Abstract
Deciphering the neuronal code - the rules by which neuronal circuits store and process information - is a major scientific challenge1,2. Currently, these efforts are impeded by a lack of experimental tools that are sensitive enough to quantify the strength of individual synaptic connections and also scalable enough to simultaneously measure and control a large number of mammalian neurons with single-cell resolution3,4. Here, we report a scalable intracellular electrode platform based on vertical nanowires that affords parallel electrical interfacing to multiple mammalian neurons. Specifically, we show that our vertical nanowire electrode arrays (VNEAs) can intracellularly record and stimulate neuronal activity in dissociated cultures of rat cortical neurons and can also be used to map multiple individual synaptic connections. The scalability of this platform, combined with its compatibility with silicon nanofabrication techniques, provides a clear path toward simultaneous, high-fidelity interfacing with hundreds of individual neurons.
To date, most microfabricated neuronal interfaces have been based on electrodes that reside outside of the cellular membrane, preventing their use for measuring sub-threshold events and also prohibiting precise cell-to-electrode registration3,5–12. Recently, emerging nanofabrication techniques have enabled the development of new micro- and nanoscale devices that exhibit significantly improved electrical coupling between cells and electrodes. Notably, gold mushroom-shaped microelectrodes have been used to perform “intracellular-like” recordings from Aplysia neurons and mammalian cell lines13,14, and true intracellular recordings from several mammalian cell lines and cardiomyocytes have been realized using microfabricated planar patch clamp devices15–17 and kinked nanowire probes18. Although these examples represent major advances in cell-electrode coupling, they have been either too large to interface to mammalian neurons13–17, or lack the scalability required to perform simultaneous measurements of multiple cells18.
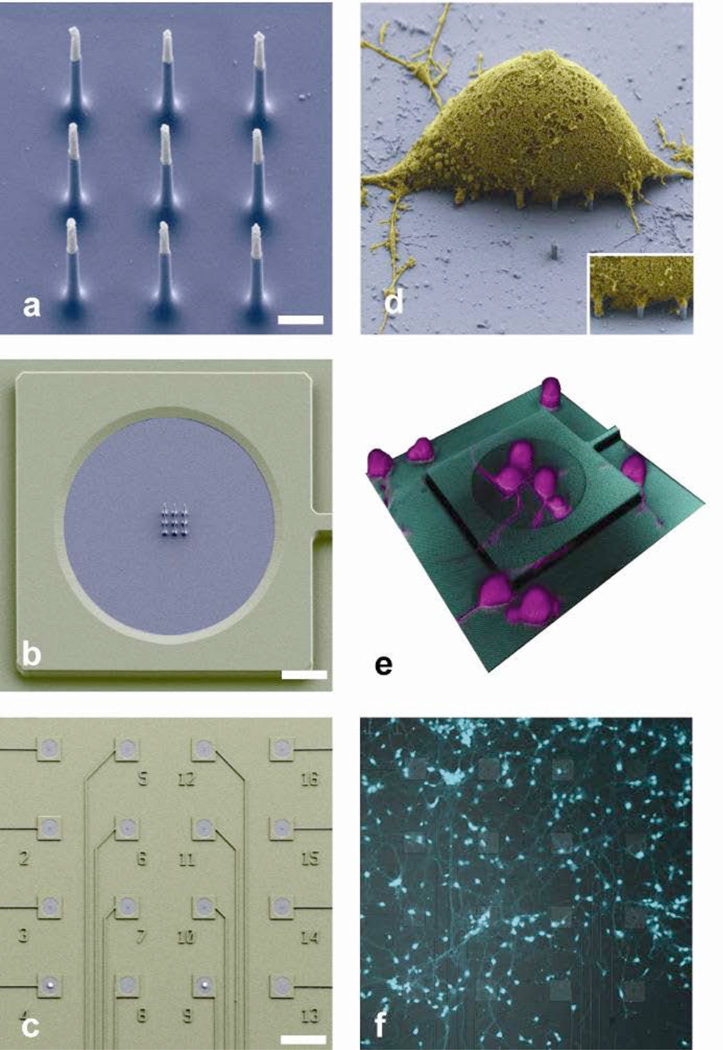
Our VNEA is specifically designed to address these issues by leveraging the same nanofabrication technology that enables mass production of integrated silicon electronic circuits. Figure 1 shows a prototype VNEA with 16 stimulation/recording pads. The entire device was fabricated from a silicon-on-insulator (SOI) substrate so that each pad could be independently addressed electrically (see Methods and Supplementary Fig. 1 for additional fabrication details). At the center of each stimulation/recording pad lies a 3 × 3 array of 9 silicon nanowires (~150 nm in diameter, 3 µm in length, at a 2-µm pitch). Each nanowire in the array consists of a degenerately doped silicon core encapsulated by a silicon dioxide (glass) shell, and is capped with a sputter-deposited metal tip (Ti/Au: see Fig. 1a): the silicon core and metal tip provide electrical access to the cell’s interior, whereas the glass shell plays the dual role of preventing current leakage through the nanowire sidewalls and serving as a material for making tight seals to the cell membrane4. The geometry of each nanowire array (a 4-µm square) was chosen to be smaller than the size of a typical neuronal cell body so as to increase the probability of single-neuron coupling.
Figure 1. Vertical nanowire electrode array (VNEA) for interrogating neuronal networks.
a, Scanning electron microscope (SEM) image of the 9 silicon nanowires that constitute the active region of a VNEA. Dimensions of the nanowire electrodes were designed to facilitate single-cell intracellular electrical coupling. False coloring shows metal-coated tips (gray) and insulating silicon oxide (blue). Scale bar, 1 µm. b, SEM image of a VNEA pad. False coloring indicates additional insulation by Al2O3 (green). Scale bar, 10 µm. c, SEM image of a device consisting of 16 stimulation/recording pads for parallel multi-site interrogation of neuronal circuits. Scale bar, 120 µm. d, SEM image of a rat cortical cell (3 days in vitro (DIV), false colored yellow) on top of a VNEA pad (false colored blue), showing nanowires interfacing with the cellular membrane (inset). e, Reconstructed three-dimensional confocal microscope image of rat cortical neurons cultured on a VNEA pad (3 DIV). f, Representative optical microscope image of calcein AM-labeled rat cortical neurons (cyan) cultured on a VNEA (5 DIV).
The top-down nanofabrication technology employed here enables precise control over the physical dimensions of the nanowires, as well as the size and the configuration of the stimulation/recording pads. Moreover, the number of pads can easily be scaled up to the thousands. By virtue of its planar integrated geometry, the VNEA is well suited for studying in vitro dissociated neuronal circuits and quasi-2D ex vivo preparations, like brain slices or retina. It can also be readily coupled with fluorescence microscopy19,20, optogenetic techniques21, and conventional patch measurements4, allowing truly multiplexed interrogation of neuronal circuits. As an example, Fig. 1f shows a typical network of dissociated rat cortical neurons on top of a VNEA after five days in vitro (DIV). Images obtained via confocal microscopy demonstrate that the neurons sitting directly on top of the nanowires envelop them and, at times, appear penetrated (Fig. 1e and Supplementary Fig. 2). Meanwhile, whole cell patch clamp measurements show that neurons cultured on the VNEAs have similar electrophysiological characteristics as those cultured on glass cover slips, as previously observed22.
We optimized operation protocols for our VNEA devices using HEK293 cells as a model system. HEK293 cells are advantageous for establishing stimulation and recording procedures because they require a short culture time before electrical interrogation (only a few hours), and their membrane resistance remains constant within 15 mV of the resting membrane potential23. To determine the device parameters that characterize the VNEA-cell interface, we performed whole-cell patch-clamp recordings of HEK293 cells residing directly on top of the nanowires and monitored voltages and currents simultaneously using both a patch pipette and a VNEA pad (Fig. 2a). In more than half of the cases, the membrane potential changed immediately upon VNEA current injection, suggesting that the nanowires had spontaneously penetrated the cell’s membrane. When the membrane potential of a cell did not change significantly upon current injection (i.e. when the nanowires were not inside the cell), we used a short voltage pulse (~ ±3 V, 100 ms duration) to permeabilize the cell membrane24 and promote nanowire penetration (see Methods).
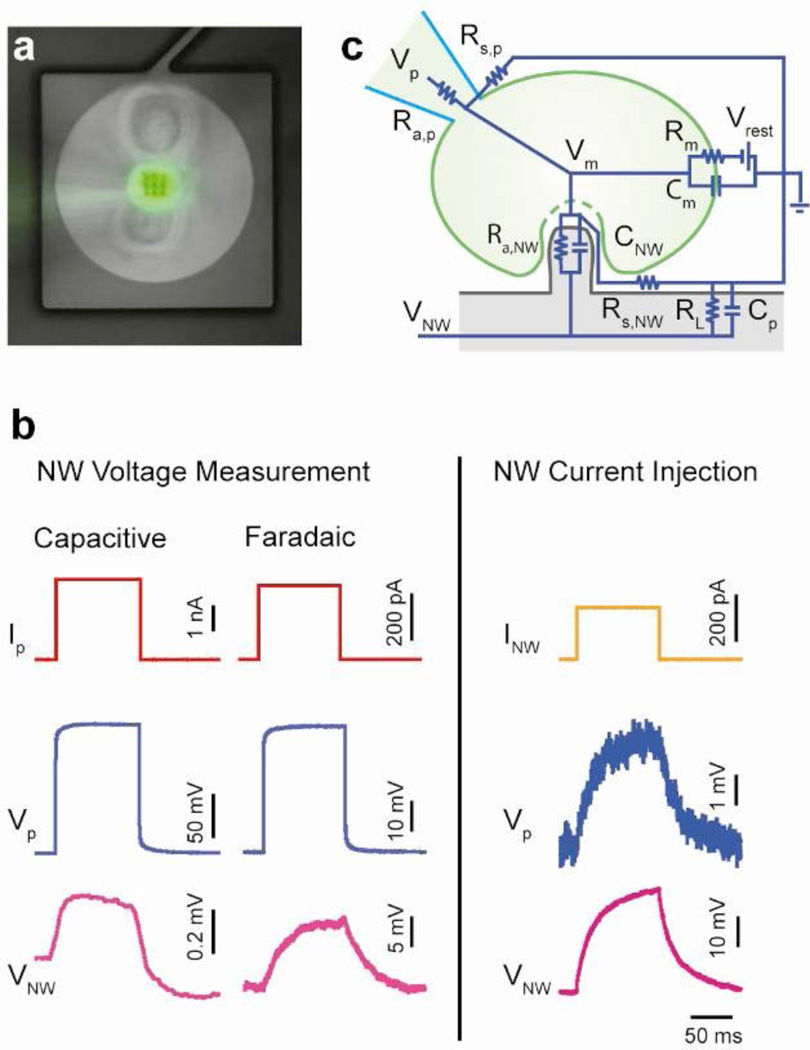
Figure 2. Characterization of the VNEA-cell electrical interface.
a, Composite bright field and fluorescence image of a patched HEK293 cell on a VNEA pad (0 DIV). Calcein (green) was added to the intracellular recording solution to enable fluorescence imaging of the patched cell. b, In both capacitive and Faradaic modes, the voltage response of the cell due to pipette current injection (Ip) (red) was recorded simultaneously using a patch pipette (Vp, blue) and a VNEA pad (VNW, magenta). Similarly, the cell’s membrane potential could be controlled (as verified by patch pipette recordings) by injecting current through the nanowires (INW) (orange). Note that capacitive and Faradaic measurements were performed on different cells since switching between recording modes required swapping amplifier electronics. c, Equivalent circuit model of the VNEA-cell interface. Ra,NW and Rs,NW (Ra,p and Rs,p) represent the access and seal resistances for the nanowires (pipette), respectively. The capacitance of the electrical double layer at the nanowire surface is represented as CNW. The equivalent circuit also includes the leak resistance due to uncoupled nanowires or defects in electrode insulation (RL) and the parasitic capacitance due to the device and associated electronics (Cp). The cell itself has a characteristic membrane resistance (Rm), capacitance (Cm) and resting potential (Vrest), all of which combine to determine the potential across the cell membrane (Vm). The values of these circuit elements were determined based on simultaneous patch pipette and VNEA measurements such as those shown in b.
Once the nanowires had access to the cell’s interior, the VNEA device was used to measure and control the cell’s membrane potential (Vm) by leveraging electrochemistry at the nanowire tips. Specifically, when no voltage was applied to the nanowires, the change in Vm induced by the patch pipette led to a change in the nanowire voltage (VNW) due to the charging/discharging of the electrical double layer at the metal-coated nanowire tips (the “capacitive” regime: Fig. 2b left column). Changes in Vm could also be recorded by applying a bias (~ −1.5V) to the nanowires that was sufficient to flow a small electrochemical current – in this “Faradaic” regime, the value of VNW required to maintain a fixed current tracked the changes in Vm, in a fashion similar to conventional current-clamp techniques4 (Fig. 2b center column). Finally, when current was injected through the nanowires, the voltage measured at the patch pipette (Vp) changed (Fig. 2b, right panel and Supplementary Fig. 3), indicating that NW-based control over the membrane potential is also possible (we note that because the nanowires provide only a small point source of current, changes to the membrane potential may not be uniform throughout the cell). In the Faradaic regime, when current was injected through the pipette (nanowire), voltage changes measured at the nanowire (pipette) were ~3 (~10) times smaller than those measured at the pipette (nanowire). In the capacitive regime, on the other hand, VNW was ~300 times smaller than Vp.
During capacitively coupled measurements, Vp typically rose by ~10 mV from the initial resting potential after 30 minutes of recording. Such a rise was common even for patch pipette recordings without nanowire penetration, suggesting that the duration of our measurement was limited not by the nanowire recording but rather by effects from patching. During capacitive measurements, a reduction in the signal amplitude measured at the nanowires was also observed over the course of few minutes, indicating partial recovery of the permeabilized membrane. However, this signal could be repeatedly restored to full strength by reapplying the permeabilization protocol. In the Faradaic regime, no signal reduction was observed, likely due to the constant current flow at the nanowire tips. It should be noted that, unlike capacitive measurements, the continuous recording time in the Faradaic regime was typically limited to less than 10 minutes, most likely due to prolonged electrochemical reactions damaging the cellular membrane. By performing measurements intermittently, however, the interrogation period could be extended significantly.
Analysis of the current/voltage traces measured at the patch pipette and the nanowire (Fig. 2b and Supplementary Fig. 4) using the equivalent circuit model in Fig. 2c enabled the determination of all of the parameters that specify the electrical coupling between the cell and the nanowires (see Supplementary Information (SI) for details). In particular, this analysis showed that the seal resistance (Rs,NW) between the nanowires and the cell membrane ranged between 100 and 500 MΩ, and the total double-layer capacitance at the nanowire tips (CNW)25 was typically ~1 pF. The nanowire access resistance (Ra,NW), which includes the intrinsic nanowire resistance and the resistance at the electrochemical junction and thus varies with VNW, was determined to be infinite at zero bias (no electrochemical reactions occurs at the metal tips) and ~300 MΩ at VNW ~ −1.5 V. When the measurement was performed in the Faradaic regime, this large Ra,NW, combined with the parasitic capacitance (Cp) of a typical VNEA pad and its associated electronics (~150 pF), resulted in an RC time constant on the order of 10 ms. Although this RC component filtered the voltage waveform measured at the nanowires (as compared to that measured at a patch pipette), this distortion could be easily corrected using a deconvolution procedure (see SI and Supplementary Fig. 5). In the capacitive regime, the change in VNW originated from the charging and discharging of the electrical double layer and thus accurately followed fast changes in Vm. The magnitude of this response, however, was attenuated due to capacitive voltage division (~CNW/Cp).
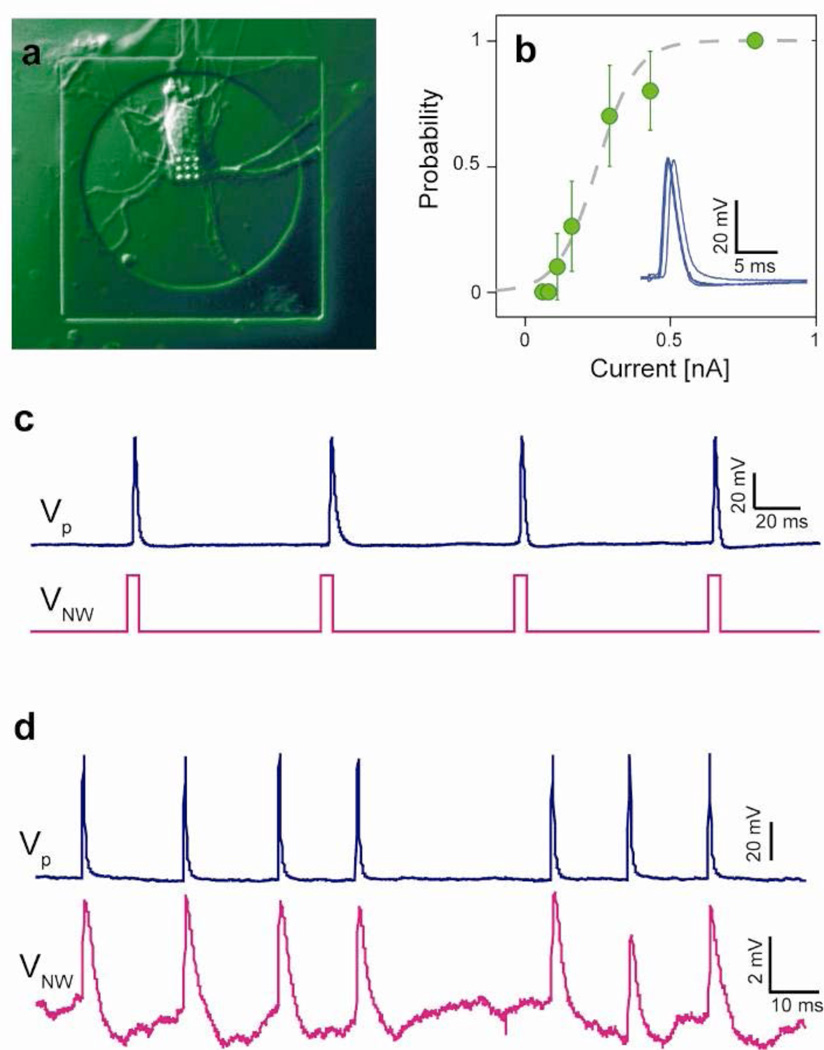
Once the device characterization and protocol optimization were complete, we utilized the VNEA to perform high-fidelity intracellular stimulation and recording of rat cortical neurons (Fig. 3 and Supplementary Fig. 6). Typically, these neurons were interrogated after 6 to 14 DIV to allow electrophysiological development and the formation of synaptic connections26,27. As shown in Fig. 3c and Supplementary Fig. 6, current pulses injected into neurons via the nanowires reliably evoked neuronal action potentials (APs) as recorded via simultaneous wholecell patch clamp. The stimulation probability followed a sigmoidal dependence on the magnitude of the nanowire-injected current (Fig. 3b), similar to that reported for patch pipette stimulation28. Moreover, the VNEA operating in current-clamp mode could be used to monitor individual APs evoked by the patch pipette. For single-shot AP measurements, the signal-to-noise ratio for VNEA recordings was typically 100 or greater (Fig. 3d). When we averaged multiple waveforms obtained under identical experimental conditions, we could improve the signal-to-noise ratio to >1000 (Supplementary Fig. 5). This suggests that by averaging the response to repeated presynaptic stimulations, postsynaptic potentials (PSPs) could be measured using the VNEA.
Figure 3. Stimulation and recording of rat cortical neurons using a VNEA.
a, Representative differential interference contrast micrograph of a rat cortical neuron cultured on a VNEA pad (6 DIV). b, Probability of action potential (AP) excitation plotted as a function of current injected by nanowires shows a sigmoidal dependence (dashed line), which is similar to AP excitation elicited via intracellular patch pipettes. Probabilities were calculated for 20 trials and plotted as a function of the stimulation current. Error bars represent 95% confidence intervals. inset, 5 consecutive time-aligned APs stimulated by nanowire current injection show less than 1-ms jitter. c, APs were reliably stimulated by voltage pulses at the VNEA pad (magenta) and recorded using a patch pipette (blue). d, Similarly, APs were stimulated using a patch pipette (blue) and recorded by the VNEA pad in the Faradaic mode (magenta). VNEA measurements show good agreement with those obtained simultaneously via patch pipette.
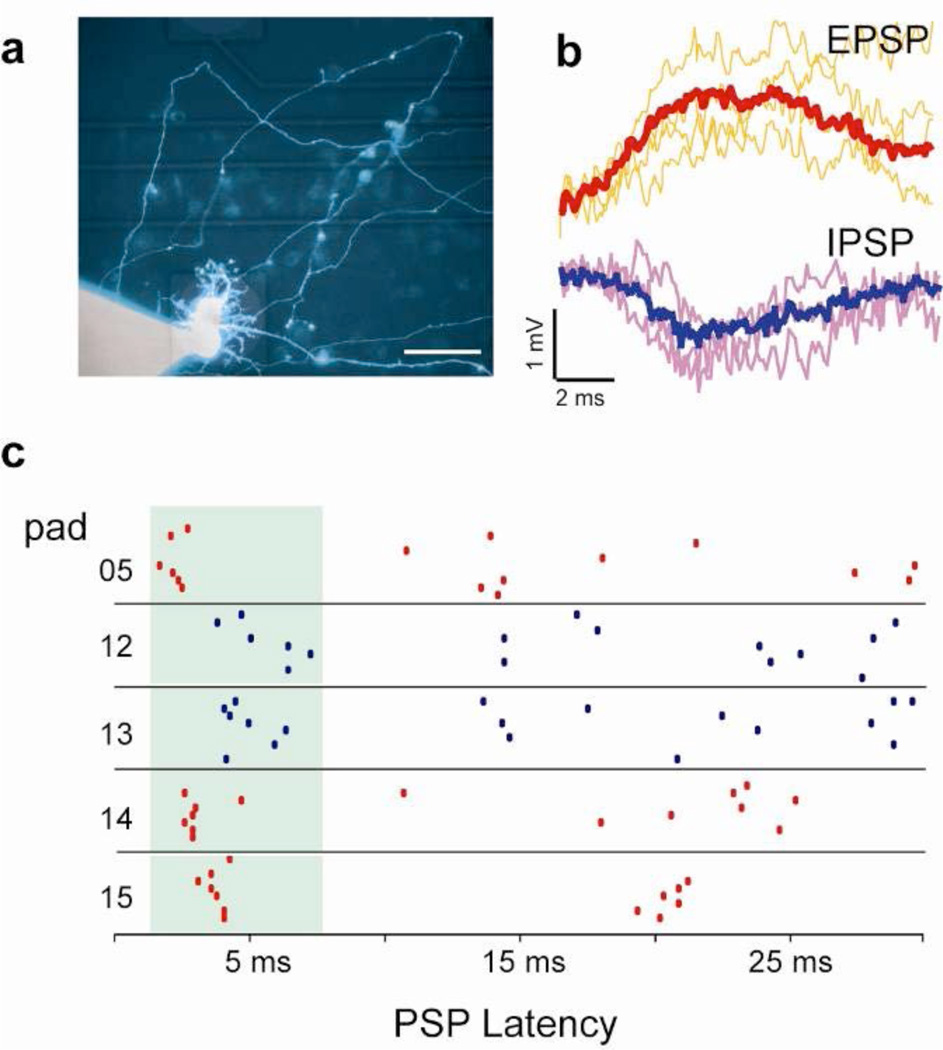
The multiplexed stimulation and recording capabilities of the VNEA platform and its compatibility with conventional patch-clamp and fluorescence microscopy techniques enable comprehensive examination of the functional connectivity in neuronal circuits beyond traditional connected-pair studies29. To illustrate the potential of such measurements, we used our VNEA platform to map multiple individual synaptic connections onto a single postsynaptic neuron. Specifically, we used a patch pipette to measure the PSPs of a single neuron in a dissociated neuronal culture while inducing APs in other neurons using neighboring VNEA pads (Fig. 4a). By monitoring PSP responses upon repeated stimulations at each pad, we found that excitation of some of the nearby neurons reproducibly generated either excitatory (EPSPs) or inhibitory PSPs (IPSPs) in the patch-clamped cell (Figs. 4b and 4c). The latency window of these EPSPs and IPSPs ranged from 2 to 8 ms, indicative of a monosynaptic connection30. We note that performing this type of measurements using conventional patch clamp techniques would require each pre-synaptic cell to be individually identified and patched separately. Using our VNEA platform, this process can be significantly simplified and expedited. Together, the magnitude, sign, and latency of these PSPs define the functional connectivity of a neuronal circuit. By monitoring changes in this connectivity as a function of time and combining it with structural reconstruction techniques, we should be able to investigate the relationships between the architecture, dynamics, and function of neuronal circuits in unprecedented detail.
Figure 4. Identification of functional synaptic connectivity using a VNEA and a patch pipette.
a, Composite bright field and fluorescence image of a cortical neuron patched and backfilled with calcein (14 DIV). b, Representative EPSPs (top) and IPSPs (bottom) were averaged (bold) and used as templates for identifying other PSPs recorded via a patch pipette. To measure these PSP events, we injected a constant current through the patch pipette to hold the membrane potential near −40 mV. At this resting potential, EPSPs and IPSPs produce positive or negative changes in the membrane potential respectively, allowing them to be distinguished from one another. c, Raster plots of EPSPs (red) and IPSPs (blue) identified by patch-clamp recording and plotted as a function of their latency following stimulation at the specified VNEA pads. Each of the five pads shown here evoked reproducible PSPs in the patched cell within a latency window of 2 – 8 ms (green), suggesting monosynaptic connectivity.
The present study demonstrates that the VNEA platform provides new experimental methods to stimulate and record neuronal activities in a highly scalable fashion. Moreover the VNEA platform can be readily coupled with conventional patch measurements, fluorescence microscopy, and optogenetic techniques, allowing truly multiplexed interrogation of a neuronal circuit. Although the prototype demonstrated here has only 16 stimulation/recording sites, higher numbers and densities can easily be achieved using standard silicon nanofabrication processes. For instance, contemporary deep UV lithography with feature sizes near 150 nm can produce recording pad densities approaching 10,000 electrodes/mm2. The integration of complementary metal-oxide-semiconductor circuitry with a VNEA would further allow on-chip digitization, signal multiplexing, compression, and telemetry. Finally, the small dimensions and planar geometry of VNEAs should facilitate their incorporation into implantable electrodes, similar to the silicon-based microelectrode array implants currently in use31, opening up new possibilities for neuronal prosthetics and large-scale studies of neuronal circuit dynamics in vivo.
Methods
Device Fabrication
Nanowires on the VNEA devices were fabricated using electron beam lithography followed by reactive ion etching and thermal oxide thinning. Nanowire tips were metal-coated via thermal or electron-beam evaporation. Electrode tracks were defined via photolithography and reactive ion etching followed by atomic layer deposition of an aluminum oxide layer to provide electrical isolation from the culture media (see SI for more details).
Cell Culture and Imaging
Cells and cell culture media were contained within polydimethylsiloxane wells bonded to the VNEA surface (see SI for details). Epifluorescence and DIC images of live cells were overlaid and colored using ImageJ. False coloring of SEM images was performed using Photoshop CS3 (Adobe).
Membrane permeabilization and visualization
Typically, the membrane potential, as measured using a patch pipette, responded immediately to the application of current via the VNEA pad (−200 to −400 pA), suggesting that some of the nanowires formed a tight seal with the cell membrane and penetrated the cells on top. In the absence of immediate electrical coupling, permeabilization could be achieved by applying 100 ms voltage pulses with amplitudes of ~ ±3 V. Membrane permeabilization was typically accompanied by a depolarization of Vm by 10 ~ 20 mV; lowering the holding current on the recording pad or the patch pipette reversed this depolarization. Confocal microscopy imaging of cortical neurons cultured on vertical silicon nanowires (3 days in vitro (DIV)) showed that some neurons appeared penetrated by nanowires while others did not, consistent with our electrical measurements (Supplementary Fig. 3). Images were obtained by labeling the nanowires with Alexa647-SE in DMSO (1mg/mL, Invitrogen)22. After a 30-minute incubation at 37°C, samples were washed three times through water, blown dry, and used as a substrate for neuron culture. Prior to imaging, neuronal membranes were labeled by incubating the samples in extracellular solution containing 1:100 Vybrant DiI (Invitrogen) at room temperature. Images were then analyzed and reconstructed in 3D using Imaris 6.2 (Bitplane).
Patch Pipette Recording
During measurements, VNEA substrates were bathed in a solution containing [in mM]: NaCl [119], KCl [5], HEPES [20], CaCl2 [2], MgCl2 [2], glucose [30], and glycine [0.001]. The pH of the solution was 7.3 and the osmolarity was 330 mosM (adjusted with sucrose). Patch pipettes were pulled to have resistances of 2 ~ 10 MΩ and then backfilled with a solution containing [in mM]: potassium gluconate [130], KCl [10], MgCl2 [5], EGTA [0.6], HEPES [5], CaCl2 [0.06], Mg-ATP [2], GTP [0.2], leupeptine [0.2], phosphocreatine [20], and creatine phosphokinase [50 U/ml] with the pH at 7.232. For certain experiments, high-purity calcein (Invitrogen) was added (1 part in 5000) into the intracellular recording solution to enable fluorescence imaging of the patched cell. All recordings were made at room temperature with a Multiclamp 700B (Molecular Devices). Current-clamp recordings of HEK293 cells were performed with a holding current of 0 pA. During neuronal measurements, the holding current was adjusted to bring the neuron to a resting membrane potential near −70 mV for stimulation and recording experiments, and −40 mV for PSP identification. The pipette capacitance was corrected electronically. All acquisitions were performed using the pCLAMP 10 software (Molecular Devices) and analyzed and plotted using Clampfit 10 (Molecular Devices) and Matlab (Mathworks).
Protocols for VNEA stimulation and recording
For VNEA stimulation and recording experiments, the devices were mounted in a custom-built aluminum enclosure designed to provide both electrical connection to the VNEA pads and electromagnetic shielding. The connection between the VNEA and stimulation/measurement electronics was achieved using conductive elastomeric connectors (ZEBRA, Fuji-poly) and a custom-built printed circuit board.
Neuronal stimulation was achieved using a 32-channel analog output module (National Instruments PXI-6723) and control software written in LabView (National Instruments). During VNEA stimulation, capacitive electronic cross-talk between the patch pipette and VNEA was removed by subtracting the characteristic transients. Current-clamp measurements were performed using a custom-built current-clamp circuit with stability on the order of 10 pA and compliance values between +/−10 V and +/− 5nA. Currents were controlled using our analog output module, and recorded voltages were amplified using AM Systems Model 1800 (gain: 100×, bandwidth: 1 Hz – 20 kHz). Both pre- and post-amplified voltages were collected and digitized. Capacitively-coupled measurements were recorded directly with the AM Systems amplifier, and digitally filtered to remove 120Hz line noise. To preserve the simultaneity of the pipette and VNEA measurements, all signals were recorded and digitized simultaneously using the pCLAMP 10 software and a 16-channel analog data acquisition system (Axon Digidata 1322A, Molecular Devices).
Steady-state control of the membrane potential (as measured by the patch pipette (Vp)) could be achieved for several seconds by changing the offset voltage applied to the nanowires (VNW) (Supplementary Fig. 3).
PSP identification
To identify excitatory (EPSPs) and inhibitory PSPs (IPSPs), we patched a rat cortical neuron (14 DIV) in whole-cell current-clamp mode. Several representative EPSPs/IPSPs were manually identified and averaged to form a template (Fig. 4B). Once a template was obtained, automatic event detection was performed using Clampfit 10. Latencies were calculated as the interval between the rising edge of the stimulus and the peak of the PSP. Data was then compiled and plotted using Matlab.
Supplementary Material
Acknowledgements
We thank J. MacArthur, E. Soucy, J. Greenwood, L. DeFeo, N. Sanjana, A. Dibos, G. Lau, B. Ilic, M. Metzler, L. Xie, and E. Macomber for scientific discussions and technical assistance. The VNEA fabrication and characterization were performed in part at the Center for Nanoscale Systems at Harvard University. This work was supported by an NIH Pioneer award (5DP1OD003893-03) and an NSF EFRI award (EFRI-0835947).
Footnotes
Author Contributions:
H.P. and J.T.R. conceived and designed the experiments. J.T.R., M.J., A.K.S., and R.S.G. performed experiments, and M.-H.Y. helped with the experimental setup and the initiation of the experiments. J.T.R., M.J., and A.K.S. analyzed the data. H.P. supervised the project. J.T.R., M.J., A.K.S., and H.P. wrote the manuscript, and everyone read and discussed it extensively.
Additional Information:
Supplementary information accompanies this paper at www.nature.com/naturenanotechnology.
References
- 1.Yuste R. Circuit Neuroscience: The Road Ahead. Front. Neurosci. 2008;2:6–9. doi: 10.3389/neuro.01.017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock DD, et al. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;471:177–182. doi: 10.1038/nature09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pine J. Advances in Network Electrophysiology. 2006:3–23. [Google Scholar]
- 4.Molleman A. Patch clamping. John Wiley and Sons: 2003. [Google Scholar]
- 5.Rolston JD, Gross RE, Potter SM. A low-cost multielectrode system for data acquisition enabling real-time closed-loop processing with rapid recovery from stimulation artifacts. Front. Neuroengineering. 2009;2:12–12. doi: 10.3389/neuro.16.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voelker M, Fromherz P. Signal Transmission from Individual Mammalian Nerve Cell to Field-Effect Transistor. Small. 2005;1:206–210. doi: 10.1002/smll.200400077. [DOI] [PubMed] [Google Scholar]
- 7.Kim D-H, et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010;9:511–517. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viventi J, et al. A Conformal, Bio-Interfaced Class of Silicon Electronics for Mapping Cardiac Electrophysiology. Sci. Transl. Med. 2010;2:24ra22–24ra22. doi: 10.1126/scitranslmed.3000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patolsky F, et al. Detection, Stimulation, and Inhibition of Neuronal Signals with High-Density Nanowire Transistor Arrays. Science. 2006;313:1100–1104. doi: 10.1126/science.1128640. [DOI] [PubMed] [Google Scholar]
- 10.Eschermann JF, et al. Action potentials of HL-1 cells recorded with silicon nanowire transistors. App. Phys. Lett. 2009;95:083703–083703. [Google Scholar]
- 11.Wang K, Fishman HA, Dai H, Harris JS. Neural Stimulation with a Carbon Nanotube Microelectrode Array. Nano Lett. 2006;6:2043–2048. doi: 10.1021/nl061241t. [DOI] [PubMed] [Google Scholar]
- 12.McKnight TE, et al. Resident Neuroelectrochemical Interfacing Using Carbon Nanofiber Arrays. J. Phys. Chem. B. 2006;110:15317–15327. doi: 10.1021/jp056467j. [DOI] [PubMed] [Google Scholar]
- 13.Hai A, et al. Spine-shaped gold protrusions improve the adherence and electrical coupling of neurons with the surface of micro-electronic devices. J. R. Soc. Interface. 2009;6:1153–1165. doi: 10.1098/rsif.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hai A, Shappir J, Spira ME. In-cell recordings by extracellular microelectrodes. Nat. Meth. 2010;7:200–202. doi: 10.1038/nmeth.1420. [DOI] [PubMed] [Google Scholar]
- 15.Lau AY, Hung PJ, Wu AR, Lee LP. Open-access microfluidic patch-clamp array with raised lateral cell trapping sites. Lab Chip. 2006;6:1510–1515. doi: 10.1039/b608439g. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Klemic KG, Reed MA, Sigworth FJ. Microfluidic System for Planar Patch Clamp Electrode Arrays. Nano Lett. 2006;6:815–819. doi: 10.1021/nl060165r. [DOI] [PubMed] [Google Scholar]
- 17.Sigworth FJ, Klemic KG. Microchip technology in ion-channel research. IEEE Trans. Nanobioscience. 2005;4:121–127. doi: 10.1109/tnb.2004.842471. [DOI] [PubMed] [Google Scholar]
- 18.Tian B, et al. Three-Dimensional, Flexible Nanoscale Field-Effect Transistors as Localized Bioprobes. Science. 2010;329:830–834. doi: 10.1126/science.1192033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolenko V, Poskanzer KE, Yuste R. Two-photon photostimulation and imaging of neural circuits. Nat. Meth. 2007;4:943–950. doi: 10.1038/nmeth1105. [DOI] [PubMed] [Google Scholar]
- 20.Peterka DS, Takahashi H, Yuste R. Imaging Voltage in Neurons. Neuron. 2011;69:9–21. doi: 10.1016/j.neuron.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalek AK, et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1870–1875. doi: 10.1073/pnas.0909350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas P, Smart TG. HEK293 cell line: A vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Rols MP, Teissié J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys. J. 1990;58:1089–1098. doi: 10.1016/S0006-3495(90)82451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moulton Studies of double layer capacitance and electron transfer at a gold electrode exposed to protein solutions. Electrochimica Acta. 2004;49:4223–4230. [Google Scholar]
- 26.Dichter MA. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978;149:279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- 27.Romijn HJ, Mud MT, Habets AM, Wolters PS. A quantitative electron microscopic study on synapse formation in dissociated fetal rat cerebral cortex in vitro. Brain Res. 1981;227:591–605. doi: 10.1016/0165-3806(81)90011-0. [DOI] [PubMed] [Google Scholar]
- 28.Kole MHP, Stuart GJ. Is action potential threshold lowest in the axon? Nat. Neurosci. 2008;11:1253–1255. doi: 10.1038/nn.2203. [DOI] [PubMed] [Google Scholar]
- 29.Dan Y, Poo M-M. Spike Timing-Dependent Plasticity: From Synapse to Perception. Physiol. Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- 30.Mason A, Nicoll A, Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J. Neurosci. 1991;11:72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolelis MAL. Brain-machine interfaces to restore motor function and probe neural circuits. Nat. Rev. Neurosci. 2003;4:417–422. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- 32.Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3[prime],5[prime]-cyclic GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.