Abstract
Dentistry has witnessed tremendous advances in all its branches over the past three decades. With these advances, the need for more precise diagnostic tools, specially imaging methods, have become mandatory. From the simple intra-oral periapical X-rays, advanced imaging techniques like computed tomography, cone beam computed tomography, magnetic resonance imaging and ultrasound have also found place in modern dentistry. Changing from analogue to digital radiography has not only made the process simpler and faster but also made image storage, manipulation (brightness/contrast, image cropping, etc.) and retrieval easier. The three-dimensional imaging has made the complex cranio-facial structures more accessible for examination and early and accurate diagnosis of deep seated lesions. This paper is to review current advances in imaging technology and their uses in different disciplines of dentistry.
Keywords: Dental X-rays, Intraoral X-rays, Dental cone beam computed tomography, Panoramic radiograph, Cephalogram
Core tip: Radiographs are a valuable diagnostic tool, as an adjunct to clinical examination in the diagnosis of dental diseases. Two dimensional periapical and panoramic radiographs are routinely used in dental practice. However, there are certain limitations of two-dimensional radiographs, which can be overcome by three-dimensional, imaging techniques such as cone beam computed tomography, magnetic resonance imaging and ultrasound. The purpose of this article is to review the advances made in digital dental imaging. Correct use of newer radiographic techniques, where indicated, can help early detection and appropriate and timely treatment for various dental and oral pathologies.
INTRODUCTION
On 8 November, 1895 Wilhelm Conrad Röntgen accidentally discovered an image cast from the cathode ray generator which was projected far beyond the possible range of the cathode rays. A week after the discovery, Röntgen discovered its medical use when he made a picture of his wife’s hand on a photographic plate formed due to unknown radiation, which he termed as X-rays. It clearly revealed her wedding ring and her bones. The first original dental roentgenogram from a portion of a glass imaging plate was taken by Dr. Otto Walkhoff in January 1896 in his own mouth for an exposure time of 25 min. Since then, dental imaging has seen tremendous progress and its applications in various fields of dentistry. Broadly, imaging techniques used in Dentistry can be categorized as: intraoral and extraoral, analogue and digital, ionizing and non-ionizing imaging, and two-dimensional (2-D) and three-dimensional (3-D) imaging.
2-D Conventional radiographs provide excellent images for most dental radiographic needs. Their primary use is to supplement the clinical examination by providing insight into the internal structure of teeth and supporting bone to reveal caries, periodontal and periapical diseases, and other osseous conditions. A significant constraint of conventional radiography is the superimposition of overlying structures, which obscures the object of interest. Eventually it results in collapsing 3-D structural information onto a 2-D image, which leads to loss of spatial information in the third dimension.
The film-based radiography requires the presence and maintenance of darkroom, chemical handling and is associated with processing errors. All these disadvantages are overcome with the advent of digital radiography. This revolution is the result of both technologic innovation in image acquisition processes and the development of networked computing systems for image retrieval and transmission.
The very first system that was introduced in digital radiography in dentistry was Radio-visio-graphy (RVG, formerly Trex-trophy Radiology Inc., Marietta, GA) by Trophy[1] in France in 1987. Digital radiography refers to a method of capturing a radiographic image using a solid-state technology sensor, breaking it into electronic pieces, and presenting and storing the image using a computer. There are currently three types of digital radiography systems available for use in dental imaging: (1) CCD-Charge-Coupled Device (direct system); (2) CMOS-Complementary Metal Oxide Semiconductor (direct system); and (3) PSP-photo-stimulable phosphor (indirect system). One of the most commonly cited positive features of digital radiography is the radiation dose reduction up to 80%, when compared with conventional plain film radiography[2]. It is estimated that the dose reduction for intraoral digital imaging is in the range of 50%[3]-60%[4] when compared to E-speed film and for extraoral digital imaging, 50%[5]-70%[6], when compared to film-screen combinations. Other obvious benefits include the short processing time, i.e., the ability to view the image more quickly, the elimination of the darkroom, processing chemicals and the errors associated with improper darkroom maintenance, chemical handling, solution replenishment and replacement, etc. It allows manipulation of the image produced such as contrast, density, sharpness and image orientation, without any additional radiation exposure to the patient or the operator.
Intraoral radiographic examination is the backbone of imaging for the general dental practitioner. It comprises of three categories: periapical, bitewing and occlusal projections.
The periapical radiograph provides detailed information about the teeth and the surrounding tissues (Figure 1A). It is mainly utilized for assessment of pulp and root canal morphology, supporting alveolar bone status in the inter-dental region, detection of periapical pathology and crown/root fractures. It is especially useful for endodontic treatment for pre-treatment evaluation of roots and root canal morphology, calcifications, curvatures, periapical lesions, working length determination, quality and extent of root canal obturation and monitoring healing after treatment. For this purpose, a special technique of periapical radiography was developed by Gordon M. Fitzgerald, called as paralleling or long cone technique. The film is placed parallel to the long axis of the tooth to be radiographed and the central beam of X-ray is directed at right angle to the film and the teeth. The long cone of the tube increases the distance between the source and the object, resulting in decreased size of focal spot. This technique reduces the geometric distortion and also avoids overlapping of other anatomic structures, which can over shadow the teeth. For monitoring the healing of periapical lesion, the X ray image needs to be standardized to keep the same horizontal and vertical angulations at every follow-up visits. Several film holding devices are available, which allow reproducing the same angulation and getting comparable images.
Figure 1.
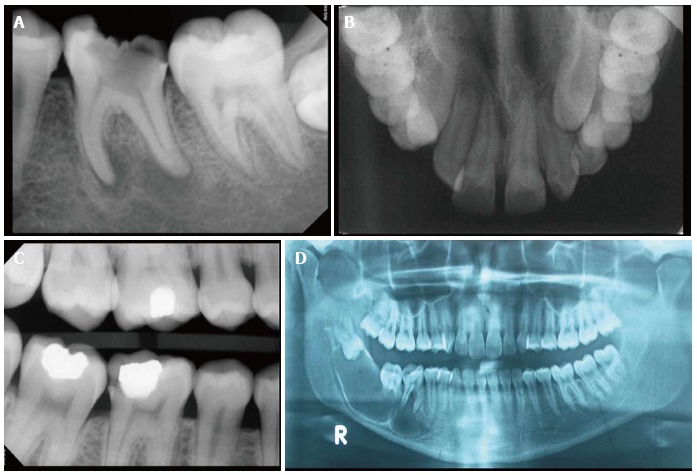
X-ray. A: An intraoral periapical X-ray helps to view the number and morphology of roots and root canals, periapical status and alveolar bone support interdentally. In this case, a grossly carious lower molar with diffuse radiolucency around both the root apices, denoting a chronic abscess is seen; B: An occlusal view of maxilla is useful to evaluate suture line of maxillary processes and extent of large pathological lesion such as a cyst and for location of impacted teeth. In this X-ray an impacted left maxillary canine can be seen; C: A bitewing X-ray is useful to examine a segment of upper and lower arch simultaneously to detect inter-proximal caries. In this X-ray, distal proximal surface of the lower 1st molar shows a carious defect; D: A panoramic radiograph gives a bird‘s-eye view of upper and lower jaws with excellent view of temporo-mandibular joint and maxillary sinuses. In this X-ray, a large, multi-locular cystic lesion, involving an impacted and inverted 3rd molar, extending up to the premolar region can be seen.
An occlusal radiograph displays a large segment of a dental arch that cannot be viewed on a periapical radiograph, such as a cyst. It helps to locate supernumerary/ impacted teeth and foreign bodies in the jaws and stones in the ducts of sub-mandibular glands (Figure 1B).
Bitewing or inter-proximal radiographs are taken to evaluate inter-proximal surfaces of 3-4 upper and lower teeth simultaneously (Figure 1C). The film has a flap on which the patient bites to keep the film in place against the crowns of upper and lower teeth simultaneously (hence called bite-wing X-ray). Bitewing films are particularly valuable for detecting inter-proximal caries in the early stages of development before it manifest clinically, reveal secondary caries below the restorations and evaluating the inter-proximal bone condition[7].
The extra-oral radiographic examination used in Dentistry includes panoramic radiographs, postero-anterior and lateral skull view, Water‘s view and postero-anterior and lateral cephalometric examinations. Extraoral radiographs help to examine larger areas of the jaws and skull, monitor growth and development of cranio-facial skeleton, to locate impacted teeth and large pathological lesions and evaluate the temporo-mandibular joint.
Panoramic imaging has become a popular and important diagnostic tool since its introduction in the 1950s. It is a specialised tomographic technique used to produce a flat representation of the curved surfaces of the jaws. The basic imaging principle is that of curved surface tomography. It visualizes the entire maxilla, mandible, temporo-mandibular joints and associated structures on a single film, i.e., gives a panoramic or bird’s eye view of the jaws[8] (Figure 1D). It is used as a preliminary screening radiograph to assess the dentition and bone support, identify impacted teeth, view the position of dental implants etc. It also gives a basic assessment of the osseous status of the temporo-mandibular joints and diagnoses maxillary and mandibular fractures. Panoramic radiographs are also being tested as a cost-effective tool to determine bone mineral density[9,10].
However, it is subject to considerable and unpredictable geometric distortion and has relatively low spatial resolution compared with intra-oral radiographs. Large differences in image projection may occur in the anterior region depending on the patient positioning and individual curvature of the jaws. Also, it does not display the fine anatomic details available on intraoral periapical radiograph. But it offers a dose advantage over large numbers of intraoral radiographs[11].
Cephalometric radiographs show the entire side of the head and help to evaluate the spatial relationships between cranial and dental structures (Figure 2). They are of value in comparing the changes in growth and development of dental and skeletal structures before, during and after orthodontic treatment, including the soft tissue profile (with lesser X-ray exposure)[12].
Figure 2.
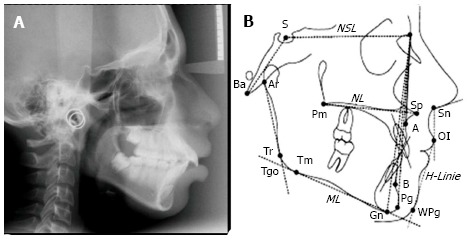
Cephalometric radiographs show the entire side of the head and help to evaluate the spatial relationships between cranial and dental structures. A: A lateral cephalometric X-ray is useful to determine cranio-facial structures and their relationship with position of the jaws and teeth; B: Different landmarks used to evaluate the planes and angles formed, to arrive at a diagnosis and treatment planning for orthodontic treatment/orthognathic surgery. Serial cephalograms can give the amount and direction of growth of facio-maxillary complex.
Digital subtraction radiography (DSR) is a technique used to determine qualitative changes that occur between two images taken at different points in time. Subtraction method was introduced by B.G. Zeides des Plantes in the 1920s. The first image is the baseline image and the second image shows the changes that have occurred since the time the first image was taken[13]. DSR cancels out the complex anatomic background against which this change occurs. In order for DSR to be diagnostically useful, it is crucial that the baseline projection geometry and image intensities be reproduced. Dove et al[14] reported that angulation errors should be limited to two degrees. DSR helps to detect the alveolar bone changes of 1%-5% per unit volume and of crestal bone height change of 0.78 mm[15,16]. Parsell et al[17] in 1998 found that digital subtraction radiography with or without enhancement improved the likelihood of a correct cancellous defect diagnosis when compared to other methods to detect oral cancellous bone lesions. However, it is only used for research purpose, as it is difficult to reproduce images with similar projection geometry every time.
Occipito-mental view, also known as Waters view, is the most favourable for visualization of maxillary sinuses, especially to compare internal radio-opacities. The frontal sinuses and ethmoid air cells can also be viewed in Waters view. When taken with open mouth position, it can help to visualize the sphenoid sinuses. The submento-vertex view is used in evaluating the lateral and posterior borders of the maxillary sinuses and the ethmoid air cells. It also visualizes the skull-base and condyles superimposed on the condylar necks and mandibular rami. It is particularly useful in diagnosis of fractures of the zygomatic arch. The Caldwell view is useful in evaluating the frontal sinuses and ethmoid air cells. Reverse-towne projection is used to determine fractures of the condylar neck of the mandible.
LIMITATIONS OF 2-D IMAGES
Radiographs provide a two-dimensional image of a three-dimensional object. Relationship of the tooth to the surrounding anatomical structures cannot be assessed accurately which limits its diagnostic performance[18]. The objects are visualized in the mesial-distal and apical- coronal plane; however the buccal-lingual plane is not possible to assess[19]. Because of the complexity of maxillofacial skeleton, 2-D radiographic images do not accurately replicate the anatomy that is being assessed. Anatomical structures surrounding the teeth may superimpose causing anatomical or background noise, leading to difficulty in interpreting periapical radiographs. 2-D radiographs show less severe bone destruction than is actually present. Radiographs do not reveal the soft-tissue to hard-tissue relationships.
All the above listed 2-D imaging techniques provide information necessary for routine dental practice. However, in case of diagnostic dilemma and treatment planning of special cases, advanced 3-D imaging modalities, revealing additional information is desirable. Various techniques have evolved in the recent past that has revolutionized the diagnosis and treatment planning in dentistry.
COMPUTED TOMOGRAPHY
The first commercial computed tomography (CT) scanner was developed in 1972 by Sir Godfrey N. Hounsfield, an engineer at EMI, Great Britain. Since then, the introduction of clinical X-ray computed tomography has transformed medical imaging and may be described as the greatest advancement in radiology, since the discovery of X-rays. Computed tomography uses a narrow fan-shaped X-ray beam and multiple exposures around an object to reveal its internal structures which helps the clinician to view morphologic features and pathology in three- dimensions[20]. It determines the mesio-distal as well as the bucco-lingual extent of the pathology.
CT scanner consists of a radiographic tube attached to a series of scintillation detectors or ionization chambers. The patient is advanced in the circular aperture in the centre of the gantry. The tube head and reciprocal detectors within the gantry either rotate synchronously around the patient, or the detectors may form a continuous ring around the patient and the X-ray tube may move in a circle within the detector ring.
There are four generations of CTs. The Hounsfield’s unit belonged to the first generation of CT scanners which used a single detector element to capture beam of X-rays. A second generation of CT systems introduced in 1975 used more than one detector and used small fan-beam, as opposed to pencil-beam scanning in the first generation. The first and second generations of CT scanners used a translate-rotate design and were used to scan only the head.
Third generation CT scanners introduced in 1976 use a large, arc-shaped detector that acquires an entire projection without the need for translation. Third generation scanners are most extensively used today. Fourth generation scanners replaced the arc-shaped detector with an entire circle of detectors. In this design, the X-ray tube rotates around the patient, while the detector stays stationary. As the fourth generation scanners were more expensive and suffer from higher levels of scatter, these are not used today. The incremental scanning approach was subject to errors relating to patient movement and limited Z-axis (vertical) image resolution resulting in loss of fracture conspicuity. The development of the power slip ring facilitated the development of spiral (or helical or volumetric) CT in the late 1980s. In spiral CT, the patient is moved continuously through the rotating gantry and image data are acquired as a “spiral” or “helix”rather than in the form of a series of slices[21]. Compared with incremental CT scanners, spiral scanners provide improved multiplanar image reconstructions, reduced exposure time (12 s vs 5 min), and a reduced radiation dose (up to 75%)[22].
Current CT scanners are called multi-slice CT scanners and have a linear array of multiple detectors (up to 64 rows) that simultaneously obtain tomographic data at different slice locations. It provides various advantages including significant reduction in scan time, reduced artifacts, and sub-millimetre resolution (up to 0.4 mm isotropic voxel)[22]. However, these scanners are extremely expensive and while beneficial for CT angiography and cardiac imaging, may have limited application in maxillofacial diagnosis.
CT was the first technology to allow visualization of both hard and soft tissues of the facial bones by image processing enhancement and the ability to acquire multiple, non- superimposed cross-sectional images. CT scans were used in medicine since 1973 but it became available for dental application only in 1987. CT provides high contrast resolution and allows differentiation of tissues with < 1% physical density difference compared to 10% required to be distinguished with conventional radiography[22]. CT images have less noise (i.e., they are less grainy), which results from superior collimation of the exit beam in CT machines. CT software programs can highlight pathologic lesions from normal anatomic structures using colour-enhancement features. CT images have the ability to show slices of a given tissue, with each slice thickness (1-2 mm) and location chosen by the operator[20].
Trope et al[23] in 1989 used CT scans to differentiate radicular cysts from granulomas based on marked difference in density between the content of the cyst cavity and granulomatous tissue.
CT is considered the gold standard imaging technique to assess injuries of the maxillofacial skeleton region. It is an excellent tool for detecting complex facial fractures, like those involving the frontal sinus, naso-ethmoidal region[24], and the orbits[25]. CT helps in defining the displacements of fractures prior to surgical reduction and fixation. It helps to diagnose undisplaced fractures of the mandible and the condyle, which are not apparent on panoramic radiographs. Markowitz et al[26] found coronal CT to be the most accurate method in the diagnosis of mandibular fractures, followed by mandibular series and panoramic radiography. CT offers superb visualization of impacted teeth and its relation to nearby anatomic structures which guides the surgeon during surgical removal of impacted teeth.
Aggarwal et al[27] used CT scans and ultrasound with power Doppler flowmetry in the diagnosis of large periapical lesions. They concluded that both, the CT scans and ultrasound with power Doppler flowmetry can provide an additional but more accurate diagnosis of periapical lesions with validity equivalent to histo-pathological diagnosis[27].
CT scan is also an excellent aid in detecting vertical root fracture or split teeth which cannot be detected on periapical radiographs, since CT is not sensitive to beam orientation unlike conventional radiograph[28].
CT helps to indentify multiple extra root canals which when missed can lead to endodontic treatment failure. Chronic apical periodontitis can be seen with the CT scan in early and established stages. It is seen as an enlargement of the periodontal space, which is seen as a small osteolytic reaction around the root tips[29]. Velvart et al[30] in 2001 compared CT scans and periapical radiographs of 50 mandibular posterior teeth scheduled for periapical surgery. They found that CT detected the presence of an apical lesion and the location of the inferior alveolar nerve in all cases, compared with 78% and 39% respectively with periapical radiographs. Robinson et al[31] evaluated mandibular first premolars on 120 routine dental. CT images for variations in root/ root canal morphology. They found that CT images identified a greater number of morphologic variations than did a panoramic radiograph[31].
CT has been used as a research tool to compare the volume of root canals before and after instrumentation with different rotary nickel-titanium systems[32] and for volumetric analysis of root filling using various obturation systems[33].
3-D images from spiral CT helped in evaluating the close relationship between maxillary sinus disease and adjacent periodontal defects and their treatment[34]. Rigolone et al[35] obtained anatomic information using low dose CT to plan peri-radicular surgery via the vestibular approach. CT scan also detects resorption of adjacent roots.
CT scan can precisely distinguish between intrinsic and extrinsic salivary tumors and is used for staging these tumors[36]. It is excellent for planning for implant placement for ear prosthesis in patients with hemi-facial microsomia[37].
The greatest disadvantage of CT imaging is the high radiation exposure. Other disadvantages of CT include high costs of the scans and scatter because of metallic objects. It has poor resolution compared to conventional radiographs. CT has limitation in the diagnosis of dental fractures (like small fissures) which are below the resolution capability of CT and may result in false-negative readings.
TUNED APERTURE COMPUTED TOMOGRAPHY
Tuned aperture computed tomography (TACT) is a relatively simple, faster method for reconstructing tomographic images, which was developed by Webber and colleagues[38]. It is based on the concept of tomo-synthesis and optical-aperture theory[39,40]. TACT uses 2-D periapical radiographs acquired from different projection angles as base images and permits retrospective generation of longitudinal tomographic slices (TACT-S) lining up in the Z axis of the area of interest. It produces true 3-D data from any number of arbitrarily oriented 2-D projections. TACT has shown to be a promising, effective alternative to other conventional modalities for a number of clinical applications. The overall radiation dose of TACT is not greater than 1 to 2 times that of a conventional periapical X-ray film. The resolution is stated to be similar with 2-D radiographs. Artefacts associated with CT, such as starburst patterns seen with metallic restorations, do not exist with TACT.
In 1998, Nair et al[41] reported TACT to be more effective imaging modality than film or individual digital images for the detection of recurrent caries. Webber et al[42] in 1999 also found TACT to be diagnostically more informative. Nance et al reported that with TACT 36% of extra canal [second mesio-buccal (MB 2)] were detected in maxillary molars and 80% of third (mesio-lingual) canals in mandibular molars[43]. TACT has proved to be effective in the determination of root fractures, especially vertical fractures.
Nair et al[44] found that TACT was a more effective and accurate imaging modality for non- destructive quantification of osseous changes within the healing bony defects. It was found to be better than planar images for the detectability of trauma-induced radicular fractures and mandibular fractures in in-vitro studies[45]. Liang et al[46] reported that TACT provides an alternative to conventional tomography for pre-surgical implant imaging. However, TACT is still at trial stage for dental applications but appears to be a promising imaging modality for the future.
Micro-computed tomography (micro-CT) is another alternative CT technique that has been used in dental imaging. However, the use of micro-CT remains a research tool limited to animal and in vitro studies on small samples. Because of the high radiation dose required, micro-CT cannot be employed for human imaging.
CONE BEAM COMPUTED TOMOGRAPHY
This imaging technique is based on a cone-shaped X-ray beam centered on a 2-D detector. It performs one rotation around the object and produces a series of 2-D images which are re- constructed in 3-D using a modification of the original cone-beam algorithm developed by Aboudara et al[47] in 1984. Major advantage of TACT over CT is the considerably lower effective radiation dose to which patients are exposed. Radiation dose of one cone beam computed tomography (CBCT) scan may be as little as 3%-20% that of a conventional CT scan, depending on the equipment used and the area scanned[22].
CBCT does not require an additional mechanism to move the patient during the acquisition. Cone beam technology significantly increases the X-ray utilization and requires far less electrical energy than fan-beam technology. X-ray tubes of cone-beam scanning are much less expensive than that for conventional CT. Images have isotropic voxels that can be as small as 0.125 mm. Subjective image quality is high, even compared to helical CT, for the highest resolution modalities. CBCT provides a high spatial resolution of bone and teeth which allows accurate understanding of the relationship of the adjacent structures.
CBCT has found varied application in all fields of dentistry. High resolution of CBCT has helps in detecting variety of cysts, tumors, infections, developmental anomalies and traumatic injuries involving the maxillo-facial structures. It has been used extensively for evaluating dental and osseous disease in the jaws and temporo-mandibular joints and treatment planning for dental implants.
CBCT is categorized into large, medium, and limited volume units based on the size of their field of view (FOV). The size of the FOV depicts the scan volume of CBCT machines. It depends on various factors like the size and shape of the detector, beam projection geometry and the ability to collimate the beam. Collimation of the beam limits the X-radiation exposure to the region of interest and ensures the most favorable FOV to be selected, based on disease presentation. Smaller scan volumes produce higher resolution images and lowers the effective radiation dose to the patient. Size of the field irradiated is the principal limitation of large FOV cone beam imaging[48].
Large field of view (FOV) units encompasses those CBVTs with a FOV from 15-23 cm. These units are mainly useful in the assessment of maxillofacial trauma, orthodontic diagnosis and treatment planning, temporo-mandibular joint (TMJ) analysis and pathologies of the jaws. Medium FOV range from 10-15 cm and are useful for mandibulo-maxillary imaging and for pre-implant planning and pathological conditions. Small FOV units (limited FOVs) of < 10 cm with some as small as 4 cm × 4 cm in size are suitable for dento-alveolar imaging and are most advantageous for endodontic applications[49].
APPLICATIONS OF CBCT IN VARIOUS BRANCHES OF DENTISTRY
Oral and maxillofacial surgery
CBCT is majorly used in oral and maxillofacial surgery for surgical evaluation and planning for surgery for impacted teeth, cysts and tumors, orthognathic and implant surgeries and diagnosis of fractures and inflammatory conditions of the jaws and the sinuses.
CBCT is largely used diagnostic technique in assessment of mid-face[50] and orbital fractures[51]. It allows easy detection of non-displaced, inter-articular fractures of the condylar head[52]. Artefacts from metal objects are lower on CBCT images[53], hence it provides better information in cases involving gun-shot wounds[54]. However, in cases of trauma to the cervical vertebrae, use of CBCT is contra-indicated, as the patient is unable to be in an upright position which is required for CBCT imaging.
Detailed visualization of the inter-occlusal relationship of 3-D virtual skull model makes CBCT a valuable tool in orthognathic surgery planning. It allows for morphological analysis and spatial relationship of the neighboring structures during follow-ups to evaluate growth, development and function. It provides pre-surgical information when planning for sinus floor augmentation in preparation for implant placement[55].
CBCT has been used for measuring the thickness of the glenoid fossa[56]. It often reveals the possible dislocation of the disk in the joint by defining the true position of the condyle and the extent of translation of the condyle in the fossa[57]. It has also been used for an image guided puncture technique of the TMJ which is a treatment modality for TMJ disk adhesion[58]. CBCT provides a dose and cost-effective alternative to helical CT for the diagnostic evaluation of osseous abnormalities of the TMJ.
Endodontics
CBCT has been extensively used in Endodontics. Numerous studies have reported its usefulness in diagnosis of periapical lesions[59-62] (Figure 3). Estrela et al[63] proposed a CBCT-based periapical index, termed as CBCT-PAI to measure and monitor periapical lesion size pre and post-endodontic treatment.
Figure 3.
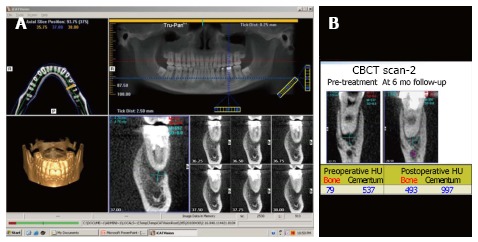
Cone beam computed tomography. A: A cone beam computed tomography scan gives a three-dimensional view of the area of interest. In this case, the periapical lesion is being evaluated; B: The image gives values in Hounsfield unit of cementum and alveolar bone density to measure post-treatment healing. CBCT: Cone beam computed tomography.
CBCT enables in the differential diagnosis of cyst from granulomas by measuring the density from the contrasted images of the periapical lesion[64,65]. Lofthag- Hansen et al[66] found that CBCT detected 62% more periapical lesions on individual roots when compared with periapical X-ray examinations. Vertical root fractures are better evaluated with CBCT images compared to periapical radiographs. CBCT can determines fractures in bucco-lingual or mesio-distal directions[67,68].
Patel et al[69] in their review of literature found CBCT to be efficacious in endodontic surgery planning and identification of root canals not seen on 2-D images. Alshehri et al[70] in their review article on CBCT reported it to be useful in cases such as inflammatory external and internal resorption. CBCT not only detects the presence of resorption, but also determines its extent. They also found CBCT useful in determining root morphology; to measure the number of roots, canals, and accessory canals and to establish their working lengths, angulations and in the location of separated instrument in the canal[70].
For most endodontic applications, limited volume CBCT is preferred over large volume CBCT for the following reasons: (1) Increased spatial resolution to improve the accuracy of endodontic-specific tasks such as the visualization of accessory canals, root fractures, apical deltas, calcifications, etc.; and (2) Decreased radiation exposure to the patient.
Implantology
CBCT has been used for preoperative and postoperative dental implant assessment. Preoperatively, it can accurately determine the quantity and quality of bone available for placement of implant[71,72]. It also provides more detailed and accurate information of the adjoining vital tissues, so that these could be protected during the placement of dental implant. Heiland et al[73] described a technique in which CBCT was used intra-operatively in two cases to navigate the implant insertion following microsurgical bone transfer.
Orthodontics
CBCT images have been used in orthodontic assessment and cephalometric analysis[74]. CBCT helps to determine root angulations, although variations are seen from the true anatomy[75]. CBCT is valuable tool to assess the facial growth, age, airway function and disturbances in tooth eruption[76]. CBCT can provide enhanced visualization of roots, making it a valuable tool for assessing pre and post-orthodontic root resorption.
CBCT evaluates the success of alveolar bone grafts in patients with cleft lip and palate by determining the bucco-palatal width and allowing the visualization of the 3-D morphology of the bone bridge[77]. Kim et al[78] used CBCT to construct placement guides for mini-implants between the roots of adjacent teeth in anatomically difficult sites.
Periodontics
CBCT has proved to be a practical clinical tool to detect intra-bony and furcation defects, dehiscence, fenestration, and periodontal cysts[79]. It provides detailed morphologic description of the bone with minimal error margins. CBCT has also been used to evaluate outcome of regenerative periodontal therapy[80].
LIMITATIONS OF CBCT
Image quality and diagnostic accuracy of CBCT is affected by the scatter and beam hardening artifacts caused by high density structures such as enamel and radiopaque materials[81]. Scatter radiation reduces the contrast and limits the imaging of soft tissues. Hence, CBCT is principally indicated for imaging hard tissues[82].
Because of distortion of Hounsfield Units, CBCT cannot be used for estimation of bone density. Scan times for CBCT are lengthy at 15-20 s and require the patient to stay completely still.
CONCERN FOR RADIATION EXPOSURE
Intraoral radiographic films are available as D, E and F. D is the slowest and F is the fastest speed films. Fast films require least radiation as compared to slow speed film. It is reported that switching from D to E speed film reduces radiation by 30%-40% and from D to F by 60%. However, due to cost consideration of fast speed films, majority of dentists prefer to use D speed films, though the cost difference is only marginal[83].
Radiation exposure for panoramic radiograph is 14.2- 24.3 mSv, for lateral cephalogram, it is 10.4 mS and for a full mouth intraoral X ray series is 13-100 mS. Digital X ray require a much lower radiation exposure, i.e., 50%-75% less than equivalent film image. Digital panoramic radiation dose is 0.020 mSv and for cephalogram it is 0.007 mSv. CBCT units have radiation exposure in the range of 87-206 mSv for a full craniofacial scan. Based on these values, it is inferred that CBCT radiation exposure is equivalent to or slightly higher than traditional imaging[84].
However, CBCT must not be used routinely for dental diagnosis or for screening purposes. The patient’s history and clinical examination must validate the use of CBCT by demonstrating that the benefits to the patient offset the potential risks. CBCT should only be used when lower dose conventional dental radiographs fails to provide adequate diagnostic information.
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is fast outpacing any other modality for in vivo viewing of soft tissues in the human body without the need to resort to any invasive procedures. MRI scan is a specialized imaging technique which does not use ionizing radiation. Most MRI machines are graded on the strength of the magnet, measured in Tesla units, which is the equivalent of 20000 times the magnetic field strength of Earth. MRI units for in vivo applications are in the range of 1.5 to 3 Tesla units.
MRI involves the behaviour of hydrogen atoms (consisting of one proton and one electron) within a strong magnetic field which is used to create the MR image. This causes the nuclei of many atoms in the body to align themselves with the magnetic field. The machine applies a radiofrequency pulse to depolarize the atoms and the energy that is released from the body is detected and used to construct the MR image by a computer. The high contrast sensitivity of MRI to soft tissue differences is the major reason MRI have replaced CT for imaging soft tissues. Hydrogen is found in abundance in soft tissue, but is lacking in most hard tissues[85].
MRI offers the best resolution of tissues of low inherent contrast. Some cases of squamous cell carcinoma of the tongue can only be visualized with MRI. Because the region of the body imaged in MRI is controlled electronically, direct multiplanar imaging is possible without reorienting the patient.
The main dental applications of MRI to date have been the investigation of soft-tissue lesions in salivary glands, TMJ and tumour staging. Its exceptional soft- tissue contrast resolution makes it ideal for detection of internal derangement of TMJ. MRI can also detect joint effusions, synovitis, erosions and associated bone marrow oedema. Odontogenic cysts and tumors can be distinguished better on MRI than on CT. It also identifies soft tissue diseases, especially neoplasia, involving tongue, cheek, salivary glands, neck and lymph nodes[86].
MRI can also accurately distinguish between solid and cystic lesions on the basis of signal characteristics and enhancement patterns. Application of specific criteria for diagnosis allows accurate distinction between the keratocystic odontogenic tumour (KCOT) and other odontogenic lesions[87]. The keratin-rich debris in a KCOT shows characteristic central drop in signal on T2-weighted images. In case of an infective lesion like a periapical abscess which expands fast in the jawbones and soft tissues, later degenerating into osteomyelitis, MRI is the diagnostic method of choice[88]. Several studies have verified the high sensitivity of MRI in detecting cancellous marrow abnormality in acute osteomyelitis. This results in reduced T1 signal, increased T2 signal and contrast enhancement of bone and the adjacent inflamed soft tissues[86].
A recent introduction in MRI technology is called SWeep Imaging with Fourier Transform to visualize dental tissues. Idiyatullin et al[89] reported that it can simultaneously image both hard and soft dental tissues with high resolution in short enough scanning times and hence is practical for clinical applications. An interesting observation was that it can determine the extent of carious lesions and simultaneously assess the status of pulpal tissue, whether reversible and irreversible pulpitis, which can impact clinical decision on treatment planning[90].
MRI has been shown to be reliable in depicting sialodochitis and sialectasia, especially when globular changes are present. A study by Browne in which 50 consecutive patients presenting with facial swelling, thought clinically to be due to salivary gland disease were chosen. Prior investigation was undertaken in 29 patients, including ortho-pantomography, ultrasound and sialography; none provided additional information than MRI. Sialography was carried out in three patients after MRI and the results agreed with MRI in all cases. They concluded that MRI diagnosis of tumour was correct in all patients and that MRI appears to be an efficient first line investigation of facial swelling[91].
Use of MRI technology has been reported to produce tooth surface digitization with an accuracy and precision sufficient for production of dental restorations[92] and to detect root resorption[93] in Orthodontic cases. Its use has been reported in characterization of the inflammation and healing processes in periodontal tissues. Schara et al[94] demonstrated through their in-vivo study that reduction of inflammation and probing depth in gingival tissues after non-surgical periodontal therapy correlated with a decrease of ratio between post- and pre-contrast signal intensity in T1 weighted MR images. They concluded that MRI could provide a new possibility to characterize the type and healing process of periodontal inflammation[94].
The presence of a strong magnetic field can potentially cause movement of ferromagnetic metals in the vicinity of the imaging magnet. Because of this, MRI may not be safe in patients with cardiac pacemakers, implantable defibrillators, some artificial heart valves, cerebral aneurysm clips, or ferrous foreign bodies in the eye.
Artifacts caused by metallic dental restorations produced a major diagnostic problem in CT examinations of malignant tumors in the maxillofacial region. Artifacts from magnetic metals also appear on MRI[95]. However it was found that severe artifacts that disturbed the interpretation of the images on MRI were only half that on CT[96]. Vijay et al[97] also reported that artifacts on MRI as a result of dental fillings were rather localized and did not degrade the entire image, unlike the streaking seen on CT images. Okano et al[98] proposed that the MRI diagnosis of the TMJ can be performed in orthodontic patients, preferably using ceramic brackets on the anterior teeth and directly bonded tubes on the molars. However, the arch wires needed to be removed[98].
MRI cannot always distinguish between benign and malignant tumours, which could lead to a false positive result[99]. Some patients suffer from claustrophobia when positioned in the close confines of an MRI machine. Other drawback of MRI is the long scanning time required. Finally, MRI is expensive compared to other conventional radiographic methods.
The use of dental MRI appears to be a safe tool for 3-D imaging without ionizing radiation. However, due to high cost of MRI imaging, its use is limited to special cases where its use is specifically indicated for correct diagnosis.
ULTRASOUND
Ultrasound (US) is a non-invasive, inexpensive and painless imaging method. Unlike X-rays, it does not cause harmful ionizing radiation. US can be used for both hard and soft tissue detection. The first data of diagnostic US in dentistry was reported in 1963 by Baum et al. They used a 15 MHz transducer to visualize the interior structures of teeth; but the quality and clarity of the resulted RF signal was not favorable.
US is based on the reflection of sound waves (echoes) with a frequency outside the range of human hearing (1-20 kHz), at the interface of tissues which have different acoustic properties. Ultrasonic waves are created by the piezoelectric effect within a transducer (probe). US waves transmit energy, as X-ray does, but it requires a medium for its transmission, unlike X-rays which pass readily through a vacuum. The echoes are detected by a transducer which converts them into an electrical signal and a real-time black, white and shades of grey picture is produced on a computer screen[100].
US can be an important diagnostic tool for patients in whom MRI is contra-indicated, such as those with cardiac pacemakers, claustrophobia and metallic prostheses. Also, US can be used repeatedly as it is free of ionizing radiation.
US is used to diagnose fractures of the orbital margin and nasal bone, zygomatic arch, and the anterior wall of the frontal sinus. It has been proposed as a complementary diagnostic procedure to augment CT in the assessment of patients with mid-facial fracture. Ultrasonography is also capable in the detection of extra-capsular sub-condylar fractures. Adeyemo and Akadiri carried out a systemic review of literature to find the diagnostic value of ultrasound in detection of maxillofacial fractures. It reported sensitivity and specificity of US in detecting orbital fractures in the range of 56%-100% and 85%-100%, respectively. Studies on nasal fractures showed sensitivity and specificity in the range of 90%-100% and 98%-100%, respectively. Sensitivity or specificity of US for detecting zygomatic fractures was higher than 90% and for mandibular sub-condylar/ ramus fractures were in the range of 66%-100% and 52%-100%, respectively[101].
US helps to differentiate solid and cystic lesions in the parotid gland. It can also detect Sialoliths in parotid, submandibular and sublingual salivary glands. These appear as echo-dense spots with a characteristic acoustic shadow[102]. US guidance can prevent injuring the facial nerve during biopsy of the parotid gland.
US can demonstrate the internal muscle structures more clearly than CT. It can also measure the thickness of muscles which can be an important tool in diagnosis and treatment for follow-up examination of inflammatory soft tissue conditions of the head and neck region and superficial tissue disorders of the maxillofacial region[103]. However, Serra et al through their review concluded that ultrasound technique generally showed lower reproducibility in relaxed than in contracted muscles.
US is a reliable diagnostic technique in determining the pathological nature (granuloma vs cysts) of periapical lesions[27,104,105]. It has been used in guided fine-needle aspiration, measurement of tongue cancer thickness, and diagnosis of metastasis to cervical lymph nodes[106]. Chandak et al[107] in their study on head and neck swellings found higher accuracy and sensitivity of US imaging than the clinical diagnosis. They concluded that US would be an important diagnostic tool in association with clinical examination to detect the nature of the swelling[107].
Rajendran et al[108] conducted a study to find out the efficacy of high-resolution ultrasound and color power Doppler as a monitoring tool in the healing of periapical lesions. They found that ultrasound with color power Doppler is an efficient tool for monitoring bone healing and would be a significant contribution to the trend toward radiation-free endodontics[108]. Tikku et al[109] also found that ultrasound and color Doppler imaging were considerably better than conventional radiography in detecting changes in the healing of periapical lesions. The authors also confirmed that only ultrasound combined with Doppler can differentiate venous from arterial flow, quantify the amount of flow, identify the anatomy of feeding vessels and offer a visual demonstration of vascularity[109].
Yoon et al[110] compared the difference in pulpal blood flow between vital and root- filled teeth by using US Doppler imaging. They found significant differences in the maximum linear velocity, average linear velocity, minimum linear velocity, pulsation index, and circulation resistance between the vital and root-filled teeth. They concluded that US Doppler imaging is an important tool to detect pulpal blood flow in vital tooth[110].
Tagtekin et al[111] while comparing DIAGNOdent (655 nm diode laser) with ultrasound for caries detection found that all measurements with US were accurate, reliable and significantly correlated between examiners. Both methods of caries detection showed high repeatability and accuracy[111].
Even though, US have limitations in detecting the periodontal ligament, Mahmoud et al[112] through their recent study found that it can be used for early diagnosis of the more severe form of periodontal disease. They used a custom-designed high-frequency (30 to 60 MHz) US imaging system to reconstruct three-dimensional surface images of periodontal defects in human[112].
US can measure soft tissue thickness which could help practitioners to select the proper orthodontic mini-screw in clinical practice[113]. Dental implant placement without incision and flap elevation require accurate determination of soft tissue thickness. Location of implant is difficult after healing, if the implants are deeply submerged after thick connective tissue grafts. US plays an important role in locating these submerged implants accurately for surgical exposure for subsequent prosthodontic rehabilitation[114].
It is an alternative diagnostic method for imaging of the TMJ disorders[115]. US showed better visualization of temporo-mandibular joint structures by using a frequency of > 12 MHz[116].
US has limited value in diagnosing undisplaced fractures, complex maxillofacial fractures, posterior orbital floor fractures and intra-capsular mandibular condyle fractures due to overlapping of zygomatic arch[101]. US are blocked by bone and therefore it can be used only if there is a bony defect over the lesion through which ultrasonic waves can traverse[105].
Though placing the US in the anterior region of the mouth is easy, positioning the probe in buccal mucosa of posterior teeth is difficult.
US examination is usually applied only to the superficial tissues in the maxillofacial region because the facial skeleton shields the deeper tissues. The correct interpretation of US images requires a trained radiologist, who has extensive training in the use and interpretation of US images.
CONCLUSION
Recent advances in imaging technologies have revolutionized dental diagnostics and treatment planning. Correct use of appropriate imaging technology and their correct interpretation, following the ALARA (As low as reasonably achievable) principles and cost-effectiveness, newer radiographic techniques can help to detect pathologies in very early stages, which ultimately help to reduce morbidity and mortality and improve the quality of life of the patients.
Footnotes
P- Reviewer: Kamburoglu K, Tomofuji T, Tsushima Y S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.Nair MK, Nair UP. Digital and advanced imaging in endodontics: a review. J Endod. 2007;33:1–6. doi: 10.1016/j.joen.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Mouyen F, Benz C, Sonnabend E, Lodter JP. Presentation and physical evaluation of RadioVisioGraphy. Oral Surg Oral Med Oral Pathol. 1989;68:238–242. doi: 10.1016/0030-4220(89)90200-4. [DOI] [PubMed] [Google Scholar]
- 3.Langland OE, Langlais RP, Preece JW. Principles of dental imaging. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 285. [Google Scholar]
- 4.Frederiksen NL. Health Physics. In: Pharoah MJ, White SC, editors. Oral Radiology Principles and Interpretation. 4th ed. Mosby: St. Louis; 2000. p. 53. [Google Scholar]
- 5.Visser H, Rödig T, Hermann KP. Dose reduction by direct-digital cephalometric radiography. Angle Orthod. 2001;71:159–163. doi: 10.1043/0003-3219(2001)071<0159:DRBDDC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Farman AG, Farman TT. Extraoral and panoramic systems. Dent Clin North Am. 2000;44:257–272, v-vi. [PubMed] [Google Scholar]
- 7.Kidd EA, Pitts NB. A reappraisal of the value of the bitewing radiograph in the diagnosis of posterior approximal caries. Br Dent J. 1990;169:195–200. doi: 10.1038/sj.bdj.4807325. [DOI] [PubMed] [Google Scholar]
- 8.Rushton VE, Horner K, Worthington HV. Routine panoramic radiography of new adult patients in general dental practice: relevance of diagnostic yield to treatment and identification of radiographic selection criteria. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:488–495. doi: 10.1067/moe.2002.121994. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi A, Ohtsuka M, Nakamoto T, Naito K, Tsuda M, Kudo Y, Motoyama E, Suei Y, Tanimoto K. Identification of post-menopausal women at risk of osteoporosis by trained general dental practitioners using panoramic radiographs. Dentomaxillofac Radiol. 2007;36:149–154. doi: 10.1259/dmfr/31116116. [DOI] [PubMed] [Google Scholar]
- 10.Bhatnagar S, Krishnamurthy V, Pagare SS. Diagnostic efficacy of panoramic radiography in detection of osteoporosis in post-menopausal women with low bone mineral density. J Clin Imaging Sci. 2013;3:23. doi: 10.4103/2156-7514.113140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JW. Assessment of panoramic radiography as a national oral examination tool: review of the literature. Imaging Sci Dent. 2011;41:1–6. doi: 10.5624/isd.2011.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintero JC, Trosien A, Hatcher D, Kapila S. Craniofacial imaging in orthodontics: historical perspective, current status, and future developments. Angle Orthod. 1999;69:491–506. doi: 10.1043/0003-3219(1999)069<0491:CIIOHP>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.van der Stelt PF. Digital radiography as a diagnostic tool. AADMRT Newsletter. Summer. Mosby: St. Louis; 2004. Available from: http://www.aadmrt.com. [Google Scholar]
- 14.Dove SB, McDavid WD, Hamilton KE. Analysis of sensitivity and specificity of a new digital subtraction system: an in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:771–776. doi: 10.1067/moe.2000.106295. [DOI] [PubMed] [Google Scholar]
- 15.Gröndahl K, Kullendorff B, Strid KG, Gröndahl HG, Henrikson CO. Detectability of artificial marginal bone lesions as a function of lesion depth. A comparison between subtraction radiography and conventional radiographic technique. J Clin Periodontol. 1988;15:156–162. doi: 10.1111/j.1600-051x.1988.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanz M, Newman MG. Advanced Diagnostic Techniques. In: Newman MG, Takei HH, Carranza FA, editors. Clinical periodontology.9th ed. Philadelphia: W.B.Saunders; 2002. pp. 487–502. [Google Scholar]
- 17.Parsell DE, Gatewood RS, Watts JD, Streckfus CF. Sensitivity of various radiographic methods for detection of oral cancellous bone lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:498–502. doi: 10.1016/s1079-2104(98)90381-x. [DOI] [PubMed] [Google Scholar]
- 18.Cotti E, Vargiu P, Dettori C, Mallarini G. Computerized tomography in the management and follow-up of extensive periapical lesion. Endod Dent Traumatol. 1999;15:186–189. doi: 10.1111/j.1600-9657.1999.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 19.Patel S, Dawood A, Whaites E, Pitt Ford T. New dimensions in endodontic imaging: part 1. Conventional and alternative radiographic systems. Int Endod J. 2009;42:447–462. doi: 10.1111/j.1365-2591.2008.01530.x. [DOI] [PubMed] [Google Scholar]
- 20.Brooks SL. Computed tomography. Dent Clin North Am. 1993;37:575–590. [PubMed] [Google Scholar]
- 21.Scarfe WC. Imaging of maxillofacial trauma: evolutions and emerging revolutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:S75–S96. doi: 10.1016/j.tripleo.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 22.White S, Pharoah M. Chapter 13. Advanced Imaging Modalities. Oral Radiology: Principles and Interpretation. 5th ed. Mosby: St Louis, MO; 2004. pp. 245–264. [Google Scholar]
- 23.Trope M, Pettigrew J, Petras J, Barnett F, Tronstad L. Differentiation of radicular cyst and granulomas using computerized tomography. Endod Dent Traumatol. 1989;5:69–72. doi: 10.1111/j.1600-9657.1989.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 24.Sargent LA, Rogers GF. Nasoethmoid orbital fractures: diagnosis and management. J Craniomaxillofac Trauma. 1999;5:19–27. [PubMed] [Google Scholar]
- 25.Davidson MJ, Daly BD, Russell JL. The use of computed tomography in the management of facial trauma by British oral and maxillofacial surgeons. Br J Oral Maxillofac Surg. 1991;29:80–81. doi: 10.1016/0266-4356(91)90085-j. [DOI] [PubMed] [Google Scholar]
- 26.Markowitz BL, Sinow JD, Kawamoto HK, Shewmake K, Khoumehr F. Prospective comparison of axial computed tomography and standard and panoramic radiographs in the diagnosis of mandibular fractures. Ann Plast Surg. 1999;42:163–169. doi: 10.1097/00000637-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal V, Logani A, Shah N. The evaluation of computed tomography scans and ultrasounds in the differential diagnosis of periapical lesions. J Endod. 2008;34:1312–1315. doi: 10.1016/j.joen.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Youssefzadeh S, Gahleitner A, Dorffner R, Bernhart T, Kainberger FM. Dental vertical root fractures: value of CT in detection. Radiology. 1999;210:545–549. doi: 10.1148/radiology.210.2.r99ja20545. [DOI] [PubMed] [Google Scholar]
- 29.Deepak BS, Subash TS, Narmatha VJ, Anamika T, Snehil TK, Nandini DB. Imaging techniques in endodontics: an overview. J Clin Imaging Sci. 2012;2:13. doi: 10.4103/2156-7514.94227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velvart P, Hecker H, Tillinger G. Detection of the apical lesion and the mandibular canal in conventional radiography and computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:682–688. doi: 10.1067/moe.2001.118904. [DOI] [PubMed] [Google Scholar]
- 31.Robinson S, Czerny C, Gahleitner A, Bernhart T, Kainberger FM. Dental CT evaluation of mandibular first premolar root configurations and canal variations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:328–332. doi: 10.1067/moe.2002.120055. [DOI] [PubMed] [Google Scholar]
- 32.Ozgur Uyanik M, Cehreli ZC, Ozgen Mocan B, Tasman Dagli F. Comparative evaluation of three nickel-titanium instrumentation systems in human teeth using computed tomography. J Endod. 2006;32:668–671. doi: 10.1016/j.joen.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Anbu R, Nandini S, Velmurugan N. Volumetric analysis of root fillings using spiral computed tomography: an in vitro study. Int Endod J. 2010;43:64–68. doi: 10.1111/j.1365-2591.2009.01638.x. [DOI] [PubMed] [Google Scholar]
- 34.Huang CH, Brunsvold MA. Maxillary sinusitis and periapical abscess following periodontal therapy: a case report using three-dimensional evaluation. J Periodontol. 2006;77:129–134. doi: 10.1902/jop.2006.77.1.129. [DOI] [PubMed] [Google Scholar]
- 35.Rigolone M, Pasqualini D, Bianchi L, Berutti E, Bianchi SD. Vestibular surgical access to the palatine root of the superior first molar: “low-dose cone-beam” CT analysis of the pathway and its anatomic variations. J Endod. 2003;29:773–775. doi: 10.1097/00004770-200311000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Stone DN, Mancuso AA, Rice D, Hanafee WN. Parotid CT sialography. Radiology. 1981;138:393–397. doi: 10.1148/radiology.138.2.7455119. [DOI] [PubMed] [Google Scholar]
- 37.Watson RM, Coward TJ, Forman GH, Moss JP. Considerations in treatment planning for implant-supported auricular prostheses. Int J Oral Maxillofac Implants. 1993;8:688–694. [PubMed] [Google Scholar]
- 38.Webber RL, Horton RA, Tyndall DA, Ludlow JB. Tuned-aperture computed tomography (TACT). Theory and application for three-dimensional dento-alveolar imaging. Dentomaxillofac Radiol. 1997;26:53–62. doi: 10.1038/sj.dmfr.4600201. [DOI] [PubMed] [Google Scholar]
- 39.Grant DG. Tomosynthesis: a three-dimensional radiographic imaging technique. IEEE Trans Biomed Eng. 1972;19:20–28. doi: 10.1109/tbme.1972.324154. [DOI] [PubMed] [Google Scholar]
- 40.Richards AG. Dynamic tomography. Oral Surg Oral Med Oral Pathol. 1976;42:685–692. doi: 10.1016/0030-4220(76)90219-x. [DOI] [PubMed] [Google Scholar]
- 41.Nair MK, Tyndall DA, Ludlow JB, May K. Tuned aperture computed tomography and detection of recurrent caries. Caries Res. 1998;32:23–30. doi: 10.1159/000016426. [DOI] [PubMed] [Google Scholar]
- 42.Webber RL, Messura JK. An in vivo comparison of diagnostic information obtained from tuned-aperture computed tomography and conventional dental radiographic imaging modalities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:239–247. doi: 10.1016/s1079-2104(99)70122-8. [DOI] [PubMed] [Google Scholar]
- 43.Nance R, Tyndall D, Levin LG, Trope M. Identification of root canals in molars by tuned-aperture computed tomography. Int Endod J. 2000;33:392–396. doi: 10.1046/j.1365-2591.2000.00330.x. [DOI] [PubMed] [Google Scholar]
- 44.Nair MK, Seyedain A, Agarwal S, Webber RL, Nair UP, Piesco NP, Mooney MP, Grondahl HG. Tuned aperture computed tomography to evaluate osseous healing. J Dent Res. 2001;80:1621–1624. doi: 10.1177/00220345010800070501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nair MK, Nair UP, Gröndahl HG, Webber RL. Accuracy of tuned aperture computed tomography in the diagnosis of radicular fractures in non-restored maxillary anterior teeth--an in vitro study. Dentomaxillofac Radiol. 2002;31:299–304. doi: 10.1038/sj.dmfr.4600712. [DOI] [PubMed] [Google Scholar]
- 46.Liang H, Tyndall DA, Ludlow JB, Lang LA. Cross-sectional presurgical implant imaging using tuned aperture computed tomography (TACT) Dentomaxillofac Radiol. 1999;28:232–237. doi: 10.1038/sj/dmfr/4600451. [DOI] [PubMed] [Google Scholar]
- 47.Aboudara CA, Hatcher D, Nielsen IL, Miller A. A three-dimensional evaluation of the upper airway in adolescents. Orthod Craniofac Res. 2003;6 Suppl 1:173–175. doi: 10.1034/j.1600-0544.2003.253.x. [DOI] [PubMed] [Google Scholar]
- 48.Scarfe WC, Levin MD, Gane D, Farman AG. Use of cone beam computed tomography in endodontics. Int J Dent. 2009;2009:634567. doi: 10.1155/2009/634567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tyndall DA, Kohltfarber H. Application of cone beam volumetric tomography in endodontics. Aust Dent J. 2012;57 Suppl 1:72–81. doi: 10.1111/j.1834-7819.2011.01654.x. [DOI] [PubMed] [Google Scholar]
- 50.Blessmann M, Pohlenz P, Blake FA, Lenard M, Schmelzle R, Heiland M. Validation of a new training tool for ultrasound as a diagnostic modality in suspected midfacial fractures. Int J Oral Maxillofac Surg. 2007;36:501–506. doi: 10.1016/j.ijom.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Zizelmann C, Gellrich NC, Metzger MC, Schoen R, Schmelzeisen R, Schramm A. Computer-assisted reconstruction of orbital floor based on cone beam tomography. Br J Oral Maxillofac Surg. 2007;45:79–80. doi: 10.1016/j.bjoms.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 52.Shintaku WH, Venturin JS, Azevedo B, Noujeim M. Applications of cone-beam computed tomography in fractures of the maxillofacial complex. Dent Traumatol. 2009;25:358–366. doi: 10.1111/j.1600-9657.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- 53.Stuehmer C, Essig H, Bormann KH, Majdani O, Gellrich NC, Rücker M. Cone beam CT imaging of airgun injuries to the craniomaxillofacial region. Int J Oral Maxillofac Surg. 2008;37:903–906. doi: 10.1016/j.ijom.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Pohlenz P, Blessmann M, Blake F, Heinrich S, Schmelzle R, Heiland M. Clinical indications and perspectives for intraoperative cone-beam computed tomography in oral and maxillofacial surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:412–417. doi: 10.1016/j.tripleo.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Naitoh M, Suenaga Y, Kondo S, Gotoh K, Ariji E. Assessment of maxillary sinus septa using cone-beam computed tomography: etiological consideration. Clin Implant Dent Relat Res. 2009;11 Suppl 1:e52–e58. doi: 10.1111/j.1708-8208.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- 56.Kijima N, Honda K, Kuroki Y, Sakabe J, Ejima K, Nakajima I. Relationship between patient characteristics, mandibular head morphology and thickness of the roof of the glenoid fossa in symptomatic temporomandibular joints. Dentomaxillofac Radiol. 2007;36:277–281. doi: 10.1259/dmfr/56344782. [DOI] [PubMed] [Google Scholar]
- 57.Tsiklakis K, Syriopoulos K, Stamatakis HC. Radiographic examination of the temporomandibular joint using cone beam computed tomography. Dentomaxillofac Radiol. 2004;33:196–201. doi: 10.1259/dmfr/27403192. [DOI] [PubMed] [Google Scholar]
- 58.Honda K, Bjørnland T. Image-guided puncture technique for the superior temporomandibular joint space: value of cone beam computed tomography (CBCT) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:281–286. doi: 10.1016/j.tripleo.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 59.Christiansen R, Kirkevang LL, Gotfredsen E, Wenzel A. Periapical radiography and cone beam computed tomography for assessment of the periapical bone defect 1 week and 12 months after root-end resection. Dentomaxillofac Radiol. 2009;38:531–536. doi: 10.1259/dmfr/63019695. [DOI] [PubMed] [Google Scholar]
- 60.Estrela C, Bueno MR, Leles CR, Azevedo B, Azevedo JR. Accuracy of cone beam computed tomography and panoramic and periapical radiography for detection of apical periodontitis. J Endod. 2008;34:273–279. doi: 10.1016/j.joen.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 61.Nakata K, Naitoh M, Izumi M, Inamoto K, Ariji E, Nakamura H. Effectiveness of dental computed tomography in diagnostic imaging of periradicular lesion of each root of a multirooted tooth: a case report. J Endod. 2006;32:583–587. doi: 10.1016/j.joen.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Patel S. New dimensions in endodontic imaging: Part 2. Cone beam computed tomography. Int Endod J. 2009;42:463–475. doi: 10.1111/j.1365-2591.2008.01531.x. [DOI] [PubMed] [Google Scholar]
- 63.Estrela C, Bueno MR, Azevedo BC, Azevedo JR, Pécora JD. A new periapical index based on cone beam computed tomography. J Endod. 2008;34:1325–1331. doi: 10.1016/j.joen.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 64.Simon JH, Enciso R, Malfaz JM, Roges R, Bailey-Perry M, Patel A. Differential diagnosis of large periapical lesions using cone-beam computed tomography measurements and biopsy. J Endod. 2006;32:833–837. doi: 10.1016/j.joen.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Tyndall DA, Rathore S. Cone-beam CT diagnostic applications: caries, periodontal bone assessment, and endodontic applications. Dent Clin North Am. 2008;52:825–841, vii. doi: 10.1016/j.cden.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Lofthag-Hansen S, Huumonen S, Gröndahl K, Gröndahl HG. Limited cone-beam CT and intraoral radiography for the diagnosis of periapical pathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:114–119. doi: 10.1016/j.tripleo.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Wang P, Yan XB, Lui DG, Zhang WL, Zhang Y, Ma XC. Detection of dental root fractures by using cone-beam computed tomography. Dentomaxillofac Radiol. 2011;40:290–298. doi: 10.1259/dmfr/84907460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hassan B, Metska ME, Ozok AR, van der Stelt P, Wesselink PR. Comparison of five cone beam computed tomography systems for the detection of vertical root fractures. J Endod. 2010;36:126–129. doi: 10.1016/j.joen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Patel S, Dawood A, Ford TP, Whaites E. The potential applications of cone beam computed tomography in the management of endodontic problems. Int Endod J. 2007;40:818–830. doi: 10.1111/j.1365-2591.2007.01299.x. [DOI] [PubMed] [Google Scholar]
- 70.Alshehri MA, Alamri H, Alshalhoub M. Applications of CBCT in Dental Practice. A Literature Review. Dental News. 2011;XVIII:26–34. [PubMed] [Google Scholar]
- 71.Hatcher DC, Dial C, Mayorga C. Cone beam CT for pre-surgical assessment of implant sites. J Calif Dent Assoc. 2003;31:825–833. [PubMed] [Google Scholar]
- 72.Hua Y, Nackaerts O, Duyck J, Maes F, Jacobs R. Bone quality assessment based on cone beam computed tomography imaging. Clin Oral Implants Res. 2009;20:767–771. doi: 10.1111/j.1600-0501.2008.01677.x. [DOI] [PubMed] [Google Scholar]
- 73.Heiland M, Pohlenz P, Blessmann M, Werle H, Fraederich M, Schmelzle R, Blake FA. Navigated implantation after microsurgical bone transfer using intraoperatively acquired cone-beam computed tomography data sets. Int J Oral Maxillofac Surg. 2008;37:70–75. doi: 10.1016/j.ijom.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Farman AG, Scarfe WC. Development of imaging selection criteria and procedures should precede cephalometric assessment with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2006;130:257–265. doi: 10.1016/j.ajodo.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 75.Van Elslande D, Heo G, Flores-Mir C, Carey J, Major PW. Accuracy of mesiodistal root angulation projected by cone-beam computed tomographic panoramic-like images. Am J Orthod Dentofacial Orthop. 2010;137:S94–S99. doi: 10.1016/j.ajodo.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 76.Shi H, Scarfe WC, Farman AG. Three-dimensional reconstruction of individual cervical vertebrae from cone-beam computed-tomography images. Am J Orthod Dentofacial Orthop. 2007;131:426–432. doi: 10.1016/j.ajodo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 77.Hamada Y, Kondoh T, Noguchi K, Iino M, Isono H, Ishii H, Mishima A, Kobayashi K, Seto K. Application of limited cone beam computed tomography to clinical assessment of alveolar bone grafting: a preliminary report. Cleft Palate Craniofac J. 2005;42:128–137. doi: 10.1597/03-035.1. [DOI] [PubMed] [Google Scholar]
- 78.Kim SH, Choi YS, Hwang EH, Chung KR, Kook YA, Nelson G. Surgical positioning of orthodontic mini-implants with guides fabricated on models replicated with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2007;131:S82–S89. doi: 10.1016/j.ajodo.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 79.Kasaj A, Willershausen B. Digital volume tomography for diagnostics in periodontology. Int J Comput Dent. 2007;10:155–168. [PubMed] [Google Scholar]
- 80.Ito K, Yoshinuma N, Goke E, Arai Y, Shinoda K. Clinical application of a new compact computed tomography system for evaluating the outcome of regenerative therapy: a case report. J Periodontol. 2001;72:696–702. doi: 10.1902/jop.2001.72.5.696. [DOI] [PubMed] [Google Scholar]
- 81.Katsumata A, Hirukawa A, Noujeim M, Okumura S, Naitoh M, Fujishita M, Ariji E, Langlais RP. Image artifact in dental cone-beam CT. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:652–657. doi: 10.1016/j.tripleo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 82.Mol A. Imaging methods in periodontology. Periodontol 2000. 2004;34:34–48. doi: 10.1046/j.0906-6713.2003.003423.x. [DOI] [PubMed] [Google Scholar]
- 83.Dental Radiography: Doses and Film Speed. U.S. Available from: http: //www.fda.gov/radiationemittingproducts/radiationsafety/nationwideevaluationofxraytrendsnext/ucm116524.htm. Accessed on 20-12-2013.
- 84.Silva MA, Wolf U, Heinicke F, Bumann A, Visser H, Hirsch E. Cone-beam computed tomography for routine orthodontic treatment planning: a radiation dose evaluation. Am J Orthod Dentofacial Orthop. 2008;133:640.e1–640.e5. doi: 10.1016/j.ajodo.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 85.Weishaupt D, Köchli VD, Marincek B. How does MRI work? An introduction to the Physics and Function of Magnetic Resonance Imaging. 2st ed. Berlin Heidelberg: Springer; 2006. [Google Scholar]
- 86.Boeddinghaus R, Whyte A. Current concepts in maxillofacial imaging. Eur J Radiol. 2008;66:396–418. doi: 10.1016/j.ejrad.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 87.van Rensburg LJ, Paquette M, Morkel JA, Nortjé CJ. Correlative MRI and CT imaging of the odontogenic keratocyst: a review of twenty-one cases. Oral Maxillofac Surg Clin North Am. 2003;15:363–382. doi: 10.1016/S1042-3699(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 88.DelBalso AM. Lesions of the jaws. Semin Ultrasound CT MR. 1995;16:487–512. doi: 10.1016/s0887-2171(06)80022-3. [DOI] [PubMed] [Google Scholar]
- 89.Idiyatullin D, Corum C, Moeller S, Prasad HS, Garwood M, Nixdorf DR. Dental magnetic resonance imaging: making the invisible visible. J Endod. 2011;37:745–752. doi: 10.1016/j.joen.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levin LG, Law AS, Holland GR, Abbott PV, Roda RS. Identify and define all diagnostic terms for pulpal health and disease states. J Endod. 2009;35:1645–1657. doi: 10.1016/j.joen.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 91.Browne RF, Golding SJ, Watt-Smith SR. The role of MRI in facial swelling due to presumed salivary gland disease. Br J Radiol. 2001;74:127–133. doi: 10.1259/bjr.74.878.740127. [DOI] [PubMed] [Google Scholar]
- 92.Schmid F, Tymofiyeva O, Rottner K, Richter E-J, Jakob PM. Dental impression using MRI. In: Proceedings of the Ninth ICMRM, Aachen, Germany; 2007. p. Abstract 103. [Google Scholar]
- 93.Tymofiyeva O, Rottner K, Jakob PM, Richter EJ, Proff P. Three-dimensional localization of impacted teeth using magnetic resonance imaging. Clin Oral Investig. 2010;14:169–176. doi: 10.1007/s00784-009-0277-1. [DOI] [PubMed] [Google Scholar]
- 94.Schara R, Sersa I, Skaleric U. T1 relaxation time and magnetic resonance imaging of inflamed gingival tissue. Dentomaxillofac Radiol. 2009;38:216–223. doi: 10.1259/dmfr/75262837. [DOI] [PubMed] [Google Scholar]
- 95.Hinshaw DB, Holshouser BA, Engstrom HI, Tjan AH, Christiansen EL, Catelli WF. Dental material artifacts on MR images. Radiology. 1988;166:777–779. doi: 10.1148/radiology.166.3.3340777. [DOI] [PubMed] [Google Scholar]
- 96.Schaefer SD, Maravilla KR, Suss RA, Burns DK, Nunnally R, Merkel MA, Close LG. Magnetic resonance imaging vs computed tomography. Comparison in imaging oral cavity and pharyngeal carcinomas. Arch Otolaryngol. 1985;111:730–734. doi: 10.1001/archotol.1985.00800130062006. [DOI] [PubMed] [Google Scholar]
- 97.Vijay MR, Adam EF, Barry MT. MRI and CT Atlas of Correlative Imaging in Otolaryngology. London: Martin Dunitz Ltd; 1992. pp. 1–11. [Google Scholar]
- 98.Okano Y, Yamashiro M, Kaneda T, Kasai K. Magnetic resonance imaging diagnosis of the temporomandibular joint in patients with orthodontic appliances. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:255–263. doi: 10.1067/moe.2003.37. [DOI] [PubMed] [Google Scholar]
- 99.Katti G, Ara SA, Shireen A. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. Int J Infereron Cytokine Mediator Res. 2011;2011:65–70. doi: 10.2147/IJICMR.S22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.White SC, Pharoah MJ. Chapter 13. Advanced Imaging. Oral Radiology. Principles and Interpretation. 6th ed. St Louis, MO: Mosby Elsevier; 2009. pp. 207–224. [Google Scholar]
- 101.Adeyemo WL, Akadiri OA. A systematic review of the diagnostic role of ultrasonography in maxillofacial fractures. Int J Oral Maxillofac Surg. 2011;40:655–661. doi: 10.1016/j.ijom.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 102.White SC, Pharoah MJ. Oral radiology, Principles and Interpretation. 6th ed. Chapter: 30 Salivary gland radiology. Mosby Elsevier; 2009. pp. 665–666. [Google Scholar]
- 103.Ariji E, Ariji Y, Yoshiura K, Kimura S, Horinouchi Y, Kanda S. Ultrasonographic evaluation of inflammatory changes in the masseter muscle. Oral Surg Oral Med Oral Pathol. 1994;78:797–801. doi: 10.1016/0030-4220(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 104.Gundappa M, Ng SY, Whaites EJ. Comparison of ultrasound, digital and conventional radiography in differentiating periapical lesions. Dentomaxillofac Radiol. 2006;35:326–333. doi: 10.1259/dmfr/60326577. [DOI] [PubMed] [Google Scholar]
- 105.Cotti E, Campisi G, Ambu R, Dettori C. Ultrasound real-time imaging in the differential diagnosis of periapical lesions. Int Endod J. 2003;36:556–563. doi: 10.1046/j.1365-2591.2003.00690.x. [DOI] [PubMed] [Google Scholar]
- 106.Wakasugi-Sato N, Kodama M, Matsuo K, Yamamoto N, Oda M, Ishikawa A, Tanaka T, Seta Y, Habu M, Kokuryo S, et al. Advanced clinical usefulness of ultrasonography for diseases in oral and maxillofacial regions. Int J Dent. 2010;2010:639382. doi: 10.1155/2010/639382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chandak R, Degwekar S, Bhowte RR, Motwani M, Banode P, Chandak M, Rawlani S. An evaluation of efficacy of ultrasonography in the diagnosis of head and neck swellings. Dentomaxillofac Radiol. 2011;40:213–221. doi: 10.1259/dmfr/68658286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rajendran N, Sundaresan B. Efficacy of ultrasound and color power Doppler as a monitoring tool in the healing of endodontic periapical lesions. J Endod. 2007;33:181–186. doi: 10.1016/j.joen.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 109.Tikku AP, Kumar S, Loomba K, Chandra A, Verma P, Aggarwal R. Use of ultrasound, color Doppler imaging and radiography to monitor periapical healing after endodontic surgery. J Oral Sci. 2010;52:411–416. doi: 10.2334/josnusd.52.411. [DOI] [PubMed] [Google Scholar]
- 110.Yoon MJ, Kim E, Lee SJ, Bae YM, Kim S, Park SH. Pulpal blood flow measurement with ultrasound Doppler imaging. J Endod. 2010;36:419–422. doi: 10.1016/j.joen.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 111.Tagtekin DA, Ozyoney G, Baseren M, Ando M, Hayran O, Alpar R, Gokalp S, Yanikoglu FC, Stookey GK. Caries detection with DIAGNOdent and ultrasound. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:729–735. doi: 10.1016/j.tripleo.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 112.Mahmoud AM, Ngan P, Crout R, Mukdadi OM. High-resolution 3D ultrasound jawbone surface imaging for diagnosis of periodontal bony defects: an in vitro study. Ann Biomed Eng. 2010;38:3409–3422. doi: 10.1007/s10439-010-0089-0. [DOI] [PubMed] [Google Scholar]
- 113.Cha BK, Lee YH, Lee NK, Choi DS, Baek SH. Soft tissue thickness for placement of an orthodontic miniscrew using an ultrasonic device. Angle Orthod. 2008;78:403–408. doi: 10.2319/051607-237.1. [DOI] [PubMed] [Google Scholar]
- 114.Culjat MO, Choi M, Singh RS, Grundfest WS, Brown ER, White SN. Ultrasound detection of submerged dental implants through soft tissue in a porcine model. J Prosthet Dent. 2008;99:218–224. doi: 10.1016/S0022-3913(08)60046-3. [DOI] [PubMed] [Google Scholar]
- 115.Manfredini D, Guarda-Nardini L. Ultrasonography of the temporomandibular joint: a literature review. Int J Oral Maxillofac Surg. 2009;38:1229–1236. doi: 10.1016/j.ijom.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 116.Bas B, Yılmaz N, Gökce E, Akan H. Diagnostic value of ultrasonography in temporomandibular disorders. J Oral Maxillofac Surg. 2011;69:1304–1310. doi: 10.1016/j.joms.2010.07.012. [DOI] [PubMed] [Google Scholar]


