Abstract
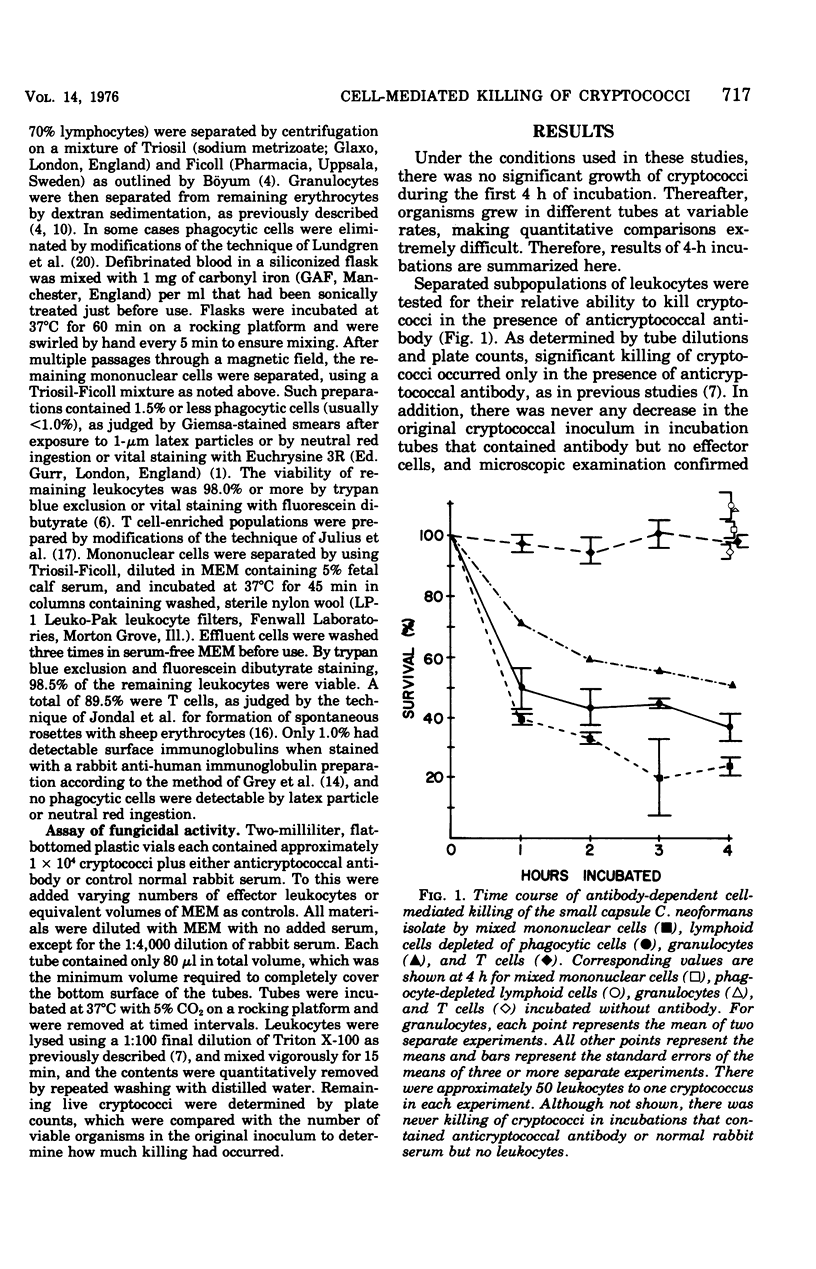
Studies were performed to identify the types of human peripheral blood leukocytes capable of killing Cryptococcus neoformans in the presence of anticryptococcal antibody in vitro. A total of 24.1 +/- 2.7% (mean +/- standard error of the mean of four experiments) of the original cryptococcal inoculum survived in a mixed mononuclear, cell preparation (approximately 30% monocytes) after 4 h of incubation at 37 degrees C with rabbit anticryptococcal antibody. When phagocytic cells were removed, there was 36.4 +/- 4.6% survival in six experiments, compared with 52.8% survival in the presence of purified granulocytes (mean of two experiments) and 96.9 +/- 1% survival in the presence of purified T cells. There was never any significant killing in control mixtures that contained leukocytes with normal rabbit serum nor in those that contained anticryptococcal antibody without effector leukocytes. Significant antibody-dependent fungicidal activity was seen with ratios of effector to target cells as low as 6.25:1. These observations indicate that multiple types of peripheral blood leukocytes, excluding T cells, are capable of antibody-dependent fungicidal activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bindschadler D. D., Bennett J. E. Serology of human cryptococcosis. Ann Intern Med. 1968 Jul;69(1):45–52. doi: 10.7326/0003-4819-69-1-45. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cardella C. J., Davies P., Allison A. C. Immune complexes induce selective release of lysosomal hydrolases from macrophages. Nature. 1974 Jan 4;247(5435):46–48. doi: 10.1038/247046a0. [DOI] [PubMed] [Google Scholar]
- Davies P., Page R. C., Allison A. C. Changes in cellular enzyme levels and extracellular release of lysosomal acid hydrolases in macrophages exposed to group A streptococcal cell wall substance. J Exp Med. 1974 May 1;139(5):1262–1282. doi: 10.1084/jem.139.5.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D. Antibody-dependent killing of Cryptococcus neopormans by human peripheral blood mononuclear cells. Nature. 1974 Jan 18;247(5437):148–150. doi: 10.1038/247148a0. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973 Feb;7(2):231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Forman J., Möller G. The effector cell in antibody-induced cell mediated immunity. Transplant Rev. 1973;17(0):108–149. doi: 10.1111/j.1600-065x.1973.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Zighelboim J. Polymorphonuclear leukocytes in antibody-dependent cellular cytotoxicity. J Immunol. 1975 Mar;114(3):1047–1051. [PubMed] [Google Scholar]
- Greenberg A. H., Shen L., Roitt I. M. Characterization of the antibody-dependent cytotoxic cell. A non-phagocytic monocyte? Clin Exp Immunol. 1973 Oct;15(2):251–259. [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm G., Hammarström S. Haemolytic activity of human blood monocytes. Lysis of human erythrocytes treated with anti-A serum. Clin Exp Immunol. 1973 Jan;13(1):29–43. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kalina M., Kletter Y., Shahar A., Aronson M. Acid phosphatase release from intact phagocytic cells surrounding a large-sized parasite. Proc Soc Exp Biol Med. 1971 Feb;136(2):407–410. doi: 10.3181/00379727-136-35275. [DOI] [PubMed] [Google Scholar]
- Larsson A., Perlmann P., Natvig J. B. Cytotoxicity of human lymphocytes induced by rabbit antibodies to chicken erythrocytes. Inhibition by normal IgG and by human myeloma proteins of different IgG subclasses. Immunology. 1973 Oct;25(4):675–686. [PMC free article] [PubMed] [Google Scholar]
- Lundgren G., Zukoski C. F., Möller G. Differential effects of human granulocytes and lymphocytes on human fibroblasts in vitro. Clin Exp Immunol. 1968 Oct;3(8):817–836. [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Howard A., Gotch F. M., Quie P. G. Effector activating determinants on IgG. I. The distribution and factors influencing the display of complement, neutrophil and cytotoxic B-cell determinants on human IgG sub-classes. Immunology. 1973 Sep;25(3):459–469. [PMC free article] [PubMed] [Google Scholar]
- Möller G., Svehag S. E. Specificiy of lymphocyte-mediated cytotoxicity induced by in vitro antibody-coated target cells. Cell Immunol. 1972 May;4(1):1–19. doi: 10.1016/0008-8749(72)90001-9. [DOI] [PubMed] [Google Scholar]
- Pearsall N. N., Sundsmo J. S., Weiser R. S. Lymphokine toxicity for yeast cells. J Immunol. 1973 May;110(5):1444–1446. [PubMed] [Google Scholar]
- Perlmann P., Perlmann H. Contactual lysis of antibody-coated chicken erythrocytes by purified lymphocytes. Cell Immunol. 1970 Sep;1(3):300–315. doi: 10.1016/0008-8749(70)90051-1. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacker J. R., Farhi F., Bulmer G. S. Intracellular fate of Cryptococcus neoformans. Infect Immun. 1972 Aug;6(2):162–167. doi: 10.1128/iai.6.2.162-167.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. E., Bennett J. E., Bailey J. W. Serologic grouping of Cryptococcus neoformans. Proc Soc Exp Biol Med. 1968 Mar;127(3):820–823. doi: 10.3181/00379727-127-32812. [DOI] [PubMed] [Google Scholar]
- Wisloff F., Michaelsen T. E., Froland S. S. Inhibition of antibody-dependent human lymphocyte-mediated cytotoxicity by immunoglobulin classes, IgG subclasses, and IgG fragments. Scand J Immunol. 1974;3(1):29–38. doi: 10.1111/j.1365-3083.1974.tb01230.x. [DOI] [PubMed] [Google Scholar]



