Abstract
The benefits of early perfusion in ST elevation myocardial infarctions (STEMI) are established; however, early perfusion of non-ST elevation myocardial infarctions has not been shown to be beneficial. In addition, ST elevation (STE) caused by conditions other than acute ischemia is common. Non-ischemic STE may be confused as STEMI, but can also mask STEMI on electrocardiogram (ECG). As a result, activating the primary percutaneous coronary intervention (pPCI) protocol often depends on determining which ST elevation patterns reflect transmural infarction due to acute coronary artery thrombosis. Coordination of interpreting the ECG in its clinical context and appropriately activating the pPCI protocol has proved a difficult task in borderline cases. But its importance cannot be ignored, as reflected in the 2013 American College of Cardiology Foundation/American Heart Association guidelines concerning the treatment of ST elevation myocardial infarction. Multiples strategies have been tested and studied, and are currently being further perfected. No matter the strategy, at the heart of delivering the best care lies rapid and accurate interpretation of the ECG. Here, we present the different patterns of non-ischemic STE and methods of distinguishing between them. In writing this paper, we hope for quicker and better stratification of patients with STE on ECG, which will lead to be better outcomes.
Keywords: Diagnosis, Electrocardiogram, Reperfusion therapy, ST segment elevation, Myocardial infarction
Core tip: At times, distinguishing between myocardial infarction with ST elevation (STEMI) from non-ischemic causes of elevation of the ST segment is difficult, especially in patients with atypical presenting symptoms. Understanding common patterns of ST elevation that are not caused by ischemia is crucial for rapid and accurate diagnosis. However, patients with baseline non-ischemic ST elevation (for example, early repolarization or repolarization changes caused by hypertrophy of the left ventricule) may develop acute myocardial infarction (true STEMI or non-ST elevation myocardial infarction with baseline ST elevation). Here we describe common patterns of non-ischemic ST elevation.
INTRODUCTION
Today, the electrocardiogram (ECG) is the most commonly used diagnostic tool for recognizing and triaging of patients with symptoms suggestive of myocardial infarction (MI). Per the “Third Universal Definition of Myocardial Infarction” document, the ECG should be acquired and interpreted within 10 min of presentation[1]. Additionally, serial ECGs every 15 to 30 min should be performed in patients with ongoing symptoms in whom the initial ECG is not diagnostic of ST elevation MI (STEMI)[1].
ST elevation (STE) is considered to reflect acute transmural ischemia caused by an occlusion of an epicardial coronary artery by a blood clot. Therefore, it is recommended that patients with suspected acute STEMI and without contraindications should be subjected as soon as possible to therapy intended to recanalize the occluded artery by either primary percutaneous coronary intervention (pPCI) or fibrinolysis. In contrast, the guidelines recommend initial conservative therapy for patients with suspected MI without STE, as active ongoing ischemia may not be present and earlier studies have not shown a benefit for reperfusion therapy in patients without STE[2].
As per the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, ST elevation myocardial infarction is a clinical syndrome that compromizes typical symptoms of acute ischemia of the heart muscle in conjunction with elevation of the ST segment and increased blood levels of biomarkers that indicate necrosis of the cardiac muscle. By these guidelines, pPCI is recommended for those with symptoms indicative of ischemia of the heart muscle that began 12 h or less before medical encounter who have elevation of the ST segment[3]. Although the innovation of cardiac troponin (cTn) assays specific to the myocardium is changing the overall diagnosis of MI, the decision to proceed with angiography or give thrombolytics is made based on STE on the ECG and is usually reached before troponins are detectable in the blood. Further, the elderly, patients of female gender, and diabetic patients frequently present with symptoms that are not typical, further emphasizing the role of the presenting ECG for diagnosis and triage of such individuals[4-6].
In most of individuals without prior cardiac disease, the ST segment is at the level of the preceding P-R segment and/or the following T-P segment (so called isoelectric). Deviation of the ST-segment (elevation or depression compared to the isoelectric line) can be a sign of ischemia of the heart muscle. However, deviations of the ST segment relative to the isoelectric line due to nonischemic etiologies are often seen. Elevation of the ST segment due to non-ischemic etiologies was reported up to 15% in the general population. One study[7] found that 91% of 6014 men who served in the United State Air Force, between 16 and 58 years of age, without any apparent cardiac disease had elevation of the ST segment of 0.1 to 0.3 mV in more than one of the precordial leads (most commonly seen in lead V2). Another study suggested that elevation of the ST segment above 0.1 mV in one or more leads (V1 to V4) in 529 men without apparent cardiac disease could be found in 93% among those who were between 17 and 24 years of age. As age progresses, the prevalence of elevation of the ST segment declined[8]. Thus, most men have elevation of the ST segment greater than 0.1 mV in the precordial leads. Therefore, elevation of the ST segment should be regarded as a normal finding and is often termed “male pattern”. On the other hand, only fifth of patients of female gender have elevation of the ST segment above 0.1 mV, and this percentage is not influenced by the age of the female patients[9]. These thresholds are discussed in the “Third Universal Definition of Myocardial Infarction” document[1].
Different cutoffs for the amount of STE are causing confusion. The cutoffs for abnormal elevation of the ST segment, per the “Third Universal Definition of Myocardial Infarction” document for leads V2-V3, are elevation of the ST segment at the J-point of above 0.2 mV in men 40 years of age or older, 0.25 mV or above in men below 40 years of age, and 0.15 mV or above in women and/or 0.1 mV or above in all other leads in patients without hypertrophy of the left ventricule or block of the left bundle branch[1]. These criteria are based on the 2% extreme outside of the mean calculated from a population of 1321 Caucasians from the city of Glasgow and the region of Strathclyde in Scotland[9]. The 2013 ACCF/AHA STEMI guidelines have simplified these recommendations. In these guidelines STE at the J point in 2 contiguous leads or more of 0.2 mV or more in males or 0.15 mV or more in women in leads V2-V3 and/or of 0.1 mV or more in all other leads is the threshold[3]. Considering the ethnic homogeneity and the decreasing STE magnitude with age, these cutoffs should appreciated in this context[9]. It is unclear whether the same thresholds for STE can be used in populations of different ethnicity, as higher magnitude of STE was reported in Nigerian healthy men[10]. It is plausible that if the thresholds, endorsed by the “Third Universal Definition of Myocardial Infarction” document are used, the reported incidence of anterior STEMI would decrease, especially in men younger than 40 years of age. Moreover, currently there are no guidelines as to what are considered “normal” STE for patients whose ECG shows criteria for hypertrophy of the left ventricular, left bundle branch block or other forms of advanced intraventricular conduction defects.
As abovementioned, many patients presenting with typical symptoms have elevation of the ST segment due to non-ischemic etiologies (NISTE)[3-6]. Physicians must use all tools at their disposable to reach accurate diagnosis and reduce the risk of false activation of the pPCI protocol or exposure to thrombolytic therapy from one hand, while not missing cases of true STEMI. There are patterns of NISTE that are frequent and typical and can be easily recognized and distinguished from ST elevation myocardia infarction. Yet, there are individuals with pre-existing ST elevation secondary to non-ischemic etiologies (e.g., hypertrophy of the left ventricle or “early repolarization”) that can develop superimposed acute MI (ST elevation myocardial infarction or non-STEMI (NSTEMI)); therefore, presence of benign patterns of NISTE does not always rule out acute coronary syndrome (ACS) and even STEMI.
The differential diagnosis of elevation of the ST segment is wide, including conditions with secondary ischemia of the myocardium (for example, dissection of the aortic wall), pre-existing elevation of the ST segment without acute ischemia, and instances with new elevation of the ST segment with chest pain but without evidence of ischemia of the heart muscle (for example, myocarditis or pericarditis, pulmonary embolus, electrolyte imbalance, rate-related repolarization changes, etc.). Obviously, with the current emphasis on diagnosing and triaging acute ST elevation myocardial infarction rapidly, the probability of over-diagnosing ST elevation myocardial infarction and false activation of the pPCI protocols or administration of fibrinolytic therapy may increase.
Failure to identify NISTE has its costs. It may delay treatment for the original medical condition (i.e., aortic dissection, pulmonary embolus, peptic disease, etc.) and may expose the patient to unnecessary irradiation and exposure to contrast agents, in addition to increased health care costs and exhaustion of the catheterization laboratory personnel.
False-positive activation of the catheterization laboratory (no culprit lesion) have been reported in 9% to 14% of the patients[11,12]. More importantly, inappropriate activation rate, where the cardiologist did not perform an emergent coronary angiogram, is varied from 5% to 23%[12], largely depending on the training of the activator (paramedic or ED physician).
In this paper, we describe different patterns of STE and their underlying causes. We intend to provide insight into pathological vs non-pathological STE (Table 1). A better understanding of STE will lead to faster and the more appropriate treatment, lower false-positive and inappropriate activation of urgent reperfusion protocols (fibrinolytic therapy or pPCI), ensuring the best patient outcomes.
Table 1.
Common patterns of nonischemic ST elevation
| ST elevation secondary to LVH |
| ST elevation secondary to conduction defect (such as left bundle branch block and non-specific intracardiac conduction delay) |
| Early repolarization pattern (notched J-point typically in anterolateral leads) |
| Normal variant of ST elevation (ST elevation mostly in leads V2-V3) |
| Concave ST elevation |
| Spontaneously reperfused STEMI |
| Aneurysm/old myocardial infarction |
| Pericarditis/myocarditis |
| Wolf-Parkinson-White syndrome (pre-excitation) |
| Brugada pattern |
| Takotsubo (apical ballooning) syndrome |
| Hyperkalemia |
| Hypercalcemia |
LVH: Left ventricular hypertrophy; STEMI: ST elevation myocardial infarctions.
“CONVEX” VS “CONCAVE” PATTERNS OF STE
As mentioned above, the ST segment is normally isoelectric. ST elevation with convex or straight pattern is traditionally considered as indicative of STEMI in contrast to a concave pattern, which is typically considered to be secondary to nonischemic etiologies. The 2004 ACCF/AHA guidelines supported this belief[13]; however, this recommendation has been omitted from the current 2013 ACCF/AHA guidelines[1].
Wang et al[14] also emphasized the importance of the concave tracing in establishing the male pattern, left bundle branch block (LBBB), and LVH forms of STE over STEMI. However, concavity verses convexity must be analyzed carefully and should not be relied on as the sole criteria for distinguishing NISTE from STEMI. Brady et al[15] reported 77% sensitivity, 97% specificity, 94% positive predictive value, and 88% negative predictive value for a non-concave STE morphology in acute MI diagnosis. Given the use of ECG for screening, such suboptimal sensitivity would yield poor patient outcomes. Figure 1 depicts an ECG of a man with acute anterior wall ST elevation myocardial infarction presenting with concave form of ST elevation in the precordial leads. Angiography of the coronary arteries revealed total occlusion of the left anterior descending coronary artery (LAD) and the STE resolved after pPCI.
Figure 1.
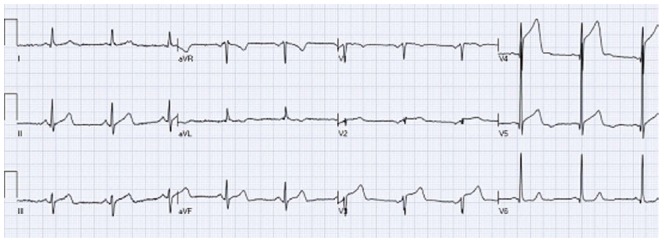
A patient with acute anterior wall ST elevation myocardial infarctions with concave form of ST elevation in the precordial leads (V3-V5). Coronary angiography revealed mid left anterior descending occlusion and primary percutaneous coronary intervention was performed.
EARLY REPOLARIZATION
The “early repolarization” pattern is usually found 1% to 5% of the population. Most commonly found in young, athletic, black males[16,17]. In the past, early repolarization pattern of NISTE was considered a benign pattern[17]. More recently, however, early repolarization pattern has been associated with cardiac arrhythmia and sudden cardiac mortality, mainly if there is 0.2 mV or more elevation of the ST segment. Nevertheless, this pattern is not caused by acute ischemia mandating emergent reperfusion therapy. The typical pattern appears as no S wave in V3; 1-4 mm concave elevation of the ST-segment in leads V2-V5 (most prominent in V3) and sometimes the inferior leads; and notching of the downstroke of the R waves (“J” wave), most distinct in lead V5 and V6[16-18]. However, other authors have used different definitions. Figure 2 is an example of early repolarization pattern.
Figure 2.
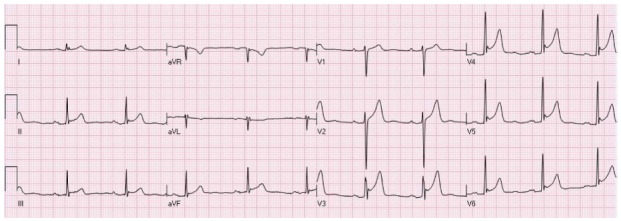
An example of ST elevation due to “early repolarization”. ST elevation with notched J waves is seen in the inferior and anterolateral leads.
In many cases of “early repolarization”, elevation of the ST segment is not lasting and decreases or disappears when the heart rate increases or if the patient hyperventilates. Therefore, significant changes in the magnitude of ST elevation are not necessarily diagnostic for acute myocardial ischemia. At times, concomitant inversion of the T-waves may be present in the precordial leads, which are due to “juvenile T wave pattern” in younger subjects. These changes could be mistaken for acute myocardial ischemia[16].
Hypothermia may cause prominent J-point notch (Osborne waves)[19] that must be distinguished from “early repolarization” pattern. Hypothermia frequently causes slow heart rate and muscle shiver. Osborne waves with elevation of the ST segment are occasionally seen in patients with severe hypercalcemia or disorders of the central nervous system. Low body temperature usually causes prolongation of the QT interval. On the other hand, hypercalcemia usually induces shortening of the QT interval[20]. Hyperkalemia can also cause elevation of the ST segment. In addition, hyperkalemia often presents with QRS widening and changes can be seen in the P waves and the PR segments. Another entity that can be mistaken for notching of the J-point (so called “epsilon waves”) is typically observed in “Arrhythmogenic Right Ventricular Dysplasia”. In arrhythmogenic right ventricular dysplasia, however, epsilon waves are commonly present in the precordial leads V1-V3[21].
A “NORMAL-VARIANT” PATTERN OF NISTE
A “normal-variant” ST elevation typically presents as elevation of the ST segments mainly in the precordial leads V1 to V3 (Figure 3)[14]. It is typically seen in young persons, mainly in Hispanic or African American males. QRS criteria for left ventricular hypertrophy are not met and concomitant depression of the ST segments and T waves changes in the lateral leads are not seen. There are investigators who do not make the difference between a “normal variant” pattern and “early repolarization” pattern, grouping them together under the “early repolarization” umbrella. It should be remembered that “early repolarization” and “normal variant” patterns are frequently present in the same patients.
Figure 3.
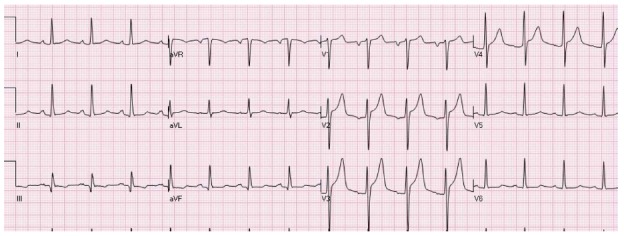
An electrocardiogram of a young male with a “normal variant” concave pattern of ST elevation in leads V2-V4.
ELEVATION OF THE ST SEGMENTS DUE TO HYPERTROPHY OF THE LEFT VENTRICLE
Just as the QRS complex amplitude may increase by a more massive left ventricle, changes in the ST segments can be amplified[22]. NISTE due to hypertrophy of the left ventricle (LVH) is usually seen in leads V1-V3. Typically, there are QRS amplitude criteria for hypertrophy of the left ventricle and associated depression of the ST segments in the leads facing the lateral wall (V5-V6 and I and aVL) (Figure 4). Frequently elevation of the ST segment is seen in lead aVR. It is crucial not to misdiagnose this pattern as the pattern thought to represent left main induced- or circumferential- subendocardial ischemia (elevation of the ST segments in leads V1 and aVR with accompanying depression of the ST segments in the inferior as well as the anterolateral leads). Per the Third Global MI Task Force consensus paper[1], the cutoffs for the absolute amplitude of the ST segment elevation do not apply for patients with hypertrophy of the left ventricle. Yet; hypertension is an established risk factor for atherosclerotic heart disease, including acute MI. It should be remembered, however, that at times hypertrophy of the left ventricle may present with atypical configurations of ST elevation (Figure 5). Furthermore, frequently patients are presenting with more than one pattern of NISTE (LVH + early repolarization or nonspecific intraventricular conduction delay [IVCD] + LVH and even STEMI on top of ST segment deviations induced by LVH).
Figure 4.
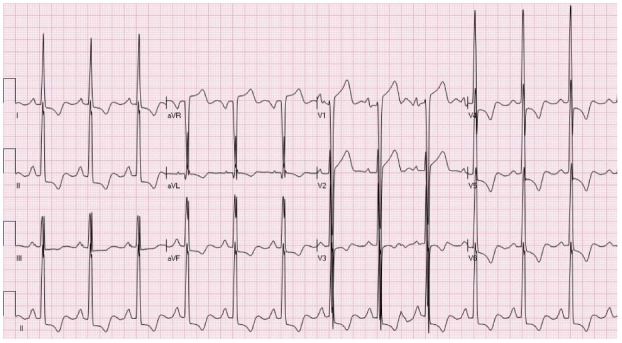
An electrocardiogram showing typical pattern of ST elevation due to hypertrophy of the left ventricular with secondary repolarization changes. There is ST elevation in leads V1-V2 and ST depression with T wave inversion in the inferolateral leads.
Figure 5.
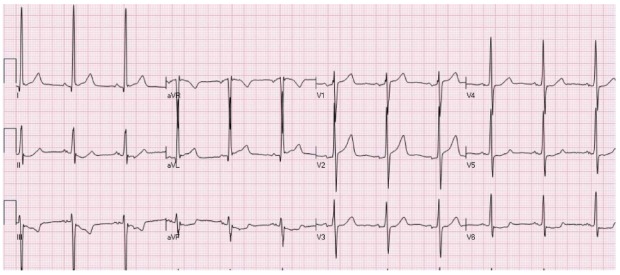
An electrocardiogram of a patient with atypical form of ST elevation secondary to left ventricular hypertrophy. ST elevation is present in leads I, aVL, V1-V2. Mild ST depression is present in the inferior leads and V5-V6.
ACUTE PERICARDITIS
STE may be seen in the acute or first stage of pericarditis, which occurs in the first few days and may last up to weeks. In most cases, diffuse STE is seen in all the ECG leads, except in leads aVR and V1, that typically have reciprocal depression of the ST segments (Figure 6). This pattern is often associated with PR depression in all ECG leads, except leads V1 and aVR, which occasionally depict reciprocal PR elevation[23]. Focal pericarditis (for example, after acute myocardial infarction or heart surgery), however, may induce more regional and non-typical forms of STE, which at times could be associated with depression of the ST segments in leads other than V1 and aVR . These atypical patterns could be mistaken for STEMI.
Figure 6.
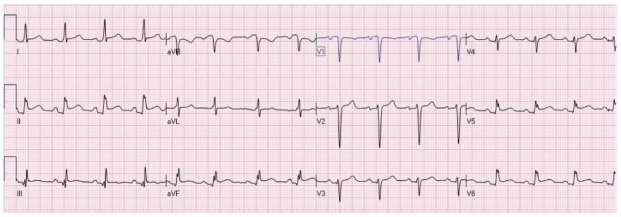
Diffuse ST elevation secondary to acute pericarditis. There is typical depression of the PR segment (seen mainly in leads II and aVF. There is ST elevation in the inferolateral leads with ST depression in lead aVR.
STE SECONDARY TO LEFT BUNDLE BRANCH BLOCK
LBBB typically causes marked ST changes (Figure 7), making it difficult to recognize STEMI when the LBBB pattern is present.
Figure 7.
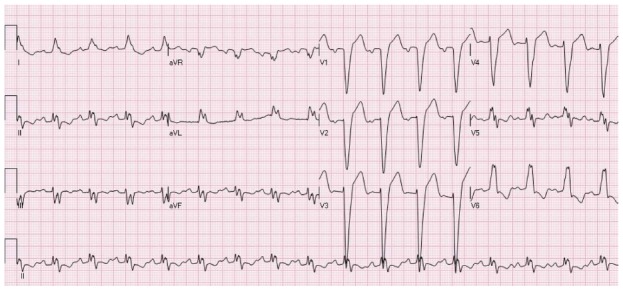
ST elevation secondary to left bundle branch block. The ST segment vector is directed opposite to the QRS vector. STE is present in the leads with negative QRS deflection (mainly leads V1-V3). There is typical ST depression in the leads with positive QRS deflection (the inferolateral leads).
New or presumably new LBBB was regarded in the past as an STEMI equivalent[13]. However, the majority of cases with LBBB at the time of presentation, are “not known to be old”, simply because an ECG prior to the index presentation is not available for comparison. Presumably new LBBB and even new LBBB at presentation occurs infrequently, is interfering with the analysis of the ECG, and according to the current STEMI guidelines are not considered diagnostic of acute myocardial infarction without the presence of typical clinical symptoms[3].
Only 1% to 9% of patients suspected of an acute myocardial infarction have LBBB (new or old) on their ECG[24]. Of the patients with LBBB on whom the STEMI protocol was initiated, 39% had a final diagnosis of true ACS, 36% had cardiac diagnoses other than ACS (hypertensive emergency, acute heart failure, atrial fibrillation, complete heart block, severe aortic stenosis, etc.) and 25% had non-cardiac chest pain[25,26].
LBBB pattern inherently has a masking feature that hides STEMIs. ST deviation typically is directed opposite to the direction of the QRS complex. Acute STEMI, on the other hand, typically presents with ST segment deviations that are concordant with the QRS complex deflections. As patients with LBBB typically show negative QRS complexes in leads V1- V3 (deep S waves), they typically have elevations of the ST segment in the precordial leads V1-V3. This pattern must not be confused with anterior STEMI.
Criteria for recognizing STEMI in patients with LBBB were published by Sgarbossa and colleagues: (1) STE more than 0.1 MV that is concordant with the vector of the QRS complex; (2) ST depression of more than 0.1 mV in lead V1, V2, or V3; and (3) STE of more than 0.5 mV that is directed opposite to the QRS direction[27,28]. Patients are given a point for each of the above criteria and can be stratified on the likelihood of having STEMI based on their Sgarbossa score.
These criteria have been validated in multiple studies[24]. These criteria are reported to have high specificity; however, their sensitivity for identifying acute myocardial infarction in patients presenting with LBBB is low[24,29]. In a recent meta-analysis, a three point Sgarbossa criteria score (≥ 0.1 mV of concordant STE or ≥ 0.1 mV ST depression in leads V1 to V3) had a sensitivity of 20% and specificity of 98%. If the third original criterion of discordant STE ≥ 0.5 mV in leads is added, the reported sensitivity is ranging between 20% and 79% and specificity between 61% and 100%[29].
Smith and colleagues[30] suggest replacing the third criteria of > 5mm absolute deviation in leads with discordant QRS complex with an ST/S ratio ≤ -0.25. Doing so increased the sensitivity from 67% to 91%, but the specificity remained unchanged at 90%. This modified Sgarbossa criteria needs to be validated with further studies[30].
The absolute magnitude of the deviation of the ST segments in patients with LBBB is influence by the degree of aberrancy and could change secondary to changes in the QRS axis, duration or heart rate. In addition, the absolute magnitude of deviation of the ST segment could change between different ECGs secondary to different electrode placement; this is often observed in the anterolateral precordial leads (V4-V6) in patients showing axis deviation to the left.
There is no data as to the thresholds of ST segment deviation in cases with incomplete LBBB (iLBBB; QRS duration of < 120 msec). Especially, it is unclear what are the cutoff values of “normal” STE in the precordial leads V1-V3 in cases with iLBBB.
STE SECONDARY TO OTHER INTRAVENTRICULAR CONDUCTION DELAYS
Patients with nonspecific intraventricular conduction delay may also diplay ST changes secondary to repolarization abnormalities (Figure 8). The pattern and magnitude of ST segment elevation or depression in such patients is highly variable, and the right diagnosis of STEMI can often be made only with comparison the index ECG and previous tracings or following changes over time in followup ECGs. Once more, the absolute magnitude of ST deviation can change as the degree of conduction delay changes (QRS width and axis) and may also depend on the heart rate.
Figure 8.
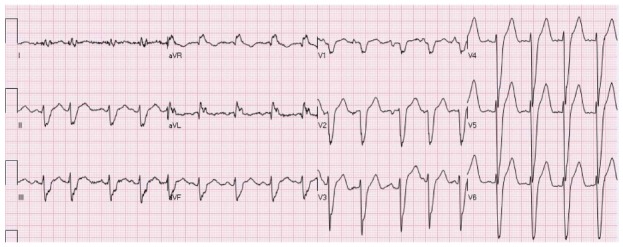
A patient with intraventricular conduction delay. There is mild elevation of the ST segment in the inferior leads and marked ST elevation in V2-V6. The patient is known to have non-ischemic dilated cardiomyopathy and this is his chronic electrocardiogram pattern.
Right bundle branch block (RBBB) is considered not to affect the interpretation of ST elevation or depression. Tachycardia, however, may cause depression of the ST segments in the right precordial leads (V1-V3) in patients with RBBB. These dynamic changes in the ST segments are often mistakenly diagnosed as true inferolateral (posterior) STEMI equivalent.
Pre-excitation (Wolf-Parkinson-White pattern) is occasionally associated with NISTE that are secondary repolarization alterations. The absolute magnitude of ST segment deviation is highly affected by the degree of pre-excitation.
BRUGADA SYNDROME
The Brugada pattern includes a pattern resembling RBBB with elevation of the ST segment in the precordial leads (V1-V2)[31,32]. The Brugada syndrome is linked to an increased risk of ventricular arrhythmia and sudden cardiac death. Type 1 Brugada pattern is defined by a coved elevation of the ST segment smore than 0.2 mV, associated T wave inversion in more than one of the right precordial leads (V1-V3. This pattern can be seen spontaneously or only after the administration of a sodium channel blocker. The diagnosis of Brugada syndrome depends on the presence of ECG Brugada pattern in a patient with documented history of ventricular fibrillation or polymorphic ventricular tachycardia, or a history of sudden cardiac death in family members that are younger than 45 years, comparable ECG configuration in relatives, unexplained syncope, ability to induce of ventricular tachycardia with programmed electrical stimulation, or agonal respiration at night time[33]. Type 2 Brugada pattern typically presents with a saddleback pattern of STE of more than 0.2 mV that attenuates in the middle and distal partof the ST segment with a positive or biphasic T waves in the precordial leads V1 to V3. Type 3 Brugada pattern shows a saddleback or coved pattern of elevation of the ST segment (less than 0.1 mV). Type 2 and 3 Brugada patterns should not be used to diagnose the Brugada syndrome and are not associated with increased risk of ventricular arrhythmia and sudden death. The ECG changes associated with the Brugada syndrome fluctuate with time, with diverse patterns and magnitude of elevation of the ST segments seen on different ECG tracings[33]. Figure 9 is an example of type 1 Brugada pattern.
Figure 9.
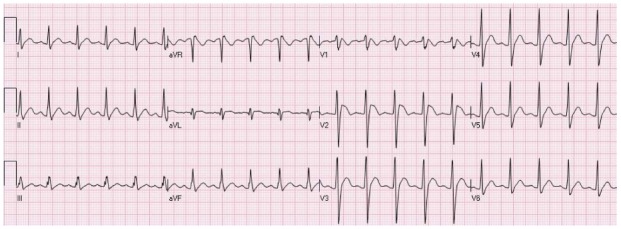
A patient showing a Brugada pattern with RsR’, elevation of the ST segment and negative T waves in leads V1-V2.
TAKOTSUBO SYNDROME (APICAL BALLOONING SYNDROME)
Takotsubo syndrome is seen mainly in females after menopause. Typically, the syndrome follows acute physiologic or emotional stress. The subjects frequently experience chest pain or dyspnea. The presenting ECG typically shows elevation of the ST segment in the majority (up to 81.6%) of the patients. STE is typically seen in the precordial leads. In addition, abnormalities of the T waves (64.3%) and Q waves (31.8%) can be detected. Takotsubo syndrome is typically associated with mild elevation of the cardiac markers (up to 86.2% of the patients)[34].
In the acute phase, the ECG of Takotsubo patients classically show marked depression of the ST segment in lead aVR, no or minimal elevation of the ST segment in lead V1 and concomitant diffuse STE in the other ECG leads (Figure 10)[35].
Figure 10.
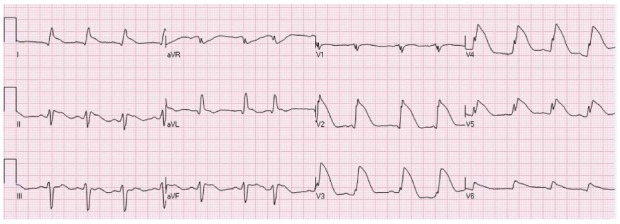
An elderly female patient with ST elevation secondary to Takotsubo. Angiography of the coronary arteries did not demonstrate significant coronary artery narrowings.
Frequently, the initial ECG pattern is mistaken for anterior STEMI (especially the type caused by distal occlusion of a wrapping long LAD that results in concomitant STE in both the inferior and precordial leads). Echocardiogram may show regional wall motion abnormalities that are confined to the apex. This pattern is not typical of the common type of anterior STEMI, but may be seen in patients with apical occlusion of a wrapping LAD. It was suggested that the ECGs of patients with Takotsubo have more marked depression of the ST segment in lead aVR in conjunction with less elevation of the ST segment in the precordial lead V1 relative to the ECGs of patients with typical acute anterior STEMI[36].
Because of the variations in presentation, Takotsubo’s may be confused for other STE causes as well. Since many patients present with PR depression, Takotsubo cardiomyopathy often resembles acute pericarditis on EKG[35].
Acute stroke (particularly subarachnoid hemorrhage)[37,38] and pheochromocytoma[39,40] occasionally present with ECG and regional wall motion dysfunction findings that are indistinguishable from Takotsubo cardiomyopathy.
SPONTANEOUSLY REPERFUSED STEMI
The current STEMI guidelines advocate that subjects presenting with symptoms suggestive of ACS within the 12 h before presentation who have elevation of the ST segment in 2 or more adjacent ECG leads should undergo reperfusion therapy as soon as possible[3]. However, a significant percentage of patients probably have (partial) decrease in the severity of symptoms by the time of arrival to the hospital, more often after receiving chewable aspirin on route to the hospital. In many patients with spontaneous reperfusion the ECG depicts (incomplete) decline in STE with concomitant inversion of the last part of the T waves, as shown in Figure 11. This entity is not recognized by the current guidelines and there are no recommendations whether coronary angiography and revascularization can be delayed in patients with clinical suspicion of “spontaneous reperfusion” if they present within 12 h of onset of symptoms and still have some degree of STE. On the other hand, urgent pPCI is not recommended to asymptomatic patients who are hemodynamically stable despite having STE, if they present > 12 h of onset of symptoms[3].
Figure 11.
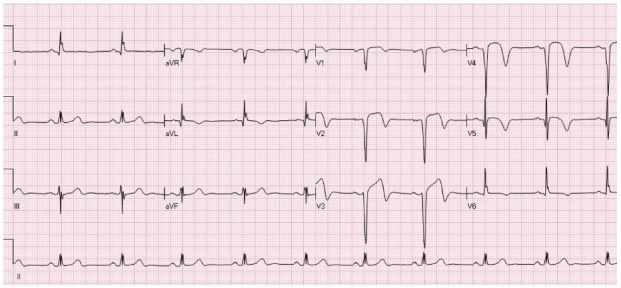
A patient with ST elevation associated with inversion of the terminal portion of the T waves in leads aVL, V1-V5 due to recent anterior ST elevation myocardial infarction. On presentation symptoms have already subsided.
LEFT VENTRICULAR ANEURYSM
Left ventricular aneurysm may result in persistent elevation of the ST segment after a previous MI. Freuqntly, the ECG may be very similar to that of acute STEMI. In fact, STE secondary to aneurysm may be the most frequently misinterpreted pattern in patients presenting to the emergency room with pain in their chest or dyspnea[41]. In Brady and colleagues’ study, where 11 hypothetical patients and accompanying EKGs were presented to 458 Emergency room physicians, left ventricular aneurysm was misdiagnosed 72% of the time, making it the most commonly misinterpreted STE pattern[42]. Diagnosis is extremely difficult when previous ECGs are unavailable for comparison. Typically, the ECGs of patients with left ventricular aneurysm depict abnormal Q waves in the ECG leads showing elevation of the ST segment. Figure 12 is an example of persistent STE due to aneurysm in a patient three months after acute MI.
Figure 12.
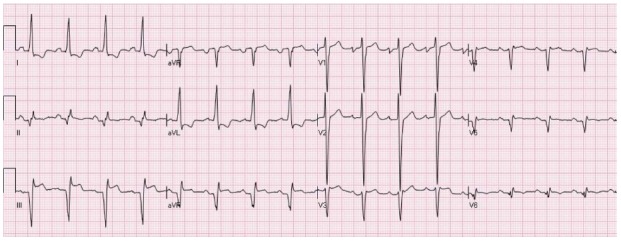
A patient with ST elevation secondary to aneurysm. There are Q waves in the inferior leads + V5-V6 and tall R waves in V1-V2, secondary to old inferolateral ST elevation myocardial infarctions. There is elevation of the ST segments in the inferior leads associated with reciprocal ST depression in leads I and aVL. The electrocardiogram (ECG) is compatible with both acute evolving inferior ST elevation myocardial infarctions or aneurysm. Comparison to previous ECG showed that this pattern is chronic and compatible with aneurysm.
MIXED PATTERNS
In a large number of patients the ECG may show more than one pattern of elevation of the ST segments that makes the precise distinction between NISTE and STEMI extremely hard. At times patients with preexisting benign pattern of NISTE may present with chest pain secondary to NSTEMI. This is termed “pseudo”-STEMI and frequently is mistaken for true STEMI. Some forms of NISTE could show wide variations in the magnitude and extent of STE (for example, the Brugada syndrome or early repolarization). These changes over time; however, differ from the typical pattern of ECG evolution after STEMI.
CONCLUSION
The physician receiving the patient with symptoms compatible with STEMI at the first encounter should make reperfusion decisions as soon as possible after interpreting the initial ECG[1,3]. This goal was set up because the benefits of reperfusion therapy decline rapidly as the duration of ischemia is prolonged. Rapid interpretation of the ECG is crucial to shorten door-to-balloon time[43]. In an attempt to shorten the time to reperfusion, new approaches are currently being tested[44].
One of the more successful strategies is pre-hospital activation of the coronary catheterization laboratory by emergency medical services. Systems have been established in which in addition to interpreting the transmitted ECG, the interpreting physician can directly communicate with the emergency medical system (EMS) team or even the patient via a mobile phone[44]. The pre-hospital activation strategy is associated with a door to balloon time reduction of 15.4 min[44]. In the systems used in the United States, on the other hand, the electrogradiographer does not have the advantage of a face-to-face history taking and physical examination of the patient. In addition, even if previous ECGs are stored in the (electronic) medical records, as a result of privacy issues, ECGs are transmitted without any identifier details, including names. Hence, the interpreter is not able to compare the transmitted ECG with preceding ECGs, even if they are readily available. Diercks and colleagues have shown an improvement in mortality for patients in whom pre-hospital activation system was used (6.7%), vs in patients without prehospital activation (9.5%)[45]. Although such approach might increase the sensitivity of detecting STE, the specificity and false activation remains a problem. The reported false-positive rate ranges from 5.6% to 25%[43,46-48]. These data suggest there is significant room for improvement.
To evaluate the capability of experienced experts in ECG reading to differentiate between STEMI to NISTE, 15 experienced ECG readers analyzed 116 ECGs showing elevation of the ST segments. The readers were asked whether the catheterization laboratory should be activated for possible STEMI if patients had symptoms suggestive of ACS[49]. In this set of ECGs, only 7% had adjudicated STEMI and 8 more patients had elevation of the heart muscle markers without clinical indication of STEMI. The number of cases for which acute reperfusion therapy was suggested by each of the ECG experts ranged between 7.8% to 33%. There were wide differences in sensitivity [50% to 100%, (average 75%)] and specificity [73% to 97%, (average 85%)] of the individual readers[49]. This study suggested that there is a need for refining the criteria for differentiating between NISTE and STEMI in different population setting and that the available criteria for diagnosing STEMI should be refined and standardized in order to maximize the accuracy of ECG interpretation.
Footnotes
Supported by John S Dunn Chair in Cardiology Research and Education
P- Reviewer: Vermeersch P S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 2.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. 1994;343:311–322. [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Canto JG, Shlipak MG, Rogers WJ, Malmgren JA, Frederick PD, Lambrew CT, Ornato JP, Barron HV, Kiefe CI. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA. 2000;283:3223–3229. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- 5.Deedwania PC, Carbajal EV. Silent myocardial ischemia. A clinical perspective. Arch Intern Med. 1991;151:2373–2382. [PubMed] [Google Scholar]
- 6.Kyker KA, Limacher MC. Gender differences in the presentation and symptoms of coronary artery disease. Curr Womens Health Rep. 2002;2:115–119. [PubMed] [Google Scholar]
- 7.Hiss RG, Lamb LE, Allen MF. Electrocardiographic findings in 67,375 asymptomatic subjects. X. Normal values. Am J Cardiol. 1960;6:200–231. doi: 10.1016/0002-9149(60)90047-3. [DOI] [PubMed] [Google Scholar]
- 8.Surawicz B, Parikh SR. Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J Am Coll Cardiol. 2002;40:1870–1876. doi: 10.1016/s0735-1097(02)02492-0. [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane PW. Age, sex, and the ST amplitude in health and disease. J Electrocardiol. 2001;34 Suppl:235–241. doi: 10.1054/jelc.2001.28906. [DOI] [PubMed] [Google Scholar]
- 10.Katibi I, Clark EN, Devine B, Lloyd SM, Macfarlane PW. Normal limits of the electrocardiogram in Nigerians. J Electrocardiol. 2013;46:289–295. doi: 10.1016/j.jelectrocard.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Larson DM, Menssen KM, Sharkey SW, Duval S, Schwartz RS, Harris J, Meland JT, Unger BT, Henry TD. “False-positive” cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA. 2007;298:2754–2760. doi: 10.1001/jama.298.23.2754. [DOI] [PubMed] [Google Scholar]
- 12.Rokos IC, French WJ, Mattu A, Nichol G, Farkouh ME, Reiffel J, Stone GW. Appropriate cardiac cath lab activation: optimizing electrocardiogram interpretation and clinical decision-making for acute ST-elevation myocardial infarction. Am Heart J. 2010;160:995–1003, 1003.e1-8. doi: 10.1016/j.ahj.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, et al. ACC/AHA Guidelines for the Management of Patients With ST-elevation Myocardial Infarction. J Am Coll Cardiol. 2004;44:E1–211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349:2128–2135. doi: 10.1056/NEJMra022580. [DOI] [PubMed] [Google Scholar]
- 15.Brady WJ, Syverud SA, Beagle C, Perron AD, Ullman EA, Holstege C, Riviello RJ, Ripley A, Ghaemmaghami CA. Electrocardiographic ST-segment elevation: the diagnosis of acute myocardial infarction by morphologic analysis of the ST segment. Acad Emerg Med. 2001;8:961–967. doi: 10.1111/j.1553-2712.2001.tb01094.x. [DOI] [PubMed] [Google Scholar]
- 16.Klatsky AL, Oehm R, Cooper RA, Udaltsova N, Armstrong MA. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med. 2003;115:171–177. doi: 10.1016/s0002-9343(03)00355-3. [DOI] [PubMed] [Google Scholar]
- 17.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 18.Macfarlane PW, Clark EN, Heng JS. J wave patterns--morphology, prevalence and nomenclature. J Electrocardiol. 2013;46:505–509. doi: 10.1016/j.jelectrocard.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Spodick DH. Hypothermia with J (Osborne) waves. Am Heart Hosp J. 2006;4:156. doi: 10.1111/j.1541-9215.2006.04916.x. [DOI] [PubMed] [Google Scholar]
- 20.Nishi SP, Barbagelata NA, Atar S, Birnbaum Y, Tuero E. Hypercalcemia-induced ST-segment elevation mimicking acute myocardial infarction. J Electrocardiol. 2006;39:298–300. doi: 10.1016/j.jelectrocard.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Nasir K, Bomma C, Tandri H, Roguin A, Dalal D, Prakasa K, Tichnell C, James C, Spevak PJ, Marcus F, et al. Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: a need to broaden diagnostic criteria. Circulation. 2004;110:1527–1534. doi: 10.1161/01.CIR.0000142293.60725.18. [DOI] [PubMed] [Google Scholar]
- 22.Estes EH, Jackson KP. The electrocardiogram in left ventricular hypertrophy: past and future. J Electrocardiol. 2009;42:589–592. doi: 10.1016/j.jelectrocard.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Punja M, Mark DG, McCoy JV, Javan R, Pines JM, Brady W. Electrocardiographic manifestations of cardiac infectious-inflammatory disorders. Am J Emerg Med. 2010;28:364–377. doi: 10.1016/j.ajem.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Cai Q, Mehta N, Sgarbossa EB, Pinski SL, Wagner GS, Califf RM, Barbagelata A. The left bundle-branch block puzzle in the 2013 ST-elevation myocardial infarction guideline: from falsely declaring emergency to denying reperfusion in a high-risk population. Are the Sgarbossa Criteria ready for prime time? Am Heart J. 2013;166:409–413. doi: 10.1016/j.ahj.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Jain S, Ting HT, Bell M, Bjerke CM, Lennon RJ, Gersh BJ, Rihal CS, Prasad A. Utility of left bundle branch block as a diagnostic criterion for acute myocardial infarction. Am J Cardiol. 2011;107:1111–1116. doi: 10.1016/j.amjcard.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Mehta N, Huang HD, Bandeali S, Wilson JM, Birnbaum Y. Prevalence of acute myocardial infarction in patients with presumably new left bundle-branch block. J Electrocardiol. 2012;45:361–367. doi: 10.1016/j.jelectrocard.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Sgarbossa EB. Value of the ECG in suspected acute myocardial infarction with left bundle branch block. J Electrocardiol. 2000;33 Suppl:87–92. doi: 10.1054/jelc.2000.20324. [DOI] [PubMed] [Google Scholar]
- 28.Sgarbossa EB, Pinski SL, Barbagelata A, Underwood DA, Gates KB, Topol EJ, Califf RM, Wagner GS. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996;334:481–487. doi: 10.1056/NEJM199602223340801. [DOI] [PubMed] [Google Scholar]
- 29.Tabas JA, Rodriguez RM, Seligman HK, Goldschlager NF. Electrocardiographic criteria for detecting acute myocardial infarction in patients with left bundle branch block: a meta-analysis. Ann Emerg Med. 2008;52:329–336.e1. doi: 10.1016/j.annemergmed.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012;60:766–776. doi: 10.1016/j.annemergmed.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 31.Antzelevitch C, Nof E. Brugada syndrome: recent advances and controversies. Curr Cardiol Rep. 2008;10:376–383. doi: 10.1007/s11886-008-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brugada P, Brugada R, Brugada J. The Brugada syndrome. Curr Cardiol Rep. 2000;2:507–514. doi: 10.1007/s11886-000-0035-0. [DOI] [PubMed] [Google Scholar]
- 33.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 34.Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–1529. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 35.Zhong-qun Z, Chong-quan W, Sclarovsky S, Nikus KC, Chao-rong H, Shan M. ST-segment deviation pattern of takotsubo cardiomyopathy similar to acute pericarditis: diffuse ST-segment elevation. J Electrocardiol. 2012;46:84–89. doi: 10.1016/j.jelectrocard.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Kosuge M, Ebina T, Hibi K, Morita S, Okuda J, Iwahashi N, Tsukahara K, Nakachi T, Kiyokuni M, Ishikawa T, et al. Simple and accurate electrocardiographic criteria to differentiate takotsubo cardiomyopathy from anterior acute myocardial infarction. J Am Coll Cardiol. 2010;55:2514–2516. doi: 10.1016/j.jacc.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 37.Rahimi AR, Katayama M, Mills J. Cerebral hemorrhage: precipitating event for a tako-tsubo-like cardiomyopathy? Clin Cardiol. 2008;31:275–280. doi: 10.1002/clc.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santana-Cabrera L, Rodríguez Escot C, Medina Gil JM, Pérez Ortiz C. Takotsubo cardiomyopathy associated with acute subarachnoid hemorrhage. J Emerg Med. 2012;42:586–587. doi: 10.1016/j.jemermed.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 39.Lassnig E, Weber T, Auer J, Nömeyer R, Eber B. Pheochromocytoma crisis presenting with shock and tako-tsubo-like cardiomyopathy. Int J Cardiol. 2009;134:e138–e140. doi: 10.1016/j.ijcard.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Takizawa M, Kobayakawa N, Uozumi H, Yonemura S, Kodama T, Fukusima K, Takeuchi H, Kaneko Y, Kaneko T, Fujita K, et al. A case of transient left ventricular ballooning with pheochromocytoma, supporting pathogenetic role of catecholamines in stress-induced cardiomyopathy or takotsubo cardiomyopathy. Int J Cardiol. 2007;114:e15–e17. doi: 10.1016/j.ijcard.2006.07.125. [DOI] [PubMed] [Google Scholar]
- 41.Engel J, Brady WJ, Mattu A, Perron AD. Electrocardiographic ST segment elevation: left ventricular aneurysm. Am J Emerg Med. 2002;20:238–242. doi: 10.1053/ajem.2002.32634. [DOI] [PubMed] [Google Scholar]
- 42.Brady WJ, Perron AD, Chan T. Electrocardiographic ST-segment elevation: correct identification of acute myocardial infarction (AMI) and non-AMI syndromes by emergency physicians. Acad Emerg Med. 2001;8:349–360. doi: 10.1111/j.1553-2712.2001.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 43.Camp-Rogers T, Kurz MC, Brady WJ. Hospital-based strategies contributing to percutaneous coronary intervention time reduction in the patient with ST-segment elevation myocardiaI infarction: a review of the “system-of-care” approach. Am J Emerg Med. 2012;30:491–498. doi: 10.1016/j.ajem.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, Magid DJ, et al. Strategies for reducing the door-to-balloon time in acute myocardial infarction. N Engl J Med. 2006;355:2308–2320. doi: 10.1056/NEJMsa063117. [DOI] [PubMed] [Google Scholar]
- 45.Diercks DB, Kontos MC, Chen AY, Pollack CV, Wiviott SD, Rumsfeld JS, Magid DJ, Gibler WB, Cannon CP, Peterson ED, et al. Utilization and impact of pre-hospital electrocardiograms for patients with acute ST-segment elevation myocardial infarction: data from the NCDR (National Cardiovascular Data Registry) ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry. J Am Coll Cardiol. 2009;53:161–166. doi: 10.1016/j.jacc.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 46.Adams GL, Campbell PT, Adams JM, Strauss DG, Wall K, Patterson J, Shuping KB, Maynard C, Young D, Corey C, et al. Effectiveness of prehospital wireless transmission of electrocardiograms to a cardiologist via hand-held device for patients with acute myocardial infarction (from the Timely Intervention in Myocardial Emergency, NorthEast Experience [TIME-NE]) Am J Cardiol. 2006;98:1160–1164. doi: 10.1016/j.amjcard.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 47.Campbell PT, Patterson J, Cromer D, Wall K, Adams GL, Albano A, Corey C, Fox P, Gardner J, Hawthorne B, et al. Prehospital triage of acute myocardial infarction: wireless transmission of electrocardiograms to the on-call cardiologist via a handheld computer. J Electrocardiol. 2005;38:300–309. doi: 10.1016/j.jelectrocard.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Sadeghi HM, Grines CL, Chandra HR, Mehran R, Fahy M, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Stuckey TD, et al. Magnitude and impact of treatment delays on weeknights and weekends in patients undergoing primary angioplasty for acute myocardial infarction (the cadillac trial) Am J Cardiol. 2004;94:637–640, A9. doi: 10.1016/j.amjcard.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 49.Jayroe JB, Spodick DH, Nikus K, Madias J, Fiol M, De Luna AB, Goldwasser D, Clemmensen P, Fu Y, Gorgels AP, et al. Differentiating ST elevation myocardial infarction and nonischemic causes of ST elevation by analyzing the presenting electrocardiogram. Am J Cardiol. 2009;103:301–306. doi: 10.1016/j.amjcard.2008.09.082. [DOI] [PubMed] [Google Scholar]


