Abstract
Positron emission tomography (PET) is a minimally invasive technique which has been well validated for the diagnosis, staging, monitoring of response to therapy, and disease surveillance of adult oncology patients. Traditionally the value of PET and PET/computed tomography (CT) hybrid imaging has been less clearly defined for paediatric oncology. However recent evidence has emerged regarding the diagnostic utility of these modalities, and they are becoming increasingly important tools in the evaluation and monitoring of children with known or suspected malignant disease. Important indications for 2-deoxy-2-(18F)fluoro-D-glucose (FDG) PET in paediatric oncology include lymphoma, brain tumours, sarcoma, neuroblastoma, Langerhans cell histiocytosis, urogenital tumours and neurofibromatosis type I. This article aims to review current evidence for the use of FDG PET and PET/CT in these indications. Attention will also be given to technical and logistical issues, the description of common imaging pitfalls, and dosimetric concerns as they relate to paediatric oncology.
Keywords: Positron emission tomography, Computed tomography, Fluorodeoxyglucose F18, Paediatrics, Oncology, Technical issues, Dosimetry
Core tip: Positron emission tomography/computed tomography has emerged as a powerful and important tool in the assessment of a variety of childhood cancers and can impact significantly on patient management. Further prospective studies will more clearly delineate the precise role of this modality in the assessment of individual malignancies. Accurate image interpretation requires a thorough understanding of the normal variants of uptake unique to children.
INTRODUCTION
Although the incidence of childhood malignancy remains relatively stable, survival rates have significantly improved over the past 30 years[1]. In addition to improved treatment strategies, survival gains have relied upon continuous improvements in the accurate detection, staging and follow-up of these cancers.
Positron emission tomography (PET) is a minimally invasive technique whereby a labelled radiopharmaceutical is injected into a patient and the resulting distribution used to generate molecular information. In practice, PET-alone scanners have largely been replaced by the hybrid modality of PET/computed tomography (CT) which combines the functional data of PET with the morphological information of CT. There has also been ongoing interest in the utility of fusing PET with magnetic resonance imaging (PET/MR)[2].
The use of PET and PET/CT in adult oncology is well established for the purpose of diagnosis, staging, monitoring of response to therapy, and disease surveillance[3,4]. In contrast to this, the value of these modalities in paediatric oncology has traditionally been much less clearly defined. More recently however, evidence has emerged regarding the diagnostic utility of PET and an important role has been reported for many paediatric malignancies[5,6]. Hybrid imaging with PET/CT has demonstrated superiority to PET alone in characterising childhood cancers, primarily by increasing diagnostic confidence and reducing equivocal findings[7-9]. PET/CT has become a central component of paediatric oncological practice.
PET imaging has now superseded 67Ga and 201Tl scintigraphy for many oncological applications[10,11]. In addition to improved accuracy and having broader clinical application, advantages of PET over 67Ga and 201Tl scintigraphy include lower radiation dose, reduced scanning time, same day imaging and improved anatomical localisation. Through calculation of the standardised uptake value (SUV), PET is also able to generate quantitative data on treatment response.
Although there are a number of radiopharmaceuticals available, the majority of clinical PET imaging is performed with 2-deoxy-2-(18F)fluoro-D-glucose (FDG). FDG has many characteristics that make it ideal for use in PET imaging. 18F has a relatively short half-life of 110 min, provides relatively low radiation exposures for diagnostic purposes, and is widely commercially available. FDG mimics glucose in its cellular uptake and thereby serves as a marker of glucose utilisation. FDG is therefore not a tumour-specific entity and can accumulate in a number of physiological and pathological processes. The use of dual time-point imaging, which exploits unique characteristics of malignant cells, can help improve the specificity of FDG imaging[12].
Common indications for PET in paediatric oncology include lymphoma, sarcoma, neuroblastoma and primary brain malignancy. Other important indications include Langerhans cell histiocytosis, Wilms tumor, and neurofibromatosis type I[13]. This article aims to review current evidence for the use of PET and PET/CT in these indications. Imaging with the radiopharmaceutical FDG will be assumed unless noted otherwise. Attention will also be given to technical and logistical issues, the description of common imaging pitfalls, and dosimetric concerns as they relate to paediatric oncology.
LYMPHOMA
Non-Hodgkin’s and Hodgkin’s lymphoma collectively account for between 10% and 15% of paediatric malignancies. Evaluation of lymphoma remains the most frequent indication of PET/CT imaging in children. Its use in this context has recently been reviewed[14].
Whilst there is a large body of evidence supporting the use of PET/CT in adults, data remains more limited in relation to paediatric lymphoma. Nevertheless, available studies in children suggest that PET/CT is both diagnostically accurate and of significant clinical importance in these children[15-17].
In two large retrospective studies PET/CT demonstrated superiority over conventional imaging modalities [CT, ultrasound, magnetic resonance imaging (MRI) or bone scintigraphy] in the primary staging of lesions due to both Hodgkin’s and non-Hodgkin’s disease (Figures 1A and 2A)[18,19]. Calculated sensitivities and specificities for initial disease staging was greater than 95% and 99% respectively[18,19]. Furthermore, PET/CT modified staging in 27% of cases, with approximately equal instances of upstaging and down-staging[19].
Figure 1.
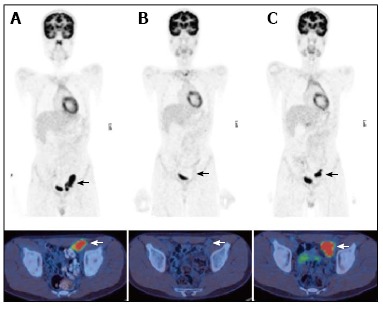
A 13 year-old male with nodular sclerosing Hodgkin’s disease. PET/CT at staging (A) demonstrated disease in the left external iliac region. After completion of chemotherapy 4 mo later (B) there was a complete metabolic response with no activity in the residual lymph node mass. PET/CT performed 6 mo later (C) for surveillance demonstrated a recurrence at the same site. PET/CT: Positron emission tomography/computed tomography.
Figure 2.
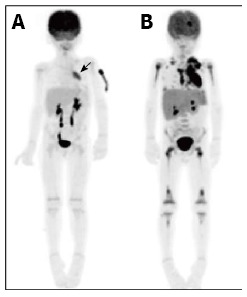
A 5-year-old male patient with relapsed T-cell lymphoblastic lymphoma. Restaging PET at the time of relapse 1 year after initial therapy demonstrated an FDG-avid mediastinal mass (A, arrow). Repeat PET performed following 1 cycle of FLAG-Ida chemotherapy demonstrated no response to treatment and progression of disease to stage IV with extensive bone marrow involvement (B). PET: Positron emission tomography; FDG: 2-deoxy-2-(18F)fluoro-D-glucose.
PET has been shown to demonstrate superior sensitivity in the detection of bone marrow involvement due to Hodgkin’s disease when compared with bone marrow biopsy[20-23]. Marrow involvement in Hodgkin’s disease is generally unifocal or multifocal and thus can easily be missed on biopsy. PET should therefore be employed as a first-line study in the detection of marrow involvement prior to directed bone marrow biopsy. There is accumulating evidence that a negative PET may replace the need for bone marrow biopsy in patients with Hodgkin’s lymphoma[20,21].
PET/CT has also demonstrated efficacy in the evaluation of treatment response, and performs significantly better than conventional imaging modalities in the evaluation of both early and post-completion chemotherapy responses (Figures 1B and 2B)[18]. Multiple studies have demonstrated that a complete metabolic response early in the course of chemotherapy is associated with an excellent prognosis in children with Hodgkin’s disease[24-26]. Ongoing trials are aiming to utilise this information to enable distinct treatment protocols for these patients and therefore minimise treatment-related toxicity[14].
Finally, PET/CT may be a useful modality for the follow-up of children with lymphoma, and a negative study during routine follow-up has a high negative predictive value[16,27,28]. PET/CT was found to be superior to conventional imaging during long-term follow-up of children with lymphoma[19]. Therefore although conventional imaging continues to be used as part of the follow-up assessment in these patients, PET/CT may eventually replace this and come to be used as a single imaging modality for routine surveillance (Figure 1C). It should be noted that the use of PET/CT for this indication is yet to be fully elucidated in paediatric patients. Although most Hodgkin’s disease recurrence is FDG-avid, surveillance PET/CT can have high false-positive rates[29]. Furthermore, because prognosis is often favourable among children with lymphoma, radiation exposure secondary to diagnostic procedures has significant relevance in children. Current evidence for follow-up PET/CT studies in paediatric lymphoma is best when relapse is clinically suspected or demonstrated by other imaging modalities.
BRAIN TUMOURS
Brain tumours represent the most common solid neoplasms in childhood and are a leading cause of cancer-related death in children[30]. As a group they represent 25% of all childhood cancers[31]. Common paediatric brain tumours include medulloblastoma, cerebellar astrocytoma, ependymoma and brain stem glioma[32]. Management of these tumours is challenging and diverse, and requires extensive multidisciplinary collaboration. Survivors of childhood brain tumours often have severe neurological, neurocognitive and psychosocial sequelae[33].
Conventional modalities for the anatomic assessment of brain tumours are CT and MRI. A major disadvantage of these modalities is that interpretation is often confounded by brain changes secondary to surgery, chemotherapy and radiotherapy. In these circumstances normal post-treatment change may be incorrectly identified as viable tumour. Functional imaging techniques are therefore of particular importance for monitoring treatment effects and recurrence.
PET has shown increasing application in paediatric neuro-oncology. Both FDG and L-methyl-(11C)methionine (CMET) radiotracers have shown utility in brain tumour grading, profiling, and for detecting residual, recurrent or progressive disease in children[34-36]. The fusion of PET data with MR images has been shown to assist with stereotactic biopsy and navigation-based resection among children in whom MR images alone were considered insufficient[37].
FDG PET has traditionally been used to distinguish low-grade from high-grade tumours, and FDG avidity is a demonstrated predictor of outcome in adults with high-grade astrocytoma[38]. Furthermore, FDG hypermetabolism has been shown to predict higher risk for disease progression among children with low-grade astrocytoma[39]. FDG PET is an established technique for the differentiation of viable tumour and surgical change in the postoperative milieu[40,41].
CMET is a marker of amino acid uptake and protein synthesis. It has been found useful in the differentiation of low-grade tumours (astrocytomas, oligodendrogliomas and dysembryoplastic neuroepithelial tumours), measurement of tumour boundaries, elucidation of treatment response, and prediction of patient outcome[36,42-45].
SARCOMA
Ewing’s sarcoma and osteosarcoma are the two primary bone malignancies of childhood, with osteosarcoma being more common[46]. Rhabdomyosarcoma is the most common soft tissue malignancy of childhood[47]. Collectively, bone and soft tissue sarcomas account for around 13% of childhood malignancies with soft tissue disease being slightly more prevalent[46,47]. Treatment is multimodal and may involve chemotherapy, radiotherapy or surgery.
There is a growing body of literature related to the use of PET/CT in paediatric sarcoma, although standard use of the modality has not been extensively validated[48,49]. Nevertheless, PET/CT does appear to demonstrate utility in the staging, monitoring of disease response to therapy, and detection of recurrent disease among these children (Figure 3)[50,51]. North American consensus guidelines have recommended whole-body PET at the initial diagnosis of osteosarcoma and Ewing sarcoma in children[52]. A previous survey has shown that PET is considered a helpful study by referrers of paediatric sarcoma patients in the vast majority of instances[5]. The diagnostic accuracy of PET/CT has been found to be greater than either PET alone or conventional imaging in the detection of distant sarcomatous metastases in children[53].
Figure 3.
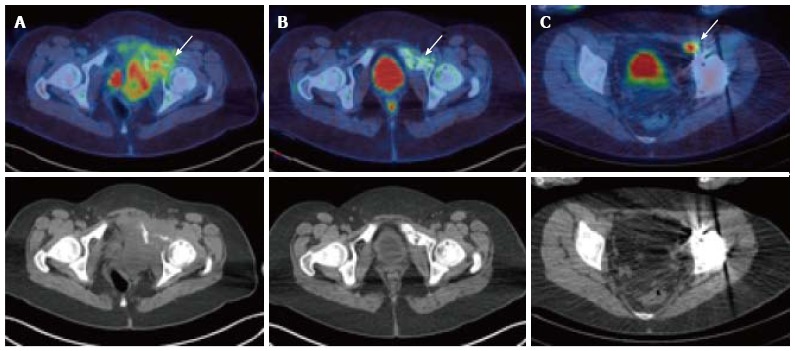
A 17-year-old female with Ewing’s sarcoma involving the left superior pubic ramus. Staging PET/CT showed extensive disease (A, arrow) with bone destruction and a large FDG-avid pelvic mass. Following chemotherapy, at 5 mo after diagnosis, there was an excellent metabolic response with minimal residual FDG uptake (B, arrow). Patient underwent surgical resection and extracorporeal radiotherapy to the bone. Sixteen months after completion of treatment, surveillance PET/CT demonstrated recurrence in a left external iliac lymph node (C) which was not detectable on the CT or MRI due to marked metal artefact. PET/CT: Positron emission tomography/computed tomography; FDG: 2-deoxy-2-(18F)fluoro-D-glucose; MRI: Magnetic resonance imaging.
Conventional staging of paediatric oncology patients includes bone scintigraphy, CT and MRI. PET has been found equal or superior to bone scintigraphy in the detection of osseus metastases from sarcoma[54,55]. Such metastases are detected in Ewing sarcoma with a sensitivity ranging from 88%-100%[54,55]. Detection of nodal metastases may also be improved with PET as compared with conventional imaging modalities[56].
Pulmonary metastases are common among children with sarcoma, being present in up to one quarter of patients at the time of diagnosis[50]. Prompt identification and treatment is crucial to effective management. PET alone has been proven less sensitive than diagnostic CT in the identification of sarcomatous pulmonary metastases[56,57]. The sensitivity of PET in detecting pulmonary metastases has been reported to be as low as 24% for lesions smaller than 1 cm[56,58]. This reduced sensitivity is multifactorial and can be attributed to technical limitations such as the finite spatial resolution of the scanner, which dictates that a lesion smaller than 3-4 mm may not be identified; as well as the partial volume effect (PVE) and respiratory movement during emission acquisition.
PVE can result in significant qualitative and quantitative changes to PET studies. In practical terms it results in the signals of small avid lesions being spread over larger volumes. It typically occurs whenever the avid lesion is smaller than 3 times the full width at half maximum, and is exacerbated when surrounding tissue uptake is particularly low (as is seen in lung tissue). PVE results in small lesions appearing larger in size but much less avid[59].
Respiratory movements affect PET images due to the long acquisition times involved[60]. The movement of lung lesions during respiration results in overestimated tracer-avid volumes and reduced apparent tracer uptake[61]. It is particularly seen in lung tissue closest to the diaphragm. Respiratory gating is a technique whereby the respiratory cycle is divided into multiple phases and the acquired events sorted into temporal bins. It aims to improve the spatial resolution of thoracic PET images at the expense of increased image noise. Respiratory gating has been shown to result in more accurate SUV and volume measurements of pulmonary nodules[62]. It remains an ongoing area of research but may have future applications, particularly in the area of radiation therapy treatment planning[63].
Aside from those technical limitations which affect small pulmonary metastases, PET sensitivity is reduced compared with conventional imaging even for pulmonary metastases greater than 1 cm in size, including those where the primary tumour is intensely avid. The reason for this finding is poorly understood but may relate to reduced perfusion of lesions, down-regulation of glucose receptors or altered glucose metabolism[58].
Fused PET/CT improves detection of pulmonary metastases above PET alone[64]. However, because PET/CT is generally undertaken with a reduced dose CT protocol, image quality may still not be sufficient to detect small pulmonary metastases. It is known that small pulmonary nodules are no more likely to be benign than larger ones among paediatric oncology patients[65]. A diagnostic quality CT of the chest therefore remains an essential part of the staging and follow-up of paediatric sarcoma[50].
PET has additionally been used to identify local and distant recurrence of sarcoma. Reliable follow-up however requires the use of other imaging modalities[66]. Additionally, there has been much interest in the potential use of PET as a prognostic tool. A correlation between the FDG SUV as measured prior to treatment, and clinically important indices such as disease progression and survival has been described[67]. Furthermore, PET/CT demonstrates potential in the prediction of treatment response. Because tumour bulk may not significantly change in response to neoadjuvant chemotherapy, CT and MRI have limited value for such assessment. A number of studies have demonstrated that changes in FDG avidity in high-grade sarcoma following neoadjuvant therapy can identify patients at risk of relapse and therefore has potential as a non-invasive surrogate marker in the prediction of patient response[68-70].
It should be noted that the degree of FDG avidity in sarcoma depends greatly on individual pathology and can range from low grade to markedly avid. This is particularly the case for soft tissue sarcomas including rhabdomyosarcoma, and needs to be appreciated when FDG is used for this indication[71]. Furthermore, there may be heterogeneity of disease-related FDG uptake even within the same patient. Being able to identify the most aggressive area within a heterogenous tumour, and direct biopsy accordingly may be of clinical benefit[71].
The use of PET and PET/CT among children with sarcoma continues to increase. As discussed above, these modalities appear to be useful in the evaluation and staging, monitoring of therapeutic response, and detection of tumour recurrence. However the exact role of this modality in the routine care of children with sarcoma remains unclear. Ongoing prospective studies are required to delineate the precise role of PET and PET/CT in their management.
NEUROFIBROMATOSIS TYPE 1
A number of studies have confirmed the utility of PET and PET/CT in the detection of malignant transformation of neurofibromatosis type 1 (NF1)[72,73]. Patients with NF1 have an approximate 10% lifetime risk of developing malignant peripheral nerve sheath tumours (MPNST)[74,75]. Such transformation is unreliably detected via conventional imaging[76-78]. In one prospective study patients with symptomatic neurofibromas were assessed with early and delayed PET/CT imaging[76]. This modality was found to be highly sensitive and specific in the detection of MPNSTs. In addition to there being significant differences in uptake between malignant and benign lesions, delayed imaging demonstrated a continued divergence of FDG avidity which highlights the value of dual time-point imaging for this indication.
NEUROBLASTOMA
Neuroblastoma is an embryonic tumour arising from neural crest cells of the sympathetic nervous system[79]. It is the most common extracranial solid malignancy in children and accounts for around 8% of all childhood cancers. The clinical course is highly variable, yet the disease accounts for around 15% of all cancer deaths in children[80,81]. Half of all patients have distant haematogenous spread at diagnosis[82].
The catecholamine analogue 123I-metaiodobenzylguanidine (MIBG) is widely used to image neuroendocrine tumours and is well established for use in the staging and post-treatment evaluation of neuroblastoma[83,84]. MIBG scintigraphy has a specificity of nearly 100% for neuroblastoma diagnosis and staging[85,86]. Uptake of MIBG requires the presence of a type I catecholamine transport system[87], which is usually but not uniformly present on neuroblastoma cells. In around 8% of patients MIBG scanning gives a false-negative result at diagnosis[88]. False negative results may also lead to incorrect down-staging of disease. Other disadvantages of MIBG scintigraphy include limited spatial resolution, limited sensitivity in small lesions, the need for multiple and prolonged acquisition sessions and a delay between the start of examination and result.
In addition to MIBG, neuroblastoma imaging utilises the modalities of bone scintigraphy, sonography, CT and MR. There is also interest in the use of FDG and other radiopharmaceuticals for PET imaging. Because FDG PET uptake reflects glucose metabolism by cancer cells, neuroblastoma which fails to accumulate MIBG due to reduced expression of transporter proteins might be expected to be more sensitively assessed using this modality. Further potential advantages of PET over MIBG scintigraphy include improved spatial resolution, single acquisition sessions and shorter scanning times which have the potential to reduce the need for sedation[89].
A number of studies have compared MIBG scintigraphy with PET in neuroblastoma[90-93]. MIBG appears overall to be superior to PET in the evaluation of stage 4 neuroblastoma, primarily due to improved detection of skeletal disease. However PET appears to demonstrate superior detection in stage 1 and 2 neuroblastoma and in tumours which only weakly accumulate MIBG (Figure 4)[90,92,93]. These results suggest that PET may be important in the context of discrepant or inconclusive findings on MIBG and morphological imaging.
Figure 4.
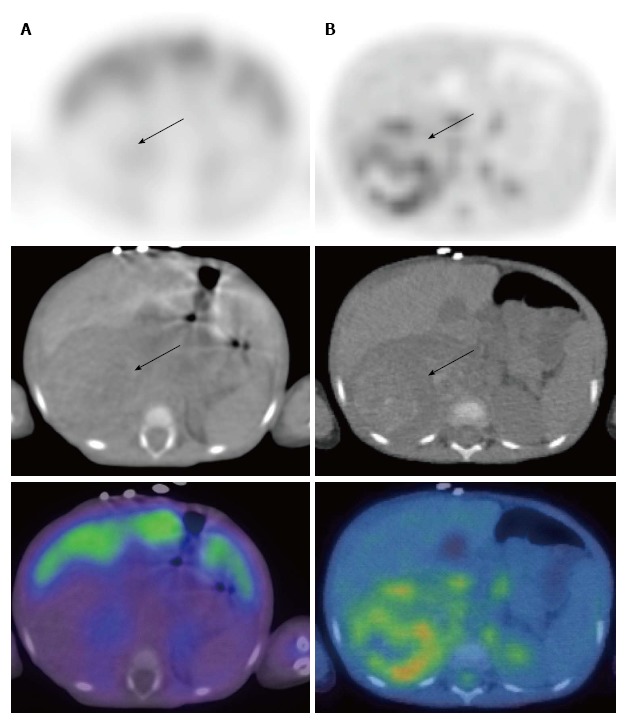
Neuroblastoma in a 2-year-old female. 123I-metaiodobenzylguanidine (MIBG) single positron emission tomography/computed tomography (SPECT/CT) images (A) demonstrated a large right suprarenal mass displacing the organs which was not MIBG-avid (arrows). FDG PET/CT (B) showed moderate heterogenous metabolic activity within the mass. PET/CT: Positron emission tomography/computed tomography; FDG: 2-deoxy-2-(18F)fluoro-D-glucose.
To summarise, compared with PET, MIBG remains the optimal modality for the noninvasive staging of children with neuroblastoma. Overall, available evidence suggests that PET is most useful in defining the distribution of disease that either fails to concentrate MIBG or does so poorly. In particular, PET should be considered when MIBG scintigraphy reveals less disease than suggested by clinical symptoms or conventional imaging modalities. During follow-up assessment of MIBG-negative neuroblastoma, PET/CT represents the imaging modality of choice. New radiopharmaceuticals for PET imaging, including 18F-dihydroxyphenylalanine and 68Ga-octreotate, are currently under evaluation[94,95].
WILMS TUMOUR
Renal tumours comprise 6% of all childhood cancers. Of these, around 95% are Wilms tumours (nephroblastomas)[96]. The molecular genetics of Wilms tumour is complex and involves multiple loci involved with WNT signalling[97]. Mutations in the WT gene are identified in 10%-15% of sporadic cases. More than 10% of children with Wilms tumour have associated abnormalities, including cryptorchidism, hypospadias, hemihypertrophy and aniridia[98]. Synchronous bilateral Wilms tumour is present in 5% of patients[99]. Although prognosis is generally favourable, higher stage disease carries significant mortality and treatment related morbidity.
Imaging at diagnosis typically involves ultrasound, CT and MRI. Although FDG uptake has been described in Wilms tumour[100-102] the role of PET/CT has not been clearly established. A particular obstacle to accurate FDG PET imaging of the primary lesion is physiological excretion of tracer via the kidneys. Such physiological activity may generate uncertainty when attempting to distinguish pathology. The use of hybrid PET/CT scanners may be of some benefit in this regard.
In a small series of 12 patients PET/CT was seen to be concordant with conventional imaging in primary staging and superior to conventional imaging for the detection of residual and recurrent disease[100]. In another small series of 27 patients Wilms tumour appeared to concentrate FDG, however small pulmonary metastases were not consistently identified on PET scan[101]. There is evidence that FDG avidity in Wilms tumour may correlate with higher histological risk[102].
These studies demonstrate that FDG PET/CT may be a useful adjunct to conventional imaging modalities in Wilms tumour, particularly for the detection of residual and recurrent disease. Further investigation is required to confirm this. The use of functional MRI with diffusion-weighted imaging is another new technique which shows promise in the evaluation of Wilms tumour.
LANGERHANS CELL HISTIOCYTOSIS
Langerhans cell histiocytosis (LCH) is a rare disease which involves the clonal proliferation of activated dendrocytes and macrophages. These clonal cells form infiltrates in a variety of organs along with other inflammatory cells[103]. LCH exists as a heterogenous and complex disease with a broad spectrum of clinical manifestations which range from spontaneous resolution to rapid progression and death[104]. It may occur at any age but generally affects children between 1 and 15 years. Skeletal survey and bone scintigraphy are established techniques in LCH disease evaluation. In addition, CT and MRI modalities are increasingly used in the detection of skeletal and visceral disease[105].
The use of PET and PET/CT in the assessment of patients with LCH has been reported in a number of studies and its use is now well established. PET has been shown to be superior to bone scintigraphy and skeletal survey for overall lesion detection (Figure 5)[106-108]. Furthermore, PET has demonstrated efficacy in post-treatment lesion assessment and provides information on treatment response earlier than plain film radiography or CT[106-110].
Figure 5.
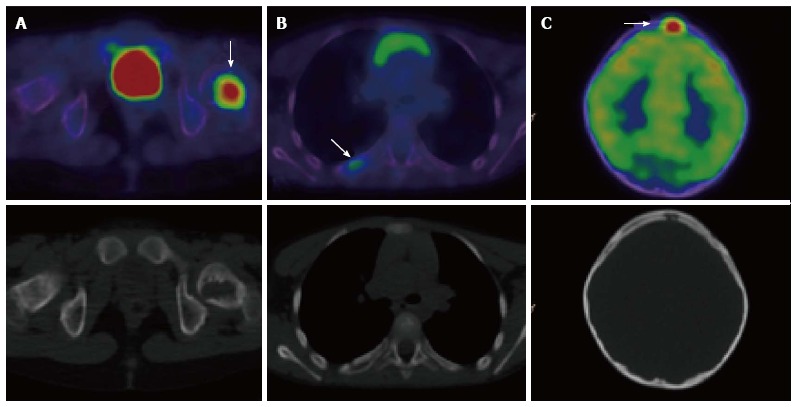
A 7-year-old male with Langerhans cell histiocytosis. Skeletal survey demonstrated an isolated left femoral lesion, confirmed on PET/CT (A, arrow). Additional lesions (arrows) in the right 6th rib posteriorly (B) and in the skull (C) were also identified on the PET/CT scan. Top panel: fused PET/CT images, bottom panel: low dose CT component of the scan. PET/CT: Positron emission tomography/computed tomography.
A recent study has compared the use of PET with MRI in paediatric patients with biopsy-proven histiocytosis[111]. In this study PET was found to be more accurate than MRI for post-treatment evaluation due to lower false-positive rates. MRI showed a higher sensitivity and was important for primary staging and evaluation of CNS disease. Interestingly, whilst there was no clear advantage for combined PET/MRI analysis during follow-up evaluation, the combination did improve sensitivity of primary staging through a reduced false-negative rate. These results suggest that whilst PET alone may be sufficient for post-treatment disease monitoring in LCH, the combination of PET and MRI is preferred for primary disease investigation. A potential future role therefore exists for combined PET/MRI in the primary investigation of paediatric LCH.
Taken together, these studies suggest that PET is a valuable modality in the primary assessment, therapeutic monitoring and detection of reactivation of patients with LCH.
PRACTICAL ASPECTS
PET/CT requires consideration of particular challenges that are common to paediatric nuclear medicine but uncommon to adult imaging. These include consent, fasting, intravenous access and the question of sedation[112,113]. Patient cooperation is a significant issue. Successful imaging can be facilitated by providing a relaxed, child-oriented environment, and staff experienced in paediatric venipuncture and the management of children. Available data suggests that PET/CT artefacts due to movement, poor co-registration or non-compliance are no more frequent among paediatric patients than among adult ones[114].
Paediatric sedation is a complex issue and requires case-by-case assessment. Often, the child’s parents will be aware of previous cooperation difficulties and can assist with decision making. When sedation is required, it should be limited to the scanning phase of the study[112]. The use of sedation in paediatrics is not without risk and has been associated with a 0.4% incidence of adverse respiratory events[115]. Sedation protocols vary by institution, but guidelines are available[116,117].
COMMON PITFALLS
The accuracy of PET can vary depending on tumour type, location and scan timing in relation to treatment. Furthermore, variant patterns of physiologic FDG uptake often differ in children as compared with adults and may be mistakenly interpreted as disease. A thorough understanding of normal variant FDG uptake is therefore essential for accurate image interpretation, preventing unnecessary studies or procedures and improving patient care. Image co-registration with hybrid PET/CT systems is helpful in distinguishing normal variation from pathology through the precise localisation of functional data.
Sites of physiologic FDG uptake that can differ in children as compared with adults include brown adipose tissue[118,119], thymus[120], brain[121] and epiphyseal plates[122]. Each of these potential pitfalls to image interpretation is discussed below. Other potential sites of increased FDG uptake in the paediatric patient include the pharyngeal lymphatic tissue of Waldeyer’s ring, salivary glands and haematopoietic bone marrow[123]. Additionally, paediatric patients suffer from a multitude of community acquired infections which may prompt intense reactive nodal avidity and thus mimic malignancy.
Brown adipose tissue
FDG uptake by brown adipose tissue in paediatric patients has recently been reviewed[124]. The primary function of brown fat is non-shivering thermogenesis[125]. This metabolic process involves expression of mitochondrial uncoupling proteins, including UCP-1, which separate oxidative phosphorylation from ATP synthesis and thereby generate energy dissipated as heat[126].
Brown fat FDG uptake has been reported in up to 20% of children and adolescents, but is a rare occurrence among adults[119,124]. Uptake is usually distributed symmetrically within the neck and supraclavicular regions, axillae, paraspinal regions of the posterior mediastinum, adjacent to the adrenal glands and upper abdominal wall (Figure 6). Such uptake may mimic malignancy or obscure pathological lesions[119,127]. This is particularly true for paediatric lymphoma patients, as brown fat is located adjacent to regions commonly involved in the disease.
Figure 6.
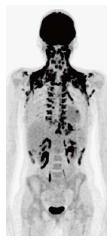
An extreme case of widespread brown fat activity in a 16-year-old female with treated Hodgkin’s disease.
Significant reductions in brown fat tracer uptake are made possible by controlling the environmental temperature and stress levels of the patient prior to injection and during the tracer uptake phase[128]. Additional blankets are often employed to provide further increases in warmth. In addition, the administration of oral diazepam may reduce brown fat uptake and is recommended by several groups[129]. Beta blockade with propranolol[130] or intravenous fentanyl[131] has also been advocated by some. Image co-registration with PET/CT is helpful to distinguish brown adipose tissue from pathological causes of FDG uptake[127].
Thymus
FDG-avid thymic tissue has been demonstrated in 34% of healthy young adults on screening PET scans[132]. Among paediatric oncology patients thymic uptake is observed in around 75% of cases irrespective of whether chemotherapy has been administered[120]. The increased activity among paediatric oncology patients is thought to be due to reactive thymic hyperplasia secondary to stress, infection or treatment. These changes may appear late and can persist for many months following the completion of treatment.
Familiarity with normal appearances is essential to distinguish between physiologic thymic uptake and active disease in the mediastinum. This is particularly important in children with potentially curable diseases such as lymphoma, as they may often have residual non-malignant tissue in the mediastinum following treatment. The normal pattern of thymic uptake is a homogenous lambda-shaped structure in the anterior mediastinum, although multiple variants are seen (Figure 7). Very intense or heterogenous uptake raises suspicion for thymic or other anterior mediastinal disease[133] (Figure 8).
Figure 7.
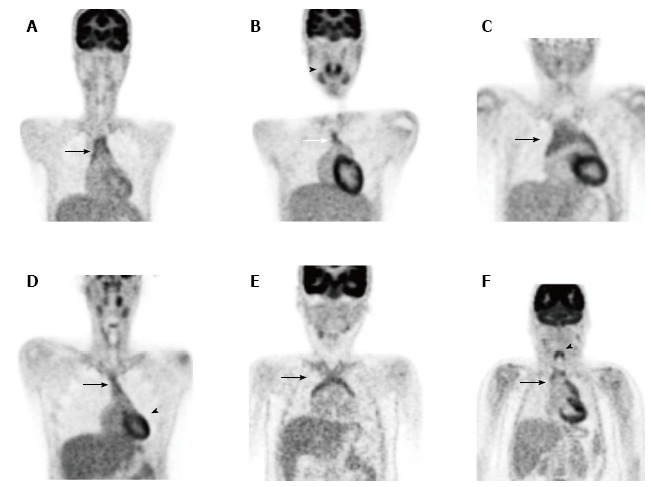
Normal physiological variants: various patterns of thymic uptake (A-F, arrow), tonsillar uptake (B, arrowhead), cardiac uptake (D, arrowhead) and laryngeal uptake (F, arrowhead).
Figure 8.
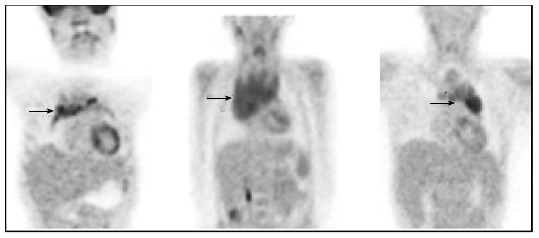
Examples of abnormal thymic uptake due to malignant disease (arrows).
Brain
It is important to recognise maturational changes in cerebral glucose metabolism to allow the identification of pathological alterations. A recent study describing cerebral FDG uptake in children demonstrated that all cerebral regions show increasing avidity with age, with rates of change being regionally specific[121]. The most metabolically active areas in early childhood are the parietal and occipital lobes. By age 7 these regions have less uptake than the frontal lobes, and by age 10 they have less uptake than the thalamus. Changes in the relative pattern of cortical FDG uptake appear to continue up until at least 16 years of age.
Epiphyseal plate
Skeletally immature paediatric patients exhibit physiologic linear uptake in physes and apophyses (Figure 9)[122]. Such uptake has the potential to obscure small skeletal lesions, or it may be mistaken for pathological activity. In addition, loss of the normal sharp demarcation of uptake in the physes may reflect bone marrow infiltration or activation and should be recognised. Avid FDG uptake may occasionally occur in benign skeletal lesions (Figure 10). These include fibroosseus defects, which are very common in childhood[122], and osteochondromas. An understanding that benign skeletal lesions may take up FDG, and knowledge of the typical appearances seen on the CT component of the scan will reduce false positive findings.
Figure 9.
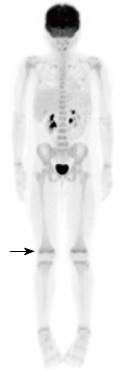
Normal 2-deoxy-2-(18F)fluoro-D-glucose positron emission tomography scan of an 8-year-old female showing physiological growth plate uptake (arrow).
Figure 10.
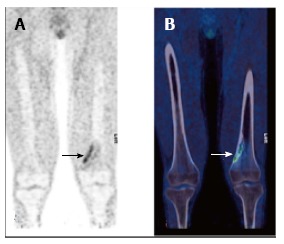
Non–ossifying fibroma (arrow) in the left distal femur of a 17-year-old male patient with Langerhans cell histiocytosis. FDG PET (A) showing avid uptake, CT (B) showing typical benign radiological features. PET: Positron emission tomography; CT: Computed tomography; FDG: 2-deoxy-2-(18F)fluoro-D-glucose.
DOSIMETRIC CONCERNS
As with all imaging modalities that utilise ionising radiation, there are special dosimetric considerations for children undergoing PET/CT scanning. Particular attention is required to keep radiation exposure as low as is reasonably achievable (ALARA principle) in view of the increased lifetime cancer mortality risk in children compared with adults[134]. The higher risk of radiation-induced malignancy in paediatric patients is a consequence of both the longer average post-exposure survival of children as compared with adults, and an intrinsic increase in radiosensitivity of children[135,136].
As risk from radiation exposure is cumulative[137], both nuclear medicine and CT components need to be considered when applying ALARA principles. In this context patient exposure may be adjusted through changes to the dose of administered radiopharmaceutical, CT protocol employed, anatomic area covered and frequency of imaging performed[138]. Notably, the end-organ for radiotracer administration is the bladder and therefore the bladder wall tends to receive the greatest radiation dose[139]. This can be minimised by encouraging fluid intake and frequent voiding following scan completion.
PET and PET/CT radiopharmaceutical dosage guidelines have been established by the European Association of Nuclear Medicine (EANM) Dosimetry and Paediatrics Committees[140,141] and the Pediatric Nuclear Medicine Dose Reduction Workgroup (North American Consensus Guidelines)[142]. Although there are some differences between these guidelines (Figure 11) both make recommendations in line with the ALARA principles. The EANM guidelines aim for the administration of FDG as a weight independent effective dose. Doses are calculated using normalisation factors which are derived from biological behaviour and patient weight. The aim is to deliver the same effective radiopharmaceutical dose to paediatric patients regardless of body weight, provided a minimum activity is administered[140]. The North American Consensus Guidelines recommend a standard FDG administered activity per kilogram of body weight[142].
Figure 11.
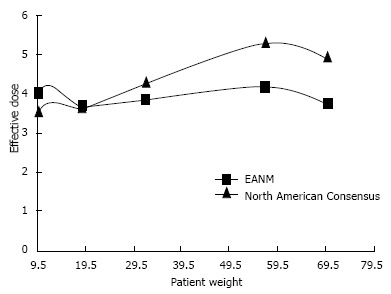
Effective dose vs patient weight for 2-deoxy-2-(18F)fluoro-D-glucose (European Association of Nuclear Medicine and North American Consensus Guidelines). Image courtesy of Bruce McBride, Chief Physicist, Department of Nuclear Medicine and PET, The Prince of Wales and Sydney Children’s Hospitals. PET: Positron emission tomography; EANM: European Association of Nuclear Medicine.
Effective doses to patients from CT images are dependent on tube current (mA), tube potential (kVp), rotation speed, pitch, slice thickness, patient mass and the anatomical volume included in the scan. CT exposures in hybrid PET/CT scanning may be tailored to meet the diagnostic needs of the patient. Commonly, diagnostic quality images are not required and CT exposure factors may be reduced significantly, with the production of images primarily for localisation and attenuation correction. These low dose protocols may reduce CT exposures by 50%-65% compared with typical diagnostic levels[143].
CONCLUSION
PET and PET/CT have emerged as powerful and important imaging techniques in the assessment of a variety of childhood malignancies, with PET/CT being the preferred modality. Although a number of radiopharmaceuticals exist, FDG remains the most widely used and clinically important. PET/CT scanning should occur in the context of rational and evidence-based imaging decisions, and ongoing large prospective trials will further clarify its role in the assessment of individual malignancies. It is important that a thorough understanding of variant childhood uptake patterns is obtained for accurate image interpretation.
ACKNOWLEDGMENTS
The authors would like to acknowledge Mr. Bruce McBride, Chief Physicist, Department of Nuclear Medicine and PET, The Prince of Wales and Sydney Children’s Hospitals for assistance in preparing the “Dosimetry” section of this manuscript.
Footnotes
P- Reviewer: Brasic JR, Cheng Z, Morbelli SD, Muller HW, Storto G, Treglia G S- Editor: Song XX L- Editor: A
E- Editor: Lu YJ
References
- 1.Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, Pastore G, Peris-Bonet R, Stiller CA; EUROCARE Working Group. Survival of European children and young adults with cancer diagnosed 1995-2002. Eur J Cancer. 2009;45:992–1005. doi: 10.1016/j.ejca.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang J, Li Q, El Fakhri G. Magnetic resonance-based motion correction for positron emission tomography imaging. Semin Nucl Med. 2013;43:60–67. doi: 10.1053/j.semnuclmed.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 4.Storto G, Nicolai E, Salvatore M. [18F]FDG-PET-CT for early monitoring of tumor response: when and why. Q J Nucl Med Mol Imaging. 2009;53:167–180. [PubMed] [Google Scholar]
- 5.Wegner EA, Barrington SF, Kingston JE, Robinson RO, Ferner RE, Taj M, Smith MA, O’Doherty MJ. The impact of PET scanning on management of paediatric oncology patients. Eur J Nucl Med Mol Imaging. 2005;32:23–30. doi: 10.1007/s00259-004-1645-3. [DOI] [PubMed] [Google Scholar]
- 6.Goo HW. Regional and whole-body imaging in pediatric oncology. Pediatr Radiol. 2011;41 Suppl 1:S186–S194. doi: 10.1007/s00247-011-2050-2. [DOI] [PubMed] [Google Scholar]
- 7.Bar-Sever Z, Keidar Z, Ben-Barak A, Bar-Shalom R, Postovsky S, Guralnik L, Ben Arush MW, Israel O. The incremental value of 18F-FDG PET/CT in paediatric malignancies. Eur J Nucl Med Mol Imaging. 2007;34:630–637. doi: 10.1007/s00259-006-0253-9. [DOI] [PubMed] [Google Scholar]
- 8.Yeung HW, Schoder H, Smith A, Gonen M, Larson SM. Clinical value of combined positron emission tomography/computed tomography imaging in the interpretation of 2-deoxy-2-[F-18]flouro-D-glucose-positron emission tomography studies in cancer patients. Mol Imaging Biol. 2005;7:229–235. doi: 10.1007/s11307-005-4113-y. [DOI] [PubMed] [Google Scholar]
- 9.Stauss J, Franzius C, Pfluger T, Juergens KU, Biassoni L, Begent J, Kluge R, Amthauer H, Voelker T, Højgaard L, Barrington S, Hain S, Lynch T, Hahn K; European Association of Nuclear Medicine. Guidelines for 18F-FDG PET and PET-CT imaging in paediatric oncology. Eur J Nucl Med Mol Imaging. 2008;35:1581–1588. doi: 10.1007/s00259-008-0826-x. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins DS, Rajendran JG, Conrad EU, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002;94:3277–3284. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- 11.Bar-Shalom R, Yefremov N, Haim N, Dann EJ, Epelbaum R, Keidar Z, Gaitini D, Frenkel A, Israel O. Camera-based FDG PET and 67Ga SPECT in evaluation of lymphoma: comparative study. Radiology. 2003;227:353–360. doi: 10.1148/radiol.2272020195. [DOI] [PubMed] [Google Scholar]
- 12.Costantini DL, Vali R, Chan J, McQuattie S, Charron M. Dual-time-point FDG PET/CT for the evaluation of pediatric tumors. AJR Am J Roentgenol. 2013;200:408–413. doi: 10.2214/AJR.12.8930. [DOI] [PubMed] [Google Scholar]
- 13.Jadvar H, Connolly LP, Fahey FH, Shulkin BL. PET and PET/CT in pediatric oncology. Semin Nucl Med. 2007;37:316–331. doi: 10.1053/j.semnuclmed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Kluge R, Kurch L, Montravers F, Mauz-Körholz C. FDG PET/CT in children and adolescents with lymphoma. Pediatr Radiol. 2013;43:406–417. doi: 10.1007/s00247-012-2559-z. [DOI] [PubMed] [Google Scholar]
- 15.Kabickova E, Sumerauer D, Cumlivska E, Drahokoupilova E, Nekolna M, Chanova M, Hladikova M, Kodet R, Belohlavek O. Comparison of 18F-FDG-PET and standard procedures for the pretreatment staging of children and adolescents with Hodgkin’s disease. Eur J Nucl Med Mol Imaging. 2006;33:1025–1031. doi: 10.1007/s00259-005-0019-9. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Pampaloni M, Takalkar A, Yu JQ, Zhuang H, Alavi A. F-18 FDG-PET imaging and correlation with CT in staging and follow-up of pediatric lymphomas. Pediatr Radiol. 2006;36:524–531. doi: 10.1007/s00247-006-0152-z. [DOI] [PubMed] [Google Scholar]
- 17.Depas G, De Barsy C, Jerusalem G, Hoyoux C, Dresse MF, Fassotte MF, Paquet N, Foidart J, Rigo P, Hustinx R. 18F-FDG PET in children with lymphomas. Eur J Nucl Med Mol Imaging. 2005;32:31–38. doi: 10.1007/s00259-004-1604-z. [DOI] [PubMed] [Google Scholar]
- 18.London K, Cross S, Onikul E, Dalla-Pozza L, Howman-Giles R. 18F-FDG PET/CT in paediatric lymphoma: comparison with conventional imaging. Eur J Nucl Med Mol Imaging. 2011;38:274–284. doi: 10.1007/s00259-010-1619-6. [DOI] [PubMed] [Google Scholar]
- 19.Riad R, Omar W, Kotb M, Hafez M, Sidhom I, Zamzam M, Zaky I, Abdel-Dayem H. Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:319–329. doi: 10.1007/s00259-009-1276-9. [DOI] [PubMed] [Google Scholar]
- 20.Purz S, Mauz-Körholz C, Körholz D, Hasenclever D, Krausse A, Sorge I, Ruschke K, Stiefel M, Amthauer H, Schober O, et al. [18F]Fluorodeoxyglucose positron emission tomography for detection of bone marrow involvement in children and adolescents with Hodgkin’s lymphoma. J Clin Oncol. 2011;29:3523–3528. doi: 10.1200/JCO.2010.32.4996. [DOI] [PubMed] [Google Scholar]
- 21.Moulin-Romsee G, Hindié E, Cuenca X, Brice P, Decaudin D, Bénamor M, Brière J, Anitei M, Filmont JE, Sibon D, et al. (18)F-FDG PET/CT bone/bone marrow findings in Hodgkin’s lymphoma may circumvent the use of bone marrow trephine biopsy at diagnosis staging. Eur J Nucl Med Mol Imaging. 2010;37:1095–1105. doi: 10.1007/s00259-009-1377-5. [DOI] [PubMed] [Google Scholar]
- 22.Pelosi E, Penna D, Deandreis D, Chiappella A, Skanjeti A, Vitolo U, Bisi G. FDG-PET in the detection of bone marrow disease in Hodgkin’s disease and aggressive non-Hodgkin’s lymphoma and its impact on clinical management. Q J Nucl Med Mol Imaging. 2008;52:9–16. [PubMed] [Google Scholar]
- 23.Cheng G, Chen W, Chamroonrat W, Torigian DA, Zhuang H, Alavi A. Biopsy versus FDG PET/CT in the initial evaluation of bone marrow involvement in pediatric lymphoma patients. Eur J Nucl Med Mol Imaging. 2011;38:1469–1476. doi: 10.1007/s00259-011-1815-z. [DOI] [PubMed] [Google Scholar]
- 24.Furth C, Steffen IG, Amthauer H, Ruf J, Misch D, Schönberger S, Kobe C, Denecke T, Stöver B, Hautzel H, et al. Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin’s lymphoma: analysis of a prospective multicenter trial. J Clin Oncol. 2009;27:4385–4391. doi: 10.1200/JCO.2008.19.7814. [DOI] [PubMed] [Google Scholar]
- 25.Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, Buus S, Keiding S, D’Amore F, Boesen AM, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–59. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 26.Kostakoglu L, Coleman M, Leonard JP, Kuji I, Zoe H, Goldsmith SJ. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin’s disease. J Nucl Med. 2002;43:1018–1027. [PubMed] [Google Scholar]
- 27.Miller E, Metser U, Avrahami G, Dvir R, Valdman D, Sira LB, Sayar D, Burstein Y, Toren A, Yaniv I, et al. Role of 18F-FDG PET/CT in staging and follow-up of lymphoma in pediatric and young adult patients. J Comput Assist Tomogr. 2006;30:689–694. doi: 10.1097/00004728-200607000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes MM, Delbeke D, Whitlock JA, Martin W, Kuttesch JF, Frangoul HA, Shankar S. Utility of FDG-PET/CT in follow-up of children treated for Hodgkin and non-Hodgkin lymphoma. J Pediatr Hematol Oncol. 2006;28:300–306. doi: 10.1097/01.mph.0000212912.37512.b1. [DOI] [PubMed] [Google Scholar]
- 29.Crocchiolo R, Fallanca F, Giovacchini G, Ferreri AJ, Assanelli A, Verona C, Pescarollo A, Bregni M, Ponzoni M, Gianolli L, et al. Role of 18FDG-PET/CT in detecting relapse during follow-up of patients with Hodgkin’s lymphoma. Ann Hematol. 2009;88:1229–1236. doi: 10.1007/s00277-009-0752-4. [DOI] [PubMed] [Google Scholar]
- 30.Ramanan M, Chaseling R. Paediatric brain tumours treated at a single, tertiary paediatric neurosurgical referral centre from 1999 to 2010 in Australia. J Clin Neurosci. 2012;19:1387–1391. doi: 10.1016/j.jocn.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Fleming AJ, Chi SN. Brain tumors in children. Curr Probl Pediatr Adolesc Health Care. 2012;42:80–103. doi: 10.1016/j.cppeds.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Kumar R, Shandal V, Shamim SA, Halanaik D, Malhotra A. Clinical applications of PET and PET/CT in pediatric malignancies. Expert Rev Anticancer Ther. 2010;10:755–768. doi: 10.1586/era.10.12. [DOI] [PubMed] [Google Scholar]
- 33.Sønderkaer S, Schmiegelow M, Carstensen H, Nielsen LB, Müller J, Schmiegelow K. Long-term neurological outcome of childhood brain tumors treated by surgery only. J Clin Oncol. 2003;21:1347–1351. doi: 10.1200/JCO.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Herholz K, Hölzer T, Bauer B, Schröder R, Voges J, Ernestus RI, Mendoza G, Weber-Luxenburger G, Löttgen J, Thiel A, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology. 1998;50:1316–1322. doi: 10.1212/wnl.50.5.1316. [DOI] [PubMed] [Google Scholar]
- 35.Pirotte B, Levivier M, Morelli D, Van Bogaert P, Detemmerman D, David P, Baleriaux D, Brotchi J, Goldman S. Positron emission tomography for the early postsurgical evaluation of pediatric brain tumors. Childs Nerv Syst. 2005;21:294–300. doi: 10.1007/s00381-004-1071-7. [DOI] [PubMed] [Google Scholar]
- 36.Utriainen M, Metsähonkala L, Salmi TT, Utriainen T, Kalimo H, Pihko H, Mäkipernaa A, Harila-Saari A, Jyrkkiö S, Laine J, et al. Metabolic characterization of childhood brain tumors: comparison of 18F-fluorodeoxyglucose and 11C-methionine positron emission tomography. Cancer. 2002;95:1376–1386. doi: 10.1002/cncr.10798. [DOI] [PubMed] [Google Scholar]
- 37.Pirotte BJ, Lubansu A, Massager N, Wikler D, Van Bogaert P, Levivier M, Brotchi J, Goldman S. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg Pediatr. 2010;5:486–499. doi: 10.3171/2010.1.PEDS09481. [DOI] [PubMed] [Google Scholar]
- 38.De Witte O, Lefranc F, Levivier M, Salmon I, Brotchi J, Goldman S. FDG-PET as a prognostic factor in high-grade astrocytoma. J Neurooncol. 2000;49:157–163. doi: 10.1023/a:1026518002800. [DOI] [PubMed] [Google Scholar]
- 39.Kruer MC, Kaplan AM, Etzl MM, Carpentieri DF, Dickman PS, Chen K, Mathieson K, Irving A. The value of positron emission tomography and proliferation index in predicting progression in low-grade astrocytomas of childhood. J Neurooncol. 2009;95:239–245. doi: 10.1007/s11060-009-9922-4. [DOI] [PubMed] [Google Scholar]
- 40.Valk PE, Budinger TF, Levin VA, Silver P, Gutin PH, Doyle WK. PET of malignant cerebral tumors after interstitial brachytherapy. Demonstration of metabolic activity and correlation with clinical outcome. J Neurosurg. 1988;69:830–838. doi: 10.3171/jns.1988.69.6.0830. [DOI] [PubMed] [Google Scholar]
- 41.Glantz MJ, Hoffman JM, Coleman RE, Friedman AH, Hanson MW, Burger PC, Herndon JE, Meisler WJ, Schold SC. Identification of early recurrence of primary central nervous system tumors by [18F]fluorodeoxyglucose positron emission tomography. Ann Neurol. 1991;29:347–355. doi: 10.1002/ana.410290403. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan AM, Lawson MA, Spataro J, Bandy DJ, Bonstelle CT, Moss SD, Manwaring KH, Reiman EM. Positron emission tomography using [18F] fluorodeoxyglucose and [11C] l-methionine to metabolically characterize dysembryoplastic neuroepithelial tumors. J Child Neurol. 1999;14:673–677. doi: 10.1177/088307389901401009. [DOI] [PubMed] [Google Scholar]
- 43.Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS, Jeong JM, Lee DS, Jung HW, Lee MC. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:176–182. doi: 10.1007/s00259-001-0690-4. [DOI] [PubMed] [Google Scholar]
- 44.Galldiks N, Kracht LW, Berthold F, Miletic H, Klein JC, Herholz K, Jacobs AH, Heiss WD. [11C]-L-methionine positron emission tomography in the management of children and young adults with brain tumors. J Neurooncol. 2010;96:231–239. doi: 10.1007/s11060-009-9953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nariai T, Tanaka Y, Wakimoto H, Aoyagi M, Tamaki M, Ishiwata K, Senda M, Ishii K, Hirakawa K, Ohno K. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg. 2005;103:498–507. doi: 10.3171/jns.2005.103.3.0498. [DOI] [PubMed] [Google Scholar]
- 46.Heare T, Hensley MA, Dell’Orfano S. Bone tumors: osteosarcoma and Ewing’s sarcoma. Curr Opin Pediatr. 2009;21:365–372. doi: 10.1097/MOP.0b013e32832b1111. [DOI] [PubMed] [Google Scholar]
- 47.Kumar R, Chauhan A, Vellimana AK, Chawla M. Role of PET/PET-CT in the management of sarcomas. Expert Rev Anticancer Ther. 2006;6:1241–1250. doi: 10.1586/14737140.6.8.1241. [DOI] [PubMed] [Google Scholar]
- 48.Quartuccio N, Treglia G, Salsano M, Mattoli MV, Muoio B, Piccardo A, Lopci E, Cistaro A. The role of Fluorine-18-Fluorodeoxyglucose positron emission tomography in staging and restaging of patients with osteosarcoma. Radiol Oncol. 2013;47:97–102. doi: 10.2478/raon-2013-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Treglia G, Salsano M, Stefanelli A, Mattoli MV, Giordano A, Bonomo L. Diagnostic accuracy of ¹⁸F-FDG-PET and PET/CT in patients with Ewing sarcoma family tumours: a systematic review and a meta-analysis. Skeletal Radiol. 2012;41:249–256. doi: 10.1007/s00256-011-1298-9. [DOI] [PubMed] [Google Scholar]
- 50.McCarville MB. PET-CT imaging in pediatric oncology. Cancer Imaging. 2009;9:35–43. doi: 10.1102/1470-7330.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mody RJ, Bui C, Hutchinson RJ, Yanik GA, Castle VP, Frey KA, Shulkin BL. FDG PET imaging of childhood sarcomas. Pediatr Blood Cancer. 2010;54:222–227. doi: 10.1002/pbc.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benz MR, Tchekmedyian N, Eilber FC, Federman N, Czernin J, Tap WD. Utilization of positron emission tomography in the management of patients with sarcoma. Curr Opin Oncol. 2009;21:345–351. doi: 10.1097/CCO.0b013e32832c95e2. [DOI] [PubMed] [Google Scholar]
- 53.Tateishi U, Hosono A, Makimoto A, Sakurada A, Terauchi T, Arai Y, Imai Y, Kim EE. Accuracy of 18F fluorodeoxyglucose positron emission tomography/computed tomography in staging of pediatric sarcomas. J Pediatr Hematol Oncol. 2007;29:608–612. doi: 10.1097/MPH.0b013e318142b5ab. [DOI] [PubMed] [Google Scholar]
- 54.McCarville MB, Christie R, Daw NC, Spunt SL, Kaste SC. PET/CT in the evaluation of childhood sarcomas. AJR Am J Roentgenol. 2005;184:1293–1304. doi: 10.2214/ajr.184.4.01841293. [DOI] [PubMed] [Google Scholar]
- 55.Franzius C, Sciuk J, Daldrup-Link HE, Jürgens H, Schober O. FDG-PET for detection of osseous metastases from malignant primary bone tumours: comparison with bone scintigraphy. Eur J Nucl Med. 2000;27:1305–1311. doi: 10.1007/s002590000301. [DOI] [PubMed] [Google Scholar]
- 56.Völker T, Denecke T, Steffen I, Misch D, Schönberger S, Plotkin M, Ruf J, Furth C, Stöver B, Hautzel H, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol. 2007;25:5435–5441. doi: 10.1200/JCO.2007.12.2473. [DOI] [PubMed] [Google Scholar]
- 57.Franzius C, Daldrup-Link HE, Sciuk J, Rummeny EJ, Bielack S, Jürgens H, Schober O. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann Oncol. 2001;12:479–486. doi: 10.1023/a:1011111322376. [DOI] [PubMed] [Google Scholar]
- 58.Iagaru A, Chawla S, Menendez L, Conti PS. 18F-FDG PET and PET/CT for detection of pulmonary metastases from musculoskeletal sarcomas. Nucl Med Commun. 2006;27:795–802. doi: 10.1097/01.mnm.0000237986.31597.86. [DOI] [PubMed] [Google Scholar]
- 59.Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932–945. doi: 10.2967/jnumed.106.035774. [DOI] [PubMed] [Google Scholar]
- 60.Allen-Auerbach M, Yeom K, Park J, Phelps M, Czernin J. Standard PET/CT of the chest during shallow breathing is inadequate for comprehensive staging of lung cancer. J Nucl Med. 2006;47:298–301. [PubMed] [Google Scholar]
- 61.Erdi YE, Nehmeh SA, Pan T, Pevsner A, Rosenzweig KE, Mageras G, Yorke ED, Schoder H, Hsiao W, Squire OD, et al. The CT motion quantitation of lung lesions and its impact on PET-measured SUVs. J Nucl Med. 2004;45:1287–1292. [PubMed] [Google Scholar]
- 62.Werner MK, Parker JA, Kolodny GM, English JR, Palmer MR. Respiratory gating enhances imaging of pulmonary nodules and measurement of tracer uptake in FDG PET/CT. AJR Am J Roentgenol. 2009;193:1640–1645. doi: 10.2214/AJR.09.2516. [DOI] [PubMed] [Google Scholar]
- 63.Biehl KJ, Kong FM, Dehdashti F, Jin JY, Mutic S, El Naqa I, Siegel BA, Bradley JD. 18F-FDG PET definition of gross tumor volume for radiotherapy of non-small cell lung cancer: is a single standardized uptake value threshold approach appropriate? J Nucl Med. 2006;47:1808–1812. [PubMed] [Google Scholar]
- 64.Gerth HU, Juergens KU, Dirksen U, Gerss J, Schober O, Franzius C. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. J Nucl Med. 2007;48:1932–1939. doi: 10.2967/jnumed.107.045286. [DOI] [PubMed] [Google Scholar]
- 65.McCarville MB, Lederman HM, Santana VM, Daw NC, Shochat SJ, Li CS, Kaufman RA. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239:514–520. doi: 10.1148/radiol.2392050631. [DOI] [PubMed] [Google Scholar]
- 66.Johnson GR, Zhuang H, Khan J, Chiang SB, Alavi A. Roles of positron emission tomography with fluorine-18-deoxyglucose in the detection of local recurrent and distant metastatic sarcoma. Clin Nucl Med. 2003;28:815–820. doi: 10.1097/01.rlu.0000089523.00672.2b. [DOI] [PubMed] [Google Scholar]
- 67.Schwarzbach MH, Hinz U, Dimitrakopoulou-Strauss A, Willeke F, Cardona S, Mechtersheimer G, Lehnert T, Strauss LG, Herfarth C, Büchler MW. Prognostic significance of preoperative [18-F] fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging in patients with resectable soft tissue sarcomas. Ann Surg. 2005;241:286–294. doi: 10.1097/01.sla.0000152663.61348.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schuetze SM, Rubin BP, Vernon C, Hawkins DS, Bruckner JD, Conrad EU, Eary JF. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer. 2005;103:339–348. doi: 10.1002/cncr.20769. [DOI] [PubMed] [Google Scholar]
- 69.Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU, Eary JF. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–8834. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- 70.Benz MR, Czernin J, Allen-Auerbach MS, Tap WD, Dry SM, Elashoff D, Chow K, Evilevitch V, Eckardt JJ, Phelps ME, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2009;15:2856–2863. doi: 10.1158/1078-0432.CCR-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Portwine C, Marriott C, Barr RD. PET imaging for pediatric oncology: an assessment of the evidence. Pediatr Blood Cancer. 2010;55:1048–1061. doi: 10.1002/pbc.22747. [DOI] [PubMed] [Google Scholar]
- 72.Treglia G, Taralli S, Bertagna F, Salsano M, Muoio B, Novellis P, Vita ML, Maggi F, Giordano A. Usefulness of whole-body fluorine-18-fluorodeoxyglucose positron emission tomography in patients with neurofibromatosis type 1: a systematic review. Radiol Res Pract. 2012;2012:431029. doi: 10.1155/2012/431029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai LL, Drubach L, Fahey F, Irons M, Voss S, Ullrich NJ. [18F]-Fluorodeoxyglucose positron emission tomography in children with neurofibromatosis type 1 and plexiform neurofibromas: correlation with malignant transformation. J Neurooncol. 2012;108:469–475. doi: 10.1007/s11060-012-0840-5. [DOI] [PubMed] [Google Scholar]
- 74.Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62:1573–1577. [PubMed] [Google Scholar]
- 75.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warbey VS, Ferner RE, Dunn JT, Calonje E, O‘Doherty MJ. [18F]FDG PET/CT in the diagnosis of malignant peripheral nerve sheath tumours in neurofibromatosis type-1. Eur J Nucl Med Mol Imaging. 2009;36:751–757. doi: 10.1007/s00259-008-1038-0. [DOI] [PubMed] [Google Scholar]
- 77.Ferner RE, Golding JF, Smith M, Calonje E, Jan W, Sanjayanathan V, O’Doherty M. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): a long-term clinical study. Ann Oncol. 2008;19:390–394. doi: 10.1093/annonc/mdm450. [DOI] [PubMed] [Google Scholar]
- 78.Brenner W, Friedrich RE, Gawad KA, Hagel C, von Deimling A, de Wit M, Buchert R, Clausen M, Mautner VF. Prognostic relevance of FDG PET in patients with neurofibromatosis type-1 and malignant peripheral nerve sheath tumours. Eur J Nucl Med Mol Imaging. 2006;33:428–432. doi: 10.1007/s00259-005-0030-1. [DOI] [PubMed] [Google Scholar]
- 79.Bousvaros A, Kirks DR, Grossman H. Imaging of neuroblastoma: an overview. Pediatr Radiol. 1986;16:89–106. doi: 10.1007/BF02386629. [DOI] [PubMed] [Google Scholar]
- 80.Spix C, Aareleid T, Stiller C, Magnani C, Kaatsch P, Michaelis J. Survival of children with neuroblastoma. time trends and regional differences in Europe, 1978--1992. Eur J Cancer. 2001;37:722–729. doi: 10.1016/s0959-8049(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 81.Taggart D, Dubois S, Matthay KK. Radiolabeled metaiodobenzylguanidine for imaging and therapy of neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52:403–418. [PubMed] [Google Scholar]
- 82.DuBois SG, Matthay KK. Radiolabeled metaiodobenzylguanidine for the treatment of neuroblastoma. Nucl Med Biol. 2008;35 Suppl 1:S35–S48. doi: 10.1016/j.nucmedbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boubaker A, Bischof Delaloye A. MIBG scintigraphy for the diagnosis and follow-up of children with neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52:388–402. [PubMed] [Google Scholar]
- 84.Claudiani F, Stimamiglio P, Bertolazzi L, Cabria M, Conte M, Villavecchia GP, Garaventa A, Lanino E, De Bernardi B, Scopinaro G. Radioiodinated meta-iodobenzylguanidine in the diagnosis of childhood neuroblastoma. Q J Nucl Med. 1995;39:21–24. [PubMed] [Google Scholar]
- 85.Gelfand MJ. Meta-iodobenzylguanidine in children. Semin Nucl Med. 1993;23:231–242. doi: 10.1016/s0001-2998(05)80104-7. [DOI] [PubMed] [Google Scholar]
- 86.Troncone L, Rufini V, Montemaggi P, Danza FM, Lasorella A, Mastrangelo R. The diagnostic and therapeutic utility of radioiodinated metaiodobenzylguanidine (MIBG). 5 years of experience. Eur J Nucl Med. 1990;16:325–335. doi: 10.1007/BF00842788. [DOI] [PubMed] [Google Scholar]
- 87.Shulkin BL, Shapiro B. Current concepts on the diagnostic use of MIBG in children. J Nucl Med. 1998;39:679–688. [PubMed] [Google Scholar]
- 88.Biasotti S, Garaventa A, Villavecchia GP, Cabria M, Nantron M, De Bernardi B. False-negative metaiodobenzylguanidine scintigraphy at diagnosis of neuroblastoma. Med Pediatr Oncol. 2000;35:153–155. doi: 10.1002/1096-911x(200008)35:2<153::aid-mpo18>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 89.Mueller WP, Coppenrath E, Pfluger T. Nuclear medicine and multimodality imaging of pediatric neuroblastoma. Pediatr Radiol. 2013;43:418–427. doi: 10.1007/s00247-012-2512-1. [DOI] [PubMed] [Google Scholar]
- 90.Shulkin BL, Hutchinson RJ, Castle VP, Yanik GA, Shapiro B, Sisson JC. Neuroblastoma: positron emission tomography with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose compared with metaiodobenzylguanidine scintigraphy. Radiology. 1996;199:743–750. doi: 10.1148/radiology.199.3.8637999. [DOI] [PubMed] [Google Scholar]
- 91.Kushner BH, Yeung HW, Larson SM, Kramer K, Cheung NK. Extending positron emission tomography scan utility to high-risk neuroblastoma: fluorine-18 fluorodeoxyglucose positron emission tomography as sole imaging modality in follow-up of patients. J Clin Oncol. 2001;19:3397–3405. doi: 10.1200/JCO.2001.19.14.3397. [DOI] [PubMed] [Google Scholar]
- 92.Sharp SE, Shulkin BL, Gelfand MJ, Salisbury S, Furman WL. 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J Nucl Med. 2009;50:1237–1243. doi: 10.2967/jnumed.108.060467. [DOI] [PubMed] [Google Scholar]
- 93.Melzer HI, Coppenrath E, Schmid I, Albert MH, von Schweinitz D, Tudball C, Bartenstein P, Pfluger T. ¹²³I-MIBG scintigraphy/SPECT versus ¹⁸F-FDG PET in paediatric neuroblastoma. Eur J Nucl Med Mol Imaging. 2011;38:1648–1658. doi: 10.1007/s00259-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 94.Piccardo A, Lopci E, Conte M, Garaventa A, Foppiani L, Altrinetti V, Nanni C, Bianchi P, Cistaro A, Sorrentino S, et al. Comparison of 18F-dopa PET/CT and 123I-MIBG scintigraphy in stage 3 and 4 neuroblastoma: a pilot study. Eur J Nucl Med Mol Imaging. 2012;39:57–71. doi: 10.1007/s00259-011-1938-2. [DOI] [PubMed] [Google Scholar]
- 95.Gains JE, Bomanji JB, Fersht NL, Sullivan T, D’Souza D, Sullivan KP, Aldridge M, Waddington W, Gaze MN. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J Nucl Med. 2011;52:1041–1047. doi: 10.2967/jnumed.110.085100. [DOI] [PubMed] [Google Scholar]
- 96.Swinson S, McHugh K. Urogenital tumours in childhood. Cancer Imaging. 2011;11 Spec No A:S48–S64. doi: 10.1102/1470-7330.2011.9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Md Zin R, Murch A, Charles A. Pathology, genetics and cytogenetics of Wilms’ tumour. Pathology. 2011;43:302–312. doi: 10.1097/PAT.0b013e3283463575. [DOI] [PubMed] [Google Scholar]
- 98.Geller E, Kochan PS. Renal neoplasms of childhood. Radiol Clin North Am. 2011;49:689–709, vi. doi: 10.1016/j.rcl.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Buckley KS. Pediatric genitourinary tumors. Curr Opin Oncol. 2012;24:291–296. doi: 10.1097/CCO.0b013e32835265c9. [DOI] [PubMed] [Google Scholar]
- 100.Misch D, Steffen IG, Schönberger S, Voelker T, Furth C, Stöver B, Hautzel H, Henze G, Amthauer H, Denecke T. Use of positron emission tomography for staging, preoperative response assessment and posttherapeutic evaluation in children with Wilms tumour. Eur J Nucl Med Mol Imaging. 2008;35:1642–1650. doi: 10.1007/s00259-008-0819-9. [DOI] [PubMed] [Google Scholar]
- 101.Moinul Hossain AK, Shulkin BL, Gelfand MJ, Bashir H, Daw NC, Sharp SE, Nadel HR, Dome JS. FDG positron emission tomography/computed tomography studies of Wilms’ tumor. Eur J Nucl Med Mol Imaging. 2010;37:1300–1308. doi: 10.1007/s00259-010-1396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Begent J, Sebire NJ, Levitt G, Brock P, Jones KP, Ell P, Gordon I, Anderson J. Pilot study of F(18)-Fluorodeoxyglucose Positron Emission Tomography/computerised tomography in Wilms’ tumour: correlation with conventional imaging, pathology and immunohistochemistry. Eur J Cancer. 2011;47:389–396. doi: 10.1016/j.ejca.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 103.Azouz EM, Saigal G, Rodriguez MM, Podda A. Langerhans’ cell histiocytosis: pathology, imaging and treatment of skeletal involvement. Pediatr Radiol. 2005;35:103–115. doi: 10.1007/s00247-004-1262-0. [DOI] [PubMed] [Google Scholar]
- 104.Abla O, Egeler RM, Weitzman S. Langerhans cell histiocytosis: Current concepts and treatments. Cancer Treat Rev. 2010;36:354–359. doi: 10.1016/j.ctrv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 105.Dogan AS, Conway JJ, Miller JH, Grier D, Bhattathiry MM, Mitchell CS. Detection of bone lesions in Langerhans cell histiocytosis: complementary roles of scintigraphy and conventional radiography. J Pediatr Hematol Oncol. 1996;18:51–58. doi: 10.1097/00043426-199602000-00010. [DOI] [PubMed] [Google Scholar]
- 106.Phillips M, Allen C, Gerson P, McClain K. Comparison of FDG-PET scans to conventional radiography and bone scans in management of Langerhans cell histiocytosis. Pediatr Blood Cancer. 2009;52:97–101. doi: 10.1002/pbc.21782. [DOI] [PubMed] [Google Scholar]
- 107.Kaste SC, Rodriguez-Galindo C, McCarville ME, Shulkin BL. PET-CT in pediatric Langerhans cell histiocytosis. Pediatr Radiol. 2007;37:615–622. doi: 10.1007/s00247-007-0467-4. [DOI] [PubMed] [Google Scholar]
- 108.Blum R, Seymour JF, Hicks RJ. Role of 18FDG-positron emission tomography scanning in the management of histiocytosis. Leuk Lymphoma. 2002;43:2155–2157. doi: 10.1080/1042819021000032908. [DOI] [PubMed] [Google Scholar]
- 109.Binkovitz LA, Olshefski RS, Adler BH. Coincidence FDG-PET in the evaluation of Langerhans’ cell histiocytosis: preliminary findings. Pediatr Radiol. 2003;33:598–602. doi: 10.1007/s00247-003-0943-4. [DOI] [PubMed] [Google Scholar]
- 110.Lee HJ, Ahn BC, Lee SW, Lee J. The usefulness of F-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with Langerhans cell histiocytosis. Ann Nucl Med. 2012;26:730–737. doi: 10.1007/s12149-012-0635-y. [DOI] [PubMed] [Google Scholar]
- 111.Mueller WP, Melzer HI, Schmid I, Coppenrath E, Bartenstein P, Pfluger T. The diagnostic value of 18F-FDG PET and MRI in paediatric histiocytosis. Eur J Nucl Med Mol Imaging. 2013;40:356–363. doi: 10.1007/s00259-012-2278-6. [DOI] [PubMed] [Google Scholar]
- 112.Kaste SC. Issues specific to implementing PET-CT for pediatric oncology: what we have learned along the way. Pediatr Radiol. 2004;34:205–213. doi: 10.1007/s00247-003-1111-6. [DOI] [PubMed] [Google Scholar]
- 113.Roberts EG, Shulkin BL. Technical issues in performing PET studies in pediatric patients. J Nucl Med Technol. 2004;32:5–9; quiz 10-1. [PubMed] [Google Scholar]
- 114.Franzius C, Juergens KU, Schober O. Is PET/CT necessary in paediatric oncology? For. Eur J Nucl Med Mol Imaging. 2006;33:960–965. doi: 10.1007/s00259-006-0114-6. [DOI] [PubMed] [Google Scholar]
- 115.Sanborn PA, Michna E, Zurakowski D, Burrows PE, Fontaine PJ, Connor L, Mason KP. Adverse cardiovascular and respiratory events during sedation of pediatric patients for imaging examinations. Radiology. 2005;237:288–294. doi: 10.1148/radiol.2371041415. [DOI] [PubMed] [Google Scholar]
- 116.Weiss S. Sedation of pediatric patients for nuclear medicine procedures. Semin Nucl Med. 1993;23:190–198. doi: 10.1016/s0001-2998(05)80101-1. [DOI] [PubMed] [Google Scholar]
- 117.Pintelon H, Dejonckheere M, Piepsz A. Pediatric nuclear medicine: a practical approach. Q J Nucl Med. 1997;41:263–268. [PubMed] [Google Scholar]
- 118.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 119.Yeung HW, Grewal RK, Gonen M, Schöder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med. 2003;44:1789–1796. [PubMed] [Google Scholar]
- 120.Brink I, Reinhardt MJ, Hoegerle S, Altehoefer C, Moser E, Nitzsche EU. Increased metabolic activity in the thymus gland studied with 18F-FDG PET: age dependency and frequency after chemotherapy. J Nucl Med. 2001;42:591–595. [PubMed] [Google Scholar]
- 121.London K, Howman-Giles R. Normal cerebral FDG uptake during childhood. Eur J Nucl Med Mol Imaging. 2014;41:723–735. doi: 10.1007/s00259-013-2639-9. [DOI] [PubMed] [Google Scholar]
- 122.Goodin GS, Shulkin BL, Kaufman RA, McCarville MB. PET/CT characterization of fibroosseous defects in children: 18F-FDG uptake can mimic metastatic disease. AJR Am J Roentgenol. 2006;187:1124–1128. doi: 10.2214/AJR.06.0171. [DOI] [PubMed] [Google Scholar]
- 123.Shammas A, Lim R, Charron M. Pediatric FDG PET/CT: physiologic uptake, normal variants, and benign conditions. Radiographics. 2009;29:1467–1486. doi: 10.1148/rg.295085247. [DOI] [PubMed] [Google Scholar]
- 124.Hong TS, Shammas A, Charron M, Zukotynski KA, Drubach LA, Lim R. Brown adipose tissue 18F-FDG uptake in pediatric PET/CT imaging. Pediatr Radiol. 2011;41:759–768. doi: 10.1007/s00247-010-1925-y. [DOI] [PubMed] [Google Scholar]
- 125.Himms-Hagen J. Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J. 1990;4:2890–2898. [PubMed] [Google Scholar]
- 126.Del Mar Gonzalez-Barroso M, Ricquier D, Cassard-Doulcier AM. The human uncoupling protein-1 gene (UCP1): present status and perspectives in obesity research. Obes Rev. 2000;1:61–72. doi: 10.1046/j.1467-789x.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 127.Truong MT, Erasmus JJ, Munden RF, Marom EM, Sabloff BS, Gladish GW, Podoloff DA, Macapinlac HA. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR Am J Roentgenol. 2004;183:1127–1132. doi: 10.2214/ajr.183.4.1831127. [DOI] [PubMed] [Google Scholar]
- 128.Garcia C, Bandaru V, Van Nostrand D, Chennupati S, Atkins F, Acio E, Kulkarni K, Majd M. Effective reduction of brown fat FDG uptake by controlling environmental temperature prior to PET scan: an expanded case series. Mol Imaging Biol. 2010;12:652–656. doi: 10.1007/s11307-010-0298-9. [DOI] [PubMed] [Google Scholar]
- 129.Barrington SF, Maisey MN. Skeletal muscle uptake of fluorine-18-FDG: effect of oral diazepam. J Nucl Med. 1996;37:1127–1129. [PubMed] [Google Scholar]
- 130.Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med. 2007;32:351–357. doi: 10.1097/01.rlu.0000259570.69163.04. [DOI] [PubMed] [Google Scholar]
- 131.Gelfand MJ, O’hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol. 2005;35:984–990. doi: 10.1007/s00247-005-1505-8. [DOI] [PubMed] [Google Scholar]
- 132.Nakahara T, Fujii H, Ide M, Nishiumi N, Takahashi W, Yasuda S, Shohtsu A, Kubo A. FDG uptake in the morphologically normal thymus: comparison of FDG positron emission tomography and CT. Br J Radiol. 2001;74:821–824. doi: 10.1259/bjr.74.885.740821. [DOI] [PubMed] [Google Scholar]
- 133.Gawande RS, Khurana A, Messing S, Zhang D, Castañeda RT, Goldsby RE, Hawkins RA, Daldrup-Link HE. Differentiation of normal thymus from anterior mediastinal lymphoma and lymphoma recurrence at pediatric PET/CT. Radiology. 2012;262:613–622. doi: 10.1148/radiol.11110715. [DOI] [PubMed] [Google Scholar]
- 134.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 135.Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990. Radiat Res. 1996;146:1–27. [PubMed] [Google Scholar]
- 136.Brenner DJ. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol. 2002;32:228–231; discussion 228-231. doi: 10.1007/s00247-002-0671-1. [DOI] [PubMed] [Google Scholar]
- 137.Gelfand MJ. Dose reduction in pediatric hybrid and planar imaging. Q J Nucl Med Mol Imaging. 2010;54:379–388. [PubMed] [Google Scholar]
- 138.Kaste SC. PET-CT in children: where is it appropriate? Pediatr Radiol. 2011;41 Suppl 2:509–513. doi: 10.1007/s00247-011-2096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stabin MG, Gelfand MJ. Dosimetry of pediatric nuclear medicine procedures. Q J Nucl Med. 1998;42:93–112. [PubMed] [Google Scholar]
- 140.Lassmann M, Biassoni L, Monsieurs M, Franzius C, Jacobs F, EANM Dosimetry and Paediatrics Committees. The new EANM paediatric dosage card. Eur J Nucl Med Mol Imaging. 2007;34:796–798. doi: 10.1007/s00259-007-0370-0. [DOI] [PubMed] [Google Scholar]
- 141.Lassmann M, Biassoni L, Monsieurs M, Franzius C, EANM Dosimetry and Paediatrics Committees. The new EANM paediatric dosage card: additional notes with respect to F-18. Eur J Nucl Med Mol Imaging. 2008;35:1666–1668. doi: 10.1007/s00259-008-0799-9. [DOI] [PubMed] [Google Scholar]
- 142.Gelfand MJ, Parisi MT, Treves ST; Pediatric Nuclear Medicine Dose Reduction Workgroup. Pediatric radiopharmaceutical administered doses: 2010 North American consensus guidelines. J Nucl Med. 2011;52:318–322. doi: 10.2967/jnumed.110.084327. [DOI] [PubMed] [Google Scholar]
- 143.Gelfand MJ, Lemen LC. PET/CT and SPECT/CT dosimetry in children: the challenge to the pediatric imager. Semin Nucl Med. 2007;37:391–398. doi: 10.1053/j.semnuclmed.2007.05.002. [DOI] [PubMed] [Google Scholar]


