Abstract
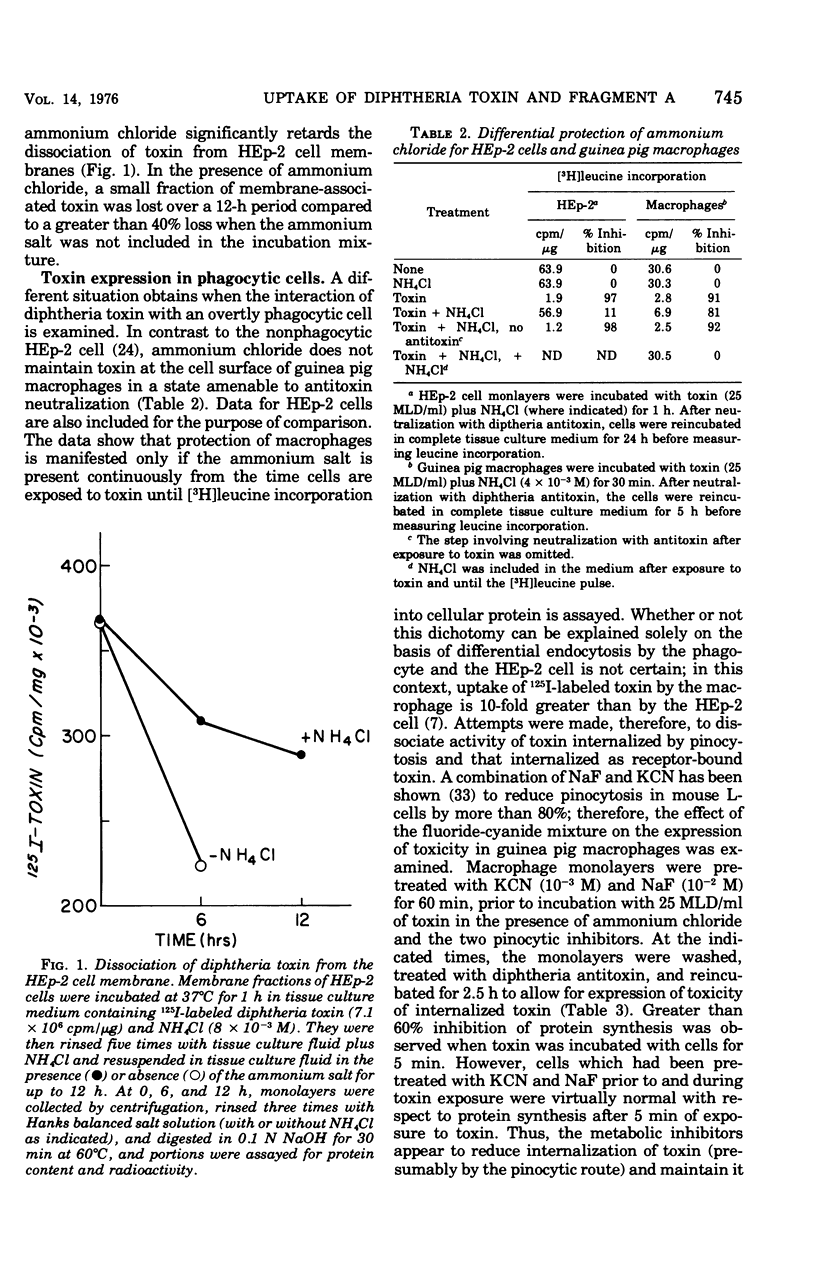
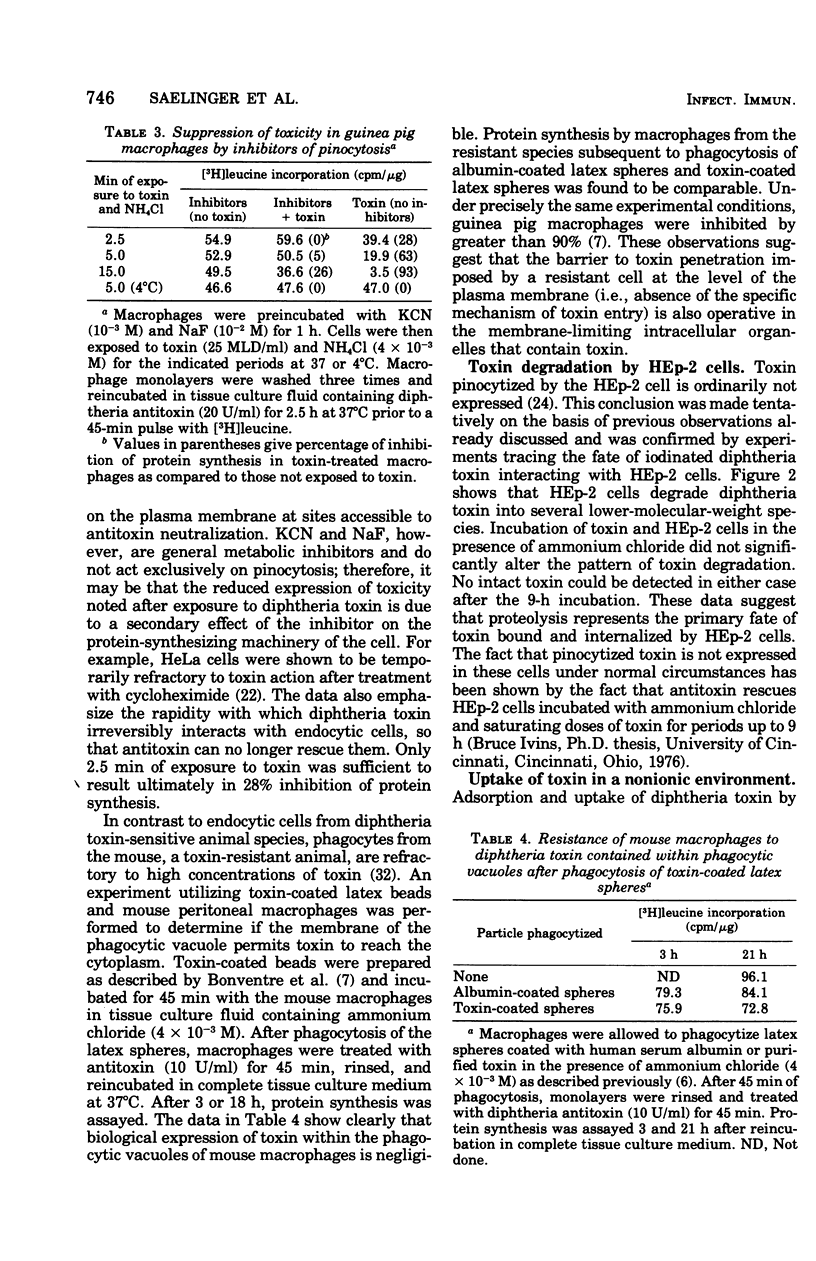
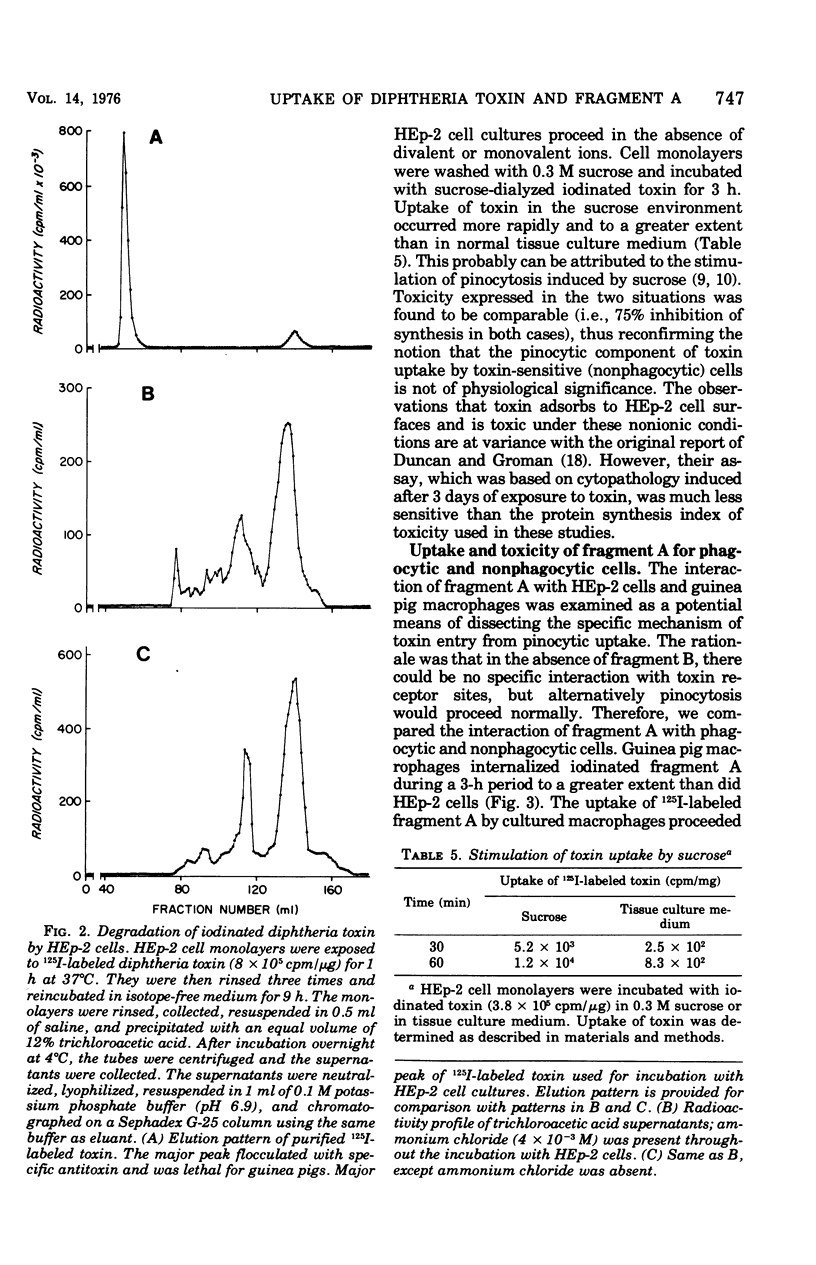
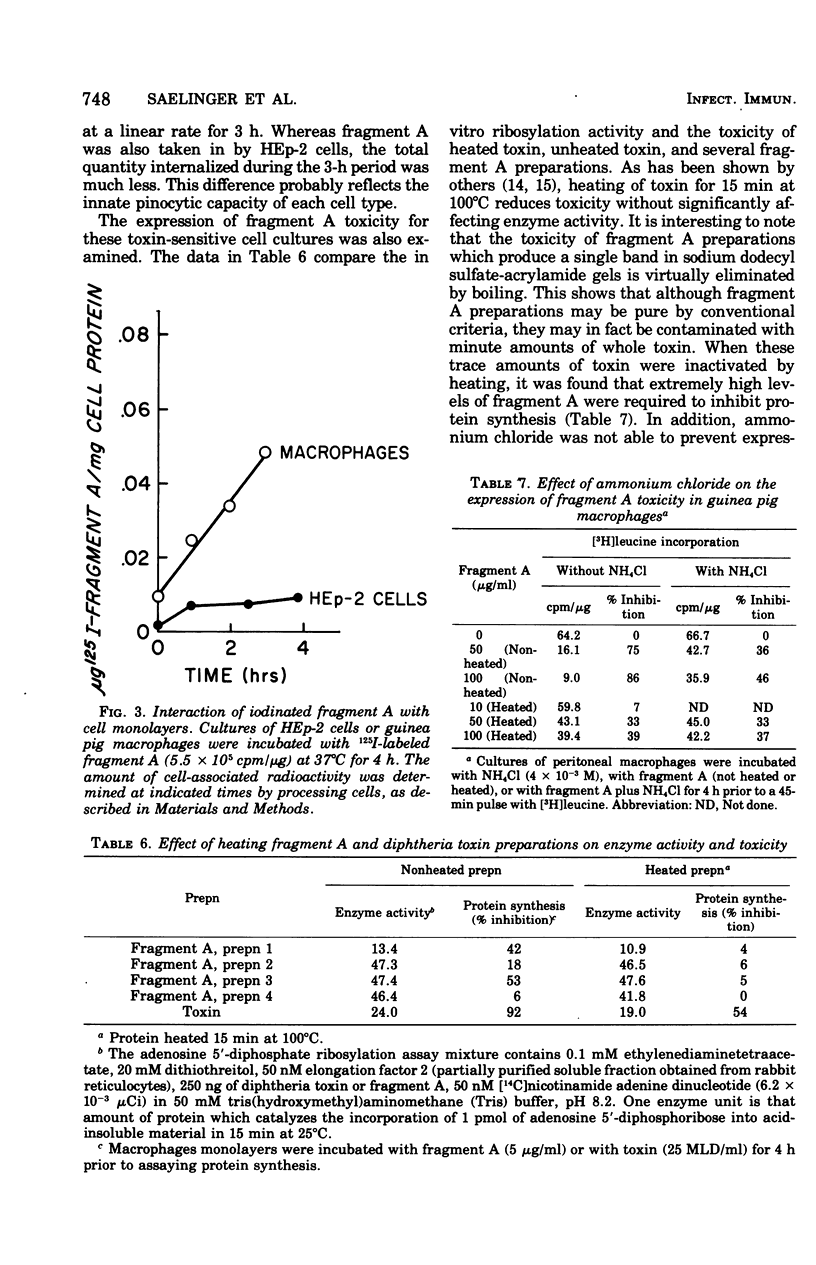
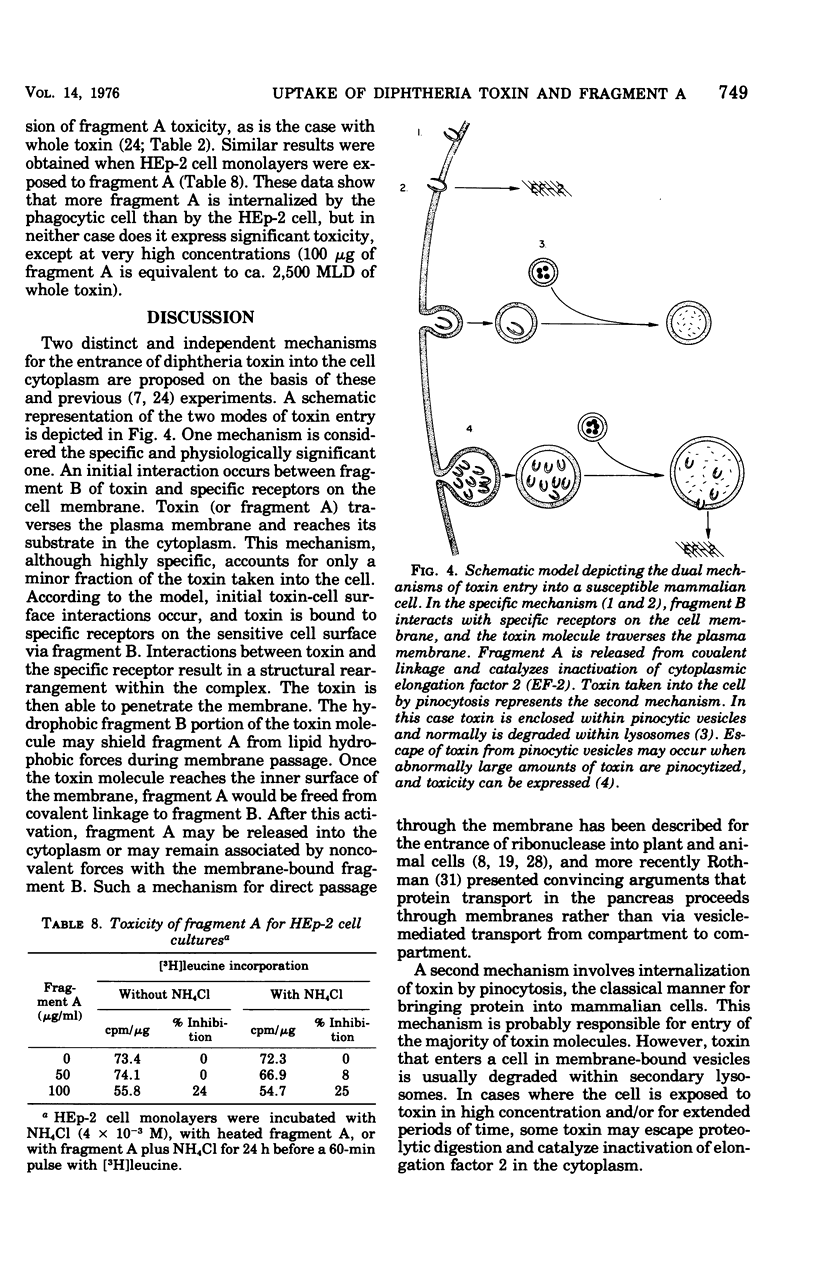
Evidence suggesting that diphtheria toxin reaches the cytoplasm of susceptible mammalian cells by two independent mechanisms is presented. A schematic model describing the two processes of toxin entry into the cell is developed. One process of toxin uptake considered to by physiologically significant is passage of the protein toxin through the plasma membrane. This most likely happens by binding of fragment B to receptors on the membrane and by subsequent toxin-membrane interaction so that ultimately fragment A, the enzymatically active moiety, is transported tothe cell interior. This process, which ultimately leads to cessation of protein synthesis and cell death, involves a comparatively small number of toxin molecules. A second mechanism of toxin uptake is by classical pinocytosis. The majority of toxin taken into the cell is accomplished by this process. The fate of toxin taken into HEp-2 cells via pinocytosis is proteolysis by lysosomal enzymes. Thus, such vesicle-bound toxin is ordinarily not expressed biologically. Evidence suggesting that ammonium chloride provides total protection to diphtheria toxin-susceptible cells by preventing entry of toxin by the specific receptor-associated process is also provided; data showing that the ammonium salt immobilizes bound toxin on the plasma membrane of HEp-2 cells are presented. Finally, it is suggested that actively endocytic cells such as guinea pig macrophages interact with toxin in a significantly different manner than do nonphagocytic cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKSDALE L., GARMISE L., HORIBATA K. Virulence, toxinogeny, and lysogeny in Corynebacterium diphtheriae. Ann N Y Acad Sci. 1960 Nov 21;88:1093–1108. doi: 10.1111/j.1749-6632.1960.tb20099.x. [DOI] [PubMed] [Google Scholar]
- BRACHET J. Effects of ribonuclease on the metabolism of living root-tip cells. Nature. 1954 Nov 6;174(4436):876–877. doi: 10.1038/174876a0. [DOI] [PubMed] [Google Scholar]
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Blackman K. E., Bubel H. C. Origin of the vaccinia virus hemagglutinin. J Virol. 1972 Feb;9(2):290–296. doi: 10.1128/jvi.9.2.290-296.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Mukkada A. J. Augmentation of glucose transport in macrophages after particle ingestion. Infect Immun. 1974 Dec;10(6):1391–1396. doi: 10.1128/iai.10.6.1391-1396.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Saelinger C. B., Ivins B., Woscinski C., Amorini M. Interaction of cultured mammalian cells with [125I] diphtheria toxin. Infect Immun. 1975 Apr;11(4):675–684. doi: 10.1128/iai.11.4.675-684.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Ehrenreich B. A. The uptake, storage, and intracellular hydrolysis of carbohydrates by macrophages. J Exp Med. 1969 Jan 1;129(1):201–225. doi: 10.1084/jem.129.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J Exp Med. 1967 Feb 1;125(2):213–232. doi: 10.1084/jem.125.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Cukor G., Moolten F. L. Unusual sensitivity of rabbit cells to a nonbinding diphtheria toxin fragment. Infect Immun. 1975 Aug;12(2):446–447. doi: 10.1128/iai.12.2.446-447.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukor G., Solotorovsky M., Kuchler R. J. Biological activity of heated diphtheria toxin. J Bacteriol. 1973 Jul;115(1):277–283. doi: 10.1128/jb.115.1.277-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- Duncan J. L., Groman N. B. Studies of the activity of diphtheria toxin. I. Poliovirus replication in intoxicated HeLa cells. J Exp Med. 1967 Mar 1;125(3):489–500. doi: 10.1084/jem.125.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GABLIKS J., SOLOTOROVSKY M. Cell culture reactivity to diphtheria, Staphylococcus, tetanus and Escherichia coli toxins. J Immunol. 1962 Apr;88:505–512. [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. Observations on the structure of diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1485–1491. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr, Baseman J. B. Studies on transferase II using diphtheria toxin. Cold Spring Harb Symp Quant Biol. 1969;34:595–602. doi: 10.1101/sqb.1969.034.01.068. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr, Uchida T. Diphtheria toxin, protein synthesis, and the cell. Fed Proc. 1973 Apr;32(4):1508–1515. [PubMed] [Google Scholar]
- Ivins B., Saelinger C. B., Bonventre P. F., Woscinski C. Chemical modulation of diphtheria toxin action on cultured mammalian cells. Infect Immun. 1975 Apr;11(4):665–674. doi: 10.1128/iai.11.4.665-674.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W., Kuchler R. J., Solotorovsky M. Site in cell-free protein synthesis sensitive to diphtheria toxin. J Bacteriol. 1968 Oct;96(4):1089–1098. doi: 10.1128/jb.96.4.1089-1098.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Groman N. B. In vitro inhibition of diphtheria toxin action by ammonium salts and amines. J Bacteriol. 1965 Dec;90(6):1552–1556. doi: 10.1128/jb.90.6.1552-1556.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Groman N. B. Mode of inhibition of diphtheria toxin by ammonium chloride. J Bacteriol. 1965 Dec;90(6):1557–1562. doi: 10.1128/jb.90.6.1557-1562.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDOUX L. Action of ribonuclease on certain ascites tumours. Nature. 1955 Feb 5;175(4449):258–259. doi: 10.1038/175258b0. [DOI] [PubMed] [Google Scholar]
- Merkel C. G., Kwan S., Lingrel J. B. Size of the polyadenylic acid region of newly synthesized globin messenger ribonucleic acid. J Biol Chem. 1975 May 25;250(10):3725–3728. [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Rothman S. S. Protein transport by the pancreas. Science. 1975 Nov 21;190(4216):747–753. doi: 10.1126/science.1105785. [DOI] [PubMed] [Google Scholar]
- STRAUSS N., HENDEE E. D. The effect of diphtheria toxin on the metabolism of HeLa cells. J Exp Med. 1959 Feb 1;109(2):145–163. doi: 10.1084/jem.109.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelinger C., Bonventre P. F., Imhoff J. Interaction of toxin of Corynebacterium diphtheriae with phagocytes from susceptible and resistant species. J Infect Dis. 1975 Apr;131(4):431–438. doi: 10.1093/infdis/131.4.431. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Harper A. A. Reconstitution of diphtheria toxin from two nontoxic cross-reacting mutant proteins. Science. 1972 Feb 25;175(4024):901–903. doi: 10.1126/science.175.4024.901. [DOI] [PubMed] [Google Scholar]
- VONHIPPEL P. H., WONG K. Y. NEUTRAL SALTS: THE GENERALITY OF THEIR EFFECTS ON THE STABILITY OF MACROMOLECULAR CONFORMATIONS. Science. 1964 Aug 7;145(3632):577–580. doi: 10.1126/science.145.3632.577. [DOI] [PubMed] [Google Scholar]



