Abstract
Research suggests that use and abuse of marijuana can be especially harmful if it occurs during adolescence, a period of vast developmental changes throughout the brain. We examined the effects of 2 mg/kg Δ9-tetrahydrocannabinol (THC) administered daily via intra-peritoneal injections during juvenile/early adolescence (postnatal day 22–40) or late adolescence (postnatal day 41–60) on locomotor activity, development of tolerance, and acquisition/retention of spatial avoidance in adulthood. THC caused locomotor depression in both male and female animals dosed during early adolescence but only in female animals dosed during late adolescence. Evidence of reverse tolerance to THC was seen in early adolescent animals only. In the active place avoidance test (APA), male and female animals administered THC during early adolescence made more errors on the reversal trial requiring flexibility in learning, but in animals dosed during late adolescence there were no significant sex or treatment differences. The results of the locomotor activity study indicate that females may be more sensitive to the effects of THC than males, while results of both locomotor activity and APA studies suggest that early adolescents appear to be more vulnerable to these effects than late adolescents/young adults.
Keywords: tetrahydrocannabinol, locomotor activity, avoidance, rodent, cannabinoid, adolescence
1. Introduction
Marijuana (cannabis sativa) remains one of the most widely used illegal drugs, with adolescents being particularly vulnerable to its use and abuse [46]. The percentage of adolescents reporting lifetime use of marijuana rises steadily from 14.2% in eighth grade to 41.8% by twelfth grade, with a majority of eighth through twelfth graders reporting that they are at a ‘great risk’ to try marijuana regularly [22]. Increased risk taking and novelty seeking behaviors during adolescence [46] may increase the tendency to try drugs of abuse. In humans, the use of marijuana causes a variety of different effects including short- and long-term memory impairment, antinociception, perceptual changes, motor incoordination, poor executive function, and long-term oculomotor impairment. Similarly, in rodents the drug has been found to induce memory and cognitive deficits, motor dysfunction, hypoactivity, immobility, and antinociception (see [2] and [21] for review). The increased tendency towards drug use during adolescence occurs during a period in which there are marked developmental changes occurring in the brain. Juvenile and adolescent brain imaging studies indicate changes in both gray and white matter volume as well as the development and pruning of fiber tracts in the cerebellum, basal ganglia and hippocampus, changes which indicate improved cognitive capabilities, movement and motor control (amongst other abilities) [7, 24, 28, 45]. The ubiquitous changes occurring in the brain during the adolescent period, including changes in the endogenous cannabinoid system, make the brain vulnerable to disruption in its development by tetrahydrocannabinol (THC) [7].
THC, the main psychoactive component in marijuana, targets cannabinoid type 1 (CB1) receptors within the brain, lungs, liver and kidneys, and cannabinoid type 2 (CB2) receptors primarily in t-cells and macrophages. Within the central nervous system, the greatest density of CB1 receptors is found in the cerebellum, basal ganglia and CA1, CA3 and dentate gyrus areas of the hippocampal formation [18, 19]. Normally stimulated by endogenous cannabinoids (endocannabinoids), the stimulation of presynaptic G-protein coupled receptors can alter the release of neurotransmitters at the synapse and is responsible for many of the behavioral effects seen with marijuana consumption. The interaction of CB1 receptors and THC may be especially detrimental during adolescence, a time period in which neuronal connections are still undergoing plastic changes. This interaction can alter neuronal circuits, potentially causing long-lasting functional alterations in multiple systems. For example, Rubino et al. [40] found significantly decreased levels of both CB1 receptor binding and CB1 receptor/G-protein coupling in several brain areas of adult rats (between postnatal day 75 to 80) exposed to THC (an increasing dose ranging from 2.5–10 mg/kg intraperitoneally) during adolescence (postnatal day 35 to 45). These areas include the nucleus accumbens, amygdala and ventral tegmental area in females and amygdala and hippocampus in males, indicating altered neuronal activity in the brains of these rats persisting into adulthood. Additionally, Whitlow, Freedland and Porrino [52] found decreased rates of glucose utilization in the nucleus accumbens, caudate, basolateral amygdala, globus pallidus, and CA1 and CA3 regions of the hippocampus after 7 and 21 days of daily 10mg/kg daily THC exposure, in male adult rats weighing between 300–375g. These changes in neuronal function may underlie the behavioral alterations observed following chronic THC exposure. It is important to note that while we are administering THC in our research, street marijuana consists of numerous cannabinoid compounds in addition to THC including cannabidiol and cannabigerol. These compounds may interact with each other, or with tobacco and nicotine found in blunts, to produce effects that will not occur with THC alone. Additionally, the concentration of THC in marijuana cigarettes varies from year to year (see Cooper and Haney [12] and Hunault et al. [20]). We are using THC alone because of its role as the main psychoactive component in marijuana and think it is important to examine its effects before we embark upon studying marijuana with various cannabinoid components as it is available outside on the street.
Previous studies have demonstrated the role of the cannabinoid system in locomotor depression as well as memory impairment in adolescent and adult rats and mice [11, 35, 41, 42, 51]. More specifically, both acute and chronic doses of THC have been shown to cause impairment in attention and motor coordination in a maze task in humans [49], inhibition of movement and basal ganglia neuronal activity in adult rats [43], decreased locomotor activity in rats [51], and impaired spatial and non-spatial memory in adolescent and adult rats and mice [8, 27, 31, 34]. These effects can become increasingly severe the earlier in life the individual begins to use marijuana [33, 34], and are often greater in adolescents than in adults [8].
Tolerance to many of the behavioral effects of THC has been shown to occur in mice, rats and humans [1, 3, 31]. Receptor downregulation and internalization are two of the potential mechanisms responsible for the development of tolerance [38]. Breivogel et al. [5] found pronounced decreases in cannabinoid-stimulated G-protein binding and CB1 receptor binding in the rat hippocampus, and less pronounced changes in the striatum and globus pallidus after chronic THC, an indication that with time a greater amount of THC might be needed to elicit the same behavioral effects.
Although numerous studies have investigated the effects of THC on motor activity and memory acquisition, retrieval and reversal training, fewer have looked at these effects in adolescent animals, and fewer still have investigated any differential effects based upon sex. For this reason, we looked at the effects of chronic THC exposure during either the juvenile/early adolescent period or late adolescence in male and female rats on locomotor activity, the development of tolerance during drug administration and on visuo-spatial learning and memory in adulthood.
2. Methods and Materials
All Procedures were carried out in accordance with NIH-approved standards under IACUC approval.
2.1 Subjects and Drugs
2.1.1 Group 1
Male and female Sprague Dawley rats (Charles River, Wilmington, MA) were housed in a reverse 12 hr light/dark cycle with lights off at 11:00am and ad libitum access to food and water. The reverse light cycle was chosen for this study because we anticipated subtle effects of THC on locomotor activity and wanted to maintain relatively natural conditions. Animals arrived at the lab between postnatal days (PND) 2–5 in natural litters. Animals were weaned at PND 21, housed in same-sex cages, and were then dosed daily from PND 22–40, a time period approximating the juvenile period to early adolescence in humans (age delineations were adapted from Spear, 46). Each rat was dosed with 2 mg/kg Δ9-tetrahydrocannabinol (THC) (RTI, Research Triangle Park, North Carolina) in pluronic acid (Sigma-Aldrich, Inc., St. Louis, MO)/saline or pluronic acid/saline vehicle via intra-peritoneal (IP) injection. For each litter, THC was assigned randomly to approximately half of the male and half of the female rats; the other half received the vehicle. This dose had previously been shown to produce effects on locomotor activity by our lab (data not shown), but does not produce catalepsy, as is demonstrated by Wiley et al. [53].
2.1.2 Group 2
Subjects were male and female Sprague Dawley rats (Charles River, Wilmington, MA) weaned at PND 21 and housed in the same conditions as Group 1. Natural litters arrived at the lab on PND 21 with dams and were housed in the reverse light cycle. Rats were administered 2 mg/kg Δ9-THC or vehicle, as above; however the daily dosing period was from PND 41–60, a period approximating late adolescence in humans. Treatment was assigned to rats in each litter as described above.
While both group 1 and group 2 rats remained in our housing conditions for approximately the same amount of time before injections began, shipping group 2 rats at this later age, and weaning on the same day as arrival, is expected to produce a different stress level than shipping group 1 rats at PND 2–5. However, we felt that the duration of housing in our vivariam under reversed light cycle conditions should be the same for both groups. Additionally, this difference in groups 1 and 2 would have little effect on the results since the control groups within each exposure group were litter mates of the treated rats and received identical housing conditions as the THC-treated rats.
2.2 Locomotor Activity
Locomotor activity was assessed using Accuscan equipment consisting of clear Plexiglas boxes placed inside the Accuscan recording chambers, recording the interruption of photobeams. Animals were placed into the chambers immediately after injection with the daily dose of THC or vehicle and movement was recorded during 12 5-minute time blocks, for a total of an hour. Upon completion, animals were removed from the Accuscan chamber and placed back into the home cage. Chambers were cleaned with a 30% ethanol/water solution after each trial. Group 1 locomotor activity was assessed on PND 22, 29 and 40 (see Table 1). Nine litters were used for group 1, with 8 rats per litter, for a total of 72 rats (36 males and 36 females). Half of the male and female rats were given THC, and half of the male and female rats were given the vehicle. Group 2 locomotor activity was assessed on PND 41, 48 and 60 (see Table 1). Six litters were used for group 2, with 8 rats per litter, for a total of 48 rats (24 males and 24 females). Half of the male and female rats were given THC, and the other half were controls.
Table 1.
Timeline of Dosing and Experiments.
| Age Group | ||
|---|---|---|
| Dosing | Juvenile/Early Adolescence Daily PND 22–40 | Mid adolescence – young adulthood Daily PND 41–60 |
| Locomotor Activity Testing | Postnatal days 22, 29, 40 | Postnatal days 41, 48, 60 |
| Active Place Avoidance Testing | Postnatal day 73 | Postnatal day 76 |
2.3 Active Place Avoidance
Equipment used was a Bio-Signal Group (Denver, USA) active place avoidance task. The experiment took place inside of a small (92”× 77” × 93”), darkened room. Various visual cues (e.g., a large white circle, large white square) were located about the room. The testing equipment consisted of an arena, a metal disk (32.5” diameter) that rotated at the rate of 1 Hz carrying a stationary animal into the shock zone at the same rate. A segment of the arena, fixed with respect to the room, was designated the ‘shock zone’ – an electrified segment in which the animal would receive a mild 1µA footshock, with a duration of 500ms and an inter-shock interval of 1500ms. A tall Perspex wall enclosed the arena so that the animal could not escape, yet could see the cues located about the room. The animals’ position on the arena was tracked by an infrared camera which monitored an LED light clipped to the rat’s back, recording the animal’s position in the arena and the room. Animals were placed onto the arena on the side opposite the shock zone. Group 1 Active Place Avoidance (APA) performance was assessed after PND 73, while Group 2 APA performance was assessed after PND 76, both during adulthood (see Table 1). A total of four litters was used for group 1, and four litters for group 2. There were 8 rats per litter, for a total of 32 rats (16 males and 16 females). Half of the male and female rats were given THC, and the other half were controls. There were three phases to the study, as follows.
Acquisition
On day 1, animals were given seven 10-minute acquisition trials (each separated by a 10-minute rest interval) in which to learn to avoid the shock zone. Total number of entries into shock zone, total number of shocks, time to 1st entry, and the maximum time spent avoiding the shock zone were calculated as measures of performance.
Retention
On day 2, animals were given one 10-minute retention trial with shock off. Total number of entries into shock zone, total number of shocks, time to first entry and the maximum time spent avoiding the shock zone were calculated as measures of performance.
Reversal
Following the retention phase on day 2, animals were given a rest interval of 10-minutes. They were then given a 20-minute shift trial, with shock on, in which the shock zone was shifted 180 degrees. Total number of entries into shock zone, total number of shocks, and the maximum time spent avoiding the shock zone were calculated for the first and second 10 minutes of the 20-minute session as measures of performance. Data for the first 10 minutes and second 10 minutes were analyzed separately since the effects of reversal on performance are maximal in the initial reversal period.
2.4 Statistical Analysis
Data were analyzed by SAS Statistical Software, v. 9.2 (SAS Institute Inc., Cary, NC). For all tests, a significance level of p<0.05 was used.
2.4.1 Locomotor activity analysis
A separate mixed linear model analysis was done for each of the four sex-dose period strata. In each stratum, the dependent variable was the distance moved (square root-transformed to reduce skew and stabilize variance since the statistical model requires normally distributed data); fixed factor terms were within session (min 1–30, min 31–60), treatment (0, 2), and day (1, 2, 3), along with their respective interactions. Litter was introduced as a random factor; within-subject covariance was modeled as compound-symmetric both across days and (as a separate term) within days. About 1% of observations were excluded as outliers. Satterthwaite corrections to denominator degrees of freedom were applied in order to balance out data in cases with unequal sample sizes or missing data (which were minimal in this case). Simple effects post-hoc analyses were conducted to clarify significant interaction effects.
2.4.2 Active place avoidance analysis
For the 7th acquisition trial, a Cox Regression (a general method of modeling right-censored outcomes) was used to analyze the maximum time spent avoiding the shock zone, and an exact logistic regression analysis with the number of shocks/entries dichotomized as 0 versus greater than 0 as the dependent variable, treatment and sex as independent variables, and litter as random independent variable was used to analyze the number of entries/shocks. For the retention and reversal trials, a Cox regression analysis was used to analyze the maximum time spent avoiding the shock zone. Additionally, a linear model analysis with the square root of number of entries as the dependent variable, treatment and sex as independent variables, and litters as a random independent variable was used to analyze the number of entries into the shock zone and the number of shocks received.
3. Results
3.1 Group 1
A total of 9 litters, or 72 rats, was tested for locomotor activity. A total of 4 litters, or 32 rats, was tested for Active Place Avoidance performance.
3.1.1 Locomotor Activity
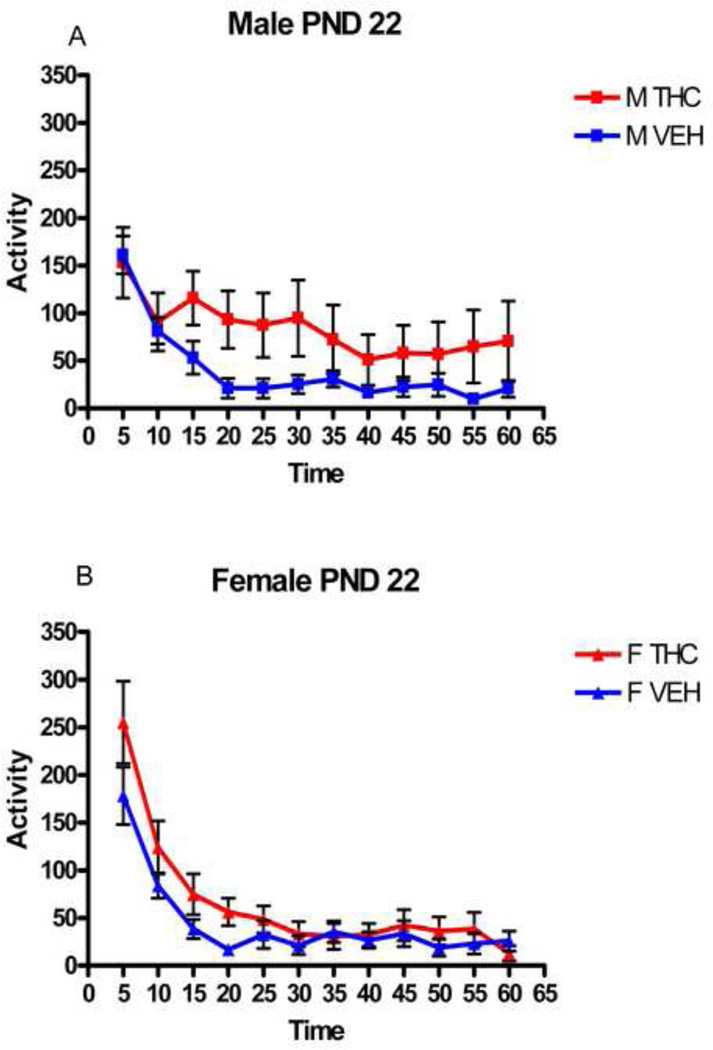
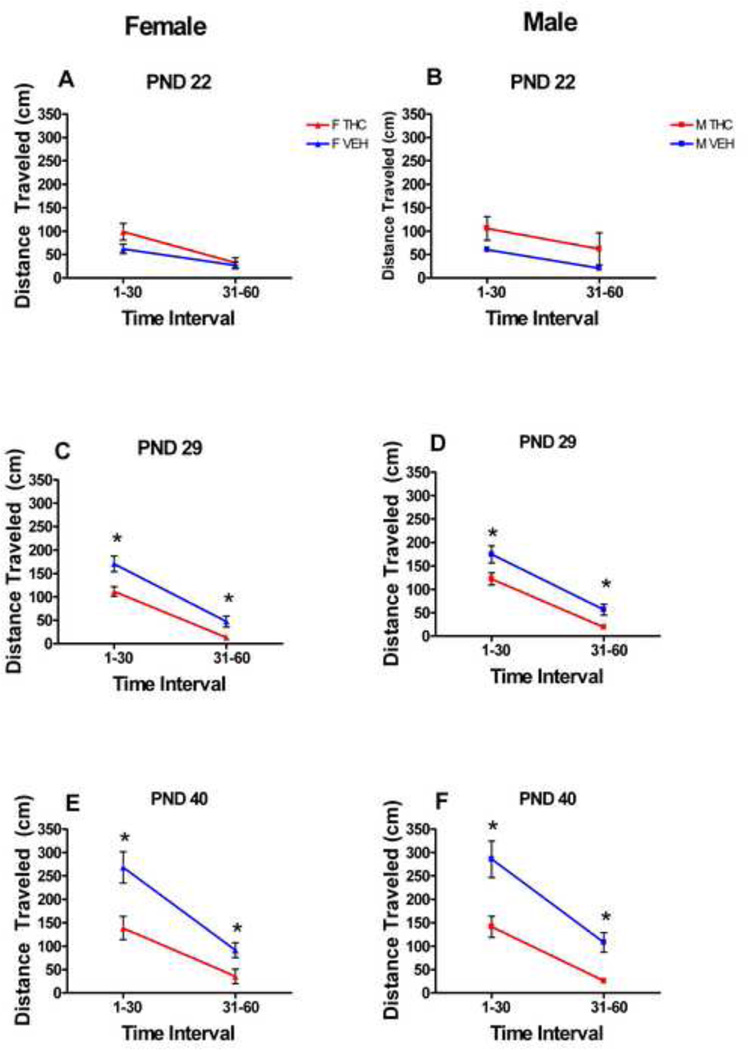
Locomotor activity, originally recorded in 12 5-minute time blocks, was combined into two time blocks for analysis and presentation: minutes 1–30 and minutes 31—60. Figures 1 A and B portray a typical locomotor activity curve for the 12 time points across the hour as originally collected while figures 2 A–F show the averaged locomotor activity as was analyzed.
Figure 1.
Figure 2.
For animals dosed during early adolescence, the administration of THC caused significant locomotor depression for both female and male rats on two of the three testing days. On PND 22, the first day of treatment, THC had no significant effect on locomotor activity in either sex. However, there were main effects of treatment on both PND 29 and PND 40 (the second and last days of testing) [(F[1,60]=26.0, p<0.001), (F[1,58]=50.8, p<0.001), respectively] (Fig. 2 C–F). No tolerance was seen; instead, a type of reverse-tolerance or sensitization was seen: the locomotor activity of male and female THC rats on both of these days was significantly depressed compared to that of controls. Overall, the locomotor activity increased with increasing age. THC treatment blunted these increases in both male and female rats.
Additionally, at each age, there were significant main effects of time block on PND 22 (F[1,60]=146, p<0.001), PND 29 (F[1,66]=505, p<0.001) and PND 40 (F[1,3]=317, p<0.001), with the locomotor activity of both males and females decreasing, in general, across the one hour session on all three test days. There was also an interaction of treatment by time on PND 22 (F[1,60]=8.00, p=0.006).
3.1.2 Active Place Avoidance
Acquisition
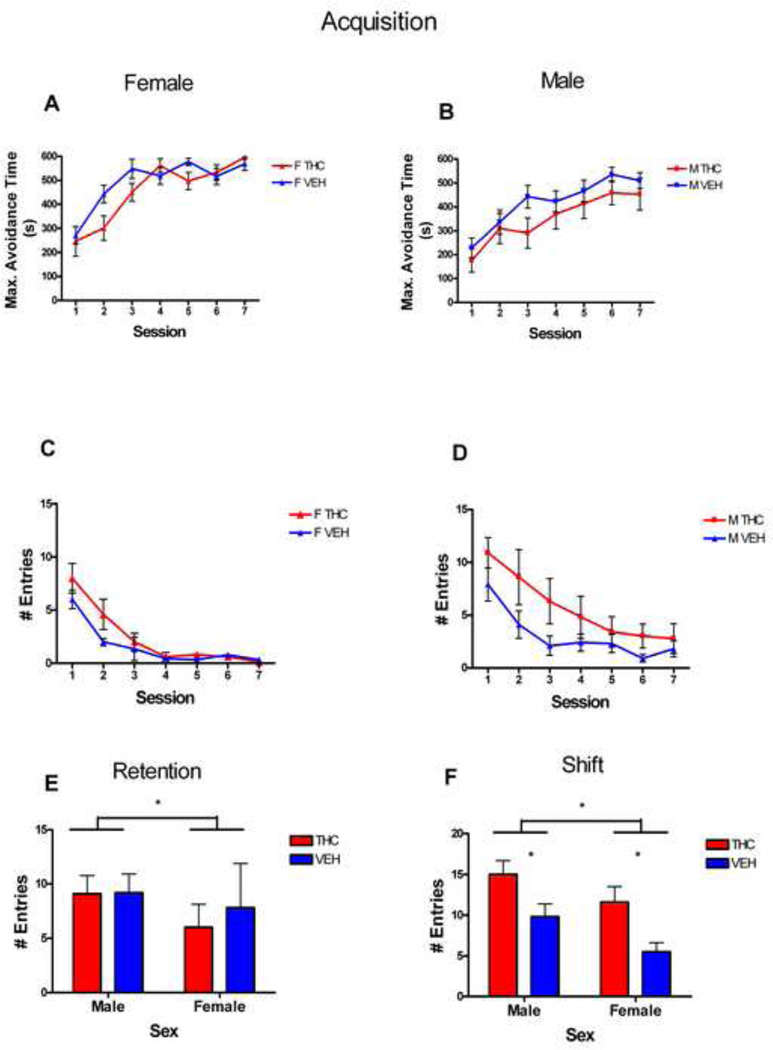
All groups were able to learn the task so that they received less than 5 entries into the shock zone by the 7th trial (Fig. 3, A–D). A Cox regression analysis of the maximum time spent avoiding the shock zone on the 7th trail revealed no treatment effects (χ2(1)=4.66, p=0.926), as well as no sex by treatment interaction (χ2(1)=0.323, p=0.570). There was a significant effect of sex with females spending a greater maximum time avoiding the shock zone than males by the 7th trial (χ2(1)=4.66, p=0.031).
Figure 3.
Retention
While there were no significant treatment effects, analysis revealed a main effect of sex (F[1,22]=9.07, p<0.001) for number of entries into the shock zone (Fig. 3E), with females showing fewer entries during retention than males. Female animals also received significantly fewer shocks (F[1,22]=9.07, p=0.006) and had a greater time to first entry (F[1,22]=8.728, p=0.006) than males.
Reversal
There were no significant treatment effects in the second half of the test and so the data are not shown. In the first 10 minutes, there were significant effects of treatment (F[1,32]=11.59, p=0.002), with treated animals making more entries into the shock zone than controls (Fig. 3F). There was also a significant effect of sex (F[1,32]=5.06, p=0.031), with male animals making more entries into the shock zone than female animals (Fig. 3F). Similarly, there were main effects of treatment (F[1,33]=12.34, p=0.001) and sex (F[1,33]=10.67, p=0.003) for the number of shocks received during the shift session, with treated animals receiving more shocks than controls, and male animals receiving more shocks than females.
3.2 Group 2
A total of 6 litters, or 48 rats, was tested for their locomotor activity. A total of 4 litters, or 32 rats, was tested for their active place avoidance performance.
3.2.1 Locomotor Activity
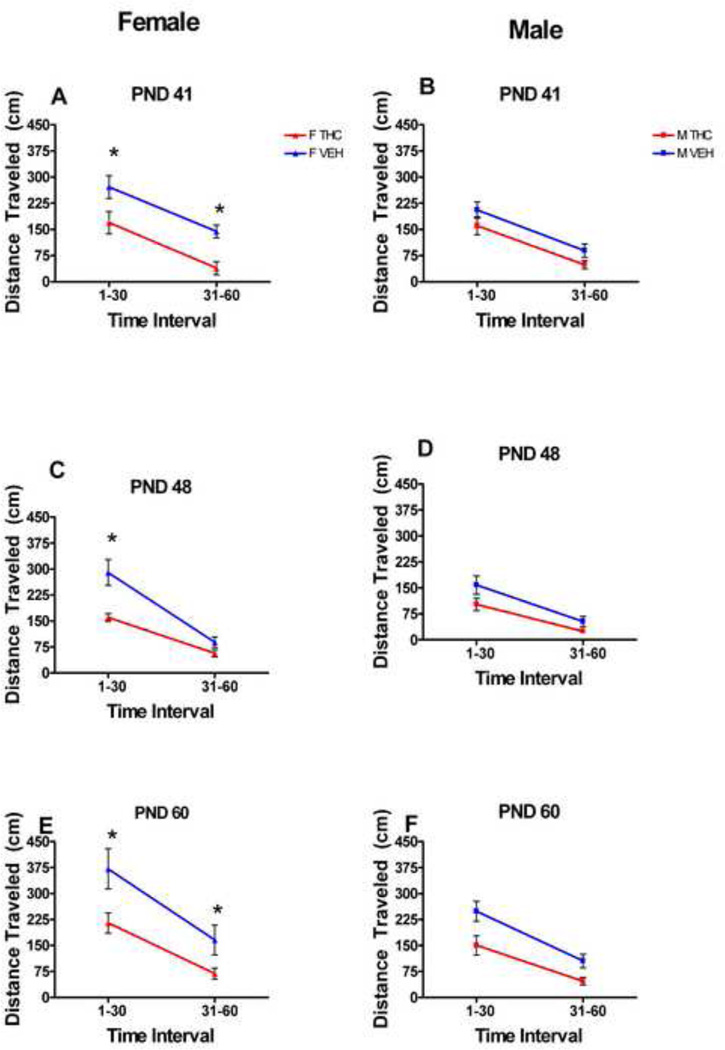
Locomotor activity was again combined into two time blocks for analysis: minutes 1–30 and minutes 31–60 (figures 4A–F).
Figure 4.
There was a significant sex by treatment interaction on the first day of dosing, PND 41 (F[1,42]=4.08, p=0.050), and significant main effects of sex on PND 48 (F[1,41]=19.2, p<0.001) and on PND 60 (F[1,4]=4.13, p=0.048). There were also significant main effects of time on both PND 48 (F[1,44]=195, p<0.001) and PND 60 (F[1,44]=11, p<0.001), with the locomotor activity of all groups decreasing, in general, across the testing hour (Fig. 4A–F).
For animals dosed during late adolescence, the administration of THC caused significant locomotor depression in the females, but not males. Female treated rats’ locomotor activity was significantly depressed when compared to female controls during minutes 1–30 and 31–60 on PND 41 (the first day of dosing/testing), minutes 1–30 on PND 48 (the second day of testing and 7th day of dosing), and minutes 1–30 and 31–60 on PND 60 (the last day of both dosing and testing) (Fig. 4, A,C,E). The locomotor activity of both the treated and control females, on the whole, did not change significantly over the three testing days. Locomotor activity in the treated group remained depressed at all but one time interval compared to controls. Therefore, there was no development of tolerance or sensitization in the treated females. There were no significant differences between treated male locomotor activity and control male locomotor activity on any of the three testing days (Fig. 4, B, D, F).
3.2.2 Active Place Avoidance (data not shown)
Acquisition
All animals learned to avoid the shock zone by the 7th trial. Statistical analysis of performance by the end of the acquisition trials on day 1, approximated by number of shocks in trial 7, number of entries into the shock zone, and the maximum time spent avoiding the shock zone showed no effects of treatment, sex, nor a treatment by sex interaction.
Retention
Cox regression analysis of the maximum time spent avoiding the shock zone revealed no effects of treatment, sex, or a treatment by sex interaction. Mixed linear model analysis of number of shocks and number of entries into the shock zone similarly showed no treatment effects, sex effects, or treatment by sex interactions, nor did an ANOVA of time to first entry.
Reversal
Cox regression analysis of the maximum time spent avoiding the shock zone revealed no effects of treatment, sex, or a treatment by sex interaction. Mixed linear model analysis of number of shocks and number of entries into the shock zone showed no treatment effects, no sex effects, and no treatment by sex interaction.
4. Discussion
4.1
In the present study, we looked at the effects of THC on locomotor activity and the development of tolerance in 22–40 and 41–60 day old rats. We confirmed that THC is a locomotor depressant in both male and female animals dosed during the juvenile period/early adolescence. However, for animals dosed during late adolescence, THC caused locomotor depression only in female animals with no significant effects seen in males. Reverse tolerance, or greater locomotor depression with repeated dosing, was seen in treated male and female younger animals, but not in male or female older animals. We also examined the long-term effects of THC exposure on visual spatial learning and memory in adulthood using the active place avoidance (APA) test. The results of our active place avoidance study indicate that THC administration during early adolescence had no effect on acquisition but decreased performance on the reversal trial in both males and females. In both the retention and reversal trials, performance of females was superior to that of males overall. However, THC administration during late adolescence produced no significant effects on the APA test. Therefore, for both locomotor activity and active place avoidance tests, early adolescence appeared to be more sensitive to the effects of THC than late adolescence. These results indicate that the time period during adolescence in which THC is administered can have a great influence on the effects it produces.
4.2 Locomotor activity
The Accuscan recording chamber is an open field environment that enables free exploration. We found that, overall, the greatest amount of locomotor activity (the greatest total distance travelled) occurred within the first 20 minutes of the one-hour test. Additionally, exploration of the recording chamber increased overall with age and repeated exposure to the testing chamber, especially in the control animals. Others have reported that exploratory activity that occurs when a rat is placed in a novel environment increases during the peri-adolescent period (see [46] for review). Therefore, our control data are in general agreement with the increase in locomotor activity with increasing age during adolescence.
Due to the large number of cannabinoid receptors located throughout the basal ganglia, the endocannabinoid system plays a crucial role in motor control. Within this area associated with selecting, filtering, and initiating motor programs such as those involving locomotion [17], CB1 receptors are located on the presynaptic terminals of medium spiny GABAergic neurons [44]. In our experiment THC generally reduced locomotor activity for animals dosed during early adolescence (Group 1). While THC caused no alteration in locomotor activity on PND 22, female treated rats showed locomotor depression compared to female controls after several days of dosing. In fact, female control locomotor activity increased across the three testing days while locomotor activity in female treated rats remained depressed. Similarly, male treated rats also showed locomotor depression compared to male control rats on PND 29 and 40, with male control rats’ locomotor activity increasing significantly across the 3 testing days while male treated rats’ activity remained at a depressed level. Due to persistent locomotor depression over the dosing period, there is no evidence for the development of tolerance in this group of animals. Instead, these data support the development of reverse-tolerance, or sensitization; treated animals did not show the progressively increasing activity across the three testing days that was seen with control animals, suggesting that the locomotor depression was greater as dosing continued. Sensitization to the behavioral effects of THC has been previously demonstrated in rodents [39] and may be attributed in part to changes in dopaminergic transmission in the mesolimbic pathway, particularly in the nucleus accumbens core and shell [6].
In contrast, only female animals dosed with THC during late adolescence (Group 2) showed significant locomotor depression compared to controls, occurring on all three testing days. No effect of THC on locomotor activity was seen in males. Therefore, there was no development of tolerance or reverse tolerance in either female or male animals dosed during late adolescence. This is in contrast to previous research that has demonstrated THC-induced tolerance after chronic dosing. For example, in a very comprehensive study, Wiley et al. [53] found tolerance in juvenile (PND 22–25) and adult (>PND65) rats exposed to an initial IP injection of from 1 – 300 mg/kg THC, and then a subcutaneous injection of 10mg/kg THC twice daily for nine days (until a final dose on the morning of the 10th day) in a variety of different measures including catalepsy and locomotor activity. This discrepancy may be due to differences in the age of rats, length of administration, or the pharmacokinetic effects of subcutaneous injection rather than IP injection. Another potential explanation for the lack of tolerance to the locomotor-depressing effects of THC administration in both the juvenile period/early adolescence and late adolescence is that the dose of 2 mg/kg THC is not adequate to produce maximum CB1 receptor downregulation or internalization. Alternatively, the particular G-protein subunits being affected may be more resistant to the development of tolerance than others [51]. Certainly, compensatory effects operating at this age may be playing a role here as well.
There is also indication that female rats (at least those exposed to THC during late adolescence) are more sensitive to the effects of THC than male rats. Results of previous studies reporting the differential effects of THC on the sexes are mixed. Animal studies have often shown sex differences in the metabolic processing and behavioral effects of THC. For example, adult female rats metabolize Δ9-THC preferentially to 11-hydroxy-Δ9-THC (a form equivalent or greater to Δ9-THC in its potency), while adult male rats metabolize Δ9-THC to a number of different compounds [30], a factor that could explain the greater behavioral effects often seen in females when compared to males. Additionally, brain concentrations of THC and its metabolites have been found to be greater in adult female rats than males 120 minutes after IP injection of 5µCi/kg of THC. Rubino et al. [40] found significantly reduced levels of CB1 receptor density and G-protein coupling in the ventral tegmental area, nucleus accumbens, and amygdala in PND 46 adolescent female rats dosed with THC from PND 35–45, while adolescent male rats displayed smaller changes, again suggesting that females are especially vulnerable to the effects of THC during adolescence. Adding to this, male rats have more body fat (especially during late adolescence), a tissue in which THC is sequestered [29].
Hormones may also exert some control over the differences in behavioral effects seen between males and females [13]. Rodriguez de Fonseca et al. [37] found that both CB1 receptor density and affinity fluctuated in female rats across the estrous cycle in different areas of the brain (for example, the density within the medial basal hypothalamus was higher in diestrus than estrus, while the affinity in the limbic forebrain was increased during diestrus and lower in estrus). Males also had a greater receptor affinity in the striatum and limbic forebrain than females. However, Wiley et al. [53] found no significant differences between male and female adolescent and adult rats (within each age group) exposed to 1 mg/kg – 300 mg/kg THC via IP injections 30 minutes before testing on a number of different pharmacological and behavioral assays, including those for antinociception, hypothermia and locomotor suppression. However, the degree of the change in several of the effects studied between adolescence and adulthood was not equivalent across the sexes. Since results from human studies are varied, with THC sometimes eliciting sex differences, and other times not [25], possible sex differences in the THC response should always be considered in study design.
4.3 Active Place Avoidance
The results of the Active Place Avoidance (APA) paradigm also show differential effects based upon sex and period of adolescent THC exposure. This is a test that taps into an animal’s ability to learn and retrieve spatial information, as well as flexibility of learning. The memory for specific places based upon cues in the surrounding environment has been shown to be a hippocampal-dependent behavior in the rat [10, 15, 50]. Because of the large density of CB1 receptors located throughout the hippocampus [4], it would be reasonable to assume that any disruption of the natural developmental pattern of these receptors by pharmacological agents such as tetrahydrocannabinol might have detrimental effects on many aspects of spatial learning and memory such as task acquisition and recall ability. Additionally, because CB1 receptors are also located throughout the prefrontal cortex (PFC), THC administration could also impact executive control and decision making [23]. Interestingly, the ability to perform the APA test develops earlier in males (PND 23–24) than in females (PND 33–34), though both age ranges occur during the time course of drug administration for group 1 in the present experiment [9].
We were somewhat surprised that THC at the psychoactive dose used in our experiments did not produce impairment in task acquisition. By the 7th trial on the acquisition day, both THC animals and controls entered the shock zone less than 5 times during the 10 minute trial. Similarly, both the number of shocks and the maximum time spent avoiding the shock zone had decreased to a level indicative of learning by the 7th trial. There were also no differences seen on performance after the 24-hour retention interval in either the early or late adolescent treated groups. Perhaps if THC did disrupt hippocampal function during the period of administration, this effect may have normalized by the time the spatial navigation occurred. Also, a different dose of THC might have revealed different long-term effects.
The sole treatment effect seen in the APA test was on the second day of testing in the group exposed to THC during early adolescence. This treatment effect occurred during the reversal trial, a type of task segregation, requiring the rat to discard information about the location of the previously learned shock zone, and to learn to avoid the new shock zone, shifted 180 degrees from the previous one. To be successful on the reversal trial, the rat had to actively suppress the previously learned responses in order to perform the newly appropriate ones. Here, both male and female rats that had been administered THC during early adolescence showed an impaired ability to learn the new shock zone when compared to the control rats, entering the shock zone significantly more times and receiving a significantly greater number of shocks than the control animals. It is important to highlight the fact that only animals dosed with THC during early adolescence showed impaired performance on the reversal trial of the active place avoidance task. These results support the assertion that early adolescence may be a time period of heightened vulnerability to the effects of THC; as noted above, early adolescence is the age during which the ability to perform this task is maturing.
The importance of the cannabinoid system in the ability to shift from a previously learned task to a similar, but different, task has been documented. THC has been shown to both impair behavior flexibility (including the capability for intradimensional shifts in attention) as well as the ability to inhibit ongoing behavior in humans and rats [14, 26]. Varvel and Lichtman [48] found impaired ability in CB1 knockout mice to locate the position of a moved platform in the reversal phase of a water maze, indicating the importance of the cannabinoid system in such performance. Despite repeatedly being shown the position of the new platform, the CB1 knockout mice kept returning to the originally learned location. Senn et al. [42] similarly found decreases in performance level on the ‘reversal’ phase of a water maze in mice that had been given an extremely low dose of THC. One possible explanation for this is that THC-induced activation of cannabinoid receptors in the PFC might modulate glutamate and dopamine levels (by decreasing GABAergic inhibition), which could alter set-shifting capabilities, decision making and inhibitory control (for review, see [32]). Our data would support an effect of THC on this PFC circuit producing sub-optimal reversal performance observed with both cannabinoid agonists and in knockout mice.
4.4 Distinct Developmental Time Periods
The fact that different amounts of locomotor depression were seen in early versus late adolescence indicates that the age of THC administration has an impact on the extent of locomotor depression. This is also true of the impaired reversal ability on the APA task seen in animals dosed during early but not late adolescence. One possible explanation for this difference is that early and late adolescence may be portioned into two distinct phases in terms of cannabinoid receptor development. Evidence for this comes from the finding by Rodriguez de Fonseca et al. [36] that in males the density of cannabinoid receptors in the limbic forebrain, striatum and ventral mesencephalon increases until around PND 40, and then subsequently undergoes a pruning process until reaching adult receptor levels by PND 60. In the current study, THC was administered during the period of receptor increases (up to PND 40) as well as decreases (from PND 40 to 60) with the first exposure period producing the greatest effects. Furthermore, Ellgren et al. [16] found the amount of anandamide (an endocannabinoid) in the nucleus accumbens after exposure to 1.5 mg/kg THC every third day between PND 28 and 49 to be dependent both upon age and THC treatment. Anandamide levels rose from PND 29 (defined here as early adolescence) to a peak at PND 38 (mid-adolescence), and then declined through PND 50 (late adolescence). Additionally, CB1 receptor density in the pre-frontal cortex was shown to decrease from early to late adolescence. Though no similar data exist for CB1 levels in the striatum, Rubino et al. [40] did find reduced CB1 receptor density and G-protein coupling in the cerebellum, substantia nigra and globus pallidus in adolescent animals dosed twice daily with THC from PND 35–46 24 hours after the last injection. Taken together, these results indicate that the effects of THC administration can differ depending upon the age of the animal, and further, that the administration of THC during the period of endocannabinoid expansion produces greater long-term effects than during the period of retraction/pruning.
4.5 Limitations
There is possible concern that animals dosed with THC during the early adolescent period might exhibit lower activity levels in the Active Place Avoidance task, and therefore be more likely to be carried into the shock zone and receive more shocks. However, while male and female early adolescent and female late adolescent animals dosed with THC did show significant locomotor depression during the dosing period, there were no differences seen between the locomotor activity levels of male and female THC animals and controls during adulthood(unpublished results), ruling out simple hypoactivity as a confounding factor in the APA test. Additionally, all animals were able to learn the task equally well, an indication that they were able to move to avoid the shock zone during the initial training. One limitation to both the locomotor activity and APA studies is that only one dose of THC was tested. Although this is a psychoactive dose of THC (as evidenced by the locomotor depression it elicited), it is possible that greater long-term effects may have been found if a higher, or perhaps lower, THC dose had been tested.
4.6 Conclusions
In general, the behavioral effects of THC administration were greater following early exposure than following late exposure and females were more sensitive to the locomotordepressing effects of THC than males. While long-term effects on spatial cognition were seen only in reversal learning, adolescent THC exposure likely affects the function of other systems not studied herein. In conclusion, the results of the present study substantiate the need to not only study the behavioral and pharmacological effects of THC in adolescents, but also to look carefully at the period within adolescence during which the drug is administered as well as the sex of the subject.
Acknowledgements
The authors would like to thank Dr. Jeremy Weedon for his statistical analysis, and Stacy Stephenson and April Jackson for their technical assistance. This work was supported by NIH grant RO1 DA019348.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abood ME, Sauss C, Fan F, Tilton CL, Martin BR. Development of behavioral tolerance to delta 9-THC without alteration of cannabinoid receptor binding or mRNA levels in whole brain, Pharmacol. Biochem. Behav. 1993;46:575–579. doi: 10.1016/0091-3057(93)90546-6. [DOI] [PubMed] [Google Scholar]
- 2.Ameri A. The effects of cannabinoids on the brain. Prog. Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 3.Bass CE, Martin BR. Time course for the induction and maintenance of tolerance to Delta(9)-tetrahydrocannabinol in mice. Drug Alcohol Depend. 2000;60:113–119. doi: 10.1016/s0376-8716(99)00150-7. [DOI] [PubMed] [Google Scholar]
- 4.Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol. Teratol. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- 5.Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J. Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- 6.Cadoni C, Valentini V, Di Chiara G. Behavioral sensitization to Delta(9)-tetrahydrocannabinol and cross-sensitization with morphine: differential changes in accumbal shell and core dopamine transmission. J. Neurochem. 2008;106:1586–1593. doi: 10.1111/j.1471-4159.2008.05503.x. [DOI] [PubMed] [Google Scholar]
- 7.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats, Pharmacol. Biochem. Behav. 2006;83:448–455. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Cimadevilla JM, Fenton AA, Bures J. Transient sex differences in the between-sessions but not in the within-session memory underlying an active place avoidance task in weanling rats. Behav. Neurosci. 2001;115:695–703. doi: 10.1037//0735-7044.115.3.695. [DOI] [PubMed] [Google Scholar]
- 10.Cimadevilla JM, Arias JL. Different vulnerability in female's spatial behaviour after unilateral hippocampal inactivation. Neurosci. Lett. 2008;439:89–93. doi: 10.1016/j.neulet.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Clarke JR, Rossato JI, Monteiro S, Bevilaqua LR, Izquierdo I, Cammarota M. Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long-term memory. Neurobiol. Learn. Mem. 2008;90:374–381. doi: 10.1016/j.nlm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 2009;103:107–113. doi: 10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of Delta9-tetrahydrocannabinol in male and female rats. Eur. J. Pharmacol. 2008;578:37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Egerton A, Brett RR, Pratt JA. Acute delta9-tetrahydrocannabinol-induced deficits in reversal learning: neural correlates of affective inflexibility. Neuropsychopharmacology. 2005;30:1895–1905. doi: 10.1038/sj.npp.1300715. [DOI] [PubMed] [Google Scholar]
- 15.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur. Neuropsychopharmacol. 2008;18:826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs--roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunault CC, Mensinga TT, de Vries I, Kelholt-Dijkman HH, Hoek J, Kruidenier M, Leenders ME, Meulenbelt J. Delta-9-tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg of THC. Psychopharmacology. 2008;201:171–181. doi: 10.1007/s00213-008-1260-2. [DOI] [PubMed] [Google Scholar]
- 21.Iverson L. Cannabis and the brain. Brain. 2003;126(6):1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- 22.Johnston LD, O'Malley PM. The recanting of earlier reported drug use by young adults. NIDA Res. Monogr. 1997;167:59–80. [PubMed] [Google Scholar]
- 23.Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci. Biobehav. Rev. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- 24.Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb. Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 25.Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD. Low doses of delta-9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology. 2006;31:462–470. doi: 10.1038/sj.npp.1300871. [DOI] [PubMed] [Google Scholar]
- 26.McDonald J, Schleifer L, Richards JB, de WH. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- 27.Mishima K, Egashira N, Hirosawa N, Fujii M, Matsumoto Y, Iwasaki K, Fujiwara M. Characteristics of learning and memory impairment induced by delta9-tetrahydrocannabinol in rats. Jpn. J. Pharmacol. 2001;87:297–308. doi: 10.1254/jjp.87.297. [DOI] [PubMed] [Google Scholar]
- 28.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 29.Nahas G, Leger C, Tocque B, Hoellinger H. The kinetics of cannabinoid distribution and storage with special reference to the brain and testis. J. Clin. Pharmacol. 1981;21:208S–214S. doi: 10.1002/j.1552-4604.1981.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 30.Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of delta 9-tetrahydrocannabinol in the rat. Biochem. Pharmacol. 1991;41:1187–1194. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- 31.Oviedo A, Glowa J, Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1993;616:293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- 32.Pattij T, Wiskerke J, Schoffelmeer AN. Cannabinoid modulation of executive functions. Eur. J. Pharmacol. 2008;585:458–463. doi: 10.1016/j.ejphar.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 33.Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 34.Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA, Matsuda-Matsumoto H, Iwazaki T, McGregor IS. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- 35.Robinson L, Goonawardena AV, Pertwee RG, Hampson RE, Riedel G. The synthetic cannabinoid HU210 induces spatial memory deficits and suppresses hippocampal firing rate in rats. Br. J. Pharmacol. 2007;151:688–700. doi: 10.1038/sj.bjp.0707273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- 38.Romero J, Berrendero F, Manzanares J, Perez A, Corchero J, Fuentes JA, Fernandez-Ruiz JJ, Ramos JA. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse. 1998;30:298–308. doi: 10.1002/(SICI)1098-2396(199811)30:3<298::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Rubino T, Vigano D, Massi P, Parolaro D. The psychoactive ingredient of marijuana induces behavioral sensitization. Eur. J. Neurosci. 2001;14:884–886. doi: 10.1046/j.0953-816x.2001.01709.x. [DOI] [PubMed] [Google Scholar]
- 40.Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 41.Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191:867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- 42.Senn R, Keren O, Hefetz A, Sarne Y. Long-term cognitive deficits induced by a single, extremely low dose of tetrahydrocannabinol (THC): behavioral, pharmacological and biochemical studies in mice. Pharmacol. Biochem. Behav. 2008;88:230–237. doi: 10.1016/j.pbb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Shi LH, Luo F, Woodward DJ, Chang JY. Dose and behavioral context dependent inhibition of movement and basal ganglia neural activity by Delta-9-tetrahydrocannabinol during spontaneous and treadmill locomotion tasks in rats. Synapse. 2005;55:1–16. doi: 10.1002/syn.20088. [DOI] [PubMed] [Google Scholar]
- 44.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev. Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 45.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 46.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 47.Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in Delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav. Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 48.Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J. Pharmacol. Exp. Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- 49.Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, Chisin R, Mechoulam R, Bar-Hamburger R, Freedman N, Even-Sapir E. Brain imaging study of the acute effects of Delta9-tetrahydrocannabinol (THC) on attention and motor coordination in regular users of marijuana. Psychopharmacology (Berl) 2008;196:119–131. doi: 10.1007/s00213-007-0940-7. [DOI] [PubMed] [Google Scholar]
- 50.Wesierska M, Dockery C, Fenton AA. Beyond memory, navigation, and inhibition: behavioral evidence for hippocampus-dependent cognitive coordination in the rat. J. Neurosci. 2005;25:2413–2419. doi: 10.1523/JNEUROSCI.3962-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitlow CT, Freedland CS, Porrino LJ. Metabolic mapping of the time-dependent effects of delta 9-tetrahydrocannabinol administration in the rat. Psychopharmacology (Berl) 2002;161:129–136. doi: 10.1007/s00213-002-1001-x. [DOI] [PubMed] [Google Scholar]
- 52.Whitlow CT, Freedland CS, Porrino LJ. Functional consequences of the repeated administration of Delta9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 2003;71:169–177. doi: 10.1016/s0376-8716(03)00135-2. [DOI] [PubMed] [Google Scholar]
- 53.Wiley JL, O'connell MM, Tokarz ME, Wright MJ., Jr Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J. Pharmacol. Exp. Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]