Abstract
Substance abuse is a risk factor for HIV infection and progression to AIDS. Recent evidence establishes that cocaine use promotes brain perivascular macrophage infiltration and microglia activation. The lysosomal protease cathepsin B is increased in monocytes from patients with HIV dementia and its secretion induces 10-15% of neurotoxicity. Here we asked if cocaine potentiates cathepsin B secretion from HIV-infected monocyte-derived macrophages (MDM) and its effect in neuronal apoptosis. Samples of plasma, CSF, and post-mortem brain tissue from HIV positive patients that used cocaine were tested for cathepsin B and its inhibitors to determine the in vivo relevance of these findings. MDM were inoculated with HIV-1ADA, exposed to cocaine, and the levels of secreted and bioactive cathepsin B and its inhibitors were measured at different time-points. Cathepsin B expression (p<0.001) and activity (p<0.05) increased in supernatants from HIV-infected cocaine treated MDM compared with HIV-infected cocaine negative controls. Increased levels of cystatin B expression was also found in supernatants from HIV-cocaine treated MDM (p<0.05). A significant increase in 30% of apoptotic neurons was obtained that decreased to 5% with the specific cathepsin B inhibitor (CA-074) or with cathepsin B antibody. Cathepsin B was significantly increased in the plasma and post-mortem brain tissue of HIV/cocaine users over non-drug users. Our results demonstrated that cocaine potentiates cathepsin B secretion in HIV-infected MDM and increase neuronal apoptosis. These findings provide new evidence that cocaine synergize with HIV-1 infection in increasing cathepsin B secretion and neurotoxicity.
Keywords: Cocaine, MDM, Cathepsin B, Cystatin B, Cystatin C, post-mortem brain tissue
Introduction
Cocaine represents the second most popular illicit drug used in the United States (Hughes et al. 2011). Studies have demonstrated that cocaine use represents a risk factor for acquisition of HIV-infection independently associated with the progression to AIDS (Larrat and Zierler; Fiala et al. 1998; Webber et al. 1999). Cocaine triggers inflammation increasing acute phase proteins such as C-reactive protein (CRP) and serum amyloid A (SAA) (Samikkannu et al. 2013).
In vitro studies have demonstrated that cocaine increases HIV-1 replication in PBMCs (Peterson et al. 1991), CD4+T cells (Pandhare et al. 2014), macrophages (Dhillon et al. 2007; Gaskill et al. 2009; Gaskill et al. 2013) , microglia (Gekker et al. 2004; Gekker et al. 2006) and potentiates astrocyte toxicity after activation by HIV-1 gp120 (Yao et al. 2010). Proteomics analyses have revealed the enhancement of HIV-replication in normal human astrocytes after exposure to cocaine (Reynolds et al. 2006). The interaction of HIV-1 with cocaine has also been evaluated using an in vivo mouse model with severe combined immunodeficiency which revealed that cocaine enhances the HIV-replication (Roth et al. 2002; Griffin et al. 2007).
HIV associated neurocognitive disorders (HAND) still remain a common complication in viral infection despite the use of combined antiretroviral therapy (CART). Nowadays, it is widely accepted that neurodegeneration is one of the principal hallmarks of HAND that occurs without neuronal infection. Brain degeneration and consequently neuronal apoptosis is triggered by signaling of viral products such as Tat and gp120 (Kaul and Lipton 1999; Bansal et al. 2000; Gurwell et al. 2001; Kaul et al. 2001; Hisaka et al. 2004; Thomas et al. 2009; Merino et al. 2011; Zhang et al. 2011), cytokines and chemokines (Lee et al. 2011; Vazquez-Valls et al. 2011; Yan et al. 2011; Johansson et al. 2013; Lombardelli et al. 2013), or by toxins produced from infected macrophages including lysosomal protease cathepsin B(Ciborowski and Gendelman 2006; Tian et al. 2008; Turchan-Cholewo et al. 2009; Sun et al. 2010; Luo et al. 2010; Rodriguez-Franco et al. 2012; Tovar-Y-Romo et al. 2013; Malla et al. 2014). Furthermore, studies demonstrated that cocaine can amplify the immune response and cause neuroinflammation (Clark et al. 2013) via dysfunction of the BBB (Dhillon et al. 2008; Gandhi et al. 2010) through alterations of tight junction proteins (Dhillon et al. 2007), increased glial activation, and induction of neuroinflammatory pathways (Yao et al. 2011; Kousik et al. 2012).
Recently, we reported that HIV-1 infection induces cathepsin B in plasma of HIV infected patients (Cantres-Rosario et al. 2013) and that cathepsin B secreted from HIV-1 infected MDM contributes to neuronal apoptosis (Rodriguez-Franco et al. 2012), but, the effects of cocaine in cathepsin B secretion were unknown. In this work, we hypothesized that cocaine potentiates the detrimental effects of HIV-1 in MDM and promotes an increase of cathepsin B secretion and neuronal apoptosis. To understand the effects of cocaine in HIV-1 infected MDM, primary macrophages were isolated from healthy donors, infected with HIV-1ADA, and treated in presence or absence of cocaine for over 12 days. Results show an increased cathepsin B secretion and activity from HIV-infected and cocaine-treated MDM supernatants. Increased levels of cathepsin B inhibitor, cystatin B were also observed. To determine the detrimental effects of cocaine exposed MDM in neurons, supernatants from HIV-infected cocaine-treated MDM following serum-free media exchange for 24 hr were added to cultured neurons and apoptosis was measured. Increased levels of apoptosis were observed in neurons treated with HIV-infected cocaine treated MDM supernatants compared to supernatants from HIV-infected cocaine negative MDM controls. Apoptosis was reversed using a cathepsin B specific inhibitor and with a cathepsin B antibody.
To determine the clinical relevance of these findings, plasma samples from HIV-seropositive Hispanic women that were cocaine users were compared to non-drug users. Results revealed an increased cathepsin B concentration and activity in plasma but not in the CSF of HIV-1 seropositive women that were cocaine users. The cathepsin B natural inhibitor, cystatin B also increased in the plasma of these women but not in the CSF. These data suggest that cocaine enhances HIV-1 infection in MDM increasing cathepsin B secretion and activity that causes detrimental effects in neuronal function by triggering apoptosis.
Materials and Methods
Human Subjects
Plasma and CSF samples were obtained from a repository of samples of the Hispanic Women cohort followed at the Specialized NeuroAIDS Research Program of the University of Puerto Rico Medical Sciences Campus from 2001-2009 (Wojna et al. 2006; Cantres-Rosario et al. 2013) following approval by the Institutional Human Subject Review Board (IRB #0720109, 0720102). Patient demographics were as follows: HIV-1 infected women with combined antiretroviral therapy (cART), ages 26-48 years, and nondrug users defined by a urine toxicology screen (n=6). Samples that were positive for cocaine (n=6) by urine toxicology were stored and selected for this study following consent of the subjects in the original studies. Four HIV seronegative donors were recruited for MDM isolation for HIV-infection studies.
Isolation and Culture of Primary Macrophages
Primary Human macrophages were isolated from peripheral blood mononuclear cells (PBMC) of healthy donors. Blood was collected in ACD tubes and PBMC isolated using Ficoll density gradient separation. Adherent monocytes were cultured in T-25 flasks at a concentration of 1.5 × 106 cells/ml and grown in RPMI supplemented with 20% heat-inactivated fetal bovine serum (FBS), 10% heat-inactivated human serum and 1% Pen/Strep (all from Sigma, St. Louis, MO). Half of the medium was changed every 3 days for all cultures and incubated at 37°C, 5% CO2. Seven days later, adherent cells were differentiated as >90% MDM (Rodriguez-Franco et al, 2011).
HIV-1 infection and cocaine exposure of Monocyte Derived Macrophages
After 7 days in culture, MDM were inoculated with HIV-1ADA (University of Nebraska Medical Center) at a multiplicity of infection (MOI) of 0.1 or with serum free media (uninfected controls) (Ghorpade et al. 1998; Rodriguez-Franco et al. 2012). After overnight incubation, unbound virus was removed using RPMI and fresh serum containing medium was added. HIV-1 infected and uninfected MDM were cultured in the presence/absence of cocaine for 12days. Cocaine was removed on day 12 post-infection, and replaced with serum free cocaine-free media that was added to MDM for 24 hrs (macrophage conditioned media or MCM). The concentration of cocaine was selected after titration with 10μM, and 100μM, following determination of cell viability by MTT assay. The range of cocaine concentration tested were based on previous studies reported in the literature (Peterson et al. 1991; Gekker et al. 2006; Dhillon et al. 2007; Yang et al. 2010; Mantri et al. 2012).Cocaine (10 μM) was added fresh with every change of cell media. Morphology of macrophages remained intact at 10 uM of cocaine with a viability of 85% but appeared to be disrupted at higher concentrations of cocaine (100uM), decreasing the viability to 79%. Therefore we selected 10uM of cocaine for the study. MDM culture supernatants were removed at 3, 6 and 12 days post-infection (dpi), centrifuged and stored at −80°C (Rodriguez-Franco et al. 2012) for HIV p24 antigen ELISA according to the manufacturer’s instructions (Express BioTech, Maryland, USA). Following media removal at 3,6, and 12 dpi, cells were washed and serum free media added for 24 hrs for neurotoxicity assays. This media is known as MDM conditioned medium (MCM).
Human neuroblastoma SK-N-SH cell cultures
SK-N-SH (HTB-11) cells (human neuroblastoma cell line, ATCC), were grown and plated in 4-well chamber slides (Fisher Scientific, Suwannee, GA) at a density of 2 ×105 cells per well and cultured in Eagle’s MEM (EMEM) supplemented with 10% fetal bovine serum (FBS), 1% sodium pyruvate and 1% non-essential amino acids (all from Sigma) and incubated for 3-5 days at 37°C, 5% CO2 until 75-80% confluence.
Expression of cathepsin B in plasma, cerebrospinal fluid (CSF) and MDM supernatants
Sandwich ELISA (R&D Systems, Minnesota, USA) was used for the quantitative determination of human cathepsin B concentrations from cell lysates and culture supernatants, as well as patient plasma and CSF following to the manufacturer’s instructions. Briefly, 100μ L of MDM supernatants (1:100 dilution), plasma (1:20 dilution), and CSF (1:10 dilution) were incubated for 2 hours in a plate pre-coated with anti-human cathepsin B. After incubation, the plate was washed and incubated with 200μ L of conjugate for 2 hours, washed, 200μ L of substrate added, and incubated for 30min in the dark. Samples from 6 different donors were assayed in duplicates and read at 450nm in a Dinex MRX Revelation Microplate Reader (Chantilly, VA).
Expression of cystatin B and cystatin C in plasma, cerebrospinal fluid (CSF) and MDM supernatants
Cystatin B expression levels were measured with an ELISA (USCN Life Science Inc., Wuhan, China) according to the manufacturer’s instructions with a final dilution of 1:10 for plasma, 1:20 for CSF, and 1:100 for MDM supernatants. Briefly, 100μ L of the diluted samples were incubated in a human anti-cystatin B pre coated plate for 2 hours at 37°C. After incubation, 100μ L of detection reagent A and detection reagent B for 30 min were added separately. Finally, 90μ L of substrate solution were added and incubated for 30 min in the dark. Cystatin C ELISA (BioVendor, Candler, North Carolina, USA) was performed following the manufacturer’s instructions using CSF at 1:600, plasma and MDM supernatants at 1:400 dilution. Samples from 6 different donors were assayed in duplicates and read at 450nm in a Dinex MRX Revelation Microplate Reader (Chantilly, VA).
Intracellular expression of cathepsin B and cystatins B and C by Western blots
Twenty micrograms of total protein from cell lystates were lyophilized at 4°C and rehydrated in 10 μl of sample buffer (4.75μL of Laemmli sample buffer, 0.25 μL of β-mercaptoethanol [BioRad] and 5.00 μl of ultrapure water (HPLC), and heated at 70°C for 10 minutes. Samples were loaded into 4%-20% Tris-HCL 15-well Ready Gels (BioRad), together with a 10 μl of molecular weight marker Invitrogen See Blue Ladder and positive controls for cystatin B, cystatin C and cathepsin B (isolated human protein from liver, Bio Vision, New Minas, N Nova Scotia, Canada). Proteins were separated at 150V for one hour according to the NuPAGE® Protocol (Life Technologies, Grand Island, NY ). After electrophoresis, proteins were transferred to a PVDF membrane using the semi-dry transfer method on a BioRad Trans Blot Turbo Transfer System for 7 minutes at 1.3 A-25V per gel. After transfer, membranes were blocked with non-fat dry milk in 1X T-TBS for 1 hour at room temperature with shaking. Membranes were incubated overnight at 4 °C with 1° antibody; these are mouse anti-human cathepsin B (1:500), mouse anti-human cystatin B (1:2000) and rabbit GAPDH (1:500) followed by secondary anti-mouse Ig G-HRP (Sigma) and by secondary anti-rabbit Ig G-HRP (Sigma) or anti-mouse IgG-HRP (Sigma). All incubations with primary antibodies were conducted overnight at 4°C; all incubations with secondary antibodies were conducted for 1 hr at room temperature. Following incubations with primary and secondary antibodies, membranes were washed with 1X T-TBS at room temperature, for five times at 5-10 minutes each time. Chemiluminescence (Super Signal West Femto Detection Kit; Pierce, Rockford, IL) was used for signal detection at BioRad Chemi Doc XR. The density of protein bands was determined using the Image Lab software (BioRad) and normalized against monoclonal rabbit anti-GAPDH (Sigma).
Cathepsin B Activity Assay
The cathepsin B activity was determined using the cathepsin B Activity Assay Kit (Bio Vision, California, USA). This is a fluorescence-based assay that utilizes the preferred cathepsin-B substrate sequence RR labeled with AFC (amino-4-trifluoromethyl coumarin). Cell lysates or other samples that contain cathepsin-B will cleave the synthetic substrate RR-AFC to release free AFC. The released AFC can easily be quantified using a fluorescence plate reader. MDM supernatants collected from 3, 6, and 12dpi were assayed in duplicates following the manufacturer’s instructions. The signal was quantified using a fluorescence plate reader (Varioskan Flash; Thermo Fisher Scientific) with excitation at 400 nm and emission at 505 nm.
Cathepsin B Neurotoxicity Assay
Cathepsin B neurotoxic potential was determined according to Rodriguez-Franco et. al 2012. Briefly, confluent SK-N-SH neurons were washed with Phosphate Buffered Saline (PBS) and incubated with MDM conditioned medium (MCM). The MCM consist of fresh serum-free supernatants from HIV-infected, uninfected, uninfected cocaine-treated and lastly HIV-infected cocaine-treated MDM from four different donors and diluted 1:4 with plain EMEM. The MCM was added with or without the specific cathepsin B inhibitor (CA-074) at 50μM. In addition to CA-074, a mouse anti-human monoclonal cathepsin B antibody (Sigma) was used at 1:500, which binds 50X of secreted cathepsin B and inhibits the enzyme. For apoptosis determination with TUNEL assay, SK-N-SK were grown in 4-well chamber slides at a density of 2×105 cells per well and incubated at 37°C, 5% CO2 until 75-80% confluence. MCM was added to confluent neurons and incubated at 37°C, 5% CO2 for 24 hours. The next day, neurons were washed with PBS and fixed with 4% paraformaldehyde (PFA) for 1 hour. To quench auto-fluorescence, fixed neurons were incubated with 3% hydrogen peroxide with methanol for 10 minutes and permeabilized on ice with 0.1% TritonX-100 in 1.0% sodium citrate for 10 minutes. The in situ cell death detection Kit; also named as TUNEL (TdT-mediated dUTP-X nick end labeling) (ROCHE®) was performed by incubating neurons with TUNEL reaction mix for 1 hour at 37°C in a humidity chamber in the dark. Cells were washed 3 times with PBS and covered with anti-fade mounting media (Vectashield®). The positive control consists of cells incubated with recombinant DNase I (30U/Ml) for 10 minutes at room temperature to induce DNA breaks. The negative control consisted of cells incubated with the label solution without the enzyme. Confocal microscopy was performed using a Zeiss confocal microscope Anxiovert 200M with LSM 510 under an excitation wavelength of 488nm, 20× magnification. Analysis of percentage of apoptosis was determined using the Image J program software (National Institutes of Health).
Immunohistochemistry of Frozen Human Post-Mortem Brain Tissues
Brain tissue samples of basal ganglia in paraffin embedded slides were obtained from the National NeuroAIDS Tissue Consortium (NNTC). Serial sections of formalin-fixed, paraffin-embedded frontal cortex tissues from HIVE patients or normal controls were placed on electromagnetically charged glass slides. Sections were deparaffinized in xylene and rehydrated through descending grades of ethanol and water. After non-enzymatic antigen retrieval in 0.01 M sodium citrate buffer (pH 6.0) for 30 min at 97°C in a vacuum oven, slides were washed with 1 X PBS and placed in blocking solution (5% normal goat serum; Vector Laboratories) for 2 h. Primary antibodies utilized included IBA (1: 100; Wako, Richmond, VA), cathepsin-B (1: 100; Sigma), and cystatin B (1:100 Sigma). Sections were incubated with primary antibody overnight in a dark, humidified chamber at room temperature, rinsed 3X with PBS, and incubated with fluorescein isothiocyanate (FITC) (1: 200; Vector Laboratories) or Rhodamine Red (1: 200; Vector Laboratories)-conjugated secondary antibodies for 1 h at room temperature in the dark. Sections were again washed 3 X with PBS, cover-slipped with an aqueous based mounting medium containing DAPI for nuclear labeling (Vectashield; Vector Laboratories), visualized with a Nikon Eclipse E400, camera SPOT Insight QE and Fluorescence X-Cite Series 120.
Statistical Analysis
Data from each experiment were tested for normal distribution using the Shapiro-Wilk test of normality. For those data that were normally distributed, descriptive statistics included mean and standard deviation. Differences between, HIV-negatives cocaine-treated/untreated and HIV-positives cocaine-treated/untreated samples over days of infection were assessed using analysis of variance (ANOVA). For those data that were not normally distributed, descriptive statistics included median and interquartile range (25th and 75th percentile of the distribution). Differences between groups and over days of infection were assessed using median one-way analysis of variance one way ANOVA. Statistical significance was considered at p< 0.05 for all comparisons. All statistics were performed using Graph pad Prism (version 5.0, La Jolla, CA;USA).
Results
Patients
This study analyzed stored samples of plasma and CSF from 6 HIV-seropositive patients (controls) and 6 HIV-seropositive positive for cocaine from our Hispanic women cohort. Most of the patients (>80%), including the cocaine users were on combined antiretroviral therapy (cART). The mean of CNS penetration index (CPE) was also calculated; 8.2 for HIV-infected patients and 5.7 for HIV-infected cocaine-users. No statistical significance in viral load in plasma and CSF was found between HIV-infected and HIV-infected cocaine-users (Table 1). No differences were found for the CD4 count levels between cocaine and non-cocaine users (Supplementary figure 1). Significant differences in the CSF viral load exist between plasma and CSF compartments from both groups of patients (p<0.001).
Table 1.
Demographics and viral-immune status of Hispanic women cohort with cART
| Viral Load (Log10
copies/mL) |
Treatment | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean Age/ Years |
CD4 cells/mm3 |
Plasma | CSF | cART1 | CPE index2 | |
| HIV- Cocaine negative |
6 | 38.2 [7.5] |
563.5 [408.8] |
2.4 [0.7] |
1.9 [0.4] |
100% | 8.2 [1.3] |
| HIV + Cocaine positive |
6 | 39.0 [4.2] |
303.3 [196.3] |
2.6 [0.8] |
1.6 [0.1] |
83.3% | 5.7 [2.8] |
Values represent mean + [standard deviation].
cART: combined antiretroviral therapy.
CPE Antiretroviral therapy CNS penetration effectiveness (CPE) is the degree of antiretroviral penetration across the blood-brain barrier to the CNS reaching therapeutic drug concentrations in cerebrospinal fluid. This depends on the characteristics of the antiretrovirals (molecular weight, lipophilicity, protein binding) and on their capacity to be substrate for efflux transporters. No significant differences were found in all of the above parameters between HIV-1 infected cocaine users and non-drug users. Significant differences existed in the viral load between plasma and CSF compartments (p<0.001).
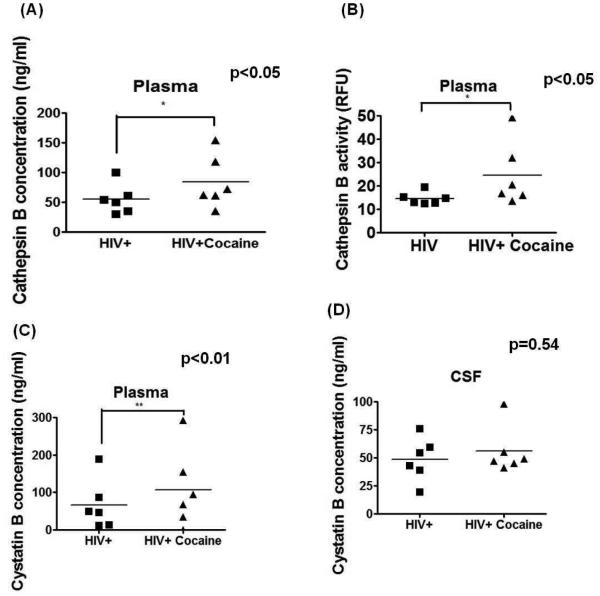
Plasma cathepsin B secretion and activity is increased in HIV-seropositive cocaine-users It has been reported that cathepsin B increases in plasma of HIV-seropositive women as compared to HIV seronegative controls (Cantres-Rosario et al. 2013). We next asked if cocaine use affected cathepsin B is expression and activity. Our results show a significantly increased (p<0.05) cathepsin B concentration and activity in the plasma of HIV-seropositive women cocaine-users compared with HIV-seropositive women (Figures 1A and B). No difference of cathepsin B secretion and activity was observed in the CSF between both groups (Data not shown).
Figure 1. Increased cathepsin B and cystatin B in plasma samples of HIV-infected cocaine-abuse patients.
Cathepsin B levels and activity were measured in plasma and CSF of HIV-seropositive patients and HIV-seropositive patients that are cocaine abusers. There was a significant increase (p<0.05) of cathepsin B expression (A) and activity (B) in plasma of HIV-seropositive women cocaine-users compared with non-cocaine users. No significant differences in cathepsin B levels and activity were observed in the CSF of these women patients (Data not shown). Cystatin B and Cystatin C levels were measured in the plasma and CSF of HIV-seropositive women (n=6) and HIV-seropositive women cocaine-users (n=6) Increased levels of cystatin B were observed in plasma of HIV-seropositive women that are cocaine abusers (C) (p<0.01). No differences in cystatin C were observed neither in plasma (D) samples. No differences in cystatins B or C were found in CSF samples (Data not shown).
Cystatin B expression is increased in plasma of HIV-seropositive cocaine-users
Under normal conditions, cathepsin B is highly regulated by its cysteine protease inhibitors cystatin B and cystatin C in the lysosomes (Brix et al. 2008). Our group has previously reported increased cathepsin B and its inhibitor, cystatin B in the plasma of HIV-1 seropositive women compared to uninfected controls demonstrating a dysregulation of cathepsin B induced by HIV-1 infection (Cantres-Rosario et al. 2013). To determine if cocaine use affect the expression of cystatin B and C in plasma of HIV-infected women cocaine-users an ELISA was performed for both proteins. Our results show an increase in plasma cystatin B of HIV-infected cocaine users women compared with HIV-seropositive women that do not use cocaine (Figure 1C). Differences in cystatin B in the CSF were not observed (Figure 1D). Median cystatin C concentration in plasma and CSF was 750 ng/mL. No significant differences in cystatin C were found in plasma or CSF between HIV seropositive women cocaine users and non-drug users (Supplementary figure 2). These data suggest that cocaine and HIV-1 increase the levels of cathepsin B and its inhibitor cystatin B in the plasma of HIV-seropositive women.
Cocaine potentiates cathepsin B secretion and activity in HIV-infected MDM
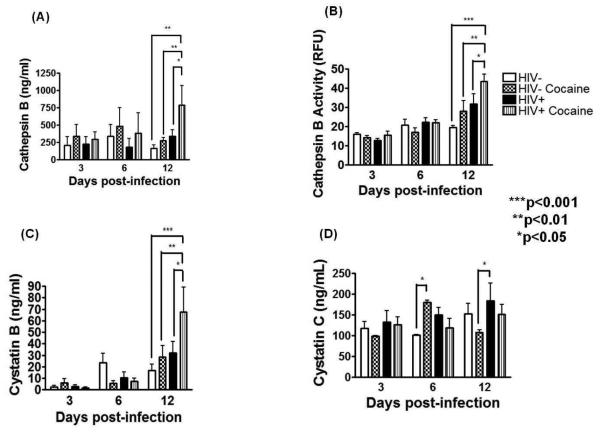
To determine the effect of cocaine in macrophage-derived cathepsin B and its inhibitors, MDM were infected with HIV-1ADA and followed over time in culture. A productive HIV-1 infection was obtained in the three experiments that increased even further with cocaine use at 12 days post-infection (Supplementary figure 3).
Cathepsin B was measured in the supernatants of HIV-infected cocaine exposed MDM as compared to HIV positives and HIV negative cultures. Results show a significant increased (p<0.001) cathepsin B secretion in HIV-infected cultures over uninfected controls (Figure 2A). The levels of cathepsin B in the HIV-infected cocaine-treated MDM increased significantly over the HIV-infected cocaine negative cultures (p<0.001). Cathepsin B activity also increased in HIV positive cocaine exposed MDM compared to HIV-infected cocaine negative cultures (p<0.05) (Figure 2B). These data suggest that cocaine enhances HIV to potentiate the secretion of cathepsin B in MDM. Cocaine also induced the expression of the cathepsin B inhibitor cystatin B (Figure 2C) but not cystatin C in MDM (Figure 2D). Levels of intracellular cathepsin B and its inhibitors, cystatin B and cystatin C were also measured by Western blots and compared to GAPDH loading control by densitometry. No significant differences were found between HIV-infected cocaine treated and cocaine negative controls for cathepsin B, cystatin B, and cystatin C expression (Supplementary Figure 3).
Figure 2. Increased cathepsin B and cystatin B in HIV-infected cocaine treated MDM.
Supernatants of uninfected, uninfected cocaine-treated, HIV-infected and HIV-infected cocaine-treated were collected at 3, 6, and 12dpi to measure the levels of cathepsin B extracellular expression by ELISA. A significantly increased cathepsin B expression (p<0.001) (A) and activity (p<0.05) (B) was found in HIV-infected cocaine-treated supernatants compared with MDM supernatants from HIV positive, cocaine negative cultures. The levels of cathepsin B inhibitors; cystatin B and cystatin C in MDM supernatants at 3, 6, and 12dpi were determined by ELISA. Significantly increased levels of cystatin B were observed in supernatant of MDM HIV-infected cocaine treated compared with HIV-infected without the drug (p<0.05), HIV-uninfected cocaine-treated (p<0.01) or HIV-uninfected cultures (p<0.001) (C). Results are representative of three different experiments. No differences were found in cystatin C expression between the two groups (D).
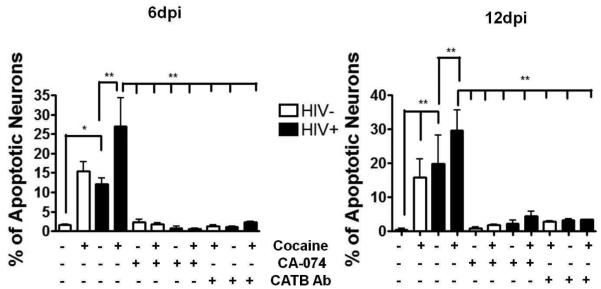
Cocaine potentiates the neurotoxic effect of cathepsin B in HIV-infected MDM
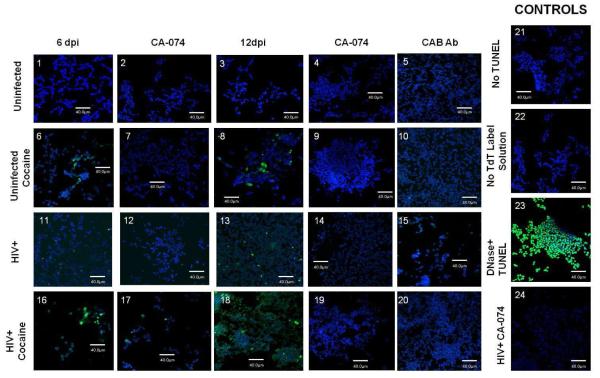
We next asked if cocaine enhances with HIV in the induction of neurotoxic potential by MDM derived cathepsin B. To test this hypothesis, differentiated SK-N-SH human neuroblastoma cells were cultured until confluence followed by addition of optimal concentration of macrophage condition media (MCM) (1:4 dilution) from cocaine exposed HIV-infected MDM and controls (Rodriguez-Franco et al. 2012). Neuronal apoptosis was determined using TUNEL labeling and the images were analyzed by confocal microscopy (Figure 3A). Results of TUNEL labeling indicate increased apoptosis after addition of MCM from HIV infected MDM as demonstrated previously with HIV-infection (Rodriguez-Franco et al, 2012). Some apoptosis was also observed after addition of MCM from cocaine exposed MDM. Apoptosis was enhanced in neurons that were incubated with MCM from cocaine exposed HIV infected MDM (Figure 3A; boxes 6 and 8) over cocaine negative HIV-1 infected cells (Figure 3A; boxes 11 and 12). The combination of HIV-1 infection and cocaine exposure of MDM resulted in enhancement of neuronal apoptosis by MCM at 6 and 12dpi (Figure 3A; boxes 16 and 18) when compared to MCM from HIV-1infected MCM, or cocaine exposed MDM. Apoptosis was reduced with cathepsin B inhibitor and antibody against cathepsin B (Figure 3A;boxes 19 and 20).
Figure 3. Cocaine potentiates cathepsin B neurotoxicity in HIV-infected MDM.
Neuronal apoptosis was measured using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay adding serum-free MDM supernatant at 6 and 12 days post-infection as shown by green fluorescence in neurons (apoptosis) and the nucleus stained with DAPI (blue). The results are representative of four experiments (Panel A). The SK-N-SH were exposed to MCM from uninfected (1and 3), uninfected cocaine-treated (6 and 8), HIV-infected (11 and 13) and HIV-infected cocaine-treated (16 and 18) with the cathepsin B inhibitor ( 2, 7, 12, and 17) and cathepsin B antibody at 1:500 dilution (5, 10, 15, and 20) do not show apoptosis. Controls are shown in panels 21-24. Neuronal apoptosis increased after the exposure of either HIV infected MCM or cocaine-treated MCM (panels 13 and 18 respectively) in later points of infection. Quantitative analysis of staining ratio between apoptotic (green)/non-apoptotic (blue) cells were calculated using Image-based Tool for Counting Nuclei (ITCN) from Image J software (NIH) (Panel B). Results revealed a significant increased percentage of apoptosis in neurons treated with HIV-infected supernatant compared with HIV-infected cocaine-treated at 6 and 12 days post-infection (p<0.01). A significant decrease in neuronal apoptosis was observed in neurons pre-treated with cathepsin B inhibitor and cathepsin B antibody at 6 and 12 dpi.(p<0.001) .
Quantification of neuronal apoptosis at 6dpi was determined using Image J software (Figure 3B). Our results show a significant increase in percentage of apoptotic neurons from HIV-1 infected cocaine exposed MCM (30%) at day 6 and 12 dpi compared with HIV-infected MCM cocaine negative controls (15% apoptosis) and to neurons treated with uninfected MCM (1-3% apoptosis) (Figure 3B). Moreover, significant reduction (p<0.001) in the percentage of neuronal apoptosis upon pre-treatment with cathepsin B inhibitor (CA-074) (Figure 3B, panels 4, 9, 14, and 19) or cathepsin B antibody (Figure 3B, panels 5, 10, 15, and 20) indicates that cathepsin B is inducing neuronal apoptosis. These results are representative of four different experiments.
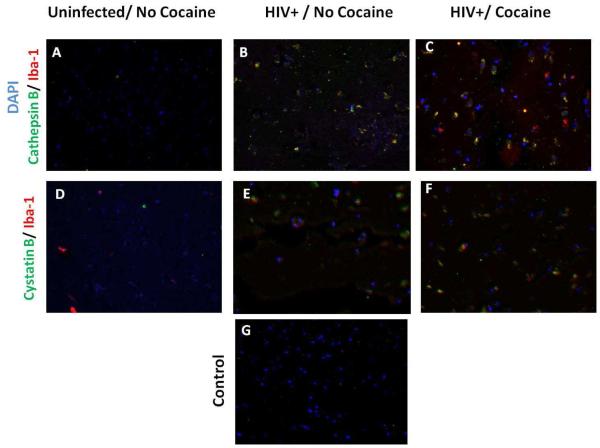
Increased cathepsin B in HIV/Cocaine post-mortem brains
We performed an analysis of cathepsin B, cystatin B expression in macrophages from the basal ganglia derived from post-mortem brain tissue of three uninfected cocaine negative, three HIV-infected cocaine negative, and three HIV-infected cocaine positive patients (Table 2). Results show that the levels of cathepsin B are undetectable in the basal ganglia of the uninfected cocaine negative group (Figure 4A). The same results were observed for cystatin B in the same group (Figure 4D). In contrast, increased levels of cathepsin B and cystatin B were seen in the basal ganglia of an HIV-1 positive individual with no cocaine history (Figure 4B and 4E). Double-staining of tissue with an antibody to the macrophage/microglia marker Iba-1 shows a significant overlap with cathepsin B and cystatin B staining in the HIV+ cocaine positive cases (Figure 4C and 4F), suggesting that cocaine induces expression of both proteins, cathepsin B and cystatin B in macrophages from basal ganglia of brain tissue derived from cocaine users. Few Iba1 were consistently observed in the post-mortem tissue from HIV seronegative non-drug users. This could be attributed to lack of macrophages/microglia activation in HIV-seronegatives as compared to HIV-infected patients. Iba1 has the actin-bundling activity and participates in membrane ruffling, phagocytosis and is upregulated during the activation of these cells (Ohsawa et al. 2004). In addition HIV-1 promotes inflammation and disruption of blood-brain barrier that results in increased migration of Iba-1 positive macrophages. This does not occur in healthy HIV-1 seronegative controls.
Table 2.
Post-mortem paraffin embedded brain tissues received from the National NeuroAIDS Tissue Consortium.
| Case ID1 | Age2 | CD43 | CD84 | Plasma VL5 | CSF VL6 | HIV-infected7 | Cocaine8 | Neurocognitive function9 |
|---|---|---|---|---|---|---|---|---|
| UCLA1113 | 68 | 427 | 1095 | 40 | 40 | Yes | Positive | 0 |
| UCLA5007 | 37 | 91 | 1155 | 750000 | N/A | Yes | Positive | 2 |
| UCLA2005 | 43 | 66 | 508 | 750000 | 249 | Yes | Positive | 1 |
| UCLA2012 | 48 | 273 | 640 | 400 | 50 | Yes | Positive | 0 |
| UCLA4129 | 59 | 61 | N/A | 40 | N/A | Yes | Negative | 4 |
| UCLA4148 | 52 | 160 | N/A | 700 | N/A | Yes | Negative | 2 |
| UCLA1008 | 54 | 491 | 379 | 207 | 50 | Yes | Negative | 4 |
| UCLA3002 | 65 | N/A | N/A | N/A | N/A | No | Unknown | 0 |
| UCLA3030 | 59 | 1191 | 190 | 400 | N/A | No | Unknown | 0 |
| UCLA3039 | 70 | N/A | N/A | N/A | N/A | No | Unknown | 0 |
Brain tissue of MHBB patients 10 and 20 micron thick sections slides of deep frontal basal ganglia. Not data available are identified as N/A. Patients identification (1) were stratified by age (2) and different immune and viral parameters such as CD4 and CDS lymphocyte cell count (3 and 4 respectively), plasma and CSF viral load in copies per mL (5 and 6). Samples were tested also for HIV-infection (7) and cocaine toxicology (8). Neurocognitive function (9) was classified by NNTC in a scale of 0-9:
0= Neurocognitive normal
= Neuropsychological impairment
= Possible MCMD
= Probable MCMD
= Possible HAD
= Probable HAD
= Probable CMVE
= Neuropsychological impairment or dementia
= Neuropsychological impairment, cause uncertain
= Test not done
Figure 4. Expression of cathepsin B and cystatin B in Basal Ganglia of Post-Mortem brain tissues.
Basal ganglia tissue from HIV-seronegative (A, and D), HIV seropositive cocaine negative (B and E), and HIV-seropositive cocaine positive patients were stained with mouse anti-human cathepsin B followed by Alexa 488 conjugate goat anti-mouse (green). For cystatin B staining, mouse anti-human cystatin B followed by Alexa 488 conjugate goat anti-mouse (green) was used. For microglia staining, rabbit anti-human Iba-1 followed by Alexa 546 goat anti-rabbit (red), and nuclear staining by DAPI (blue) was used. Secondary antibody was used as control of non-specific staining as shown in G. Magnification: 40X.
Discussion
Substance abuse, such as cocaine remains as one of the major barriers of HIV eradication, because drugs, specifically cocaine acts as a cofactor increasing the levels of virus through remodeling of brain vascular endothelial cells (Vittinghoff et al. 2001; Fiala et al. 2005). Studies have established that cocaine can enhance HIV-replication in CD4+ T cells (Mantri et al. 2012; Pandhare et al. 2014), blood peripheral monocytes (Peterson et al. 1991), microglia (Gekker et al. 2006) and astrocytes (Yang et al. 2010). Moreover, recent studies have determined that cocaine enhances HIV-1 induced CD4+ T cell apoptosis and contributes to disease progression among drug users (Pandhare et al. 2014). Proteomics analyses of monocyte-derived mature dendritic cells demonstrated the effects of cocaine in enhancing HIV-replication with over-expression of important proteins such as aldolase, enolase, cathepsin S, HSP90, MEK1, MEK2 among others (Reynolds et al. 2009). Studies in human astrocytes exposed to cocaine also demonstrated that increased enolase, aldolase, HSP60, Anexin I among others (Reynolds et al. 2006). Recent studies from our laboratory demonstrated that HIV-1 replication alters the interactions of cathepsin B with its inhibitors cystatins B and C and induces cathepsin B secretion from MDM which increases neuronal apoptosis (Rodriguez-Franco et al. 2012). In this study we now asked if cocaine enhances cathepsin B secretion from HIV-infected MDM. To determine the clinical relevance of our findings, studies were also conducted using samples of plasma and CSF from the Hispanic HIV-seropositive women and from post-mortem brain tissue obtained from the NNTC. We observed a significant increase in the levels of cathepsin B secretion and activity in plasma of HIV-infected cocaine users compared with non-drug users. This increased cathepsin B in HIV-1 infected cocaine users parallels to previous clinical findings from studies of plasma samples of patients with HIV associated neurocognitive disorders (HAND) (Rivera et al. 2014). Similar results were obtained in the CSF of HAND patients and in HIV seropositive patients that are cocaine users where cathepsin B secretion and activity remained at the same levels between experimental and control groups. It has been established that HIV-1 transforms the monocyte plasma membrane proteome (Kadiu et al. 2009) and proteomics analysis of CSF demonstrated that reduction of antioxidants enzymes such as Cu/Zn SOD and GPx, have been associated with disease severity (Laspiur et al. 2007; Velazquez et al. 2009; Angel et al. 2012). It is quite possible that an antioxidant imbalance could promote secretion and neurotoxic activity of cathepsin B in the CNS. Despite lack of differences in cathepsin B expression and activity in the CSF, we observed increased cathepsin B in Iba+ macrophages and in some Iba-cells from HIV-seropositive patients that were cocaine users. These results demonstrate that cocaine is also inducing cathepsin B expression in the CNS of HIV-1 infected patients.
It is well known that cathepsin B is highly regulated by two principals natural inhibitors; cystatins B and C (Kopitar-Jerala 2006; Brix et al. 2008). However, previous studies in plasma and CSF of a Hispanic women cohort reported increased cathepsin B and its inhibitors cystatin B and C when compared HIV-infected patients with uninfected controls (Cantres-Rosario et al. 2013). Our results also show an increased cathepsin B and cystatin B secretion during HIV-infection that is potentiated by cocaine. The increased expression of both cathepsin B and its inhibitor, cystatin B can be explained by previous studies that revealed that the delicate balance/regulation between cathepsin B and its inhibitors cystatins B and C is disrupted by HIV-1 infection of MDM (Rodriguez-Franco et al. 2012). This is also observed in other conditions such as ovarian cancer (Kolwijck et al. 2010). We observed a significant increased cystatin B but not cystatin C, from plasma of HIV-infected patients that used cocaine compared with non-drug users. No differences were observed in the cystatins B and C concentration in the CSF. In previous studies we have found that cystatin C increases in the plasma of HIV infected patients compared to HIV seronegative controls (Cantres-Rosario et al. 2013). However, cystatin C did not increase further with HAND (Cantres-Rosario et al. 2013) The current results with 6 patients suggest that cystatin C does not increase with cocaine use in HIV infected patients but additional studies with more patients may be necessary to confirm our findings.
Drug abuse in HIV-infected patients, such as cocaine results in the acceleration and progression from HIV to AIDS (Nair et al. 2000; Vittinghoff et al. 2001), increased neuroinvasion (Fiala et al. 1998) thought remodeling brain microvascular endothelial cells (Fiala et al. 2005), and opens the blood brain barrier to HIV-1 invasion (Zhang et al. 1998; Silverstein et al. 2012). We have demonstrated previously, that HIV-1 infection in MDM promotes cathepsin B secretion and neuronal apoptosis. Now, we wanted to determine the effects of cocaine in cathepsin B secretion from MDM during HIV-infection. In order to answer this question, we used monocytes differentiated into macrophages from healthy donors and infected these with HIV and thereafter exposed them to cocaine. Supernatants of uninfected, uninfected cocaine-treated, HIV-infected and HIV-infected cocaine-treated were collected at 3, 6 and 12 dpi in order to measure the levels of secretion of cathepsin B by ELISA. In the present study we found that cocaine induced a 60% increase in the levels of cathepsin B secretion from HIV-infected cocaine-treated MDM supernatants compared to HIV-infected non-cocaine controls at 12dpi. Levels of bioactive cathepsin B were also measured in order to determine how cocaine affects the activity of this enzyme. We also found 60% higher levels of active cathepsin B in supernatants HIV-infected cocaine treated compared with supernatant HIV. These data suggest an enhancement of cathepsin B secretion and activity in HIV infection and cocaine exposure that could result in increased brain damage.
Studies of cathepsin B endogenous inhibitor; cystatin B show the importance of this protein in HIV-1 replication (Luciano-Montalvo and Meléndez 2009; Rivera-Rivera et al. 2012) and in other conditions such as progressive myoclonus epilepsy (Lehtinen et al. 2009). Our results show increased levels of secreted cystatin B from HIV-infected cocaine-treated MDM cultures compared to cocaine negative HIV infected cultures at 12dpi that parallels with the peak HIV replication. These data may also suggest that cocaine enhances secretion of cystatin B in order to regulate the levels of cathepsin B. However, the inhibition of cathepsin B by cystatin B during HIV infection has demonstrated to be ineffective (Rodriguez-Franco et al. 2012) . Identification of cathepsin B as a mediator of cell death induced by amyloid beta-activated microglial cells (Kingham and Pocock 2001; Gan et al. 2004) and MDM (Rodriguez-Franco et al. 2012) has been demonstrated and additional studies further support the role of this protein in neuronal apoptosis (Yamashima et al. 1998; Kingham and Pocock 2001; Sun et al. 2010; Luo et al. 2010; Rodriguez-Franco et al. 2012; Cho et al. 2013). However, only a single study has shown the opposite, a neuroprotective effect for cathepsin B (Gray et al. 2001; Nagai et al. 2005).
Our current study demonstrates that cocaine enhances HIV-1 infection, promoting the secretion of toxic levels of the protease cathepsin B from MDM inducing neuronal apoptosis. To determine the specificity of neuronal death by this enzyme, we used the specific cathepsin B inhibitor CA-074 (Cho et al. 2013) and a monoclonal anti-cathepsin B antibody. We observed decreased levels of neuronal death in neurons treated with MCM pre-treated either with CA-074 or cathepsin B antibody. These data reveal that cocaine enhances cathepsin B secrtion during HIV-1 inducing detrimental effects in neurons. Therefore targeted extracellular cathepsin B inhibitors could be a potential complementary therapy to cART in order to ameliorate the neurotoxic effect of cocaine in HIV-1 infected patients.
In conclusion, our results demonstrate that cocaine enhances HIV-1 infection promoting an increased cathepsin B in patient peripheral blood, and in brain macrophages/microglia as shown in the results of post-mortem brain tissue from the NNTC. In addition our in vitro studies demonstrated cocaine induces increased cathepsin B secretion from HIV-infected MDM that results in neuronal apoptosis and could be prevented by using the specific inhibitor of cathepsin B CA-074 or the cathepsin B antibody. Future experiments will be aimed to determine the effect of cocaine in cathepsin B secretion from HIV-infected microglia / astrocytes co-cultures and its effect in neurons.
Supplementary Material
Acknowledgements
Sponsored by grants from the National Institutes of Health R01MH083516 (L.M.M.), U54NS043011, RISE Program R25GM061838 (FZ.), SNRP-NINDS-1-U54NS431, INBRE P20RR016470-12, NIMHHD 8G12-MD007600 Translational Proteomics Center, and DBI-0923132 to establish and grants ISI0 RR-13705-01 and DBI-0923132 to establish and upgrade the Confocal Microscopy Facility at the University of Puerto Rico (CIF-UPR). UPR Vice President (M.P.), UPR Medical Sciences Campus Chancellor, and the Associate Deanship of Biomedical Sciences provided additional funding to complete this study. We thank the PI of the Hispanic Women cohort, Dr. Valerie Wojna for following the study patients. This publication was made possible from NIH funding through the NIMH and NINDS Institutes by the following grants:
Manhattan HIV Brain Bank: U01MH083501, R24MH59724
Texas NeuroAIDS Research Center U01MH083507, R24 NS45491
National Neurological AIDS Bank 5U01MH083500, NS 38841
California NeuroAIDS Tissue Network U01MH083506, R24MH59745
Statistics and Data Coordinating Center U01MH083545, N01MH32002
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NNTC or NIH.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interests.
References
- Angel TE, Jacobs JM, Spudich SS, et al. The cerebrospinal fluid proteome in HIV infection: change associated with disease severity. Clinical proteomics. 2012;9:3. doi: 10.1186/1559-0275-9-3. doi: 10.1186/1559-0275-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, et al. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain research. 2000;879:42–9. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Brix K, Dunkhorst A, Mayer K, Jordans S. Cysteine cathepsins: cellular roadmap to different functions. Biochimie. 2008;90:194–207. doi: 10.1016/j.biochi.2007.07.024. doi: 10.1016/j.biochi.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Cantres-Rosario Y, Plaud-Valentín M, Gerena Y, et al. Cathepsin B and cystatin B in HIV-seropositive women are associated with infection and HIV-1-associated neurocognitive disorders. AIDS (London, England) 2013;27:347–56. doi: 10.1097/QAD.0b013e32835b3e47. doi: 10.1097/QAD.0b013e32835b3e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Yoon SY, Choi JE, et al. CA-074Me, a cathepsin B inhibitor, decreases APP accumulation and protects primary rat cortical neurons treated with okadaic acid. Neuroscience letters. 2013;548:222–7. doi: 10.1016/j.neulet.2013.05.056. doi: 10.1016/j.neulet.2013.05.056. [DOI] [PubMed] [Google Scholar]
- Ciborowski P, Gendelman HE. Human immunodeficiency virus-mononuclear phagocyte interactions: emerging avenues of biomarker discovery, modes of viral persistence and disease pathogenesis. Current HIV research. 2006;4:279–91. doi: 10.2174/157016206777709474. [DOI] [PubMed] [Google Scholar]
- Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotoxicity research. 2013;23:174–88. doi: 10.1007/s12640-012-9334-7. doi: 10.1007/s12640-012-9334-7. [DOI] [PubMed] [Google Scholar]
- Dalvi P, Wang K, Mermis J, et al. HIV-1/Cocaine Induced Oxidative Stress Disrupts Tight Junction Protein-1 in Human Pulmonary Microvascular Endothelial Cells: Role of Ras/ERK1/2 Pathway. PloS one. 2014;9:e85246. doi: 10.1371/journal.pone.0085246. doi: 10.1371/journal.pone.0085246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, Peng F, Bokhari S, et al. Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood-brain barrier: implications for HIV-dementia. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2008;3:52–6. doi: 10.1007/s11481-007-9091-1. doi: 10.1007/s11481-007-9091-1. [DOI] [PubMed] [Google Scholar]
- Dhillon NK, Williams R, Peng F, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. Journal of neurovirology. 2007;13:483–95. doi: 10.1080/13550280701528684. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- Fiala M, Eshleman AJ, Cashman J, et al. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. Journal of neurovirology. 2005;11:281–91. doi: 10.1080/13550280590952835. doi: 10.1080/13550280590952835. [DOI] [PubMed] [Google Scholar]
- Fiala M, Gan XH, Zhang L, et al. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine’s connection to AIDS dementia and vasculitis? Advances in experimental medicine and biology. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- Gan L, Ye S, Chu A, et al. Identification of cathepsin B as a mediator of neuronal death induced by Abeta-activated microglial cells using a functional genomics approach. The Journal of biological chemistry. 2004;279:5565–72. doi: 10.1074/jbc.M306183200. doi: 10.1074/jbc.M306183200. [DOI] [PubMed] [Google Scholar]
- Gandhi N, Saiyed ZM, Napuri J, et al. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. Journal of neurovirology. 2010;16:294–305. doi: 10.3109/13550284.2010.499891. doi: 10.3109/13550284.2010.499891. [DOI] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Coley JS, Berman JW. Drug induced increases in CNS dopamine alter monocyte, macrophage and T cell functions: implications for HAND. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:621–42. doi: 10.1007/s11481-013-9443-y. doi: 10.1007/s11481-013-9443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, et al. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. The American journal of pathology. 2009;175:1148–59. doi: 10.2353/ajpath.2009.081067. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekker G, Hu S, Sheng WS, et al. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. International immunopharmacology. 2006;6:1029–33. doi: 10.1016/j.intimp.2005.12.005. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gekker G, Hu S, Wentland MP, et al. Kappa-opioid receptor ligands inhibit cocaine-induced HIV-1 expression in microglial cells. The Journal of pharmacology and experimental therapeutics. 2004;309:600–6. doi: 10.1124/jpet.103.060160. doi: 10.1124/jpet.103.060160. [DOI] [PubMed] [Google Scholar]
- Ghorpade a, Xia MQ, Hyman BT, et al. Role of the beta-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. Journal of virology. 1998;72:3351–61. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Haran MM, Schneider K, et al. Evidence that inhibition of cathepsin-B contributes to the neuroprotective properties of caspase inhibitor Tyr-Val-Ala-Asp-chloromethyl ketone. The Journal of biological chemistry. 2001;276:32750–5. doi: 10.1074/jbc.M103150200. doi: 10.1074/jbc.M103150200. [DOI] [PubMed] [Google Scholar]
- Griffin WC, Middaugh LD, Tyor WR. Chronic cocaine exposure in the SCID mouse model of HIV encephalitis. Brain research. 2007;1134:214–9. doi: 10.1016/j.brainres.2006.11.059. doi: 10.1016/j.brainres.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, et al. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–63. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisaka T, Desmouliere A, Taupin JL, et al. Expression of leukemia inhibitory factor (LIF) and its receptor gp190 in human liver and in cultured human liver myofibroblasts. Cloning of new isoforms of LIF mRNA. Comp Hepatol. 2004;3:10. doi: 10.1186/1476-5926-3-10. doi: 1476-5926-3-10 [pii] 10.1186/1476-5926-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CE, Lancaster K, Spicer B. How do Australian news media depict illicit drug issues? An analysis of print media reporting across and between illicit drugs, 2003-2008. The International journal on drug policy. 2011;22:285–91. doi: 10.1016/j.drugpo.2011.05.008. doi: 10.1016/j.drugpo.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Johansson JU, Pradhan S, Lokteva LA, et al. Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:16016–32. doi: 10.1523/JNEUROSCI.2203-13.2013. doi: 10.1523/JNEUROSCI.2203-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiu I, Wang T, Schlautman JD, et al. HIV-1 transforms the monocyte plasma membrane proteome. Cellular immunology. 2009;258:44–58. doi: 10.1016/j.cellimm.2009.03.012. doi: 10.1016/j.cellimm.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8212–6. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingham PJ, Pocock JM. Microglial secreted cathepsin B induces neuronal apoptosis. Journal of neurochemistry. 2001;76:1475–84. doi: 10.1046/j.1471-4159.2001.00146.x. [DOI] [PubMed] [Google Scholar]
- Kolwijck E, Kos J, Obermajer N, et al. The balance between extracellular cathepsins and cystatin C is of importance for ovarian cancer. European journal of clinical investigation. 2010;40:591–9. doi: 10.1111/j.1365-2362.2010.02305.x. doi: 10.1111/j.1365-2362.2010.02305.x. [DOI] [PubMed] [Google Scholar]
- Kopitar-Jerala N. The role of cystatins in cells of the immune system. FEBS letters. 2006;580:6295–301. doi: 10.1016/j.febslet.2006.10.055. doi: 10.1016/j.febslet.2006.10.055. [DOI] [PubMed] [Google Scholar]
- Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Frontiers in pharmacology. 2012;3:121. doi: 10.3389/fphar.2012.00121. doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrat EP, Zierler S. Entangled epidemics: cocaine use and HIV disease. Journal of psychoactive drugs. 25:207–21. doi: 10.1080/02791072.1993.10472272. [DOI] [PubMed] [Google Scholar]
- Laspiur JP, Anderson ER, Ciborowski P, et al. CSF proteomic fingerprints for HIV-associated cognitive impairment. Journal of neuroimmunology. 2007;192:157–70. doi: 10.1016/j.jneuroim.2007.08.004. doi: 10.1016/j.jneuroim.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Suk K, Kang Y, et al. Neurotoxic factors released by stimulated human monocytes and THP-1 cells. Brain research. 2011;1400:99–111. doi: 10.1016/j.brainres.2011.05.021. doi: 10.1016/j.brainres.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Tegelberg S, Schipper H, et al. Cystatin B deficiency sensitizes neurons to oxidative stress in progressive myoclonus epilepsy, EPM1. J Neurosci. 2009;29:5910–5915. doi: 10.1523/JNEUROSCI.0682-09.2009. doi: 29/18/5910 [pii] 10.1523/JNEUROSCI.0682-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardelli L, Aguerre-Girr M, Logiodice F, et al. HLA-G5 induces IL-4 secretion critical for successful pregnancy through differential expression of ILT2 receptor on decidual CD4+ T cells and macrophages. Journal of immunology (Baltimore, Md : 1950) 2013;191:3651–62. doi: 10.4049/jimmunol.1300567. doi: 10.4049/jimmunol.1300567. [DOI] [PubMed] [Google Scholar]
- Luciano-Montalvo C, Ciborowski P, Duan F, et al. Proteomic analyses associate cystatin B with restricted HIV-1 replication in placental macrophages. Placenta. 2008;29:1016–1023. doi: 10.1016/j.placenta.2008.09.005. doi: S0143-4004(08)00307-X [pii] 10.1016/j.placenta.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano-Montalvo C, Meléndez LM. Cystatin B associates with signal transducer and activator of transcription 1 in monocyte-derived and placental macrophages. Placenta. 2009;30:464–7. doi: 10.1016/j.placenta.2009.03.003. doi: 10.1016/j.placenta.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C-L, Chen X-P, Yang R, et al. Cathepsin B contributes to traumatic brain injury-induced cell death through a mitochondria-mediated apoptotic pathway. Journal of neuroscience research. 2010;88:2847–58. doi: 10.1002/jnr.22453. doi: 10.1002/jnr.22453. [DOI] [PubMed] [Google Scholar]
- Malla R, Gopinath S, Alapati K, et al. Correction: Downregulation of uPAR and Cathepsin B Induces Apoptosis via Regulation of Bcl-2 and Bax and Inhibition of the PI3K/Akt Pathway in Gliomas. PloS one. 2014 doi: 10.1371/journal.pone.0013731. doi: 10.1371/annotation/bb2f92ec-2ac1-4952-b744-0519aeb10d1c. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mantri CK, Pandhare Dash J, Mantri JV, Dash CC. Cocaine enhances HIV-1 replication in CD4+ T cells by down-regulating MiR-125b. PloS one. 2012;7:e51387. doi: 10.1371/journal.pone.0051387. V. doi: 10.1371/journal.pone.0051387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino JJ, Montes ML, Blanco A, et al. [HIV-1 neuropathogenesis: therapeutic strategies against neuronal loss induced by gp120/Tat glycoprotein in the central nervous system] Revista de neurologia. 2011;52:101–11. [PubMed] [Google Scholar]
- Nagai A, Ryu JK, Terashima M, et al. Neuronal cell death induced by cystatin C in vivo and in cultured human CNS neurons is inhibited with cathepsin B. Brain research. 2005;1066:120–8. doi: 10.1016/j.brainres.2005.10.063. doi: 10.1016/j.brainres.2005.10.063. [DOI] [PubMed] [Google Scholar]
- Nair MP, Chadha KC, Hewitt RG, et al. Cocaine differentially modulates chemokine production by mononuclear cells from normal donors and human immunodeficiency virus type 1-infected patients. Clinical and diagnostic laboratory immunology. 2000;7:96–100. doi: 10.1128/cdli.7.1.96-100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Sasaki Y, Kohsaka S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. Journal of neurochemistry. 2004;88:844–56. doi: 10.1046/j.1471-4159.2003.02213.x. [DOI] [PubMed] [Google Scholar]
- Pandhare J, Addai AB, Mantri CK, et al. Cocaine Enhances HIV-1-Induced CD4(+) T-Cell Apoptosis: Implications in Disease Progression in Cocaine-Abusing HIV-1 Patients. The American journal of pathology. 2014 doi: 10.1016/j.ajpath.2013.12.004. doi: 10.1016/j.ajpath.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Chao CC, et al. Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor-beta. Journal of immunology (Baltimore, Md : 1950) 1991;146:81–4. [PubMed] [Google Scholar]
- Reynolds JL, Mahajan SD, Aalinkeel R, et al. Proteomic analyses of the effects of drugs of abuse on monocyte-derived mature dendritic cells. Immunological investigations. 2009;38:526–50. doi: 10.1080/08820130902874110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JL, Mahajan SD, Bindukumar B, et al. Proteomic analysis of the effects of cocaine on the enhancement of HIV-1 replication in normal human astrocytes (NHA) Brain research. 2006;1123:226–36. doi: 10.1016/j.brainres.2006.09.034. doi: 10.1016/j.brainres.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera LE, Colon K, Cantres-Rosario YM, et al. Macrophage Derived Cystatin B / Cathepsin B in HIV Replication and Neuropathogenesis. Current HIV research. 2014 doi: 10.2174/1570162X12666140526120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Rivera L, Perez-Laspiur J, Colón K, Meléndez LM. Inhibition of interferon response by cystatin B: implication in HIV replication of macrophage reservoirs. Journal of neurovirology. 2012;18:20–9. doi: 10.1007/s13365-011-0061-2. doi: 10.1007/s13365-011-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Franco EJ, Cantres-Rosario YM, Plaud-Valentin M, et al. Dysregulation of Macrophage-Secreted Cathepsin B Contributes to HIV-1-Linked Neuronal Apoptosis. PloS one. 2012;7:e36571. doi: 10.1371/journal.pone.0036571. doi: 10.1371/journal.pone.0036571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Tashkin DP, Choi R, et al. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. The Journal of infectious diseases. 2002;185:701–5. doi: 10.1086/339012. doi: 10.1086/339012. [DOI] [PubMed] [Google Scholar]
- Samikkannu T, Rao KVK, Arias AY, et al. HIV infection and drugs of abuse: role of acute phase proteins. Journal of neuroinflammation. 2013;10:113. doi: 10.1186/1742-2094-10-113. doi: 10.1186/1742-2094-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein PS, Shah A, Weemhoff J, et al. HIV-1 gp120 and drugs of abuse: interactions in the central nervous system. Current HIV research. 2012;10:369–83. doi: 10.2174/157016212802138724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu Z, Baba M, et al. Cathepsin B-dependent motor neuron death after nerve injury in the adult mouse. Biochemical and biophysical research communications. 2010;399:391–5. doi: 10.1016/j.bbrc.2010.07.084. doi: 10.1016/j.bbrc.2010.07.084. [DOI] [PubMed] [Google Scholar]
- Thomas S, Mayer L, Sperber K. Mitochondria influence Fas expression in gp120-induced apoptosis of neuronal cells. The International journal of neuroscience. 2009;119:157–65. doi: 10.1080/00207450802335537. doi: 10.1080/00207450802335537. [DOI] [PubMed] [Google Scholar]
- Tian C, Erdmann N, Zhao J, et al. HIV-infected macrophages mediate neuronal apoptosis through mitochondrial glutaminase. Journal of neurochemistry. 2008;105:994–1005. doi: 10.1111/j.1471-4159.2007.05197.x. doi: 10.1111/j.1471-4159.2007.05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Y-Romo LB, Kolson DL, Bandaru VVR, et al. Adenosine triphosphate released from HIV-infected macrophages regulates glutamatergic tone and dendritic spine density on neurons. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:998–1009. doi: 10.1007/s11481-013-9471-7. doi: 10.1007/s11481-013-9471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga VM, Gupta S, et al. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-Tat. Antioxidants & redox signaling. 2009;11:193–204. doi: 10.1089/ars.2008.2097. doi: 10.1089/ARS.2008.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Valls E, Flores-Soto ME, Chaparro-Huerta V, et al. HIF-1α expression in the hippocampus and peripheral macrophages after glutamate-induced excitotoxicity. Journal of neuroimmunology. 2011;238:12–8. doi: 10.1016/j.jneuroim.2011.06.001. doi: 10.1016/j.jneuroim.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Velazquez I, Plaud M, Wojna V, et al. Antioxidant enzyme dysfunction in monocytes and CSF of Hispanic women with HIV-associated cognitive impairment. Journal of neuroimmunology. 2009;206:106–11. doi: 10.1016/j.jneuroim.2008.10.013. doi: 10.1016/j.jneuroim.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittinghoff E, Hessol NA, Bacchetti P, et al. Cofactors for HIV disease progression in a cohort of homosexual and bisexual men. Journal of acquired immune deficiency syndromes (1999) 2001;27:308–14. doi: 10.1097/00126334-200107010-00015. [DOI] [PubMed] [Google Scholar]
- Webber MP, Schoenbaum EE, Gourevitch MN, et al. A prospective study of HIV disease progression in female and male drug users. AIDS (London, England) 1999;13:257–62. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- Wojna V, Skolasky RL, Hechavarria R, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12:356–364. doi: 10.1080/13550280600964576. doi: W1R11W6329X631NM [pii] 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- Yamashima T, Kohda Y, Tsuchiya K, et al. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on “calpain-cathepsin hypothesis”. The European journal of neuroscience. 1998;10:1723–33. doi: 10.1046/j.1460-9568.1998.00184.x. [DOI] [PubMed] [Google Scholar]
- Yan Y-F, Wang Z-Y, Pu S-S, et al. HIV-1B gp120 genes from one patient with AIDS dementia complex can affect the secretion of tumor necrosis factor and interleukin 1β in glial cells. Chinese medical journal. 2011;124:4217–22. [PubMed] [Google Scholar]
- Yang Y, Yao H, Lu Y, et al. Cocaine Potentiates Astrocyte Toxicity Mediated by Human Immunodeficiency Virus ( HIV-1 ) Protein gp120. 2010 doi: 10.1371/journal.pone.0013427. doi: 10.1371/journal.pone.0013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Buch S. Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability. Blood. 2011;117:2538–47. doi: 10.1182/blood-2010-10-313593. doi: 10.1182/blood-2010-10-313593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Yang Y, Kim KJ, et al. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115:4951–62. doi: 10.1182/blood-2010-01-266221. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu J, Katafiasz B, et al. HIV-1 gp120-induced axonal injury detected by accumulation of β-amyloid precursor protein in adult rat corpus callosum. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2011;6:650–7. doi: 10.1007/s11481-011-9259-6. doi: 10.1007/s11481-011-9259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Looney D, Taub D, et al. Cocaine opens the blood-brain barrier to HIV-1 invasion. Journal of neurovirology. 1998;4:619–26. doi: 10.3109/13550289809114228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.