Abstract
Objectives
To examine contribution of social cognitive constructs to meeting physical activity (PA) recommendations in rural breast cancer survivors (BCS).
Methods
Rural BCS (N = 483) completed a mail-based survey. PA, fatigue, barriers and exercise self-efficacy, environment, social support, and perceived barriers to PA were assessed. PA was dichotomized into either meeting guidelines (150+minutes/week) or not.
Results
Our model fit the data well with less fatigue, greater efficacy, and lower barriers being associated with PA (χ²=804.532(418), p < .001, CFI=.948, RMSEA=.044, SRMR=.046).
Conclusions
Fatigue, self-efficacy, and perceived barriers are key targets for future interventions designed to increase PA in rural BCS. Enhancing self-efficacy and overcoming barriers will require strategies unique and relevant to BCS living in rural settings.
Keywords: cancer survivorship, behavioral medicine, physical activity, self-efficacy, rural health
INTRODUCTION
Physical activity improves emotional, physical, and physiological treatment side-effects and overall quality of life in breast cancer survivors.1–4 Physical activity is related to lower risk of all-cause mortality, breast cancer mortality and cancer recurrence for breast cancer survivors.5,6 Meeting or exceeding recommended levels of physical activity, ie, ≥ 150 minutes per week, has been associated with 50% reduction in recurrence and all-cause mortality.6,7
Despite the well-documented benefits of physical activity, it is estimated that 19% to 33% of breast cancer survivors meet the public health guidelines of 150+ minutes/week of moderate-to-vigorous physical activity, a threshold volume of physical activity associated with significant health benefits.8–10 Most breast cancer survivors experience a marked, often permanent, decline in physical activity levels within 12 months of diagnosis.11,12 Moreover, a mere 19% of breast cancer survivors living in rural, or non-metropolitan and non-suburban, areas report meeting physical activity guidelines. Such low participation levels could be related to environmental barriers, such as further distance to exercise facilities and lack of appropriate activity programs; social barriers, such as a lack of social support; or barriers relating to low confidence or lack of education related to cancer survivorship.13 Few studies have reported correlates of physical activity in rural breast cancer survivors.14–16 After breast cancer diagnosis, rural populations, who constitute one of the largest medically underserved groups of breast cancer survivors,14,15 are more likely to be sedentary and suffer poor health status.
To design interventions that address low physical activity adherence, several studies have reported correlates and determinants of physical activity in breast cancer survivors. However, it is noteworthy that little is known about rural breast cancer survivors specifically. Rural populations (not cancer specific) are less likely to be physically active and more likely to suffer poor health status.17 Similarly, health disparities potentially responsive to increasing physical activity exist for rural breast cancer survivors who suffer from poorer quality of life and functional well-being, when compared with their urban counterparts.15 Moreover, correlates of physical activity differ for urban and rural populations in non-cancer studies.18 Therefore, further research is needed to identify factors associated with physical activity in rural breast cancer survivors.
Adopting and sustaining regular physical activity requires a wealth of psychological and environmental resources. Social Cognitive Theory (SCT) models triadic reciprocity among an individual, his/her environment, and behavior and specifies a set of psychosocial determinants useful for understanding, explaining, and predicting physical activity behavior.19 Self-efficacy, the belief in personal abilities to successfully carry out a course of action, is the core construct of SCT and influences activity choice, amount of energy devoted to accomplishing tasks, and degree of perseverance in the face of barriers.19,20 Indeed among constructs of SCT, self-efficacy is one of the most consistent correlates of physical activity participation.21 Although self-efficacy is theorized to have a direct effect on behavior, it is also proposed to have indirect influences through sociostructural factors that can act as barriers and/or facilitators to executing the behavior. Additionally, there are a number of factors unique to cancer survivors that could act on personal efficacy beliefs. These include disease and treatment-related symptoms, such as fatigue, lack of social support, and environmental factors.22 For rural breast cancer survivors, such environmental conditions include lower access to treatment and recreation facilities, farther driving distances, and lack of sidewalks as compared to urban, micro-urban, or suburban survivors.
The purpose of the current study was to examine social cognitive correlates of meeting the public health recommendations of 150+ minutes/week of physical activity in rural breast cancer survivors. We hypothesized that perceptions of fatigue, social support, and environmental factors would influence exercise and barriers self-efficacy, which in turn would have a direct effect on meeting physical activity guidelines, and an indirect effect, via perceived barriers, to physical activity. We present data that support a framework for understanding and explaining adherence to physical activity recommendations in rural breast cancer survivors.
METHODS
Study Design
Sample selection, response rate, survey administration, and missing data for this cross-sectional study have been described elsewhere.23 Breast cancer survivors living in the most rural counties of a Midwest, U.S. state, completed a mail survey (see Table 1 for demographic information). A statewide cancer registry sent study information to 1598 eligible survivors; 560 agreed to allow the registry to release their contact information to investigators and were subsequently mailed the study survey. Approximately 86% returned completed surveys (N = 483). Overall response rate was 30%.24 The study protocol and consent waiver were approved by an institutional review board and the state cancer registry obtained its own review board approval and consent for release of potential participants’ contact information.
Table 1.
Demographic and Treatment-Related Sample Characteristics
| Sample (N = 483)
|
|||
|---|---|---|---|
| Characteristic | No. | % | |
| Age | |||
| <55 years | 128 | 26.6 | |
| 55 to 69 years | 208 | 43.3 | |
| ≥70 years | 145 | 30.1 | |
| Race | |||
| Caucasian | 468 | 96.9 | |
| African American | 9 | 1.9 | |
| Other | 9 | 1.2 | |
| Ethnicity | |||
| Hispanic | 5 | 1.0 | |
| Not Hispanic | 434 | 89.9 | |
| Declined | 44 | 9.1 | |
| Education | |||
| Less than high school | 42 | 8.8 | |
| High school graduate | 209 | 43.8 | |
| Some college | 119 | 24.6 | |
| College graduate | 55 | 11.4 | |
| Graduate degree | 52 | 10.9 | |
| Income | |||
| < $10,000 | 36 | 7.5 | |
| $10,000 to $19,9999 | 78 | 16.1 | |
| $20,000 to $34,999 | 103 | 21.3 | |
| $35,000 to $49,999 | 79 | 16.4 | |
| >$50,000 | 152 | 31.5 | |
| Received adjuvant therapy | |||
| Yes | 275 | 56.9 | |
| No | 208 | 43.1 | |
| Cancer stage | |||
| Stage 1 | 158 | 32.7 | |
| Stage 2 | 86 | 17.8 | |
| Stage 3 | 42 | 8.7 | |
| Stage 4 | 16 | 3.3 | |
| Unreported | 181 | 37.5 | |
| Beale Code: County Population | |||
| 1 (1 million +) | 22 | 4.6 | |
| 2 (250K to 1 million) | 31 | 6.4 | |
| 3 (metro areas <250K) | 21 | 4.3 | |
| 4 (20K +, adjacent to metro) | 36 | 7.5 | |
| 5 (20K +, non-adjacent to metro) | 14 | 2.9 | |
| 6 (2.5K to 19.9K adjacent to metro) | 164 | 34.0 | |
| 7 (2.5K to 19.9K, non-adjacent to metro) | 122 | 25.3 | |
| 8 (<2.5K, adjacent to metro) | 12 | 2.5 | |
| 9 (<2.5K, non-adjacent to metro) | 61 | 12.6 | |
Measures
Physical activity
Physical activity was measured by self-report using the Godin Leisure-time Exercise Questionnaire.25 Participants reported frequency and duration of mild, moderate, and strenuous physical activity (of at least 15 minutes per bout) per week on average during the past month. Duration was multiplied by frequency to calculate weekly minutes spent engaging in each exercise intensity level. Total minutes per week of moderate plus strenuous activity were summed and a categorical variable reflecting whether participants met recommendations defined as reporting ≥ 150 weekly minutes of moderate + strenuous activity per week was calculated.9 We did not separate time spent in aerobic versus resistance training but rather calculated total time. Meeting the physical activity recommendations was coded as ‘1’ and failure to meet recommendations was coded as ‘0’.
Demographic, cancer-specific, and medical variables
Participants self-reported age in years, ethnicity, race, annual household income, and years of education. As noted previously by Rogers and colleagues,23 education rather than income was used as the marker of socioeconomic status because all participants provided years of education but 28 refused to report their annual household income. Due to the representation of only 2 races/ethnicities, race was dichotomized as White (N = 465, 96.3%) or African-American (N = 18; 3.7%). Rurality was assessed, based on participant zip code, using Beale Codes (also known as Rural-Urban Continuum Codes), which was developed by the United States Department of Agriculture and ranges from 0 (counties in metro areas of > 1 million population) to 9 (countries in rural areas of < 2500 population and not adjacent to a metropolitan area). Participants were asked to report breast cancer stage (~30% of participants did not know their cancer stage), time since diagnosis, whether the participant underwent surgery for their breast cancer, and type of non-surgical breast cancer treatment (ie, none, chemotherapy, radiation therapy, anti-hormone therapy, or other). Due to transition in the coding system related to cancer stage used by the state cancer registry at the time of registry data extraction, cancer stage data was not available directly from the registry but were obtained using self-report.
Fatigue
Fatigue was assessed using the sum of the 13-item Functional Assessment of Cancer Therapy-Fatigue (FACT-F) scale (0 to 4 Likert scale for a possible range of 0 to 52).26 The FACT-F assesses physical fatigue and the effects of fatigue on daily life including productivity, eating, social interaction, and sleep quality. Example statements include: “I feel fatigued”, “I am too tired to eat”, “I have trouble finishing things because I am tired”, and “I have to limit my social activities because I am too tired.” Responses range from “0-not at all” to “4-very much”. A higher score indicates greater fatigue in this report. These data indicated scale reliability was excellent (ω = .916). The omega reliability coefficient (ω = (Σλi)²/([Σλi]²+Σδii) was calculated from standardized estimates based on loadings (λ) and residual variances (δii).
Social cognitive theory constructs
To measure barriers self-efficacy, 8 items asked participants how confident they were that they could exercise in different situations (ie, lack discipline, exercise not a priority, weather is bad, tired, not interested, do not enjoy exercise, and do not have someone to encourage me to exercise).27 The 4 items measuring exercise self-efficacy included confidence in 1) ability to walk briskly for 20 minutes, 2) run or jog for 10 minutes, 3) climb 3 flights of stairs without stopping, and 4) exercise for 20 minutes at a level hard enough to cause a large increase in heart rate and breathing.27 For both self-efficacy scales, Likert-type responses of 0% (not at all confident) to 100% (extremely confident) in 10% increments were used. Reliability omega coefficients for exercise (ω = .90) and barrier (ω = .94) self-efficacy were excellent.
Four items assessed exercise social support (ie, how often friends or family offered to exercise with them or gave them encouragement to stick with their exercise program) on a Likert scale of 0 (none) to 4 (very often).22,28 Participants could answer “does not apply” if they were currently not participating in physical activity. Reliability was good (ω = .870).
Perceived barriers to exercise were assessed with a 20-item questionnaire whereby participants rated whether specific barriers, such as lack of time, lack of enjoyment, weather, etc., interfered with exercise since their cancer diagnosis on a Likert scale of 1 (disagree) to 5 (agree).29 Reliability of the scale was good (ω = .877).
An assessment of perceived environmental facilitators was obtained by asking participants to rate agreement with 7 different statements related to their neighborhood on a 4-point Likert scale, ie, 1=strongly agree to 4=strongly disagree.30,31 These statements asked about easy walking distance from home to retail businesses, home within a 10–15 minute walk to transit stop, presence of sidewalks, bicycle/pedestrian paths in or near neighborhood, recreational facilities, crime rate, and trust in neighbors. Reliability omega coefficient was acceptable (ω = .68).
Data Analysis
Analyses were conducted within a latent variable framework using Mplus version 7.0 with full information maximum likelihood robust (MLR) estimation.32 We conducted confirmatory factor analysis for all primary study variables including fatigue, social support, perceived environmental facilitators, task self-efficacy, barrier self-efficacy, and perceived barriers to physical activity. When evaluating the fit of the model to the data, multiple indices were considered including the chi-square statistic (significant p values indicating a better fit), the comparative fit index (CFI; > .95 suggests good model-data fit), the root mean square error of approximation (RMSEA; values < .06 suggests a good fit), and the square root mean residual (SRMR; < .06 suggests a good fit). Measurement models were assessed for each construct individually before they were entered into an overall theoretical model. All possible paths were tested and the significant paths reported in the final models. We tested 2 structural models of examining social cognitive correlates of 1) total physical activity and 2) meeting physical activity guidelines. Finally, logistic regression was used to estimate odds ratios among predictors and the categorical outcome of meeting physical activity guidelines.
A preliminary series of analyses included the following covariates in the structural models: race, education, residential Beale code, time since diagnosis, and whether participants ever received chemotherapy, radiation, and/or adjuvant therapy (therapy in addition to original treatment such as hormone therapy). These analyses indicated that none of the cancer-related variables were associated with any of the model constructs and were subsequently dropped in the final analyses for parsimony.
RESULTS
Descriptive Statistics
As previously reported, 483 female, rural breast cancer survivors (Mage = 63±12 years) participated.23 The vast majority of the sample was White Non-Hispanic and most participants had received at least a high school education. Mean months since diagnosis was 39±21.5. Nearly all of the participants (93%) had received adjuvant therapy and 56.9% were currently receiving adjuvant therapy. The mean number of comorbidities was 2.6±2.2. Participants came from counties coded from 6 to 9 using the Beale Code (range is from ‘0’- completely urban to ‘9’-completely rural). The majority of the sample (77%) came from somewhat rural locations with county population between 2500 to 19,999 individuals. The remaining (23%) lived in completely rural counties with less than 2500 residents. Mean minutes of moderate-to-vigorous physical activity per week were 78.28 ± 6.7 and 19.2% of the sample was meeting physical activity recommendations. Means and standard deviations for all demographic variables are presented in Table 1.
Measurement Model
Initially, we conducted a series of confirmatory factor analyses of each measure to assess the factor structure of untested scales. Uni-dimensional models for fatigue (χ² = 5.653 (1), p < .05, CFI = .995, RMSEA = .098, SRMR = .006), social support (χ² = 3.182 (1), p = .07, CFI = .991, RMSEA = .067, SRMR = .019), perceived environmental facilitators (χ² = 18.422 (4), p < .01, CFI = .953, RMSEA = .088, SRMR = .036), exercise self-efficacy (χ² = .036 (2), p = .982, CFI = 1.00, RMSEA = .000, SRMR = .001), and barrier self-efficacy (χ² = 55.672 (19), p < .001, CFI = .975, RMSEA = .064, SRMR = .024) fit the data well. However, preliminary results suggested that barriers to exercise did not reflect a single dimension (χ² = 1162.386 (170), p < .001, CFI = .683, RMSEA = .111, SRMR = .097). To ensure that we tested theoretical pathways across uniform levels of analysis, we proceeded to examine the scale structure via exploratory structural equation modeling.33 This resulted in dropping 7 of the original 20 items. The remaining items represented 4 distinct factors including external, self-regulation, physical, and time, which reflect barriers consistent with prior physical activity research on women’s barriers in a variety of demographic populations.34,35 The external factor may be especially important for rural breast cancer survivors as items included lack of equipment, no facilities or space, and cost of exercising. Further information regarding items constituting these factors is available upon request. In addition, the 4 emergent factors in our sample are identical to the 4 dimensions in the Physical Activity Barrier Scale by Sechrist, Walker, and Pender,36 used with young, middle-aged, and older adult men and women. This model fit the data well (χ² = 135.268 (58), p < .001, CFI = .955, RMSEA = .053, SRMR = .046) and was used as the basis for computing composite indicators of a uni-dimensional latent barriers to exercise construct (χ² = 148.717(60), p = .000, CFI = .948, RMSEA = .056, SRMR = .053).
Next, we assessed the full measurement model, including fatigue, social support, perceived environmental facilitators, task self-efficacy, barrier self-efficacy, and perceived barriers to exercise. According to the majority of fit indices, this model fits the data well (χ² = 628.674 (56), p < .001, CFI = .958, RMSEA = .043, SRMR = .046). Therefore, we proceeded with testing pathways.
Structural Model
The full model, including the measurement model (ie, 6 latent factors) and the structural model (ie, hypothesized direct and indirect pathways) was tested, with physical activity as both a continuous variable (model 1) and categorical variable of either meeting or not meeting physical activity guidelines (model 2-represented in Figure 1). Structural equation modeling with a categorical primary outcome provides path relationship information but no fit indices. In order to examine model fit indices, we ran the model first with continuous physical activity as the primary outcome. Model 1 fit the data well (χ² = 804.532 (418), p < .001, CFI = .948, RMSEA = .044, SRMR = .046). The results, path estimations and R2 values, were consistent when we re-ran the model (2) with meeting physical activity recommendations as a categorical dependent outcome.
Figure 1.
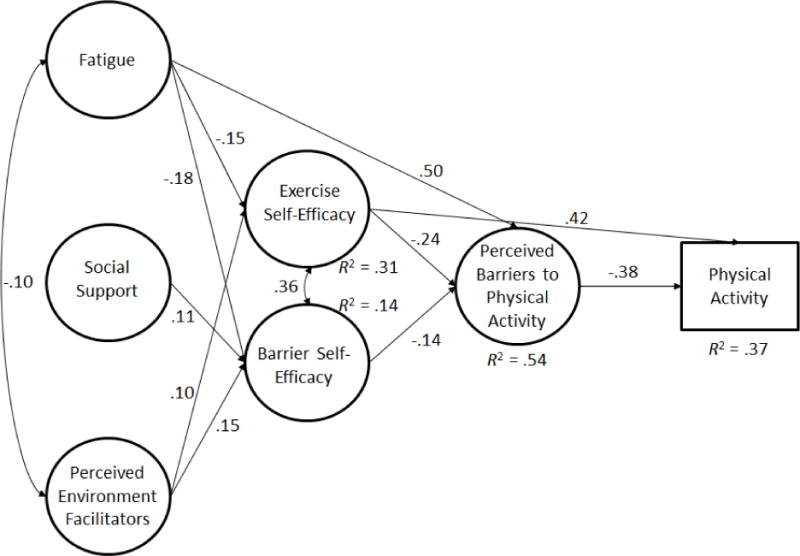
Structural Model of Social Cognitive Correlates of Meeting Physical Activity Guidelines
Note. All paths were significant, p < .05
Consistent with SCT, we found significant direct effects of fatigue on exercise self-efficacy (β = −.15, p < .01) and barrier self-efficacy (β = −.18, p < .01); an effect of social support on barrier self-efficacy (β = .11, p < .05); and perceived environmental facilitators had a direct effect on exercise self-efficacy (β = .11, p < .05) and barrier self-efficacy (β = .15, p < .01). Additionally, fatigue had a direct effect on perceived barriers to exercise (β = .50, p < .001).
Exercise self-efficacy, in turn, had a direct effect on perceived barriers to exercise (β = −.24, p < .001), as did barrier self-efficacy (β = −.13, p < .05). Again, only exercise self-efficacy had a direct effect on physical activity (β = .37, p < .001). Perceived barriers to exercise was directly, and negatively, associated with physical activity (β = −.18, p < .05).
There was a significant indirect effect of fatigue on physical activity through exercise self-efficacy (β = −.07, p < .01) and through barriers (β = −.12, p < .05). Perceived environmental facilitators had an indirect effect on physical activity through exercise self-efficacy (β = .06, p < .05). Overall, model 1 predicted 20% of the variance in physical activity.
We re-ran the model with meeting physical activity recommendations as a categorical outcome variable (model 2; 0 = not meeting recommendations, 1 = meeting recommendations). The relationships observed in model 2 were identical to model 1 and of similar magnitudes, although the direct effect of barriers on meeting physical activity recommendations was substantially larger than in Model 1 (β = −.38, p < .01). Logistic regression odds ratio analysis indicated that exercise self-efficacy alone had a direct effect on meeting physical activity recommendations, where every unit increase in efficacy was associated with a 2.266 increase in the odds of meeting physical activity recommendations (see model 2). Overall, model constructs accounted for 37% of the variance in meeting physical activity recommendations (See Figure 1).
There were several significant associations among our covariates and other model constructs. Participants with higher levels of education had higher barrier (β = .08, p < .05) and exercise (β = .13, p < .001) self-efficacy. Number of co-morbidities was significantly correlated with: barrier (β = −.05, p < .05) and task (β = −.18, p < .001) self-efficacy; social support (β = −.06, p < .01); fatigue (β = .17, p < .001); and perceived environmental facilitators (β = −.06, p < .05). Finally, a higher Beale code (more rural) was associated with lower social support (β = −.05, p < .05) and lower perceived environmental facilitators (β = −.06, p < .05). Somewhat surprisingly, physical activity was positively correlated with number of comorbidities (β = .16, p < .01).
DISCUSSION
The public health guidelines for physical activity provide a threshold for gaining substantial health benefits in both healthy and diseased populations. Understanding which factors influence adherence to these recommendations is important for the design of interventions and behavior change programs. Rural individuals represent an underserved group in terms of healthcare, as evidenced by health indicators such as mortality and morbidity,23 and are at higher risk of compromised quality of life15 and low physical activity levels.9,10 Due to differences in access to healthcare programs and other environmental barriers, increasing physical activity levels in rural breast cancer survivors could require specific strategies. Our data underscore the importance of social cognitive approaches to the promotion of physical activity behavior in rural breast cancer survivors and, importantly, identify factors influencing self-efficacy that may reflect the specific needs of rural breast cancer survivors.
Fatigue is commonly identified as a barrier to physical activity in breast cancer survivors37,38 and was indeed the strongest correlate of perceived barriers to physical activity in these data. In an earlier report from this dataset,39 we reported that fatigue levels were associated with differing levels of physical activity whereby those with higher fatigue also reported lower levels of physical activity, especially gardening, leisure-time activity, and moderate physical activity. Individuals with the lowest fatigue levels engaged in the most physical activity (≥500 metabolic equivalent (MET)-minutes/week). However, it is proposed that fatigue may respond to exercise, in a linear manner, due to gradual increases in muscle strength and fitness.23 Additionally, Courneya and colleagues40 reported that long-term adherence to public health physical activity guidelines, after participation in an exercise trial for breast cancer patients, was associated with better quality of life and less fatigue at a 6 month follow-up. Clearly, there are reciprocal relationships between fatigue and physical activity. Although our cross-sectional data are open to other interpretations relative to directionality, we believe that they provide the foundation for testing these relationships in a longitudinal, multi-time point manner.
Environmental conditions experienced by rural breast cancer survivors can be markedly different from those experienced by urban or suburban survivors. Rural breast cancer survivors have different physical activity programming needs due to differing social capital structure and environmental conditions.16 Our measure of perceived environmental facilitators included assessment of the walkability to shops, stores, and markets; the presence of sidewalks; the presence of areas for biking, such as bike paths; and the local accessibility to recreation facilities or public playgrounds, swimming pools, etc. The combination of these environmental characteristics contributed to both barrier and exercise self-efficacy. Duncan, Spence, & Mummery41 reported physical environment characteristics such as walkability to shops, the presence of sidewalks, and perceived accessibility to fitness centers, were associated with greater rates of physical activity. A supportive physical environment is necessary but, in and of itself, is insufficient to increase community physical activity levels.42,43 Such an environment must be combined with individual and social determinants to effectively promote physical activity behavior. Our data suggest that perceptions of the environment are associated with meeting physical activity guidelines through self-efficacy, which may explain the requirement yet insufficiency of environmental factors to elicit physical activity. Bandura suggests 2 aspects of behavioral control which are integral to successful behavior change: 1) level and strength of personal efficacy to produce changes and 2) the modifiability of the environment. Granted, some aspects of environment are difficult, if not impossible, to change. For example, participants reported lack of access to easy shopping (70.1%), public transport (92.1%), neighborhood sidewalks (61.8%), places to bike (69.8%), and free or low-cost recreational facilities (70.0%). However, most individuals believed that the neighborhood was safe enough to walk around in (82.9%) and that their neighbors could be trusted (86.7%). When environmental factors cannot be modified, SCT proposes a shift towards personal agency to accomplish behavior change goals.
It is important to note that those who have firm beliefs in their efficacy find ways of exercising some control, even in environments containing limited opportunities and multiple constraints.20 Individuals with high efficacy for regular physical activity tend to persevere in the face of barriers such as poor weather, lack of motivation, distance, and time constraints; indeed, self-efficacy has greater predictive ability under more challenging circumstances.21,44,45 Interestingly, this sample of rural breast cancer survivors rated “lack of discipline,” “procrastination,” “exercise not a priority,” and “exercise not in routine” as the most common barriers to physical activity and “fear of injury,” “lack of knowledgeable exercise staff,” “cost of exercising,” and “no facilities or space,” as the least commonly experienced physical activity barriers. Environmental factors can influence physical activity behavior indirectly via self-efficacy.46
These data also suggest that social support is another key contributor to physical activity behavior. As modeled, social support is a source of efficacy information for this population47 and this may be of particular importance for breast cancer survivors living in rural areas.16,48 Given their lower accessibility to medical centers and health programming, rural breast cancer survivors need more emotional support after diagnosis.48 Social support is multifaceted49 and determining which types of support are more likely to influence physical activity in rural breast cancer survivors would be useful for designing interventions to maximize physical activity.
Commonly used strategies for facilitating social support among breast cancer survivors include connecting with other breast cancer survivors, getting support from friends and family, and having a good relationship with their medical provider.48 A recent meta-analytic review indicated that social support is a consistent determinant of physical activity in cancer survivors of all types and concluded that more strategies need to be developed to enhance social support in physical activity programming.50 Our data suggest this relationship to be an indirect effect via self-efficacy. Self-efficacy mediates the relationship between social support and perceived barriers to physical activity and ultimately to physical activity itself. It is important to understand and foster social support in light of its targeted influence on self-efficacy. Focusing on social interaction, which builds efficacy, would be instrumental to lower perceived barriers and increase adherence to physical activity recommendations. The social capital of rural individuals can be increased via the strengthening and leveraging of existing social ties. Increased number of connections could be fostered through internet-, text-, or phone-based social support for cancer survivors. In recent years, the internet has been increasingly used to foster social interaction and relationships. The accessibility and use of internet in a variety of populations (eg, older adults and rural individuals) is increasing.51 The majority of our sample reported having access to internet (66.9%) and most of them had internet access from home (61.5%). Indeed, breast cancer patients have reported feelings of empowerment related to internet-based cancer-specific social support groups.52 The internet and social media may represent an important resource for fostering social support and improved self-efficacy in rural breast cancer survivors.
Our data indicate that both barrier and exercise self-efficacy are associated with fewer perceived barriers, which is not surprising. Self-efficacy to overcome physical activity barriers, such as lack of time or bad weather, alters the perception of those barriers.53 Consistent with previous research, the direct path from exercise self-efficacy to physical activity was significant. Rural breast cancer survivors face unique challenges related to self-efficacy. Physical distance and isolation can limit access, not only to cancer centers and fitness facilities but also social support and cancer-specific social modeling of physical activity behavior.
To increase physical activity levels in this population, it is crucial to decrease perceived barriers to physical activity. Targeting self-efficacy, through both 1) the classical self-efficacy information sources and 2) perceived environmental facilitators, fatigue, and social support, may be a powerful strategy for decreasing perceived barriers and ultimately increasing physical activity levels in rural breast cancer survivors. The results from this study clarify the relational effects of social support, perceived environmental factors, and fatigue on adherence to public health physical activity guidelines through self-efficacy among rural breast cancer survivors. Several pertinent practical applications are evident. Targeting social cognitive constructs is relatively low-cost and feasible, compared to changing the environment, and can be achieved by: increasing social support for rural breast cancer survivors—perhaps through virtual breast cancer support groups; designing programs that gradually increase volume of physical activity allowing individuals to experience mastery; providing education to breast cancer survivors about how their bodies will respond to physical activity; and teaching individuals to notice and take advantage of environmental facilitators to physical activity (such as walkable or paved roads with low traffic) that are accessible to rural individuals. Whereas these approaches often require significant financial and human resources, they provide a more feasible solution compared to changing environmental or system-level factors and a more ethical solution ignoring the needs of underserved populations. The degree of impact is an important question. The presented model accounts for 54% of the variance in perceived barriers to physical activity and 37% of the variance in reported physical activity. While caution is warranted because these are subjectively reported outcomes, there is potential for a large effect on physical activity behavior in the population through targeting the model’s constructs.
We note several limitations to this report. The study sample primarily consisted of White Non-Hispanic individuals residing in central Illinois. Despite the fact that this study applies a novel extension of the application of social cognitive theory, through exploring influences of environment, social support, and fatigue on self-efficacy, on rural breast cancer survivors’ physical activity patterns, the results may not be applicable to rural racial/ethnic minorities. In addition, all measures are subject to the same biases inherent in self-report. Additionally, the environment scale needs further validation in rural populations as the wording (eg, being within 10–15 minutes travel distance) may not be best suited to rural individuals. The psychometric testing presented here is a preliminary step. However, specific scales require further longitudinal invariance testing in rural populations.
A significant limitation is the subjective nature of the Godin Leisure Time Questionnaire in measuring physical activity behavior. Subjective assessments of physical activity can differ greatly from objective measurements. Finally, the study design was cross-sectional in nature, preventing any conclusions regarding causal inference. Although causal modeling analysis was utilized,54 results should not be interpreted as causal effects. Future research is warranted to establish whether the working model can be expanded upon to help explain temporal relationships and long-term adherence to physical activity recommendations.
In conclusion, the results from this study may have important implications for understanding adherence to public health guidelines for physical activity in rural breast cancer survivors. Several pertinent implications for practice are evident including understanding the relationships of social support, environment, fatigue, and self-efficacy to physical activity behavior in rural breast cancer survivors. Recommendations for clinicians and public health professionals include: 1) targeting self-efficacy through the classic sources of self-efficacy information—skill mastery, social persuasion, social modeling, and interpretation of physiological and psychological states20; 2) targeting self-efficacy through cognitive reframing of fatigue, enhancing social support, and teaching individuals to notice and take advantage of the physical activity supportive features of the rural environment; 3) creating multi-media modes for reaching rural breast cancer survivors and fostering multiple types of social support; and 4) designing interventions with physical activity prescriptions that gradually increase in difficulty (time and intensity) in order to not overly fatigue patients.
Table 2.
Loadings for the 4-factor Solution to the Barriers to Exercise Scale
| Item | Barrier | External Factor 1 | Self – Regulation Factor 2 | Physical Factor 3 | Time Factor 4 |
|---|---|---|---|---|---|
| 1 | lack of interest | ||||
| 2 | lack of self-discipline | .80 | |||
| 3 | lack of time | .50 | |||
| 4 | lack of company | ||||
| 5 | lack of enjoyment | ||||
| 6 | lack of equipment | .78 | |||
| 7 | Weather | ||||
| 8 | lack of skills | ||||
| 9 | no facilities or space | .80 | |||
| 10 | pain or discomfort | .79 | |||
| 11 | fear of injury | .62 | |||
| 12 | cost of exercising | .62 | |||
| 13 | fatigue, lack of energy | .72 | |||
| 14 | exercise is boring | ||||
| 15 | lack of knowledgeable exercise staff | ||||
| 16 | inconvenient exercise schedule | .79 | |||
| 17 | Procrastination | .79 | |||
| 18 | not a priority | .77 | |||
| 19 | family responsibilities | .51 | |||
| 20 | not in routine | .79 |
Acknowledgments
This research was supported by American Cancer Society, Illinois Division #PSB05-03; National Cancer Institute R01 CA136859; National Cancer Institute R01 CA136859; the National Institute on Aging R01AG020118; National Heart Lung and Blood Institute R21 HL11341001; National Institute on Aging F31 AG042232; Canada Research Chairs Program; American Institute of Cancer Research Grant 10A-048.
Footnotes
Human Subjects Statement
The protocol was approved by the Springfield Committee for Research Involving Human Subjects (SCRIHS) at Southern Illinois University School of Medicine (#04-012).
Conflict of Interest Statement
All authors declare no conflict of interest.
Contributor Information
Erin A. Olson, Research Assistant, University of Illinois at Urbana-Champaign, Department of Kinesiology and Community Health, Urbana, IL.
Sean P. Mullen, Assistant Professor, University of Illinois at Urbana-Champaign, Department of Kinesiology and Community Health, Urbana, IL.
Laura Q. Rogers, Professor, University of Alabama at Birmingham, Department of Nutrition Sciences, Birmingham, AL.
Kerry S. Courneya, Professor & Canada Research Chair, University of Alberta, Faculty of Physical Education and Recreation, Edmonton, AB.
Steven Verhulst, Professor, Southern Illinois University School of Medicine, Springfield, IL.
Edward McAuley, Professor, University of Illinois at Urbana-Champaign, Department of Kinesiology and Community Health, Urbana, IL.
References
- 1.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108:279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 3.Milne HM, Gordon S, Guilfoyle A, et al. Association between physical activity and quality of life among Western Australian breast cancer survivors. Psychooncology. 2007;16:1059–1068. doi: 10.1002/pon.1211. [DOI] [PubMed] [Google Scholar]
- 4.Alfano CM, Smith AW, Irwin ML, et al. Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: a prospective analysis. Journal of Cancer Survivorship. 2007;1:116–128. doi: 10.1007/s11764-007-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friendenreich CM, Gregory J, Kopciuk KA, et al. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer. 2009;124:1954–1962. doi: 10.1002/ijc.24155. [DOI] [PubMed] [Google Scholar]
- 6.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 7.Sternfeld B, Weltzien E, Quesenberry CP, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18:87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484–1491. doi: 10.1249/01.MSS.000007460.03001.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskell WL, Lee I-M, Pate RR, et al. American College of Sports Medicine and American Heart Association. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONNAHA.1071185649. [DOI] [PubMed] [Google Scholar]
- 10.Reis JP, Bowles HR, Ainsworth BE, et al. Nonoccupatioal physical activity by degree of urbanization and U.S. geographic region. Med Sci Sports Exerc. 2004;36:2093–2098. doi: 10.1249/01.mss.0000147589.98744.85. [DOI] [PubMed] [Google Scholar]
- 11.Devoogdt N, Van Kampen M, Geraerts I, et al. Physical activity levels after treatment for breast cancer: one-year follow-up. Breast Cancer Res Treat. 2010;123:417–425. doi: 10.1007/s10549-010-0997-6. [DOI] [PubMed] [Google Scholar]
- 12.Littman AJ, Tang M-T, Rossing MA. Longitudinal study of recreational physical activity in breast cancer survivors. Journal of Cancer Survivorship. 2010;4:119–127. doi: 10.1007/s11764-009-0113-2. [DOI] [PubMed] [Google Scholar]
- 13.Rogers LQ, Markwell SJ, Courneya KS, et al. Physical activity type and intensity among rural breast cancer survivors: patterns and associations with fatigue and depressive symptoms. Journal of Cancer Survivorship. 2011;5:54–61. doi: 10.007/s11764-010-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettencourt BA, Schlegal RJ, Talley AE, Molix LA. The breast cancer experience of rural women: a literature review. Psychooncology. 2007;16:875–887. doi: 10.1002/pon.1235. [DOI] [PubMed] [Google Scholar]
- 15.Redi-Arndt SA, Cox CR. Does rurality affect quality of life following treatment for breast cancer? Journal of Rural Health. 2010;26:402–405. doi: 10.1111/j.1748-0361.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallance JK, Lavallee C, Culos-Reed NS, Trudeau MG. Predictors of physical activity among rural and small town breast cancer survivors: an application of the theory of planned behaviour. Psychology, Health & Medicine. 2014;17(6):685–697. doi: 10.1080/13548506.2012.659745. [DOI] [PubMed] [Google Scholar]
- 17.Eberhardt MS, Pamuk ER. The importance of place of residence: examining health in rural and nonrural areas. Am J Public Health. 2004;94(10):1682–1686. doi: 10.2105/ajph.94.10.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parks SE, Housemann RA, Brownson RC. Differential correlates of physical activity in urban and rural adults of various socioeconomic backgrounds in the United States. J Epidemiol Community Health. 2003;57:29–35. doi: 10.1136/jech.57.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandura A. Social Foundations of Thoughts and Action. Englewood Cliff, NJ: Prentice Hall; 1986. [Google Scholar]
- 20.Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: Freeman; 1997. [Google Scholar]
- 21.McAuley E, Blissmer B. Self-efficacy determinants and consequences of physical activity. Exerc Sport Sci Rev. 2000;28:85–88. [PubMed] [Google Scholar]
- 22.Rogers LQ, McAuley E, Courneya KS, Verhulst SJ. Correlates of physical activity self-efficacy among breast cancer survivors. American Journal of Health Behavior. 2008;36:594–603. doi: 10.5555/ajhb.2008.32.6.594. [DOI] [PubMed] [Google Scholar]
- 23.Rogers LQ, Markwell SJ, Verhulst S, et al. Rural breast cancer survivors: exercise preferences and their determinants. Psychooncology. 2009;18:412–421. doi: 10.1002/pon.1497. [DOI] [PubMed] [Google Scholar]
- 24.Rogers LQ, Markwell SJ, Courneya KS, et al. Physical activity type and intensity among rural breast cancer survivors: patterns and associations with fatigue and depressive symptoms. Journal of Cancer Survivorship. 2011;5:54–61. doi: 10.1007/s11764-010-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77:359–362. [PubMed] [Google Scholar]
- 26.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 27.Rogers LQ, Courneya KS, Verhulst SS, et al. Exercise barrier and task self-efficacy in breast cancer patients during treatment. Support Care Cancer. 2006;14:84–90. doi: 10.1007/s00520-005-0851-2. [DOI] [PubMed] [Google Scholar]
- 28.Sallis JF, Grossman RM, Pinski RB, et al. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 29.Rogers LQ, Courneya KS, Shah P, et al. Exercise stage of change, barriers, expectations, values and preferences among breast cancer patients during treatment: a pilot study. European Journal of Cancer Care. 2007;16(1):55–66. doi: 10.1111/j.1365-2354.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 30.Brownson RC, Chang JJ, Eyler AA, et al. Measuring the environment for friendliness toward physical activity: a comparison of the reliability of 3 questionnaires. Am J Public Health. 2004;94:473–483. doi: 10.2105/ajph.94.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirtland KA, Porter DE, Addy CL, et al. Environmental measures of physical activity supports: perception versus reality. Am J Prev Med. 2003;24:323–331. doi: 10.1016/S0749-3797(03)00021-7.. [DOI] [PubMed] [Google Scholar]
- 32.Muthén L, Muthén B. Mplus User’s Guide [Computer software and manual] Muthén & Muthén; Los Angeles, CA: 1998–2012. p. 7. [Google Scholar]
- 33.Asparouhov T, Muthén B. Exploratory structural equation modeling. Structural Equation Modeling. 2009;16:397–438. [Google Scholar]
- 34.Cerin E, Leslie E, Sugiyama T, Owen N. Perceived barriers to leisure-time physical activity in adults: an ecological perspective. Journal of Physical Activity & Health. 2010;7:451–459. doi: 10.1123/jpah.7.4.451. [DOI] [PubMed] [Google Scholar]
- 35.Salmon J, Owen N, Crawford D, et al. Physical activity and sedentary behavior: a population-based study of barriers, enjoyment, and preference. Health Psychol. 2003;22:178–188. doi: 10.1037//0278-6133.22.2.178. [DOI] [PubMed] [Google Scholar]
- 36.Sechrist KR, Walker SN, Pender NJ. Development and psychometric evaluation of the exercise benefits/barriers scale. Res Nurs Health. 1987;10:357–365. doi: 10.1002/nur.4770100603. [DOI] [PubMed] [Google Scholar]
- 37.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 38.Courneya KS, Friedenreich CM, Reid RD, et al. Predictors of follow-up exercise behavior 6 months after a randomized trial of exercise training during breast cancer chemotherapy. Breast Cancer Res Treat. 2009;114:179–187. doi: 10.1007/s10549-008-9987-3. [DOI] [PubMed] [Google Scholar]
- 39.Rogers LQ, Markwell SJ, McAuley E, et al. Physical activity type and intensity among rural breast cancer survivors: patterns and associations with fatigue and depressive symptoms. Journal of Cancer Survivorship. 2011;5:54–61. doi: 10.1007/s11764-010-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Courneya KS, Segal RJ, Gelmon K, et al. Six-month follow-up of patient-rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiol Biomarkers Prev. 2007;16:2572–2578. doi: 10.1158/1055-9965.EPI-07-0413. [DOI] [PubMed] [Google Scholar]
- 41.Duncan MJ, Spence JC, Mummery WK. Perceived environment and physical activity: a meta-analysis of selected environmental characteristics. International Journal of Behavioral Nutrition & Physical Activity. 2005;2:11. doi: 10.1186/1479-5868-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giles-Corti B, Donovan RJ. The relative influence of individual, social and physical environment determinants of physical activity. Soc Sci Med. 2002;54:1793–1812. doi: 10.1016/s0277-9536(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 43.Nagel CL, Carlson NE, Bosworth M, Michael YL. The relation between neighborhood built environment and walking activity among older adults. American Journal of Epidemiology. 2008;168:461–468. doi: 10.1093/aje/kwn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dishman RK, Motl RW, Sallis JF, et al. Self-management strategies mediate self-efficacy and physical activity. Am J Prev Med. 2005;29(1):10–18. doi: 10.1016/j.amepre.2005.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAuley E, Szabo A, Gothe N, Olson EA. Self-efficacy: implications for physical activity, function, and functional limitations in older adults. American Journal of Lifestyle Medicine. 2011;5(4):361–369. doi: 10.1177/1559827610392704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNeill LH, Wyrwich KW, Brownson RC, et al. Individual, social environmental, and physical environmental influences on physical activity among black and white adults: a structural equation analysis. Ann Behav Med. 2006;31:36–44. doi: 10.1207/s15324796abm3101_7. [DOI] [PubMed] [Google Scholar]
- 47.Duncan TE, McAuley E. Social support and efficacy cognitions in exercise adherence: a latent growth curve analysis. J Behav Med. 1993;16:199–218. doi: 10.1007/BF00844893. [DOI] [PubMed] [Google Scholar]
- 48.Wilson SE, Andersen MR, Meischke H. Meeting the needs of rural breast cancer survivors: what still needs to be done. Journal of Women’s Health & Gender-Based Medicine. 2000;9:667–677. doi: 10.1089/15246090050118198. [DOI] [PubMed] [Google Scholar]
- 49.Weiss RS. The provisions of social relations. In: Rubin Z, editor. Doing unto others. Englewood Cliffs, NJ: Prentice Hall; 1974. [Google Scholar]
- 50.Barber FD. Social support and physical activity engagement by cancer survivors. Clinical Journal of Oncology Nursing. 2012;16:E84–E98. doi: 10.1188/12.CJON.E84-E98.. [DOI] [PubMed] [Google Scholar]
- 51.Pew Research Center. Older adults and technology use. 2014 Apr; Available at: http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use/. Accessed Accessed July 8, 2014.
- 52.Høybye MT, Johansen C, Tjørnhøj-Thomsen T. Online interaction. Effects of storytelling in an internet breast cancer support group. Psychooncology. 2005;14:211–220. doi: 10.1002/pon.837. [DOI] [PubMed] [Google Scholar]
- 53.Rogers LQ, Markwell SJ, Hopkins-Price P, et al. Reduced barriers mediated physical activity maintenance among breast cancer survivors. Journal of Sport & Exercise Psychology. 2011;33:235–254. doi: 10.1123/jsep.33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearl J. Causal inference in statistics: an overview. Statistics Surveys. 2009;3:96–146. doi: 10.1214/09-SS057.. [DOI] [Google Scholar]


