Abstract
The aim of this study was to survey the work-relatedness of symptoms and diseases among engineered nanomaterials handling workers by questionnaire. A total of 258 exposed workers and 200 comparison workers were recruited from 14 nanomaterials handling factories in Taiwan. In addition to current disease status (prevalence), we classified the diseases worsened by employment (worsened by work). The control banding nanotool risk level matrix was adopted to categorize the severity and probability of nanomaterial exposure. The work-relatedness of symptoms was also self-reported in the questionnaire. The only symptom identified as significantly work-related was sneezing (5.88% in risk level 2 and 7.91% in risk level 1 vs. 2.00% in controls, p=0.04). The prevalences of work-related dry cough (p=0.06) and productive cough (p=0.09) in nanomaterials handling workers were also higher than those in controls. The only disease significantly worsened by work was allergic dermatitis (4.20% in risk level 2, 0% in risk level 1 vs. 0.50% in control, p=0.01). The incidence of angina in nanoworkers was also higher than in controls (p=0.06). In addition to allergic diseases, cardiopulmonary symptoms such as cough and angina may be used as screening tools for medical surveillance of people handling engineered nanomaterials.
Keywords: Allergic dermatitis, Sneezing, Risk levels, Nanomaterial handling workers, Questionnaire
Introduction
Potential routes of nanoparticle exposure include inhalation, ingestion, and dermal absorption. Among them, inhalation is the most important exposure route1,2,3). Previous studies revealed that nanoparticles less than 100 nm in size have several toxic characteristics, including nanoparticles depositing mainly in the alveoli, nanoparticles clearing from the lungs slower than fine particles, and inhaled nanoparticles migrating from the lungs into the circulation, brain, interstitial tissues, and regional lymph nodes1,2,3). Even so, the health effects of engineered nanoparticles are uncertain. Evidence of human toxicity of nanoparticles, for example, oxidative damage, lung inflammation, asthma, possibly lung cancer, atherosclerosis, and worsening of heart disease, came from epidemiological studies of unintentionally or naturally produced ultrafine particles generated from traffic pollution and combustion processes such as diesel exhaust and welding fumes4,5,6,7,8,9). Epidemiological studies have shown positive correlation between the particulate matter in air pollution and increased morbidity and mortality in adults and children4, 5). Epidemiological studies also show links between respiratory illnesses and the number of ambient ultrafine particles4, 5).
Little was known about exposure assessment and health risk assessment of people exposed to engineered nanoparticles until suspected occupational diseases due to polyacrylate nanoparticles were reported in China10). Although health hazards induced by engineered nanoparticles have never been confirmed in humans, there is cumulative evidence from animal studies that exposure to some nanomaterials is harmful. The health effects induced by engineered nanoparticles in animal inhalation studies included oxidative stress, pulmonary inflammation, granuloma formation, lung fibrosis, cardiovascular effects, pleural plaque formation, mesothelioma-like effects, and lung cancer1,2,3,4,5).
There are increasing public, governmental, and scientific concerns about the potential adverse health effects of nanoparticle exposure. Depression of antioxidant enzymes (superoxide dismutase and glutathione peroxidase) and increased expression of cardiovascular markers (fibrinogen and intercellular adhesion molecules) have been found among workers handling nanomaterials11). Although no human illness to date is confirmed to be attributed to engineered nanoparticles, occupational epidemiological study is needed to verify the health effects of engineered nanoparticles. We emphasize that our survey of workers handling engineered nanomaterials is not to answer “What are the health effects of nanoparticles?” Instead, we sought to answer “What are the potential health hazards among workers handling nanomaterials who are exposed to nanoparticles?” Therefore, the objective of this study was to survey symptoms complained and diseases that developed or worsened after work among workers handling engineered nanomaterials.
Subjects and Methods
Study population
We have conducted a survey of the nanotechnology factories in Taiwan. Among the lists of nanomaterials handling factories from the Environment, Health and Safety project, some were selling nanomaterials only but not handling raw nanomaterials, were shut-down, were not currently using nanomaterials, or had never used nanomaterials. We estimated that about 70 factories were manufacturing or applying nanomaterials in Taiwan. The total number of workers handling nanomaterials was estimated to be about 1,000 workers.
Among the 70 factories manufacturing or applying nanomaterials, we visited 39 factories and collected brief industrial hygiene information. There were 14 factories that agreed to participate in this study and provide detailed information. The basic information of these 13 factories except for one research institute is listed in Table 1. Among them, 5 factories manufacture nanomaterials and 12 factories use nanomaterials to manufacture other products. The physicochemical properties of nanomaterials manufactured and/or used in these factories are also listed in Table 1.
Table 1 . The characteristics of nanomaterials used or manufactured in these 13 factories.
| Factory number |
Type
of nanomaterial handling |
Nanomaterial used/mfg |
Major nanomaterial used/mfg |
Size (nm) | Amount used/mfg (mg/time) |
Duration of use/mfg (hour/time) |
Frequency of use/mfg (times/week) |
Type of nanomaterials used/mfg |
|---|---|---|---|---|---|---|---|---|
| 1 | Use | Nano-silver | Nano-silver | Unknown | 20 | 5 | 5 | Liquid solution |
| 2 | Use | Fe2O3 | Fe2O3 | 6–10 | 5,000 | 2 | 1 | Liquid solution |
| Nano-gold | 3–40 | 19 | 0.1 | 1 | Liquid solution | |||
| Nano-silver | 5–10 | 2.1 | 0.1 | 1 | Liquid solution | |||
| Mfg | Fe2O3 | Fe2O3 | 6–10 | 5,000 | 0.2 | 8 | Liquid solution | |
| Nano-gold | 3–40 | 10 | 0.25 | 8 | Liquid solution | |||
| Nano-silver | 5–10 | 21 | 0.1 | 8 | Liquid solution | |||
| 3 | Use | Titanium dioxide | Titanium dioxide | 15–20 | 10,000 | 2.5 | 2.5 | Powder and liquid solution |
| Mfg | Titanium dioxide | Titanium dioxide | 15–20 | 1,000,000 | 1 | 8 | Powder and liquid solution | |
| 4 | Use | Nano-silver | Nano-silver | Commercial secret | Commercial secret | 3 | 7 | Liquid solution |
| Mfg | Nano-silver | Nano-silver | Commercial secret | Commercial secret | 7 | 3 | Liquid solution | |
| 5 | Use | Titanium dioxide | Titanium dioxide | 20 | 50,000 | 4 | 1 | Liquid solution |
| 6 | Use | Silicon dioxide | Silicon dioxide | 10 | 50,000 | 1 | 1 | Liquid solution |
| Nano-silver | 100 | 50 | 1 | 1 | Liquid solution | |||
| 7 | Use | Carbon nanotube | Carbon nanotube | 40 | 100 | 1 | 1 | Powder and liquid solution |
| Silicon dioxide | Silicon dioxide | 100 | 50,000 | 1 | 1 | Powder | ||
| 8 | Use | Carbon nanotube | Carbon nanotube | 0.5 | Commercial secret | Commercial secret | Commercial secret | Liquid solution |
| 9 | Use | Silicon dioxide | Silicon dioxide | 12–17 | 300 | 0.2 | 4 | Powder |
| 10 | Mfg | Silicon dioxide | 160 | 2,500 | 5 | 1 | Liquid solution | |
| Silicon dioxide | Silicon dioxide | 100–200 | 60,000 | 5 | 8 | Colloid | ||
| 11 | Use | Carbon nanotube | Carbon nanotube | 110 | 5,000 | 0.5 | 4.5 | Powder, liquid solution, and gel |
| Mfg | Carbon nanotube | Carbon nanotube | 110 | 4,000,000 | 4 | 12 | Powder and liquid solution | |
| 12 | Use | Carbon nanotube | Carbon nanotube | 100 | >20 | 1 | 3.5 | Powder |
| Nano-silver | >100 | >40 | 1 | 3.5 | Liquid solution | |||
| 13 | Use | Titanium dioxide | Titanium dioxide | Unknown | 5 | 6 | 0.33 | Liquid solution |
mfg: manufacturing
This is a cross-sectional study design. Both nanomaterial handling workers and non-exposed controls were recruited from 14 above-mentioned nanomaterial handling factories. In order to have comparable geographic area and socioeconomic status, the controls were selected from volunteers at the same factories as the exposed workers, but who did not handle nanomaterials. We recruited 258 nanomaterial handling workers and 200 non-exposed controls to participate in this cross-sectional study.
Work-relatedness of symptoms and diseases worsened by work
For each participant, a self-administered questionnaire was distributed to collect work history, personal habits, detailed symptom history, and detailed past and current disease history after informed consent. This study has been reviewed and approved by our institution’s ethics review board. Health examinations were then performed and the symptom complained or the diseases developed were checked by an occupational medicine physician.
The symptom complained and diseases developed before (I had this symptom or disease before doing the job) or after (I didn’t have this symptom or disease before doing the job) working in nanomaterial handling factories in this study population were collected by questionnaire. The symptom complaints collected included respiratory, cardiovascular, skin, and neurological symptoms. Work-relatedness of symptoms was self-reported in the questionnaire, along with current status (prevalence). The diseases collected included respiratory, cardiovascular, and skin diseases. In addition to current disease status (prevalence), participants further reported I had this disease after doing the job (incidence), as well as this disease got worse after doing the job (worsened by work).
Exposure assessment
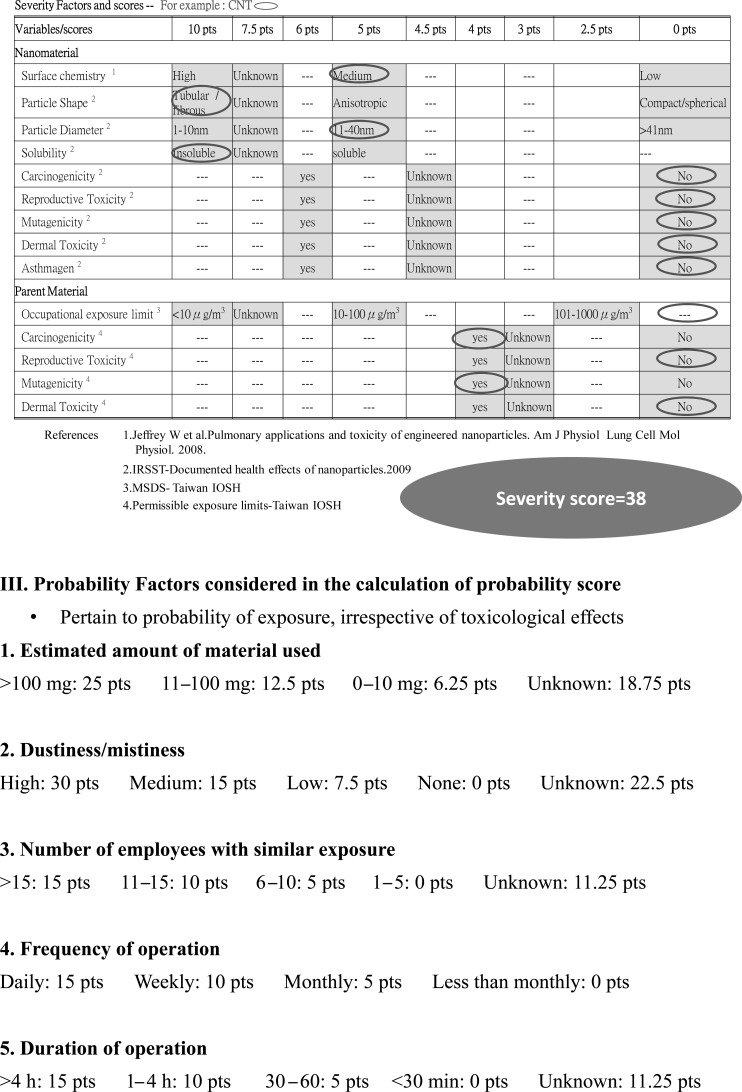
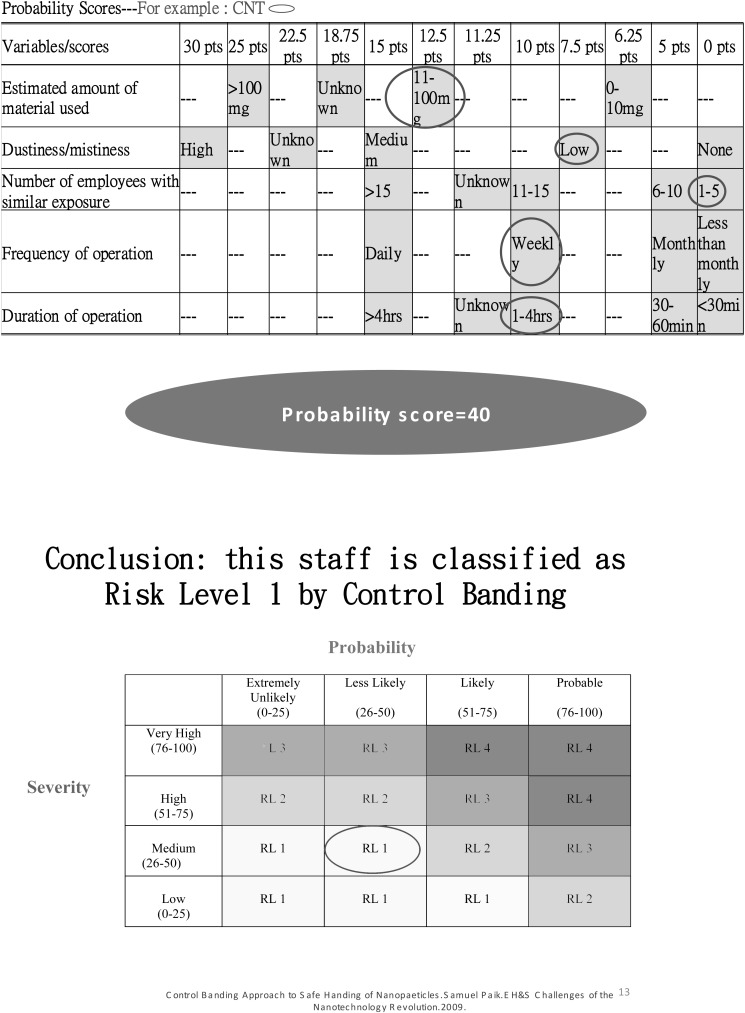
Due to a lack of harmonization of measurement strategies for exposure to engineered nanoparticles, we adopted the control banding nanotool risk level matrix proposed by Dr. Paik and his colleagues12,13,14) to categorize the risk level for each participant. An example elaborated detailed calculation of severity score, probability score and risk level was shown in Appendix 1.
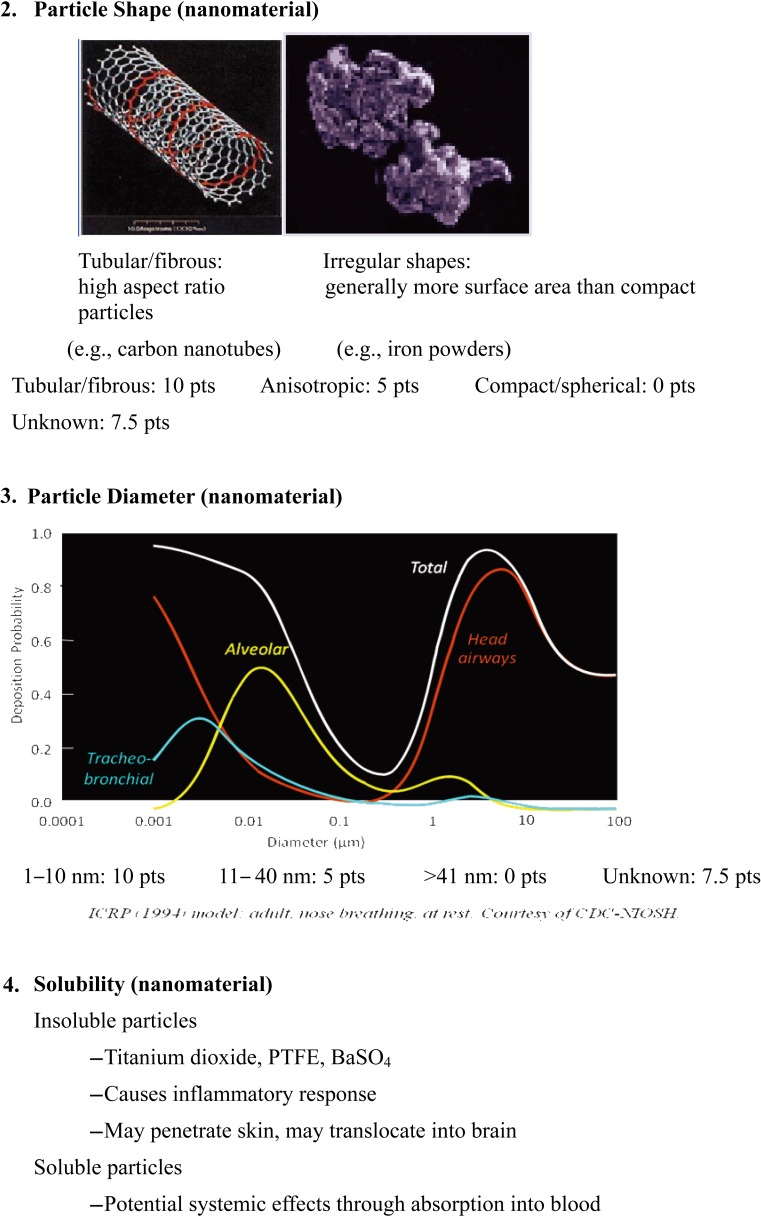
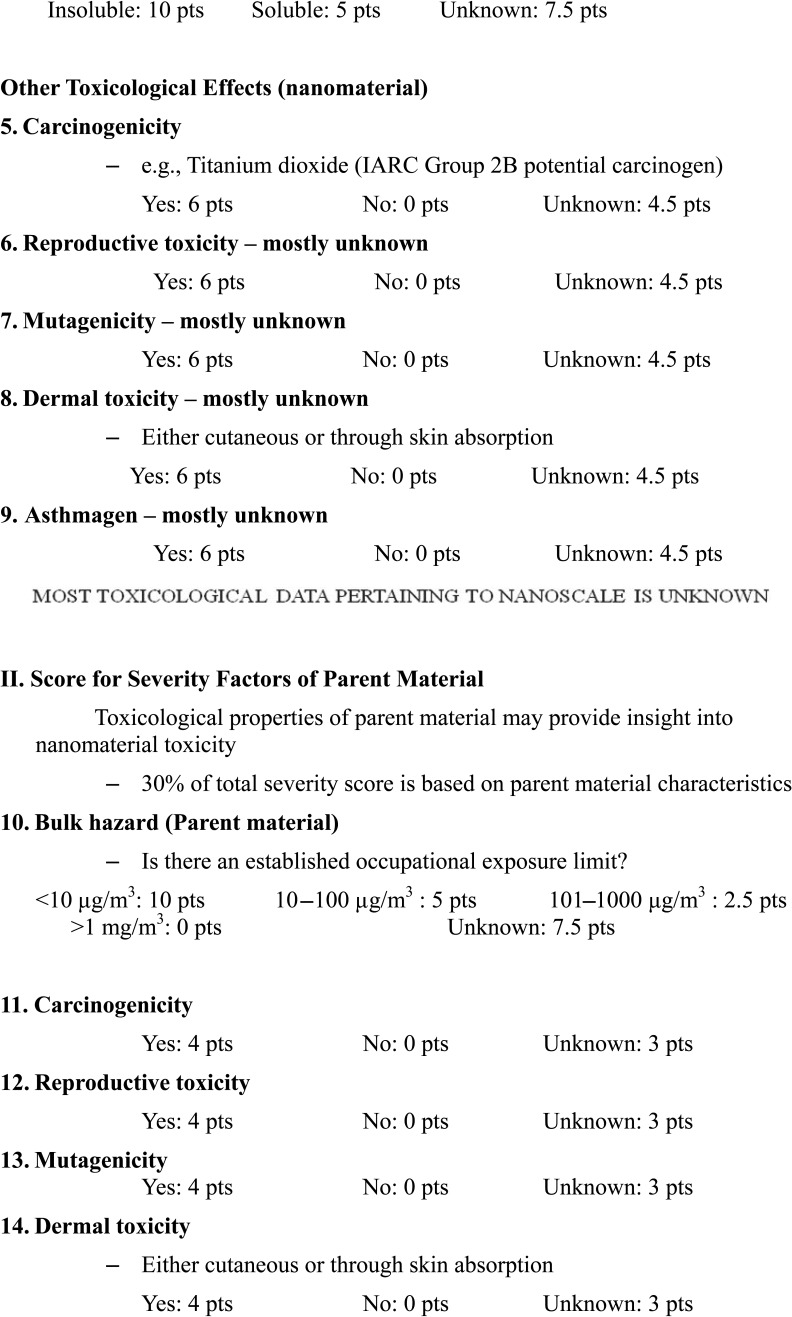
Briefly, the risk level matrix was calculated based on the severity score of the nanomaterial toxicity and the score of the exposure probability. The factors considered in the calculation of the severity score included nanomaterial (70% of severity score) and parent material (30% of severity score). The factors considered in the calculation of the severity score of the nanomaterials included surface chemistry (10 points), particle shape (10 points), particle diameter (10 points), solubility (10 points), carcinogenicity (6 points), reproductive toxicity (6 points), mutagenicity (6 points), dermal toxicity (6 points), and asthmagenicity (6 points). The factors considered in the calculation of the severity score of parent material included occupational exposure limit (10 points), carcinogenicity (4 points), reproductive toxicity (4 points), mutagenicity (4 points), dermal toxicity (4 points), and asthmagenicity (4 points). The variables considered in the exposure probability included the estimated amount of material used (25 points), dustiness/mistiness (30 points), number of employees with similar exposure (15 points), frequency of operation (15 points), and duration of operation (15 points).
The exposure probability scores were collected by questionnaire from personal interview of individual workers exposed to the various nanomaterials. In order to obtain consistent scores, the nanomaterial toxicity severity score was based on the summary reports of a review document15). The cross-table of the severity scores and probability scores was used to generate the risk levels (1 to 4) for each individual. The higher the risk level, the higher the severity of nanomaterial toxicity and/or the higher the exposure probability.
Data analysis
Percentages were used to describe the distributions of categorical variables. The χ2 test was used to test differences among categorical variables. In the data analysis, we compared the differences of prevalence, incidence and worsen by work among risk level 2 (RL2) workers (we combined RL3 and RL2 into RL2 in the data analysis), risk level 1 (RL1) workers, and non-exposed control workers. We also performed a trend analysis to test the dose-response gradients among control, RL1, and RL2 groups.
After identification of significant outcomes, multivariate logistic regression was used to adjust for confounders, including age, gender, smoking, history of respiratory diseases, and history of dust exposure. In addition to variables mentioned above, we also collected some other circumstance with potential exposed to incidential ultrafine particles. We found that there was no difference among RL2, RL1 and control groups in the distribution of frequency of types of transportation, resident closed to traffic roads, resident closed to factories within 50 meters, burning incense in the house, and burning anti-mosquito coil in the house. Therefore, the incidental ultrafine particles or nanoparticles exposure was not adjusted in our model.
Results
Distribution of risk levels and nanomaterials handled
The distribution of risk levels among the exposed study population (n=258) were 139 (53.9%) subjects in RL1, 110 (42.6%) in RL2, and 9 (3.5%) in RL 3. Since RL3 had such a small number of subjects, we combined RL3 and RL2 into RL2 (n=119, 46.1%) for the data analysis.
The types of nanomaterials handled by the 258 exposed individuals were carbon nanotubes, titanium dioxide, silica dioxide, nanosilver, and other nanomaterials including nanoresins, nanogold, nanoclay, nanoalumina, and metal oxides. Since most factories used several types of nanomaterials, most of our study population were multiple exposure to mixed types of nanomaterials (n=99, 38.4%) (Table 1). Besides, carbon nanotube was the most prevalent single exposure nanomaterial (n=60, 23.3%), followed by silica dioxide (n=37, 14.3%), titanium dioxide (n=21, 8.1%), nanosilver (n=15, 5.8%), and others (including nanoresin, nanoclay, etc.) (n=26, 10.1%).
Distribution of characteristics among the study population
The distribution of characteristics among the study population stratified by risk levels is shown in Table 2. The distribution of gender, education, alcohol drinking, and betel nut chewing differed significantly by risk level. RL2 had more men, high educational level, more alcohol drinkers, and more betel nut chewers, while the control group had more women, more university educated subjects, fewer alcohol drinkers, and fewer betel nut chewers. The difference in age distribution and smoking status among the three groups were not significant.
Table 2. Distribution of characteristics among study population stratified by risk levels.
| Variables | Risk Levels |
p-value* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n=200) |
Risk Level 1 (n=139) |
Risk Level 2 (n=119) |
||||||||
| n | (%) | n | (%) | n | (%) | |||||
| Age | ||||||||||
| ≤40 | 135 | 67.5 | 101 | 72.7 | 93 | 78.2 | 0.12 | |||
| >40 | 65 | 32.5 | 38 | 27.3 | 26 | 21.9 | ||||
| Gender | ||||||||||
| Female | 80 | 40.0 | 35 | 25.2 | 26 | 21.9 | <0.01 | |||
| Male | 120 | 60.0 | 104 | 74.8 | 93 | 78.2 | ||||
| Ethnicity | ||||||||||
| Taiwanese | 157 | 79.3 | 109 | 78.4 | 92 | 77.3 | 0.55 | |||
| Hakka | 22 | 11.1 | 15 | 10.8 | 19 | 16.0 | ||||
| Mainlander and Aborigine | 19 | 9.6 | 15 | 10.8 | 8 | 6.7 | ||||
| Education | ||||||||||
| High school and less | 38 | 19.2 | 18 | 13.1 | 20 | 16.8 | 0.03 | |||
| University | 104 | 52.5 | 62 | 45.3 | 47 | 39.5 | ||||
| Graduate school | 56 | 28.3 | 57 | 41.6 | 52 | 43.7 | ||||
| Smoking | ||||||||||
| No | 174 | 87.9 | 118 | 85.5 | 97 | 81.5 | 0.30 | |||
| Yes | 24 | 12.1 | 20 | 14.5 | 22 | 18.5 | ||||
| Alcohol drinking | ||||||||||
| No | 180 | 90.5 | 133 | 95.7 | 103 | 86.6 | 0.04 | |||
| Yes | 19 | 9.6 | 6 | 4.3 | 16 | 13.5 | ||||
| Betel nut chewing | ||||||||||
| No | 196 | 98.5 | 139 | 100.0 | 112 | 94.1 | <0.01 | |||
| Yes | 3 | 1.5 | 0 | 0.0 | 7 | 5.9 | ||||
*p value for χ2 test
Prevalence of work-related symptoms
The prevalence of self-reported respiratory and cardiovascular symptoms and work-related symptoms are listed in Table 3 (The dematological and neurological symtoms were shown in Appendix 2). The prevalence of unexpected chest pain without resolution after 10 to 15 min of rest in the RL2 group (5.88%) was significantly higher than that of the control (0.51%) and RL1 groups (0.72%) (p<0.001). In contrast, the prevalence of shortness of breath in the exposed workers (1.45% in RL1 and 2.52% in RL2) were significantly lower than that of the controls (7.04%).
Table 3. Self-reporting work-related respiratory and cardiovascular symptoms developed after employed in nanomaterials handling plants stratified by risk levels.
| Variables | Prevalence |
Self-reporting work-related |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n=200) |
Risk Level 1 (n=139) |
Risk Level 2 (n=119) |
p-value* | Control (n=200) |
Risk Level 1 (n=139) |
Risk Level 2 (n=119) |
p-value* | ||||||||||||
| n | (%) | n | (%) | n | (%) | n | % | n | % | n | % | ||||||||
| Respiratory symptoms (not related to URI) | |||||||||||||||||||
| Dry cough | 24 | 12.1% | 19 | 13.7% | 20 | 16.8% | 0.49 | 3 | 1.5% | 8 | 5.8% | 7 | 5.9% | 0.06 | |||||
| Productive cough | 14 | 7.0% | 16 | 11.6% | 11 | 9.2% | 0.35 | 0 | 0.0% | 3 | 2.2% | 3 | 2.5% | 0.09 | |||||
| Wheezing | 4 | 2.0% | 1 | 0.7% | 1 | 0.8% | 0.51 | 0 | 0.0% | 0 | 0.0% | 1 | 0.8% | 0.24 | |||||
| Chest tightness | 20 | 10.1% | 8 | 5.8% | 10 | 8.4% | 0.37 | 7 | 3.5% | 3 | 2.2% | 4 | 3.4% | 0.76 | |||||
| Shortness of breath | 14 | 7.0% | 2 | 1.5% | 3 | 2.5% | 0.02 | 2 | 1.0% | 1 | 0.7% | 2 | 1.7% | 0.75 | |||||
| Sneezing | 35 | 17.6% | 25 | 18.0% | 23 | 19.3% | 0.93 | 4 | 2.0% | 11 | 7.9% | 7 | 5.9% | 0.04 | |||||
| Rhinitis | 22 | 11.1% | 14 | 10.1% | 15 | 12.6% | 0.81 | 2 | 1.0% | 6 | 4.3% | 3 | 2.5% | 0.15 | |||||
| Nose obstruction | 30 | 15.1% | 23 | 16.6% | 21 | 17.7% | 0.83 | 5 | 2.5% | 7 | 5.0% | 4 | 3.4% | 0.46 | |||||
| Rhinorrhea | 30 | 15.1% | 18 | 13.0% | 19 | 16.0% | 0.77 | 3 | 1.5% | 8 | 5.8% | 5 | 4.2% | 0.10 | |||||
| Cardiovascular symptoms | |||||||||||||||||||
| Chest oppression, pain, burning sensation | 10 | 5.1% | 7 | 5.0% | 8 | 6.7% | 0.79 | 3 | 1.5% | 1 | 0.7% | 3 | 2.5% | 0.50 | |||||
| Chest pain with radiation to left arm, shoulder, chin or back | 6 | 3.0% | 5 | 3.6% | 4 | 3.4% | 0.96 | 4 | 2.0% | 3 | 2.2% | 3 | 2.5% | 0.95 | |||||
| Sweating, nausea, vomiting, dizziness and irritable, in addition to chest pain | 5 | 2.5% | 1 | 0.7% | 6 | 5.1% | 0.09 | 2 | 1.0% | 1 | 0.7% | 4 | 3.4% | 0.16 | |||||
| Unexpected chest pain, without resolve after 10 to 15 min rest | 1 | 0.5% | 1 | 0.7% | 7 | 5.9% | <0.001 | 0 | 0.0% | 1 | 0.7% | 1 | 0.8% | 0.45 | |||||
*p value for χ2 test
Sneezing was the only work-related symptom complained by the nanomaterials handling workers (7.9% in RL1 and 5.9% in RL2 vs. 2.0% in control, p=0.04) (Table 3). Sneezing symptoms revealed a dose-dependent gradient by risk levels (p=0.05). Multivariate logistic regression was used to adjust for confounders, including age, gender, smoking, history of respiratory diseases, and history of dust exposure (Table 4). The adjusted odds ratio of sneezing in RL1 was 4.99 (95% CI = 1.47–16.90), while it was 3.58 (95% CI = 0.97–13.25) in RL2. If duration of exposure to nanomaterials was used as surrogate of exposure, the regression models after adjusting for confounders showed that there was no significant association between work-related sneezing and duration of exposure (data not shown). If stratified by types of nanomaterial handling, we found sneezing was increased in workers handling carbon nanotube, titanium dioxide, and silicon dioxide, but not in workers handling nanosilver and other nanomaterials (data not shown). Therefore, sneezing was associated with nanomaterials handling in total population and in workers who handled specific nanomaterials.
Table 4. Multiple logistic regression of risk levels on work-related sneezing adjusted for confounders.
| Variables | B | SE | Exp(B) (Odds ratio) | 95% CI for Odds ratio | p value | |
|---|---|---|---|---|---|---|
| Age | 0.03 | 0.02 | 1.03 | 0.98 | 1.08 | 0.21 |
| Gender (male vs. female) | 0.24 | 0.56 | 1.27 | 0.42 | 3.80 | 0.67 |
| Smoking (yes vs. no) | –0.27 | 0.69 | 0.77 | 0.20 | 2.95 | 0.70 |
| Respiratory disease history (yes vs. no) | 2.22 | 0.49 | 9.16 | 3.54 | 23.71 | <0.001 |
| Dust exposure history (yes vs. no) | 0.35 | 0.88 | 1.41 | 0.25 | 7.98 | 0.69 |
| Risk Levels | ||||||
| Risk Level 1 vs. control | 1.61 | 0.62 | 4.99 | 1.47 | 16.90 | 0.01 |
| Risk Level 2 vs. control | 1.28 | 0.67 | 3.58 | 0.97 | 13.25 | 0.06 |
| Constant | –6.26 | 1.23 | – | – | – | |
The self-reporting work-related prevalence of dry cough (5.8% in RL2, 5.9% in RL1, vs. 1.5% in the control group) (p=0.06) and productive cough (2.5% in RL2, 2.2% in RL1, vs. 0% in controls) (p=0.09) was higher in exposed workers than in controls (Table 3). The self-reporting work-related cardiovascular symptoms, skin symptoms and neurological symptoms (Appendix 2) were not statistically different between the exposed and control groups.
Prevalence of diseases and diseases worsened by nanomaterials work
The prevalence of diseases and diseases worsened by work in the nanomaterials handling factories (worsened by work) are shown in the Table 5, diseases that developed after employment in the nanomaterials handling factories (incidence), and the combination of incidence and worsened by work are shown in the Appendix 3. The prevalence of arrhythmia (5.88% in RL2, and 3.60% in RL1 vs. 1.01% in control, p=0.05), angina (4.20% in RL2 and 0% in RL1 vs. 0% in control, p<0.001), and allergic dermatitis (15.13% in RL2 and 5.76% in RL1 vs. 9.55% in control, p=0.04) were significantly higher in nanomaterials handling workers than in the controls (Table 5).
Table 5. The prevalence of diseases or diseases worsened by nanomaterials handling work stratified by risk levels.
| Variables | Prevalence |
Worsened by work |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n=200) |
Risk Level 1 (n=139) |
Risk Level 2 (n=119) |
p-value* | Control (n=200) |
Risk Level 1 (n=139) |
Risk Level 2 (n=119) |
p-value* | ||||||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | ||||||||
| Respiratory Diseases | |||||||||||||||||||
| Chronic Bronchitis | 11 | 5.5% | 8 | 5.8% | 6 | 5.0% | 0.96 | 0 | 0.0% | 3 | 2.2% | 2 | 1.7% | 0.13 | |||||
| Emphysema | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | |||||
| Asthma | 7 | 3.5% | 4 | 2.9% | 2 | 1.7% | 0.63 | 0 | 0.0% | 1 | 0.7% | 1 | 0.8% | 0.46 | |||||
| Tuberculosis | 1 | 0.5% | 0 | 0.0% | 0 | 0.0% | 0.52 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | |||||
| Lung cancer | 1 | 0.5% | 0 | 0.0% | 0 | 0.0% | 0.52 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | |||||
| Rhinitis | 37 | 18.7% | 27 | 19.4% | 23 | 19.3% | 0.98 | 1 | 0.5% | 5 | 3.6% | 2 | 1.7% | 0.10 | |||||
| Cardiovascular diseases | |||||||||||||||||||
| Stroke | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | |||||
| Arrhythmia | 2 | 1.0% | 5 | 3.6% | 7 | 5.9% | 0.05 | 0 | 0.0% | 0 | 0.0% | 1 | 0.8% | 0.24 | |||||
| Ischemic heart dis | 0 | 0.0% | 0 | 0.0% | 1 | 0.8% | 0.24 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | |||||
| Angina | 0 | 0.0% | 0 | 0.0% | 5 | 4.2% | <0.01 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | |||||
| Valve heart dis. | 3 | 1.5% | 2 | 1.4% | 2 | 1.7% | 0.99 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | |||||
| Hyperlipidemia | 13 | 6.6% | 9 | 6.5% | 11 | 9.2% | 0.62 | 1 | 0.5% | 1 | 0.7% | 1 | 0.8% | 0.93 | |||||
| Hypertension | 12 | 6.1% | 9 | 6.5% | 11 | 9.2% | 0.54 | 2 | 1.0% | 2 | 1.4% | 0 | 0.0% | 0.45 | |||||
| Skin Diseases | |||||||||||||||||||
| Atopic dermatitis | 9 | 4.5% | 11 | 7.9% | 9 | 7.6% | 0.37 | 0 | 0.0% | 1 | 0.7% | 1 | 0.8% | 0.46 | |||||
| Allergic dermatitis | 19 | 9.6% | 8 | 5.8% | 18 | 15.1% | 0.04 | 1 | 0.5% | 0 | 0.0% | 5 | 4.2% | 0.01 | |||||
| Pigmentation | 3 | 1.5% | 2 | 1.4% | 5 | 4.2% | 0.22 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | |||||
| Skin cancer | 0 | 0.0% | 2 | 1.4% | 1 | 0.8% | 0.26 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | . | |||||
| Folliculitis | 9 | 4.5% | 7 | 5.0% | 7 | 5.9% | 0.87 | 0 | 0.0% | 0 | 0.0% | 1 | 0.8% | 0.24 | |||||
*χ2p-value
However, the only disease significantly worsened by work was allergic dermatitis (4.20% in RL2 and 0% in RL1 vs. 0.50% in the control group, p=0.01) (Table 5). Allergic dermatitis revealed a dose-dependent gradient by risk levels (p=0.02). Multivariate logistic regression was used to adjust for confounders, including age, gender, smoking, history of respiratory diseases, and history of dust exposure (Table 6). The adjusted odds ratio was 11.12 (95% CI = 1.18–104.51) in RL2 (there were no allergic dermatitis worsened by work in risk level 1). If duration of exposure to nanomaterials was used as surrogate of exposure, allergic dermatitis worsened by work was significantly associated with duration of exposure, either by 3 yr cut-point or 5 yr cut-point (data not shown). If stratified by types of nanomaterial handled, the prevalence of worsened by work allergic dermatitis in any nanomaterial handled was not significantly higher than in controls (data not shown).
Table 6. Multiple logistic regression of risk levels on worsened by work allergic dermatitis adjusted for confounders.
| Variables | B | SE | Exp (B) (Odds ratio) |
95% CI for odds ratio |
p value | |
|---|---|---|---|---|---|---|
| Age | –0.01 | 0.05 | 0.99 | 0.90 | 1.10 | 0.91 |
| Gender (male vs. female) | –0.30 | 0.94 | 0.74 | 0.12 | 4.65 | 0.75 |
| Smoking (yes vs. no) | – | – | – | – | – | – |
| Respiratory disease history (yes vs. no) | 2.09 | 0.90 | 8.10 | 1.39 | 47.13 | 0.02 |
| Dust exposure history (yes vs. no) | – | – | – | – | – | – |
| Risk Levels | ||||||
| Risk Level 1 vs. control* | – | – | – | – | – | – |
| Risk Level 2 vs. control | 2.41 | 1.14 | 11.12 | 1.18 | 104.51 | 0.04 |
| Constant | –5.72 | 2.23 | – | – | – | |
*There were 0 allergic dermatitis worsened by work in risk level 1 and 5 allergic dermatitis cases in risk level 2.
When the diseases that developed after employment (incidence) and incidence plus the diseases worsened by the employment (incidence plus worsened by work) are listed separately (Appendix 3), there was no significant difference between exposed workers and controls for any disease surveyed. However, the incidence as well as the incidence plus worsened by work for angina was higher in exposed workers than in controls (1.68% in RL2, 0% in RL1 and. 0% in control group) (p=0.06) (Appendix 3). The incidence plus worsened by work for allergic dermatitis was also higher in exposed workers than in controls (10.92% in RL2, 4.32% in RL1 and 5.53% in the control group) (p=0.07) (Appendix 3).
Discussion
Recently, several cases of illnesses suspected of being caused by nanoparticles were reported in the medical literature. Two cases were reported in Germany and one in China. In the first case, in late March 2006, six people were admitted to the hospital with serious respiratory problems after using a new German bathroom cleaning product called Magic Nano16, 17). The second case was pleural effusion, pulmonary fibrosis, and granuloma development in a printing plant worker in China10). The third case was a female office worker with toner dust exposure from laser printers who developed submesothelial deposition of carbon nanoparticles in the peritoneum18). Although the above-mentioned cases have never been confirmed to be caused by inhalation of nanoparticles, the primary target organ affected by nanoparticles seems to be the lungs, with acute irritation to chronic inflammation, pulmonary fibrosis, and granuloma formation10, 16,17,18). In this cross-sectional survey, we found that sneezing was significantly increased in nanomaterials handling workers and was reported as work-related. In addition to sneezing, dry cough and productive cough was the second and the third most frequently reported work-related symptoms (Table 3). Our findings are compatible with previous reports and support the notion that the primary target organ affected by nanoparticles is the lungs.
Detrimental cardiovascular consequences due to ultrafine particles exposure are reported in several epidemiological studies19,20,21,22,23,24,25). Cardiovascular diseases could likely be explained by translocation of nanoparticles from the respiratory epithelium into the circulation, with subsequent toxicity to the vascular endothelium, alteration of blood coagulation, eventually leading to atherosclerosis23,24,25). Nanoparticles can also trigger autonomic nervous system reflexes and alter cardiac frequency and function20, 21). Although cardiovascular diseases significantly worsen by work were not revealed in this study, the prevalence of arrhythmia and angina were significantly higher in nanomaterials handling workers than in the controls (Table 5). Also, the incidence as well as the incidence plus worsened by work for angina was higher in exposed workers than in controls (p=0.06) (Appendix 3). Our previous study has shown increased expression of blood coagulation markers (i.e. fibrinogen) and vascular endothelial damage marker (i.e. intercellular adhesion molecules) among workers handling nanomaterials11). The association between nanomaterials exposure and cardiovascular diseases and its exact mechanism need further investigation.
While interaction between nanoparticles and the immune system has been demonstrated, the details of this interaction are limited. Certain nanoparticles have been shown to accumulate in regional lymph nodes, where nanoparticles can be taken up and processed by dendritic cells, interact with self-proteins and, hence, modify their antigenicity and elicit altered immune responses and even autoimmunity26). Some nanoparticles have also been found to induce allergic sensitization, for example, allergic contact dermatitis induced by palladium26). These findings suggested that nanoparticles acted as adjuvants and induce specific patterns of cytokines, antibodies, and cells that favored allergic sensitization to environmental allergens, but nanoparticles unlikely acted as haptens, inducing specific immunoglobulin E production26). In addition, the immunotoxicity of both airborne and engineered nanoparticles may act as exacerbating factors in hypersusceptible subjects27). Our findings that allergic dermatitis being worsened by nanomaterials handling work but not being significantly increased in nanomaterials handling workers were consistent with the above-mentioned findings. A review article concluded that further mechanistic studies were required to improve our understanding of the physicochemical parameters of nanoparticles and their effects on the immune system28).
There are several limitations to this epidemiologic study. First, the significant findings found in this study may be due to chance or by random. Second, self-reported questionnaire cannot avoid misclassification and overestimation of health hazards. Third, the heterogeneity of nanomaterials made it difficult to find a sufficiently large group of workers exposed to the same particles to represent potential health effects of any individual nanomaterial. Fourth, validation of the control banding tools for nanomaterials exposure needs to be clarified13). Four of the five operations evaluated in that study were found to have implemented controls consistent with what was recommended by the Control Banding Nanotool13). The CB Nano Tool outcomes have been also compared with occupational hygienists’ evaluations and showed a good agreement14). By developing this dynamic Control Banding Nanotool within the realm of the scientific information available, this application of control banding appears to be a useful approach for assessing the risk of nanomaterial operations, providing recommendations for appropriate engineering controls and facilitating the allocation of resources to the activities that most need them13).
Until recently, information regarding the health hazards of nanoparticles has been lacking. In order to prevent the hazards of handling nanomaterials, the introduction of strict preventive measures, such as local ventilation and personal protective equipment, is currently the only way to prevent any risk of occupational disease in workers who handle nanomaterials. Periodic health examinations of workers handling nanomaterials, allergic diseases such as allergic dermatitis and angina as well as cardiopulmonary symptoms such as sneezing and cough may be used as screening tool. However, most of the symptoms identified in this study are not specific for nanoparticle exposure. A more sophisticated study design is needed to validate the sensitivity and specificity of these symptoms used for screening.
Conclusions
Sneezing and allergic dermatitis were significantly increased in engineered nanomaterials handling workers. In addition to allergic diseases, cardiopulmonary symptoms such as cough and angina may be used as screening tools for medical surveillance of people handling engineered nanomaterials.
Acknowledgments
This study was partly supported by the National Health Research Institutes (grants 98A1-EOSP03-014, 99A1-EOSP03-014, 00A1-EOSP03-014, and 01A1-EOSP03-014) and the Institute of Occupational Safety and Health (grants IOSH98-M323, IOSH99-M323, IOSH100-M323, and IOSH101-M323), Taiwan, ROC.
Appendix 1.
Example of risk level calculation
Appendix 2.
Self-reporting work-related dermatological and neurological symptoms developed after employed in nanomaterials handling plants stratified by risk levels (Table A1)
| Variables | Prevalence |
Self-reporting work-related |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n=200) |
Risk Level 1 (n=139) |
Risk Level 2 (n=119) |
p-value* | Control (n=200) |
Risk Level 1 (n=139) |
Risk Level 2 (n=119) |
p-value* | ||||||||||||
| n | (%) | n | (%) | n | (%) | n | % | n | % | n | % | ||||||||
| Dermatological symptoms | |||||||||||||||||||
| Itching | 34 | 17.26% | 27 | 19.42% | 26 | 21.85% | 0.60 | 10 | 5.00% | 8 | 5.76% | 13 | 10.92% | 0.11 | |||||
| Red swelling | 11 | 5.58% | 10 | 7.19% | 7 | 5.88% | 0.82 | 4 | 2.00% | 4 | 2.88% | 6 | 5.04% | 0.31 | |||||
| Papule | 4 | 2.03% | 1 | 0.72% | 3 | 2.52% | 0.51 | 3 | 1.50% | 1 | 0.72% | 1 | 0.84% | 0.76 | |||||
| Loss of hair | 28 | 14.14% | 17 | 12.23% | 14 | 11.76% | 0.79 | 7 | 3.50% | 9 | 6.47% | 4 | 3.36% | 0.35 | |||||
| Neurological symptoms | |||||||||||||||||||
| Dizziness | 25 | 12.56% | 18 | 12.95% | 20 | 16.81% | 0.54 | 8 | 4.00% | 11 | 7.91% | 11 | 9.24% | 0.14 | |||||
| Headache | 35 | 17.59% | 23 | 16.67% | 27 | 22.69% | 0.41 | 12 | 6.00% | 11 | 7.91% | 9 | 7.56% | 0.76 | |||||
| Fatigue | 60 | 30.15% | 41 | 29.71% | 39 | 32.77% | 0.85 | 34 | 17.00% | 23 | 16.55% | 23 | 19.33% | 0.82 | |||||
| Anxiety | 39 | 19.80% | 18 | 12.95% | 21 | 17.65% | 0.26 | 24 | 12.00% | 13 | 9.35% | 12 | 10.08% | 0.72 | |||||
| Loss of memory | 46 | 23.12% | 34 | 24.46% | 27 | 22.69% | 0.94 | 12 | 6.00% | 8 | 5.76% | 10 | 8.40% | 0.63 | |||||
| Insomnia | 26 | 13.13% | 18 | 12.95% | 12 | 10.08% | 0.70 | 18 | 9.00% | 9 | 6.47% | 5 | 4.20% | 0.26 | |||||
| Nightmare | 8 | 4.02% | 11 | 7.97% | 4 | 3.36% | 0.16 | 2 | 1.00% | 6 | 4.32% | 3 | 2.52% | 0.15 | |||||
| Night sweating | 3 | 1.51% | 4 | 2.90% | 2 | 1.68% | 0.64 | 2 | 1.00% | 3 | 2.16% | 1 | 0.84% | 0.57 | |||||
*χ2p-value
Appendix 3.
The incidence of respiratory, cardiovascular and skin diseases or incidence plus worsened by nanomaterials handling work stratified by risk levels (Table A2)
| Variables | Incidence |
Incidence + worsened by work |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n=200) |
Risk Level 1 (n=139) |
Risk Level 2 (n=119) |
p-value* | Control (n=200) |
Risk Level 1 (n=139) |
Risk Level 2 (n=119) |
p-value* | ||||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | ||||||||
| Respiratory Diseases | |||||||||||||||||||
| Chronic Bronchitis | 4 | 2.01% | 2 | 1.45% | 1 | 0.84% | 0.71 | 4 | 2.01% | 5 | 3.62% | 3 | 2.52% | 0.66 | |||||
| Emphysema | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | . | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | . | |||||
| Asthma | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | . | 0 | 0.00% | 1 | 0.72% | 1 | 0.84% | 0.46 | |||||
| Tuberculosis | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | . | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | . | |||||
| Lung cancer | 1 | 0.51% | 0 | 0.00% | 0 | 0.00% | 0.52 | 1 | 0.51% | 0 | 0.00% | 0 | 0.00% | 0.52 | |||||
| Rhinitis | 11 | 5.56% | 6 | 4.32% | 2 | 1.68% | 0.25 | 12 | 6.06% | 11 | 7.91% | 4 | 3.36% | 0.30 | |||||
| Cardiovascular diseases | |||||||||||||||||||
| Stroke | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | . | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | . | |||||
| Arrhythmia | 2 | 1.01% | 2 | 1.44% | 3 | 2.52% | 0.56 | 2 | 1.01% | 2 | 1.44% | 4 | 3.36% | 0.28 | |||||
| Ischemic heart dis | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | . | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | . | |||||
| Angina | 0 | 0.00% | 0 | 0.00% | 2 | 1.68% | 0.06 | 0 | 0.00% | 0 | 0.00% | 2 | 1.68% | 0.06 | |||||
| Valve heart dis. | 1 | 0.51% | 1 | 0.72% | 1 | 0.84% | 0.93 | 1 | 0.51% | 1 | 0.72% | 1 | 0.84% | 0.93 | |||||
| Hyperlipidemia | 9 | 4.55% | 6 | 4.32% | 5 | 4.20% | 0.99 | 10 | 5.05% | 7 | 5.04% | 6 | 5.04% | 1.00 | |||||
| Hypertension | 7 | 3.55% | 5 | 3.60% | 8 | 6.72% | 0.35 | 9 | 4.57% | 7 | 5.04% | 8 | 6.72% | 0.70 | |||||
| Skin Diseases | |||||||||||||||||||
| Atopic dermatitis | 4 | 2.01% | 4 | 2.88% | 3 | 2.52% | 0.87 | 4 | 2.01% | 5 | 3.60% | 4 | 3.36% | 0.64 | |||||
| Allergic dermatitis | 10 | 5.03% | 6 | 4.32% | 8 | 6.72% | 0.68 | 11 | 5.53% | 6 | 4.32% | 13 | 10.92% | 0.07 | |||||
| Pigmentation | 1 | 0.50% | 1 | 0.72% | 3 | 2.52% | 0.22 | 1 | 0.50% | 1 | 0.72% | 3 | 2.52% | 0.22 | |||||
| Skin cancer | 0 | 0.00% | 1 | 0.72% | 0 | 0.00% | 0.32 | 0 | 0.00% | 1 | 0.72% | 0 | 0.00% | 0.32 | |||||
| Folliculitis | 4 | 2.01% | 3 | 2.16% | 3 | 2.52% | 0.96 | 4 | 2.01% | 3 | 2.16% | 4 | 3.36% | 0.73 | |||||
*χ2p-value
References
- 1.Oberdörster G, Oberdörster E, Oberdörster J. (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113, 823–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, Schins R, Stone V, Kreyling W, Lademann J, Krutmann J, Warheit D, Oberdorster E. (2006) The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol 3, 11–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern ST, McNeil SE. (2008) Nanotechnology safety concerns revisited. Toxicol Sci 101, 4–21. [DOI] [PubMed] [Google Scholar]
- 4.Hesterberg TW, Long CM, Bunn WB, Sax SN, Lapin CA, Valberg PA. (2009) Non-cancer health effects of diesel exhaust: a critical assessment of recent human and animal toxicological literature. Crit Rev Toxicol 39, 195–227. [DOI] [PubMed] [Google Scholar]
- 5.Hesterberg TW, Long CM, Lapin CA, Hamade AK, Valberg PA. (2010) Diesel exhaust particulate (DEP) and nanoparticle exposures: what do DEP human clinical studies tell us about potential human health hazards of nanoparticles? Inhal Toxicol 22, 679–94. [DOI] [PubMed] [Google Scholar]
- 6.Oberdörster G, Elder A, Rinderknecht A. (2009) Nanoparticles and the brain: cause for concern? J Nanosci Nanotechnol 9, 4996–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberdörster G. (2010) Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Intern Med 267, 89–105. [DOI] [PubMed] [Google Scholar]
- 8.Bonner JC. (2010) Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc Am Thorac Soc 7, 138–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simkó M, Mattsson MO. (2010) Risks from accidental exposures to engineered nanoparticles and neurological health effects: a critical review. Part Fibre Toxicol 7, 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Y, Li X, Du X. (2009) Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J 34, 559–67. [DOI] [PubMed] [Google Scholar]
- 11.Liou SH, Tsou TC, Wang SL, Li LA, Chiang HC, Li WF, Lin PP, Lai CH, Lee HL, Lin MH, Hsu JH, Chen CR, Shih TS, Liao HY, Chung YT. (2012) Epidemiological study of health hazards among workers handling engineered nanomaterials. J Nanopart Res 14, 878–92. [Google Scholar]
- 12.Zalk DM, Nelson DI. (2008) History and evolution of control banding: a review. J Occup Environ Hyg 5, 330–46. [DOI] [PubMed] [Google Scholar]
- 13.Paik SY, Zalk DM, Swuste P. (2008) Application of a pilot control banding tool for risk level assessment and control of nanoparticle exposures. Ann Occup Hyg 52, 419–28. [DOI] [PubMed] [Google Scholar]
- 14.Zalk DM, Paik SY, Swuste P. (2009) Evaluating the control banding nanotool: a qualitative risk assessment method for controlling nanoparticle exposures. J Nanopart Res 11, 1685–704. [Google Scholar]
- 15.Ostiguy C, Soucy B, Lapointe G, Woods C, Ménard L, Trottie M. (2008) Health Effects of Nanoparticles, 2nd ed, Chemical Substances and Biological Agents, Studies and Research Projects, R-589, The Institut de recherche Robert-Sauvé en santé et en sécurité du travail (IRSST), Canada. [Google Scholar]
- 16.Pauluhn J, Hahn A, Spielmann H. (2008) Assessment of early acute lung injury in rats exposed to aerosols of consumer products: attempt to disentangle the “Magic Nano” conundrum. Inhal Toxicol 20, 1245–62. [DOI] [PubMed] [Google Scholar]
- 17.Wolinsky H. (2006) Nanoregulation: a recent scare involving nanotech products reveals that the technology is not yet properly regulated. EMBO Rep 7, 858–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theegarten D, Boukercha S, Philippou S, Anhenn O. (2010) Submesothelial deposition of carbon nanoparticles after toner exposition: case report. Diagn Pathol 5, 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wichmann HE, Spix C, Tuch T, Wölke G, Peters A, Heinrich J, Kreyling WG, Heyder J. (2000) Daily mortality and fine and ultrafine particles in Erfurt, Germany part I: role of particle number and particle mass. Res Rep Health Eff Inst 98, 5–86, discussion 87–94. [PubMed] [Google Scholar]
- 20.Donaldson K, Stone V, Seaton A, MacNee W. (2001) Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect 109 Suppl 4, 523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frampton MW. (2007) Does inhalation of ultrafine particles cause pulmonary vascular effects in humans? Inhal Toxicol 19 Suppl 1, 75–9. [DOI] [PubMed] [Google Scholar]
- 22.Peters A, Breitner S, Cyrys J, Stölzel M, Pitz M, Wölke G, Heinrich J, Kreyling W, Küchenhoff H, Wichmann HE. (2009) The influence of improved air quality on mortality risks in Erfurt, Germany. Res Rep Health Eff Inst 137, 5–77, discussion 79–90. [PubMed] [Google Scholar]
- 23.Araujo JA, Nel AE. (2009) Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol 6, 24–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. (2008) Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res 102, 589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan C, Sun Q, Lippmann M, Chen LC. (2010) Comparative effects of inhaled diesel exhaust and ambient fine particles on inflammation, atherosclerosis, and vascular dysfunction. Inhal Toxicol 22, 738–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Gioacchino M, Petrarca C, Lazzarin F, Di Giampaolo L, Sabbioni E, Boscolo P, Mariani-Costantini R, Bernardini G. (2011) Immunotoxicity of nanoparticles. Int J Immunopathol Pharmacol 24 Suppl, 65S–71S. [PubMed] [Google Scholar]
- 27.Inoue K. (2011) Promoting effects of nanoparticles/materials on sensitive lung inflammatory diseases. Environ Health Prev Med 16, 139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolnik BS, González-Fernández A, Sadrieh N, Dobrovolskaia MA. (2010) Nanoparticles and the immune system. Endocrinology 151, 458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]