Abstract
The B6.Cg-Tg(Thy1-YFP)16Jrs/J transgenic mouse strain, widely used to study neuronal development and regeneration, expresses the yellow fluorescent protein (YFP) in the peripheral nerves and the central nervous system under the control of regulatory sequences of the Thy1 gene. The Thy1 (CD90) cell surface glycoprotein is present on many cell types besides neurons, and is known to be involved in cell adhesion, migration and signal transduction. We hypothesized that Thy1-activating conditions could probably activate the truncated Thy1 regulatory sequences used in the Thy1-YFP construct, resulting in YFP transgene expression outside the nervous system. We demonstrated that the stroma of subcutaneous tumours induced by the injection of 4T1 or MC26 carcinoma cells into BALB/c(Thy1-YFP) mice, carrying the same construct, indeed expressed the YFP transgene. In the tumour mass, the yellow-green fluorescent stromal cells were clearly distinguishable from 4T1 carcinoma cells stably transfected with red fluorescent protein. Local inflammation induced by subcutaneous injection of complete Freund's adjuvant, as well as the experimental wound-healing milieu, also triggered YFP fluorescence in both the BALB/c(Thy1-YFP) and B6.Cg-Tg(Thy1-YFP)16Jrs/J mice, pointing to eventual overlapping pathways of wound-healing, inflammation and tumour growth.
The cell surface glycoprotein Thy1 (CD90) is expressed on many mammalian cell types, including neurons, thymocytes, T cells, endothelial cells and fibroblasts. It also serves as a human mesenchymal stem cell (MSC) marker. Its expression pattern is controlled by different promoter and enhancer elements and exhibits tissue-, developmental stage- and species-specific differences. Thy1 has several cellular functions, including the promotion of T cell activation and the inhibition of neurite outgrowth. It also functions as a cell adhesion molecule, has roles in leukocyte interaction with the vascular endothelium, and in fibroblast and melanoma cell migration, and is involved in apoptotic signalling, tumour suppression, fibroblast proliferation and lipofibroblast development1. Regulatory sequences responsible for the neuronal expression of the Thy1 gene have been used to establish different transgenic mouse strains to direct the transgene expression only to neuronal cells. Neurons in the central and the peripheral nervous systems were fluorescently labelled in this way, and the developmental effects of different growth factors and cytokines have been examined under the control of these elements2,3,4. The JAX transgenic mouse strain B6.Cg-Tg(Thy1-YFP)16Jrs/J (http://jaxmice.jax.org/) expresses the fluorescent YFP protein in the motor and sensory neurons and in subsets of the central neurons, and is widely used to study neuronal development and regeneration5,6,7,8,9. In this model, the YFP transgene expression is directed by regulatory elements from the 5′ portion of the Thy1 gene, extending from the promoter to the intron following exon 4. Exon 3 and its flanking introns, known to be required for expression in non-neural cells, were deleted10. It was reported that no YFP fluorescent signal was detected outside of the nervous system in these transgenic mice, but Thy1-activating conditions have not been systematically studied in non-neuronal cells.
Among others, the Thy1 protein is known to be an activation-associated, inducible cell adhesion molecule on human endothelial cells. It is upregulated in human prostate cancer stroma, and could serve as a tumour prognostic marker11. Thy1 has also been shown to influence fibroblast adhesion and migration. Moreover, fibroblast and stem cell activation are important not only in inflammation and wound-healing, but through their contribution to the tumour stroma formation, they also play a role in tumour progression. We hypothesized that the YFP marker gene expression may additionally occur in cells where Thy1 gene activation otherwise occurs. To examine this hypothesis, we have started to test this transgenic mouse strain in tumourigenesis, wound-healing and inflammation studies.
Results
The analysis of the transgenic mice under fluorescent microscope revealed, as expected, the expression of the transgene was restricted to the brain and the nerves; no other tissues proved to be fluorescent, either on in vivo microscopic observation, or in tissue sections. Due to their thin skin and lack of hair, the newborn mice were especially useful for excluding the non-neuronal expression of the transgene. Moreover, no YFP fluorescence was detected in an adipose-derived mesenchymal stem cell culture established by standard protocols from the visceral and subcutaneous fat of adult mice (Supplementary Figure S1).
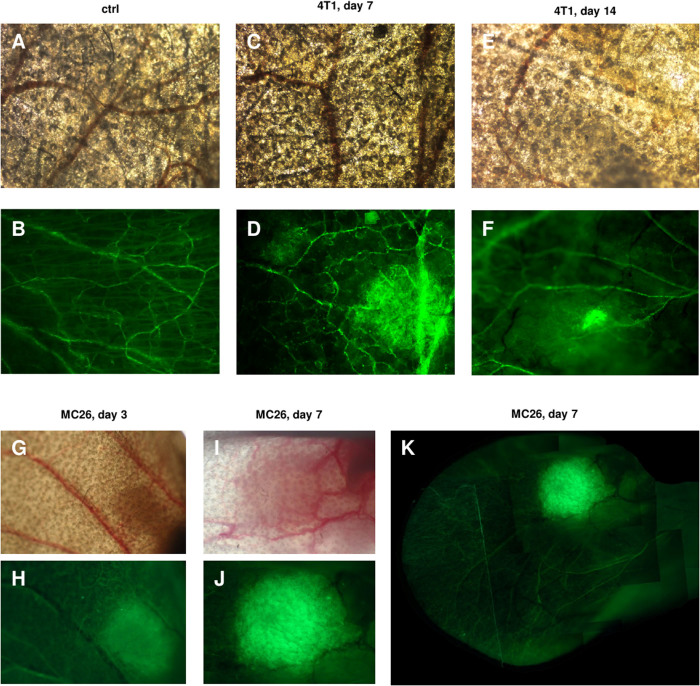
The effect of tumour growth was tested in BALB/c(Thy1-YFP) mice created by back-crossing the original B6.Cg-Tg(Thy1-YFP)16Jrs/J mouse strain to BALB/c genetic background. Two tumour cell lines were tested, the 4T1 mammary carcinoma and the MC26 colon carcinoma, respectively. In the case of s.c. tumour injections in the ear pinnae, in the first few days the injection site was surrounded by a yellow fluorescent “halo” of cells. From about day 5, a more circumscribed fluorescence was strongly co-localized with the tumour mass. The 4T1 and the MC26 tumours behaved similarly in this respect. YFP expression was detected at an unchanged intensity up to the termination of the experiments, i.e. for at least 35 days (Figure 1). Surprisingly, no YFP fluorescence was detected in the case of metastases of the lung, kidney or peritoneum, probably as an indication of tissue-specific differences in the expression pattern even for the truncated Thy1 gene. For visual partitioning of the tumour cells from the stroma, we established a red fluorescent 4T1 cell line, stably transfected with pEF1α-tdTomato plasmid (Tsien Lab). We injected these cells s.c. into the ears of mice and, before the tumour could cause any considerable discomfort, the mice were sacrificed. Frozen sections from the tumour area were then examined by fluorescent microscopy. The red tumour cells were clearly distinguishable from the yellow-green tumour matrix, suggesting that the fluorescence-based cell sorting of tumour- derived stromal cells for functional studies, including adoptive transfer experiments, or in vivo analyses of tumour stroma formation, would be possible. Importantly this model also could be used for the in vivo imaging of a wide array of non-genetically labelled tumours in immunocompetent or immunodeficient transgenic mice (Figure 2).
Figure 1. Thy1 promoter activation in subcutaneous tumors in Thy1-YFP transgenic mice.
BALB/c mice carrying the YFP transgene driven by the regulatory elements of the Thy1 gene were injected with 4T1 mammary carcinoma or MC26 colon carcinoma cells s.c. in the ear pinna. Photographs of the ears were made under light anaesthesia, using a Nikon Eclipse E600 fluorescent microscope (A–F): 20× magnification objective lens, (G–K): 10× magnification objective lens). (A,B): uninjected control transmitted light and fluorescent microscopic image (C,D): transmitted light and fluorescent microscopic image of the Thy1-YFP mice ear 7 days after 4T1 injection. A diffuse halo of YFP fluorescent cells formed around the 4T1 tumor. (E,F): transmitted light and fluorescent microscopic image of the Thy1-YFP mice ear 14 days after 4T1 injection; the YFP fluorescence was strictly localized within the 4T1 tumor mass. (G,H): transmitted light and fluorescent microscopic image of the Thy1-YFP mice ear 3 days after MC26 colon carcinoma cell injection. (I,J): transmitted light and fluorescent microscopic image of the Thy1-YFP mouse ear 7 days after MC26 injection. (K): fluorescent microscopic image of the Thy1-YFP mice ear 7 days after MC26 injection. Multiple images were combined to depict the whole ear. The same phenomenon was observed in the case of MC26 injection. Because of the intensity of the YFP fluorescence induced, the ears of tumour-bearing non-transgenic mice appeared almost completely dark in images shot with the same settings.
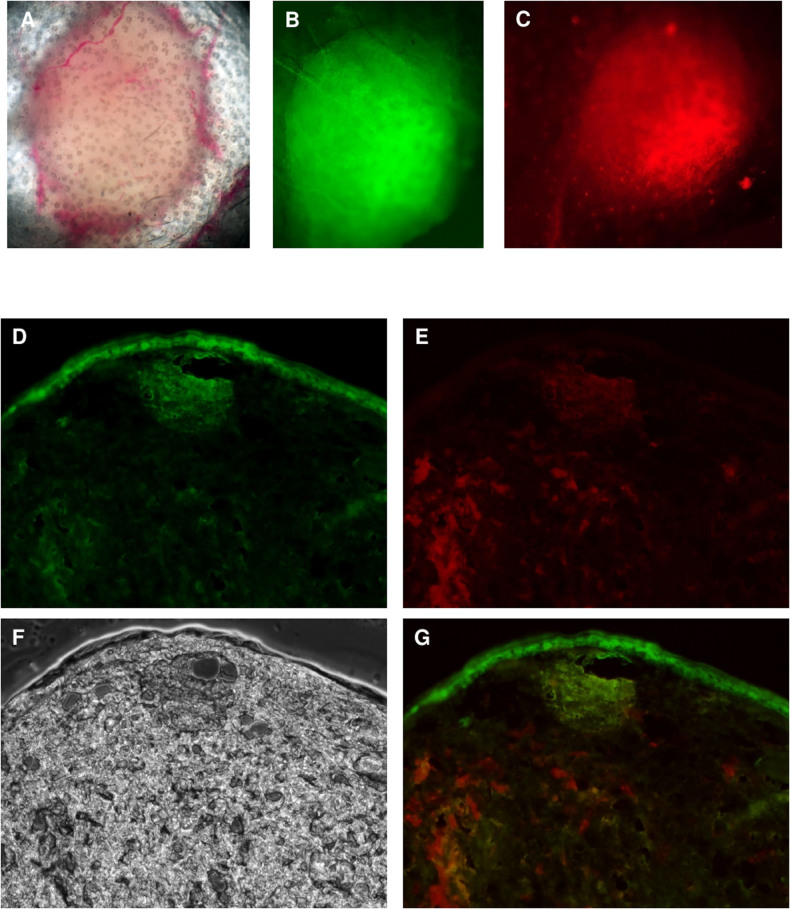
Figure 2. Examination of the tumor cell and Thy1 promoter activity co-localization.
(A–C): Transmitted light, pseudo-green and red fluorescent microscopic image of the tumor formed 35 days after 4T1-red tumor cell injection into the ear pinna of BALB/c(Thy1-YFP) transgenic mice. Photographs of the mice ears were made under light anaesthesia, using a Nikon Eclipse E600 fluorescent microscope. The YFP fluorescence in BALB/c(Thy1-YFP) mice shows co-localization with the red fluorescence of RFP-expressing tumor cells (4× magnification objective lens). (D–G): Transmitted light (F), green (D), red (E) fluorescent and combined (G) microscopic image of the sections of the Thy1-YFP mice ear 7 days after tdTomato expressing 4T1 tumor cell injection into the ear pinna. The red fluorescence of tumor cells is only partly co-localized with YFP fluorescence revealing the different origins of the cells comprising the tumor body. Photographs of the sections were made using a Zeiss Axiovision Z1 fluorescent microscope (20× magnification objective lens).
To see if the activation of the Thy1 promoter might occur outside of the tumour microenvironment, we tested inflammatory tissue, as well. We induced local inflammation in both B6.Cg-Tg(Thy1-YFP)16Jrs/J and BALB/c(Thy1-YFP) mice with a s.c. injection of complete Freund's adjuvant. In contrast with the tumour experiments the inflamed tissue displayed a homogeneous, well circumscribed YFP fluorescence starting from day 2, which indicates that MSC migration and/or fibroblast activation (involving Thy1 activation) might be part of the Freund's adjuvant induced inflammation as well (Figure 3). The fluorescence intensity of the YFP transgene was much above the autofluorescence of non-transgenic mice receiving the same treatment (Supplementary Figure 2).
Figure 3. Inflammation activates the Thy1 promoter in Thy1-YFP mice.
BALB/c(Thy1-YFP) transgenic mice were subcutaneously injected in the ear pinnae with complete Freund's adjuvant. The inflamed tissue showed a diffuse pseudo-green fluorescence. The photos illustrate the ears of two individual mice 5 days after the injections. Photographs of the mouse ears were made under light anaesthesia, using a Nikon Eclipse E600 fluorescent microscope (10× magnification objective lens).
Vascular endothelial cells in the vicinity of carcinomas also seemed to demonstrate intense YFP fluorescence, which means that these tumour cells could trigger Thy1 expression (Figure 1.D). Thy1 on activated microvascular endothelial cells is known to promote melanoma cell migration through blood and lymphatic vessels, and other type of tumour cells could probably also use this metastatic route.
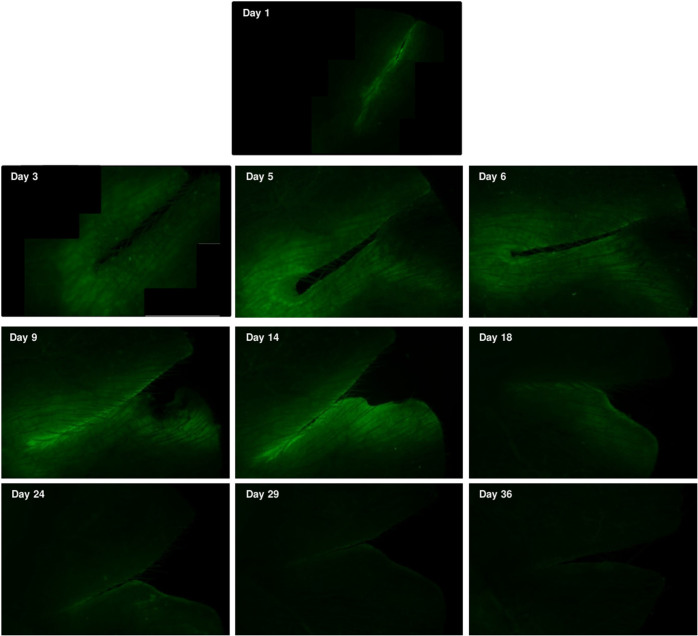
In response to tissue injury, and during the subsequent wound-healing process, sequential and overlapping events take place. First, the active bleeding must be stopped, then inflammatory, proliferative and tissue remodelling phases occur, which include the activation and migration of distinct sets of immune cells, stem cells, fibroblasts and keratinocytes. Since Thy1 is known to affect cell migration into the wound area, we followed the changes in YFP fluorescence during the healing process. One day after sharp superficial cuts were made in the ear of either B6.Cg-Tg(Thy1-YFP)16Jrs/J or BALB/c(Thy1-YFP) mice by scalpel in anesthesia, a faint fluorescent halo appeared along the wound, which expanded until day 14. At the end of day 3, a more intense fluorescent margin also emerged around the wound, which remained significantly brighter until the end of the experiment. The fluorescence area around the wound gradually decreased from day 21, and had almost entirely disappeared by the end of day 36 (Figure 4). As in the case of the local inflammation model, the fluorescence intensity of the YFP transgene was much above the autofluorescence of non-transgenic mice receiving the same treatment (Supplementary Figure 2).
Figure 4. Wound healing activates the Thy-1 promoter in Thy1-YFP mice.
The ear pinnae of BALB/c(Thy-1-YFP) mice were cut with a scalpel under aseptic conditions, under anaesthesia, and the wound healing was then followed by fluorescent microscopy. The wound-healing process was accompanied by temporary upregulation of the YFP fluorescence, gradually fading after 2 weeks. Photographs of the mouse ears were made under light anaesthesia, using a Nikon Eclipse E600 fluorescent microscope (either 20× magnification objective lens or 10× magnification objective lens). Some of the pictures were generated by the combination of multiple images to depict the whole wound surface.
Discussion
Thy1 is a glycosylphosphatidylinositol (GPI)-anchored, cell surface glycoprotein, which belongs to the immunoglobulin superfamily. Thy1 functions as a cell adhesion molecule, and is involved in cell-cell and cell-extracellular matrix interactions. Its occurrence on many cell types suggests the possibility of different physiological roles, which depend on the surrounding tissues and circumstances. Thy1 was first discovered as a thymocyte differentiation marker antigen12,13,14. It mediates thymocyte adhesion to the thymic epithelium, and regulates T cell activation15. It is also important in the adhesion of leukocytes and monocytes to endothelial cells and fibroblasts, and it is upregulated on the surface of activated endothelial cells. Neuronal Thy1 induces astrocyte adhesion through αvβ3 integrin in a cooperative interaction with the proteoglycan syndecan-416. Thy1 participates in several signalling cascades, including signalling via integrins, cytokins, growth factors, and tyrosine kinases, such as focal adhesion kinase17. There are pronounced differences in the focal adhesion structure, stress fiber and cytoskeleton arrangement between Thy1(+) and Thy1(−) lung fibroblast populations, which correlates with their migration capacity. Thy1(+) fibroblasts have well developed focal adhesions and actin stress fibers and migrate less efficiently than Thy1(−) fibroblasts, which harbor only smaller focal complexes and fewer stress fibers18. Leukocytes and melanoma cells also attach via integrins to the Thy1 adhesion molecule on activated endothelial cells. The strong induction of Thy1 on blood and lymphatic vessels in melanoma is induced by vascular endothelial growth factor (VEGF) and TNF-α. Moreover, the number of melanoma metastasis from primary subcutaneous tumour was greatly reduced in Thy1(-/-) mice, suggesting a significant role of the Thy1 mediated attachment of melanoma cells to microvascular EC during their migration19.
In our experiments, the tumour stroma of primary s.c. carcinomas also expressed the YFP transgene, as a marker of Thy1 promoter activation. Tumours are thought to develop stroma from several sources: the recruitment of resident tissue stem cells, the epithelial to mesenchymal transition of the tumour parenchyma, fibroblast recruitment into the tumour stroma, and the recruitment of bone marrow-derived cells from the circulation20. Tumour cells interact with their microenvironment by producing different hormones, cytokines and chemokines. The induction of an elevated cell surface expression of Thy1 on stromal cells could be important in the tumour promoting remodelling of the surrounding cells, but there could be tissue-specific differences, because the lung, liver and kidney metastases derived from primary subcutaneous carcinoma, didn't show any elevated YFP transgene expression. As a model for visualizing subcutaneous tumours in vivo, mice carrying the Thy1-YFP transgene have obvious advantages. Depending on the genetic background, these mice might be used as a tool for visualizing the growth of non-genetically modified transplantable syngeneic tumours. After back-crossing on an immunodeficient, nude, SCID or RAG-/- genetic background, this strain might also be used for the visualization of xenogeneic tumours, which are widely used in the preclinical testing of anti-tumour drugs. In comparison with the genetic labelling of individual tumour cell lines, the advantages of this model are evident. No genetic manipulation is needed for the preparation of the tumour transplant, and genetic changes are therefore avoided, e.g. those caused by the integration of the transgene into the host chromosomes, induced during the introduction of the transgene. It also prevents the “genetic drift” resulting from the selection and cloning of transfectants with established transgene expression. A further advantage of this model is the stability of the fluorescent signal. It is well known that transfected tumour cells are genetically unstable, and can lose or down-regulate the transgene. In contrast, in this case, the Thy1 transgene is expressed by genetically stable, diploid somatic cells, which do not tend to eliminate the marker gene.
Thy1-YFP transgenic mice could be promising tools for studying MSC biology. It might be used to visualize tumour matrix MSC recruitment or in situ activation. In mice, unlike in humans, Thy1 is absent on cultured, non-activated MSC. The differences in the tissue-specific expression of Thy1 in the case of subcutaneous and internal organ metastases might also serve as a starting point of further studies. Additionally, the immunofluorescent FACS sorting of dissociated tissue samples would yield pure populations of tumour MSCs for in vitro or in vivo adoptive transfer experiments. It might also be used for the visualization, or possibly the objective quantification of inflammatory reactions, adaptable for both basic research and preclinical pharmacology.
Microvascular EC does not express Thy1 in healthy adult tissue, but it does so at the sites of inflammation or in tumours, and this can be followed by YFP expression in these transgenic mice. Activated microvascular endothelial cells in the vicinity of the primary carcinoma presented really high expression of YFP in our experiments, as a marker of the activation of the Thy1 gene. This suggests the existence of interacting signals between the carcinoma and endothelial cells that can facilitate their metastatic behaviour, similarly as for melanoma cells19. As the Thy1 protein may show up on activated microvascular EC, this mouse strain can also be used in angiogenesis studies.
Following a tissue injury, cellular debris and potential infective agents must be removed by a distinct set of immune cells, such as neutrophils, macrophages, and mast cells. Fibroblasts and mesenchymal stem cells then enter to the wound site, and the fibroblasts become activated and differentiate into myofibroblasts. The newly differentiated myofibroblasts contract the wound area, and produce extracellular matrix proteins21. It has been shown that myofibroblast differentiation requires Thy1 expression, and impaired wound-healing was detected in Thy1(-/-) mice, and temporally blocking Thy1 in skin wound regions worsens the quality of healing22,23. Moreover, only Thy1(+) human myometrial and orbital fibroblasts were capable of myofibroblast differentiation24. In contrast, Thy1 promotes the development of lipofibroblasts, and not myofibroblasts in the mouse lung25,26,27. The well circumscribed fluorescent margin around the healing area probably comprises mainly myofibroblasts and MSCs, but migrating keratinocytes, immune cells and microvascular epithelial cells may also be present28,29. We therefore propose the B6.Cg-Tg(Thy1-YFP)16Jrs/J mice, or the same Thy1-YFP transgene carrying mouse strains as a model in wound- healing studies, where impaired healing or the therapeutic effects of various pharmacological agents could be investigated. Further these results also support the view of authors who regard tumours as “over-healing” wounds30.
It seems that an elevated cell surface expression of Thy1 contributes to cell-cell interactions, where migrating cells have to be attached to their target, such as different immune cells at the site of inflammation, or fibroblasts and stem cells in the wound area. Thy1 then serves as a receptor for other cell surface proteins on adjacent or “trapped” migrating cells, and could also initiate signalling events itself. Moreover, Thy1 facilitates the metastasis of certain tumour cells through activated microvascular EC. We could track this elevated expression by following the higher fluorescent signal of YFP. Since YFP expression is not controlled by all of the regulatory elements of the wild type Thy1 gene, and exon 3 and its flanking introns (known to be responsible for Thy1 expression in non-neural cells) were deleted, the promoter and the remaining intragenic regulatory sequences could still direct higher YFP expression in some non-neural cells, as a response to activating signals. This could be especially interesting in studies of different tumours, where the high diversity of the tumour and stromal cells and the surrounding tissue could also affect tumour development and its metastatic capabilities. Impaired wound healing could also be followed through the different expression of the YFP marker gene, because some of the immune cells, keratinocytes, activated fibroblasts and stem cells respond to injury with an elevated expression of Thy1 gene.
As the Thy1 cell surface glycoprotein affects adhesion, signalling and motility in many cell types, and its elevated expression was demonstrated at the site of inflammation, in the formation of the tumour stroma, and during tissue remodelling in the case of injury, the elevated expression of the YFP transgene could serve as a fluorescent marker that follows these events. We plan further studies to dissect the activation parameters of the Thy1-YFP transgene, as well detailed analysis of the cell types expressing it. In conclusion, we propose that these mouse strains could also be used as an inflammation, wound-healing and tumour model, over and above its well exploited potential in neuronal development studies.
Methods
Animals
The JAX mouse strain B6.Cg-Tg(Thy1-YFP)16Jrs/J was obtained from The Jackson Laboratory. The mice were kept in a conventional animal house. Originally the transgene was present on the heavily pigmented B6 mouse strain genetic background. To facilitate the in vivo observation of the YFP fluorescence, transgenic mice were back-crossed on non-pigmented BALB/c for at least 6 generations. The BALB/c mice were obtained from Charles River Hungary. The presence of the transgene in the back-crossed generations was visually verified by in vivo fluorescent microscopy of the newborn mice by a long-working-distance stereo microscope, making genetic testing unnecessary. To obtain a homogeneous fluorescence level, all the experimental mice were hemizygous for the transgene.
Ethics
All experimental protocol were performed in accordance with national (1998. XXVIII; 40/2013) and European (2010/63/EU) animal ethics guidelines. The were approved by the Animal Experimentation and Ethics Committee of the Biological Research Centre of the Hungarian Academy of Sciences and the Hungarian National Animal Experimentation and Ethics Board (clearance numbers: XVI./03047-1/2008; SI/01/1489-5/2014.)
Cell cultures
The 4T1 mouse mammary carcinoma cell line was obtained from ATCC and kept in RPMI 1640 tissue culture medium supplemented with 10% FCS (Lonza, Basel, Switzerland), and 1 mM pyruvate (Sigma, St. Louis, USA). Red fluorescent clones were established after the transfection of 4T1 cells with pEF1α-tdTomato plasmid (a kind gift of the Tsien Lab), and then selected on 400 μg/ml G418 (Sigma, St. Louis, USA) containing medium. The MC26 BALB/c colon carcinoma cell line was originally obtained from the National Institute of Radioprotection and Radiobiology, Hungary, and kept in RPMI 1640 tissue culture medium supplemented with 10% FCS and 1 mM pyruvate.
In vivo tumourigenesis, inflammation and wound healing
4T1 cells were injected subcutaneously (s.c.) either in the mammary fat pad of the flank, or s.c. into the ear pinna of the BALB/c(Thy1-YFP) or BALB/c mice. The orthotopically growing 4T1 tumour line forms metastases in the lung, while other organs are mostly metastasis-free. To obtain mice with metastases in other organs, in some of the experiments the mice were injected intravenously with 105 4T1 tumour cells. In these cases, the numerous lung metastases were accompanied by liver, kidney and, rarely, heart metastases. MC26 cells were injected s.c. into the ear pinna. The tumour-bearing mice were euthanized before the large tumours could cause discomfort. The effects of non-tumour specific inflammation on the Thy1 expression was also tested by injecting the ears of both B6.Cg-Tg(Thy1-YFP)16Jrs/J and BALB/c(Thy1-YFP) mice with 20 μl complete Freund's adjuvant (Sigma, St. Louis, USA) or sterile physiological saline solution s.c. In another series of experiments, superficial cuts were made with a sterile scalpel blade in the ear pinnae of the mice under sodium pentobarbital (Nembutal) anaesthesia. The wound- healing process was followed by fluorescent microscopy for 36 days.
Imaging studies
The photographs were made with a Nikon Eclipse E600 fluorescent microscope, using the B-2A filter block (EX:450; DM > 505; BA:520). The micrographs were made with a SPOT RT-SE™ Digital Camera (Diagnostic Instruments) or with a Nikon D5000 camera. Frozen tissue sections were analyzed by Zeiss Axio Imager Z1 fluorescent microscope and Zeiss AxioVision 4.6.3 software. The contrast of the micrographs was adjusted with the Adobe Photoshop software. The same corrections were applied for the experimental samples and their appropriate controls. The YFP fluorescence was visualized with a pseudo-green colour.
Author Contributions
K.J. and C.V. conceived the experiments, Z.W., A.M. and L.P. performed the animal experimentation, R.L.K., K.J. and L.P. performed the in vitro experiments, A.B. and Z.W. prepared the images, K.J., G.S., Z.O. and C.V. wrote the manusctript.
Supplementary Material
Supplementary information, Josvay et al.
Acknowledgments
This work was supported by the following grants: FP7-HEALTH-2012-INNOVATION-1, Proposal No: 305341-2, CTCtrap; GOP-1.1.1-11-2011-0003; ERC_HU_09 3D_TRPV1; and OMFB-01813/2009. The authors would like to express their appreciation to David Durham, Ph.D., for proof reading the manuscript.
References
- Rege T. A. & Hagood J. S. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta 1763, 991–999 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. A., Misgeld T., Lichtman J. W. & Sanes J. R. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J Neurosci 23, 11479–11488 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre F. M., Kerschensteiner M., Misgeld T. & Sanes J. R. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med 11, 1355–1360 (2005). [DOI] [PubMed] [Google Scholar]
- Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods 71, 3–9 (1997). [DOI] [PubMed] [Google Scholar]
- Feng G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000). [DOI] [PubMed] [Google Scholar]
- Keller-Peck C. R. et al. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron 31, 381–394 (2001). [DOI] [PubMed] [Google Scholar]
- Nguyen Q. T., Sanes J. R. & Lichtman J. W. Pre-existing pathways promote precise projection patterns. Nat Neurosci 5, 861–867 (2002). [DOI] [PubMed] [Google Scholar]
- Chen Y. S., Chung S. S. & Chung S. K. Noninvasive monitoring of diabetes-induced cutaneous nerve fiber loss and hypoalgesia in thy1-YFP transgenic mice. Diabetes 54, 3112–3118 (2005). [DOI] [PubMed] [Google Scholar]
- Porrero C., Rubio-Garrido P., Avendano C. & Clasca F. Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice. Brain Res 1345, 59–72 (2010). [DOI] [PubMed] [Google Scholar]
- Vidal M., Morris R., Grosveld F. & Spanopoulou E. Tissue-specific control elements of the Thy-1 gene. EMBO J 9, 833–840 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- True L. D. et al. CD90/THY1 is overexpressed in prostate cancer-associated fibroblasts and could serve as a cancer biomarker. Mod Pathol 23, 1346–1356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A. E. & Allen J. M. The Akr Thymic Antigen and Its Distribution in Leukemias and Nervous Tissues. J Exp Med 120, 413–433 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A. E. & Allen J. M. Mouse nervous tissue iso-antigens. Nature 209, 523 (1966). [DOI] [PubMed] [Google Scholar]
- Reif A. E. & Allen J. M. Mouse thymic iso-antigens. Nature 209, 521–523 (1966). [DOI] [PubMed] [Google Scholar]
- Haeryfar S. M. & Hoskin D. W. Thy-1: more than a mouse pan-T cell marker. J Immunol 173, 3581–3588 (2004). [DOI] [PubMed] [Google Scholar]
- Avalos A. M. et al. Neuronal Thy-1 induces astrocyte adhesion by engaging syndecan-4 in a cooperative interaction with alphavbeta3 integrin that activates PKCalpha and RhoA. J Cell Sci 122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker T. H. & Hagood J. S. Getting a grip on Thy-1 signaling. Biochim Biophys Acta 1793, 921–923 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker T. H. et al. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res 295, 488–496 (2004). [DOI] [PubMed] [Google Scholar]
- Schubert K., Gutknecht D., Koberle M., Anderegg U. & Saalbach A. Melanoma cells use Thy-1 (CD90) on endothelial cells for metastasis formation. Am J Pathol 182, 266–276 (2013). [DOI] [PubMed] [Google Scholar]
- Spaeth E. L. et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 4, e4992 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol 127, 526–537 (2007). [DOI] [PubMed] [Google Scholar]
- Rege T. A. & Hagood J. S. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J 20, 1045–1054 (2006). [DOI] [PubMed] [Google Scholar]
- Lee M. J., Shin J. O. & Jung H. S. Thy-1 knockdown retards wound repair in mouse skin. J Dermatol Sci 69, 95–104 (2013). [DOI] [PubMed] [Google Scholar]
- Koumas L., Smith T. J., Feldon S., Blumberg N. & Phipps R. P. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol 163, 1291–1300 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagood J. S. et al. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol 167, 365–379 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varisco B. M., Ambalavanan N., Whitsett J. A. & Hagood J. S. Thy-1 signals through PPARgamma to promote lipofibroblast differentiation in the developing lung. Am J Respir Cell Mol Biol 46, 765–772 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis K., Liu X. & Hagood J. S. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med 13, e27 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y. et al. Expression of CD90 on keratinocyte stem/progenitor cells. Br J Dermatol 154, 1062–1070 (2006). [DOI] [PubMed] [Google Scholar]
- Barisic-Dujmovic T., Boban I. & Clark S. H. Fibroblasts/myofibroblasts that participate in cutaneous wound healing are not derived from circulating progenitor cells. J Cell Physiol 222, 703–712 (2010). [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315, 1650–1659 (1986). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information, Josvay et al.