Abstract
AIM: To study changes produced within the inferior vena cava (IVC) during respiratory movements and identify their possible clinical implications.
METHODS: This study included 100 patients (46 women; 54 men) over 18 years of age who required an abdominal computed tomography (CT) and central venous access. IVC cross-sectional areas were measured on CT scans at three levels, suprarenal (SR), juxtarenal (JR) and infrarenal (IR), during neutral breathing and again during the Valsalva maneuver. All patients were instructed on how to perform a correct Valsalva maneuver. In order to reduce the total radiation dose in our patients, low-dose CT protocols were used in all patients. The venous blood pressure (systolic, diastolic and mean) was invasively measured at the same three levels with neutral breathing and the Valsalva maneuver during venous port implantation. From CT scans, three-dimensional models of the IVC were constructed and a collapsibility index was calculated for each patient. These data were then correlated with venous pressures and cross-sectional areas.
RESULTS: The mean patient age was 51.64 ± 12.01 years. The areas of the ellipse in neutral breathing were 394.49 ± 85.83 (SR), 380.10 ± 74.55 (JR), and 342.72 ± 49.77 mm2 (IR), and 87.46 ± 18.35 (SR), 92.64 ± 15.36 (JR) and 70.05 ± 9.64 mm2 (IR) during the Valsalva (Ps < 0.001). There was a correlation between areas in neutral breathing and in the Valsalva maneuver (P < 0.05 in all areas). Large areas decreased more than smaller areas. The collapsibility indices were 0.49 ± 0.06 (SR), 0.50 ± 0.04 (JR) and 0.50 ± 0.04 (IR), with no significant differences in any region. Reconstructed three-dimensional models showed a flattening of the IVC during Valsalva, adopting an ellipsoid cross-sectional shape. The mean pressures with neutral breathing were 9.44 ± 1.78 (SR), 9.40 ± 1.44 (JR) and 8.84 ± 1.03 mmHg (IR), and 81.08 ± 21.82 (SR), 79.88 ± 19.01 (JR) and 74.04 ± 16.56 mmHg (IR) during Valsalva (Ps < 0.001). There was a negative correlation between cross-sectional caval area and venous blood pressure, but this was not statistically significant in any of the cases. There was a significant correlation between diastolic and mean pressures measured during neutral breathing and in Valsalva.
CONCLUSION: Respiratory movements have a major influence on IVC dynamics. The increase in intracaval pressure during Valsalva results in a significant decrease in the IVC cross-sectional area.
Keywords: Inferior vena cava, Morphology, Respiratory movements, Size, Valsalva, Venous hemodynamics
Core tip: This study is focused on the poorly investigated and controversial field of vena caval dynamics. It provides findings about possible correlations between respiratory movements and caval hemodynamic characteristics. This knowledge may have many clinical applications for therapeutic minimally invasive strategies, especially for the study of intravenous devices.
INTRODUCTION
The relationship between diameter and morphology of the inferior vena cava (IVC) during respiration and in Valsalva is poorly understood. In the medical literature, there is no agreement on whether the IVC increases or decreases in diameter during the Valsalva maneuver[1-7]. In a study on the diameter of the IVC measured by ultrasound, Grant et al[1] acknowledged this controversy and observed a decrease of IVC caliber during the Valsalva maneuver with a maximum increase at the end of the forced inspiration. Miraflor et al[8] attempted to relate IVC diameter and vascular filling (volume status) in hypotension, but did not observe a strong correlation.
There is little data in the literature relating biometrics and hemodynamics of the IVC[9-11]. Kaura et al[12] studied the biometrics of the IVC and concluded that venography alone is not an ideal method to assess true IVC diameters. Various authors recommend ultrasonography[5-7,13], intravascular ultrasonography[2], computed tomography (CT)[14-16], and even magnetic resonance imaging[9,17] for its morphologic study. To our knowledge, there are no prior studies that correlate the variation in venous blood pressure with caliber and morphology of IVC during respiration and Valsalva maneuver. We present a prospective study of biometric, geometric and hemodynamic changes in the IVC during respiratory movements and Valsalva in 100 patients.
MATERIALS AND METHODS
Study participants
This work has been carried out in accordance with the Declaration of Helsinki (1964, as revised in 2004) of the World Medical Association. This study was approved by the Ethics Committee of the Regional Government. All patients gave their informed consent prior to their inclusion in the study.
From January until December 2013, we measured morphologic, geometric and hemodynamic changes in the inferior vena cava in 100 patients during neutral breathing and then in Valsalva maneuver. Inclusion criteria were: patients over 18 years-old scheduled for implantation of a subcutaneous venous port, required an abdominal CT for evaluation of their underlying disease, and gave informed consent. Patients under 18 years-old, a body mass index over 30 kg/m2, unable to follow instructions, diagnosed with cardiovascular or pulmonary pathology with prior abdominal surgery in the last two years, or with any pathology of the abdominal walls were excluded from the study. All patients were instructed on how to perform a correct Valsalva maneuver, while the operator controlled their inspiration and manually assessed the abdominal pressure. Three rehearsals were made before each of the two maneuvers.
Data collection
Intravascular blood pressure data were collected during the central venous catheter implantation in the angio suite. Systolic, diastolic and mean pressures were recorded at the same three levels, juxtarenal (JR), suprarenal (SR) and infrarenal (IR), both during neutral respiration and in Valsalva using a pressure monitor (Vitara PA 8060; Dragër Medical Hispania, Madrid, Spain). We used a 100 cm, 5F pig-tail catheter (Cook Medical, Limerick, Ireland) to obtain three measurements in each of the locations, and calculated their average value.
Abdominal CT was also performed in Valsalva and in neutral respiration. CT studies were performed in a 64-slice Brilliance CT scanner (Philips Electronics, Amsterdam, Netherlands) with 100-120 cc of intravenous ioversol (Optiray Ultraject 240; Covidien, Barcelona, Spain) and reconstructed with a slice thickness of 5 mm. In order to reduce total radiation dose, low-dose CT protocols (120 kV and 30 mAs) were used[18] in all patients.
After the third rehearsal, contrast was injected and a delay of 60 s was scheduled. At 55 s, the patient was instructed to start the Valsalva maneuver. After the first acquisition, they were instructed to breath normally and the second scan was acquired.
IVC measurements
The cross-sectional area of the IVC was calculated using the software program Philips ViewForum R6.3V1L7 SP1-2010 (Philips Medical Systems Nederland B.V. Best, Netherlands) in three planes along the IVC: JR, at the renal veins junction; SR, 3 cm above; and IR, 3 cm below, as shown in Figure 1. The validation of the calculations was performed with axial CT measurements of the elliptical cross-sectional areas using the major and the minor diameters of the IVC: A = π (0.5 major diameter × 0.5 minor diameter). The software automatically calculated all areas shown in results, but we used the classic formula for validation. The collapsibility index (CI) was calculated as the difference between the value of the maximum diameter and the minimum diameter, divided by the maximum of the two values[19-22], and ranged from 0 (non-collapsible) to 1 (fully collapsible). All patients were monitored with pulse oximetry and electrocardiogram during every procedure.
Figure 1.
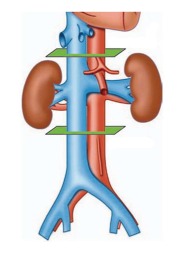
Inferior vena cava studied levels. The juxtarenal level is the site of the entrance of the renal veins; at 3 cm to the juxtarenal level are the suprarenal (above) and the infrarenal (below) levels, marked in green.
Three-dimensional reconstruction
Reconstruction was performed using the commercial software MIMCS (Materialise, Leuven, Belgium). Longitudinal and orthogonal vena caval cross-sections were used to build a three-dimensional model that was exported as a stereolithography and then imported into the commercial CAD package SolidWorks (Dassault Systèmes, Vélizy-Villacoublay, France) as shown in Figures 2 and 3. Using this software, the three-dimensional model was finally smoothed and refined in order to avoid possible irregularities of the geometry caused by the previous manual segmentation.
Figure 2.
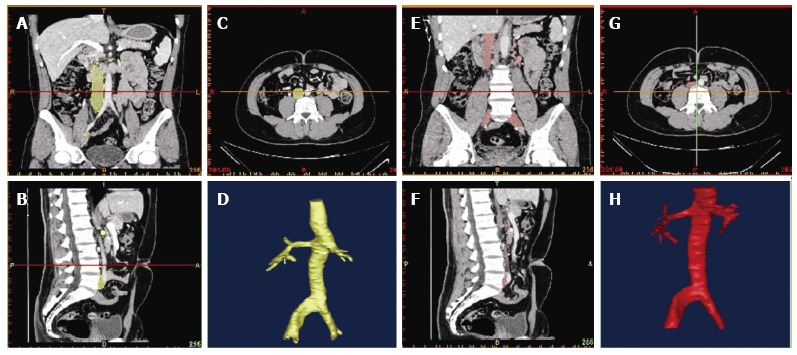
Three-dimensional inferior vena cava reconstruction in neutral breathing and in Valsalva. The figure shows different perspectives in order to get the different shapes of the vena cava cross-section. The inferior vena cava is marked in yellow during neutral breathing (A-D) and in red during the Valsalva maneuver (E-H). A, E: Coronal cross-section; B, F: Sagittal cross-section; C, G: Axial cross-section; D, H: Reconstruction of the inferior vena cava.
Figure 3.
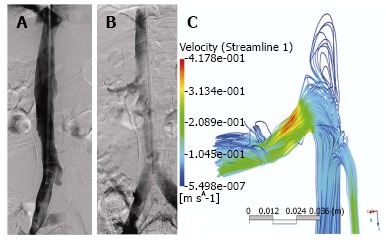
Distribution forces of the contrast medium in the inferior vena cava. A: Phlebography in neutral breathing; B: Phlebography during the Valsalva maneuver that shows distribution of the contrast medium to renal and iliac veins; C: Computational study of inferior vena cava migration forces using an experimental set up and finite element simulation during the Valsalva maneuver. When the patient is asked to perform a Valsalva maneuver, the inferior vena cava blurs as the contrast is displaced to lower pressure areas, such as iliacs and renal veins.
Statistical analysis
Statistical analysis was performed with the SPSS 17 software (SPSS Inc., Chicago, IL, United States). A P < 0.05 and a power of 90% was established for the statistical analysis. The data were tested for normality using the Kolmogorov-Smirnov test and means were compared using Student’s t-tests for paired samples. For correlation between areas and pressure, the Pearson’s correlation coefficient was used. Data are presented as mean ± SD.
RESULTS
The mean patient age was 51.64 ± 12.01 years (range: 32-72 years), 46% were women and the average body mass index was 22.58 ± 3.48 kg/m2 (range: 18.0-28.3 kg/m2). Intravenous blood pressure measurement added 5.35 ± 2.31 min to the total time of port-a-cath implantation. Total fluoroscopy time was 0.74 ± 0.28 min with an increase of 105.4 mGy/cm2 on the average dose area product for implantation of a central venous catheter in our department. The procedure was technically successful in 100% of the patients and no complications were observed in any of the patients studied.
The IVC areas measured by CT in each region during neutral respiration and in Valsalva are depicted in Figure 4. Significant differences (P < 0.001) were observed between both measurements in all three levels. The IVC areas in neutral breathing were 394.49 ± 85.83 (SR), 380.10 ± 74.55 (JR), and 342.72 ± 49.77 mm2 (IR), and 87.46 ± 18.35 (SR), 92.64 ± 15.36 (JR) and 70.05 ± 9.64 mm2 (IR) in Valsalva (P < 0.001). The area of the IVC in Valsalva was 22.17% (range: 16.43%-27.91%) of the area in neutral respiration at the SR level, 24.37% (range: 19.21%-29.54%) at the JR level, and 20.44% (range: 16.62%-24.26%) at the IR level. There was a correlation between areas in neutral breathing and in Valsalva (P < 0.05 in all levels) and larger areas decreased more than smaller areas.
Figure 4.
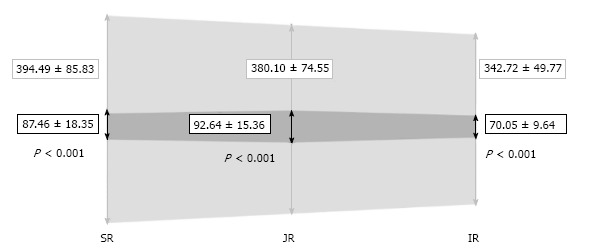
Mean area values in each level and their standard deviation (mm2). During neutral breathing (in light grey) and during Valsalva (in dark grey); SR: Suprarenal; JR: Juxtarenal; IR: Infrarenal.
The CI was constant and independent from the final pressure in Valsalva: 0.49 ± 0.06 (SR), 0.50 ± 0.04 (JR), and 0.50 ± 0.04 (IR). No significant differences were observed between the three different levels.
The IVC showed an ellipsoid shape during neutral breathing and adopted a crescent morphology during Valsalva, as shown is Figures 5 and 6.
Figure 5.
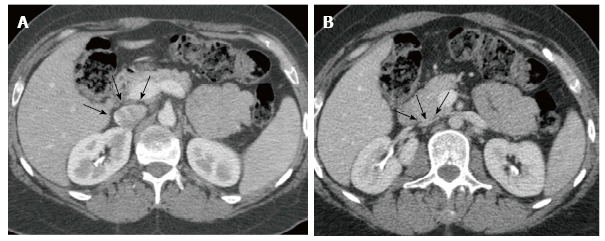
Angio computed tomography. Images showing the suprarenal inferior vena cava (arrows). A: In neutral breathing; B: In the Valsalva maneuver.
Figure 6.
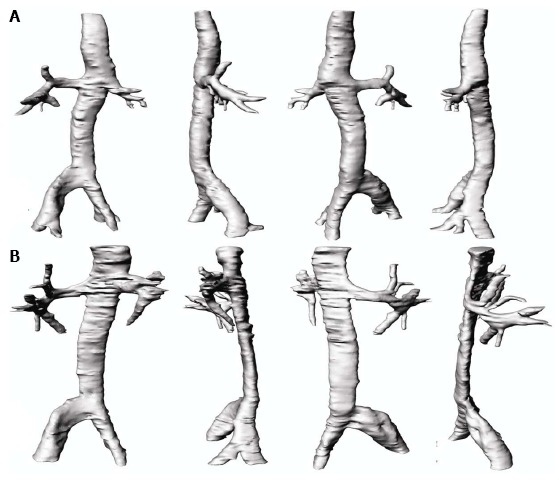
Three-dimensional reconstruction of the inferior vena cava. Modeling at various perspectives showing the different shapes of the vena cava cross-section in A: Neutral breathing; B: During the Valsalva maneuver.
Venous blood pressures at the three different levels are shown in Table 1. The standard deviation in Valsalva measurements was significantly higher than in neutral breathing (P < 0.001). The venous pressure in Valsalva maneuver was up to five times higher than in neutral breathing at all three levels (P < 0.001).
Table 1.
Venous blood pressures (mmHg)
| Inferior vena cava level | Venous pressure | Neutral breathing | Valsalva maneuver | P |
| Suprarenal | Systolic | 11.84 ± 1.55 | 97.96 ± 30.27 | < 0.001 |
| Diastolic | 7.16 ± 1.86 | 62.92 ± 12.48 | < 0.001 | |
| Mean | 9.44 ± 1.78 | 81.08 ± 21.82 | < 0.001 | |
| Juxtarenal | Systolic | 11.64 ± 1.32 | 96.69 ± 27.01 | < 0.001 |
| Diastolic | 7.24 ± 1.64 | 62.20 ± 11.33 | < 0.001 | |
| Mean | 9.40 ± 1.44 | 79.88 ± 19.01 | < 0.001 | |
| Infrarenal | Systolic | 11.28 ± 1.06 | 88.12 ± 24.09 | < 0.001 |
| Diastolic | 6.92 ± 1.19 | 58.68 ± 11.58 | < 0.001 | |
| Mean | 8.84 ± 1.03 | 74.04 ± 16.56 | < 0.001 |
There was a negative correlation between cross-sectional caval area and venous blood pressure, but this was not statistically significant in any of the cases. There was a significant correlation of diastolic and mean pressures between neutral breathing and in Valsalva, but systolic pressures showed higher variations and did not correlate with respirations. There was no influence of patient body mass index, age, or sex.
DISCUSSION
While reviewing the literature for this work, we encountered a controversy regarding variability of IVC size during respiration. Some authors claim that the IVC diameter increases in Valsalva based on measurements obtained under general anesthesia using a forced inspiration maneuver with positive pressure ventilation[2]. The Valsalva maneuver is defined as a forceful attempted exhalation against a closed airway, and we believe this cannot be simplified as a forced inspiration, which would cause an increased IVC diameter.
In our study, we observed no relation between size of the IVC and age, sex, or height and weight of the individual, which is consistent with previous studies[14]. Some studies have attempted to relate the biometrics of the IVC with various clinical applications, especially in the field of IVC filters[12,14,15,23] and as an indirect assessment of intravascular volume[14,24-26]. The majority of authors have concluded that three-dimensional CT is the most accurate method to study filter position and to assess safety against migration, as two projections are not adequate to evaluate filter anchoring to the vein wall. Interestingly, the classical concept of megacava (transverse diameter > 28-30 mm)[27-29] as measured in two-dimensional venography is still used as a contraindication for filter placement, but we believe this definition should be revised based on more accurate diameter assessments measured by CT[12].
In the current study, we identified a significant decrease in IVC size during Valsalva maneuvers to just 20%-25% of the full IVC area observed during neutral breathing. This finding was less pronounced in the JR IVC, and may be due to stabilization by the renal veins. We also observed that larger veins decreased in size more than smaller veins, and the CI remained constant and independent from the final pressures achieved in Valsalva. Interestingly, three-dimensional geometry revealed that this size decrease is not concentric, but instead involves a flattening of the vein that could influence future development of implantable intravenous devices.
There are few studies on the size of the IVC and its relationship to respiratory movements[1,2,10] or with central venous pressure[7,11,20], and no studies correlating these data with the intravenous abdominal pressure. Using real-time intravenous pressure measurements, we found that the mean pressure during the Valsalva maneuver was up to five times higher than in neutral breathing, and this pressure change was similar along all three IVC levels.
All patients were trained to perform a Valsalva maneuver while the doctor kept his hand on the patient’s abdomen to feel the contraction of the abdominal muscles. We noted that in women, abdominal contraction was not as remarkable as in men, coinciding with observations by Grant et al[1], but there were not significant differences in calculated IVC area or in invasive venous pressures between the sexes. However, Valsalva maneuvers differ greatly depending on the patient. In our investigation, we observed higher standard deviations during Valsalva compared with neutral breathing in calculated cross-sectional areas (70.05 vs 14.45 mm2) and in venous pressure measurements (19.27 vs 1.43 mmHg). This may be considered a limitation of the study, as our measurements were partially dependent on the cooperation of the patient and their ability to perform full Valsalva maneuvers. Another study limitation is that venous pressures and CT studies were not performed in the same session, but Valsalva maneuvers were standardized during these sessions.
In summary, we successfully correlated respiratory movements to IVC morphology and dynamics. We found a significant increase in intracaval pressure during Valsalva that resulted in a significant decrease in IVC cross-sectional area. This new knowledge of IVC physiology and geometry may be applied to the future design of intravenous devices such as IVC filters, to better withstand the dynamic caval environment.
COMMENTS
Background
The inferior vena cava (IVC) is the largest vein that returns blood from the lower body to the heart. Nevertheless, caval hemodynamics are poorly investigated and many aspects remain unclear, such as the influence of respiratory movements on size, morphology and pressure. Various intravascular devices, such as IVC filters, are deployed within it, so it is important to understand its changes in morphology and pressure to better design these types of medical devices.
Research frontiers
A person may perform the Valsalva maneuver many times during a day, voluntarily (e.g., during defecation, urination, lifting something heavy) or involuntarily (e.g., coughing). It causes changes in blood pressure and heart rate that can be dangerous for patients with cardiovascular disease. This maneuver also causes important biometric, geometric and hemodynamic changes in the abdominal cava that are not well known. This study tries to explain what happens in the normal IVC during neutral respiratory movements and during Valsalva.
Innovations and breakthroughs
In the medical literature, there is no consensus as to whether the IVC diameter increases or diminishes during the Valsalva maneuver. Furthermore, there is little published data relating biometrics and hemodynamics of the IVC. To our knowledge, this study is the only work that investigates possible correlations between the intravenous abdominal pressure and the size of the vena cava. This study demonstrates that when performing the Valsalva maneuver there is a great decrease in size of the vena cava, a flattening of the vein and an increase of venous pressure.
Applications
The findings of this study may be used in clinical applications for venous therapeutic strategies, especially in minimally invasive techniques, such as for the designing of vena cava filters or stents.
Terminology
Valsalva maneuver is defined as a forceful attempted exhalation against a closed airway, which increases intrathoracic pressure and impedes venous return of blood to the heart. Vena cava filters are devices designed to prevent blood clots from traveling from the veins in the legs or pelvis to the lungs, typically remaining inside the IVC for weeks or months.
Peer review
This manuscript presents a very interesting study evaluating the influence of neutral breathing and Valsalva maneuver on the diameters and intravascular pressure of the IVC at the suprarenal, juxtarenal and infrarenal levels. The study is sound and may be a valuable contribution to the literature.
Footnotes
P- Reviewer: Chen JH, Cebi N, Widmann G S- Editor: Gong XM L- Editor: AmEditor E- Editor: Lu YJ
References
- 1.Grant E, Rendano F, Sevinc E, Gammelgaard J, Holm HH, Grønvall S. Normal inferior vena cava: caliber changes observed by dynamic ultrasound. AJR Am J Roentgenol. 1980;135:335–338. doi: 10.2214/ajr.135.2.335. [DOI] [PubMed] [Google Scholar]
- 2.Murphy EH, Johnson ED, Arko FR. Evaluation of wall motion and dynamic geometry of the inferior vena cava using intravascular ultrasound: implications for future device design. J Endovasc Ther. 2008;15:349–355. doi: 10.1583/08-2424.1. [DOI] [PubMed] [Google Scholar]
- 3.Leopold GR. Gray scale ultrasonic angiography of the upper abdomen. Radiology. 1975;117:665–671. doi: 10.1148/117.3.665. [DOI] [PubMed] [Google Scholar]
- 4.Taylor KJ. Ultrasonic investigation of inferior vena-caval obstruction. Br J Radiol. 1975;48:1024–1026. doi: 10.1259/0007-1285-48-576-1024. [DOI] [PubMed] [Google Scholar]
- 5.Brennan JM, Ronan A, Goonewardena S, Blair JE, Hammes M, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749–753. doi: 10.2215/CJN.00310106. [DOI] [PubMed] [Google Scholar]
- 6.Mintz GS, Kotler MN, Parry WR, Iskandrian AS, Kane SA. Reat-time inferior vena caval ultrasonography: normal and abnormal findings and its use in assessing right-heart function. Circulation. 1981;64:1018–1025. doi: 10.1161/01.cir.64.5.1018. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Luan H, Zhu P, Feng J, Cui J, Zhao Z. Does ultrasonographic measurement of the inferior vena cava diameter correlate with central venous pressure in the assessment of intravascular volume in patients undergoing gastrointestinal surgery? J Surg Res. 2014;191:339–343. doi: 10.1016/j.jss.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Miraflor E, Yeung L, Strumwasser A, Sadjadi J, Victorino GP. Correlation between IVC dimensions and volume status on CT scan. J Surg Res. 2011;170:291–296. doi: 10.1016/j.jss.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi J, Horie T, Yamamoto K, I Muro, K. Kuroda. The evaluation of inferior vena caval hemodynamics by magnetic resonance imaging. Cardiovasc Intervent Radiol. 2010;33 Suppl 2:S250. [Google Scholar]
- 10.Natori H, Tamaki S, Kira S. Ultrasonographic evaluation of ventilatory effect on inferior vena caval configuration. Am Rev Respir Dis. 1979;120:421–427. doi: 10.1164/arrd.1979.120.2.421. [DOI] [PubMed] [Google Scholar]
- 11.Citilcioglu S, Sebe A, Ay MO, Icme F, Avci A, Gulen M, Sahan M, Satar S. The relationship between inferior vena cava diameter measured by bedside ultrasonography and central venous pressure value. Pak J Med Sci. 2014;30:310–315. doi: 10.12669/pjms.302.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaura DR, Gray RR, Sadler DJ, So CB, Saliken JC. Value of frontal caval measurement in the placement of inferior vena cava filters. Can Assoc Radiol J. 1999;50:301–305. [PubMed] [Google Scholar]
- 13.Chen L, Kim Y, Santucci KA. Use of ultrasound measurement of the inferior vena cava diameter as an objective tool in the assessment of children with clinical dehydration. Acad Emerg Med. 2007;14:841–845. doi: 10.1197/j.aem.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Bonnichon P, Gaudard F, Ouakil E, Lebozec P, de Labrouhe C, Bonnin A, Aaron C, Chapuis Y. Biometry of infrarenal inferior vena cava measured by cavography. Clinical applications. Surg Radiol Anat. 1989;11:149–154. doi: 10.1007/BF02096473. [DOI] [PubMed] [Google Scholar]
- 15.Bonnichon P, Gaudard F, Lecam B, Shilder J, Pariente D, Sarfati PO, Chapuis Y. Biometry of the infrarenal inferior vena cava measured by computed tomography. Clinical applications. Surg Radiol Anat. 1992;14:265–269. doi: 10.1007/BF01794951. [DOI] [PubMed] [Google Scholar]
- 16.Murphy EH, Arko FR, Trimmer CK, Phangureh VS, Fogarty TJ, Zarins CK. Volume associated dynamic geometry and spatial orientation of the inferior vena cava. J Vasc Surg. 2009;50:835–842; discussion 842-843. doi: 10.1016/j.jvs.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Thornton MJ, Ryan R, Varghese JC, Farrell MA, Lucey B, Lee MJ. A three-dimensional gadolinium-enhanced MR venography technique for imaging central veins. AJR Am J Roentgenol. 1999;173:999–1003. doi: 10.2214/ajr.173.4.10511166. [DOI] [PubMed] [Google Scholar]
- 18.Poletti PA, Platon A, Rutschmann OT, Schmidlin FR, Iselin CE, Becker CD. Low-dose versus standard-dose CT protocol in patients with clinically suspected renal colic. AJR Am J Roentgenol. 2007;188:927–933. doi: 10.2214/AJR.06.0793. [DOI] [PubMed] [Google Scholar]
- 19.Thanakitcharu P, Charoenwut M, Siriwiwatanakul N. Inferior vena cava diameter and collapsibility index: a practical non-invasive evaluation of intravascular fluid volume in critically-ill patients. J Med Assoc Thai. 2013;96 Suppl 3:S14–S22. [PubMed] [Google Scholar]
- 20.Moreno FL, Hagan AD, Holmen JR, Pryor TA, Strickland RD, Castle CH. Evaluation of size and dynamics of the inferior vena cava as an index of right-sided cardiac function. Am J Cardiol. 1984;53:579–585. doi: 10.1016/0002-9149(84)90034-1. [DOI] [PubMed] [Google Scholar]
- 21.Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, Vieillard-Baron A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30:1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- 22.Kimura BJ, Dalugdugan R, Gilcrease GW, Phan JN, Showalter BK, Wolfson T. The effect of breathing manner on inferior vena caval diameter. Eur J Echocardiogr. 2011;12:120–123. doi: 10.1093/ejechocard/jeq157. [DOI] [PubMed] [Google Scholar]
- 23.McLoney ED, Krishnasamy VP, Castle JC, Yang X, Guy G. Complications of Celect, Günther tulip, and Greenfield inferior vena cava filters on CT follow-up: a single-institution experience. J Vasc Interv Radiol. 2013;24:1723–1729. doi: 10.1016/j.jvir.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40:845–853. doi: 10.1016/j.ultrasmedbio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Kawataki M, Hirakawa A, Toyoshima K, Kato T, Itani Y, Hayakawa M. The diameter of the inferior vena cava provides a noninvasive way of calculating central venous pressure in neonates. Acta Paediatr. 2013;102:e241–e246. doi: 10.1111/apa.12247. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JJ, Garwe T, Albrecht RM, Adeseye A, Bishop D, Fails RB, Shepherd DW, Lees JS. Initial inferior vena cava diameter on computed tomographic scan independently predicts mortality in severely injured trauma patients. J Trauma Acute Care Surg. 2013;74:741–745; discussion 741-745. doi: 10.1097/TA.0b013e3182827270. [DOI] [PubMed] [Google Scholar]
- 27.Brown DB, Labuski MR, Cardella JF, Singh H, Waybill PN. Determination of inferior vena cava diameter in the angiography suite: comparison of three common methods. J Vasc Interv Radiol. 1999;10:143–147. doi: 10.1016/s1051-0443(99)70456-2. [DOI] [PubMed] [Google Scholar]
- 28.Baron HC, Klapholz A, Nagy AA, Wayne M. Bilateral iliac vein filter deployment in a patient with megacava. Ann Vasc Surg. 1999;13:634–636. doi: 10.1007/s100169900313. [DOI] [PubMed] [Google Scholar]
- 29.Van Ha TG, Dillon P, Funaki B, Zangan S, Lorenz J, Piano G, Regalado S. Use of retrievable filters in alternative common iliac vein location in high-risk surgical patients. J Vasc Interv Radiol. 2011;22:325–329. doi: 10.1016/j.jvir.2010.09.033. [DOI] [PubMed] [Google Scholar]


