Abstract
A heterodimeric bispecific biological recombinant drug was synthesized by splicing DNA fragments from two fully humanized single-chain variable-fragment (scFV) antibody fragments forming a novel drug simultaneously recognizing the CD16 natural killer (NK) cell marker and the cancer marker epithelial cell adhesion molecule (EpCAM). The drug precipitously enhanced the killing of human carcinomas of the prostate, breast, colon, head, and neck even at very low effector:target ratios. The drug EpCAM16 rendered even nonactivated NK cell-proficient killers and activated them to kill via degranulation and cytokine production. Studies show that bispecific antibodies can be used to induce proficient killing of the carcinoma targets that ordinarily are resistant to NK-mediated killing. Apparently, the innate immune system can be effectively recruited to kill cancer cells using the bispecific antibody platform and EpCAM targeting.
Key words: ADCC, anti-CD16, bispecific antibody, carcinoma, human NK cells
Introduction
Epithelial cell adhesion molecule (EpCAM), also known as CD326 or 17-1A antigen, is well known for its expression on human carcinomas.1 Originally considered a pedestrian cell surface protein meditating homotypic cell adhesion,2–4 it is now understood that EpCAM is involved in more complex pathways that culminate in switching on cancer cells and cancer stem cells (CSC).5–8 For example, studies show that EpCAM plays a role in cell proliferation, wnt signal transduction, and as a proto-oncogene.9–11 Its expression correlates with a poor survival prognosis.1,12 Because it is a high-profile carcinoma marker, EpCAM has been selected as a target for various immunotherapeutic approaches.1,13,14 The first monoclonal antibody tested in cancer patients was a murine anti-EpCAM antibody, tested mostly in colorectal cancer cases.15,16 Initial studies showed a significant clinical benefit,17–19 but this could not be confirmed in larger studies in Europe and the United States.20–22 Thus, studies with fully human anti-EpCAM monoclonal antibodies (mAbs) with reduced immunogenicity and improved serum half-life continue.
Natural killer (NK) cells are a type of cytotoxic lymphocytes critical to the innate immune system (reviewed by Vivier et al.23). NK cells play a different role compared to the CD3+ cytotoxic T-cells, since they provide a rapid response to virally infected cells and tumor formation without antigen priming. They have the ability to recognize stressed cells in the absence of MHC, allowing for a much faster killing. NK cells kill by delivering toxic stores of membrane-penetrating and apoptosis-inducing granules that contain perforin and granzymes. They highly express CD16, the FcγRIII receptor, and are not very effective in killing carcinoma cells.
Investigators have shown that it is possible to use cancer cell markers as targets for the NK immune system, and this was particularly appealing to our cancer center, which has an established NK cell therapy program.24,25 Thus, a bispecific NK cell engager capable of killing EpCAM-expressing carcinoma cells was created by splicing a gene encoding an anti-human CD16 single-chain variable fragment (scFV) to a human scFV-recognizing human EpCAM. The anti-CD16 was derived from a human phage-display library.26 The purpose of the hybrid recombinant protein EpCAM16 was to create a bispecific bridge that would expedite NK-mediated killing of carcinoma cells. We have found EpCAM16 to be highly efficient in killing several targets thus far. In this article, we show that EpCAM16 renders NK cell-proficient carcinoma killers and activates them to kill via degranulation and cytokine production. Our studies show that this single agent can be used to engage the innate immune system to kill multiple types of carcinomas, including cancer of the prostate, breast, colon, head, and neck.
Methods
Construction of bispecific EpCAM16
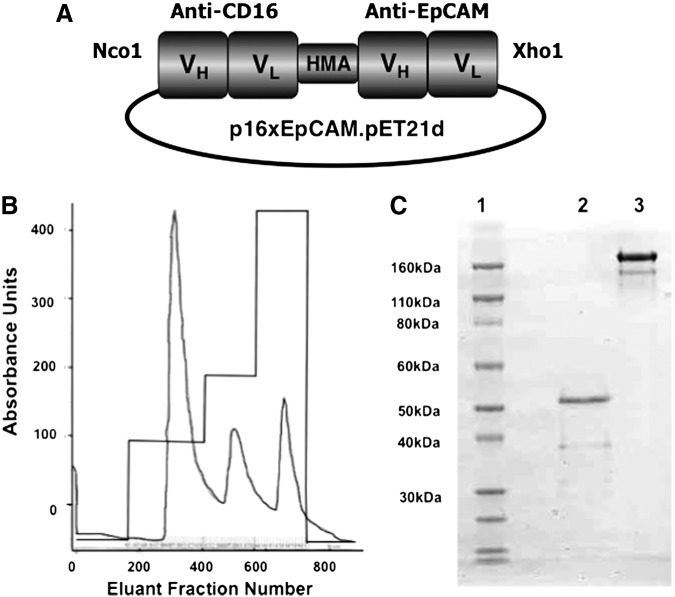
Synthesis and assembly of hybrid genes encoding the single-chain bispecific scFV EpCAM16 were accomplished using the DNA-shuffling and DNA ligation techniques. The construct is illustrated in Figure 1A. The fully assembled gene (from 5′-end to 3′-end) consisted of an Nco1 restriction site, an ATG initiation codon, the VH and VL regions of anti-human CD16 (NM3E2) derived from a phage display library produced by McCall et al.,26 a 20-amino-acid segment of human muscle aldolase (PSGQAGAAASESLFVSNHAY), the VH and VL regions of humanized anti-EpCAM (MOC31), and finally, a XhoI restriction site. The resultant 1560-bp NcoI/XhoI fragment gene was spliced into the pET21d expression vector under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible T7 promoter (Fig. 1B). DNA sequencing analysis (Biomedical Genomics Center, University of Minnesota) was used to verify that the gene was correct in sequence and had been cloned in frame. A separate hybrid gene encoding DTEpCAM was constructed. Genes for monospecific anti-CD16 scFV and anti-EpCAM were created in the same manner.
FIG. 1.
EpCAM16 was successfully purified from bacterial inclusion bodies. (A) A diagram shows the plasmid p16xEpCAM.pET21 containing the EpCAM16 construct consisting of an Nco1 restriction site, the VH and VL regions of anti-human CD16 (NM3E2), a segment of human muscle aldolase, the VH and VL regions of humanized anti-EpCAM (MOC31), and a XhoI restriction site. (B) The absorbance trace of the refolded protein eluted from the Q-sepharose ion-exchange column using a stepwise NaCl gradient. (C) The gel stained with Coomasie blue showing a single band of about 54 kDa eluted from the second column that we ran (size-exclusion column). Lane 1, molecular-weight standards; Lane 2, nonreduced EpCAM16; Lane 3, nonreduced anti-Ly5.1 monoclonal antibody control. EpCAM, epithelial cell adhesion molecule.
Inclusion body isolation
For bacterial protein expression, plasmids were transformed into the Escherichia coli strain BL21(DE3) (EMD millipore). After an overnight culture, bacteria were grown in 800 mL Luria broth containing 50 mg/mL carbenicillin. Gene expression was induced when the culture medium reached an OD600 of 0.65 with the addition of IPTG (Fischer Biotech). Two hours after induction, bacteria were harvested and then homogenized in a buffer solution (50 mM Tris, 50 mM NaCl, and 5 mM ethylenediaminetetraacetic acid [EDTA], pH 8.0). After sonication and centrifugation, the pellets were extracted with 0.3% sodium deoxycholate, 5% Triton X-100, 10% glycerin, 50 mM Tris, 50 mM NaCl, and 5 mM EDTA (pH 8.0) and washed.
Refolding and purification
For refolding proteins from inclusion bodies (IB), IB were dissolved at 20:1 (mg wet weight/mL) in a solubilization buffer (7 M guanidine hydrochloride, 50 mM tris, 50 mM NaCl, 5 mM EDTA, and 50 mM DTT, pH 8.0). After a 1-hour incubation at 37°C, the pellets were removed by centrifugation. The supernatant was diluted 20-fold with a refolding buffer and incubated at 4°C for 2 days. The refolding buffer consisted of 50 mM Tris–HCl, 50 mM NaCl, 0.8 mM l-arginine, 20% glycerin, 5 mM EDTA, and 1 mM GSSG, pH 8.0. The buffer was removed by 10-fold dialysis against 20 mM Tris–HCl, pH 9.0. in 20 mM Tris–HCl, pH 9.0, over four column volumes (Fig. 1B). Sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis was performed, and the fusion proteins were stained with Coomasie brilliant blue.
NK cells
PBMCs were isolated from adult blood (Memorial Blood Center) by centrifugation using a Histopaque gradient (Sigma-Aldrich). NK cells were enriched by negative selection using the magnetic activated cell-sorting NK Cell Isolation Kit as per the manufacturer's protocol (Miltenyi Biotec). Samples were obtained after informed consent and in accordance with the University of Minnesota human subjects Institutional Review Board and the Declaration of Helsinki.
Cell lines
The following human cancer cell lines (and cancer types) were obtained from American Type Culture Collection: BT-474 (breast), SK-BR-3 (breast), MDA-MB-231 (breast), MDA- MB-468 (breast), PC-3 (prostate), DU-145 (prostate), UMSCC-11B (head and neck), NA (head and neck), HT-29 (colorectal), CaCo-2 (colorectal), Daudi (B-cell lymphoma), Raji (B-cell lymphoma), and U-87MG (glioma). Table 1 describes the species and tissue of origin for all cell lines. All carcinoma and glioblastoma cell lines were grown as monolayers in tissue culture flasks, and the Daudi cells were grown in suspension. Cells were maintained in either RPMI-1640 (HT-29, CaCo-2, SK-BR-3, BT-474, DU-145, Daudi, Raji, MDA-MB-231, MDA- MB-468, UMSCC-11B, or NA) or DMEM (U-87MG) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. In addition to the preceding supplements, the BT-474 medium contained 10 μg/mL insulin. Cell cultures were incubated in a humidified 37°C atmosphere containing 5% CO2. When cells were 80%–90% confluent, they were passaged using trypsin–EDTA for detachment. All cells were counted using a standard hemocytometer, and only cells with a viability >95%, as determined by trypan blue exclusion, were used for experiments.
Table 1.
Epithelial Cell Adhesion Molecule Expression on Various Cell Lines Determined by Flow Cytometry
| % Positive cells | |||
|---|---|---|---|
| Cell line | Cancer type | EpCAM | CD19 |
| SK-BR-3 | Human breast cancer | 97 | 3 |
| BT-474 | Human breast cancer | 93 | 1 |
| MDA-MB-231 | Human breast cancer | 14 | 2 |
| MDA-MB-468 | Human breast cancer | 89 | 5 |
| PC-3 | Human prostate cancer | 98 | 3 |
| DU-145 | Human prostate cancer | 63 | 2 |
| UMSCC-11B | Human head neck cancer | 97 | 1 |
| NA | Human head neck cancer | 92 | 0 |
| Daudi | Human B cell lymphoma | 2 | 97 |
| Raji | Human B cell lymphoma | 1 | 96 |
| U87 | Human glioma | 3 | 2 |
| HT-29 | Human colorectal cancer | 95 | 1 |
| CaCo-2 | Human colorectal cancer | 92 | — |
EpCAM expression was measured on various human carcinoma lines by flow cytometry. The anti-EpCAM scFV was tagged with FITC and then reacted with various human carcinoma, lymphoma, and glioma cell lines. Gates established from viable untreated cells were used to establish percentages of EpCAM- and CD19 FITC-positive cells. The percentage of FITC-positive cells was determined from analysis of 10,000 events. As a further negative control, CD19 expression was measured, since CD19 is mostly restricted to normal and malignant hematopoietic B-cells.
EpCAM, epithelial cell adhesion molecule; scFV, single-chain variable fragment; FITC, fluorescein isothiocyanate.
Flow cytometry
For NK cell analysis, single-cell suspensions were stained with the following mAbs: PE/Cy7-conjugated CD56 (HCD56; BioLegend), ECD-conjugated CD3 (UCHT1; Beckman Coulter), PerCP/Cy5.5-conjugated anti-human CD107a (LAMP-1) (H4A3; BioLegend), and Pacific Blue-conjugated anti-human interferon-γ (IFN-γ) (4S.B3; BioLegend). The cells were phenotypically acquired on the LSRII (BD Biosciences) and analyzed with FlowJo software (Tree Star, Inc.). For cancer cell analysis in Table 1, the cells were stained with EpCAM scFV–fluorescein isothiocyanate (FITC) or control anti-CD19-FITC. To determine the dissociation constant (Kd) and the maximum number of binding sites (Bmax), the mean fluorescence intensity was plotted versus the drug concentration and analyzed with Prism software (GraphPad Software).
Cytokine production and CD107a degranulation assay
Our use of this assay has been reported.27 Purified peripheral blood NK cells were incubated overnight at 37°C, 5% CO2, in a basal medium (RPMI supplemented with 10% fetal calf serum and 1% penicillin/streptomycin). Cells were washed in 1× phosphate-buffered saline, treated with 10 μg/mL of EpCAM16 or anti-CD16 scFv (negative control) or anti-EpCAM Fc (negative control), and incubated for 15 minutes at 37°C. The anti-human CD107a mAb was added and further incubated for 1 hour, after which target cells (SKBR3; effector:target [E:T] ratio 2:1), BD GolgiStop (1:1500), and BD GolgiPlug (1:1000; both from BD Biosciences) were added, and the cells were further incubated for 5 hours. The cells were then harvested and stained with mAb CD56 and CD3 before fixation and permeabilization. The permeabilized cells were then stained for intracellular IFN-γ using anti-human IFN-γ mAb. IFN-γ and CD107a expression was evaluated by fluorescence-activated cell-sorting analysis.
Recombinant interleukin (IL)-12 (Peprotech) was used at 10 ng/mL for NK cells. IL-18 (R&D Systems) was used at 100 ng/mL.
51-Chromium-release cytotoxicity assay
UMSCC head and neck cancer cells and HT-29 colorectal cancer cells were labeled for 1 hour with 1 μCi of 51Cr per 1×105 target cells at 37°C, 5% CO2. The target cells were washed to remove excess 51Cr, and 5×103 labeled target cells were added to the wells of 96-well round-bottom plates. Resting effector NK cells or resting effector NK cells coated with anti-CD16/anti-EpCAM or negative controls were added to the plates at E:T cell ratios ranging between 20:1 and 0.08:1. Effector target cells were incubated for 4 hours in a 37°C–5% CO2 incubator. The amount of 51Cr released, which corresponds to target cell death, was measured by a gamma-scintillation counter, and the percent target cell lysis was calculated as follows: [(experimental lysis−spontaneous lysis)/(maximal lysis−spontaneous lysis)]×100.
To determine maximal lysis, 51Cr-labeled target cells were treated with 3% Triton-X for 4 hours.
Results
The construct EpCAM16
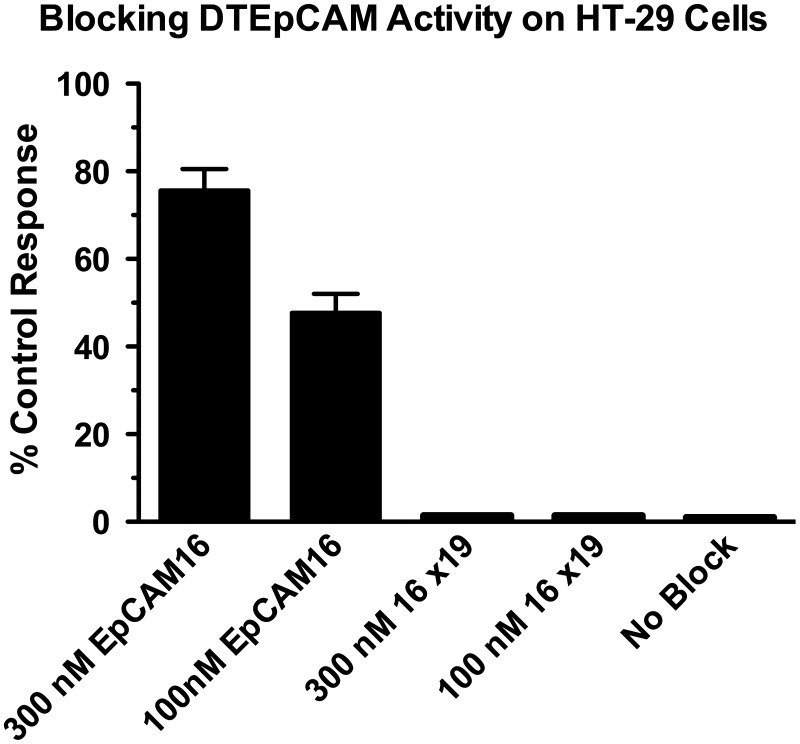
The purity of all purified hybrid proteins was between 80% and 95% when analyzed by SDS-PAGE (Fig. 1). The various drug constructs used in this manuscript were identity-tested by first verifying that the construct was the appropriate molecular weight and then employing blocking assays to prove that both scFV components of the bispecific were indeed operational. Figure 2 shows that EpCAM16 was able to block the activity of a targeted toxin DTEpCAM constructed of the same anti-EpCAM scFV bound to truncated diphtheria toxin against the EpCAM+ target HT-29. Control bispecific scFV 16×19 was not able to block killing.
FIG. 2.
The EpCAM scFV of EpCAM16 binds EpCAM-expressing HT-29 colorectal cancer cells. HT-29 cells were incubated with a targeted toxin DTEpCAM consisting of anti-EpCAM scFV spliced to diphtheria toxin. The addition of 100 or 300 nM EpCAM16-bispecific antibody blocked 1 nM DTEpCAM killing, but the addition of 100 or 300 nM of 16×19 bispecific antibody did not. Killing was measured as inhibition of thymidine uptake. scFV, single-chain variable fragment.
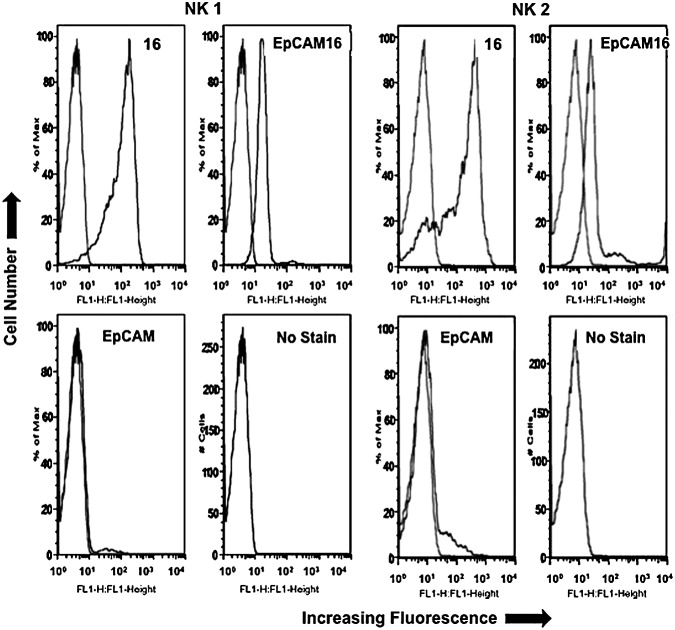
Both EpCAM16 and monospecific anti-CD16 scFV were tested for their ability to bind human NK cells using flow cytometry (Fig. 3). Figure 3 shows that the anti-CD16scFV construct binds well to the purified human NK cells. When the Kd was determined against enriched NK cells, anti-CD16 scFV had the lower Kd value of 175, while EpCAM16 measured 227. Thus, the monospecific form of anti-CD16 binds more intensely than the bispecific anti-CD16 construct. The same anti-EpCAM scFV used in the bispecific drug did not bind human NK cells at all as expected, since EpCAM is an epithelial marker not found on hematopoietic cells.
FIG. 3.
Enriched NK cells from 2 normal human donors express CD16. Enriched NK cells from donors NK1 and NK2 were treated with 10 μg/mL EpCAM16-FITC or anti-CD16-FITC in a direct immunofluorescence assay using flow cytometry. Cells were highly positive compared to the untreated cell (no stain) or anti-B-cell control DT2219ARL-FITC. In every panel, the histogram from the experimental sample is compared to the untreated blank control. NK, natural killer; FITC, fluorescein isothiocyanate.
EpCAM16 kills carcinoma cell lines
Carcinoma cell lines are known for their resistance to NK cell-mediated killing. Therefore, to determine the ability of EpCAM16 to facilitate the killing of EpCAM-expressing carcinoma cells, it was tested against several EpCAM-positive carcinoma cells. EpCAM is an epithelial marker and on all carcinomas derived from epithelial tissue origins by definition. Most, but not all, carcinoma cell lines that we have tested in our laboratory express EpCAM with a very high frequency. Table 1 shows that SK-BR-3, BT-474, and MDA-MB-468 breast cancer cell lines, prostate DU-145 and PC-3 prostate cancer cell lines, UMSCC-11B and NA head and neck cancer cell lines, and the HT-29 head and neck colorectal cancer cell lines all express EpCAM at high levels. The lymphomas and gliomas did not.
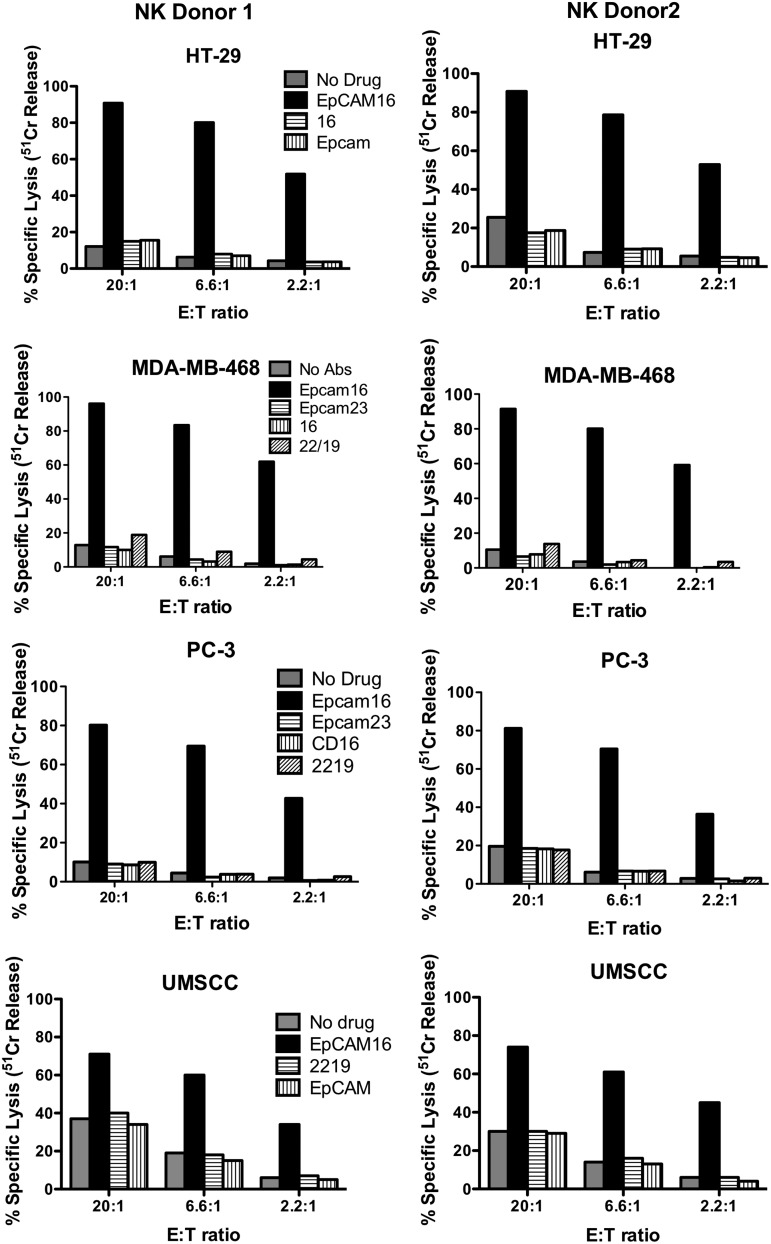
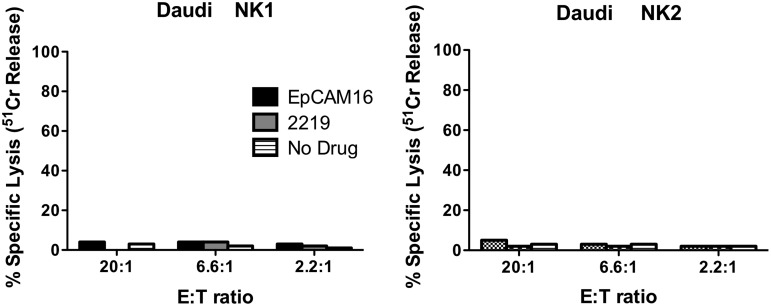
The 51Cr release assay is typically used to measure the killing of target cells by NK cells, since the assay measures the release of 51Cr from the isotope-pulsed targets upon their lysis. If EpCAM16 was able to facilitate NK killing, then it would also be able to facilitate the release of 51Cr from various carcinoma target cells. Indeed, this was the case for all the carcinoma lines tested that were treated with 20 μg/mL EpCAM16. EpCAM16 facilitated the killing of PC-3, UMSCC-11B, MDA-MB-231, and HT-29 (Fig. 4). In some cases, killing exceeded 90%. In all instances, high levels of killing were observed even at low E:T ratios, indicating that addition of the bispecific antibody efficiently enhances NK cell cytotoxicity against targets. The same levels of kill were obtained using EpCAM16 at 2 μg/mL (not shown). EpCAM-negative Daudi cells did not show enhanced killing, further demonstrating that killing was specific and correlated with the presence of EpCAM on the cell surface (Fig. 5).
FIG. 4.
EpCAM16 engages enriched human NK cells to kill various carcinoma cells in vitro. EpCAM-expressing HT-29 colorectal, MDA-MD-468 breast, PC-3 prostate, and UMSCC-11B head and neck cancer cells were tested in different experiments. Cancer cells were mixed with enriched NK cells from 2 different normal donors (NK donor 1 and NK donor 2) and EpCAM16 in plastic plates. After 4 hours, the cells were pulsed with 51Cr, and the NK activity was calculated based on the isotope release from lysed target cells. In all instances, EpCAM16 showed vastly enhanced NK-killing activity. Control wells where cells were incubated with anti-CD16 alone, anti-EpCAM alone, control anti-EpCAM×Her2/Neu (EpCAM23), or no drug at all did not enhance NK activity.
FIG. 5.
EpCAM16 does not engage enriched human NK cells to kill EpCAM-negative Daudi B-cell lymphoma cells. Daudi cells were mixed with enriched NK cells from 2 different normal donors (NK donor 1 and NK donor 2) and EpCAM16 in plastic plates. Killing was calculated from 51Cr release. EpCAM16 did not enhance the NK-killing activity.
EpCAM16 and NK cells
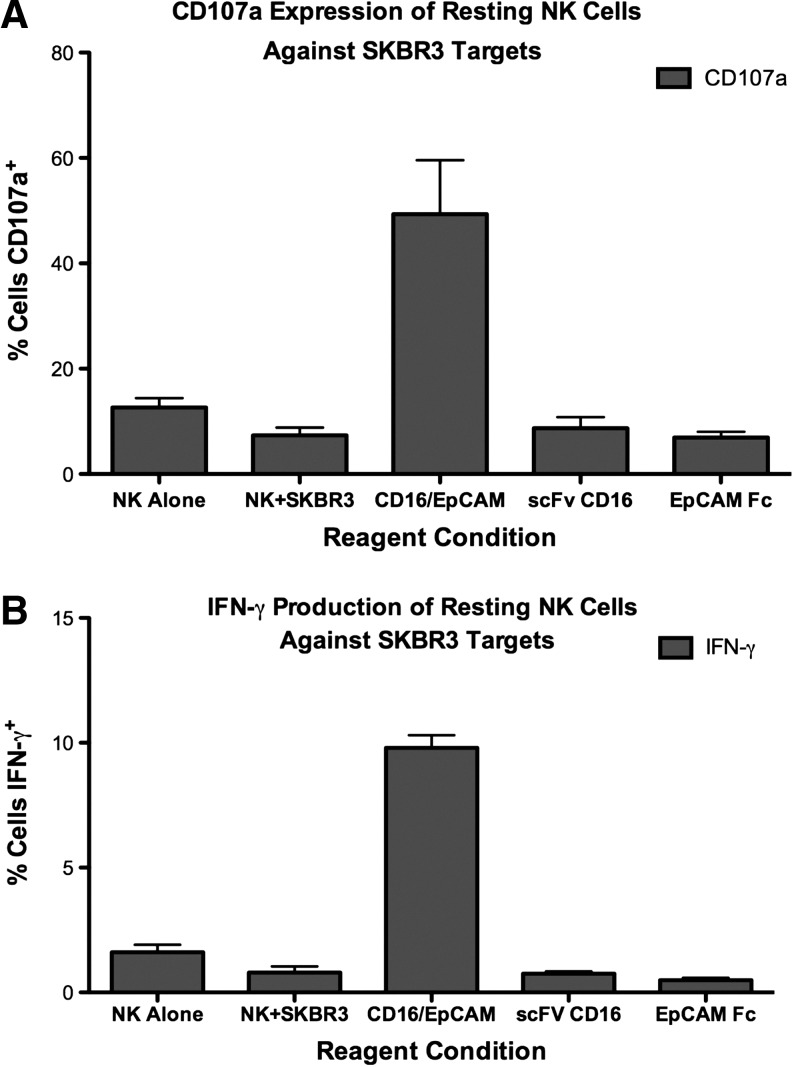
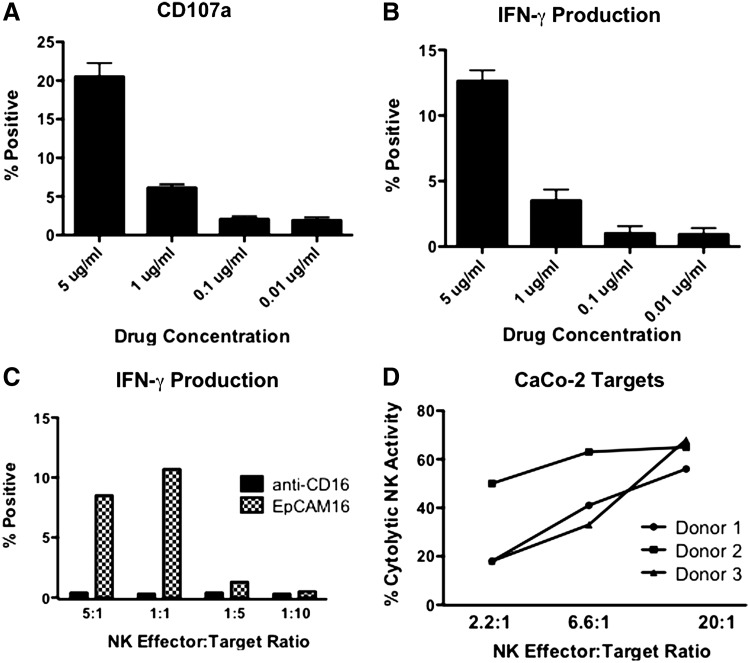
To determine if EpCAM16 caused the degranulation of NK cells, a surrogate marker for NK cell killing, human NK cells were treated with 20 μg/mL EpCAM16 and then tested for CD107a expression by flow cytometry. Figure 6A shows that only EpCAM16, but not any of the controls, including monospecific anti-CD16 and anti-EpCAM scFV, caused a significant increase in CD107a expression. Figure 6B shows that only EpCAM16 caused a simultaneous elevation in IFN-γ production, demonstrating the ability of EpCAM16 to induce both target-specific cytotoxicity and cytokine production. Figure 7A and B show that an EpCAM16-induced effector function occurs at a concentration of 5 μg/mL, but the response drops off at 1 μg/mL, indicating dose dependency. Interestingly, Figure 7C shows that when cells are treated at 5 μg/mL, high levels of activity still occur at E:T ratios as low as 1:1, indicating that even one NK effector cell can respond against one NK target in the presence of the bispecific antibody. Thus, the response is quite potent. Figure 7D shows that resting NK cells treated with 20 μg/mL of EpCAM16 and cocultured with EpCAM-positive CaCo-2 targets efficiently induced target cell lysis.
FIG. 6.
EpCAM16 markedly enhances the expression of CD107a, considered a marker of NK cell activity and expression of interferon-γ (IFN-γ). SKBR3 human breast cancer cells were incubated with enriched resting NK cells in the presence of 20 μg/mL EpCAM16. (A) Cells were studied by flow cytometry to determine the expression of CD107a, also called lysosomal-associated membrane protein. Levels were elevated, but not in cells treated with control anti-EpCAM, anti-CD16 scFV, or untreated cells. (B) Cells were studied by flow cytometry to determine the expression of IFN-γ. The levels were elevated, but not in cells treated with control anti-EpCAM, anti-CD16 scFV, or untreated cells.
FIG. 7.
EpCAM16 is dose-dependent and active at very low E:T ratios. CaCo2 human colorectal cancer cells were incubated with enriched resting NK cells in the presence of EpCAM16. (A, B) Decreasing concentrations of drug were added beginning at 5 μg/mL. A drop-off in activity was observed at the 1 μg/mL dose level. (C) Cells treated at 5 μg/mL were tested at very low E:T ratios. Activity was still observed at an E:T of 1:1. (D) Three NK donors were tested simultaneously at the 20 μg/mL dose level. High NK activity occurred with all 3 donors. E:T, effector:target.
EpCAM16 and IFN-γ production
Since it is not possible to assess cytokine storm in vivo, we checked for the ability of EpCAM16 to induce the supraphysiologic IFN-γ in vitro. Others have shown that high levels of IL-12/1L-18 precipitously enhance NK-cell IFN-γ production.28 Thus, CD56-enriched NK cells were stimulated in the presence of CaCo-2 cells with high-dose IL-12/IL-18 or 5 μg (high dose) EpCAM16 (Table 2). High-dose IL-12/IL-18 showed supraphysiologic stimulation of IFN-γ. EpCAM16 treatment revealed IFN-γ increases, but they were lower than those obtained with IL-12/IL-18. Measurement of CD107a in the same cells indicated NK cell degranulation and killing in the EpCAM16-treated cells, but not in the IL-12-/IL-18-treated cells. Together, these findings suggest that EpCAM16 does not induce release of an NK cell cytokine storm while NK cells kill their EpCAM+ targets.
Table 2.
EpCAM16 Degranulates Natural Killer Cells, But Does Not Induce a Supraphysiologic Interferon-γ Stimulus
| % IFN-γ+ | % CD107a+ | |||||
|---|---|---|---|---|---|---|
| Treatment | Donor 1 | Donor 2 | Donor 3 | Donor 1 | Donor 2 | Donor 3 |
| None | 0.5 | 0.2 | 1.0 | 1.6 | 1.7 | 2.0 |
| IL12/IL18 | 22.0 | 34.8 | 24.6 | 4.3 | 3.7 | 2.8 |
| EpCAM16 | 9.6 | 7.6 | 8.3 | 23.2 | 17.2 | 21.1 |
| 2219 scFVs | 0.4 | — | — | 2.7 | — | — |
| anti-CD16 scFV | 0.5 | — | — | 4.0 | — | — |
IFN-γ+ cells and CD107a (NK cell degranulation) were measured by flow cytometry on CD56- enriched human NK cells in the presence of CaCo-2 targets. IFN-γ and CD107a were measured in the same experiment. The percentage of FITC-positive cells was determined from analysis of 10,000 events.
IFN, interferon; NK, natural killer.
Discussion
Clinical studies have verified the value of BiTE technology and have resulted in complete responses in carcinoma therapy.29,30 BiTEs are single-chain bispecific scFV antibodies that simultaneously recognize T-cells via the T-cell receptor and a cancer cell marker. Although engaging T-cells show promise, past successes have taught us that the populations of NK cells also can readily be expanded in patients, and NK cells can be powerful killers of tumor cells.31 By themselves, NK cells are marginally effective against carcinomas. However, using the bispecific scFV platform that we call BiKEs, NK cells can be effectively recruited to kill even those carcinomas that have shed class 1 histocompatibility markers to avoid T-cell killing.32 The purpose of these studies was to develop a BiKE platform that would permit the development of new BiKEs for the use in our program at the University of Minnesota, currently providing cellular NK cell therapy to 20 patients/year.
In this study, we show that a completely humanized bispecific scFV simultaneously targeting the carcinoma marker EpCAM and CD16 expressed on the surface of human NK cells powerfully boosts the killing of various human carcinoma cell lines, including cancers of the prostate, breast, colon, and head and neck. The original contribution is the data showing that the drug is capable of NK activation and the induction of an IFN-γ response, which facilitates the antitumor response. Our data indicates that although very effective, binding of the EpCAM moiety of EpCAM16 was decreased in the bispecific drug as compared to the monospecific construct made with the same anti-EpCAM scFV. The decrease in the ability of bispecific EpCAM16 to bind NK cells as compared to monospecific anti-CD16 scFV may indicate that our bispecific framework may be putting an undo stress on the scFV causing mispairing. Alternatively, the same number of FITC molecules was conjugated to both the bispecific and monospecific molecules. Since the bispecific is twice the size of the monospecific, fewer molecules might bind to the cell, reducing its signal. Future studies will be necessary to determine this. Regardless, the bispecific EpCAM16 molecule is impressive in its ability to enhance the NK cell activity.
A more recent study of EpCAM targeting BiTEs indicate that anti-EpCAM targets CSC.33 The BiTE was affectively redirected to destroy the tumor initiating CSC in a pancreatic cancer model in vivo. Increasing evidence indicates that CSC may play a decisive role in the development and progression of different human tumors.34,35 The current consensus definition is that a CSC is a minority population within a tumor that is able to self-renew and produce the heterogeneous lineages of cancer cells that comprise the majority of the tumor mass.35 Since tumorigenic CSC are highly resistant to standard chemotherapy, such cells may be the source of the inevitable relapse of carcinomas. Thus, directing NK cells using this drug may be a powerful method of directing nonselective NK cells to specifically kill CSC.
How do these bispecific drugs work? The mechanism is not fully understood, but clearly the drug functions to bring NK cells into close proximity with EpCAM+ carcinoma cells. In a second stage of interaction, an immune lytic synapse forms between the NK effector cells and tumor targets, resulting in the LFA-1/ICAM interactions that accelerate tumor cell killing. Findings in this article showed that CD107a was precipitously elevated. Others have shown that CD107a expression is an indicator of NK cell functional activity identifying a large fraction of activated NK cells.36 CD107a is expressed on degranulating NK cells that secrete cytokines as well as a large subset of NK cells that do not secrete cytokine after stimulation. Thus, we also tested for IFN-γ release, which requires a higher threshold to induce, and this was increased as well.
Studies have addressed the temporal aspects of cytokine secretion and show that CD16 NK cell receptor engagement releases proinflammatory cytokines and chemokines, and that IFN-γ release occurs later in the process.37 Thus, IFN-γ secretion is indicative of a cascade that functions to activate signal transducers and activators of transcription complexes that regulate the expression of the immune system genes and enhance the anticancer response. Together, the data suggest that bispecific scFV interactions activated NK cells to kill both directly and indirectly, explaining the impressive anticancer effects that have been observed clinically.
Overproduction of cytokine or cytokine storm can lead to unwanted toxicity. The following points suggest that cytokine storm may not be a problem with EpCAM16. (1) High-dose IL12/IL-18 was used in this article to induce supraphysiologic IFN-γ responses in enriched NK cells mimicking cytokine storm in vitro. In comparison, treatment with EpCAM-16 did not. (2) The lack of the Fc domain in EpCAM16 may limit formation of multiple cell bridging, a requisite for cytokine storm.26 (3) Schmitt et al. studied a trifunctional hybrid recognizing EpCAM, CD3, and CD16 in vivo.38 ELISPOT analysis indicated that the construct did not induce cytokine storm.
One advantage of EpCAM16 is that it is entirely humanized, so an HAMA response will be unlikely. Anti-human EpCAM and anti-human CD16 do not cross-react with rodent EpCAM or rodent CD16. Thus, safety testing and establishing maximum tolerated dose are options in primate models. In fact, an anti-EpCAM/anti-CD3 hybrid protein (Catumaxomab) was developed and tested for toxicity in standard animal models, including mouse, rat, rabbit, guinea pig, and cynomolgus monkey.39 Administration of drug at doses exceeding the human therapeutic dose range did not result in abnormal or substance-related acute toxicity or local intolerability at the administration site. In a multicenter clinical study, 24 patients with EpCAM-positive GI cancer received an MTD of catumaxomab, and 11 of 17 evaluable patients (65%) were progression-free at the final examination. One patient had a CR, and 3 patients had a PR (discussed by Seimetz et al.39). Regarding side effects, in a different phase I/II clinical trial of catumaxomab for malignant pleural effusion, the most common side effects in 24 patients were pyrexia, elevated liver enzymes, nausea, and decreased lymphocytes.40 Acceptable safety profiles may result because BiTEs are not as toxic as drug or toxin conjugates, and therefore some normal cell damage occurs, but is reversible.
In summary, heterodimeric EpCAM16 is a potent engager of the innate immune system and may be highly useful in a clinical NK cell therapy program. Since chemotherapy resistance is the major problem in today's clinical carcinoma treatment, this bispecific drug may be very valuable because of its different mechanism of action. Based on these data, EpCAM16 could be tested against any EpCAM+ carcinoma and would be particularly valuable in clinical programs in which the NK cell therapy is currently in use.
Acknowledgments
These studies were supported in part by P01 CA65493, P30 CA77598, the Minnesota Masonic Charities, the U.S. Public Health Service Grants R01-CA36725, the Randy Shaver Foundation, and the Mayo Partnership.
Disclosure Statement
No competing financial interests exist.
References
- 1.Baeuerle PA. Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96:417. doi: 10.1038/sj.bjc.6603494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litvinov SV. Velders MP. Bakker HA, et al. Ep-CAM: A human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litvinov SV. Bakker HA. Gourevitch MM, et al. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesion. Cell Adhes Commun. 1994;2:417. doi: 10.3109/15419069409004452. [DOI] [PubMed] [Google Scholar]
- 4.Balzar M. Briaire-de Bruijn IH. Rees-Bakker HA, et al. Epidermal growth factor-like repeats mediate lateral and reciprocal interactions of Ep-CAM molecules in homophilic adhesions. Mol Cell Biol. 2001;21:2570. doi: 10.1128/MCB.21.7.2570-2580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munz M. Baeuerle PA. Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 6.Gires O. Klein CA. Baeuerle PA. On the abundance of EpCAM on cancer stem cells. Nat Rev Cancer. 2009;9:143. doi: 10.1038/nrc2499-c1. [DOI] [PubMed] [Google Scholar]
- 7.Visvader JE. Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 8.Marhaba R. Klingbeil P. Nuebel T, et al. CD44 and EpCAM: Cancer-initiating cell markers. Curr Mol Med. 2008;8:784. doi: 10.2174/156652408786733667. [DOI] [PubMed] [Google Scholar]
- 9.Osta WA. Chen Y. Mikhitarian K, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64:5818. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 10.Maetzel D. Denzel S. Mack B, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 11.Munz M. Kieu C. Mack B, et al. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene. 2004;23:5748. doi: 10.1038/sj.onc.1207610. [DOI] [PubMed] [Google Scholar]
- 12.Stoecklein NH. Siegmund A. Scheunemann P, et al. Ep-CAM expression in squamous cell carcinoma of the esophagus: A potential therapeutic target and prognostic marker. BMC Cancer. 2006;6:165. doi: 10.1186/1471-2407-6-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudry MA. Sales K. Ruf P, et al. EpCAM an immunotherapeutic target for gastrointestinal malignancy: Current experience and future challenges. Br J Cancer. 2007;96:1013. doi: 10.1038/sj.bjc.6603505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stish BJ. Chen H. Shu Y, et al. Increasing anticarcinoma activity of an anti-erbB2 recombinant immunotoxin by the addition of an anti-EpCAM sFv. Clin Cancer Res. 2007;13:3058. doi: 10.1158/1078-0432.CCR-06-2454. [DOI] [PubMed] [Google Scholar]
- 15.Sears HF. Herlyn D. Steplewski Z, et al. Effects of monoclonal antibody immunotherapy on patients with gastrointestinal adenocarcinoma. J Biol Response Mod. 1984;3:138. [PubMed] [Google Scholar]
- 16.Sears HF. Atkinson B. Mattis J, et al. Phase-I clinical trial of monoclonal antibody in treatment of gastrointestinal tumours. Lancet. 1982;1:762. doi: 10.1016/s0140-6736(82)91811-6. [DOI] [PubMed] [Google Scholar]
- 17.Ragnhammar P. Fagerberg J. Frodin JE, et al. Effect of monoclonal antibody 17- 1A and GM-CSF in patients with advanced colorectal carcinoma–longlasting, complete remissions can be induced. Int J Cancer. 1993;53:751. doi: 10.1002/ijc.2910530508. [DOI] [PubMed] [Google Scholar]
- 18.Riethmuller G. Schneider-Gadicke E. Schlimok G, et al. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes' C colorectal carcinoma. German Cancer Aid 17-1A Study Group. Lancet. 1994;343:1177. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 19.Riethmuller G. Holz E. Schlimok G, et al. Monoclonal antibody therapy for resected Dukes' C colorectal cancer: Seven-year outcome of a multicenter randomized trial. J Clin Oncol. 1998;16:1788. doi: 10.1200/JCO.1998.16.5.1788. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg RM. Lessons learned from the edrecolomab story: How a checkered past became a checkered flag for monoclonal antibodies in colorectal cancer therapy. Onkologie. 2005;28:311. doi: 10.1159/000085570. [DOI] [PubMed] [Google Scholar]
- 21.Fields AL. Keller A. Schwartzberg L, et al. Adjuvant therapy with the monoclonal antibody Edrecolomab plus fluorouracil-based therapy does not improve overall survival of patients with stage III colon cancer. J Clin Oncol. 2009;27:1941. doi: 10.1200/JCO.2008.18.5710. [DOI] [PubMed] [Google Scholar]
- 22.Schmoll HJ. Arnold D. When wishful thinking leads to a misty-eyed appraisal: The story of the adjuvant colon cancer trials with edrecolomab. J Clin Oncol. 2009;27:1926. doi: 10.1200/JCO.2008.20.6284. [DOI] [PubMed] [Google Scholar]
- 23.Vivier E. Raulet DH. Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein C. Kellner C. Kügler M, et al. Novel conjugates of single-chain Fv antibody fragments specific for stem cell antigen CD123 mediate potent death of acute myeloid leukaemia cells. Br J Haematol. 2010;148:879. doi: 10.1111/j.1365-2141.2009.08033.x. [DOI] [PubMed] [Google Scholar]
- 25.Singer H. Kellner C. Lanig H, et al. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010;33:599. doi: 10.1097/CJI.0b013e3181dda225. [DOI] [PubMed] [Google Scholar]
- 26.McCall AM. Adams GP. Amoroso AR, et al. Isolation and characterization of an anti-CD16 single-chain Fv fragment and construction of an anti-HER2/neu/anti-CD16 bispecific scFv that triggers CD16-dependent tumor cytolysis. Mol Immunol. 1999;36:433. doi: 10.1016/s0161-5890(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 27.Gleason MK. Lenvik TR. McCullar V, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119:3064. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadakis KA. Prehn JL. Landers C, et al. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172:7002. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 29.Topp MS. Kufer P. Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 30.Ströhlein MA. Lordick F. Rüttinger D, et al. Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: An open-label, multicenter, phase I/II trial. Onkologie. 2011;34:101. doi: 10.1159/000324667. [DOI] [PubMed] [Google Scholar]
- 31.Geller MA. Miller JS. Use of allogeneic NK cells for cancer immunotherapy. Immunotherapy. 2011;3:1445. doi: 10.2217/imt.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf E. Hofmeister R. Kufer P, et al. BiTEs: Bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today. 2005;10:1237. doi: 10.1016/S1359-6446(05)03554-3. [DOI] [PubMed] [Google Scholar]
- 33.Cioffi M. Dorado J. Baeuerle P, et al. EpCAM/CD3-Bispecific T-cell engaging antibody MT110 eliminates primary human pancreatic cancer stem cells. Clin Cancer Res. 2012;18:465. doi: 10.1158/1078-0432.CCR-11-1270. [DOI] [PubMed] [Google Scholar]
- 34.Jordan CT. Guzman ML. Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 35.Clarke MF. Dick JE. Dirks PB, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 36.Alter G. Malenfant JM. Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Fauriat C. Long EO. Ljunggren H-G, et al. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt M. Schmitt A. Reinhardt P, et al. Opsonization with a trifunctional bispecific (alphaCD3×alphaEpCAM) antibody results in efficient lysis in vitro and in vivo of EpCAM positive tumor cells by cytotoxic T lymphocytes. Int J Oncol. 2004;25:841. [PubMed] [Google Scholar]
- 39.Seimetz D. Lindhofer H. Bokemeyer Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM×anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36:458. doi: 10.1016/j.ctrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Sebastian M. Kiewe P. Schuette W, et al. Treatment of malignant pleural effusion with a trifunctional antibody Catumaxomab (Removab) (anti-EpCAM x anti-CD3): Results of a phase 1/2 study. J Immunother. 2009;32:195. doi: 10.1097/CJI.0b013e318195b5bb. [DOI] [PubMed] [Google Scholar]