Abstract
Background:
Cerebral arteriovenous malformations (AVMs) have been long thought to be a congenital anomaly of vasculogenesis in which arteries and veins form direct connections forming a vascular nidus without an intervening capillary bed or neural tissue. Scattered case reports have described that AVMs may form de novo suggesting they can become an acquired lesion.
Case Description:
The current case report describes a patient who presented with new-onset seizures with an initial negative magnetic resonance imaging (MRI) of the brain and subsequently developed an AVM on a MRI 9 years later.
Conclusion:
This case joins a small, but growing body of literature that challenges the notion that all AVMs are congenital.
Keywords: AVM, arteriovenous malformation, AVM formation, adult onset AVM, de novo AVM
INTRODUCTION
Cerebral arteriovenous malformations (AVMs) are abnormal vascular connections that form between arteries and veins, without an intervening capillary bed or neuronal tissues.[6] They are a heterogeneous group of vascular lesions varying in locations, sizes, and venous drainages. The incidence of cerebral AVMs in the general population is less than 1%. AVMs may be clinically silent or present with hemorrhages, often in the second or third decade of life.[2] Less commonly, AVMs may manifest other symptoms such as seizures and those related to mass effect or ischemic steal.[7] The pathogenesis of AVMs remains incompletely elucidated and has traditionally been thought to be congenital. We present a case of symptomatic de novo cerebral AVM in a patient with an initial negative magnetic resonance imaging (MRI) of the brain at the age of 15, but 9 years later developed a left parietal AVM.
CASE REPORT
History and presentation
The patient is a 24-year-old right-handed male with a past medical history significant for a seizure disorder. He experienced his first seizure at age 15, resulting in a severe facial burn after falling on a home heater. An initial MRI of the brain was performed, which was negative [Figure 1]. He continued to have intermittent seizures until age 17 when he was placed on antiepileptic medications. At the age of 24, he suffered a 4-min generalized tonic-clonic seizure. Further workup including an MRI of the brain revealed a 2 × 1.7 × 1.8 cm left parietal AVM [Figure 2], which was not present on the MRI 9 years ago. Digital subtraction cerebral angiogram (DSA) showed a Spetzler–Martin grade II AVM in the left parietal region with arterial feeders from the left middle cerebral artery (MCA) and the left posterior cerebral artery (PCA) and early venous drainage into the superior sagittal sinus [Figure 3].
Figure 1.
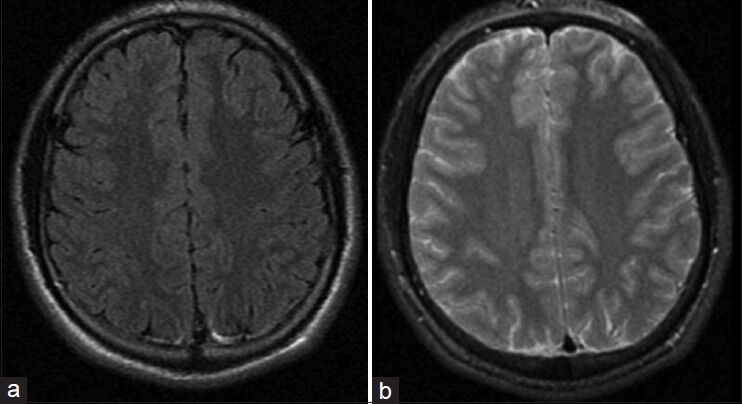
Axial Flair (a) and T2 weighted (b) MRI performed December 06, 2003 without evidence of vascular malformation
Figure 2.
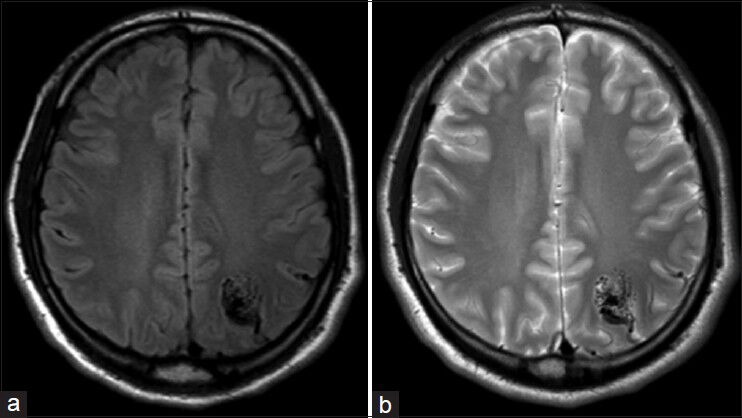
Axial Flair (a) and T2 weighted (b) MRI performed July 21, 2012 demonstrating left parietal AVM
Figure 3.
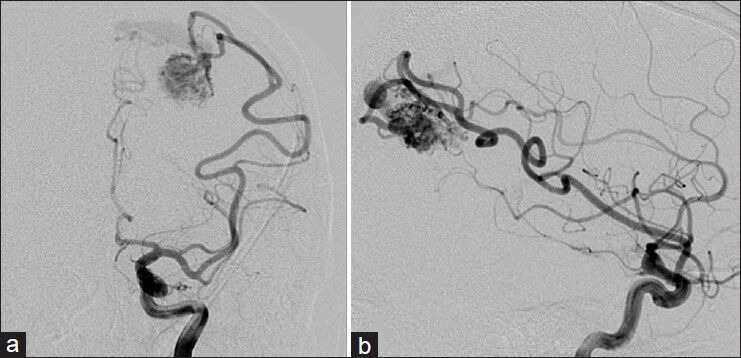
AP (a) and Lateral (b) left internal carotid injections demonstrating left parietal AVM
Procedure
After a thorough discussion about the natural history and treatment options (observation, microsurgical, and radiosurgery), the patient elected to have the AVM resected. The patient underwent presurgical Onyx (eV3 Neurovascular Inc., Irvine, CA) embolization of the AVM followed by a left parietal craniotomy and resection of the AVM without complication or morbidity. Postoperative cerebral angiography demonstrated no residual AVM nidus or early venous drainage. The hospital course was unremarkable and the patient was discharged home on postoperative day three. At 6 months follow-up, the patient remained seizure free and not on antiepileptic medications.
DISCUSSION
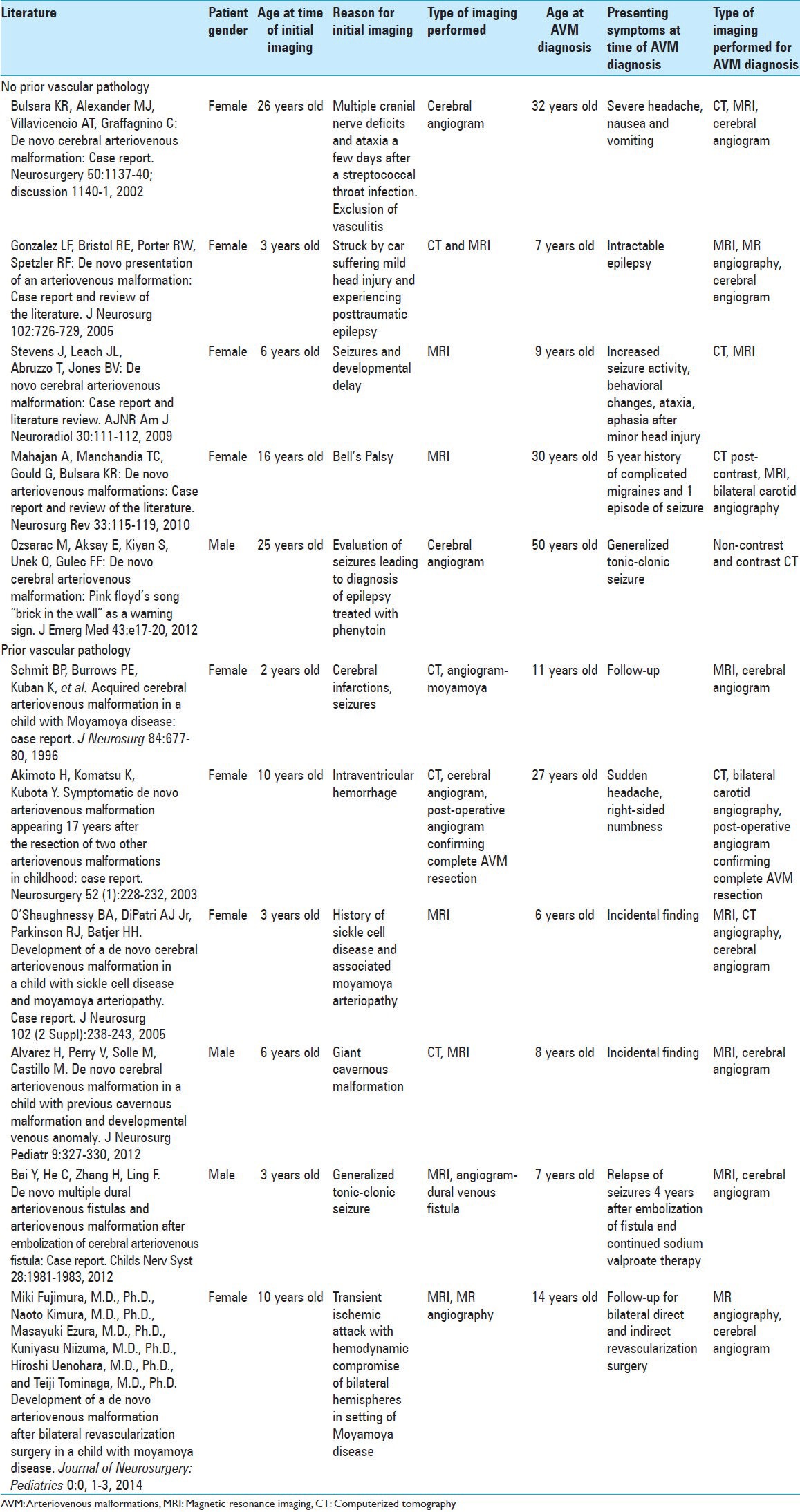
Although cerebral AVMs have traditionally been considered congenital lesions, there is an increasing number of case reports of de novo AVMs [Table 1]. To date, there are nine other cases that have reported these phenomena. Of these nine cases, seven reported the occurrence of de novo AVMs in patients that had other vascular pathology, such as prior resection of AVMs in different locations during childhood.[1,3,4,13,16,17,18] The majority of those cases were documented in pediatric patients. The literature review yielded five cases that reported de novo AVM development in patients without vascular pathology on prior MRI, as is the case in this patient.[5,9,11,14,20] Of these de novo AVM cases, only two of the reported cases involved adult patients.[5,14] Together, with the case we have presented in this paper, these reports comprised a very small, but growing body of literature that challenged the traditional view of the pathogenesis of cerebral AVMs.
Table 1.
Previously reported cases of de novo AVM formation
The pathogenesis of AVMs remains ill defined. It is a widespread belief that AVMs are congenital, arising between weeks 4-8 of embryological growth.[8] The symptomatic presentation of AVMs in adults before the age of 40, in addition to the de novo AVMs reported in children, support the concept of the temporal vulnerability of vascular elements to a physiologic or environmental trigger.[20] These triggers, which can be mechanical, inflammatory, thrombogenic, ischemic/hypoxic, or hormonal, generally lead to hemodynamic stress.[5,20] Disturbances of the venous drainage system may contribute to the formation of cerebral AVMs. Venous stenosis, occlusion, or agenesis during embryology or chronic venous hypertension during childhood and adulthood can result in tissue hypoxia and drive the impetus for angiogenesis.[12,15,21,22,25] However, the role of the venous hypertension in the growth of an AVM remains indeterminate. Other vascular lesions such as dural or pial arteriovenous fistula in the brain and spine can develop after trauma, infection, or inflammation further support the notion of environmental influences stimulating angiogenesis. Many of these injuries result in the release or increased expression of transforming growth factor (TGF) and vascular endothelial growth factor (VEGF), which play important roles in angiogenesis. The overstimulation of angiogenesis due to these stressors leads to vascular remodeling and other changes resulting in the maturation of AVMs.[24]
Genetic etiologies for cerebral AVMs are also currently being explored. AVMs have been known to occur in the context of certain genetic disorders, such as hereditary hemorrhagic telangiectasia (HHT), Wyburn–Mason syndrome, and Sturge–Weber syndrome. Through studies of knockout mice modeled after the loss-of-function mutations found in HHT, a genetic two-hit mechanism has been proposed for the development of AVMs.[10] In this two-hit model, a loss-of-function mutation of various receptors in the TGF-β family or a dysregulation of nitric oxide synthase activity results in vascular destabilization. This leaves the vasculature more vulnerable to a second hit by environmental factors.
The growing number of sporadic cases of cerebral AVMs still warrants an explanation. Some studies suggest that germ-line mutations of certain proteins may be involved in AVM development.[10,19] Potential target proteins include endothelial angioprotein receptor Tie-2, TGF-β, nitric oxide synthase, vascular endothelial growth factor (VEGF), and fibroblast growth factor. A recent study has also suggested an association between single nucleotide polymorphisms and sporadic cerebral AVM susceptibility, implicating proteins, and cytokines involved in the inflammatory cascade, angiogenesis, vascular remodeling, and stabilization.[23]
Thus the pathomechanism of cerebral AVMs remains incompletely elucidated. The theory of congenital formation of all cerebral AVMs is being challenged as de novo AVMs are increasingly being reported. This case report documents a rare occurrence of a symptomatic de novo AVM in an adult male with no prior vascular cerebral pathology with an initial negative MRI at age 15 who subsequently developed an AVM on later imaging, providing further evidence that not all cerebral AVMs are congenital.
ACKNOWLEDGMENTS
The authors have no personal or institutional interest with regard to the authorship and/or publication of this manuscript.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/148/142796
Contributor Information
Jayson A. Neil, Email: Jayson.neil@gmail.com.
Daphne Li, Email: daphne.li@gmail.com.
Michael F. Stiefel, Email: StiefelM@wcmc.com.
Yin C. Hu, Email: HuY@wcmc.com.
REFERENCES
- 1.Akimoto H, Komatsu K, Kubota Y. Symptomatic de novo arteriovenous malformation appearing 17 years after the resection of two other arteriovenous malformations in childhood: Case report. Neurosurgery. 2003;52:228–31. doi: 10.1097/00006123-200301000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shahi R, Warlow C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain. 2001;124:1900–26. doi: 10.1093/brain/124.10.1900. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez H, Perry V, Solle M, Castillo M. De novo cerebral arteriovenous malformation in a child with previous cavernous malformation and developmental venous anomaly. J Neurosurg Pediatr. 2012;9:327–30. doi: 10.3171/2011.12.PEDS11312. [DOI] [PubMed] [Google Scholar]
- 4.Bai Y, He C, Zhang H, Ling F. De novo multiple dural arteriovenous fistulas and arteriovenous malformation after embolization of cerebral arteriovenous fistula: Case report. Childs Nerv Syst. 2012;28:1981–3. doi: 10.1007/s00381-012-1878-6. [DOI] [PubMed] [Google Scholar]
- 5.Bulsara KR, Alexander MJ, Villavicencio AT, Graffagnino C. De novo cerebral arteriovenous malformation: Case report. Neurosurgery. 2002;50:1137–40. doi: 10.1097/00006123-200205000-00036. [DOI] [PubMed] [Google Scholar]
- 6.Farhat HI. Cerebral arteriovenous malformations. Dis Mon. 2011;57:625–37. doi: 10.1016/j.disamonth.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356:2704–12. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 8.Garretson H. New York: McGraw-Hill; 1996. Intracranial arteriovenous malformations, in Wilkins RH RS: Neurosurgery; pp. 2433–42. [Google Scholar]
- 9.Gonzalez LF, Bristol RE, Porter RW, Spetzler RF. De novo presentation of an arteriovenous malformation: Case report and review of the literature. J Neurosurg. 2005;102:726–9. doi: 10.3171/jns.2005.102.4.0726. [DOI] [PubMed] [Google Scholar]
- 10.Leblanc GG, Golanov E, Awad IA, Young WL. Biology of Vascular Malformations of the Brain NINDS Workshop Collaborators: Biology of vascular malformations of the brain. Stroke. 2009;40:e694–702. doi: 10.1161/STROKEAHA.109.563692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahajan A, Manchandia TC, Gould G, Bulsara KR. De novo arteriovenous malformations: Case report and review of the literature. Neurosurg Rev. 2010;33:115–9. doi: 10.1007/s10143-009-0227-z. [DOI] [PubMed] [Google Scholar]
- 12.Nussbaum ES, Heros RC, Madison MT, Awasthi D, Truwit CL. The pathogenesis of arteriovenous malformations: Insights provided by a case of multiple arteriovenous malformations developing in relation to a developmental venous anomaly. Neurosurgery. 1998;43:347–51. doi: 10.1097/00006123-199808000-00103. [DOI] [PubMed] [Google Scholar]
- 13.O'shaughnessy BA, DiPatri AJ, Jr, Parkinson RJ, Batjer HH. Development of a de novo cerebral arteriovenous malformation in a child with sickle cell disease and moyamoya arteriopathy: Case report. J Neurosurg. 2005;102(2 Suppl):S238–43. doi: 10.3171/jns.2005.102.2.0238. [DOI] [PubMed] [Google Scholar]
- 14.Ozsarac M, Aksay E, Kiyan S, Unek O, Gulec FF. De novo cerebral arteriovenous malformation: Pink floyd's song “brick in the wall” as a warning sign. J Emerg Med. 2012;43:e17–20. doi: 10.1016/j.jemermed.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Pietila TA, Zabramski JM, Thellier-Janko A, Duveneck K, Bichard WD, Brock M, et al. Animal model for cerebral arteriovenous malformation. Acta Neurochir (Wien) 2000;142:1231–40. doi: 10.1007/s007010070019. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Arias C, Martinez R, Rey G, Bravo G. Recurrence in a different location of a cerebral arteriovenous malformation in a child after radiosurgery. Childs Nerv Syst. 2000;16:363–5. doi: 10.1007/s003810050532. [DOI] [PubMed] [Google Scholar]
- 17.Schmit BP, Burrows PE, Kuban K, Goumnerova L, Scott RM. Acquired cerebral arteriovenous malformation in a child with moyamoya disease: Case report. J Neurosurg. 1996;84:677–80. doi: 10.3171/jns.1996.84.4.0677. [DOI] [PubMed] [Google Scholar]
- 18.Song JK, Niimi Y, Kupersmith MJ, Berenstein A. Postnatal growth and development of a cerebral arteriovenous malformation on serial magnetic resonance imaging in a child with hemangiomatosis: Case report. J Neurosurg. 2007;106(5 Suppl):S384–7. doi: 10.3171/ped.2007.106.5.384. [DOI] [PubMed] [Google Scholar]
- 19.Stapf C, Mohr JP. New concepts in adult brain arteriovenous malformations. Curr Opin Neurol. 2000;13:63–7. doi: 10.1097/00019052-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Stevens J, Leach JL, Abruzzo T, Jones BV. De novo cerebral arteriovenous malformation: Case report and literature review. AJNR Am J Neuroradiol. 2009;30:111–2. doi: 10.3174/ajnr.A1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streeter G. The development of the venous sinus of the dura mater in the human embryo. Am J Anat. 1915;18:145–78. [Google Scholar]
- 22.Streeter G. The developmental alterations in the vascular system of the brain of the human embryo. Contrib Embryol. 1918;8:5–38. [Google Scholar]
- 23.Sturiale CL, Puca A, Sebastiani P, Gatto I, Albanese A, Di Rocco C, et al. Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: Where do we stand? Brain. 2013;136:665–81. doi: 10.1093/brain/aws180. [DOI] [PubMed] [Google Scholar]
- 24.Sure U, Butz N, Schlegel J, Siegel AM, Wakat JP, Mennel HD, et al. Endothelial proliferation, neoangiogenesis, and potential de novo generation of cerebrovascular malformations. J Neurosurg. 2001;94:972–7. doi: 10.3171/jns.2001.94.6.0972. [DOI] [PubMed] [Google Scholar]
- 25.Wilson CB. Cryptic vascular malformations. Clin Neurosurg. 1992;38:49–84. [PubMed] [Google Scholar]


