Abstract
The liver is one of the most important organs in the body, performing a fundamental role in the regulation of diverse processes, among which the metabolism, secretion, storage, and detoxification of endogenous and exogenous substances are prominent. Due to these functions, hepatic diseases continue to be among the main threats to public health, and they remain problems throughout the world. Despite enormous advances in modern medicine, there are no completely effective drugs that stimulate hepatic function, that offer complete protection of the organ, or that help to regenerate hepatic cells. Thus, it is necessary to identify pharmaceutical alternatives for the treatment of liver diseases, with the aim of these alternatives being more effective and less toxic. The use of some plants and the consumption of different fruits have played basic roles in human health care, and diverse scientific investigations have indicated that, in those plants and fruits so identified, their beneficial effects can be attributed to the presence of chemical compounds that are called phytochemicals. The present review had as its objective the collecting of data based on research conducted into some fruits (grapefruit, cranberries, and grapes) and plants [cactus pear (nopal) and cactus pear fruit, chamomile, silymarin, and spirulina], which are consumed frequently by humans and which have demonstrated hepatoprotective capacity, as well as an analysis of a resin (propolis) and some phytochemicals extracted from fruits, plants, yeasts, and algae, which have been evaluated in different models of hepatotoxicity.
Keywords: Hepatoprotection, Grapefruit, Vaccinium spp, Vitis vinifera L, Opuntia ficus-indica, Spirulina, Propolis, Glucans, Alcoholic liver disease
Core tip: Currently, diverse natural products have been studied, in attempts to identify protective effects against agents that cause disease. In this paper, we conduct a review of diverse natural products which, by means of their antioxidant activity, protect the liver from the damage caused by substances such as ethanol, acetaminophen, carbon tetrachloride, thioacetamide, etc.
INTRODUCTION
The liver is one of the most important organs of the body. It performs a fundamental role in the regulation of diverse physiological processes, and its activity is related to different vital functions, such as metabolism, secretion, and storage. Its capacity to detoxify endogenous (waste metabolites) and/or exogenous (toxic compounds) substances of organisms, as well as for synthesize useful agents, has been analyzed since the 1970s by many researchers[1-4].
The liver is also involved in the biochemical processes of growing, providing nutrients, supplying energy, and reproducing. In addition, it aids in the metabolism of carbohydrates and fats, in the secretion of bile, and in the storage of vitamins[5].
Because of all of these functions, hepatic diseases continue to among the principal threats to public health, and they are a problem worldwide[4,6]. Hepatic disease is a term that indicates damage to the cells, tissues, structure, or liver function, and this damage can be induced by biological factors (bacteria, virus, and parasites) and autoimmune diseases (immune hepatitis, primary biliary cirrhosis), as well as by the action of different chemicals, such as some drugs [high doses of paracetamol (PCM) and antitubercular drugs], toxic compounds [carbon tetrachloride (CCl4), thioacetamide, dimethylnitrosamine (DMN), D-galactosamine/lipopolysaccharide (GalN/LPS)], and unquestionably, excessive consumption of alcohol[7-9]. Despite enormous advances in modern medicine, there are no completely effective drugs that stimulate hepatic function, offer complete protection to the organ, or aid in regenerating hepatic cells[10]. Additionally, some drugs can induce adverse or side effects. Thus, it is necessary to identify alternative pharmaceuticals for the treatment of hepatic diseases, with the aim of these agents being more effective and less toxic.
The use of some plants and the consumption of different fruits have played fundamental roles in human health care. Approximately 80% of the world’s population has employed traditional medicine for health care, which is based predominantly on plant materials[4,9]. Diverse scientific investigations of medicinal plants and the ingestion of fruits have indicated that the properties that are responsible for their beneficial effects could be attributed to the presence of chemical compounds or substances that are biologically active and that are non-essential nutrients for life, called phytochemicals[11].
In the literature, studies can be found that have examined the impact that different phytochemicals exert on health Among the most frequently cited examples, we find the following: (1) the vinca alkaloids (vincristine, vinblastine, and vindesine); (2) the betalain pigments (betanin and indicaxanthine); (3) the anthocyanins (cranberries); and (4) and resveratrol; all of these have generally been analyzed based on their chemoprotective properties against cancer[4,12-14]. All of the medicinal plants, as well as the ingestion of certain fruits, have demonstrated different effects on living systems. Although there have been diverse studies directed toward the evaluation of their hepatoprotective potential, the majority of investigations have been directed at analysis of their sedative, analgesic, antipyretic, cardioprotective, antibacterial, antiviral, antiprotozoal, and anticarcinogenic capacities[15].
In addition to these studies, empirical evidence for the use of natural remedies for the treatment of hepatic diseases has a long history, and this field has become an innovative field of study, with the principal aim of analyzing the consumption of traditional fruits and medicinal plants by a great number of people and the different phytochemicals that are extracted from these foods. In general, liver-protective fruits, as well as plants, contain a variety of chemical compounds, such as phenols, coumarins, lignans, essential oils, monoterpenes, glycosides, alkaloids, carotenoids, flavonoids, organic acids, and xanthines[16].
This present review had as its objective the gathering of data based on works conducted in some fruits and plants that are consumed frequently by humans and that have demonstrated hepatoprotective capacity, as well as analysis of a resin and some phytochemicals extracted from fruits, plants, yeasts, and algae that have been evaluated in different models of hepatotoxicity. With these goals in mind, the authors of this paper have attempted to provide information and bibliographic support to researchers who are exploring compounds with this potential and to encourage the development of new investigations in this area of study.
HEPATOPROTECTIVE FRUITS
Grapefruit (Citrus paradisi)
Overview: The grapefruit is an important member of the genus Citrus of the Rutaceae family, the scientific name of which is Citrus paradisi. The grapefruit originated on Barbados Island, but it is currently cultivated in Mexico, Spain, Morocco, Israel, Jordan, South Africa, Brazil, and Jamaica and on the Asian continent[17]. In addition to its being consumed as a seasonal fruit or in juice to accompany other foods, it has been used in many countries in popular and traditional medicine as an antimicrobial, antifungal, anti-inflammatory, antioxidant, and antiviral, as an astringent solution, and as a preservative agent[18]. Studies conducted over past decades have suggested that the grapefruit might be active in cellular regeneration, cholesterol reduction, the detoxifying process, and the maintenance of heart health, in rheumatoid arthritis, for the control of body weight, and in cancer prevention[17-19]. Grapefruit juice is an excellent source of many phytochemicals and nutrients that contribute to a healthy diet. It contains significant levels of vitamin C, folic acid, phenolic acid, potassium, calcium, iron, limonoides, terpenes, monoterpenes, and D-glucaric acid. The red and pink varieties also contain beta-carotene and lycopene, antioxidants that the body can convert into vitamin A[17]. However, the flavonoid that has the greatest concentration is naringin, which humans metabolize into naringenin[20,21].
Hepatoprotective evidence for naringin and naringenin: Despite the common consumption of the grapefruit as a fresh fruit or in juice, there have been no studies that have directly analyzed its protective effects against the damage produced by hepatotoxic substances. The main evidence that is able to suggest its hepatoprotective potential has emerged from the study of naringenin and naringin.
One of the first studies was the investigation performed by Parmar[22], who evaluated in rats the antiulcer activity of inhibiting histidine decarboxylase in retention ulcers and in phenylbutazone- and aspirin-induced pyloric ligatures. Their results showed that naringenin significantly reduced mucosa damage in both models, with a greater effect observed in ulcers with pyloric ligation, suggesting that the protective mechanism of naringenin involved inhibition of the formation and release of endogenous histamine in the gastric mucosa of rats.
These results motivated other investigations specifically into hepatoprotective effects; thus, the protective capacity of naringenin on dimethylnitrosamine (DMN)-induced hepatic damage in rats was investigated in 2004. Oral administration of naringenin (20 and 50 mg/kg daily over 4 wk) notably diminished DMN-induced damage when the weight of the liver was evaluated, as well as alanine transaminase (ALAT), aspartate transaminase (ASAT), alkaline phosphatase (ALP), and bilirubin levels. Naringerin also restored natural protein levels in serum and albumin and hepatic malondialdehyde (MDA) levels. In conclusion, it was demonstrated that naringenin had antifibrinogenic and hepatoprotective effects, suggesting that it could be useful in the treatment of hepatic fibrosis[23].
Taken together, the results of the two most recent studies indicated that this flavonoid is a biologically active compound that is capable of significantly reducing levels of serum ALAT and ASAT, gamma-glutamyl transpeptidase (GGT), thiobarbituric acid-reactive substances (TBARS) tissue, conjugated dienes, lipid hydroperoxides, protein carbonyl content, bilirubin, ALP, lactate dehydrogenase (LDH), and phase I enzymes and of increasing the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GRx), glutathione-S-transferase (GST), alcohol dehydrogenase (ADH), and aldehyde dehydrogenase (ALDH), as well as the levels of vitamins C and E in tissues and phase II enzymes in rats with ethanol-treated hepatic damage in comparison to animals not receiving treatment[24,25].
With respect to naringin, the sole study that found evidence of the hepatoprotective capacity of this flavonoid was the investigation of Seo et al[26], who analyzed the role of a naringin supplement in the regulation of lipid and ethanol metabolism in male Sprague-Dawley rats. These animals were organized into six groups based on the following six dietary categories: ethanol- and naringin-free; ethanol (50 g/L) plus low-naringin (0.05 g/L); ethanol plus high-naringin (0.125 g/L); and three corresponding pair-fed groups. The pair-fed control rats received an isocaloric diet containing dextrin-maltose instead of ethanol for 5 wk. Among the ethanol-treated groups, naringin supplements significantly lowered the plasma ethanol concentration, with simultaneous increases in ADH and/or ALDH activity. However, among the ethanol-treated groups, naringin supplementation resulted in a significant decrease in hepatic triglycerides (TGs) and plasma and hepatic total cholesterol (TC), compared with the naringin-free group. Naringin supplementation significantly increased high-density lipoprotein-c (HDL-cholesterol) and HDL-c/TC ratio, while lowering the AI value among the ethanol-treated groups. Hepatic lipid accumulation was also significantly reduced in the naringin-supplemented groups compared with the naringin-free group among ethanol-treated groups, while no differences were found among the pair-fed groups. Among the ethanol-treated groups, low-naringin supplementation resulted in a significant decrease in the levels of plasma and hepatic TBARS, whereas it resulted in higher SOD and GPx activities and higher glutathione (GSH) levels in the liver. Accordingly, naringin would appear to contribute to alleviating the adverse effects of ethanol ingestion by enhancing ethanol and lipid metabolism, as well as promoting the hepatic antioxidant defense system.
Blueberries/cranberries (Vaccinium spp)
Overview: Among the small, soft-fleshed, colorful fruits, berries make up the largest proportion consumed in our diets. These fruits are not only ingested in their fresh and/or frozen forms, but they also appear in processed products, such as canned fruits, yogurts, beverages, jams, and jellies. Thus, in recent years, the consumption of berry extracts has increased as an ingredient in functional foods and dietary supplements, which might or might not be combined with other colored fruits, plants, and herbal extracts. The berry fruits that are habitually consumed in North America include blackberries (Rubus spp.), black raspberries (Rubus occidentalis), red raspberries (Rubus idaeus), strawberries (Fragaria X ananassa), blueberries (Vaccinium corymbosum), and cranberries (Vaccinium macrocarpon)[14].
At the 2007 International Berry Health Benefits Symposium, diverse research from Asia, Europe, New Zealand, Mexico, and North and South America were presented that demonstrated the potential benefits that could be obtained by consuming these fruits. In general, it was concluded that these benefits could be observed in cardiovascular diseases, neurodegenerative diseases, and other diseases associated with aging, in obesity, and in some human cancers (mainly esophageal and gastrointestinal)[27]. It was determined that the agents responsible for these biological properties were diverse phenolic-type phytochemicals, among which the following were highlighted (1) flavonoids (anthocyanines, flavonols, and flavanols); (2) tannins [condensed tannins (proanthocyanidins) and hydrolyzable tannins (ellagitannins and gallotannins)]; (3) stilbenoids; and (4) phenolic acids[14]. Of all of these compounds, those that have been most studied are the anthocyanins (pigments are responsible for the berries’ attractive colors), which have demonstrated antioxidant, anticarcinogenic, and anti-inflammatory biological activity[14,28].
Hepatoprotective evidence for the berry fruits and proanthocyanidins: Oxidative stress (OS) and dysfunction of cellular immunity are important indicators in the pathogenesis of hepatic diseases caused by diverse xenobiotics[29]. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is an important transcription factor that regulates OS reactions and is in charge of controlling two antioxidant enzymes: HO-1 and Nqo1[30-32]. This antecedent motivated Wang et al[32] to evaluate the effects of the blueberry on liver protection and cellular immune function. The authors randomly submitted male Kunming mice 6-8 wk of age to two experiments: in the first, they extracted samples of hepatic RNA with the Trizol reagent (Invitrogen, United States) and determined the expression of Nrf2, HO-1, and Nqo1 by real-time reverse transcription-polymerase chain reaction, as well as the quantification of SOD and MDA after administering blueberries orally (0.6 g/10 g) over 21 d; in the second experiment, the animals received blueberries over 35 d with the aim of evaluating cellular immune function and quantifying the percentage of CD3+, CD4+, and CD8+ T lymphocyte subgroups in peripheral blood by flow cytometry, as well as the thymus index and lymphocyte proliferation in the spleen by MTT tetrazolium-dye assay. The results indicated that treatment with this fruit significantly increased the expression of Nrf2, HO-1, and Nqo1, as well as the percentages of the CD3+ and CD4+ T lymphocyte subsets. It also increased the spleen index, improving the proliferation of lymphocytes deriving from this organ, increasing hepatic SOD, and reducing MDA. In conclusion, the authors suggested that consuming this berry type protected hepatocytes from OS and could modulate the function of T cells.
As mentioned in the overview, different types of berries are consumed frequently, and among these, the cranberry was the topic of an experiment with the primary objective of evaluating its antioxidant and hepatoprotective potential against liver mitochondrial damage induced by acute [0.8 g/kg body weight (bw), single injection] and chronic (1.6 g/kg bw, 30 d, biweekly injections) CCl4 intoxication in rats. Both acute and chronic intoxication negatively affected the mitochondrial respiratory parameters in the liver, and the enzymatic activities of succinate dehydrogenase, GPx, and cytoplasmic catalase were significantly inhibited. However, administration of cranberries (7 mg/kg) was effective in diminishing the toxic effects of CCl4, normalizing ALAT and ASAT activity and bilirubin concentrations. Similarly, it prevented accumulation of membrane lipid peroxidation products in the rat liver, resulting in apparent preservation of the mitochondrial ultrastructure[33].
It is important to remember that the anthocyanins and proanthocyanidins are widely available compounds in fruits, vegetables, and seeds of natural origin. Specifically, the proanthocyanidins are a class of phenolic compounds that have demonstrated a broad range of biological effects; therefore, Shin et al[34] investigated the protective effects of these compounds against DMN-induced hepatic lesions in rats. Treatment with DMN caused significant increases in the levels of serum ALAT, ASAT, ALP, and bilirubin, but on administering proanthocyanidins orally (20 mg/kg daily over 4 wk), these authors observed a significant reduction in these parameters, as well as the normalization of serum albumin and total protein levels and a reduction in the hepatic level of MDA. In contrast, accumulation of DMN-induced collagen, observed in the histological analysis of Sirius red-stained tissue, was reduced in rats treated with proanthocyanidins. In conclusion, the authors’ results demonstrated that these phenols showed hepatoprotective potential in vivo and antifibrogenic effects against DMN-induced hepatic lesions, suggesting that the proanthocyanidins (derived from cranberries, as well as from other fruits) could be useful in the prevention of hepatic fibrosis.
Grape (Vitis vinifera L)
Overview: The grape (the scientific name of which is Vitis vinifera) is a woody climbing plant that, when growing in its free state, can reach up to 30 m; however, due to the human action of annual pruning, it often remains reduced to a small, 1-m shrub. The grape is a fruit of the vine, it is edible, and it is the raw material for the manufacture of wine and other alcoholic beverages. Viticulture began in Asia and Southeastern Europe, and as a result of this development, the grape is an indispensable element in the feeding of humans, and its cultivation has extended to the American and African continents. There are approximately 3000 varieties of grapes in the world, although not all of these are equally appreciated. According to their final use, grapes are classified into two large groups: (1) those designated for consumption with meals (table grapes); and (2) wine grapes, which are employed for the creation of wine[35,36]. The leaves, as well as the fruit, are a stupendous source of vitamins and minerals and other active ingredients (Table 1); medicinal properties have been attributed to these components, which is why some authors have considered the grape to be a drug-food[37,38]. From the different parts of this plant, particularly from the fruit, different preparations have been obtained that have been used in popular and traditional medicine, highlighting laxative, astringent, diuretic, cicatrisant, immunological stimulant, anti-inflammatory, and hypocholesterolemic activities, as well as chemopreventive activity against cardiovascular disease and some cancers (mainly prostate and colon)[39,40].
Table 1.
Main active ingredients of the grape
| Active ingredient | Compounds |
| Carbohydrates | Glucose, fructose, sacarose,dextrose, yeast, and levulose |
| Vitamins | Vitamin C and vitamin B6 |
| Beta-carotene | Vitamin A |
| Tannins | Resveratrol |
| Minerals | Potassium, magnesium, calcium, sulfur, iron, and manganese |
| Flavonoids | Quercetin |
Hepatoprotective evidence for the grape and its phytochemicals: Diverse studies have shown that grape juice and grape seeds (GS) are rich sources of flavonoids, such as catechins, epicatechins, anthocyanidins, proanthocyanidins, and resveratrol[39,40], compounds, which, similar to the fruit and juice, have aroused great interest due to their biological effects. The first studies on hepatoprotection conducted on grapes were focused on the analysis of the antioxidant capacity of the skin and seed using TBARS testing in laboratory animals intoxicated with alcohol. The experimental design, developed by Peruvian scientists of the Universidad Nacional Mayor de San Marcos, consisted of comparing a group of animals that freely consumed a triturated and dry mixture of grape skin-seeds, which they ingested from a drinking fountain in a 5% alcohol solution, against two experimental lots of animals treated only with 5% alcohol (positive control) or with the dry mixture of grape seeds. The evaluation of hepatic tissue to measure OS levels consisted of extracting the animals’ livers, weighing them, evaluating them for hepatomegaly, and analyzing their lipoperoxidation by means of TBARS at 24, 48, and 72 h, as well as later, on days 5 and 7 of treatment. Their results showed that hepatomegaly presented from 24 h (36.68% increase in hepatic mass) in the alcohol group, which was less than in the skin-seeds lot. A very similar phenomenon was seen with the TBARS test, and additionally, the skin-seeds plus alcohol group exhibited a reduction in this parameter as the treatment time advanced, obtaining the best protection on days 5 and 7[39]. These results motivated further exploration in this field of study; thus, in 2008, the antioxidant activity of two different types of grape was investigated (the juice of the organic purple grape and the juice of the conventional purple grape). The antioxidant activity of both juices was evaluated using an animal model of three groups: control, organic, and conventional. After 30 d, the animals were sacrificed, and their blood and livers were recovered to evaluate lipid peroxidation levels (TBARS assay), protein oxidative levels (carbonyl assay), and CAT and superoxide dismutase (SOD) activities. The group treated with organic grape juice exhibited the highest SOD and CAT activity in the plasma and liver, compared with the conventional and control groups. Both types of juice were capable of reducing carbonyl and lipid peroxidation levels in the plasma and liver. However, in the plasma, the organic group showed lower carbonyl and TBARS levels, compared to the conventional grape juice group. This finding demonstrated that the ingestion of black grape juice, especially of organic juice, induced better antioxidant effects compared with conventional juice and that this difference could be an important problem for future investigations in the area of functional biochemical foods. Similarly, secondary data from this same investigation showed that in plasma, there was a positive correlation among SOD and CAT activities, resveratrol, and the content of anthocyanins; thus, it was suggested that these polyphenols could be, at least in part, responsible for this increase in antioxidant defense and protection against alcohol[41].
In a study recently performed by Dogan and Celik[42], the authors evaluated the hepatoprotective effects and antioxidant role of GS against ethanol-induced OS by measuring the liver damage serum marker enzymes ASAT, ALAT, GGT, and LDH and antioxidant defense systems, such as GSH, GRx, SOD, GST, GPx, and MDA, in rats. The results that the authors obtained from the four experimental groups [I (control), II (20% ethanol), III (15% GS), and IV (20% ethanol + 15% GS)] indicated that the levels of serum marker enzymes were significantly increased in the ethanol-treated group (II) compared with group I and that this level notably decreased with both compounds (group IV). Additionally, they observed that administration of GS-supplemented food restored ethanol-induced MDA, which was increased to near the control level. These results newly confirmed that GS, as well as grape juice, could be an important antioxidant supplement in the diet for the prevention of oxidative damage to tissues, by reducing lipoperoxidation or inhibiting the production of ethanol-induced free radicals.
With regard to the grape’s phytochemicals, resveratrol has been the most studied of these compounds as a possible hepatoprotective agent. In this regard, there has been evidence that resveratrol exerts a protective effect by controlling chronic intoxication due to ethanol[43] and by reducing the damage caused by hepatocarcinogens, such as azoxymethane (AOM)[44]. In the first study, the authors evaluated whether a dietary supplement of resveratrol could attenuate lipid peroxidation, the result of oxidative damage induced by the chronic administration of 35% ethanol. The results that the authors obtained after daily intraperitoneal (ip) administration of ethanol for 6 wk were that MDA levels increased in liver, heart, brain, and testicles. However, when the animals were treated with ethanol plus resveratrol (5 g/kg), the increase in MDA levels was significantly reduced in all of the organs. Based on these results, the authors concluded that this tannin was capable of inhibiting ethanol-induced lipoperoxidation, and it exerted a protective effect against oxidative damage. Very likely with the aim of confirming these results, Gürocak et al[44] conducted a new study, the principal objective of which was to evaluate whether resveratrol could protect liver tissues against the effects of AOM, a chemical carcinogen that causes de novo hepatic damage. Their study was organized into four groups, each of which consisted of seven rats; group 1 only received AOM (twice weekly, 5 mg/kg); group 2 was treated with AOM plus resveratrol (twice weekly, 20 mg/kg); group 3 was considered the control group; and the animals in group 4 were administered resveratrol alone. At the end of the seven week, the animals were sacrificed to perform a biochemical analysis of MDA, nitric oxide (NO), and GSH levels in the liver. In general, it was found that the group concomitantly administered resveratrol had significantly reduced MDA and NO levels and that they had increased GSH, resulting in the new conclusion that this tannin most likely could exert protective effects against the oxidative damage caused by chemical carcinogens, including AOM.
HEPATOPROTECTIVE PLANTS
Nopal (Cactus pear) and tuna (Cactus pear fruit) “Opuntia ficus-indica”
Overview: Plants of the genus Opuntia are the most abundant of the Cactaceae family, which is cultivated throughout the American continent, as well as in the central zone of the Mediterranean, Europe, Asia, Africa, and Australia. The majority of Opuntia spp. present flat stems called pencas or cladodes (paddles), and the most common variety is the cactus pear (nopal)[45]. The fruits of this plant [called cactus pear fruits (tunas) or prickly pear fruits] are oval berries with a large number of seeds and a semihard bark containing thorns, and they are grouped by their different colors (red, purple, orange/yellow, and white). Generally, fruit with white pulp and green skin is preferred for consumption as food, and its domestic production corresponds to nearly 95% of the total production worldwide. Mexico is the primary producer of cactus pear fruits, representing more than 45% of world production; however, only 1.5% of this production is exported[45]. Cactus pear fruit, as well as prickly pear fruit, has been employed for a long time in traditional medicine as a treatment for different pathologies, such as ulcers, dyspnea, and glaucoma, as well as for liver diseases, wounds, and fatigue[45,46].
Different studies performed in European and Asiatic prickly pear varieties have demonstrated antioxidant activities, with significant reductions in OS in patients and prevention of chronic pathologies[45]. Some preparations of the fleshy stems (cladodes) have been tested for the treatment of the symptomatology of diabetes in humans and in animal models[47-49]. Some authors have also reported that the fresh stems and the prickly pear are good sources of fiber and that these materials also reduce blood sugar and cholesterol levels in the plasma[50,51]. The prickly pear fruit can be considered a functional food, which is a property that has been attributed to its bioactive compounds, such as vitamin C and vitamin E, polyphenols, carotenoids, flavonoid compounds (for example, kaempherol, quercetin, and isorhamnetin), taurine, and pigments[45,52-54]. The betalains are water-soluble pigments. Two betalainic derivatives are present in prickly pears: betacyanin, responsible for its purplish-red color; and betaxanthine, responsible for its yellowish-orange color. These pigments have demonstrated beneficial effects in the redox-regulated pathways implicated in cellular growth and inflammation, and no toxic effects have been observed in humans[45,55,56].
Hepatoprotective evidence for Opuntia ficus-indica: Despite the diverse therapeutic uses that, for many years, have been attributed to plants of the genus Opuntia, prickly pear and cactus pear fruits (tunas) were ignored by the scientific world until the beginning of the 1980s, when they started to receive attention, and different investigations and symposia were initiated, resulting in publications, books, and book chapters. However, the scientific research into the hepatoprotective capacity of this food continues to be insufficient[57]. The first scientific evidence for the use of Opuntia ficus-indica against hepatotoxic substances was described by Wiese et al[58], who reported that this cactaceous species was useful for reducing the symptoms of hangovers after consuming alcohol in excess. Their data indicated that the severity of the hangover produced by alcohol consumption might be caused by inflammation induced by the impurities in beverages or by alcoholic metabolism subproducts and that an extract of Opuntia ficus-indica could diminish nausea, dry mouth, and anorexia, which are characteristic signs of alcoholic hangovers in humans.
Years later, Ncibi et al[59] demonstrated that an extract of Opuntia ficus-indica cladodes (CCE) could reduce the hepatic toxicity of the organophosphorous insecticide chlorpyrifos (CPF), which was a conclusion that these authors obtained in observing whether combining CPF plus CCE could significantly normalize biochemical parameters, such as ALAT, ASAT, ALP, LDH, cholesterol, and albumin, in contrast with animals treated with the pesticide alone, in which the biochemical parameters were notably affected. In more recent years, investigations developed by the el Hassen Bacha scientific group explored the hepatoprotective effects of a CCE against two compounds with hepatocarcinogenic capacity in Balb/C mice: benzo(a)pyrene [B(a)P][60] and aflatoxin B1 (AFB1)[61]. In both studies, it was observed that the two carcinogens significantly altered markers of oxidative stress, such as MDA level and CAT activity, and increased of the expression of heat shock proteins (Hsp 70 and Hsp 27). In addition, they induced fragmentation of the DNA in the liver and chromosomal aberrations in bone marrow cells, increased the expression of the bcl2 antiapoptotic proteins, and decreased the expression of bax. Additionally, the authors proved that the treatment with CCE prior to or after treatment with B(a)P or AFB1 resulted in a total reduction in oxidative damage induced in all of the markers tested, and they demonstrated that CCE had an antigenotoxic effect by reducing the damage to the genetic material of the liver and to the bone marrow caused by both toxins. Similarly, it was observed that CCE was capable of inhibiting the toxic effects of B(a)P and/or AFB1 by differential modulation of the expression of p53, which increased in its associated genes, such as bax and bcl2. Based on these results, the scientific group concluded that the cactus cladode extract was effective in protecting against the dangers of both carcinogens and suggested that Opuntia ficus-indica could be considered a plant with hepatoprotective capacity.
Due to the majority of the studies available in the literature, including those previously mentioned, being centered on analysis of the extracts of the fruit, the nopal or the cladodes and these generally being consumed as fresh fruit or juice and, to a lesser degree, as dried fruits, candies, jams, jellies, and/or wines, an experiment was undertaken in 2012 with the aim of investigating the effects of Opuntia ficus-indica f. inermes prickly pear juice (PPJ) against ethanol-induced liver injury in rats. Chronic ethanol administration (3 g/kg bw) over 90 d in Wistar rats significantly increased liver lipid and protein oxidation, reduced glutathione (GSH) content and the activities of liver antioxidant enzymes, such as SOD, CAT, and GPx, and conversely elevated liver injury biochemical markers, such as ASAT, ALAT, ALP, GGT, LDH, cholesterol, and TGs, thereby causing severe histopathologic injury. In contrast, pre-treatment of ethanol-fed rats with PPJ (20 and 40 mL/kg bw, orally) interestingly reduced liver lipid and protein oxidation, decreased histopathologic lesions, and inhibited alterations in antioxidant enzymes and the release of biochemical markers. The hepatoprotective effects of PPJ could be due to its capacity to end free radical chain reactions or to enhance endogenous antioxidant activities[62].
Chamomile (Matricaria chamomilla or Chamomilla recutita)
Overview: While there is an extensive literature suggesting health benefits associated with drinking teas prepared with Camilla sinensis (i.e., black and green teas)[63], evidence-based information regarding the effects of the majority of herbal teas, or tisanes, has been quite limited. One of the most commonly consumed single-ingredient herbal teas is chamomile[64].
Chamomile (Matricaria chamomilla or Chamomilla recutita) is an Asteraceae plant native to Europe, and it is distributed throughout the world, except in tropical and polar regions. This plant has been used for its curative properties since the ancient Egyptian and Greek civilizations, and it currently is frequently used as an antiseptic, antiphlogistic, diuretic, expectorant, febrifuge, sedative, anti-inflammatory, and anticarcinogenic[18]. The pharmacological activities of various components of the plant have been reported, for example, the anti-inflammatory capacity and the modulating effects of Hsp on apigenin and quercetin flavonoids, as well as the anti-inflammatory, antioxidant, and antiseptic activities detected in α-bisabolol, guargazulene, and chamazulene[18,65,66]. The essential oil extracted from the chamomile flower ranges from 0.42% to 2% and consists of compounds such as bisabolol, chamazulene, cyclic sesquiterpenes, bisabolol oxides, and other azulenes and terpenes[18,67].
Madrigal-Bujaidar et al[68] conducted an investigation with the primary purpose of evaluating the chemoprotective capacity of chamomile. These authors determined the inhibitory effect of three doses of chamomile essential oil (CEO) on the sister chromatid exchanges (SCE) produced by daunorubicin and methyl methanesulfonate in mouse bone marrow cells. Initially, the authors obtained the CEO from flowers of Chamomilla recutita by steam distillation, and then they analyzed this oil by gas chromatography to identify the chemical species. Thirteen compounds were determined with this assay, including bisabolol and its oxides, β-farnecene, chamazulene, germacrene, and sesquiterpenes (Table 2). In general, the results indicated dose-dependent inhibition of SCE formed by both mutagens and that its antioxidant capacity could induce this effect. This study, in addition to others that have provided evidence for its spectrum of biological activities, as well as the more than 100 components identified to date, promote chamomile as an ideal candidate for possessing hepatoprotective capacity.
Table 2.
Main components of the essential oil of chamomile
| Component | RTa | Area in the plant |
| (E)-β-farnecene | 38.46 | 28.17% |
| Germacrene-D | 39.23 | 2.19% |
| Unidentified sesquiterpene | 40.07 | 1.40% |
| Unidentified sesquiterpene | 41.17 | 0.78% |
| (Z,E)-α-farnecene | 41.35 | 1.59% |
| Unidentified sesquiterpene | 48.52 | 0.71% |
| α-bisabolol oxide A | 54.46 | 41.77% |
| α-bisabolol oxide B | 49.28 | 4.31% |
| α-bisabolol oxide | 50.65 | 5.30% |
| α-bisabolol | 51.18 | 2.31% |
| Chamazulene | 52.80 | 2.39% |
| 1,6-dioxaspiro[4,4]non-3-ene,2-(2,4 hexadyn-1-ylidene) | 60.73 | 2.19% |
| Hexatriacontane | 67.49 | 0.50% |
RTa: Retention time obtained with gas chromatography.
Hepatoprotective evidence for chamomile and its phytochemicals: As mentioned in the general comments, chamomile is one of the most popular teas in the world, consumed as a sole herbal ingredient, or tisane, and it has been utilized traditionally with medicinal aims. It has been studied by a great number of investigators with the purpose of fully demonstrating its therapeutic potential. With regard to its gastroprotective potential, this property began to be explored in the 1980s, when the antispasmodic effects were analyzed of different preparations of chamomile in isolated guinea-pig ileum. In agreement with Achterrath-Tuckermann et al[69], the compounds contained in aqueous and oil extracts of the plant were effective antispasmodics in this experimental model. In comparison with papaverine, a smooth muscle relaxant drug, α-bisabolol was 91% as effective in barium chlorate-induced spasms, while bisabolol A and B oxides were 46%-50% effective. Utilizing the same experimental model, the effectiveness of an ethanolic extract of chamomile in histamine- and acetylcholine-induced spasms. At 2.5- and 10-mL/L doses (of a 31% w/w solution), the chamomile extract shifted to the right the dose response curves of both acetylcholine and histamine, reflecting an increase in their median effective doses, compared with an ethanol control solution. Atropine (7.0 μg/L) similarly shifted the dose response curves, but unlike chamomile, this antispasmodic drug was unable to decrease the maximal contraction in response to either acetylcholine or histamine. In rats, both apigenin and α-bisabolol inhibited the development of gastric ulcers induced by indomethacin, stress, and alcohol[67].
Similar to black or green tea, chamomile tea demonstrated that it could modulate the activity of hepatic cytochrome P450. Maliakal et al[70] provided Wistar rats with free access to a solution of 2% chamomile tea over 4 wk, finding that CYP1A2 isoform activity was 39% lower in the control group, which received only water. Based on these data, Gupta et al[71] evaluated the hepatoprotective activity of an aqueous ethanolic extract of Chamomile recutita capitula against PCM-induced damage in albino rats. To determine this protection and to ascertain the possible mechanism of action, the extract was evaluated via blood and liver glutathione, Na+ K+-ATPase activity, serum marker enzymes, bilirubin, glycogen, and TBARS assay. Their results confirmed that the chamomile extract had reversal effects on the levels of the above-mentioned parameters of paracetamol hepatotoxicity, and the authors concluded that the extract served as a hepatoprotective agent and that this hepatoprotective activity of chamomile might be due to the normalization of impaired membrane function activity. Because paracetamol (PCM) is not the only hepatotoxic compound to which humans have free access, Al-Hashem (2010) investigated the gastroprotective effects of an orally administered aqueous extract of Chamomilla recutita (ACE) against ethanol-induced gastric ulcers in male Wistar rats, which were organized into five experimental lots: one group was treated with deionized water alone (control group), and four groups received 0, 0.5, 1.0, and 2.0 g/kg ACE, respectively, for 27 d. Stomach ulcerations were induced by orally administering a single dose of 70% ethanol on day 28. Lesions in the gastric mucosa were examined macroscopically to calculate the ulcer index (UI) and the estimated GSH for each animal. On comparing the control group with the remainder of the experimental lots, it was observed that the UI was significantly reduced in a dose-dependent manner. In contrast, GSH levels decreased after treatment with ethanol, and this reduction was impeded by treatment with ACE, principally at the highest dose (2.0 g/kg). Histological examination revealed that ACE treatment alleviated, or completely resolved, ethanol-induced degenerative alterations, including disorganization of cell nuclei and gland morphology with erosion in the gastric mucosa, and it interrupted muscularis mucosa. In conclusion, this study provided evidence for the regulation of ACE-mediated gastroprotection against ethanol-induced ulceration by GSH[72]. Finally, a study that evaluated another extract of Matricaria chamomilla L. on lipid peroxidation and antioxidant enzyme systems (ASAT, ALAT, MDA, SOD, GSH levels, GPx, and CAT) in rats treated with CCl4 for 14 d, demonstrating that depending on the dose of the extract administered (50, 100, and 200 mg/kg), the extract reduced the damage induced by CCl4 and oxidative stress, thereby positively modifying the antioxidant system[73].
Silymarin (Silybum marianum)
Overview: Silybum marianum is the scientific name for milk thistle (Mt) or St. Mary’s thistle. It is a plant that is native to the Mediterranean region and that belongs to the Asteraceae family. It is characterized by thorny branches, milky sap, and oval leaves that reach up to 30 cm; its flowers are bright pink and can measure up to 8 cm in diameter[18,74]. Milk thistle (Mt) grows in its wild form in southern Europe, North Africa, and in the Middle East, but it is cultivated in Hungary, China, and in South American countries, such as Argentina, Venezuela, and Ecuador. In Mexico, it has been consumed as a food supplement for many years[18,75].
In the 1960s, German scientists performed a chemical investigation of the milk thistle fruit, isolating a crude extract formed by active compounds with hepatoprotective capacity; this group of compounds was called silymarin. In 1975, it was found that the main components of silymarin were silybin A, silybin B, isosilybin A, isosilybin B, silychristine A, silychristine B, and silydianine[18,76]. Currently, it is known that the chemical constituents of silymarin are flavonolignans, i.e., a combination composed of flavonoids and lignin structures[18,77].
Mt is one of the most investigated plant extracts, with known mechanisms of action for oral treatment of toxic liver damage. Silymarin has been used as a protective treatment in acute and chronic liver diseases[18,78]. Its protective capacity is related to different mechanisms, such as suppressing toxin penetration into hepatic cells, increasing SOD activity, increasing the glutathione tissue level, inhibiting lipid peroxidation, and enhancing hepatocyte protein synthesis. The hepatoprotective activity of silymarin can be explained based on its antioxidant properties due to the phenolic nature of its flavonolignans. It also acts by stimulating liver cell regeneration and cell membrane stabilization to prevent hepatotoxic agents from entering hepatocytes[18,79]. Silymarin has also been beneficial for reducing the chances for developing certain cancers[18,80]. The molecular targets of silymarin for cancer prevention have been studied. Mt interferes with the expression of cell cycle regulators and of proteins involved in apoptosis to modulate the imbalance between cell survival. These compounds and two other related analogs present in extremely minute quantities were evaluated for antiproliferative/cytotoxic activity against human prostate cancer cell lines. Isosilybin B exhibited the most potent activity[18,79]. The isolation of six isomers afforded a preliminary analysis of the structure-activity relationship in terms of prostate cancer prevention. The results suggested that an ortho relationship for the hydroxyl and methoxy substituents in silybin A, silybin B, isosilybin A, and isosilybin B was more favorable than a meta relationship for the same substituents in the minor flavonolignans. Silymarin suppressed ultraviolet A (UVA)-induced oxidative stress, which can induce skin damage. Therefore, topical application of silymarin could be a useful strategy for protection against skin cancer[18,80,81].
Hepatoprotective evidence of silymarin: As previously noted, silymarin is one of the most investigated plant extracts, with known mechanisms of action for oral treatment of toxic liver damage[82]. Silymarin has been employed as a protective treatment in acute and chronic liver diseases[83]. In addition, it supports liver cells through multifactorial action, including binding to cell membranes to suppress toxin penetration into hepatic cells, increasing SOD activity[79], increasing glutathione tissue levels[84], inhibiting lipid peroxidation[85,86], and enhancing hepatocyte protein synthesis[87]. The hepatoprotective activity of silymarin can be explained by its antioxidant properties deriving from the phenolic nature of flavonolignans. It also acts by stimulating liver cell regeneration and cell membrane stabilization to prevent hepatotoxic agents from entering hepatocytes[88]. It has been shown that flavonolignans inhibited leukotriene production; this inhibition explains the anti-inflammatory and antifibrotic activity of flavonolignans[89].
Due to the large number of studies that have demonstrated its hepatoprotective activity and the insufficient space in this review to analyze all of them, we will focus on those investigations in which the combination of silymarin with other compounds has been explored. One of these studies had as its objective the investigation of silymarin, and misoprostol or the co-administration of both against CCl4-induced hepatic lesion in rats. Misoprostol (MSP) at 10, 100, and 1000 μg/kg, silymarin (25 mg/kg) and the combination of MSP (100 μg/kg) + silymarin (25 mg/kg) were orally administered once daily simultaneously with CCl4 over 15 d. The authors’ results showed that MSP (at all of the doses) exhibited significant protection against the hepatotoxic activity of CCl4 in the rats, observing reductions in serum ALAT levels of 24.7%, 42.6%, and 49.4%, in comparison to the control group. MSP at doses of 100 and 1000 μg/kg diminished ASAT in 28% and 43.6% and ALP in 19.3% and 53.4%, respectively, while silymarin reduced ALAT, ASAT, and ALP levels, in 62.7%, 66.1%, and 65.1%, respectively. In contrast, co-administration of both compounds resulted in reductions of 61.4%, 66.1%, and 57.5% in ALAT, ASAT, and ALP levels, respectively.
Histopathological alterations and depletion of hepatocyte glycogen and DNA content by CCl4 were markedly reduced after treatment with MSP, silymarin, or the combination thereof. Image analysis of liver specimens revealed a marked reduction in liver necrosis, with areas of damage of 32.4%, 24%, and 10.2% after MSP (10, 100, or 1000 μg/kg), of 7.2% after silymarin, and of 10.9% after treatment with both compounds, compared with the CCl4 control group (46.7%). These results indicate that treatment with MSP and silymarin protected against CCl4-induced hepatocellular necrosis. In general, this study suggests that the combination of both compounds demonstrated therapeutic potential for reducing hepatic damage[90].
Another study that has also attracted attention was performed by Kim et al[91], who evaluated the protective effects of a mixture of Aloe vera and Silybum marianum (the ACTIValoe N-931 complex) against CCl4-induced acute hepatotoxicity and liver fibrosis. Acute hepatotoxicity was induced by an ip injection of CCl4 (50 μL/kg), and ACTIValoe complex N-931 was administered orally, at doses of 85, 170, and 340 mg/kg, at 48, 24, and 2 h before and 6 h after the injection of CCl4. Hepatic fibrosis was induced by an ip injection of CCl4 over 8 wk (0.5 mL/kg, twice weekly), and the mice were treated with the ACTIValoe N-931 complex at the same doses once daily. In acute hepatotoxicity, as well as in hepatic fibrosis, serum aminotransferase and lipid peroxidation levels increased, while the hepatic glutathione content was observed as diminished. These changes were impeded by the ACTIValoe N-931 complex, because it attenuated the tumor necrosis factor-α (TNF-alpha) levels in nitric oxide synthase, in cyclooxygenase 2 and in the expression of messenger RNA (mRNA) involved in acute hepatotoxicity. In antifibrotic experiments, tissue inhibitor of metalloprotease-1 mRNA expression was attenuated by treatment with the complex. Similarly, hepatic hydroxyproline content and transforming growth factor-beta 1 levels decreased. In summary, these results suggested that the mixture of Aloe vera and Silybum marianum had hepatoprotective effects against chronic and acute lesions induced by the organochlorine (OC) compound.
More recently, a study in which two plants extracts were combined, namely Ginkgo biloba (employed for centuries because of its therapeutic action, especially by Chinese traditional medicine) and Silybum marianum, demonstrated that this combination could increase chemoprevention against hepatocarcinogenesis induced by N-nitrosodiethyl-amine (NDEA) through its antioxidant properties and its antigenotoxic and antiangiogenic activity. El Mesallamy et al[92] evaluated these activates after organizing male Wistar albino rats into six experimental groups, which included: a control lot; a second group in which NDEA was administered intragastrically (ig) at a 10-mg/kg dose five times weekly over 12 wk to induce hepatocellular carcinoma (HCC); two groups (3 and 4) pre-treated with silymarin and Ginkgo biloba, respectively; and finally, two lots (5 and 6) of animals post-treated with each of the extracts. The parameters investigated in the serum were ALAT, ASAT, GGT, and vascular endothelial growth factor (VEGF), while the parameters investigated in hepatic tissue were MDA, GSH, SOD, GPx, GRx, and the Comet assay. On finalizing the experiment, the authors determined that in the NDEA group, the MDA level was elevated, with a subsequent decrease in the GSH level and in the SOD, GPx, and GRx activities. In addition, the NDEA group revealed a significant increase in serum ALAT, ASAT, and GGT levels and in the VEGF level. Furthermore, NDEA-administered animals showed a marked increase in Comet assay parameters. These biochemical alterations induced by NDEA were confirmed by histopathological examination of rat livers intoxicated with NDEA, which disclosed obvious cellular damage and well-differentiated HCC. In contrast, the silymarin + NDEA-treated groups (3 and 5) and the Ginkgo biloba + NDEA-treated groups (4 and 6) exhibited a significant decrease in MDA levels and a significant increase in GSH content and in SOD, GPx, and GRx activities, compared with the NDEA group. Silymarin and/or Ginkgo biloba also beneficially downregulated the increases in serum ALAT, ASAT, and GGT activities and in the VEGF level induced by NDEA. In addition, both extracts significantly decreased Comet assay parameters and induced an improvement in liver architecture.
Blue green algae spirulina (Spirulina maxima, Spirulina platensis, and Spirulina fusiformis)
Overview: Spirulina is a microscopic blue-green filamentous alga that floats freely; it grows in fresh water, as well as in alkaline salt water. It is a cyanobacterium belonging to the class Cyanophyceae and the order Oscillatoriales. It is an organism with the capacity to store different bioactive molecules, among which are the following: (1) proteins (60%-65% dry weight) with essential amino acids; (2) polyunsaturated fatty acids, such as linoleic acid; (3) vitamins (B12 and E); (4) polysaccharides; (5) minerals (Na, K, Ca, Fe, Mn, and Se); and (6) pigments (chlorophyll, c-phycocyanin, allophycocyanin, β-carotene, lutein, and zeaxanthin). Generally, the content of the compounds varies in species-to-species proportions, but the phytochemicals that are always present in their biomass are c-phycocyanin (with a content of 12.6% in dry spirulina) and high percentages of dietary zeaxanthin[93].
Spirulina is the sole blue-green alga that is cultivated commercially for food use; there are various species, but those of the greatest commercial importance are Spirulina maxima, Spirulina platensis, and Spirulina fusiformis[93]. Spirulina has been consumed for centuries in many parts of the world, ranking from the Aztec civilization in Latin America to the tribes that inhabit the Lake Chad region of central Africa. In 1996, the United Nations World Health Organization declared spirulina the best food for the future, and it has gained in popularity as a food supplement in recent years thanks to its high content of proteins and natural vitamins[94]. Throughout its long history of use as a food and as a result of contemporary scientific discoveries, spirulina is considered safe for human consumption; thus, it has become an important ingredient in many nutraceutical formulations[93-95]. Currently, there is varying scientific evidence for its biological effects against health problems. In summary, the results indicated that it was antioxidant, anti-inflammatory, hypolipemic, antihypertensive, antidiabetic, antimicrobial, neuroprotective, antianemic, immunostimulant, and anticarcinogenic and a hepatoprotector.
Hepatoprotective evidence for spirulina: It is curious that, despite spirulina having been consumed for centuries, studies that have demonstrated its hepatoprotective effects began only in this century, specifically in 2005, when carotenoids were extracted from a mixture of hexane alcohol:isopropyl alcohol (1:1 vol/vol) in two microalgae, among these S. platensis. These carotenoids were mixed with olive oil and were administered orally to Wistar rats at a dose of 100 μg/kg bw/d (in terms of carotenoids). The degree of hepatoprotection was measured by means of estimating biochemical parameters, such as serum transaminases [serum glutamate oxaloacetate transaminase (GOT) and serum glutamate pyruvate transaminase (GPT)], serum ALP, total albumin, and total protein. The results were compared with those of a control group, a CCl4-induced hepatic damage group, and a group treated with synthetic β-carotene at the same dose. The protein content of the CCl4-treated group, which received a normal diet, showed a significant decrease (3.92 mg/mL), while the carotenoids from spirulina raised this value to 6.32 mg/mL. The CCl4-treated group showed higher transaminase activity (128.68 units/mL GPT and 171.52 units/mL GOT). However, the activity of GPT was 76.83 units/mL with spirulina. For serum ALP, the standard beta-carotene value was 81.52 units/mL, compared with 84.46 units/mL for the CCl4-treated group; however, natural algal carotenoids yielded 44.73 units/mL. Similarly, the authors observed this same decrease in the parameter corresponding to total albumin. In summary, their results indicated that the carotenoids derived from spirulina had greater antihepatotoxic effects, compared to synthetic beta-carotene[96]. Later, Kuriakose et al[97] evaluated the hepatoprotector effect of an ethanolic extract of Spirulina lonar (EESLO) on the damage produced by paracetamol (PCM) in rats. These scientists considered analyzing this extract due a in vitro prior study having found better antioxidant activity compared to methanolic and aqueous extracts. As in other studies, the authors evaluated parameters such as the degree of lipid peroxidation (TBARS), antioxidant enzymes (SOD, CAT, GPx, and GST) and the activity of ASAT and ALAT, which tends to be altered by high doses of PCM. In general, these indicators of hepatic damage recovered, and consequently, the results suggested that EESLO could act as a hepatoprotective agent and that its mechanism of action was related to antioxidant phenomena.
These findings motivated other investigators to explore the spectrum of protection, evaluating this property against other compounds, including mercuric chloride (HgCl2), cisplatin, and CCl4. In the case of the first compound, it was found that albino rats treated with HgCl2 showed significant increases in the levels of blood hydroperoxide, ASAT, ALAT, ALP, TC, TG, very-low-density lipoprotein-cholesterol (VLDL-c), and MDA. In the same manner, intoxication with mercury induced pathological alterations in the liver, such as necrosis and cytoplasmic vacuolization. In contrast, treatment with S. platensis confirmed that the increases in the previously mentioned parameters diminished notably; this treatment attenuated HgCl2-induced hepatotoxicity and modified lipid profiles through its antioxidant properties[98].
In contrast, these same species of spirulina, combined with vitamin C and/or administered independently, were capable of reducing the same hepatic markers that were altered after treatment with cisplatin. In the same manner, optic microscopy showed that the hepatic lesions and histopathological abnormalities induced by treatment with cisplatin were impeded by spirulina and by the combination of vitamin C with spirulina. These data newly suggested that the protector effect was related to the antioxidant capacities of the alga and the vitamin[99]. The most recent study providing hepatoprotective evidence for spirulina was performed by Kepekçi et al[100], who believe that phenolic compounds constitute the main secondary metabolites, with high pharmaceutical potential, and there have been reports that microalgae contain low amounts of these compounds. These authors decided to investigate the hepatoprotective potential of the biomass of S. platensis enriched with phenolic compounds (SP1) and with large amounts of phenolic compounds (SP2) against acute CCl4-induced hepatotoxicity in rats. The increases in ALAT, ASAT, and MDA levels, together with the decreases in SOD and CAT activities, were significantly improved by SP2. The histopathological examinations revealed that SP2 was more potent than SP1 in protecting the liver from the toxic lesions caused by CCl4, although both compounds preserved the hepatocyte ultrastructure. Lesions including necrosis, lymphocyte infiltration, ballooning degeneration, and hepatocyte injury, as well as irregular lamellar organization, dilation of the endoplasmic reticula, and the presence of great numbers of cytoplasmic vacuolizations, were reversed by SP2.
NATURAL RESIN
Propolis (bee glue)
Overview: Propolis is a resinous substance of natural origin, gathered by bees from different parts of plants, shoots, and exudates[101]. Bees utilize it as a sealant for their hives[102] and to avoid the decomposition of creatures that have been killed by the bees after hive invasions[103]. Chemically, propolis is a lipophilic material that is hard and fragile when cold but soft, flexible, and very sticky when hot, hence the name bee glue. It has an agreeable aromatic odor, and it varies in color depending on its origin and maturation[104,105]. The compounds identified in propolis derive from three sources: plant exudate collected by bees; substances secreted by the metabolism of the bees; and materials that are introduced during the creation of the resin[104]. The ethanolic extract of propolis (known as propolis balsam) is the most common, but there are other solvents used to separate and identify many of its components. Among the types of chemical substances found in propolis are waxes, resins, balsams/balms, aromatic oils, pollen, flavonoids, terpenoids, and other organic materials (Table 3). The proportions of these substance are variable and depend on the place and time the propolis is obtained[104].
Table 3.
Major compounds identified in propolis resin
| Chemical groups | Compounds |
| Alcohols | Benzene methanol, cinnamyl alcohol, glycerol, α-glycerol phosphate, hydroquinone, isobutenol, phenethyl alcohol |
| Aldehydes | Benzaldehyde, caproic aldehyde, p-hydroxybenzaldehyde, isovanillin, vanillin |
| Aliphatic acids and aliphatic esters | Acetic acid, angelic acid, butyric acid, crotonic acid, fumaric acid, isobutyric acid, methylbutyric acid, isobutyl acetate, isopentyl acetate |
| Amino acids | Alanine, β-alanine, α-aminobutyric acid, γ-aminobutyric acid, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, ornithine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, valine |
| Aromatic acids | Benzoic acid, caffeic acid, cinnamic, coumaric, acid, ferulic acid, gallic acid, gentisic acid, hydroxycinnamic acid, p-hydroxybenzoic acid, isoferulic acid, 4-methoxy cinnamic acid, salicylic acid, vanillic acid |
| Aromatic esters | Benzyl acetate, benzyl benzoate, benzyl caffeate, benzyl coumarate, benzyl ferulate, benzyl isoferulate, benzyl salicylate, butenyl caffeate, butyl caffeate, cinnamyl benzoate, cinnamyl caffeate, ethyl benzoate |
| Chalcones | Alpinetin chalcone, naringenin chalcone, pinobanksin chalcone, pinocembrin chalcone, sakuranetin chalcone |
| Flavanones | Naringenin, pinobanksin, pinobanksin-3-acetate, pinobanksin-3-butyrate, pinobanksin-3-hexanoate, pinobanksin-3-methyl ether, pinobanksin-3-pentanoate |
| Flavones and flavonols | Acacetin, apigenin, apigenin-7-methyl ether, galagin, galagin-3-methyl ether, izalpinin, isorhamnetin, kaempferol, quercetin, ramnetin, ramnocitrin |
| Waxy acids | Arachid acid, behenic acid, cerotic acid, lauric acid, linoleic acid, lignoceric acid, montanic acid, myristic acid, oleic acid, palmitic acid, stearic acid |
| Ketones | Acetophenone, dihydroxy-acetophenone, methylacetophenone |
| Terpenoids and other compounds | α-acetoxybetulenol, β-bisabolol, 1,8-cineole, α-copaene, cymene, limonene, styrene, xanthorreol, naphthalene, sesquiterpene alcohol, sesquiterpene diol |
| Steroids | Calinasterol acetate, b-dihydrofucosterol acetate, ucosterol acetate, stigmasterol acetate |
| Sugars | Fructofuranose, α-D-glucopyranose, β-D-glucopyranose |
Propolis has a long history of being utilized in popular medicine, with its employment dating from at least 300 BC[104,105]; however, over the last decade, it has been found to possess diverse biological activities, and among the most prominent is its antioxidant, anti-inflammatory, antibiotic, and antifungal potential[106]. Recently, propolis has gained popularity as a health beverage; thus, it has been widely utilized in foods and drinks, with the aim of preventing alterations of the heart and chronic degenerative diseases, such as diabetes and cancer[107].
As a consequence of its more than 300 components identified to date and its broad spectrum of biological activities, it has been considered a promoter agent for human health and an ideal candidate for possessing hepatoprotector capacity.
Hepatoprotective evidence for propolis: The hepatoprotective effects of Cuban propolis were first examined González et al[108] in a mouse model of acute hepatotoxicity induced by a high oral dose (600 mg/kg) of PCM; 95% ethanol extract of propolis at ip, at doses of 25, 50, and 100 mg/kg bw, was found to decrease significantly both the activity of serum ALAT and the concentration of reduced GSH in the liver, which was raised by PCM alone. Propolis extract also reduced liver damage induced by PCM, as observed by optical, as well as electron microscopic, examination. The ALAT and GSH levels were 65.1 ± 5.36 and 538 ± 0.30, respectively, at a dose of 100 mg/kg bw in the propolis-treated group, compared with ALAT (89.0 ± 3.77) and GSH levels (3.44 ± 0.47) in the PCM-treated control group of mice. Later, Remirez et al[109] further reported the hepatoprotective effects of Cuban red propolis against acetaminophen (PCM)-administered liver injury in mice. The ALAT level was found to decrease significantly at ip doses of 25, 50, and 100 mg/kg of propolis. Similarly, Czarnecki et al[110] also found protective effects of lipid flower pollen extract against PCM-induced hepatotoxicity in mice.
González et al[111] further examined a 95% ethanol extract of Cuban red propolis on CCl4-induced liver injury in rats. Propolis extract at doses of 5, 10, and 25 mg/kg administered ip decreased ALAT and MDA levels in blood serum. The ALAT level decreased to 34.1 ± 4.2 U/L in the 10-mg/kg propolis-treated group, compared with the CCl4-treated control, which had an ALAT level of 117.7 ± 3.6 U/L. The concentration of TG in the liver in the same experiment was also decreased by propolis treatment. With regard to histopathological evaluation of CCl4-induced liver injury in rats, González et al[111] found a significant reduction in ballooned cells in the livers of propolis-treated rats (25, 50, and 100 mg/kg bw), compared with the CCl4-treated group.
Similarly, Sharma et al[112] also found decreases in ALAT and ASAT, which were raised by alcohol or CCl4 (68.39 ± 1.89 and 65.89 ± 0.75 IU/L) to physiological levels (39.33 ± 0.90 and 40.66 ± 0.51 IU/L) by co-administration of propolis. Moreover, Basnet et al[113] examined the hepatoprotective activity of Brazilian propolis against CCl4-induced toxicity in rats; water extract (PWE) showed a stronger protective effect than the corresponding methanol and chloroform extracts when 200-mg/kg propolis extracts were administered orally. The GOT, GPT, and LDH levels in the blood serum of CCl4-treated control groups increased to 1132 ± 140, 395 ± 56, and 353 ± 51 U/L, respectively, 24 h after CCl4 administration. In contrast, in the PWE pre-treated group, GOT (481 ± 88 U/L), GPT (157 ± 19 U/L), and LDH (247 ± 28 U/L) were significantly decreased. The hepatoprotective effect of oral administration of PWE was also evaluated in a D-GalN/LPS-induced liver injury model in mice. In the control group, it was observed that the GPT level rapidly increased to 6901 U/L 8 h after administration of D-GalN/LPS, while the GPT levels with 200- and 100-mg/kg doses administered po to PWE-treated groups were 355 and 1204 U/L, respectively. PWE also showed significant hepatoprotective activity against CCl4-induced liver cell injury in cultured rat hepatocytes. Fractionation and chemical analysis, guided by in vitro hepatoprotective activity (CCl4-induced liver cell injury in cultured rat hepatocytes), led to the isolation of four dicaffeoylquinic acid derivatives from PWE, i.e., (1) methyl 3,4-di-O-affeoylquinate, (2) 3,4-di-O-caffeoylquinic acid; (3) methyl 4,5-di-O-caffeoylquinate; and (4) 3,5-di-O-caffeoylquinic acid. All of these dicaffeoylquinic acid derivatives possessed significant hepatocyte protective activity against CCl4-induced cell injury.
In 1999, Sugimoto et al[114] studied the hepatoprotective effect of Brazilian propolis on D-GalN-induced hepatic injury in rats. When the 95% ethanol extract was administered orally three times at a dose of 3 or 30 mg/kg at 18 h and 1 h before and 8 h after DGalN injection, the propolis extract caused increases in serum ASAT and ALAT activities induced by D-GalN injection in a dose-dependent manner. A significant effect was observed in mice treated with a dose of 30 mg/kg propolis extract.
Lin et al[115] observed that an ethanol extract of propolis at an oral dose of 10 mg/kg significantly inhibited the elevations of serum GOT, GPT, and TGs levels in an alcohol-induced liver injury model in rats. In contrast, in an in vivo model, Mahran et al[116] observed a dose-dependent hepatoprotective effect of aqueous propolis extract on isolated rat hepatocytes against CCl4-inducted toxicity in vitro. Later, Banskota et al[117] examined the hepatocyte protective effects of nine different propolis samples collected from Brazil, Peru, the Netherlands, and China on D-GalN/TNF-α-induced cell death in primary cultured mouse hepatocytes at 200- and 100-μg/mL concentrations. It was observed that nearly all of the methanol extracts possessed stronger hepatocyte protective effects than the corresponding water extracts. Moreover, all of the methanol extracts had significant hepatoprotective activity both at 100- and 200-μg/mL concentrations, with the cell survival rate ranging from 49.3% to 117.7% compared with that the controls (35.0%). These finding suggest that the aqueous and alcoholic extracts of propolis possessed hepatoprotective effects against toxic compounds, such as CCl4, paracetamol, and D-GalN, as well as immunologically, in D-GalN/LPS-induced liver injury models. In the most recent study, this capacity was explored against a more modern compound, cypermethrin. A pyrethroid pesticide was used preferably, instead of OC and OP pesticides, due to its high effectiveness, low toxicity to non-target organisms, and easy biodegradability.
Gomaa et al[118] evaluated the protective effect of propolis against cypermethrin-provoked hepatic damage utilizing 50 adult male albino rats that were classified into four groups as follows: group I (control), subdivided into (1) a negative control, in which 2.0 mL of saline solution was administered; and (2) a positive control, which received the same amount but of corn oil; group II, which received cypermethrin (14.5 mg/kg); group III, which was administered propolis (200 mg/kg) dissolved in saline solution; and finally, group IV, which received the pyrethroid pesticide combined with propolis. All of the compounds were orally administered daily and over 4 wk. At the end, blood samples were obtained to estimate the hepatic enzyme levels (ALAT, ASAT, and ALP) and to determine total proteins and levels of albumin, TC, TGs, and VLDL-c. Later, the animals were sacrificed to obtain their livers and to quantify the levels of MDA and antioxidant enzymes (CAT, SOD, and GPx), as well as for histopathological examination. Their results indicated that cypermethrin induced an increase in the levels of the hepatic enzymes TC and MDA, while the total proteins albumin, TGs, and VLDL-c and the antioxidant enzymes were reduced in comparison with the controls. Histopathological examination of the livers revealed congestion of the central and portal veins with hydrotrophic degeneration of the hepatocytes. However, in contrast, the combined administration of propolis with cypermethrin induced a significant decrease in the levels of hepatic enzymes, TC, and MDA, as well as an increase in the levels of antioxidant enzymes, total proteins, TGs, and VLDL-c. Moreover, histopathological examination of the liver revealed apparent improvement in the lesions induced by the pyrethroid pesticide. The authors’ conclusion was that propolis exerted a beneficial influence via reduction of the hepatotoxic effects of cypermethrin, suggesting that its protective spectrum was quite broad.
POLYSACCHARIDES
β-glucans
Overview: Different foods and natural products have traditionally been accepted as health remedies due to the popular belief that they cause fewer adverse effects. Therefore, it is indispensable to determine the beneficial potentials of these foods and natural products, and well as to understand the mechanism or mechanisms by which they exercise this potential.
The β-glucans belong to a group of polysaccharides that are localized in the intermediate layer of the cell wall of yeasts, algae, fungi, and some bacteria and cereals (such as barley and oatmeal)[119,120]. They mainly consist of a linear central backbone of D-glucose linked in the β (1→3) position with glucose side branches (linkage β 1→6) of various sizes (Figure 1), which occur at different intervals along the central backbone. Beta glucans are derived from cereals that present residues of glucose with β (1→3) and β (1→4) linkages[120].
Figure 1.
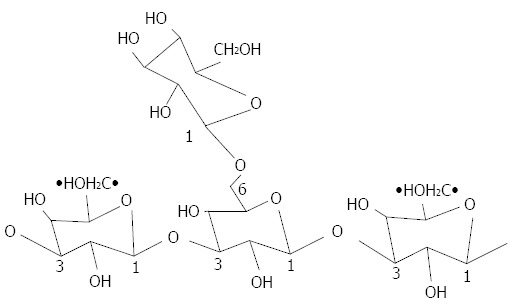
Structure of (1→-3) beta-glucans with ramifications for (1→-6).
In recent years, these polysaccharides have begun to acquire great economic importance due to their immunological activity; thus, they have been classified as biological response modifiers (BRS). However, this is not the sole property that they possess because different studies have shown their antiviral, antiparasitic, antifungal, antimicrobial, antioxidant, antigenotoxic, antitumor, antimutagenic, and anticlastogenic potential[120].
Hepatoprotective evidence for β-glucans: Despite β-glucans having shown different therapeutic effects, investigations directed toward providing evidence of its hepatoprotective potential have been insufficient because it is a field of recent study. The first studies were conducted by Neyrinck et al[121], who evaluated a diet supplemented with laminarin (LAM), a fermentable marine β (1→3) glucan, to avoid lipopolysaccharides (LPS)-induced hepatotoxicity (Escherichia coli lipopolysaccharides) through the modulation of the immune response in hepatic tissue. Wistar rats were fed a standard diet (control) and/or a diet supplemented with β (1→3) glucan, extracted from brown algae over 25 d (5% for 4 d, followed by 10% for 21 d). At the end of this period, the animals were administered LPS (10 mg/kg) ip, and after 24 h, they were sacrificed. The results of these studies showed that hypothermia, hyperglycemia, and hypertriglyceridemia, which occurred soon after the administration of LPS, were less pronounced in rats treated with LAM, compared to the controls. The increases in ALAT, ASAT, and LDH activity were diminished by the action of treatment with LAM, and similarly, the latter reduced the number of serum monocytes, nitrite (NO2), and TNF-alpha. β (1→3) glucan also modulated intrahepatic immune cells: it lowered the occurrence of peroxidase-positive cells (corresponding to monocytes/neutrophils) and, in contrast, increased the number of ED2-positive cells, corresponding to resident hepatic macrophages, i.e., Kupffer cells. In conclusion, the hepatoprotective effect of marine β (1→3) glucan during endotoxic shock could be linked to its immunomodulatory properties, suggesting that lower recruitment of inflammatory cells inside the liver and lower secretion of inflammatory mediators play important roles in the hepatoprotective effects of LAM. Thus, these effects could be attributed to a direct effect of β-glucan on the immune cells or an indirect effect through its dietary fiber properties.
In contrast, paramylon [a β (1→3)-D-glucan isolated from Euglena gracilis Z] administered orally in three doses (500, 1000, and 2000 mg/kg) to rats with previous hepatic lesions induced by ip administration of CCl4 at 50% (2.0 mL/kg of bw) demonstrated that paramylon prevented a rise in the serum levels of hepatic enzyme markers (GPT and GOT) and inhibited fatty degeneration and hepatic necrosis induced by CCl4. Pre-administration of paramylon also reduced the index of hepatic apoptosis and the enzymatic activity of SOD, CAT, and GPT. These results demonstrated that paramylon presented protective action through an antioxidant mechanism against the acute hepatic lesions induced by the organochlorine compound[122].
CONCLUSION
The present study synthesized the most accurate evidence for the hepatoprotective effects of some fruits and plants, of a natural resin, and of one of the main polysaccharides present in the cellular wall of yeasts, algae, and cereals against different toxic compounds that cause hepatic damage. In the same fashion, the investigations presented validated the use of fruits and plants in popular medicine to control hepatic damage, considered within the area of chronic degenerative diseases. The plants, fruits, and compounds described could offer novel alternatives to the limited therapeutic options that exist for the treatment of liver diseases: thus, these foods should be considered in future studies. In general, this article identified and provided evidence of some phytochemicals with hepatoprotective activity, the principal mechanisms of action of which were related to their antioxidant potential, a characteristic that should motivate and promote the search for effective protective agents, which must be evaluated later in pre-clinical and clinical assays to determine their safety and their chemopreventive capacity.
Footnotes
Supported by Proyecto SIP 20140856, ESM-IPN
P- Reviewer: Leitman M, Romani A, Simkhovich BZ, Slomiany BL S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Lin JH, Lu AY. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev. 1997;49:403–449. [PubMed] [Google Scholar]
- 2.Shanani S. Evaluation of hepatoprotective efficacy of APCL-A polyherbal formulation in vivo in rats. Indian Drugs. 1999;36:628–631. [Google Scholar]
- 3.Subramoniam A, Pushpangadan P. Development of phytomedicine for liver diseases. Indian J Pharmacol. 1999;31:166–175. [Google Scholar]
- 4.Adewusi EA, Afolayan AJ. A review of natural products with hepatoprotective activity. J Med Plants Res. 2010;4:1318–1334. [Google Scholar]
- 5.Ahsan MR, Islam KM, Bulbul IJ. Hepatoprotective activity of Methanol Extract of some medicinal plants against carbon tetrachloride-induced hepatotoxicity in rats. Global J Pharmacol. 2009;3:116–122. [Google Scholar]
- 6.Asha VV, Pushpangadan P. Preliminary evaluation of the anti-hepatotoxic activity of Phyllanthus kozhikodianus, Phyllanthus maderspatensis and Solanum indicum. Fitoterapia. 1998;59:255–259. [Google Scholar]
- 7.Casafont-Morencos F, Puente A. Pons-Romero F. Infecciones bacterianas y parasitarias del hígado. Medicine. 2008;10:563–569. [Google Scholar]
- 8.Amengual-Guedan MJ, Rodríguez Sánchez JL. Autoinmunidad en las enfermedades del hígado (I) Inmunologia. 2000;19:90–102. [Google Scholar]
- 9.Deshwal N, Sharma AK, Sharma P. Review on hepatoprotective plants. Int J Pharm Sci Rev Res. 2011;7:15–26. [Google Scholar]
- 10.Chattopadhyay RR. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: part II. J Ethnopharmacol. 2003;89:217–219. doi: 10.1016/j.jep.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Gupta SS. Prospects and perspectives of natural plant products in medicine. Indian J Pharmacol. 1994;26:1–12. [Google Scholar]
- 12.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, Gharbi N, Kamoun A, El-Fazaa S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol. 2006;41:236–239. doi: 10.1093/alcalc/agh256. [DOI] [PubMed] [Google Scholar]
- 13.Sumaya-Martínez MT, Cruz-Jaime S, Madrigal-Santillán E, García-Paredes JD, Cariño-Cortés R, Cruz-Cansino N, Valadez-Vega C, Martinez-Cardenas L, Alanís-García E. Betalain, Acid ascorbic, phenolic contents and antioxidant properties of purple, red, yellow and white cactus pears. Int J Mol Sci. 2011;12:6452–6468. doi: 10.3390/ijms12106452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madrigal-Santillán E, Fragoso-Antonio S, Valadez-Vega C, Solano-Solano G, Pérez CZ, Sánchez-Gutiérrez M, Izquierdo-Vega JA, Gutiérrez-Salinas J, Esquivel-Soto J, Esquivel-Chirino C, et al. Investigation on the protective effects of cranberry against the DNA damage induced by benzo[a]pyrene. Molecules. 2012;17:4435–4451. doi: 10.3390/molecules17044435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olaleye MT, Adegboye OO, Akindahunsi AA. Alchornea cordifolia extract protects wistar albino rats against acetaminophen inducedliver damage. Afr J Biotechnol. 2006;5:2439–2445. [Google Scholar]
- 16.Bhawna S, Kumar SU. Hepatoprotective activity of some indigenous plants. Int J Pharm Tech Res. 2009;4:1330–1334. [Google Scholar]
- 17.Gupta V, Kohli K, Ghaiye P, Bansal P, Lather A. Pharmacological potentials of citrus paradise-An overview. Int J Phytother Res. 2011;1:8–17. [Google Scholar]
- 18.Madrigal-Santillán E, Madrigal-Bujaidar E, Cruz-Jaime S, Valadez-Vega MC, Sumaya-Martínez MT, Pérez-Ávila KG, Morales-González JA. The Chemoprevention of Chronic Degenerative Disease Through Dietary Antioxidants: Progress, Promise and Evidences. In: Morales-González JA, editor. Oxidative stress and chronic degenerative diseases-a role for antioxidants. Rijeka: Croatia InTech; 2013. pp. 155–185. [Google Scholar]
- 19.Monroe KR, Murphy SP, Kolonel LN, Pike MC. Prospective study of grapefruit intake and risk of breast cancer in postmenopausal women: the Multiethnic Cohort Study. Br J Cancer. 2007;97:440–445. doi: 10.1038/sj.bjc.6603880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Dogra S, Prakash A. Protective effect of naringin, a citrus flavonoid, against colchicine-induced cognitive dysfunction and oxidative damage in rats. J Med Food. 2010;13:976–984. doi: 10.1089/jmf.2009.1251. [DOI] [PubMed] [Google Scholar]
- 21.Pereira RM, Andrades NE, Paulino N, Sawaya AC, Eberlin MN, Marcucci MC, Favero GM, Novak EM, Bydlowski SP. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules. 2007;12:1352–1366. doi: 10.3390/12071352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parmar NS. The gastric anti-ulcer activity of naringenin, a specific histidine decarboxylase inhibitor. Int J Tissue React. 1983;5:415–420. [PubMed] [Google Scholar]
- 23.Lee MH, Yoon S, Moon JO. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull. 2004;27:72–76. doi: 10.1248/bpb.27.72. [DOI] [PubMed] [Google Scholar]
- 24.Jayaraman J, Veerappan M, Namasivayam N. Potential beneficial effect of naringenin on lipid peroxidation and antioxidant status in rats with ethanol-induced hepatotoxicity. J Pharm Pharmacol. 2009;61:1383–1390. doi: 10.1211/jpp/61.10.0016. [DOI] [PubMed] [Google Scholar]
- 25.Jayaraman J, Namasivayam N. Naringenin modulates circulatory lipid peroxidation, anti-oxidant status and hepatic alcohol metabolizing enzymes in rats with ethanol induced liver injury. Fundam Clin Pharmacol. 2011;25:682–689. doi: 10.1111/j.1472-8206.2010.00899.x. [DOI] [PubMed] [Google Scholar]
- 26.Seo HJ, Jeong KS, Lee MK, Park YB, Jung UJ, Kim HJ, Choi MS. Role of naringin supplement in regulation of lipid and ethanol metabolism in rats. Life Sci. 2003;73:933–946. doi: 10.1016/s0024-3205(03)00358-8. [DOI] [PubMed] [Google Scholar]
- 27.Seeram NP. Berry fruits: compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J Agric Food Chem. 2008;56:627–629. doi: 10.1021/jf071988k. [DOI] [PubMed] [Google Scholar]
- 28.Neto CC. Cranberry and its phytochemicals: a review of in vitro anticancer studies. J Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- 29.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 30.Ade N, Leon F, Pallardy M, Peiffer JL, Kerdine-Romer S, Tissier MH, Bonnet PA, Fabre I, Ourlin JC. HMOX1 and NQO1 genes are upregulated in response to contact sensitizers in dendritic cells and THP-1 cell line: role of the Keap1/Nrf2 pathway. Toxicol Sci. 2009;107:451–460. doi: 10.1093/toxsci/kfn243. [DOI] [PubMed] [Google Scholar]
- 31.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 32.Wang YP, Cheng ML, Zhang BF, Mu M, Zhou MY, Wu J, Li CX. Effect of blueberry on hepatic and immunological functions in mice. Hepatobiliary Pancreat Dis Int. 2010;9:164–168. [PubMed] [Google Scholar]
- 33.Cheshchevik VT, Lapshina EA, Dremza IK, Zabrodskaya SV, Reiter RJ, Prokopchik NI, Zavodnik IB. Rat liver mitochondrial damage under acute or chronic carbon tetrachloride-induced intoxication: protection by melatonin and cranberry flavonoids. Toxicol Appl Pharmacol. 2012;261:271–279. doi: 10.1016/j.taap.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Shin MO, Yoon S, Moon JO. The proanthocyanidins inhibit dimethylnitrosamine-induced liver damage in rats. Arch Pharm Res. 2010;33:167–173. doi: 10.1007/s12272-010-2239-1. [DOI] [PubMed] [Google Scholar]
- 35.Arroyo-García R, Ruiz-García L, Bolling L, Ocete R, López MA, Arnold C, Ergul A, Söylemezoğlu G, Uzun HI, Cabello F, et al. Multiple origins of cultivated grapevine (Vitis vinifera L. ssp. sativa) based on chloroplast DNA polymorphisms. Mol Ecol. 2006;15:3707–3714. doi: 10.1111/j.1365-294X.2006.03049.x. [DOI] [PubMed] [Google Scholar]
- 36.Suklje K, Lisjak K, Česnik HB, Janeš L, Du Toit W, Coetzee Z, Vanzo A, Deloire A. Classification of grape berries according to diameter and total soluble solids to study the effect of light and temperature on methoxypyrazine, glutathione, and hydroxycinnamate evolution during ripening of Sauvignon blanc (Vitis vinifera L.) J Agric Food Chem. 2012;60:9454–9461. doi: 10.1021/jf3020766. [DOI] [PubMed] [Google Scholar]
- 37.Hernández-Jiménez A, Gil-Muñoz R, Ruiz-García Y, López-Roca JM, Martinez-Cutillas A, Gómez-Plaza E. Evaluating the Polyphenol Profile in Three Segregating Grape (Vitis vinifera L.) Populations. J Anal Methods Chem. 2013;2013:572896. doi: 10.1155/2013/572896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Nisco M, Manfra M, Bolognese A, Sofo A, Scopa A, Tenore GC, Pagano F, Milite C, Russo MT. Nutraceutical properties and polyphenolic profile of berry skin and wine of Vitis vinifera L. (cv. Aglianico) Food Chem. 2013;140:623–629. doi: 10.1016/j.foodchem.2012.10.123. [DOI] [PubMed] [Google Scholar]
- 39.Sandoval M, Lazarte K, Arnao I. Hepatoprotección antioxidante de la cáscara y semilla de Vitis vinifera L. (uva) An Fac Med. 2008;69:250–259. [Google Scholar]
- 40.Katalinic V, Smole Mozina S, Generalic I, Skroza D, Ljubenkov I, Klancnik A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis vinifera L. Varieties. Int J Food Prop. 2013;16:45–60. [Google Scholar]
- 41.Dani C, Oliboni LS, Pasquali MA, Oliveira MR, Umezu FM, Salvador M, Moreira JC, Henriques JA. Intake of purple grape juice as a hepatoprotective agent in Wistar rats. J Med Food. 2008;11:127–132. doi: 10.1089/jmf.2007.558. [DOI] [PubMed] [Google Scholar]
- 42.Dogan A, Celik I. Hepatoprotective and antioxidant activities of grapeseeds against ethanol-induced oxidative stress in rats. Br J Nutr. 2012;107:45–51. doi: 10.1017/S0007114511002650. [DOI] [PubMed] [Google Scholar]
- 43.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, Kamoun A, El-Fazaâ S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007;80:1033–1039. doi: 10.1016/j.lfs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 44.Gurocak S, Karabulut E, Karadag N, Ozgor D, Ozkeles N, Karabulut AB. Preventive effects of resveratrol against azoxymethane induced damage in rat liver. Asian Pac J Cancer Prev. 2013;14:2367–2370. doi: 10.7314/apjcp.2013.14.4.2367. [DOI] [PubMed] [Google Scholar]
- 45.Madrigal-Santillán E, García-Melo F, Morales-González JA, Vázquez-Alvarado P, Muñoz-Juárez S, Zuñiga-Pérez C, Sumaya-Martínez MT, Madrigal-Bujaidar E, Hernández-Ceruelos A. Antioxidant and anticlastogenic capacity of prickly pear juice. Nutrients. 2013;5:4145–4158. doi: 10.3390/nu5104145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaur M, Kaur A, Sharma R. Pharmacological actions of Opuntia ficus indica: A Review. J Appl Phar Sci. 2012;2:15–18. [Google Scholar]
- 47.Livrea MA, Tesoriere L. Antioxidant Activities of Prickly Pear (Opuntia ficus indica) Fruit and Its. Betalains, Betanin and Indicaxanthin. In: Packer L, Nam OC, Halliwell B, editors. Herbal and Traditional Medicine. Molecular Aspects of Health. New York: Marcel Dekker; 2004. pp. 537–556. [Google Scholar]
- 48.Ibañez-Camacho R, Meckes-Lozoya M, Mellado-Campos V. The hypoglucemic effect of Opuntia streptacantha studied in different animal experimental models. J Ethnopharmacol. 1983;7:175–181. doi: 10.1016/0378-8741(83)90019-3. [DOI] [PubMed] [Google Scholar]
- 49.Trejo-González A, Gabriel-Ortiz G, Puebla-Pérez AM, Huízar-Contreras MD, Munguía-Mazariegos MR, Mejía-Arreguín S, Calva E. A purified extract from prickly pear cactus (Opuntia fuliginosa) controls experimentally induced diabetes in rats. J Ethnopharmacol. 1996;55:27–33. doi: 10.1016/s0378-8741(96)01467-5. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez ML, Lin EC, Trejo A, McNamara DJ. Prickly pear (Opuntia sp.) pectin reverses low density lipoprotein receptor suppression induced by a hypercholesterolemic diet in guinea pigs. J Nutr. 1992;122:2330–2340. doi: 10.1093/jn/122.12.2330. [DOI] [PubMed] [Google Scholar]
- 51.Muñoz de Chávez M, Chávez A, Valles V, Roldán JA. The nopal: a plant of manifold qualities. World Rev Nutr Diet. 1995;77:109–134. doi: 10.1159/000424468. [DOI] [PubMed] [Google Scholar]
- 52.Sawaya WN, Khatchadourian HA, Safi WM, Al-Hammad HM. Chemical characterization of prickly pear pulp, Opuntia ficus-indica, and the manufacturing of prickly pear jam. J Food Tech. 1983;18:183–193. [Google Scholar]
- 53.Kuti JO. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004;85:527–533. [Google Scholar]
- 54.Fernández-López JA, Almela L, Obón JM, Castellar R. Determination of antioxidant constituents in cactus pear fruits. Plant Foods Hum Nutr. 2010;65:253–259. doi: 10.1007/s11130-010-0189-x. [DOI] [PubMed] [Google Scholar]
- 55.Castellar R, Obón JM, Alacid M, Fernández-López JA. Color properties and stability of betacyanins from Opuntia fruits. J Agric Food Chem. 2003;51:2772–2776. doi: 10.1021/jf021045h. [DOI] [PubMed] [Google Scholar]
- 56.Livrea MA, Tesoriere L. Antioxidative effects of cactus pear [Opuntia ficus-indica (L) Mill] fruits from Sicily and bioavailability of betalain components in healthy humans. Acta Horticult. 2009;811:197–204. [Google Scholar]
- 57.Piga A. Cactus Pear: A Fruit of Nutraceutical and Functional Cactus Pear: A Fruit of Nutraceutical and Functional. J Prof Ass Cactus Dev. 2004;6:9–22. [Google Scholar]
- 58.Wiese J, McPherson S, Odden MC, Shlipak MG. Effect of Opuntia ficus indica on symptoms of the alcohol hangover. Arch Intern Med. 2004;164:1334–1340. doi: 10.1001/archinte.164.12.1334. [DOI] [PubMed] [Google Scholar]
- 59.Ncibi S, Ben Othman M, Akacha A, Krifi MN, Zourgui L. Opuntia ficus indica extract protects against chlorpyrifos-induced damage on mice liver. Food Chem Toxicol. 2008;46:797–802. doi: 10.1016/j.fct.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 60.Brahmi D, Ayed Y, Bouaziz C, Zourgui L, Hassen W, Bacha H. Hepatoprotective effect of cactus extract against carcinogenicity of benzo(a)pyrene on liver of Balb/C mice. J Med Plants Res. 2011;5:4627–4639. [Google Scholar]
- 61.Brahmi D, Bouaziz C, Ayed Y, Ben Mansour H, Zourgui L, Bacha H. Chemopreventive effect of cactus Opuntia ficus indica on oxidative stress and genotoxicity of aflatoxin B1. Nutr Metab (Lond) 2011;8:73. doi: 10.1186/1743-7075-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alimi H, Hfaeidh N, Mbarki S, Bouoni Z, Sakly M, Ben Rouma K. Evaluation of Opuntia ficus indica f. inermis fruit juice hepatoprotective effect upon ethanol toxicity in rats. Gen Physiol Biophys. 2012;31:335–342. doi: 10.4149/gpb_2012_038. [DOI] [PubMed] [Google Scholar]
- 63.McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. 2002;21:1–13. doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- 64.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother Res. 2006;20:519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 65.Jakolev V, Issac O, Flaskamp E. Pharmacological investigation with compounds of chamazulene and matricine. Planta Med. 1983;49:67–73. [PubMed] [Google Scholar]
- 66.Viola H, Wasowski C, Levi de Stein M, Wolfman C, Silveira R, Dajas F, Medina JH, Paladini AC. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995;61:213–216. doi: 10.1055/s-2006-958058. [DOI] [PubMed] [Google Scholar]
- 67.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 68.Hernández-Ceruelos A, Madrigal-Bujaidar E, de la Cruz C. Inhibitory effect of chamomile essential oil on the sister chromatid exchanges induced by daunorubicin and methyl methanesulfonate in mouse bone marrow. Toxicol Lett. 2002;135:103–110. doi: 10.1016/s0378-4274(02)00253-9. [DOI] [PubMed] [Google Scholar]
- 69.Achterrath-Tuckermann U, Kunde R, Flaskamp E, Isaac O, Thiemer K. [Pharmacological investigations with compounds of chamomile. V. Investigations on the spasmolytic effect of compounds of chamomile and Kamillosan on the isolated guinea pig ileum] Planta Med. 1980;39:38–50. doi: 10.1055/s-2008-1074901. [DOI] [PubMed] [Google Scholar]
- 70.Maliakal PP, Wanwimolruk S. Effect of herbal teas on hepatic drug metabolizing enzymes in rats. J Pharm Pharmacol. 2001;53:1323–1329. doi: 10.1211/0022357011777819. [DOI] [PubMed] [Google Scholar]
- 71.Gupta AK, Misra N. Hepatoprotective Activity of Aqueous Ethanolic Extract of Chamomile capitula in Paracetamol Intoxicated Albino Rats. Am J Pharmacol Toxicol. 2006;1:17–20. [Google Scholar]
- 72.Al-Hashem FH. Gastroprotective effects of aqueous extract of Chamomilla recutita against ethanol-induced gastric ulcers. Saudi Med J. 2010;31:1211–1216. [PubMed] [Google Scholar]
- 73.Aksoy L, Sözbilir NB. Effects of Matricaria chamomilla L. on lipid peroxidation, antioxidant enzyme systems, and key liver enzymes in CCl4-treated rats. Toxicol Environ Chem. 2012;94:1780–1788. [Google Scholar]
- 74.Hamid S, Sabir A, Khan S, Aziz P. Experimental cultivation of Silybum marianum and chemical composition of its oil. Pak J Sci Ind Res. 1983;26:244–246. [Google Scholar]
- 75.Morazzoni P, Bombardelli E. Silybum marianum & Cardusarianum. Fitoterapia. 1995;66:3–42. [Google Scholar]
- 76.Lee DY, Liu Y. Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, Isolated from Silybum marianum (milk thistle) J Nat Prod. 2003;66:1171–1174. doi: 10.1021/np030163b. [DOI] [PubMed] [Google Scholar]
- 77.Ligeret H, Brault A, Vallerand D, Haddad Y, Haddad PS. Antioxidant and mitochondrial protective effects of silibinin in cold preservation-warm reperfusion liver injury. J Ethnopharmacol. 2008;115:507–514. doi: 10.1016/j.jep.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 78.Shaker E, Mahmoud H, Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010;48:803–806. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 79.Abou Zid S. Silymarin, Natural Flavonolignans from Milk Thistle. In: Venketeshwer R, editor. Phytochemicals-A Global Perspective of Their Role in Nutrition and Health. Rijeka: Croatia InTech; 2012. pp. 255–272. [Google Scholar]
- 80.Deep G, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin B and isosilybin A inhibit growth, induce G1 arrest and cause apoptosis in human prostate cancer LNCaP and 22Rv1 cells. Carcinogenesis. 2007;28:1533–1542. doi: 10.1093/carcin/bgm069. [DOI] [PubMed] [Google Scholar]
- 81.Svobodová A, Zdarilová A, Walterová D, Vostálová J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J Dermatol Sci. 2007;48:213–224. doi: 10.1016/j.jdermsci.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 82.Hikino H, Kiso Y, Wagner H, Fiebig M. Antihepatotoxic actions of flavonolignans from Silybum marianum fruits. Planta Med. 1984;50:248–250. doi: 10.1055/s-2007-969690. [DOI] [PubMed] [Google Scholar]
- 83.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–143. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 84.Pietrangelo A, Borella F, Casalgrandi G, Montosi G, Ceccarelli D, Gallesi D, Giovannini F, Gasparetto A, Masini A. Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology. 1995;109:1941–1949. doi: 10.1016/0016-5085(95)90762-9. [DOI] [PubMed] [Google Scholar]
- 85.Bosisio E, Benelli C, Pirola O. Effect of the flavanolignans of Silybum marianum L. on lipid peroxidation in rat liver microsomes and freshly isolated hepatocytes. Pharmacol Res. 1992;25:147–154. doi: 10.1016/1043-6618(92)91383-r. [DOI] [PubMed] [Google Scholar]
- 86.Carini R, Comoglio A, Albano E, Poli G. Lipid peroxidation and irreversible damage in the rat hepatocyte model. Protection by the silybin-phospholipid complex IdB 1016. Biochem Pharmacol. 1992;43:2111–2115. doi: 10.1016/0006-2952(92)90168-i. [DOI] [PubMed] [Google Scholar]
- 87.Sonnenbichler J, Goldberg M, Hane L, Madubunyi I, Vogl S, Zetl I. Stimulatory effect of Silibinin on the DNA synthesis in partially hepatectomized rat livers: non-response in hepatoma and other malign cell lines. Biochem Pharmacol. 1986;35:538–541. doi: 10.1016/0006-2952(86)90233-9. [DOI] [PubMed] [Google Scholar]
- 88.Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Invest. 2002;22:51–65. [Google Scholar]
- 89.Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–754. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- 90.Salam OM, Sleem AA, Omara EA, Hassan NS. Hepatoprotective effects of misoprostol and silymarin on carbon tetrachloride-induced hepatic damage in rats. Fundam Clin Pharmacol. 2009;23:179–188. doi: 10.1111/j.1472-8206.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 91.Kim SH, Cheon HJ, Yun N, Oh ST, Shin E, Shim KS, Lee SM. Protective effect of a mixture of Aloe vera and Silybum marianum against carbon tetrachloride-induced acute hepatotoxicity and liver fibrosis. J Pharmacol Sci. 2009;109:119–127. doi: 10.1254/jphs.08189fp. [DOI] [PubMed] [Google Scholar]
- 92.El Mesallamy HO, Metwally NS, Soliman MS, Ahmed KA, Abdel Moaty MM. The chemopreventive effect of Ginkgo biloba and Silybum marianum extracts on hepatocarcinogenesis in rats. Cancer Cell Int. 2011;11:38. doi: 10.1186/1475-2867-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel S, Goyal A. Current and Prospective Insights on Food and Pharmaceutical Applications of Spirulina. Curr Trends Biotechnol Pharm. 2013;7:681–695. [Google Scholar]
- 94.Deng R, Chow TJ. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc Ther. 2010;28:e33–e45. doi: 10.1111/j.1755-5922.2010.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oliveira EG, Rosa GS, Moraes MA, Pinto LA. Characterization of thin layer drying of Spirulina platensis utilizing perpendicular air flow. Bioresour Technol. 2009;100:1297–1303. doi: 10.1016/j.biortech.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 96.Murthy KN, Rajesha J, Swamy MM, Ravishankar GA. Comparative evaluation of hepatoprotective activity of carotenoids of microalgae. J Med Food. 2005;8:523–528. doi: 10.1089/jmf.2005.8.523. [DOI] [PubMed] [Google Scholar]
- 97.Kuriakose GC, Kurup MG. Hepatoprotective effect of Spirulina lonar on paracetamol induced liver damage in rats. Asian J Exp Biol Sci. 2010;1:614–623. [Google Scholar]
- 98.Bashandy SA, Alhazza IM, El-Desoky GE, Al-Othman Z. A Hepatoprotective and hypolipidemic effects of Spirulina platensis in rats administered mercuric chloride. Afr J Pharm Pharmacol. 2011;5:175–182. [Google Scholar]
- 99.Bhattacharyya S, Mehta P. The hepatoprotective potential of Spirulina and vitamin C supplemention in cisplatin toxicity. Food Funct. 2012;3:164–169. doi: 10.1039/c1fo10172b. [DOI] [PubMed] [Google Scholar]
- 100.Kepekçi RA, Polat S, Çelik A, Bayat N, Saygideger SD. Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem. 2013;141:1972–1979. doi: 10.1016/j.foodchem.2013.04.107. [DOI] [PubMed] [Google Scholar]
- 101.Ghisalberti EL. Propolis: a review. Bee World. 1979;60:59–84. [Google Scholar]
- 102.Garcia-Viguera C, Greenaway W, Whatley FR. Composition of propolis from two different Spanish regions. Z Naturforsch. 1992;47C:634–637. [Google Scholar]
- 103.Brumfitt W, Hamilton-Miller JM, Franklin I. Antibiotic activity of natural products: 1. Propolis. Microbios. 1990;62:19–22. [PubMed] [Google Scholar]
- 104.Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- 105.Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73 Suppl 1:S1–S6. doi: 10.1016/s0367-326x(02)00185-5. [DOI] [PubMed] [Google Scholar]
- 106.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–571. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 107.Farooqui T, Farooqui AA. Beneficial effects of propolis on human health and neurological diseases. Front Biosci (Elite Ed) 2012;4:779–793. doi: 10.2741/e418. [DOI] [PubMed] [Google Scholar]
- 108.González R, Remirez D, Rodriguez S, González A, Ancheta O, Merino N, Pascua C. Hepatoprotective effects of propolis extract on paracetamol-induced liver damage in mice. Phytother Res. 1994;8:229–232. [Google Scholar]
- 109.Remirez D, González A, Rodriguez S. Hepatoprotective effect of the Cuban red propolis on the toxicity induced in mice by the administration of acetaminophen. Rev Cubana Farm. 1994;28:45–54. [Google Scholar]
- 110.Czarnecki R, Librowski T, Polański M. [Hepatoprotective effect of flower pollen lipid extract in paracetamol-induced hepatotoxicity in mice] Folia Med Cracov. 1997;38:53–61. [PubMed] [Google Scholar]
- 111.González R, Corcho I, Remirez D, Rodriguez S, Ancheta O, Merino N, González A, Pascua C. Hepatoprotective effects of propolis extract on carbon tetrachloride-induced liver injury in rats. Phytother Res. 1995;9:114–117. [Google Scholar]
- 112.Sharma M, Pillai KK, Husain SZ, Giri DK. Protective role of propolis against alcohol carbon tetrachloride-induced hepatotoxicity in rats. Indian J Pharmacol. 1997;29:76–81. [Google Scholar]
- 113.Basnet P, Matsushige K, Hase K, Kadota S, Namba T. Four di-O-caffeoyl quinic acid derivatives from propolis. Potent hepatoprotective activity in experimental liver injury models. Biol Pharm Bull. 1996;19:1479–1484. doi: 10.1248/bpb.19.1479. [DOI] [PubMed] [Google Scholar]
- 114.Sugimoto Y, Tarumi T, Kaneko Y, Isayama S, Kawai N, Sugimoto H, Yamada H, Kamei C. Effect of propolis extract on D-galactosamine-induced hepatic injury in rats. Biol Pharm Bull. 1999;22:1237–1239. doi: 10.1248/bpb.22.1237. [DOI] [PubMed] [Google Scholar]
- 115.Lin SC, Lin YH, Chen CF, Chung CY, Hsu SH. The hepatoprotective and therapeutic effects of propolis ethanol extract on chronic alcohol-induced liver injuries. Am J Chin Med. 1997;25:325–332. doi: 10.1142/S0192415X97000366. [DOI] [PubMed] [Google Scholar]
- 116.Mahran LG, el-Khatib AS, Agha AM, Khayyal MT. The protective effect of aqueous propolis extract on isolated rat hepatocytes against carbon tetrachloride toxicity. Drugs Exp Clin Res. 1996;22:309–316. [PubMed] [Google Scholar]
- 117.Banskota AH, Tezuka Y, Adnyana IK, Midorikawa K, Matsushige K, Message D, Huertas AA, Kadota S. Cytotoxic, hepatoprotective and free radical scavenging effects of propolis from Brazil, Peru, the Netherlands and China. J Ethnopharmacol. 2000;72:239–246. doi: 10.1016/s0378-8741(00)00252-x. [DOI] [PubMed] [Google Scholar]
- 118.Gomaa MS, Abd Alla MA, Sameer MM. The possible protective effect of propolis (Bee glue) on cypermethrin-induced hepatotoxicity in adult albino rats. Mansoura J Forensic Med Clin Toxicol. 2011;19:17–32. [Google Scholar]
- 119.Zeković DB, Kwiatkowski S, Vrvić MM, Jakovljević D, Moran CA. Natural and modified (1--> 3)-beta-D-glucans in health promotion and disease alleviation. Crit Rev Biotechnol. 2005;25:205–230. doi: 10.1080/07388550500376166. [DOI] [PubMed] [Google Scholar]
- 120.Mantovani MS, Bellini MF, Angeli JP, Oliveira RJ, Silva AF, Ribeiro LR. beta-Glucans in promoting health: prevention against mutation and cancer. Mutat Res. 2008;658:154–161. doi: 10.1016/j.mrrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 121.Neyrinck AM, Mouson A, Delzenne NM. Dietary supplementation with laminarin, a fermentable marine beta (1-3) glucan, protects against hepatotoxicity induced by LPS in rat by modulating immune response in the hepatic tissue. Int Immunopharmacol. 2007;7:1497–1506. doi: 10.1016/j.intimp.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 122.Sugiyama A, Suzuki K, Mitra S, Arashida R, Yoshida E, Nakano R, Yabuta Y, Takeuchi T. Hepatoprotective effects of paramylon, a beta-1, 3-D-glucan isolated from Euglena gracilis Z, on acute liver injury induced by carbon tetrachloride in rats. J Vet Med Sci. 2009;71:885–890. doi: 10.1292/jvms.71.885. [DOI] [PubMed] [Google Scholar]


