Abstract
Many therapeutic targets are cell surface receptors, which can be challenging antigens for antibody generation. For many therapeutic applications, one needs antibodies that not only bind the cell surface receptor but also are internalized into the cell. This allows use of the antibody to deliver various payloads into the cell to achieve a therapeutic effect. Phage antibody technology has proven a powerful tool for the generation and optimization of human antibodies to any antigen. While applied to the generation of antibodies to purified proteins, it is possible to directly select cell binding and internalizing antibodies on cells. Potential advantages of this approach include: cell surface receptors are in native conformation on intact cells while this might not be so for recombinant proteins; antibodies can be selected for both cell binding and internalization properties; the antibodies can be used to identify their tumor associated antigens; and such antibodies can be used for human treatment directly since they are human in sequence.
This review will discuss the factors that impact the successful selection of cell binding and internalizing antibodies. These factors include the cell types used for selection, the impact of different phage antibody library formats, and the specific selection protocols used.
Keywords: Phage antibody, antibody internalization, targeted drug delivery, cell selection, flow cytometry
Introduction
Cell surface membrane proteins have proven to be important targets for the development of monoclonal antibody (mAb) therapies. At least 17 FDA approved therapeutic antibodies bind cell surface proteins with more than half binding tumor antigens and being used to treat cancers (Table 1). However cell surface proteins associated with inflammation or clotting have also been targeted to develop treatments for cardiovascular disease, transplant rejection, multiple sclerosis, Crohn's disease and rheumatoid arthritis (Table 1). Most of the antibodies are ‘naked’ IgG and work by binding the surface receptor and blocking signaling or cell-to-cell communication. This is thought to be the primary mode of action of cancer mAbs binding EGFR (cetuximab and panitumumab) [1-3], HER2 (trastuzumab) [4], as well as many of the other mAbs in Table 1. Naked IgG can also elicit antibody dependent cellular cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC), which is a major mechanism of action of anti-CD20 mAbs [5, 6] (which also induce apoptosis [7]) and may contribute to the action of trastuzumab and cetuximab [8, 9].
Table 1.
FDA approved antibodies binding cell surface proteins.
| Generic name | Brand name | Date approved | Mab Type | mAb target | Disease indication |
|---|---|---|---|---|---|
| Abciximab | ReoPro | 1994 | chimeric | inhibition of GP IIb/IIIa | Cardiovascular disease |
| Alemtuzumab | Campath | 2001 | humanized | CD52 | Chronic lymphocytic leukemia |
| Basiliximab | Simulect | 1998 | chimeric | IL-2Rα receptor (CD25) | Transplant rejection |
| Brentuximab vedotin | Adcetris | 2011 | chimeric | CD30 | Anaplastic large cell lymphoma (ALCL) and Hodgkin lymphoma (conjugated with auristatin E) |
| Cetuximab | Erbitux | 2004 | chimeric | EGFR | Colorectal cancer, Head and neck cancer |
| Daclizumab | Zenapax | 1997 | humanized | IL-2Rα receptor (CD25) | Transplant rejection |
| Gemtuzumab | Mylotarg | 2000 | humanized | CD33 | Acute myelogenous leukemia (conjugated with calicheamicin) |
| Ibritumomab tiuxetan | Zevalin | 2002 | murine | CD20 | Non-Hodgkin lymphoma (conjugated with yttrium-90 or indium-111) |
| Ipilimumab | Yervoy | 2011 | human | blocks CTLA-4 | Melanoma |
| Muromonab-CD3 | Orthoclone OKT3 | 1986 | murine | T cell CD3 Receptor | Transplant rejection |
| Natalizumab | Tysabri | 2006 | humanized | alpha-4 (α4) integrin | Multiple sclerosis and Crohn's disease |
| Ofatumumab | Arzerra | 2009 | human | CD20 | Chronic lymphocytic leukemia |
| Panitumumab | Vectibix | 2006 | human | EGFR | Colorectal cancer |
| Rituximab | Rituxan, Mabthera | 1997 | chimeric | CD20 | Non-Hodgkin lymphoma |
| Tocilizumab (or Atlizumab) | Actemra & RoActemra | 2010 | humanized | Anti- IL-6R | Rheumatoid arthritis |
| Tositumomab | Bexxar | 2003 | murine | CD20 | Non-Hodgkin lymphoma (conjugated with I131 |
| Trastuzumab | Herceptin | 1998 | humanized | ErbB2 | Breast cancer |
The next generation of “armed” tumor-specific antibodies and antibody fragments are in clinical trials and entering clinical practice. Such antibodies typically have enhanced effector activity, either via engineered Fc receptors that more efficiently active ADCC and CDC or by fusions to radionuclides, toxins or chemotherapeutic agents for targeted drug delivery [10-12]. For example, radiolabelled CD20 mAbs are approved for treating non-Hodgkins lymphoma [13], and the FDA recently approved brentuximab vedotin (anti-CD30 conjugated with auristatin E) for the treatment of anaplastic large cell lymphoma [14, 15]. For toxin or chemotherapy fusions, it is essential that the antibody not only bind to a cell surface receptor, but that the antibody and its fusion partner undergo endocytosis so that the drug or toxin payload can be delivered to the cytosol.
Antibodies currently entering clinical trials are either humanized or fully human in sequence, in order to avoid immunogenicity [16]. Humanized antibodies are derived from murine mAbs generated by rodent immunization and hyrbidoma technology [17]. Human antibodies are generated using hybridoma technology and rodents transgenic for the human immunoglobulin locus or via display technologies such as phage, yeast or ribosome display [18-21]. For example, large non-immune human (naïve) antibody gene diversity libraries displayed on filamentous phage have proven a reliable source of human antibodies to any purified protein antigen [21, 22].
One challenge of these routes to therapeutic antibodies is that purified protein is generally required. Cell surface membrane proteins, however, generally do not fold properly in the bacterial cytosol, necessitating use of the bacterial secretion system for expression. The presence of multiple disulfide bonds in the extracellular domains of type 1 and type 2 membrane proteins is typical, and their large size makes expression yields in bacteria frequently too low to be useful. This can be partially overcome by expressing protein domains, however it is often necessary to express these proteins, or domains of these proteins, in either insect or mammalian cells. These are relatively time consuming expression systems with variable yields. In addition, unique expression strategies are typically required for each different protein antigen. Multipass transmembrane proteins are even more difficult to express and purify. Their large hydrophobic transmembrane domains mean they must be harvested from membrane fractions and purified in the presence of detergents [23]. It is not uncommon for them to lose their conformation during the purification process. Loss of conformation makes generation of antibodies recognizing the native conformation unlikely. In addition, some purified proteins are poor mimics of the protein conformation present on the cell surface. Finally, many membrane proteins are evolutionarily conserved, limiting the robustness of the immune response when the protein is used as an immunogen.
Selection of cell binding antibodies from phage antibody libraries
A different approach for antibody generation is to use cells expressing the cell surface protein of interest rather than purified protein. For example, a number of mAbs from hybridomas have been generated by immunizing rodents with tumor cells [24]. Alternatively, it has proven possible to generate mAbs to cell surface antigens by directly selecting phage antibody libraries on cells [25-29]. In this approach, phage antibody libraries are incubated with target cells, non-binding phage removed by washing, and specifically bound phage eluted (Figure 1B). Eluted phage are then amplified by infecting and culturing E. coli, and the process repeated for several rounds. In the first such example, a panel of single chain Fv (scFv) antibodies were generated whose specificity could be deciphered using panels of erythrocytes with known antigen expression profiles. scFv binding and specifically agglutinating erythrocytes expressing the blood group B antigen (500,000 sites/cell), Rh(D) and Rh(E) (20,000 sites/cell) and the Kpb antigen (5,000 sites/cell) were isolated [30, 31]. The Rh(D) and Rh(E) antigens are multispanning membrane proteins with 10 and 12 transmembrane domains respectively and have hypothesized channel or pore transporter activity. Variations of direct cell selection have yielded a large number of cell type and antigen specific antibodies binding cell surface receptors (Tables 2 and 3).
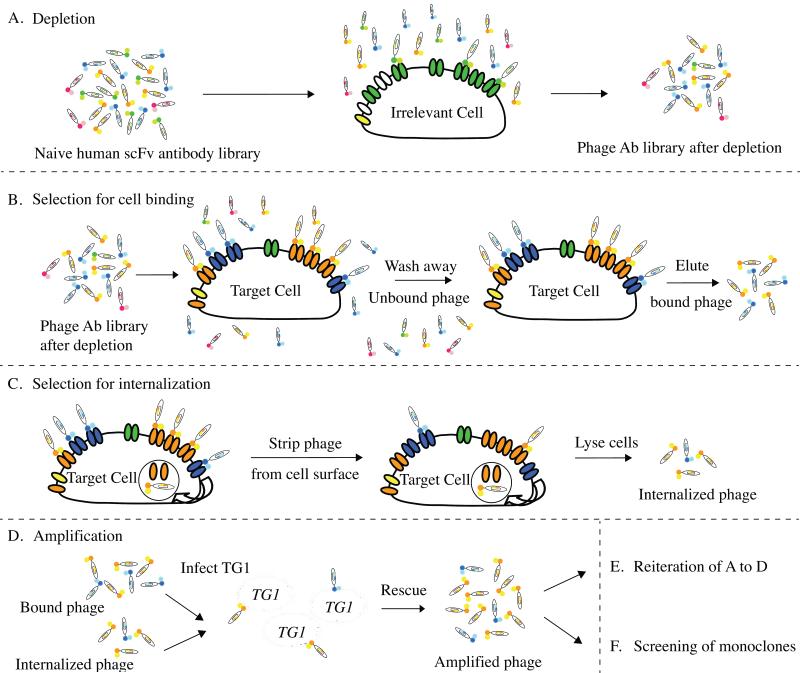
Figure 1. Strategy for selecting cell binding and internalizing phage antibodies.
(A) Deplete the naïve human scFv antibody library by incubating with the control cells; (B) incubate the depleted phage library with the target cell followed by washing the cells to remove the unbound phage and eluting the surface bound phage; alternatively, (C) after the removal of unbound phage, incubate cells at 37°C to allow endocytosis of the receptor and the bound phage followed by stripping off the surface bound phage with low pH buffer, and cell lysis to recover the endocytosed phage from inside the cells; (D) amplify phage antibodies from either B or C; (E) repeat selections for 2-3 rounds; (F) screening of monoclonal phage antibodies.
Table 2.
Published examples of selection of phage antibody libraries on cells.
| Cell type | Antibody library | Selection protocol | Specificity | Reference |
|---|---|---|---|---|
| Human red blood cels | Human naïve scFv | Cell Binding | Anti-B, D (3), HI, E, Kpb | [29] |
| Human peripheral leukocytes | Semi-synthetic scFv | Cell Binding | Sub-populations of leukocytes | [28] |
| Melanoma cells | Melanoma immunized scFv (multivalent) | Cell Binding | Melanoma-specific, tumor-specific, lineage-specific | [27] |
| CD36/CHO myoblast | Human naïve scFv (Vaughan) | Cell Binding | CD36 | [44] |
| Human breast cancer cell line SKBR3 | Human naïve scFv (Sheets, monovalent) | Cell Internalizing | HER2, Transferrin receptor | [33] |
| Tumor cell line A431 and EGFR/CHO cells | Human naïve scFv (Sheets, monovalent) | Cell Internalizing | EGFR | [32] |
| Human fetal erythroid cells | Human naïve scFv (Sheets, multivalent) | Cell Binding | Fetal nucleated red blood cells | [47] |
| Human colorectal cancer cell lines | Colorectal cancer cell immunized Fab | Density gradient centrifugation | Colorectal cancer | [43] |
| Human tumor cell line 22Rv1, SW1990, HPAFII | Human naïve scFv (Gao, 1999) | Cell Internalizing | Transferrin receptor, Integrein α3β1 | [34] |
| Human prostate Cancer cell lines | Human naïve scFv (Sheets, multivalent) | Cell Internalizing | Prostate cancer cells | [35] |
| Human Leukemia cell lines | Human naïve Fab (Dyax) | Cell Internalizing | FLT3 | [53] |
| Human tumor sections | Human naïve scFv (Sheets, multivalent) | Laser capture microdissection | Prostate cancer cells | [38] |
| EGFR/CHO Cells | Human naïve scFv (MRC) | Cell Internalizing | EGFR | [54] |
| 33 tumor cell lines | Human naïve scFv-CL-GFP (Morino) | Cell binding with organic solvent separation | 21 Tumor associated antigens | [41] |
| Mesothelioma cells | Human naïve scFv (Sheets, multivalent) | Cell Internalizing | CD146 | [39] |
| Brain tumor sphere cells | Human naïve scFv (Sheets, multivalent) | Cell Internalizing | Glioblastoma multiforme sphere cells | [37] |
| Tumor cell line MDAMB231 | Human naïve scFv (Sheets, multivalent) | Cell Internalizing | EphA2, CD44 | [36] |
Table 3.
Internalizing antibodies to specific cell surface proteins isolated by selection on cells.
| Cell surface protein | Antibody | Activity | Reference |
|---|---|---|---|
| HER2 | F5 | Targeted Liposomal drug | [33] |
| Transferrin receptor | H7, PR5 | [33], [34] | |
| EGFR | C10 and affinity F4 | [32], [54] | |
| CD146 | M1 | Targeted Liposomal drug | [39] [49] |
| CD166 | I/F8 H3 |
Immunotoxin Targeted Liposomal drug |
[51] [35], [52] |
| ICAM-1 | M10A12 | Block cell invasion | [35], [50] |
| FLT3 | EB10, A2IN, D4-3 | Suppress cell growth | [53] |
| Integrein α3β1 | SW1, PAN10 | SW1 mimic ligand | [34] |
| EphA2 | D2-1A7, D2-1A9 | [36] | |
| CD44 | F2-1A6, F2-1H9 | [36] |
Cell selections can be performed to either isolate antibodies binding a specific cell surface receptor or to generate a panel of antibodies binding any surface proteins that are over-expressed or preferentially expressed on a target cell type (for example cancer cells). To generate antibodies to a specific cell surface receptor, a cell line with high level expression of the target antigen is used. This could be an immortalized cell line, for example a tumor cell line, or a transfected cell line. For example, we generated antibodies binding EGFR and HER2 by selecting phage antibody libraries on tumor cell lines overexpressing EGFR or HER2, or a cell line transfected with EGFR [32, 33]. ELISA using recombinant antigen (EGFR or HER2) allowed identification of antigen specific phage antibodies. Alternatively, antigen specific phage antibodies can be identified by using transfected and untransfected cells.
Panels of antibodies binding any surface proteins that are over-expressed or preferentially expressed on a target cell type can also be accomplished using the cell selection strategy shown in Figure 1. One or more target cell lines of the desired phenotype (for example basal subtype breast cancer) are used for selection. In theory, the use of intact cells to select cell-binding antibodies from phage display antibody library could generate antibodies to all cell surface antigens, especially for integral membrane proteins, which are difficult to express in native forms. By contrasting the binding of antibodies to a panel of related and unrelated cell types, large panels of potential tumor targeting antibodies can be generated [27, 32-36]. To generate such mAb panels, multiple forms of target cells have been used. These forms include target transfected cells, cancer cell lines in suspension culture or adherent cell culture [27, 33-35]; primary tumor cell spheres [37], and paraffin embedded or frozen tissue sections [38]. Compared to primary tumor cells, cancer cell lines may possess surface receptors that emerged during the process of being removed from tumor environment, continuous culture and immortalization. Nevertheless, the large cohort of established cancer cell lines are useful sources of tumor-associated antigens for cell panning to isolate anti-tumor mAbs [34, 36, 39-41]
Different strategies have also been used to either reduce background binding of phage or to achieve more specific elution of bound phage. These include the use of organic solvent separation [41, 42], cell density gradient centrifugation [43], and laser capture microdissection [38]. Separation of cell bound and free phages by density gradient centrifugation can minimize the non-specific removal of bound phage by shear forces during washing, may allow rebinding of dissociated phages, and can enhance the selectivity of cell-selections. The use of laser capture microdissection may be an ideal protocol to isolate clinically relevant anti-tumor antibodies that can function in immunohistochemistry, but is complicated by the need to recover antibody gene sequences by PCR, due to the destruction of phage by the laser.
The isolation of cell type specific antibodies from large phage antibody libraries can prove technically challenging because selections often result in the generation of cross reactive antibodies binding to frequently expressed cell surface proteins [44]. Thus, prior to positive selection on the target cells, antibodies recognizing common cell surface molecules need to be removed by pre-absorbing the phage antibody library using control cells; this step is termed “depletion” (Figure 1A). The cell line chosen for depletion should be related to the cell line used for positive selection but should lack the cell surface antigen profile that has been targeted for antibody generation. For example, when generating phage antibodies binding specifically to the basal subtype of breast cancers, we used the luminal subtype breast cancer cell line for depletion. Serial repetition of the depletion process for two to six times prior to positive selection on the target cell line may be more effective at removing unwanted phage antibodies. However, we typically do not use a ‘depleting’ cell line prior to the first round of selection to avoid eliminating phage antibodies which might bind receptors overexpressed on the target cell line, but which are also present at lower density on the subtracting cell line and might be removed during the depletion process. This is especially important in the first round of selection, where each individual phage antibody is present at a relatively low copy number. Extensive depletions of antibody library with control cells have been used in the selection of cell binding antibodies yielding cell type specific antibodies (Table 2).
Selection of cell binding and internalizing antibodies from phage libraries
The ability of phage displaying short peptides to undergo receptor-mediated endocytosis into cells [26, 45] indicated that phage antibody libraries might be selected not only for cell binding but also for internalization into mammalian or other target cells. Such an approach would be especially useful for generating antibodies that could deliver drugs, toxins, or nucleic acids into a cell for therapeutic applications. The approach is similar to that used for selection of cell binding antibodies, but the incubation of target cells with phage occurs at 4°C, followed by removal of unbound phage and then warming of the cells to 37°C so internalization can occur (Figure 1C). After internalization, phage left on the cell surface are stripped by stringent washes, internalized phage recovered by cell lysis, phage amplified by infection E. coli, and the process iterated. Prior to selection, depletion on a different cell type, as described above, is typically performed.
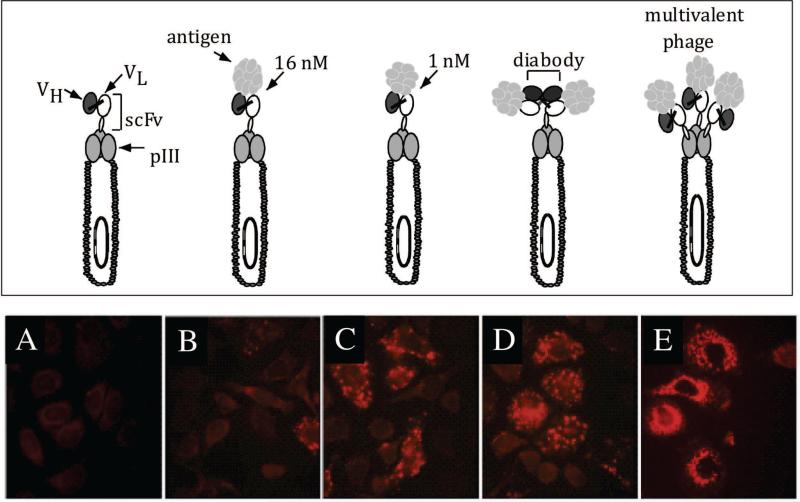
To determine that phage antibodies could be internalized into cells, and to identify the optimal library type, we studied a model system employing an anti-HER2 scFv and HER2 expressing cells (Figure 2). We showed that anti-HER2 phage could be endocytosed by HER2 expressing cells and that cellular uptake of phage required both the targeting scFv and expression of the receptor on cells (Figure 2) [46]. We also showed that enrichment ratios were greater when phage were recovered from within the cell compared to recovery from the cell surface. Enrichment ratios were also higher when the phages were capable of crosslinking the HER2 receptor, rather than merely binding (Figure 2). Crosslinking could be made to occur when either bivalent antibody fragments, such as diabodies, were displayed in a phagemid system, or when monovalent scFv antibody fragments were displayed using a multivalent true phage vector. Thus phage antibody libraries constructed using phage vector systems [47, 48] may prove more useful for generation of internalizing antibodies than antibody fragments displayed using phagemid systems, if they can be large enough in size.
Figure 2. Internalization of phage antibodies into ErbB2 expressing cells.
Top panel: different phage antibody constructs. Bottom panel: immunofluorescent staining of phage major coat protein pVIII showing the localization of phage antibodies when they are displayed in a phagemid vector with 1 copy of scFv per phage (A-D) versus in a phage vector with 3-5 copies of scFv per phage. (A) Control phage antibody (binds BoNT); (B) anti-ErbB2 scFv C6.5; (C) anti-ErbB2 scFv ML3-9 with higher affinity than C6.5; (D) diabody C6.5; (E) scFv C6.5 displayed multivalently in fd phage vector. This figure cited images from reference #46.
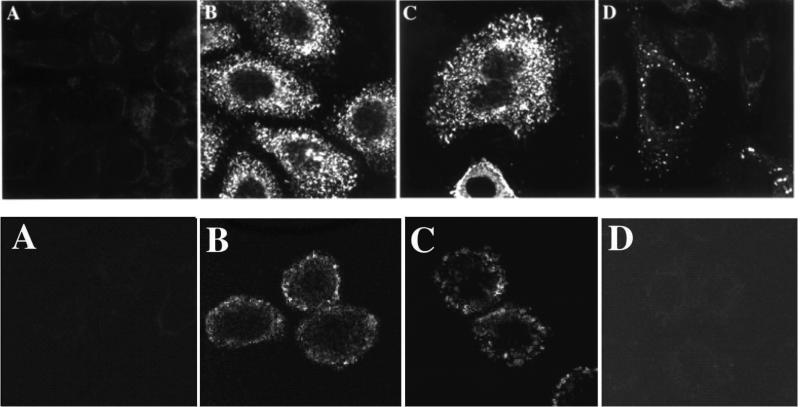
In the first example of this approach, we selected a phagemid scFv antibody library for internalization into the breast cancer cell line SKBR3 [33]. A large panel of antibodies were isolated, all of which were capable of internalizing into the target cells. Since the selecting cells expressed very high levels of HER2, we were able to identify internalizing HER2 antibodies by screening by ELISA for binding to the HER2 extracellular domain. We determined that additional antibodies bound the transferrin receptor by using the single chain Fv antibody to immunoprecipitate antigen that was then sequenced by tandem mass spectrometry [33]. We and others have used this approach to generate internalizing antibodies to a wide range of different cell surface proteins ([32, 33, 35, 36, 39, 49-54](and see Tables 2 and 3). Internalizing antibodies and antibody fragments are rapidly internalized into the target cells and can be used to construct receptor targeted drugs or nucleic acids such as HER2, EGFR, or other surface receptor targeted immunoliposomes (Figure 3) [12, 35, 40, 45, 52, 55-58]. The most advanced of these drugs are HER2 immunoliposomes targeting liposomal doxorubicin to HER2 expressing breast cancers (Nielsen, Kirpotin et al. 2002; Park, Hong et al. 2002). Anti-HER2 liposomal doxorubicin entered phase 1 clinical trial as MM-302 in 2010 [59].
Figure 3. HER2 and transferrin receptor phage antibodies and scFv are internalized into cells.
Top panel: Confocal microscopy with detection of phage antibodies using anti-pVIII antibody. A. Control phage antibody, binds botulinum neurotoxin. B. F5 anti-HER2 phage antibody selected for cellular endocytosis. C. Anti-transferrin phage antibody selected for cellular endocytosis. D. C6.5 anti-ErbB2 phage antibody selected on recombinant ErbB2 [61].
Bottom panel: Confocal microscopy with detection of scFv using an antibody to a Cterminal peptide tag on the scFv. A. Control scFv, binds botulinum neurotoxin. B. F5 scFv selected for cellular endocytosis. C. Anti-transferrin scFv H7 selected for cellular endocytosis. D. C6.5 anti-ErbB2 scFv selected on recombinant ErbB2 [61]. This figure cited images from reference #33.
Identification of antigens bound by cell binding and internalizing antibodies
One of the greatest challenges in selecting cell specific binding and internalizing antibodies is the identification of the antigen recognized by the antibodies. In some instances, it is possible to use the antibody to immunoprecipitate the antigen from cell lysates [33, 34, 40, 41, 60]. For example, we identified human transferrin receptor as the antigen bound by the H7 antibody by biotinylating the surface of the cell line used for selection, lysing the cells, immunoprecipitating the cell lysate using the H7 scFv attached to beads, running an SDS-PAGE to separate the proteins, identifying the area of interest on the gel using streptavidin-HRP, excising the band, and performing mass spectrometry sequencing [33]. This approach requires using a cell line with high-level expression of the target antigen. Screening a number of cell lines using the relevant antibody and flow cytometry analysis can identify the appropriate cell line.
Alternatively, cDNA cell surface expression libraries constructed in mammalian or yeast cells can be screened to identify the antigen bound by the antibody [49]. We have also used the antibody of interest to stain a panel of cell lines that have been transcriptionally profiled for the expression levels of 40,000 genes. The mean fluorescent intensity for cell staining is normalized and correlated with gene transcript levels to identify the most correlated genes. These genes can then be displayed on the surface of yeast or mammalian cells to determine if this is the antigen recognized by the antibodies [36]. We have also been able to direct selections for internalization to specific tumor antigens. Several rounds of selection are performed on a tumor cell line followed by selection on the desired target antigen displayed on the surface of yeast. The resulting antibodies bind the target antigen and are internalized into mammalian cells [36].
Characteristics of cell binding and internalizing antibodies
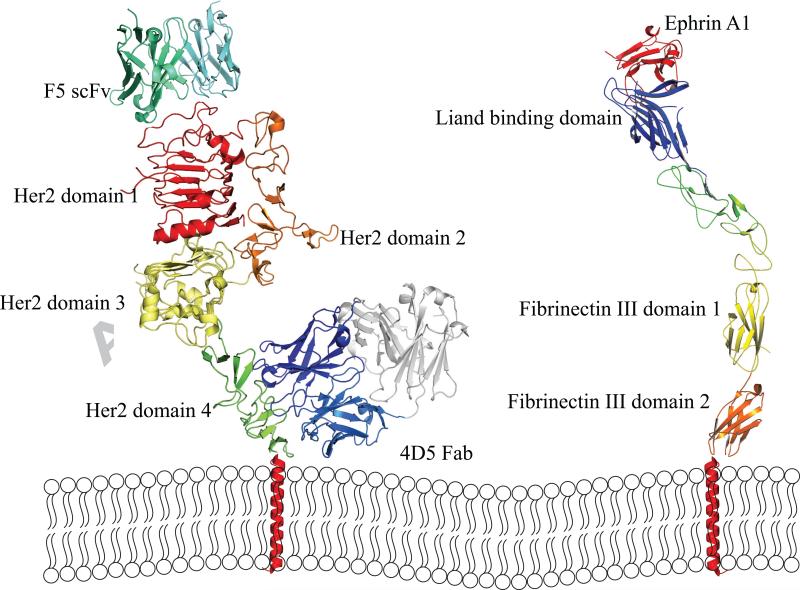
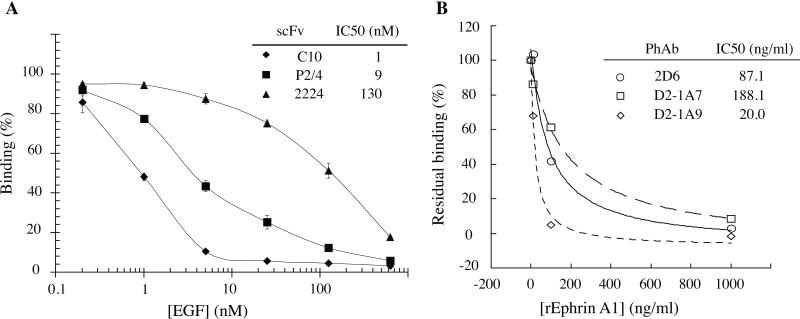
Analysis of antibodies binding cell surface receptors by selection of phage display antibody library on cells suggests a number of common antibody features [32, 33, 35, 36, 39, 49-54](Table 2). Many antibodies seem to bind dominant overlapping epitopes on the target receptors regardless of the receptor size. For example, the selection of internalizing antibodies to breast cancer SKBR3 cells from naïve human scFv library resulted in 2 dominant clones F5 and C1, which bind an overlapping epitope on HER2 [33]. No antibodies were identified to other HER2 epitopes, such as those recognized by the antibodies C6.5 or 4D5 [33]. C6.5 was isolated by biopanning of a naïve human scFv phage display library on immuno-tubes coated with recombinant ErbB2 extracellular domain [61]; while 4D5 was isolated from a hybridoma generated from a mouse immunized with NIH3T3/HER2-3400 cells [24]. The epitope on HER2 bound by F5 is on the upper surface of the HER2 domain 1, farthest from the membrane, while 4D5 binds domain 4, the closest domain to the cell membrane (Figure 4). Similarly, selection-dominant epitopes were observed for CD36 [44] and EphA2 [36]. In the case of the EphA2 antibodies, all bound the most membrane distal ligand-binding domain (Figure 4). This also appears to be a feature of other cell-selected antibodies; many bind near the ligand binding sites. For example EGFR and EphA2 monoclonal antibodies isolated from cell panning competed with cell-binding of the natural ligand EGF and Ephrin A1 respectively [32, 36, 58] (Figure 5), suggesting that such epitopes are also more accessible on the cell surface than others. It is perhaps not surprising that cell panning resulted in the selection of antibodies to a limited number of epitopes, those being epitopes that are most accessible on the cell surface. There are also instances where receptors do not display any epitopes accessible for phage antibodies to bind on cells [44]. Thus for some targets the approach may not be successful.
Figure 4. Cartoon of epitopes of internalizing phage antibodies binding HER2 and EphA2.
Left panel: Epitopes of F5 phage antibody and 4D5 Fab (Herceptin epitope). F5 binds the most membrane distal domain 1. Right panel: Epitopes of internalizing EphA2 phage antibodies. All phage antibodies compete with ephrin A1 ligand binding and thus bind to the membrane distal ligand-binding domain. The cartoon structures were adopted from PDB file 1N8Z, 2X10, and 3CZU.
Figure 5. Effect of ligand on the binding of EGFR and EphA2 antibodies to cell surface receptors.
(a) Binding of EGFR scFv C10 (◇), P2/4 (■), and 2224 (sh-utrif) to MDAMB468 cells in the presence of increasing concentrations of EGFR ligand, EGF was determined by flow cytometry. (b) EphA2 antibodies D2-1A7 (□), D2-1A9 (◇), and 2D6 (sh=cir) compete with ephrin A1 for binding to MDAMB231 cells. Ability of phage antibodies D2-1A7, D2-1A9, and 2D6 binding to MDAMB231 cells in the presence of increasing concentrations of EphA2 ligand, Ephrin A1 was determined by flow cytometry. This figure cited images from reference #36.
In conclusion, the direct selection of phage antibody libraries for cell binding or internalization is a powerful approach for generating antibodies binding cell surface proteins in their native conformations. The major challenge for this approach is the identification of the antigens bound by the antibodies. This issue can be addressed by using the antibody for immunoprecipitation followed by mass spectrometry sequencing, by screening antigen cDNA libraries, or by sequential selections on cells followed by selection on specific target antigens displayed on cells or as recombinant protein. Resulting antibodies bind cells well and when selected for internalization, appear to be residualized more within cells than antibodies generated by other approaches. This may be due to a combination of binding highly accessible epitopes and with lower affinity. We expect that this approach will continue to prove highly useful for generating cell binding and internalizing antibodies.
Selection of cell binding antibodies from phage antibody libraries
Selection of cell binding and internalizing antibodies from phage libraries
Identification of antigens bound by cell binding and internalizing antibodies
Characteristics of cell binding and internalizing antibodies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 2.Gibson TB, Ranganathan A, Grothey A. Clinical colorectal cancer. 2006;6:29–31. doi: 10.3816/CCC.2006.n.01. [DOI] [PubMed] [Google Scholar]
- 3.Weiner LM, Belldegrun AS, Crawford J, Tolcher AW, Lockbaum P, Arends RH, Navale L, Amado RG, Schwab G, Figlin RA. Clin Cancer Res. 2008;14:502–508. doi: 10.1158/1078-0432.CCR-07-1509. [DOI] [PubMed] [Google Scholar]
- 4.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 5.Dall'Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, Watier H, Thibault G. Cancer Res. 2004;64:4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien S, Osterborg A. Clin Lymphoma Myeloma Leuk. 2010;10:361–368. doi: 10.3816/CLML.2010.n.069. [DOI] [PubMed] [Google Scholar]
- 7.Shan D, Ledbetter JA, Press OW. Blood. 1998;91:1644–1652. [PubMed] [Google Scholar]
- 8.Hudis CA. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn J. Clin Cancer Res. 2000;6:747–753. [PubMed] [Google Scholar]
- 10.Kreitman RJ, Pastan I. Current drug targets. 2006;7:1301–1311. doi: 10.2174/138945006778559139. [DOI] [PubMed] [Google Scholar]
- 11.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, Vielmetter J, Carmichael DF, Hayes RJ, Dahiyat BI. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen UB, Kirpotin DB, Pickering EM, Hong K, Park JW, Refaat Shalaby M, Shao Y, Benz CC, Marks JD. Biochim Biophys Acta. 2002;1591:109–118. doi: 10.1016/s0167-4889(02)00256-2. [DOI] [PubMed] [Google Scholar]
- 13.Kaminski MS, Zelenetz AD, Press OW, Saleh M, Leonard J, Fehrenbacher L, Lister TA, Stagg RJ, Tidmarsh GF, Kroll S, Wahl RL, Knox SJ, Vose JM. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 14.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, Francisco JA, Wahl AF, Meyer DL, Senter PD. Nature biotechnology. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 15.FDA approves Adcetris to treat two types of lymphoma. Aug;19:2011. [Google Scholar]
- 16.Reichert JM, Valge-Archer VE. Nature reviews. Drug discovery. 2007;6:349–356. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 17.Kohler G, Milstein C. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 18.Feldhaus MJ, Siegel RW. Journal of immunological methods. 2004;290:69–80. doi: 10.1016/j.jim.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Hanes J, Pluckthun A. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4937–4942. doi: 10.1073/pnas.94.10.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonberg N, Huszar D. International reviews of immunology. 1995;13:65–93. doi: 10.3109/08830189509061738. [DOI] [PubMed] [Google Scholar]
- 21.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. Journal of molecular biology. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 22.Sheets MD, Amersdorfer P, Finnern R, Sargent P, Lindquist E, Schier R, Hemingsen G, Wong C, Gerhart JC, Marks JD. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6157–6162. doi: 10.1073/pnas.95.11.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daulat AM, Maurice P, Froment C, Guillaume JL, Broussard C, Monsarrat B, Delagrange P, Jockers R. Molecular & cellular proteomics : MCP. 2007;6:835–844. doi: 10.1074/mcp.M600298-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Fendly BM, Winget M, Hudziak RM, Lipari MT, Napier MA, Ullrich A. Cancer Res. 1990;50:1550–1558. [PubMed] [Google Scholar]
- 25.Andersen PS, Stryhn A, Hansen BE, Fugger L, Engberg J, Buus S. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1820–1824. doi: 10.1073/pnas.93.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barry MA, Dower WJ, Johnston SA. Nature medicine. 1996;2:299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 27.Cai X, Garen A. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:6537–6541. doi: 10.1073/pnas.92.14.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Kruif J, Terstappen L, Boel E, Logtenberg T. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3938–3942. doi: 10.1073/pnas.92.9.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks JD, Ouwehand WH, Bye JM, Finnern R, Gorick BD, Voak D, Thorpe SJ, Hughes-Jones NC, Winter G. Biotechnology (n Y) 1993;11:1145–1149. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- 30.Hughes-Jones NC, Gorick BD, Bye JM, Finnern R, Scott ML, Voak D, Marks JD, Ouwehand WH. British journal of haematology. 1994;88:180–186. doi: 10.1111/j.1365-2141.1994.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 31.Marks JD, Ouwehand WH, Bye JM, Finnern R, Gorick BD, Voak D, Thorpe S, Hughes-Jones NC, Winter G. Bio/Technology. 1993;11:1145–1149. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- 32.Heitner T, Moor A, Garrison JL, Marks C, Hasan T, Marks JD. Journal of immunological methods. 2001;248:17–30. doi: 10.1016/s0022-1759(00)00340-9. [DOI] [PubMed] [Google Scholar]
- 33.Poul MA, Becerril B, Nielsen UB, Morisson P, Marks JD. Journal of molecular biology. 2000;301:1149–1161. doi: 10.1006/jmbi.2000.4026. [DOI] [PubMed] [Google Scholar]
- 34.Gao C, Mao S, Ronca F, Zhuang S, Quaranta V, Wirsching P, Janda KD. Journal of immunological methods. 2003;274:185–197. doi: 10.1016/s0022-1759(02)00522-7. [DOI] [PubMed] [Google Scholar]
- 35.Liu B, Conrad F, Cooperberg MR, Kirpotin DB, Marks JD. Cancer Res. 2004;64:704–710. doi: 10.1158/0008-5472.can-03-2732. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Zou H, Zhang S, Marks JD. Journal of molecular biology. 2010;404:88–99. doi: 10.1016/j.jmb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X, Bidlingmaier S, Hashizume R, James CD, Berger MS, Liu B. Mol Cancer Ther. 2010;9:2131–2141. doi: 10.1158/1535-7163.MCT-09-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruan W, Sassoon A, An F, Simko JP, Liu B. Molecular & cellular proteomics : MCP. 2006;5:2364–2373. doi: 10.1074/mcp.M600246-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.An F, Drummond DC, Wilson S, Kirpotin DB, Nishimura SL, Broaddus VC, Liu B. Mol Cancer Ther. 2008;7:569–578. doi: 10.1158/1535-7163.MCT-07-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goenaga AL, Zhou Y, Legay C, Bougherara H, Huang L, Liu B, Drummond DC, Kirpotin DB, Auclair C, Marks JD, Poul MA. Mol Immunol. 2007;44:3777–3788. doi: 10.1016/j.molimm.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurosawa G, Akahori Y, Morita M, Sumitomo M, Sato N, Muramatsu C, Eguchi K, Matsuda K, Takasaki A, Tanaka M, Iba Y, Hamada-Tsutsumi S, Ukai Y, Shiraishi M, Suzuki K, Kurosawa M, Fujiyama S, Takahashi N, Kato R, Mizoguchi Y, Shamoto M, Tsuda H, Sugiura M, Hattori Y, Miyakawa S, Shiroki R, Hoshinaga K, Hayashi N, Sugioka A, Kurosawa Y. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7287–7292. doi: 10.1073/pnas.0712202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giordano RJ, Cardo-Vila M, Lahdenranta J, Pasqualini R, Arap W. Nature medicine. 2001;7:1249–1253. doi: 10.1038/nm1101-1249. [DOI] [PubMed] [Google Scholar]
- 43.Williams BR, Sharon J. Immunol Lett. 2002;81:141–148. doi: 10.1016/s0165-2478(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 44.Hoogenboom HR, Lutgerink JT, Pelsers MM, Rousch MJ, Coote J, Van Neer N, De Bruine A, Van Nieuwenhoven FA, Glatz JF, Arends JW. Eur J Biochem. 1999;260:774784. doi: 10.1046/j.1432-1327.1999.00214.x. [DOI] [PubMed] [Google Scholar]
- 45.Hart SL, Knight AM, Harbottle RP, Mistry A, Hunger HD, Cutler DF, Williamson R, Coutelle C. J Biol Chem. 1994;269:12468–12474. [PubMed] [Google Scholar]
- 46.Becerril B, Poul MA, Marks JD. Biochem Biophys Res Commun. 1999;255:386–393. doi: 10.1006/bbrc.1999.0177. [DOI] [PubMed] [Google Scholar]
- 47.Huie MA, Cheung MC, Muench MO, Becerril B, Kan YW, Marks JD. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2682–2687. doi: 10.1073/pnas.051631798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Connell D, Becerril B, Roy-Burman A, Daws M, Marks JD. Journal of molecular biology. 2002;321:49–56. doi: 10.1016/s0022-2836(02)00561-2. [DOI] [PubMed] [Google Scholar]
- 49.Bidlingmaier S, He J, Wang Y, An F, Feng J, Barbone D, Gao D, Franc B, Broaddus VC, Liu B. Cancer Res. 2009;69:1570–1577. doi: 10.1158/0008-5472.CAN-08-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conrad F, Zhu X, Zhang X, Chalkley RJ, Burlingame AL, Marks JD, Liu B. J Mol Med (Berl) 2009;87:507–514. doi: 10.1007/s00109-009-0446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piazza T, Cha E, Bongarzone I, Canevari S, Bolognesi A, Polito L, Bargellesi A, Sassi F, Ferrini S, Fabbi M. J Cell Sci. 2005;118:1515–1525. doi: 10.1242/jcs.02280. [DOI] [PubMed] [Google Scholar]
- 52.Roth A, Drummond DC, Conrad F, Hayes ME, Kirpotin DB, Benz CC, Marks JD, Liu B. Mol Cancer Ther. 2007;6:2737–2746. doi: 10.1158/1535-7163.MCT-07-0140. [DOI] [PubMed] [Google Scholar]
- 53.Williams B, Atkins A, Zhang H, Lu D, Jimenez X, Li H, Wang MN, Ludwig D, Balderes P, Witte L, Li Y, Zhu Z. Leukemia. 2005;19:1432–1438. doi: 10.1038/sj.leu.2403825. [DOI] [PubMed] [Google Scholar]
- 54.Zhao X, Dai W, Cao L, Zhu H, Yu Y, Ye Q, Wang M, Lei P, Shen G. Biotechnol Appl Biochem. 2007;46:27–33. doi: 10.1042/BA20060056. [DOI] [PubMed] [Google Scholar]
- 55.Hayes ME, Drummond DC, Hong K, Zheng WW, Khorosheva VA, Cohen JA, th CON, Park JW, Marks JD, Benz CC, Kirpotin DB. Mol Pharm. 2006;3:726–736. doi: 10.1021/mp060040v. [DOI] [PubMed] [Google Scholar]
- 56.Hayes ME, Drummond DC, Kirpotin DB, Zheng WW, Noble CO, Park JW, Marks JD, Benz CC, Hong K. Gene Ther. 2006;13:646–651. doi: 10.1038/sj.gt.3302699. [DOI] [PubMed] [Google Scholar]
- 57.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, Papahadjopoulos D, Benz CC. Clin Cancer Res. 2002;8:1172–1181. [PubMed] [Google Scholar]
- 58.Zhou Y, Drummond DC, Zou H, Hayes ME, Adams GP, Kirpotin DB, Marks JD. Journal of molecular biology. 2007;371:934–947. doi: 10.1016/j.jmb.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merrimack, MM-302. 2011 [Google Scholar]
- 60.Liu B, Conrad F, Roth A, Drummond DC, Simko JP, Marks JD. J Mol Med (Berl) 2007;85:1113–1123. doi: 10.1007/s00109-007-0208-z. [DOI] [PubMed] [Google Scholar]
- 61.Schier R, Marks JD, Wolf EJ, Apell G, Wong C, McCartney JE, Bookman MA, Huston JS, Houston LL, Weiner LM, et al. Immunotechnology : an international journal of immunological engineering. 1995;1:73–81. doi: 10.1016/1380-2933(95)00007-0. [DOI] [PubMed] [Google Scholar]
- 62.Neve RM, Nielsen UB, Kirpotin DB, Poul MA, Marks JD, Benz CC. Biochem Biophys Res Commun. 2001;280:274–279. doi: 10.1006/bbrc.2000.4104. [DOI] [PubMed] [Google Scholar]