)). The acousticolateralis hypothesis suggested that the inner ear and the lateral line system are evolutionarily related, with the inner ear most likely arising as a specialisation of the lateral line system (reviewed in Jørgensen, 1989; Streit, 2001). However, all living craniates possess a distinct inner ear and a lateral line system, and it is equally likely that the inner ear and the lateral line (both mechanosensory and electroreceptive components) evolved independently from a primitive system of epidermal ciliated sensory receptor cells, most likely mechanoreceptors (Kalmijn, 1989; Popper and Fay, 1997; Coombs and Montgomery, 2005). Lateral line neuromasts detect acceleration of water relative to the animal, while inner ear otolith organs detect the acceleration of the animal per se (reviewed in Kalmijn, 1989). Kalmijn (1989) suggests that inner ear sense organs evolved from early forms of lateral line neuromasts, and that the different functions (sense of equilibrium, detection of moving objects, etc.) evolved concurrently, rather than any one function being the most ancient or most important. The inner ear otolith organs of fish typically respond to moving objects that are further away, and are sensitive to higher frequencies, than those detected by the lateral line (Kalmijn, 1989). It was therefore suggested that a continuation of this trend might have led to detection of the propagating sound wave (Kalmijn, 1989). Conversely, it has also been proposed that the inner ear evolved first, and that lateral line placodes evolved later from regions of the otic placode that failed to invaginate (Fritzsch et al., 1997; Fritzsch, 1998). Whatever their evolutionary relationship, the separation between the two systems is evidently very ancient, since differences exist in their induction (see Baker and Bronner-Fraser, 2001; Schlosser, 2006) and the lateral line system has been lost many times in evolution (both in part and in its entirety) without affecting the inner ear (see Schlosser, 2002a, 2005).
Epibranchial placodes
The epibranchial placodes form a “ventrolateral” series above the pharyngeal (branchial) clefts (Fig. 2J,K). They form the visceral sensory neurons of the geniculate, petrosal and nodose ganglia (reviewed in Baker and Bronner-Fraser, 2001; Schlosser, 2006); these are, respectively, the distal ganglia of cranial nerves VII (facial), IX (glossopharyngeal) and X (vagal) (Fig. 2L,M). Epibranchial placode-derived neurons provide e.g. baroreceptive, osmoreceptive and chemoreceptive information from the heart, lungs, gut and other visceral organs; they form the afferent arm of the visceral medullary reflexes that control autonomic functions (Kandel et al., 2000). They are also thought to provide afferent innervation for taste buds, which are locally derived from oropharyngeal epithelium (ectoderm and/or endoderm; Barlow and Northcutt, 1995; Stone et al., 1995) and innervated by branches of the facial, glossopharyngeal and vagal nerves (reviewed in Northcutt, 2004). (These nerves also carry afferents from neural crest-derived sensory neurons, whose cell bodies are located in the proximal ganglia of the same nerves: the specific epibranchial placode origin of taste bud afferents, although generally assumed, has yet to be demonstrated experimentally; see Northcutt, 2004.) Furthermore, in anamniotes, epibranchial placode-derived afferents carried in the glossopharyngeal and vagal nerves that innervate the gills (reviewed in Sundin and Nilsson, 2002) are likely also to be important for transmitting information acquired by peripheral chemoreceptors stimulated by O2 and CO2 levels in inspired water, i.e., the external environment (see later, and also Milsom, 2002; Jonz and Nurse, 2006). Hence, epibranchial placode-derived neurons provide information about both the internal and external environment.
Epibranchial placode-derived mechanosensory organs?
The spiracular organ of non-teleost fish is a tube or pouch-like structure, containing typical lateral line neuromasts, and associated with the first gill slit (the spiracle, which forms where the outpocketing first pharyngeal pouch meets the invaginating first pharyngeal cleft). The spiracle enables breathing to continue even when the mouth is closed. Spiracular organs are found in elasmobranchs, lungfish, polypterids, chondrosteans (sturgeons, paddlefish), and non-teleost neopterygians (gars and bowfins, though they have been lost in bichirs) (Northcutt, 1989, 1997). The spiracular organ is usually considered to be a specialised lateral line organ (reviewed in Norris and Hughes, 1920; Norris, 1929; Barry and Bennett, 1989; Northcutt, 1989, 1997), although the geniculate (first epibranchial) placode, which forms above the first pharyngeal cleft, has also been suggested to give rise to the spiracular organ (Vitali, 1925, 1926; Ranzi, 1926). In both African and Australian lungfish embryos, the spiracular organ was described as lying at the anterior end of a “spiracular” or “suprabranchial” line of vestigial sense organs, each sense organ located dorsal to a branchial arch (Pehrson, 1949). This intriguing observation might even be taken to suggest that all epibranchial placodes can form sense organs. However, the usual “rule” for placode-derived sense organs is that both the sense organ and its afferent innervation arise from the same placode: the afferent innervation for the spiracular organ has variously been described as arising from the anterior lateral line nerve or, where present, the otic lateral line nerve (Barry and Bennett, 1989; Northcutt, 1997), rather than the geniculate ganglion. Furthermore, in the clearnose skate, spiracular organ afferents project to the hindbrain in the ventral root of the anterior lateral line nerve (Barry and Boord, 1984). They mostly terminate in a restricted area of the mechanoreceptive medial (intermediate) octavolateralis nucleus, though their primary projection field also overlaps with the octaval nuclei that receive information from the inner ear (Barry and Boord, 1984). This innervation pattern supports a lateral line placode origin for the spiracular organ.
In chick embryos, a mechanosensory hair cell-containing pouch in the middle ear cavity (itself derived from the first pharyngeal pouch), close to the tympanic membrane, has been fate-mapped to the geniculate placode (Vitali, 1925; Yntema, 1944; D’Amico-Martel and Noden, 1983). Elastic ligaments connect this “paratympanic organ” rostrally to the tympanic membrane and caudally to the columella-squamosal ligament (von Bartheld, 1994). Given its structure and position, the paratympanic organ is thought to be homologous to the anamniote spiracular organ; however, the lateral line system is generally considered to have been completely lost in amniotes. Furthermore, the paratympanic organ is innervated by a lateral or “paratympanic” extension of the geniculate ganglion, and paratympanic organ afferents enter the hindbrain together with the facial nerve, which carries geniculate afferents (Vitali, 1911; Yntema, 1944; von Bartheld, 1990). More recently, clonal analysis in the chick embryo using replication-defective retroviruses has shown that paratympanic organ cells and geniculate ganglion neurons can be clonally related, although it was not possible to determine whether individual geniculate ganglion neurons innervate paratympanic organ hair cells to which they are clonally related (Satoh and Fekete, 2005). Using the same logic about common origins for both a sense organ and its afferent innervation, this provides further support for a geniculate placode origin for the paratympanic organ, in addition to ablation and fate-mapping studies (Yntema, 1944; D’Amico-Martel and Noden, 1983). Arguing against this, however, is the projection of paratympanic organ afferents to vestibular brainstem nuclei (von Bartheld, 1990).
The paratympanic organ is not unique to birds: a paratympanic organ is also present in juvenile alligators (Neeser and von Bartheld, 2002), and an elongated pouch, though lacking neuromasts or innervation, was found in the middle ear of the adult Sphenodon (Simonetta, 1953, 1959). No such structure has been found in modern amphibians, or indeed most mammals: the armadillo has a ciliated vesicle in the same position, but this does not seem to contain hair cells (Neeser and von Bartheld, 2002); the monotreme echidna (Tachyglossus) has a simple non-sensory vesicle attached to the tympanic membrane, while the dolphin embryo has an innervated epithelial vesicle that is reabsorbed before birth (Simonetta, 1953, 1959). However, in the adult pipistrelle bat, Vesperuyo pipistrellus, an epithelial structure of variable appearance, from a thickened epithelium to a small vesicle, was found associated with the superior wall of the tympanic membrane and with a small fascicle containing neuron cell bodies, originating from the facial nerve (Vitali, 1924, 1926).
Hence, although lost in modern amphibians, most reptiles and mammals, the spiracular/paratympanic organ seems to be an ancient vertebrate sense organ, whose function remains enigmatic: the shark spiracular organ has been suggested to be a proprioceptor for jaw movement (Barry and Boord, 1984), while the avian paratympanic organ has been proposed to detect changes in air pressure in the middle ear (Vitali, 1921; Federici, 1927; Jørgensen, 1984; Giannessi and Pera, 1986; von Bartheld, 1994). The paratympanic organ has also been suggested to function in a reflex allowing fast opening of the Eustachian tube to equalise air pressure during rapid changes in altitude in flight (Giannessi et al., 1996). (It is interesting in this respect that falcons seem to have the largest and most complex paratympanic organs; Simonetta, 1959.) The earliest stages in the evolution of the tetrapod middle ear from the fish spiracle have recently been investigated in Panderichthys, the immediate sister taxon of tetrapods (Brazeau and Ahlberg, 2006). The results suggest that the tetrapod middle ear initially evolved as part of a spiracular breathing apparatus, supporting the hypothesis that the “otic notch” of the earliest tetrapods contained a persistent spiracle (Clack, 2002). Persistence of the spiracle in early tetrapods would also explain the persistence of the spiracular organ after the transition to life on land.
If the spiracular and paratympanic organs are indeed homologous, which is supported by their structure (a tube or pouch containing mechanosensory hair cells) and position (associated with the first pharyngeal pouch/cleft), then one would expect both organs and their afferent innervating neurons to arise from the same placode. A lateral line placode origin for the spiracular organ is supported by its innervation by anterior/otic lateral line afferents that project to the medial octavolateralis nucleus in the hindbrain. A geniculate placode origin for the paratympanic organ is supported by its innervation by geniculate afferents, though their projection to hindbrain vestibular nuclei is inconsistent with the usual projection of geniculate neurons to the solitary tract and nucleus. Perhaps an undetected remnant of the lateral line system is present in amniotes, with its sensory ganglion fused to the geniculate ganglion, or with the geniculate ganglion having “taken over” the role of providing afferent innervation to the paratympanic organ. Another possibility is that the spiracular/paratympanic organ (and its placode of origin) represents an ancient, independent specialisation of the ancestral ciliated sensory receptor system that is distinct from both lateral line and epibranchial placodes. Resolution of such speculation must await experimental investigation.
Developmental relationships with lateral line and otic placodes
In Xenopus, the epibranchial, lateral line and otic placodes all develop from a molecularly and morphologically distinct “dorsolateral placode primordium”, or “posterior placodal area” (Schlosser and Ahrens, 2004; Schlosser, 2005, 2006), from which individual placodes subsequently become distinct, with the loss of common markers and the acquisition of new, placode-specific markers. The transcription factors Pax8, Pax2, and Foxi1, which are often considered to be otic placode markers, are expressed at neural plate stages in the posterior placodal area (Schlosser and Ahrens, 2004; Schlosser, 2005, 2006). Pax2 is in fact a pan-gnathostome marker for the epibranchial placodes as well as for the otic placode (Fig. 2J,K; Baker and Bronner-Fraser, 2000; Schlosser and Ahrens, 2004; Nechiporuk et al., 2007; O’Neill et al., 2007).
Parallels have been drawn recently between the induction of the otic and epibranchial placodes (Nechiporuk et al., 2007): FGF signalling from cephalic mesoderm regulates (though in different time windows) the initial induction of both otic and epibranchial placodes from Eya+/Six+ preplacodal ectoderm (Wright and Mansour, 2003; Kil et al., 2005; Nikaido et al., 2006; Nechiporuk et al., 2007; Sun et al., 2007). (Establishment of the preplacodal domain is reviewed in Bailey and Streit, 2006.) In zebrafish, Pax2/Pax8 expression in both otic and epibranchial placodes is regulated by Foxi1, which seems to be a competence factor for the response to FGF signalling, at least for the otic placode (Lee et al., 2003; Nissen et al., 2003; Solomon et al., 2003; Hans et al., 2004; Solomon et al., 2004; Mackereth et al., 2005; Hans et al., 2007). FGF signalling is also involved in regulating Ngn1 expression and subsequent neurogenesis in both the otic and epibranchial placodes (Alsina et al., 2004; Nechiporuk et al., 2005). BMPs induce Foxi1 expression in zebrafish (Hans et al., 2007), and are also essential for neurogenesis in the epibranchial placodes in chick and zebrafish (Begbie et al., 1999; Holzschuh et al., 2005). Hence, there are many similarities between otic and epibranchial placode induction. More information on the molecular control of lateral line placode induction is urgently needed, as otherwise it is not possible to make a full comparison.
A common evolutionary origin for lateral line, otic and epibranchial placodes in the afferent arm of defence reflexes?
Froriep, who described the epibranchial placodes in detail in 1885, suggested that they were vestiges of ancestral sense organs (Froriep, 1885). Some early workers, including Vitali, thought the epibranchial placodes were directly homologous to the lateral line placodes (Vitali, 1926). However, the most primitive extant craniates possess lateral line, otic and epibranchial placodes, so, as in the discussion of the relationship between otic and lateral line placodes above, we can only speculate as to the order of their evolution. Nonetheless, given the description above of the possible formation of a mechanosensory hair cell-containing organ by the geniculate placode in amniotes, and the presence of a “spiracular line” of “vestigial sense organs” dorsal to the branchial arches in lungfish (Pehrson, 1949), as well as the parallels between induction of the otic and epibranchial placodes, it seems worth speculating about possible developmental and evolutionary relationships between lateral line, epibranchial and otic placodes. Perhaps all epibranchial placodes originally made sense organs, like those in the lateral line and inner ear, which were subsequently lost (apart from the specialised spiracular/paratympanic organ), and the afferent neurons recruited for other functions. Here, we speculate that a unifying characteristic of lateral line, otic and epibranchial placode-derived cells might be their involvement in the afferent arm of defence reflexes. The potential importance of lateral line mechanoreceptors and electroreceptors for defensive reflexes is perhaps more intuitive than that of epibranchial placode-derived neurons, but we hope to illustrate here that the latter may also play a defensive role.
Epibranchial placode-derived neurons and visceral medullary reflex circuits
The paired-like homeodomain transcription factor Phox2b, and the closely related transcription factor Phox2a, are both expressed in epibranchial placode-derived neurons (reviewed in Brunet and Pattyn, 2002). Indeed, we have recently shown that Phox2b is indeed a pan-gnathostome marker for epibranchial placode-derived neurons (Fig. 2L,M; O’Neill et al., 2007). Phox2b is a pan-autonomic regulator that, remarkably, is essential for the formation of all the neurons that participate in the medullary reflex circuits controlling autonomic (visceral) functions, no matter what their embryonic origin or neurotransmitter phenotype: (1) their afferent arm, i.e., the neural crest-derived carotid body and epibranchial placode-derived visceral sensory neurons in the geniculate, petrosal and nodose ganglia; (2) the central targets of these neurons (the nucleus of the solitary tract and associated area postrema), and (3) their efferent arm, i.e., neural crest-derived motor neurons in parasympathetic and enteric ganglia that innervate glands and smooth muscle, and the preganglionic (visceromotor) neurons of the hindbrain that synapse onto them (reviewed in Brunet and Pattyn, 2002; Brunet and Goridis, in press).
Given its otherwise tight association with visceral reflexes, it is initially perhaps surprising to learn that Phox2b is also required for the formation of all hindbrain branchiomotor neurons (Pattyn et al., 2000). Hindbrain branchiomotor neurons innervate branchial arch-derived muscles (reviewed in Chandrasekhar, 2004). In mammals, these muscles control facial expression, jaw movements, the pharynx and the larynx. Primitively, however, the function of branchial muscles was ventilation (Brunet and Pattyn, 2002): in lampreys, branchiomotor neurons are effectors of the central pattern generators for respiration (Rovainen, 1996). Since branchiomotor neurons were primitively part of the efferent arm of visceral reflex circuits, Phox2b expression in branchiomotor neurons is consistent with the Phox2b-dependence of all neurons involved, or once involved, in visceral reflexes (Brunet and Pattyn, 2002; Brunet and Goridis, in press).
Perhaps one of the most important and basic visceral reflexes is the control of ventilatory and cardiac activity in response to hypoxia and/or hypercarbia. All vertebrates have mechanoreceptors and/or chemoreceptors in their gas exchange organs and lower respiratory passages that transmit information about the rate and amplitude of ventilation for reflex control of breathing pattern, heart rate and output (reviewed in Jones and Milsom, 1982). The afferent arm of these reflexes is provided by Phox2b+ epibranchial placode-derived neurons; the efferent arm by Phox2b+ branchiomotor and visceromotor neurons.
In terrestrial vertebrates, epibranchial placode-derived neurons provide afferent innervation for the neural crest-derived (and Phox2b+) peripheral O2 chemoreceptors of the carotid and aortic bodies, and diffusely distributed paraganglia, that stimulate reflex responses to hypoxia in the blood (internal environment). In teleost fish and amphibian larvae, peripheral O2 chemoreceptive cells (termed “neuroepithelial” cells by the researchers who identified them) are distributed over the gills; they sense oxygen levels in both blood (internal environment) and inspired water (external environment) (see Smatresk, 1990; Jonz and Nurse, 2006; Saltys et al., 2006). Their embryonic origin is currently unknown, but they may be the evolutionary precursors of carotid and aortic body O2 chemoreceptors (reviewed in Jonz and Nurse, 2006). They are innervated by the glossopharyngeal and vagal nerves and thus are likely to be innervated by epibranchial placode-derived neurons, though as for taste buds, this remains to be investigated experimentally. In zebrafish, they do not appear until 5 days post-fertilisation (Jonz and Nurse, 2005); it would be very interesting to determine whether or not they express Phox2b.
Peripheral CO2 chemoreceptors on the gills have also been described in sharks and teleosts (reviewed in Milsom, 2002): like peripheral O2 chemoreceptors, they are innervated by the glossopharyngeal and vagal nerves. They elicit cardiorespiratory reflex changes (increased ventilation and decreased heart rate) in response to hypercarbia in the external water (see Milsom, 2002; Sundin et al., 2000; Gilmour et al., 2005).
It has been suggested that the original function of vagal afferents was in protective and defensive somatic reflexes, rather than homeostatic visceral reflexes, allowing escape from (and avoidance of) noxious stimuli (Andrews and Lawes, 1992). Even in mammals, stimulation of carotid and aortic chemoreceptors produces reflex effects on both the respiratory and cardiovascular system that are important components of the defence (alerting) response (Marshall, 1994, 1998). The protective/defensive function of vagal reflexes is also exemplified by coughing and vomiting, which protect the delicate surfaces of the gills/airways and gut respectively. Taste (and conditioned taste aversion) is an important component of internal defence: taste determines whether a stimulus in the mouth will lead to ingestion or rejection (Andrews and Lawes, 1992). Indeed, it is possible that the association between epibranchial placode-derived afferents for taste and reflex protection of the gills may reflect a much more ancient function in the ancestor of the vertebrates, which used its pharyngeal slits for filter-feeding rather than respiration (see also Dufour et al., 2006). Vagal afferents from the gut are also important for higher levels of behaviour: a hungry animal is active and explores its environment for food, while an animal that has fed shows reduced levels of arousal, using its energy for digestion (Andrews and Lawes, 1992).
Evidence for an ancient visceral reflex role for lateral line, otic and perhaps even trigeminal neurons?
Apart from branchiomotor and visceromotor neurons, a third class of hindbrain efferents depends on Phox2b, namely inner ear efferent neurons. In fish and amphibians, inner ear efferents are a subset of the “octavolateral” efferents that arise from the octavolateralis efferent nucleus in the hindbrain (at the rostral pole of the facial motor nucleus) and innervate mechanosensory hair cells of both the lateral line and the inner ear (reviewed in Roberts and Meredith, 1989; Simmons, 2002). Rostral lateral line efferents, like inner ear efferents, are a subset of facial branchiomotor neurons, while caudal lateral line efferents are a subset of glossopharyngeal branchiomotor neurons (Meredith and Roberts, 1986; Sapède et al., 2005). Collaterals of these efferents contact hair cells of both the inner ear and the lateral line; this may be the primitive condition for gnathostomes (reviewed in Fritsch et al., 1979; Fritzsch, 1989; also see González et al., 1993). Perhaps this ontogenetic commonality of inner ear and lateral line efferents, as well as other branchiomotor neurons, represents the residual trace of a common ancestry?
The only other neurogenic placodes that do not form sense organs in extant vertebrates are the trigeminal placodes (reviewed in Baker and Bronner-Fraser, 2001; Schlosser, 2006). Many teleost fish living in hypoxic environments exhibit an adaptive reflex response to hypoxia called aquatic surface respiration, in which they rise to the surface to access the oxygen-rich layer of water adjacent to the air (see e.g. Shingles et al., 2005; Florindo et al., 2006). A similar reflex is seen in air-gulping fish. Intriguingly, the aquatic surface respiration reflex in the neotropical teleost fish Colossoma macroponum seems to be triggered by O2 chemoreceptors innervated by the trigeminal nerve, while the cardiorespiratory response to hypoxia is mediated by the glossopharyngeal and vagal nerves (Florindo et al., 2006). In sharks (dogfish), peripheral O2 chemoreceptors in the gills and buccal cavity are innervated not only by the facial, glossopharyngeal and vagal nerves, but also by the trigeminal nerve (Butler et al., 1977). Butler et al. (1977) speculate that the receptors innervated by the glossopharyngeal and vagal nerves evolved into the carotid and aortic bodies of terrestrial vertebrates, while those innervated by the trigeminal and facial nerves were either lost, or became sensitive to other chemicals. (In mammals, for example, trigeminal nerve-innervated chemoreceptors around the mouth and in the nasopharynx cause a reflex slowing of the heart rate, the usual response to hypoxia, in response to water or noxious chemicals; see Butler et al., 1977.) Like the efferent neurons of the facial, glossopharyngeal and vagal motor nuclei, the efferent neurons of the trigeminal motor nucleus express Phox2b.
We conclude with a highly speculative proposal, namely that the original function in the vertebrate ancestor of the placodes whose evolutionary descendants now include the lateral line, otic and epibranchial placodes (and perhaps even the trigeminal placodes), was to produce neurons and sensory receptors for the afferent arm of Phox2-dependent reflex circuits (both somatic and visceral). Subsequently, selection pressures imposed on the vertebrate ancestor by the shift to an active, fast-swimming predatory lifestyle, led to the elaboration of the lateral line system for distance reception, the inner ear for detection of linear acceleration, and perhaps the spiracular organ for some unknown function, with the concomitant loss of Phox2b expression in these sensory receptors and their afferent neurons. These selection pressures also resulted in the loss of most or all epibranchial placode-derived sensory receptors, and the specialisation of epibranchial placode-derived sensory neurons for visceral reflex responses. The distinct spatial separation of octavolateralis and visceral sensory nuclei in the hindbrain of extant vertebrates may simply reflect the very ancient timing of this evolutionary divergence. Although this scenario is necessarily very speculative, we hope this novel view will stimulate debate and a closer investigation of the potential developmental and evolutionary relationships between these placodes.
Acknowledgments
Many thanks to Dr Jean-François Brunet for valuable discussions about Phox2-dependent circuits and for critical reading of the manuscript, and to two anonymous reviewers for very helpful comments. Thanks to Dr Jeremy Skepper for preparing specimens for scanning electron microscopy. Funding for this work was provided by BBSRC grants 8/G18657 and BB/D008336/1 to C.V.H.B.
REFERENCES
- Alsina B, Abello G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev Biol. 2004;267:119–134. doi: 10.1016/j.ydbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Andrews PLR, Lawes INC. A protective role for vagal afferents: an hypothesis. In: Ritter S, Ritter RC, Barnes CD, editors. Neuroanatomy and Physiology of Abdominal Vagal Afferents. CRC Press; Boca Raton: 1992. pp. 279–302. [Google Scholar]
- Bailey AP, Streit A. Sensory organs: making and breaking the pre-placodal region. Curr Top Dev Biol. 2006;72:167–204. doi: 10.1016/S0070-2153(05)72003-2. [DOI] [PubMed] [Google Scholar]
- Baker CVH. Neural Crest and Cranial Ectodermal Placodes. In: Jacobson M, Rao M, editors. Developmental Neurobiology. Kluwer Academic/Plenum Publishers; New York: 2005. pp. 67–127. [Google Scholar]
- Baker CVH, Bronner-Fraser M. The origins of the neural crest. Part II: an evolutionary perspective. Mech Dev. 1997;69:13–29. doi: 10.1016/s0925-4773(97)00129-9. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–3056. doi: 10.1242/dev.127.14.3045. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Schlosser G. Editorial: The evolutionary origin of neural crest and placodes. J Exp Zool B Mol Dev Evol. 2005;304:269–273. doi: 10.1002/jez.b.21060. [DOI] [PubMed] [Google Scholar]
- Barlow LA, Northcutt RG. Embryonic origin of amphibian taste buds. Dev Biol. 1995;169:273–285. doi: 10.1006/dbio.1995.1143. [DOI] [PubMed] [Google Scholar]
- Barry MA, Bennett MVL. Specialised lateral line receptor systems in elasmobranchs: the spiracular organs and vesicles of Savi. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line. Neurobiology and Evolution. Springer-Verlag; New York: 1989. pp. 591–606. [Google Scholar]
- Barry MA, Boord RL. The spiracular organ of sharks and skates: anatomical evidence indicating a mechanoreceptive role. Science. 1984;226:990–992. doi: 10.1126/science.6505680. [DOI] [PubMed] [Google Scholar]
- Begbie J, Brunet J-F, Rubenstein JL, Graham A. Induction of the epibranchial placodes. Development. 1999;126:895–902. doi: 10.1242/dev.126.5.895. [DOI] [PubMed] [Google Scholar]
- Bodznick D. Comparisons between electrosensory and mechanosensory lateral line systems. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line. Neurobiology and Evolution. Springer-Verlag; New York: 1989. pp. 655–678. [Google Scholar]
- Bodznick D, Montgomery JC. The physiology of low-frequency electrosensory systems. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. Springer; New York: 2005. pp. 132–153. [Google Scholar]
- Braun CB, Northcutt RG. The lateral line system of hagfishes (Craniata: Myxinoidea) Acta Zool (Stockh) 1997;78:247–268. [Google Scholar]
- Brazeau MD, Ahlberg PE. Tetrapod-like middle ear architecture in a Devonian fish. Nature. 2006;439:318–321. doi: 10.1038/nature04196. [DOI] [PubMed] [Google Scholar]
- Brunet J-F, Goridis C. Phox2b and the homeostatic brain. In: Gaultier C, editor. Genetics of respiratory control disorders. Springer-Verlag.; In press. [Google Scholar]
- Brunet J-F, Pattyn A. Phox2 genes - from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Bullock TH. Electroreception. Ann Rev Neurosci. 1982;5:121–170. doi: 10.1146/annurev.ne.05.030182.001005. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Hopkins CD, Popper AN, Fay RR. Electroreception. Springer; New York: 2005. [Google Scholar]
- Butler PJ, Taylor EW, Short S. The effect of sectioning cranial nerves V, VII, IXand X on the cardiac response of the dogfish Scyliorhinus canicula to environmental hypoxia. J Exp Biol. 1977;69:233–245. doi: 10.1242/jeb.69.1.233. [DOI] [PubMed] [Google Scholar]
- Cernuda-Cernuda R, García-Fernández JM. Structural diversity of the ordinary and specialized lateral line organs. Microsc Res Tech. 1996;34:302–312. doi: 10.1002/(SICI)1097-0029(19960701)34:4<302::AID-JEMT3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A. Turning heads: development of vertebrate branchiomotor neurons. Dev Dyn. 2004;229:143–161. doi: 10.1002/dvdy.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack JA. Gaining Ground: the Origin and Evolution of Tetrapods. Indiana University Press; Bloomington: 2002. [Google Scholar]
- Coombs S, Montgomery JC. Comparing octavolateralis sensory systems: what can we learn? In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. Springer; New York: 2005. pp. 318–359. [Google Scholar]
- D’Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Dufour HD, Chettouh Z, Deyts C, de Rosa R, Goridis C, Joly J-S, Brunet J-F. Precraniate origin of cranial motoneurons. Proc Natl Acad Sci USA. 2006 doi: 10.1073/pnas.0600805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici F. Uber die Innervation des von Vitali entdeckten Sinnesorgans in Mittelohr der Vögel (sogen. paratympanisches Organ) Anat Anz. 1927;62:241–254. [Google Scholar]
- Florindo LH, Leite CA, Kalinin AL, Reid SG, Milsom WK, Rantin FT. The role of branchial and orobranchial O2 chemoreceptors in the control of aquatic surface respiration in the neotropical fish tambaqui (Colossoma macropomum): progressive responses to prolonged hypoxia. J Exp Biol. 2006;209:1709–1715. doi: 10.1242/jeb.02199. [DOI] [PubMed] [Google Scholar]
- Freitas R, Zhang G, Albert JS, Evans DH, Cohn MJ. Developmental origin of shark electrosensory organs. Evol Dev. 2006;8:74–80. doi: 10.1111/j.1525-142X.2006.05076.x. [DOI] [PubMed] [Google Scholar]
- Fritsch HA, Van Noorden S, Pearse AG. Localization of somatostatin-, substance P- and calcitonin-like immunoreactivity in the neural ganglion of Ciona intestinalis L. (Ascidiaceae) Cell Tissue Res. 1979;202:263–274. doi: 10.1007/BF00232240. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Diversity and regression in the amphibian lateral line and electrosensory system. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line. Neurobiology and Evolution. Springer-Verlag; New York: 1989. pp. 99–114. [Google Scholar]
- Fritzsch B. Evolution of the vestibulo-ocular system. Otolaryngol. Head Neck Surg. 1998;119:182–192. doi: 10.1016/S0194-5998(98)70053-1. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Barald KF, Lomax MI. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. Springer-Verlag; New York: 1997. pp. 80–145. [Google Scholar]
- Fritzsch B, Pauley S, Beisel KW. Cells, molecules and morphogenesis: the making of the vertebrate ear. Brain Res. 2006;1091:151–171. doi: 10.1016/j.brainres.2006.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froriep A. Ueber Anlagen von Sinnesorganen am Facialis, Glossopharyngeus und Vagus, über die genetische Stellung des Vagus zum Hypoglossus, und über die Herkunft der Zungenmusculatur. Arch Anat Physiol. 1885:1–55. [Google Scholar]
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–274. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudière C. Development of the zebrafish lateral line. Curr Opin Neurobiol. 2004;14:67–73. doi: 10.1016/j.conb.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudière C. The three-sided romance of the lateral line: glia love axons love precursors love glia. BioEssays. 2005;27:488–494. doi: 10.1002/bies.20225. [DOI] [PubMed] [Google Scholar]
- Giannessi F, Fattori B, Ruffoli R, Gagliardo A. Homing experiments on pigeons subjected to bilateral destruction of the paratympanic organ. J Exp Biol. 1996;199:2035–2039. doi: 10.1242/jeb.199.9.2035. [DOI] [PubMed] [Google Scholar]
- Giannessi F, Pera L. The ultrastructure of the paratympanic organ in the domestic fowl (Gallus gallus domesticus) J Anat. 1986;147:191–199. [PMC free article] [PubMed] [Google Scholar]
- Gibbs MA. Lateral line receptors: where do they come from developmentally and where is our research going? Brain Behav Evol. 2004;64:163–181. doi: 10.1159/000079745. [DOI] [PubMed] [Google Scholar]
- Gilmour D, Knaut H, Maischein HM, Nüsslein-Volhard C. Towing of sensory axons by their migrating target cells in vivo. Nat Neurosci. 2004;7:491–492. doi: 10.1038/nn1235. [DOI] [PubMed] [Google Scholar]
- Gilmour KM, Milsom WK, Rantin FT, Reid SG, Perry SF. Cardiorespiratory responses to hypercarbia in tambaqui Colossoma macropomum: chemoreceptor orientation and specificity. J Exp Biol. 2005;208:1095–1107. doi: 10.1242/jeb.01480. [DOI] [PubMed] [Google Scholar]
- González A, Meredith GE, Roberts BL. Choline acetyltransferase immunoreactive neurons innervating labyrinthine and lateral line sense organs in amphibians. J Comp Neurol. 1993;332:258–268. doi: 10.1002/cne.903320209. [DOI] [PubMed] [Google Scholar]
- Hans S, Christison J, Liu D, Westerfield M. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol. 2007;7:5. doi: 10.1186/1471-213X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S, Liu D, Westerfield M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development. 2004;131:5091–5102. doi: 10.1242/dev.01346. [DOI] [PubMed] [Google Scholar]
- Harrison RG. Experimentelle Untersuchung über die Entwicklung der Sinnesorgane der Seitenlinie bei den Amphibien. Arch Mikrosk Anat EntwMech. 1904;63:35–149. [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuss A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–3742. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Jones DR, Milsom WK. Peripheral receptors affecting breathing and cardiovascular function in non-mammalian vertebrates. J Exp Biol. 1982;100:59–91. [Google Scholar]
- Jonz MG, Nurse CA. Development of oxygen sensing in the gills of zebrafish. J Exp Biol. 2005;208:1537–1549. doi: 10.1242/jeb.01564. [DOI] [PubMed] [Google Scholar]
- Jonz MG, Nurse CA. Ontogenesis of oxygen chemoreception in aquatic vertebrates. Respir Physiol Neurobiol. 2006;154:139–152. doi: 10.1016/j.resp.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Jørgensen JM. Fine structure of the paratympanic organ in the avian middle ear. Acta Zool (Stockh) 1984;65:89–94. [Google Scholar]
- Jørgensen JM. Evolution of octavolateralis sensory cells. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line. Neurobiology and Evolution. Springer-Verlag; New York: 1989. pp. 115–145. [Google Scholar]
- Jørgensen JM. Morphology of electroreceptive sensory organs. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. Springer; New York: 2005. pp. 47–67. [Google Scholar]
- Kalmijn AJ. Functional evolution of lateral line and inner ear sensory systems. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line. Neurobiology and Evolution. Springer-Verlag; New York: 1989. pp. 187–215. [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw-Hill; 2000. [Google Scholar]
- Kil SH, Streit A, Brown ST, Agrawal N, Collazo A, Zile MH, Groves AK. Distinct roles for hindbrain and paraxial mesoderm in the induction and patterning of the inner ear revealed by a study of vitamin-A-deficient quail. Dev Biol. 2005;285:252–271. doi: 10.1016/j.ydbio.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Kishida R, Goris RC, Nishizawa H, Koyama H, Kadota T, Amemiya F. Primary neurons of the lateral line nerves and their central projections in hagfishes. J Comp Neurol. 1987;264:303–310. doi: 10.1002/cne.902640303. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- Lee SA, Shen EL, Fiser A, Sali A, Guo S. The zebrafish forkhead transcription factor Foxi1 specifies epibranchial placode-derived sensory neurons. Development. 2003;130:2669–2679. doi: 10.1242/dev.00502. [DOI] [PubMed] [Google Scholar]
- Mackereth MD, Kwak SJ, Fritz A, Riley BB. Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development. 2005;132:371–382. doi: 10.1242/dev.01587. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Chemoreceptors and cardiovascular control in acute and chronic systemic hypoxia. Braz J Med Biol Res. 1998;31:863–888. doi: 10.1590/s0100-879x1998000700002. [DOI] [PubMed] [Google Scholar]
- Martin K, Groves AK. Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development. 2006;133:877–887. doi: 10.1242/dev.02267. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Roberts BL. Central organization of the efferent supply to the labyrinthine and lateral line receptors of the dogfish. Neuroscience. 1986;17:225–233. doi: 10.1016/0306-4522(86)90238-1. [DOI] [PubMed] [Google Scholar]
- Milsom WK. Phylogeny of CO2/H+ chemoreception in vertebrates. Respir Physiol Neurobiol. 2002;131:29–41. doi: 10.1016/s1569-9048(02)00035-6. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–623. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Raible DW. Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development. 2005;132:3717–3730. doi: 10.1242/dev.01876. [DOI] [PubMed] [Google Scholar]
- Neeser JA, von Bartheld CS. Comparative anatomy of the paratympanic organ (Vitali organ) in the middle ear of birds and non-avian vertebrates: focus on alligators, parakeets and armadillos. Brain Behav Evol. 2002;60:65–79. doi: 10.1159/000065206. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North-Holland: 1967. [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn. 2006 doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Nissen RM, Yan J, Amsterdam A, Hopkins N, Burgess SM. Zebrafish foxi one modulates cellular responses to Fgf signaling required for the integrity of ear and jaw patterning. Development. 2003;130:2543–2554. doi: 10.1242/dev.00455. [DOI] [PubMed] [Google Scholar]
- Norris HW. The distribution and innervation of the ampullae of Lorenzini of the dogfish, Squalus acanthias. Some comparisons with conditions in other plagiostomes and corrections of prevalent errors. J Comp Neurol. 1929;47:449–465. [Google Scholar]
- Norris HW, Hughes SP. The spiracular sense-organ in elasmobranchs, ganoids and dipnoans. Anat Rec. 1920;18:205–209. [Google Scholar]
- Northcutt RG. The phylogenetic distribution and innervation of craniate mechanoreceptive lateral lines. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line. Neurobiology and Evolution. Springer-Verlag; New York: 1989. pp. 17–78. [Google Scholar]
- Northcutt RG. Evolution of gnathostome lateral line ontogenies. Brain Behav Evol. 1997;50:25–37. doi: 10.1159/000113319. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Taste buds: development and evolution. Brain Behav Evol. 2004;64:198–206. doi: 10.1159/000079747. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Ontogeny of electroreceptors and their neural circuitry. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. Springer; New York: 2005b. pp. 112–131. [Google Scholar]
- Northcutt RG. The New Head hypothesis revisited. J Exp Zool B Mol Dev Evol. 2005a;304B:274–297. doi: 10.1002/jez.b.21063. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Brändle K, Fritzsch B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev Biol. 1995;168:358–373. doi: 10.1006/dbio.1995.1086. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Quart Rev Biol. 1983;58:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Holmes PH, Albert JS. Distribution and innervation of lateral line organs in the channel catfish. J Comp Neurol. 2000;421:570–592. [PubMed] [Google Scholar]
- O’Neill P, McCole RB, Baker CVH. A molecular analysis of neurogenic placode and cranial sensory ganglion development in the shark, Scyliorhinus canicula. Dev Biol. 2007;304:156–181. doi: 10.1016/j.ydbio.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Hirsch M-R, Goridis C, Brunet J-F. Control of hindbrain motor neuron differentiation by the homeobox gene Phox2b. Development. 2000;127:1349–1358. doi: 10.1242/dev.127.7.1349. [DOI] [PubMed] [Google Scholar]
- Pehrson T. The ontogeny of the lateral line system of the head of dipnoans. Acta Zool (Stockh) 1949;30:153–182. [Google Scholar]
- Popper AN, Fay RR. Evolution of the ear and hearing: issues and questions. Brain Behav Evol. 1997;50:213–221. doi: 10.1159/000113335. [DOI] [PubMed] [Google Scholar]
- Ranzi S. L’organo di senso spiracolare dei Selaci. Pubbl Staz Zool Napoli. 1926;7:37–76. [Google Scholar]
- Roberts BL, Meredith GE. The efferent system. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line. Neurobiology and Evolution. Springer-Verlag; New York: 1989. pp. 445–459. [Google Scholar]
- Roth A. Development of catfish lateral line organs: electroreceptors require innervation, although mechanoreceptors do not. Naturwissenschaften. 2003;90:251–255. doi: 10.1007/s00114-003-0424-5. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Feeding and breathing in lampreys. Brain Behav Evol. 1996;48:297–305. doi: 10.1159/000113208. [DOI] [PubMed] [Google Scholar]
- Saltys HA, Jonz MG, Nurse CA. Comparative study of gill neuroepithelial cells and their innervation in teleosts and Xenopus tadpoles. Cell Tissue Res. 2006;323:1–10. doi: 10.1007/s00441-005-0048-5. [DOI] [PubMed] [Google Scholar]
- Sapède D, Rossel M, Dambly-Chaudière C, Ghysen A. Role of SDF1 chemokine in the development of lateral line efferent and facial motor neurons. Proc Natl Acad Sci USA. 2005;102:1714–1718. doi: 10.1073/pnas.0406382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–1697. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Development and evolution of lateral line placodes in amphibians I. Development. Zoology (Jena) 2002b;105:119–146. doi: 10.1078/0944-2006-00058. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Development and evolution of lateral line placodes in amphibians. II. Evolutionary diversification. Zoology (Jena) 2002a;105:177–193. doi: 10.1078/0944-2006-00062. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Evolutionary origins of vertebrate placodes: insights from developmental studies and from comparisons with other deuterostomes. J Exp Zoolog B Mol Dev Evol. 2005;304:347–399. doi: 10.1002/jez.b.21055. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev Biol. 2004;271:439–466. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Shingles A, McKenzie DJ, Claireaux G, Domenici P. Reflex cardioventilatory responses to hypoxia in the flathead gray mullet (Mugil cephalus) and their behavioral modulation by perceived threat of predation and water turbidity. Physiol Biochem Zool. 2005;78:744–755. doi: 10.1086/432143. [DOI] [PubMed] [Google Scholar]
- Simmons DD. Development of the inner ear efferent system across vertebrate species. J Neurobiol. 2002;53:228–250. doi: 10.1002/neu.10130. [DOI] [PubMed] [Google Scholar]
- Simonetta AM. L’organo di senso dello spiracolo e l’organo paratimpanico nella sistematica dei Vertebrati. Arch It Anat Embriol. 1953;58:266–294. [PubMed] [Google Scholar]
- Simonetta AM. Distribuzione e significato dell’organo paratimpanico del Vitali. Att Soc Tosc Sci Nat Pisa. 1959;66:39–55. [Google Scholar]
- Smatresk NJ. Chemoreceptor modulation of endogenous respiratory rhythms in vertebrates. Am J Physiol. 1990;259:R887–897. doi: 10.1152/ajpregu.1990.259.5.R887. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kwak SJ, Fritz A. Genetic interactions underlying otic placode induction and formation. Dev Dyn. 2004;230:419–433. doi: 10.1002/dvdy.20067. [DOI] [PubMed] [Google Scholar]
- Stone LM, Finger TE, Tam PP, Tan SS. Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc Natl Acad Sci USA. 1995;92:1916–1920. doi: 10.1073/pnas.92.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. Origin of the vertebrate inner ear: evolution and induction of the otic placode. J Anat. 2001;199:99–103. doi: 10.1046/j.1469-7580.2001.19910099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S-K, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol. 2007;303:675–686. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Sundin L, Nilsson S. Branchial innervation. J Exp Zool. 2002;293:232–248. doi: 10.1002/jez.10130. [DOI] [PubMed] [Google Scholar]
- Sundin L, Reid SG, Rantin FT, Milsom WK. Branchial receptors and cardiorespiratory reflexes in a neotropical fish, the tambaqui (Colossoma macropomum) J Exp Biol. 2000;203:1225–1239. doi: 10.1242/jeb.203.7.1225. [DOI] [PubMed] [Google Scholar]
- Vitali G. Di un interessante derivato della prima fessura branchiale nel passero. Anat Anz. 1911;39:219–224. [Google Scholar]
- Vitali G. L’organo paratimpanico e la sua funzione. Riv Biol. 1921;3:302–316. [Google Scholar]
- Vitali G. Il comportamento dell’organo della prima fessura branchiale negli Anfibi, nei Rettili e nei Mammiferi. Ricerche di Morfologia. 1924;4:191–221. [Google Scholar]
- Vitali G. Il comportamento dell’organo della prima fessura branchiale (placode epibranchiale) nei Selaci. Monit Zool Ital. 1925;36:122–130. [Google Scholar]
- Vitali G. La façon de se comporter du placode de la première fente branchiale (placode épibranchial) dans la série des vertébrés. Arch Ital Biol. 1926;76:94–106. [Google Scholar]
- von Bartheld CS. Development and innervation of the paratympanic organ (Vitali organ) in chick embryos. Brain Behav Evol. 1990;35:1–15. doi: 10.1159/000115851. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Functional morphology of the paratympanic organ in the middle ear of birds. Brain Behav Evol. 1994;44:61–73. doi: 10.1159/000113570. [DOI] [PubMed] [Google Scholar]
- Weeg MS, Land BR, Bass AH. Vocal pathways modulate efferent neurons to the inner ear and lateral line. J Neurosci. 2005;25:5967–5974. doi: 10.1523/JNEUROSCI.0019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicht H, Northcutt RG. Ontogeny of the head of the Pacific hagfish (Eptatretus stouti, Myxinoidea): development of the lateral line system. Philos Trans R Soc Lond B. 1995;349:119–134. doi: 10.1098/rstb.1995.0098. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Yntema CL. Experiments on the origin of the sensory ganglia of the facial nerve in the chick. J Comp Neurol. 1944;81:147–167. [Google Scholar]