Abstract
Aims
To construct and evaluate rats' tolerogenic dendritic cells (DC) through induction by NF-κB Decoy method.
Methods
GM-CSF and IL-4 were used to transform rats's monocytes into DC, and DC were stimulated with LPS, NF-κB Decoy ODN, and loaded with Bovine Type II Collagen. The following methods were employed to phenotype DC: 1) Observation of cell morphology; 2) Evaluation of cell viability using trypan blue staining; 3) Purity determination of DC through detection of specific markers OX-62; 4) Evaluation of mature state of DC via the determination of the expression of CD80 and CD86; 5) Determination of stimulation capability towards the proliferation of lymphocyte and the secretion of INF-r and IL-10.
Results
The activity of DC was more than 92%, and the expression of OX-62 was more than 70%. Most of DC exhibited the phenotype of CD80+/CD86-. Compared with control group and LPS-stimulation group, the less mature adhered cells and hairlike DC were observed in NF-κB decoy group. Significant reduction (p<0.05) was observed for the positive expression and extension of CD80 and CD86 in cell surface. After loaded with calf type II collagen, the low expression of CD80 and CD86 remains to be existed. The stimulation capability of DC towards lymphocyte in NF-κB decoy group was lower than that in control group (p<0.05) and LPS stimulation group (p<0.05).
Conclusion
NF-κB Decoy ODN method can be successfully applied for construct rats' tolerogenic dendritic cells (DC) with stable morphology and phenotype. The tolerogenic DC exhibited immature immune phenotype, and low capability to stimulate lymphocytes.
Keywords: dendritic cells (DC), NF-κB Decoy ODN, calf type II collagen
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease influencing multiple joints such as hands, wrists and feet. The main pathological changes for RA patients contains infiltration of inflammatory cells, neovascularization and hyperplasia of synovial tissues [1,2]. To date, the clinical therapeutic drugs for treatment of RA commonly focus on the inhibition of immune system, which provides big risk of infectious diseases. Searching of new therapeutic methods for RA is necessary and important.
Generally, the initiation of RA has been closely related with the abnormal antigen presenting cell (APC), which will result in the activation of T cells, especially Th1 and Th17 cells [3]. Dendritic cells (DC) is a kind of APC, and play a key role in regulation of the induction of the differentiation naive T cells towards Th cells through increased expression of adhesion molecules and cytokine receptors as well as the production of cytokines [4]. Compared with mature DCs, immature DCs have been commonly loaded with antigen and manipulated to generate tolerogenic DCs and suppress autoimmune responses both in vivo and in vitro. Various manipulations have been applied to generate tolerogenic phenotype, including regulation of molecular targets on DCs, uptake of apoptotic cells, and genetic engineering strategy. For example, the immunoglobulin-like transcript (ILT) family contains a group of activating and inhibitory receptors, and some of them play a role in tolerance induction [5]. The upregulation of ILT3 and ILT4 receptors on human DCs renders them tolerogenic. Direct induction using low dose of GM-CSF and transfection of IL-4 gene can also adapted to make tolerogenic DCs.
The present study aims to induce DC using monocytes isolated from rats' spleen. The new reported method Decoy strategy was utilized, in which Nuclear factor-kappa B (NF-κB) Decoy oligodeoxynucleotide (ODN) was imported into the cells to prevent the activation of NF-κB. After loaded with calf type II collagen, some important phenotype was observed, including morphological characteristics, the expression of costimulatory molecules in cell surface, and stimulation capability towards lymphocytes.
Materials and Methods
Chemicals and reagents
Bovine Type Collagen, MTT formazan, HEPES, and Lipolysaccharides(LPS) were purchased from Sigma company (St. Louis, MO). PE conjugated anti-rat CD80(B7-1), PE conjugated anti-rat CD103(αE2,DC marker) and FITC conjugated anti-rat CD86(B7-2) were obtained from eBioscience (San Diego, CA). Recombinant rat GM-CSF and IL-4 were purchased from peprotech company. RPMI medium was obtained from GIBCO (Maryland, USA). Mitomycin C was obtained from Suolaibao company.
Isolation of rats' spleen monocytes
Female SD rats (250–320 g, 7–8 months) and male Wistar rats (300g, 7 months) were obtained from the experimental animal center of Guiyang Medical University. The spleen was taken from SD rats, and washed using RMPI1640 medium for three times. The spleen was grinded and washed with d-Hanks. The spleen cells were collected through filteration, and monocytes were obtained through density gradient centrifugation. The monocytes were cultured, and trypan blue staining was used to show the cell viability to be more than 85%.
The induction for the formation of dendritic cells (DC)
The monocytes were incubated with RMPI 1640 containing GM-CSF (30 ng/mL) and IL-4 (20 ng/mL). The cells were divided into the following groups: 1) Control group 2) LPS stimulation group: After the culture of DC cells for 7 days, 10µg/mL of LPS was added to stimulate DC. 3) Decoy induction group: 5 µmol/L of NF-κB Decoy ODN was added at the beginning of DC culture. 4) LPS stimulation+Decoy induction group: 5 µmol/L of NF-κB Decoy ODN was added at the beginning of DC culture, and 10µg/mL of LPS was added to stimulate DC after 7 day culture. 5) Loaded with Bovine Type Collagen after Decoy induction group: 5 µmol/L of NF-κB Decoy ODN was added at the beginning of DC culture, and 50µg/mL of calf type II collagen (pH=7.2). The synthesis of NF-κB Decoy ODN was according to previous description with some modifications [6].
The observation of cell morphology
The inverted phase contrast microscope was used to observe the cell morphology of DC. The smear cytology was also performed, and cell morphology was observed after staining with Giemse.
The detection of specific markers using flow cytometric analysis
The cells number was diluted 1×106 cells/tube, and the corresponding antibody was added to undergo reaction for 40 min. The added quantity of PE-OX-62, PE-CD80, and FITC-CD86 was 1.5µL/106 cells, 2µL/106 cells, and 0.8µL/106 cells, respectively.
The flow cytometric analysis was carried out as previously described [7].
Investigation of stimulation capability of DC towards lymphocytes
The DCs were cultured for 8 days, and then mitomycin C (3g/L) was added and cultured for another 30 min. The cells were diluted to 2×105/mL with RMPI1640 as stimulation cells. The spleens of wistar rats were taken, and the same method for DC preparation was used to prepare the reaction cells. The mixed ratio between stimulation cells and reaction cells was 1:5 as previously described [8]. After 68h incubation, 50 µL of supernatant was analyzed for the concentration of IFN-r and IL-10. 20 µL of MTT was added in well, and after the incubation for 4h at 37oC, the medium was discarded. 100 µL of DMSO was added, and OD values were read at 570 nm.
Statistical analysis
The statistical analysis was performed using SPSS11.5 software, and Statistical differences were evaluated using the two-tailed Student's t-test and considered significant at the *p < 0.05, **p <0.01 level.
Results
The cell morphology of DC
After 72h incubation, cell colony was formed, and the volume of some cells increased. The cell morphology was star or round, and a few cells exhibited hairlike morphology. There was no significant difference for the cell morphology. After 5 day culture, there were more adhered cells in decoy group than control group. The addition of LPS significantly increased the growth of DC. After 8 day culture, there was more hairlike DC in LPS stimulation group than that in other groups.
The expression of OX62 in cell surface
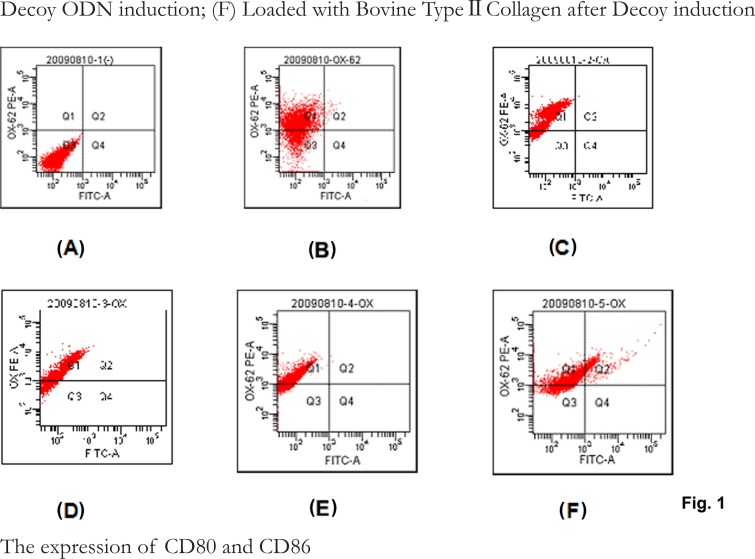
As shown in Fig. 1, the expression ratio of OX62 was 72.6%, 85.2%, 87.6%, 79.6% and 86.6% for control, LPS stimulation, Dexoy induction, LPS stimulation+Decoy induction, and Bovine Type Collagen-Decoy-DC groups, respectively.
Fig.1.
The expression of OX-62 on DCs. (A) negative control; (B) control; (C) LPS stimulation; (D) NF-κB Decoy ODN induction; (E) LPS stimulation+NF-κB Decoy ODN induction; (F) Loaded with Bovine Type II Collagen after Decoy induction
The expression of CD80 and CD86
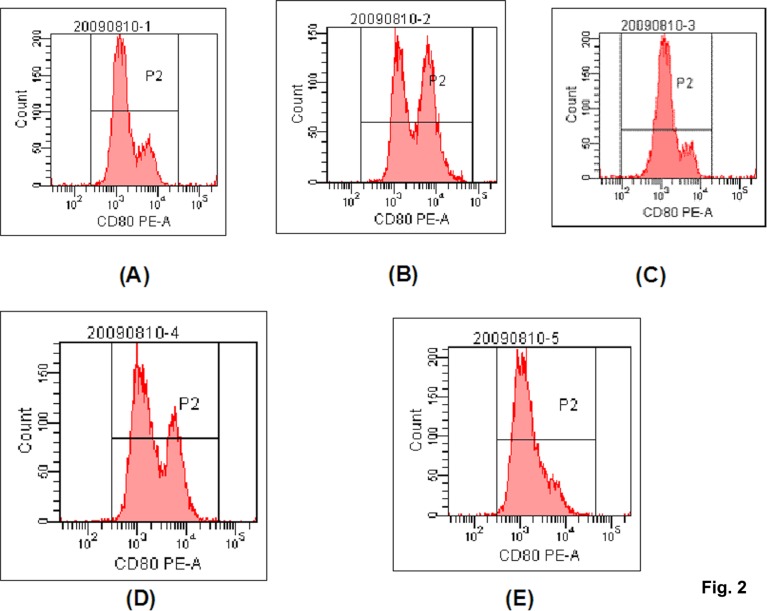
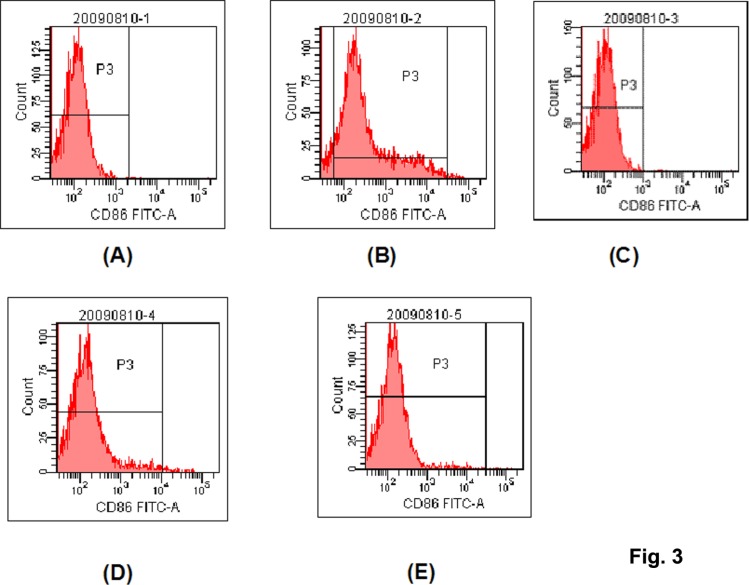
The Fig. 2 and Fig. 3 were representative figures of the expression of CD80 and CD86, and the table 1 gave a conclusion of the positive expression ratio and intensity for CD80 and CD86.
Fig. 2.
The representative flow cytometric histograms for the expression of CD80. (A) control; (B) LPS stimulation; (C) NF-κB Decoy ODN induction; (D) LPS stimulation+NF-κB Decoy ODN induction; (E) Loaded with Bovine Type II Collagen after Decoy induction.
Fig. 3.
The representative flow cytometric histograms for the expression of CD86. (A) control; (B) LPS stimulation; (C) NF-κB Decoy ODN induction; (D) LPS stimulation+NF-κB Decoy ODN induction; (E) Loaded with Bovine Type II Collagen after Decoy induction
Table 1.
The positive expression ratio and intensity of CD80 and CD86
| Groups | CD80 | CD86 | ||
| Positive expression (Meanxx±S.D.) |
Expression intensity (Meanxx±S.D.) |
Positive expression (Meanxx±S.D.) |
Expression intensity (Meanxx±S.D.) |
|
| Control | 0.74±0.05 | 3019±306 | 0.07±0.06 | 341±262 |
| LPS stimulation | 0.86±0.13 | 5427±768* | 0.41±0.20* | 1497±160* |
| NF-κB Decoy | 0.53±0.17 | 1540±339*# | 0.01±0.01# | 109±163# |
| LPS stimulation+ NF-κB Decoy |
0.65±0.17 | 2350±1100# | 0.03±0.02# | 537±266# |
| Bovine Type II Collagen-Decoy |
0.66±0.09 | 2007±422*# | 0.05±0.04# | 240±118# |
Note:
p<0.05, compared with control group;
p<0.05, compared with LPS stimulation group.
For the positive expression ratio of CD80, there was no significant among these groups. For the expression intensity, the intensity in Decoy group and Bovine Type II Collagen-Decoy group was lower than that in control group (p<0.05). Although the LPS stimulation can increase the expression intensity of CD80 for decoy induction, the intensity value in LPS stimulation+decoy induction group remained to be lower than LPS stimulation group (p<0.05). DC in the control group nearly did not express CD86. LPS stimulation significantly increased the positive expression ratio of CD86 (p<0.01). There is no significant difference between Decoy, LPS stimulation+Decoy, Bovine Type II Collagen-Decoy group and control group (p>0.05). The expression of CD86 showed low intensity in control, decoy, LPS stimulation+decoy, and Bovine Type II Collagen-decoy groups, and there were no significantly difference among these groups (p>0.05). The expression intensity of CD86 in LPS stimulation group was significantly higher than that in other group (p<0.01).
Evaluation of stimulation capability of DC towards lymphocytes
The influence of DC towards proliferation capability of lymphocytes was listed in Table 2. Compared with control group, the proliferation capability of lymphocytes in LPS stimulation group was much higher (p<0.01), and the proliferation capability of lymphocytes in decoy induction group was much lower (p<0.05). After stimulation by LPS, the stimulation capability of DC towards lymphocytes in decoy induction group increased. However, the stitulation capability remains to be lower than LPS stimulation group (p<0.001). The addition of serum of healthy person exhibited stronger stimulation capability than that for the addition of Bovine Type II Collagen (p<0.001).
Table 2.
Evaluation of stimulation capability of DC towards lymphocytes
| Groups | n | OD value (Meanxx±S.D) |
| Control | 8 | 0.346±0.056 |
| LPS stimulation | 8 | 0.538±0.084* |
| Decoy induction | 8 | 0.279±0.034*# |
| LPS stimulation+Decoy induction | 8 | 0.365±0.080#Δ |
| Bovine Type II Collagen -Decoy | 8 | 0.438±0.092 |
| Bovine Type II Collagen-Decoy(plus serum) | 8 | 0.712±0.073྿ |
Note:
p<0.05, compared with control group;
p<0.05, compared with LPS stimulation group;
p<0.05, compared with Decoy induction group;
p<0.05, compared with Bovine Type II Collagen-decoy group.
The secretion of IFN-γ and IL-10
As shown in Table 3, compared with control group, the secreted IFN-γ in LPS stimulation group was higher (p<0.05), and the secreted IFN-γ in decoy induction group was lower (p<0.05). The secreted IFN-γ in LPS stimulation+Decoy induction group exhibited no significant difference with that in the decoy induction group, but was significantly lower than that in LPS stimulation group (p<0.05). The addition of serum of healthy person exhibited stronger IFN-γ secretion than that for the addition of Bovine Type II Collagen (p<0.05).
Table 3.
The influence of DC towards the secretion of IFN-r by lymphocytes (Meanxx±S.D.)
| Groups | n | OD values | IFN-r (ng/L) |
| Control | 4 | 0.407±0.180 | 385±34 |
| LPS stimulation | 4 | 0.589±0.196 | 666±58* |
| Decoy induction | 4 | 0.274±0.187 | 179±44*# |
| LPS stimulation+Decoy induction |
4 | 0.304±0.175 | 226±27*# |
| Bovine Type II Collagen-Decoy | 4 | 0.427±0.193 | 416±54 |
| Bovine Type II Collagen-Decoy (plus serum) |
4 | 0.714±0.207 | 859±75Δ |
Note:
p<0.05, compared with control group
p<0.05, compared with LPS stimulation group
p<0.05, compared with Bovine Type II Collagen-decoy group.
For IL-10 secretion (Table 4), no significantly difference was observed between decoy induction group and control group. The secretion of IL-10 in decoy group and LPS stimulation+decoy induction group was higher than LPS stimulation group (p<0.05). The addition of serum of healthy person exhibited weaker IL-10 secretion than that for the addition of Bovine Type II Collagen (p<0.05).
Table 4.
The influence of DC towards the secretion of IL-10 by lymphocytes (Meanxx±S.D.)
| Groups | n | OD values | IL-10 (ng/LJ |
| Control | 4 | 0.411±0.172 | 9.45±0.90 |
| LPS stimulation | 4 | 0.370±0.169 | 8.50±0.77 |
| Decoy induction | 4 | 0.438±0.169 | 10.45±0.79* |
| LPS stimulation+Decoy | 4 | 0.448±0.167 | 10.78±0.69* |
| induction | |||
| Bovine Type II Collagen | 4 | 0.421±0.177 | 9.83±1.07 |
| -Decoy | |||
| Bovine Type II Collagen | 4 | 0.289±0.169 | 5.08±0.80# |
| -Decoy(plus serum) |
Note:
p<0.05, compared with LPS stimulation group
p<0.05, compared with Bovine Type II Collagen-decoy group.
Discussion
NF-κB is a transcription regulation factor which is involved in the body defense, cell differentiation and apoptosis through regulating the transcription of cytokines, adhesion molecules, and immunologic receptors. The activation of NF-κB plays a key role in the maturation of DC [9]. Decoy ODN, a recently developed genetic method, can completely block transcription factor function. Compared with more classical methods (e.g. chloramphenicol acetyltransferase, luciferase constructs, etc.), this method was inexpensive [10].
The addition of GM-CSF and IL-4 can induce the formation of immature DCs. However, this kind of immature DCs is not easily regulated, and many factors (e.g. Inflammation, LPS, IFN-r, etc.) can activate immature DCs to become mature. The present study utilized the strong capability of immature DC to absorb the antigen, and transfect NF-κB Decoy ODN into the nucleus of DC [11]. The transfection of NF-κB Decoy ODN made DC to maintain the following immature state: 1) The adhered and hairlike cells are more in decoy group than control group; 2) In NF-κB Decoy ODN group, the expression of CD80 and CD86 in cell surface is lower than that in control group. Additionally, LPS stimulation was also used to stability of immatured DCs, and the results showed the expression of CD80 and CD86 was not significantly altered, indicating that decoy-induced DC was very stable. This result was similar with the previous literatures [11,12]. The activation of lymphocytes depends the maturation of DC. The present study showed that the activation capability in decoy group was lower than control group and LPS stimulation group, and the expression of IFN-r was significantly inhibited in decoy group, furtherly indicating the stability of decoy-induced immatured DC [13].
With the increased study for rheumatoid arthritis (RA), some antigens related with RA have been detected, including type II collagen and glucose-6-phosphate isomerase (GPI) [14,15]. The tolerogenic DC vaccine with specific antigen will be a promising therapy for RA. Immature DCs have very strong capability to capture antigen. Once captured by immature DCs, the proteinase in lysosome can digest the antigen into peptides containing 14–20 amino acids which furtherly formed complex with MHG-II and were secreted into cell surface. The present study prepared the tolerogenic DC loaded with Bovine Type II Collagen antigen which did not affect the low expression of CD80 and CD86, indicating that the loaded Bovine Type II Collagen did not affect the status of tolerogenic DC.
Conclussion
The tolerogenic DC was successfully constructed through NF-κB Decoy ODN method towards isolated spleen cells. This tolerogenic DC has low stimiulation capability towards lymphocytes, and this properties can be difficult to be reversed by LPS stimulation. After loaded with Bovine Type II Collagen antigen, this tolerogenic DC vaccine can be successfully applied to RA treatment.
Acknowledgement
This work was supported by a grant from Science and Technology Department of Guizhou Province, China, No.QianKeHe LG(2012)004, and a grant from Science and Technology Department of Shannxi Province, China, No. 2009K12-02(8).
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. New England Journal of Medical. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Adorini L, Aloisi F, Galbiati F, Gately MK, Gregori S, Penna G, Ria F, Smiroldo S, Trembleau S. Targeting IL-12, the key cytokine driving Th1-mediated autoimmune diseases. Chemistry Immunology. 1997;68:175–197. doi: 10.1159/000058691. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Kabalak G, Dobberstein SB, Matthias T, Reuter S, The YH, Dorner T, Schmidt RE, Witte T. Association of immunoglobulin-like transcript 6 deficiency with Sjogren's syndrome. Arthritis Rheum. 2009;60(10):2923–2925. doi: 10.1002/art.24804. [DOI] [PubMed] [Google Scholar]
- 6.Bonham CA, Peng L, Liang X, Chen Z, Wang L, Ma L, Hackstein H, Robbins PD, Thomson AW, Fung JJ, Qian S, Lu L. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. Journal of Immunology. 2002;169(6):3382–3391. doi: 10.4049/jimmunol.169.6.3382. [DOI] [PubMed] [Google Scholar]
- 7.Fang ZZ, Nian Y, Li W, Wu JJ, Ge GB, Dong PP, Zhang YY, Qiu MH, Liu L, Yang L. Cycloartane triterpenoids from Cimicifuga yunnanensis induce apoptosis of breast cancer cells (MCF7) via p53-dependent mitochondrial signaling pathway. Phytother Research. 2011;25(1):17–24. doi: 10.1002/ptr.3222. [DOI] [PubMed] [Google Scholar]
- 8.Voisine C, Hubert FX, Trinite B, Heslan M, Josien R. Two phenotypically distinct subsets of spleen dendritic cells in rats exhibit different cytokine production and T cell stimulatory activity. Journal of Immunology. 2002;169(5):2284–2291. doi: 10.4049/jimmunol.169.5.2284. [DOI] [PubMed] [Google Scholar]
- 9.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16:257–270. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 10.Ozaki K, Makino H, Aoki M, Miyake T, Yasumasa N, Osako MK, Nakagami H, Rakugi H, Morishita R. Therapeutic effect of ribbon-type nuclear factor-κB decoy oligonucleotides in a rat model of inflammatory bowel disease. Current Gene Therapy. 2012;12(6):484–492. doi: 10.2174/156652312803519814. [DOI] [PubMed] [Google Scholar]
- 11.Xu MQ, Suo YP, Gong JP, Zhang MM, Yan LN. prolonggation of liver allograft survival by dendritic cells modified with NF-κB decoy oligodioxynucleo-tides. World Jounal of Gastroenterol. 2004;10:2361–2368. doi: 10.3748/wjg.v10.i16.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16:257–270. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 13.Speirs K, Caamano J, Goldschmidt MH, Hunter CA, Scott P. NF-kappa B2 is required for optimal CD40-induced IL-12 production but dispensable for Th1 cell Differentiation. Journal of Immunology. 2002;168(9):4406–4413. doi: 10.4049/jimmunol.168.9.4406. [DOI] [PubMed] [Google Scholar]
- 14.Renju GL, Muraleedhara Kurup G, Saritha Kumari CH. Anti-inflammatory activity of lycopene isolated from Chlorella marina on type II collagen induced arthritis in Sprague Dawley rats. Immunopharmacol Immunotoxicol. 2013;35(2):282–291. doi: 10.3109/08923973.2012.742534. [DOI] [PubMed] [Google Scholar]
- 15.Umeda N, Matsumoto I, Ito I, Kawasaki A, Tanaka Y, Inoue A, Tsuboi H, Suzuki T, Hayashi T, Ito S, Tsuchiya N, Sumida T. Anti-citrullinated glucose-6-phosphate isomerase peptide antibodies in patients with rheumatoid arthritis are associated with HLA-DRB1 shared epitope alleles and disease activity. Clinical & Experimental Immunology. 2013;172(1):44–53. doi: 10.1111/cei.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]