Abstract
Netrin-1 has been shown to regulate the function of the EGF-like protein Cripto-1 (Cr-1) and affect mammary gland development. Since Cr-1 is a target gene of Nanog and Oct4, we investigated the relationship between Netrin-1 and Cr-1, Nanog and Oct4 during different stages of development in the mouse mammary gland. Results from histological analysis show that exogenous Netrin-1 was able to induce formation of alveolar-like structures within the mammary gland terminal end buds of virgin transgenic Cripto-1 mice and enhance mammary gland alveologenesis in early pregnant FVB/N mice. Results from immunostaining and Western blot analysis show that Netrin-1, Nanog and Oct4 are expressed in the mouse embryonic mammary anlage epithelium while Cripto-1 is predominantly expressed outside this structure in the surrounding mesenchyme. We find that in lactating mammary glands of postnatal FVB/N mice, Netrin-1 expression is highest while Cripto-1 and Nanog levels are lowest indicating that Netrin-1 may perform a role in the mammary gland during lactation. HC-11 mouse mammary epithelial cells stimulated with lactogenic hormones and exogenous soluble Netrin-1 showed increased beta-casein expression as compared to control thus supporting the potential role for Netrin-1 during functional differentiation of mouse mammary epithelial cells. Finally, mouse ES cells treated with exogenous soluble Netrin-1 showed reduced levels of Nanog and Cripto-1 and higher levels of beta-III tubulin during differentiation. These results suggest that Netrin-1 may facilitate functional differentiation of mammary epithelial cells and possibly affect the expression of Nanog and/or Cripto-1 in multipotent cells that may reside in the mammary gland.
The postnatal mammary gland undergoes different stages of development and differentiation. From the initial stages of puberty in which allometric ductal growth and lateral side branching occur, through pregnancy and lactation where milk proteins such as, beta-casein are expressed, the mammary gland is subject to various degrees of proliferation and differentiation, and with apoptosis occurring during involution (Medina, 1996). For these reasons the mammary gland represents an ideal organ for the study of local factors that regulate tissue development and morphogenesis. Systemic hormonal influences and receptor status including estrogen, progesterone, prolactin and growth factors such as amphiregulin, transforming growth factor beta, fibroblast growth factors and insulin-like growth factors, as well as extracellular matrix components and the presence of certain immune cell types that reside in the mammary gland have all been shown to contribute to different phases of pre- and postnatal mammary gland development (Woodward et al., 1998; Hens and Wysolmerski, 2005). However, further insight is needed on how multipotent cells residing in the mammary gland, and factors that affect their function, are involved in controlling these developmental processes since deregulation of the homeostatic control of mammary cell proliferation and differentiation has been suggested to represent an initiating step towards mammary gland tumorigenesis (Brisken and Duss, 2007; Stingl and Caldas, 2007).
The transcription factors Oct4 and Nanog and the Nodal co-receptor Cripto-1 (Cr-1), have been shown to regulate pluripotency, self-renewal, cellular commitment and differentiation of mouse and human embryonic stem (ES) cells (Pan et al., 2002; Loh et al., 2006; Assou et al., 2007; Pan and Thomson, 2007). Expression of Oct4 and Nanog is not restricted to ES cells as previously thought but can also be detected in multipotent cells of adult tissues and/or in their direct progeny, transit amplifying cells, also referred to as committed precursor cells, and thereby possibly performing an important role in regulating genes involved in differentiation and repopulation of distinct cell types within adult organs or tissues (Tai et al., 2005; Diaz-Flores et al., 2006; Yu et al., 2006; Ratajczak et al., 2007).
Cripto-1 is an epidermal growth factor-like protein that has been extensively studied in developmental biology and shown to play a role in cellular transformation and tumorigenesis (Strizzi et al., 2005a). In fact, the overexpression of human Cripto-1 (CR-1) in transgenic mice models has been associated with an increased incidence of tumors of the mammary gland and of the uterus (Sun et al., 2005; Wechselberger et al., 2005; Strizzi et al., 2007). With respect to mammary epithelial cells, the overexpression of Cr-1, using a retroviral vector, was shown to significantly increase growth rates and cell densities of mouse mammary epithelial CID 9 cells as compared to parental and vector control cells (Niemeyer et al., 1998). Moreover, Cr-1 overexpression or treatment with exogenous recombinant Cr-1 protein reduced the expression of the milk proteins beta-casein and whey acidic protein (WAP) in mouse mammary epithelial cells in response to lactogenic hormones suggesting that Cr-1 might stimulate mammary epithelial cell proliferation at the expense of differentiation (De Santis et al., 1997).
There is little information on the expression pattern of Nanog and/or Oct4 during the development of the mammary gland. We hypothesize that since Cripto-1 has been shown to be a direct target gene in mouse and human ES cells for Nanog and Oct4 (Loh et al., 2006; Assou et al., 2007), the expression of these ES cell related transcription factors might also vary, like Cr-1, during the different stages of mammary gland development and that this variability may be regulated by extracellular factor(s) present in the mammary gland.
The neural guidance molecule, Netrin-1 has been detected in the mouse mammary gland and shown to promote adhesion between cap cells and subtending body epithelial cells in the mammary gland terminal end bud (TEB; Srinivasan et al., 2003). Netrin-1 has also been shown to antagonize Cripto-1 induced invasion and migration of mammary epithelial cells in vitro and allometric mammary ductal outgrowth in vivo (Strizzi et al., 2005b). These observations suggest that Netrin-1 may represent a morphogenic factor that is capable of regulating mammary development and/or differentiation. Since the mammary gland is embryologically derived from the neuroectoderm (Hennighausen and Robinson, 2005), it is possible that neuronal related morphogenic factor(s) may affect the development and/or function of this organ.
In this study, we describe the morphological and developmental effects of Netrin-1 in mammary glands of virgin CR-1 transgenic mice and in early pregnant FVB/N mice. In postnatal FVB/N mouse mammary glands, we find that the expression of Netrin-1 inversely correlates with that of Cr-1 and Nanog, particularly during lactation, indicating a possible role for Netrin-1 during functional differentiation of mammary epithelial cells. In fact, our results demonstrate that Netrin-1 can significantly enhance the effect of lactogenic hormones in HC-11 mouse mammary epithelial cells. The potential role for Netrin-1 during differentiation is further supported by our results showing regulation of Cr-1 and Nanog expression by Netrin-1 in mouse ES cells.
Materials and Methods
Antibodies and reagents
All antibodies were used for both immunohistochemistry (IHC; 1:100) and Western blotting (WB; 1:1,000) unless specified. The following antibodies against: type IV collagen (rabbit), Oct4 (rabbit for IHC), alpha-fetoprotein (goat) and Netrin-1 (rabbit for IHC), were all purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Netrin-1 (rabbit, Ab-1 for WB) was purchased from Oncogene Research (San Diego, CA). Antibodies against Nanog (rabbit), Oct4 (rabbit for WB) beta-III tubulin (rabbit), and Brachyury (rabbit) were purchased from Abcam (Cambridge, MA). Antibody against mouse Cr-1 (rabbit) was purchased from Rockland Immunochemicals (Gilbertsville, PA) and the antibody against CK8 (mouse) was purchased from Novocastra (Newcastle, UK). Rabbit anti-mouse milk specific protein antibody (RAM/MSP), which recognizes beta-casein as a double band at approximately 25 kDa (Morwald et al., 2007), was purchased from Nordic Immunological Laboratories (Tilburg, Netherlands). Recombinant mouse Netrin-1 (rmNetrin-1) and recombinant mouse Cripto-1 (rmCr-1) proteins were purchased from R&D Systems (Minneapolis, MN). Dexamethasone, bovine insulin, and ovine prolactin were all purchased from Sigma (St. Louis, MO).
In vivo mammary gland experiments and whole mount analysis
Tissue sections of pubescent virgin 11- to 12-week-old FVB/N (n = 4) or MMTV-CR-1 (n = 5) female mice obtained from a previous in vivo study (Strizzi et al., 2005b) were analyzed. In addition, cholesterol pellets were formulated to continuously release 50 ng/day of rmNetrin-1 or 100 ng/day of rmCripto-1 for approximately 2 weeks as previously described (Vonderhaar, 1987). A total of 22 female virgin FVB/N mice were used. The prepared pellets were surgically inserted into the right inguinal mammary gland at approximately the same distance from the mammary lymph node in the pellet implanted mice as follows: four FVB/N female mice were implanted with control cholesterol only pellets, four were implanted with rmCr-1 releasing pellets, four more were implanted with the rmNetrin-1 releasing pellets and a final group of four FVB/N mice received both rmCr-1 and rmNetrin-1 releasing pellet implants. A group of six FVB/N mice did not receive any pellets and used as pellet free controls. Three days after implantation of the pellets the female mice were allowed to mate with FVB/N male mice except for three FVB/N mice without pellets which were segregated and not allowed to mate and used as pellet free non-pregnant controls. Ten days after insertion of the pellets, the mammary glands from each group of mice were surgically removed and analyzed by whole mount morphology. For whole mount preparation, mammary glands were spread and fixed on glass slides with Carnoy’s solution (glacial acetic acid/ethanol; 1:3) for 60 min at room temperature. The glands were rehydrated and stained overnight in aluminum carmine solution. The glands were subsequently dehydrated, cleared in xylene and mounted for microscopic observation at 8× and 40× magnifications. Digital microphotographs were taken using a Polaroid DMC-1 digital camera (Polaroid, Cambridge, MA) mounted on a Leica MZ125 microscope (Leica, Wetzlar, Germany). Care and use of the experimental animals for this study was in compliance with the relevant animal welfare laws, guidelines and policies at the National Institutes of Health.
Immunohistochemistry and Western blotting
Paraffin-embedded sections were prepared and obtained from C57Bl/6 mice embryos containing the mammary anlage at days E12 (n = 4) and E13 (n = 4) and from mammary glands of postnatal FVB/N mice of 5- and 10-week-old virgin, 8- and 14-day pregnancy, 2- and 21-day lactation and 2- and 5-day involution. Sections were deparaffinized, rehydrated and IHC performed as previously described (Strizzi et al., 2005b; Sun et al., 2005). Mammary tissue samples were also collected from the same mice above and quickly frozen on dry ice and subsequently processed for WB as previously described (Strizzi et al., 2004). IHC and WB were performed on samples from at least three different animals at each developmental stage.
Induction of beta-casein in HC-11 cells
Mouse mammary HC-11 epithelial cells were cultured under conditions for induction of milk protein expression as previously described (De Santis et al., 1997). Recombinant mCr-1 (100 ng/ml) and rmNetrin-1 (100 ng/ml) alone or in combination were added to complete media (RPMI + 10% FBS) containing the lactogenic hormones dexamethasone (1 µM), insulin (5 µg/ml), and prolactin (5 µg/ml) (DIP) to confluent HC-11 cells and cultured for two days. After 2 days medium was changed and fresh DIP containing rmCr-1 and/or rmNetrin-1 medium was added and cultured for an additional 2 days. To determine beta-casein expression, the cells were then harvested for protein and RNA analysis by WB and qRT-PCR, respectively.
Mouse ES cell culture
Rosa 26 mouse ES cells (Zambrowicz et al., 1997) were cultured in ES media (Knockout DMEM containing 15% Knockout SR, 2 mM l-Glutamine, Penicillin and Streptomycin, HEPES, 0.1 mM 2-mercaptoethanol, and MEM non-essential amino acids (Invitrogen, Carlsbad, CA) and supplemented with 1,000 units of LIF (Chemicon, Temecula, CA)). The ES cells were grown for 3 days on mouse embryonic fibroblasts (MEFs) on 0.1% Gelatin coated tissue flasks. MEFs were harvested from 12.5-day-old mouse embryos and irradiated at 60 Gy 24 h before plating the ES cells. ES cells were harvested from the MEFs using collagenase IV (Sigma; 1.5 mg/ml in ES media) and 0.25% Trypsin/EDTA as to disrupt the ES aggregates into single cell suspensions and then plated at 5 × 105 cells/well in 0.1% gelatin coated 6 well tissue culture plates in ESGRO medium (Millipore, Temecula, CA) and in the absence of MEFs. Treatment of ES cells with 100 ng/ml of rmNetrin was added 24 h later and left in culture for 3 or 7 days. ES cells were then harvested and protein was extracted for WB and RNA was collected using the mini RNeasy kit (Qiagen, Valencia, CA) for quantitative real-time PCR (qRT-PCR). Experiments were performed in triplicate and repeated three times.
Quantitative real-time PCR method and primer sequences
Quantitative RT-PCR was performed using a MX3000P PCR and Brilliant SYBR Green QPCR master mix (Stratagene, La Jolla, CA) and comparative analysis between the genes of interest and the housekeeping gene GAPDH. cDNA was made from 1 µg RNA using the Retroscript kit (Ambion, Austin, TX) then diluted 1:8 in DNAse/RNAse free water. PCR was performed as follows: 40 × (10 min 95°C, 30 sec 95°C, 1 min 50°C, 1 min 72°C). The sequences of the primer sets for GAPDH, OCT4, CR-1, and Nanog are as follows: GAPDH For: 5′-TTGATGGCAACAATCTCCAC-3′ and Rev: 5′-CGTCCCGTAGACAAAATGGT-3′; Oct4 For: 5′-GGCGTTCTCTTTGGAAAGGTGTTC-3′ and Rev: 5′-CTCGAACCACATTCCTTCTCT-3′; CR-1 For: 5′-GACTGGGGAAACAGAGTGGA-3′ and Rev: 5′-ACTCGGGTGTTTGGGTTTGC-3′; Nanog For: 5′-CTCTTCAAGGCAGCCCTGAT-3′ and Rev: 5′-CCATTGCTAGTCTTCAACCAC-3′; beta-casein For: 5′-ATCCTTTCAGCTTCACCTCCTC-3′ and Rev: 5′-GTCCATGGGTCGAATTCAAATG-3′.
Results
Netrin-1 induces morphological alterations within the terminal end buds of nulliparous MMTV-CR-1 transgenic mice
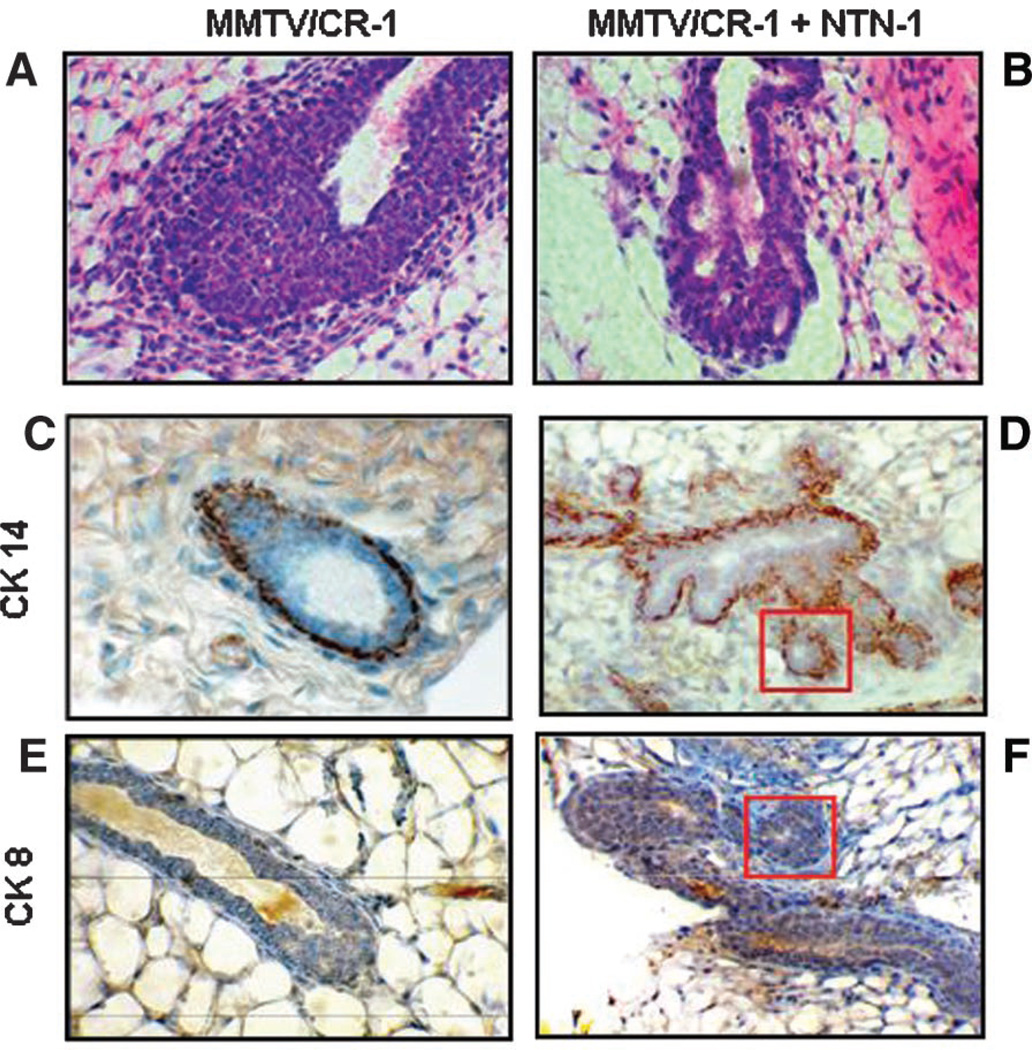
Previous experiments have shown that exogenous soluble Netrin-1 is capable of inhibiting Cr-1 induced migration and epithelial to mesenchymal transition (EMT) of mouse mammary epithelial cells in vitro and significantly reduces mammary ductal outgrowth in vivo in 7-week-old virgin MMTV-CR-1 transgenic mice (Strizzi et al., 2005b). Further histological analysis of hematoxylin and eosin (H&E) stained sections of these mouse mammary glands from the 7-week-old virgin MMTV-CR-1 transgenic mice shows that virgin MMTV-CR-1 control mice contain bud-like, multi-layered epithelium characteristic of normal mouse mammary TEBs (Fig. 1A). In contrast, mammary glands from 7-week-old virgin MMTV-CR-1 mice implanted with rmNetrin-1 releasing pellets, show multiple, circular epithelial structures contained within the TEBs (Fig. 1B). Results from immunostaining for CK14, used to detect myoepithelial cells (Smith et al., 1990; Sapino et al., 1993), shows the characteristic distribution of CK14 expression in the TEBs of control MMTV-CR-1 transgenic mice corresponding to the single row of myoepithelial cells that normally surround mammary gland TEBs (Fig. 1C). Instead, positive staining for CK14 was detected around each individual circular epithelial structure that formed within the TEBs of the Netrin-1 treated MMTV-CR-1 transgenic mice (Fig. 1D). Staining for CK8, which generally stains positive for mammary luminal cells, was very weak in the mammary TEBs from control MMTV-CR-1 transgenic mice (Fig. 1E) as compared to the mammary TEBs from the Netrin-1 treated MMTV-CR-1 mice that showed positive staining particularly in the individual circular epithelial structures (Fig. 1F). Since alveolar structures in the mammary gland consists of myoepithelial cells and luminal cells (Smith et al., 1990), the positive staining for CK14 and CK8 in the aberrant circular epithelial structures contained within the TEBs from the Netrin-1 treated MMTV-CR-1 mammary glands suggest that Netrin-1 may be regulating the differentiation and/or spatial organization of the cellular components within the mouse mammary gland in favor of mammary gland alveolar formation.
Fig. 1.
Exogenous soluble rmNetrin-1 affects the morphology and differentiation of TEBs in mammary glands of MMTV-CR-1 transgenic mice. When compared to normal TEBs of MMTV-CR-1 transgenic mice containing the control cholesterol only releasing pellets (A), the TEBs of the MMTV-CR-1 transgenic mice treated with the rmNetrin-1 releasing pellets show multiple, alveolar-like structures (B and boxes in D, F). Parts (C,D) show the distribution of myoepithelial cells as determined by immunostaining for CK14. Immunostaining for the luminal cell marker CK8 showed weak staining in the TEBs of the MMTV-CR-1 control mice (E) and strong staining in the TEBs of the Netrin-1 treated MMTV-CR-1 transgenic mice (F) suggesting an alveolar-like differentiation (CK14+; CK8+) within the TEBs of the Netrin-1 treated MMTV-CR-1 transgenic mice.
Netrin-1 enhances alveolar development in early pregnant FVB/N mice
Since Netrin-1 could induce the formation of alveolar-like structures within the mammary gland TEBs of 7-week-old virgin MMTV-CR-1 transgenic mice and because normal 7-week-old virgin FVB/N mice treated with Netrin-1 releasing pellets showed no effect on either ductal outgrowth or TEB morphology (Strizzi et al., 2005b), we next investigated whether Netrin-1 could affect the development of the mammary gland in normal FVB/N mice during early pregnancy.
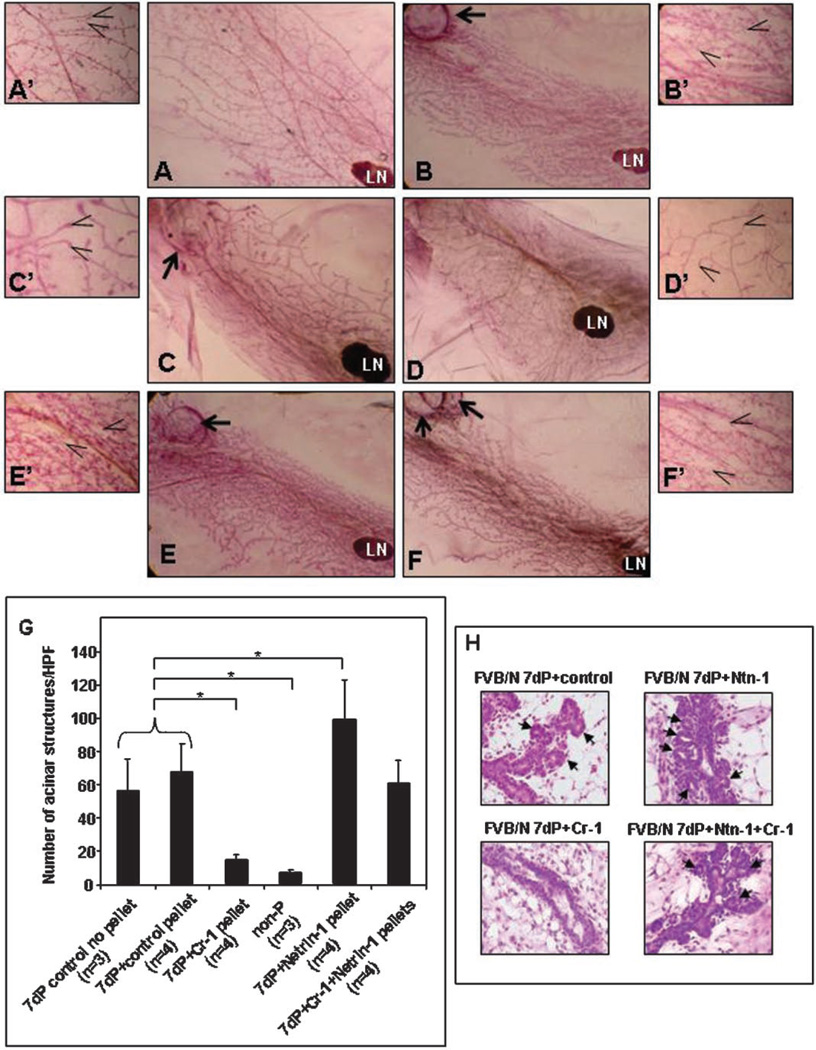
As compared to day 7 pregnant FVB/N control mice without pellet implants (Fig. 2A,A′) or day 7 pregnant FVB/N mice with control pellets (Fig. 2B,B′), the mammary glands from day 7 pregnant FVB/N mice implanted with rmCr-1 releasing pellets showed significantly reduced numbers (P < 0.05) of mammary alveolar structures (Fig. 2C,C′,G) that morphologically and quantitatively resembled the alveolar structures that are found in the mammary glands from pubescent non-pregnant FVB/N mice (Fig. 2D,D′,G). In contrast, day 7 pregnant FVB/N mice implanted with rmNetrin-1 releasing pellets showed significantly greater counts (P < 0.05) of mammary alveolar structures as compared to day 7 pregnant control mice (Fig. 2E,E′,G). Day 7 pregnant FVB/N mice implanted with both rmCr-1 and rmNetrin-1 releasing pellets showed alveolar development comparable to that observed in the FVB/N control mice (Fig. 2F,F′,G) suggesting that Netrin-1 was able to inhibit any negative effect of rmCr-1 on alveolar development as observed in FVB/N mice that had been treated with rmCr-1 releasing pellets. The histogram in Figure 2G represents the quantification of the alveolar structures in each group of day 7 pregnant FVB/N mice counted in four different fields (40×) in the mammary gland whole mounts from three separate mice for each condition. Detailed histological analysis clearly showed the numerous alveolar structures that formed in day 7 pregnant FVB/N mammary glands containing the Netrin-1 releasing pellet compared to the scarce development of alveolar structures that formed in the day 7 pregnant FVB/N mammary glands implanted with the Cr-1 releasing pellet (Fig. 2H). The experiment was also performed in midpregnant FVB/N mice where the pellets were inserted at 14 days pregnancy. The mammary glands from these more advanced pregnant FVB/N mice showed complete mammary gland maturation with no differences in the morphology or numbers of alveolar structures between the various groups (data not shown). This suggests that the morphological effect produced by Cr-1 and Netrin-1 are restricted to the initial phases of pregnancy during mammary development.
Fig. 2.
Whole mount (WM) morphology of mammary glands from FVB/N mice. Untreated mammary gland from a 7-day pregnant (dP) FVB/N mouse or with control cholesterol pellet show comparable WM morphology (A,A′ vs. B,B′) and alveolar counts (G). WM morphology of a 7 dP FVB/N mouse containing rmCR-1 releasing pellet (C,C′) shows significantly reduced (P < 0.05) alveolar counts (G) which is similar to the WM morphology (D,D′) and alveolar counts (G) of normal non-pregnant (non-P) pubescent FVB/N control mice. Treatment with rmNetrin-1 caused enhanced development (E,E′) and significant increase (P < 0.05) in alveolar counts (G) in mammary glands of 7 dP FVB/N mice. WM morphology (F,F′) and alveolar counts (G) was similar to that of control mice when both rmCR-1 and rmNetrin-1 pellets were combined. H&E sections in (H) show the histological details of the alveolar structures (arrows) that formed during the different treatment conditions. Arrows in A to F point to surgically implanted pellets. Open arrows in A′ to F′ point to alveolar structures counted for quantification in (G). (LN = lymph node; *P < 0.05). Magnification of A to F = 10×; magnification of A′ to F′ and H = 40×.
Cripto-1, Netrin-1, Nanog and Oct3/4 are expressed in the mouse mammary anlage
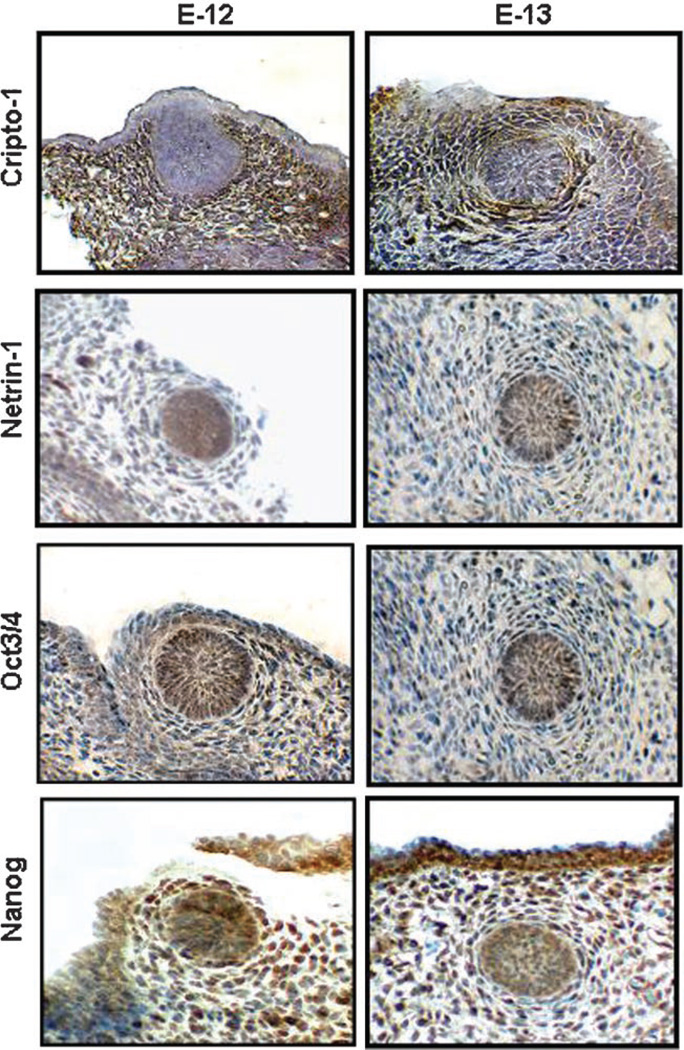
To ascertain the possible expression of Cr-1 and Netrin-1 during embryonic development in the mouse mammary gland, we performed IHC on mouse tissue sections that were obtained from day E12 and E13 mouse embryos where the primitive mammary anlage can be detected along the ventral regions. Since the ES cell related transcription factors, Nanog and Oct4, have been shown to regulate Cr-1 expression and are known to play a role during cellular differentiation in mouse and human ES cells (Loh et al., 2006; Assou et al., 2007), we also examined their expression by IHC in the mouse mammary anlage tissue sections.
Histological analysis of sagittal sections demonstrates the first appearance of the mammary anlage in late day E10 to early day E11 embryos as mammary epithelial buds or placodes and become spatially located at specific sites along the milk line on the ventral ectoderm body axis (Howard and Ashworth, 2006). By day E12 to E13 all five pairs of mammary buds are distinct. IHC revealed expression of Cr-1, Netrin-1, Nanog and Oct4 in the mouse mammary anlage at days E12 and E13 (Fig. 3). Cr-1 was detected mainly in the mesenchyme surrounding the epithelial mammary placode whereas Netrin-1 was predominantly expressed in the epithelial placodes suggesting that Cr-1 may represent a possible modifier in the development of the mammary mesenchyme as opposed to Netrin-1, which may be more important for mammary epithelial placode development. Immunostaining for Oct4 revealed nuclear expression in a few mesenchymal cells and throughout the epithelial placodes of the mammary anlage. Nanog expression was predominantly nuclear in mesenchymal cells surrounding the epithelial mammary placode and in cells within the epithelial placode. These results demonstrate the expression of ES cell related transcription factors in the mouse mammary anlage and suggest a potential role for these genes in regulating embryonic mammary gland development in the mouse.
Fig. 3.
Immunohistochemistry results show expression and localization of Cr-1, Netrin-1, Oct4 and Nanog in E12 and E13 mouse embryonic mammary placodes. Cr-1 is predominantly expressed in the placode mesenchyme while Netrin-1 appears to be expressed mostly in the epithelium. Oct4 is expressed in the nuclei of cells in both epithelium and mesenchyme of E12 and E13 mouse embryonic mammary placode whereas Nanog appears to be expressed mostly in the nuclei of cells of the mesenchyme with some expression in the nuclei of the epithelium.
Cripto-1, Netrin-1, Nanog and Oct4 are variably expressed at different stages in the postnatal mammary gland
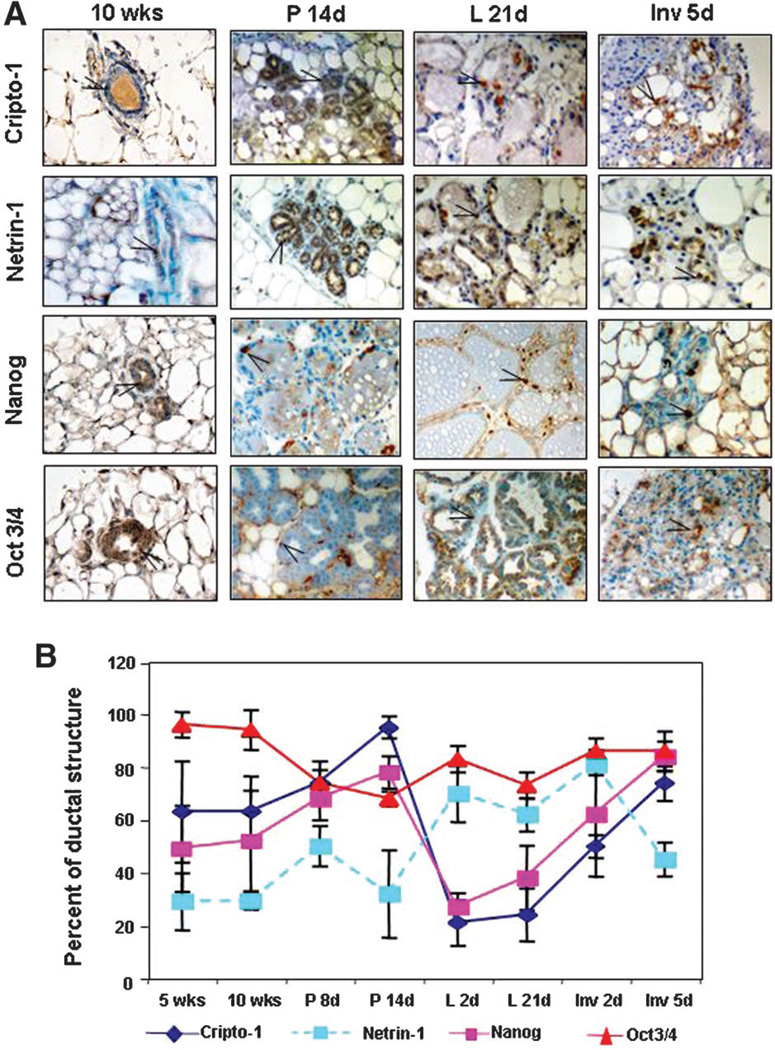
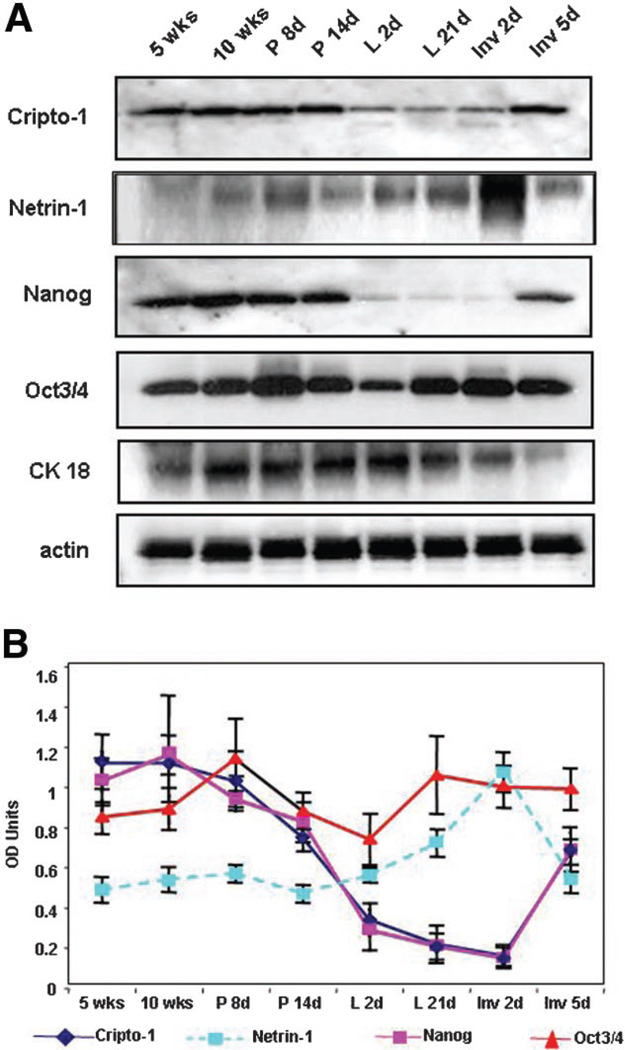
To examine the expression pattern of Cr-1, Netrin-1, Nanog and Oct4 during the postnatal development of the mouse mammary gland, we performed IHC on mammary gland tissue sections of FVB/N mice at the following stages of development: 5- and 10-week virgin; 8- and 14-day pregnant; 2- and 21-day lactation; 2- and 7-day involution.
Results from IHC demonstrate that Cr-1, Netrin-1, Nanog and Oct4 are expressed throughout the different stages of development of the postnatal mouse mammary gland (Fig. 4A). Within the mammary epithelium, Cr-1 and Netrin-1 show mostly cytoplasmic staining while Nanog and Oct3/4 generally stain the nuclei of positive cells. Occasionally, cells within the mammary gland stroma also show positive staining for Nanog and Oct4. To quantify and compare the expression of these markers, the percentage of positive epithelial cells within a mammary gland duct or alveolar structure was calculated from four separate fields at high power (40×) for each of three separate mice analyzed at each developmental stage and the mean values graphically represented (Fig. 4B). These results demonstrate lower levels of expression for Netrin-1 as compared to Cr-1 and Nanog in the mammary gland from 5- to 10-week virgin mice and in 8- and 14-day pregnant mice (Fig. 4B). However at 2 and 21 days of lactation, the percentage of Netrin-1 positive cells increases above the level observed in the virgin and pregnant mammary gland and remains high through to day 2 of involution and then declines to the levels detected before lactation. In contrast, the percentage of Cr-1 and Nanog positive epithelial cells significantly decreases at day 2 and 21 of lactation reaching values that are lower than those found in the virgin and pregnant stages. The percentage of Cr-1 and Nanog positive cells then increase at days 2 and 5 of involution, returning to approximately the same levels that were found during pregnancy and in the virgin mice. The percentage of mammary epithelial cells that stained for Oct4 remained generally constant and higher than the percentage of mammary epithelial cells expressing either Cr-1, Nanog or Netrin-1 at almost every stage of mammary gland development analyzed except at day 14 of pregnancy were Oct4 was slightly lower than the values calculated for Cr-1 and Nanog, although still remaining greater than the percentage of Netrin-1 positive cells. From these IHC results it appears that Cr-1 and Nanog are expressed in a parallel pattern during the different stages of postnatal mouse mammary gland development and both Cr-1 and Nanog change in the same direction when compared to Netrin-1 expression during pregnancy, lactation and involution. This suggests that Cr-1 and Nanog may play an opposing role to Netrin-1 during the development of the mouse mammary gland in these stages of mammary gland development.
Fig. 4.
Immunohistochemistry staining in (A) shows expression of Cr-1, Netrin-1, Nanog and Oct4 in tissue sections from postnatal mouse mammary glands at different stages of development. The graph in (B) represents the percentage of positive epithelial cells staining for Cr-1, Netrin-1, Nanog and Oct4 within a mammary gland duct or alveolar structure. The percentage of Oct4 staining remains relatively constant throughout the developmental stages analyzed where as the percentage of Cr-1 and Nanog immunostaining decreases during lactation. In contrast, the percentage of Netrin-1 immunostaining increases during lactation suggesting that Netrin-1 may play a role during the differentiation of mammary epithelial cells during lactation. Open arrow indicates example of positive staining.
Cripto-1, Netrin-1, Nanog and Oct4 are variably expressed in tissue extracts from mouse mammary glands obtained at different stages of postnatal development
The histological distinction of the epithelial component of the mammary ductal or alveolar structures from the surrounding cellular components of the stroma can be difficult, especially in pregnant and lactating glands, where these structures are tightly compressed and their cellular boundaries are not clearly defined. To obviate this technical difficulty and to more accurately quantify the level of expression of Cr-1, Netrin-1, Nanog and Oct4, we obtained mammary gland tissue samples corresponding to the same developmental stages that were analyzed by IHC and then performed Western blot analysis for Cr-1, Netrin-1, and Nanog and Oct4 for protein expression. The intensity of the bands were analyzed and quantified by densitometry.
The expression levels of Cr-1, Oct4, and Nanog and Netrin-1 proteins were found to vary according to the different stages of development in normal FVB/N mouse mammary glands as determined by WB analysis (Fig. 5A). Results from semiquantitative analysis using densitometry to measure the intensity of the bands shown in the Western blot of Figure 4A were normalized to the expression of epithelial cell content as determined by CK18 expression (Fig. 5B). The results demonstrate that Cr-1 and Nanog are expressed in a similar pattern during the different stages of mammary gland development with the highest level of expression for these two proteins being detected in the mammary glands of 5- and 10-week virgin mice and in early and mid-pregnant mice. The levels of Cr-1 and Nanog expression decreased as the mammary gland progressed through lactation, reaching the lowest level during early and late lactation and in early involution. The expression level of Oct4 was less variable as compared to that of Cr-1 and Nanog with only a modest increase in expression during early pregnancy and modest decrease during early lactation at day 2. The expression level of Netrin-1 generally remained low but constant in the mammary glands of 5- and 10-week virgin mice and during early and late pregnancy and early lactation. During late lactation and early involution, Netrin-1 levels significantly increased returning to virgin levels in late involution. The expression level of Netrin-1 was highest when the levels of Cripto-1 and Nanog were lowest, again suggesting that Netrin-1 may perform a different role, in particular during lactation, where differentiation in the mammary gland is extensive, and that this might possibly be accomplished by regulating the expression and/or activity of Cr-1 and/or Nanog.
Fig. 5.
Western blot results in (A) show the pattern of expression for Cr-1, Netrin-1, Nanog and Oct4 in tissue extracts from postnatal mouse mammary gland at different stages of development. Results from densitometric analysis of the Western blots in (A) are shown in (B). Similar to the immunostaining results, expression of Oct4 protein remains fairly constant in the mouse mammary glands during the different stages of development analyzed. Cr-1 and Nanog shows a decrease in protein expression in the mouse mammary glands during lactation and early involution whereas Netrin-1 expression is highest during these same developmental stages. Densitometric values (OD units) were normalized to the epithelial content of the mammary gland, as determined by the expression for cytokeratin CK18.
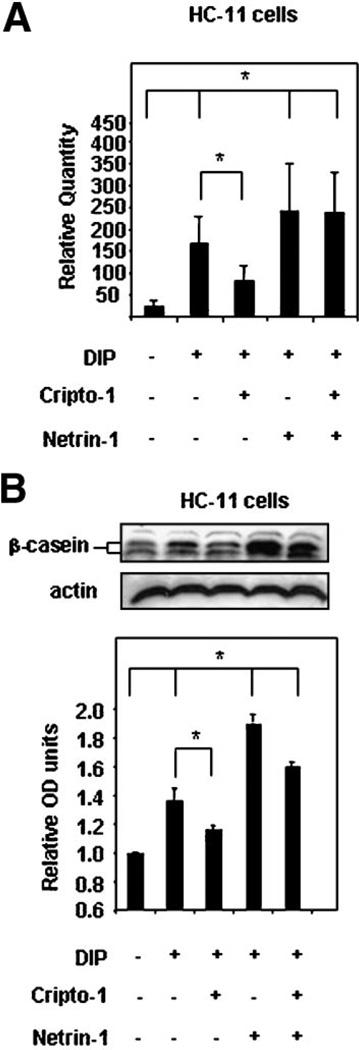
Netrin-1 accentuates the effect of dexamethasone, insulin, and prolactin (DIP) on beta-casein expression in mouse mammary epithelial HC-11 cells
Previous studies have shown that mouse mammary epithelial cells in vitro express milk proteins after treatment with DIP (Ball et al., 1988; Hynes et al., 1990). It has also been demonstrated that mouse mammary epithelial cells after DIP treatment exhibit a reduced response to beta-casein expression in the presence of exogenous soluble Cr-1 suggesting that Cr-1 is capable of inhibiting lactogenesis and therefore differentiation of mammary epithelial cells in vitro (De Santis et al., 1997; Niemeyer et al., 1998). Since Netrin-1 expression appears to increase in the mammary gland of FVB/N mice during lactation, it is possible that it may have an effect on milk protein expression in mammary epithelial cells. When HC-11 mouse mammary epithelial cells were treated with either 100 ng/ml of exogenous soluble rmCripto-1 or 100 ng/ml of exogenous soluble rmNetrin-1 there was no induction of beta-casein mRNA as determined by qRT-PCR analysis or beta-casein protein expression as determined by Western blot analysis (data not shown). However, in accordance with previous data, treatment with DIP induced the expression of beta-casein mRNA and protein expression in HC-11 cells, as determined by qRT-PCR and WB, respectively (Fig. 6A,B). The addition of exogenous soluble Netrin-1 (100 ng/ml) concomitantly with DIP enhanced the effect of DIP on induction of beta-casein mRNA and protein expression in HC-11 cells while the addition of exogenous soluble Cr-1 (100 ng/ml) reduced the effect of DIP on expression of beta-casein mRNA and protein in HC-11 cells (Fig. 6A,B). The addition of Netrin-1 with Cr-1 to the DIP treated HC-11 cells appeared to override the negative effect of Cr-1 on milk protein expression since beta-casein mRNA and protein levels were similar to those levels that were detected in HC-11 treated with DIP and Netrin-1 (Fig. 6A,B). These results suggest that in contrast to Cr-1, Netrin-1 is capable of enhancing functional differentiation of mouse mammary epithelial cells in response to lactogenic hormones.
Fig. 6.
Exogenous soluble rmNetrin-1 accentuates beta-casein mRNA (A) and protein (B) in HC-11 mouse mammary epithelial cells cultured in the presence of the lactogenic hormones dexamethasone, insulin, and prolactin (DIP). The addition of exogenous soluble Cr-1 (100 ng/ml) significantly (*P < 0.05) attenuated the effect of DIP on beta-casein mRNA and protein expression in HC-11 cells. Netrin-1 (100 ng/ml) significantly increased (*P < 0.05) the level of beta-casein mRNA and protein expression in HC-11 cells cultured in the presence of DIP. The positive effect of Netrin-1 on beta-casein expression in HC-11 cells persisted even in the presence of exogenous soluble rmCr-1 (100 ng/ml). The histogram at the bottom of part (B) shows the densitometric analysis of the Western blot results normalized to actin for equal loading.
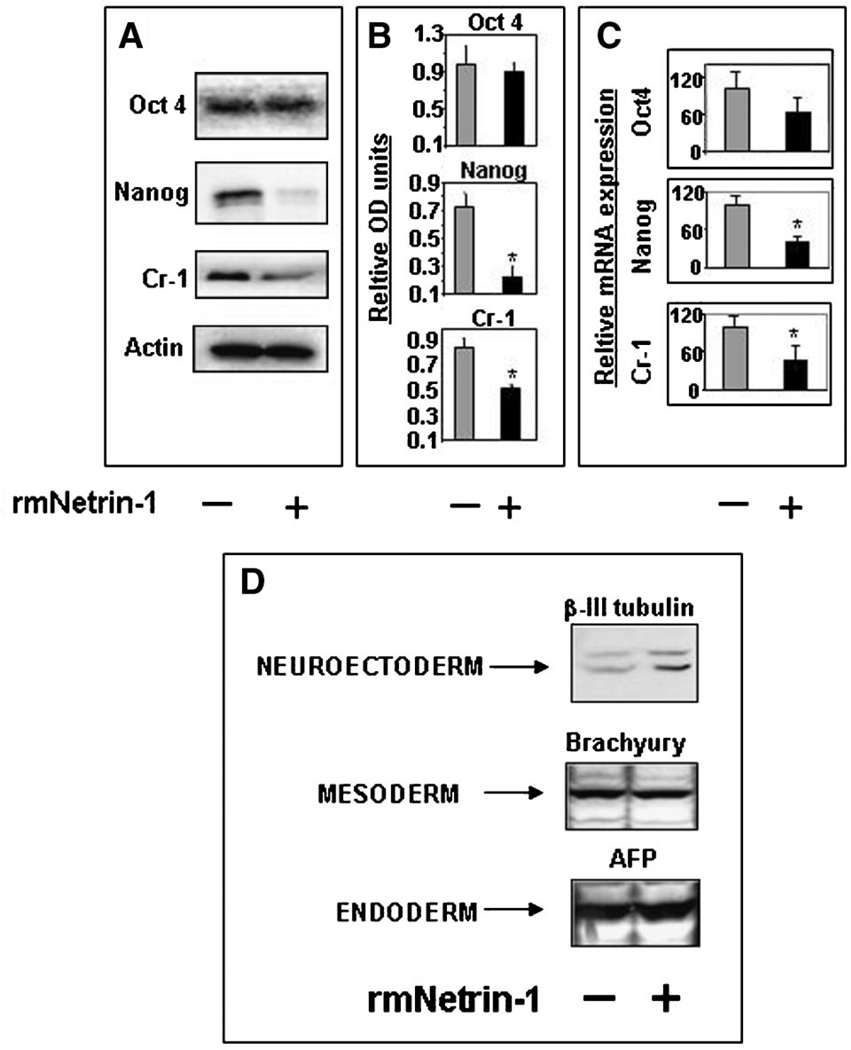
Netrin-1 negatively regulates expression of Cripto-1 and Nanog in undifferentiated mouse ES cells and enhances the expression of beta-III tubulin in differentiating mouse ES cells
Since it appears that Netrin-1 may play a role during the differentiation of mammary epithelial cells through the potential regulation of Cr-1 and Nanog, we investigated whether it could also regulate the expression of Cr-1, Nanog and Oct4 in ES cells. Western blot analysis of cell lysates obtained from ES cells that were treated with 100 ng/ml of exogenous soluble rmNetrin-1 for 3 days show significant reductions (P < 0.05) in Nanog and Cr-1 protein expression but no significant changes in the expression of Oct4 protein (Fig. 7A,B). Results from qRT-PCR analysis also show significant Netrin-1 induced reduction of Nanog and Cr-1 mRNA by at least half (59% and 53%, respectively; P < 0.05) while only reducing Oct4 mRNA level by approximately one third (37%; Fig. 7C). These results suggest that Netrin-1 may be capable of regulating Nanog and Cr-1 expression in ES cells.
Fig. 7.
When undifferentiated (Nanog, Oct4 and Cr-1 positive) mouse ES cells (A) are cultured in the presence of exogenous soluble rmNetrin-1 (100 ng/ml) the expression of Nanog and Cr-1 protein (A and B) significantly decrease (*P < 0.05) as compared to untreated control mouse ES cells. No significant change in expression of Oct4 protein was detected (A and B). Quantitative RT-PCR (C) also shows a significant decrease in Nanog and Cr-1 mRNA levels, but only a modest decrease in Oct4 mRNA, in rmNetrin-1 treated mouse ES cells compared to control. Western blot (D) of extracts from differentiating mouse ES cells (see Materials and Methods Section) treated with exogenous soluble rmNetrin-1 shows enhanced expression of beta-III tubulin but no change in the expressions of Brachyury or alpha-fetoprotein.
To determine the potential for Netrin-1 to affect ES cell fate during differentiation, mouse ES cells were maintained in culture with or without exogenous soluble rmNetrin-1 for up to 7 days. Western blot analysis of lysates show that differentiating mouse ES cells treated with Netrin-1 have increased their expression of the neuronal filament marker, beta-III tubulin (Smith et al., 2008) as compared to untreated control cells. Since ES cells grown in the absence of MEFs will express different markers of differentiation (Keller, 1995), we also examined the effect of Netrin-1 on the expression of Brachyury and alpha-fetoprotein, markers for mesoderm and visceral endoderm specification, respectively (Hyslop et al., 2005). Figure 7D shows that the ES cells grown in these conditions did initiate differentiation as shown by the expression of both Brachyury and alpha-fetoprotein but rmNetrin-1 had no effect on the levels of expression detected between treated and untreated ES cells. Additional WB analysis was performed to determine whether Netrin-1 was capable of affecting the expression of other markers such as, cytokeratin 18 and S100 for ectodermal and neuronal differentiation, respectively (Bosch et al., 1988). No expression of these molecules was detected in either Netrin-1 treated or untreated mouse ES cells (data not shown). These results suggest that Netrin-1 may regulate the expression of Cr-1 and Nanog in mouse ES cells and affect the expression of the early neuronal filament marker, beta-III tubulin in these cells as they differentiate.
Discussion
Extracellular environmental factors play important roles during the regulation of cell growth and differentiation (Kondo, 2006; Wu and Sun, 2006; Hwang et al., 2008). They can significantly influence cell fate by activating specific signaling pathways and can affect the activity of different transcription factors and therefore gene expression. Here, we investigated the role of the neural guidance molecule Netrin-1 as a potential regulator of mouse mammary gland differentiation for three reasons. First, Netrin-1 was among several genes that were identified in ES cells using a modified gene-trap approach which analyzed the in vivo function of these genes encoding secreted and membrane proteins and considered critical for normal or abnormal in vivo development (Mitchell et al., 2001). Second, Netrin-1 was found to promote adhesion between cap cells and body epithelial cells, which is necessary for proper development of the mouse mammary TEB (Srinivasan et al., 2003) and to affect branching and development of extraneuronal systems like lung and vasculature (Liu et al., 2004; Wilson et al., 2006). Third, Netrin-1 was identified as a potential regulator of Cr-1 function both in vitro and in vivo (Strizzi et al., 2005b). We hypothesized that in order for Netrin-1 to play an important role during the early development of the mouse mammary gland it would have to be expressed during embryonic development in the mammary anlage. We found Netrin-1 expression in the mouse mammary placode epithelium surrounded by Cr-1 positive mesenchyme. Cr-1 has been shown to be a target gene of the canonical Wnt/β-catenin/Lef-1 signaling pathway and this signaling pathway is known to play a major role during mammary gland development and tumorigenesis (Morkel et al., 2003; Hens and Wysolmerski, 2005; Howard and Ashworth, 2006). Our observation of the localization of Cr-1 in the mouse mammary anlage is consistent with the findings that demonstrate canonical Wnt signaling observed in the mesenchyme adjacent to the mammary bud at E12.5 and in the condensed mesenchyme surrounding the mammary placode of E13.5 (Boras-Granic et al., 2006). Nanog and Oct4 were also predominantly expressed within the epithelial cells of the E12 and E13 mouse mammary placodes suggesting that Cr-1, Nanog and Oct4 may perform a role in maintaining stem cells in the mouse mammary placodes.
In the postnatal mouse mammary gland, immunostaining for Nanog and Oct4 was more widespread than expected if these two genes are to be considered exclusively as stem cell markers. However, it is becoming increasingly evident that expression of Nanog and Oct4 is not restricted to ES cells since they can be detected in transit amplifying cells and in somatic cells that have undergone differentiation (Tai et al., 2005; Yu et al., 2006) raising the question for the role of these transcription factors within stem cells. Only in vivo functional reconstitution assays will ultimately determine whether Nanog, Oct4 or other “stem cell specific” factors can represent a meaningful set of markers that would confidently characterize their expression or signature as specific to adult tissue stem cells.
The strongest expression for Netrin-1 was observed during lactation when Cr-1 and Nanog expression levels were lowest. Since lactation represents the attainment of full functional state and developmental potential of the mammary gland, it might be expected that factors involved in the maintenance of an undifferentiated state such as, Nanog and Cr-1, would play a less significant role in the physiology of this organ at this stage of development and as a consequence, would be expressed at lower levels. In a previous study, biologically active rhCR-1 fusion protein containing the Fc domain of a human IgG1 was found to bind to a putative receptor or carrier protein in the mouse mammary gland during lactation suggesting that this binding of secreted Cr-1 may facilitate its secretion into milk (Bianco et al., 2002). In fact, high levels of CR-1 have been detected in human milk during lactation (Bianco et al., 2001). The fact that we did not detect the same stage-specific fluctuation in Oct4 expression as Cr-1 and Nanog suggests that Oct4 might affect other functions in the mouse mammary gland not related to lactation.
In experiments where Netrin-1 releasing pellets were inserted in virgin MMTV-CR-1 mice we show that Netrin-1 may play a role during mammary gland alveologenesis. This effect was mostly evident during early (7 day) pregnancy and not detected in FVB/N mice implanted with the different pellets at 14-day pregnancy (data not shown) perhaps due to the fact that during the initial phases of pregnancy, pregnancy related hormones or growth factors have not yet reached peak levels and are therefore unable yet to overcome the effects of Cr-1 or Netrin-1 on mammary duct and alveolar development. Conversely, the absence of sufficient stimulation by pregnancy related hormones may also explain why we were not able to detect appreciable differences in the expression of milk proteins in the mammary glands of the different groups of 7-day pregnant FVB/N mice (data not shown). In fact, only DIP stimulated mouse mammary epithelial HC-11 cells, which respond by expressing beta-casein (De Santis et al., 1997), showed significantly enhanced beta-casein expression when treated with exogenous soluble Netrin-1. This raises the possibility that Netrin-1 may modulate the response to lactogenic hormone stimulation of milk protein expression in mouse mammary epithelial cells by regulating the expression of factors such as, Cr-1 and Nanog that would otherwise keep these cells in a more undifferentiated, non-milk expressing state. These possibilities are supported by the ability of Netrin-1 to negatively regulate in mouse ES cells the expression of Cr-1 and Nanog, which normally play a part during the maintenance of ES cells in an undifferentiated state. Interestingly, when these ES cells were induced to differentiate, Netrin-1 treatment caused increased expression in these cells of the neuronal filament marker, beta-III tubulin. Previous studies have shown that it is possible to induce the expression of early neuroectodermal markers during differentiation in multipotent cells in which Nanog or Cr-1 expression have been reduced (Parisi et al., 2003; Rogers et al., 2007). Although studies have shown a direct role for Netrin-1 during the development and functional differentiation of neuronal structures (Lin and Isacson, 2006; Round and Stein, 2007) it was not possible, in our study, to observe the expression of additional markers of neuronal differentiation such as, S100 (data not shown) and therefore we cannot conclude whether Netrin-1 could be involved in neuroectodermal differentiation. Only advanced neurophysiologic studies will demonstrate whether Netrin-1 may play a meaningful role during neuronal differentiation.
Together, these results suggest that Netrin-1 may represent an important factor for mouse mammary gland development and differentiation by positively affecting alveologenesis and lactogenesis potentially through the regulation of the expression of genes like Nanog and Cr-1 that could otherwise maintain mammary epithelial cells in a more undifferentiated state.
Acknowledgments
Contract grant sponsor: National Institutes of Health Intramural Funding.
Literature Cited
- Assou S, LeCarrour T, Tondeur S, Strom S, Gabelle A, Marty S, Nadal L, Pantesco V, Reme T, Hugnot JP, Gasca S, Hovatta O, Hamamah S, Klein B, DeVos J. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells (Dayton, Ohio) 2007;25:961–973. doi: 10.1634/stemcells.2006-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988;7:2089–2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C, Wechselberger C, Ebert A, Khan NI, Sun Y, Salomon DS. Identification of Cripto-1 in human milk. Breast Cancer Res Treat. 2001;66:1–7. doi: 10.1023/a:1010648923432. [DOI] [PubMed] [Google Scholar]
- Bianco C, Normanno N, De Luca A, Maiello MR, Wechselberger C, Sun Y, Khan N, Adkins H, Sanicola M, Vonderhaar B, Cohen B, Seno M, Salomon D. Detection and localization of Cripto-1 binding in mouse mammary epithelial cells and in the mouse mammary gland using an immunoglobulin-cripto-1 fusion protein. J Cell Physiol. 2002;190:74–82. doi: 10.1002/jcp.10037. [DOI] [PubMed] [Google Scholar]
- Boras-Granic K, Chang H, Grosschedl R, Hamel PA. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol. 2006;295:219–231. doi: 10.1016/j.ydbio.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Leube RE, Achtstatter T, Moll R, Franke WW. Expression of simple epithelial type cytokeratins in stratified epithelia as detected by immunolocalization and hybridization in situ. J Cell Biol. 1988;106:1635–1648. doi: 10.1083/jcb.106.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Duss S. Stem cells and the stem cell niche in the breast: An integrated hormonal and developmental perspective. Stem Cell Rev. 2007;3:147–156. doi: 10.1007/s12015-007-0019-1. [DOI] [PubMed] [Google Scholar]
- De Santis ML, Kannan S, Smith GH, Seno M, Bianco C, Kim N, Martinez-Lacaci I, Wallace-Jones B, Salomon DS. Cripto-1 inhibits beta-casein expression in mammary epithelial cells through a p21 ras-and phosphatidylinositol 3’-kinase-dependent pathway. Cell Growth Differ. 1997;8:1257–1266. [PubMed] [Google Scholar]
- Diaz-Flores L, Jr, Madrid JF, Gutierrez R, Varela H, Valladares F, Alvarez-Arguelles H, Diaz-Flores L. Adult stem and transit-amplifying cell location. Histol Histopathol. 2006;21:995–1027. doi: 10.14670/HH-21.995. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Hens JR, Wysolmerski JJ. Key stages of mammary gland development: Molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 2005;7:220–224. doi: 10.1186/bcr1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B, Ashworth A. Signalling pathways implicated in early mammary gland morphogenesis and breast cancer. PLoS Genet. 2006;2:e112. doi: 10.1371/journal.pgen.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2008;60:199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NE, Taverna D, Harwerth IM, Ciardiello F, Salomon DS, Yamamoto T, Groner B. Epidermal growth factor receptor, but not c-erbB-2, activation prevents lactogenic hormone induction of the beta-casein gene in mouse mammary epithelial cells. Mol Cell Biol. 1990;10:4027–4034. doi: 10.1128/mcb.10.8.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop L, Stojkovic M, Armstrong L, Walter T, Stojkovic P, Przyborski S, Herbert M, Murdoch A, Strachan T, Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells (Dayton, Ohio) 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- Kondo T. Epigenetic alchemy for cell fate conversion. Curr Opin Genet Dev. 2006;16:502–507. doi: 10.1016/j.gde.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lin L, Isacson O. Axonal growth regulation of fetal and embryonic stem cell-derived dopaminergic neurons by Netrin-1 and Slits. Stem Cells (Dayton, Ohio) 2006;24:2504–2513. doi: 10.1634/stemcells.2006-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Stein E, Oliver T, Li Y, Brunken WJ, Koch M, Tessier-Lavigne M, Hogan BL. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;14:897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, Wong KY, Sung KW, Lee CW, Zhao XD, Chiu KP, Lipovich L, Kuznetsov VA, Robson P, Stanton LW, Wei CL, Ruan Y, Lim B, Ng HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Medina D. The mammary gland: A unique organ for the study of development and tumorigenesis. J Mammary Gland Biol Neoplasia. 1996;1:5–19. doi: 10.1007/BF02096299. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Pinson KI, Kelly OG, Brennan J, Zupicich J, Scherz P, Leighton PA, Goodrich LV, Lu X, Avery BJ, Tate P, Dill K, Pangilinan E, Wakenight P, Tessier-Lavigne M, Skarnes WC. Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat Genet. 2001;28:241–249. doi: 10.1038/90074. [DOI] [PubMed] [Google Scholar]
- Morkel M, Huelsken J, Wakamiya M, Ding J, van de Wetering M, Clevers H, Taketo MM, Behringer RR, Shen MM, Birchmeier W. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- Morwald H, Wurm S, Crailsheim K, Wechselberger C. Prion protein facilitates hormone-induced differentiation of mammary gland epithelial cells. Biochem Biophys Res Commun. 2007;360:746–751. doi: 10.1016/j.bbrc.2007.06.154. [DOI] [PubMed] [Google Scholar]
- Niemeyer CC, Persico MG, Adamson ED. Cripto: Roles in mammary cell growth, survival, differentiation and transformation. Cell Death Differ. 1998;5:440–449. doi: 10.1038/sj.cdd.4400368. [DOI] [PubMed] [Google Scholar]
- Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- Pan GJ, Chang ZY, Scholer HR, Pei D. Stem cell pluripotency and transcription factor Oct4. Cell Res. 2002;12:321–329. doi: 10.1038/sj.cr.7290134. [DOI] [PubMed] [Google Scholar]
- Parisi S, D’Andrea D, Lago CT, Adamson ED, Persico MG, Minchiotti G. Nodal-dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J Cell Biol. 2003;163:303–314. doi: 10.1083/jcb.200303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- Rogers I, Yamanaka N, Bielecki R, Wong CJ, Chua S, Yuen S, Casper RF. Identification and analysis of in vitro cultured CD45-positive cells capable of multi-lineage differentiation. Exp Cell Res. 2007;313:1839–1852. doi: 10.1016/j.yexcr.2007.02.029. [DOI] [PubMed] [Google Scholar]
- Round J, Stein E. Netrin signaling leading to directed growth cone steering. Curr Opin Neurobiol. 2007;17:15–21. doi: 10.1016/j.conb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Sapino A, Macri L, Gugliotta P, Pacchioni D, Liu YJ, Medina D, Bussolati G. Immunophenotypic properties and estrogen dependency of budding cell structures in the developing mouse mammary gland. Differentiation. 1993;55:13–18. doi: 10.1111/j.1432-0436.1993.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Smith GH, Mehrel T, Roop DR. Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ. 1990;1:161–170. [PubMed] [Google Scholar]
- Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313:107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Normanno N, Salomon D. Cripto-1: A multifunctional modulator during embryogenesis and oncogenesis. Oncogene. 2005a;24:5731–5741. doi: 10.1038/sj.onc.1208918. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Raafat A, Abdallah W, Chang C, Raafat D, Hirota M, Hamada S, Sun Y, Normanno N, Callahan R, Hinck L, Salomon D. Netrin-1 regulates invasion and migration of mouse mammary epithelial cells overexpressing Cripto-1 in vitro and in vivo. J Cell Sci. 2005b;118:4633–4643. doi: 10.1242/jcs.02574. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Hirota M, Watanabe K, Mancino M, Hamada S, Raafat A, Lawson S, Ebert AD, D’Antonio A, Losito S, Normanno N, Salomon DS. Development of leiomyosarcoma of the uterus in MMTV-CR-1 transgenic mice. J Pathol. 2007;211:36–44. doi: 10.1002/path.2083. [DOI] [PubMed] [Google Scholar]
- Sun Y, Strizzi L, Raafat A, Hirota M, Bianco C, Feigenbaum L, Kenney N, Wechselberger C, Callahan R, Salomon DS. Overexpression of human Cripto-1 in transgenic mice delays mammary gland development and differentiation and induces mammary tumorigenesis. Am J Pathol. 2005;167:585–597. doi: 10.1016/S0002-9440(10)63000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: Evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK. Local effects of EGF, alpha-TGF, and EGF-like growth factors on lobuloalveolar development of the mouse mammary gland in vivo. J Cell Physiol. 1987;132:581–584. doi: 10.1002/jcp.1041320324. [DOI] [PubMed] [Google Scholar]
- Wechselberger C, Strizzi L, Kenney N, Hirota M, Sun Y, Ebert A, Orozco O, Bianco C, Khan NI, Wallace-Jones B, Normanno N, Adkins H, Sanicola M, Salomon DS. Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene. 2005;24:4094–4105. doi: 10.1038/sj.onc.1208417. [DOI] [PubMed] [Google Scholar]
- Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TL, Xie JW, Haslam SZ. The role of mammary stroma in modulating the proliferative response to ovarian hormones in the normal mammary gland. J Mammary Gland Biol Neoplasia. 1998;3:117–131. doi: 10.1023/a:1018738721656. [DOI] [PubMed] [Google Scholar]
- Wu H, Sun YE. Epigenetic regulation of stem cell differentiation. Pediatr Res. 2006;59:21R–25R. doi: 10.1203/01.pdr.0000203565.76028.2a. [DOI] [PubMed] [Google Scholar]
- Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879–1888. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]