Abstract
Our laboratory and others have reported that Brattleboro (BRAT) rats, a Long Evans (LE) strain with a single gene mutation, have inherent deficits in prepulse inhibition (PPI) homologous to those observed in schizophrenia patients and that these deficits are reversed by antipsychotic drugs (APDs).
To further evaluate the potential predictive validity of BRAT rat PPI for APDs, we compared the effects of acute subcutaneous administration of the typical APD chlorpromazine to that of three psychotropic drugs without antipsychotic efficacy, the antidepressant imipramine, the anxiolytic diazepam and the anticonvulsant mood stabilizer valproic acid on male and female BRAT rat PPI.
Male and female BRAT rats exhibited baseline (saline treatment) PPI that was not different from each other (21.1 % and 21.3 %, respectively) and low compared to those historically exhibited by LE rats (approximately 59 %). Chlorpromazine facilitated PPI in male and female BRAT rats, whereas imipramine, diazepam, and valproic acid had no significant effect on PPI.
These results suggest that PPI in the BRAT rat responds specifically to drugs with APD efficacy but not psychotropic drugs of different therapeutic families.
Keywords: Prepulse Inhibition, Antipsychotic Drugs, Predictive Validity, Schizophrenia
Introduction
Animal models with strong predictive validity for the therapeutic effects of psychotropic drugs are essential for the development of novel therapeutics for psychiatric disorders. Prepulse inhibition (PPI) of the acoustic startle response is the basis of a number of animal models with strong predictive validity for the effects of antipsychotic drugs (APDs) (Geyer et al, 2001; Swerdlow and Geyer, 1998).
PPI of the acoustic startle response, an operational measure of sensorimotor gating, is the reduction in the startle response when the startle-eliciting stimulus is preceded immediately by a sub-threshold stimulus (Swerdlow et al, 1998). Schizophrenia patients exhibit lower PPI compared to healthy human subjects and these deficits are reversed by APD treatment In this respect, second generation APDs such as clozapine and risperidone tend to be more effective than the first generation APD, haloperidol at reversing these deficits (Kumari and Sharma, 2002).
Decreased PPI can be induced in rodents by administration of psychotomimetic drugs such as the dopamine agonists amphetamine and apomorphine (Mansbach et al, 1988), noncompetitive NMDA antagonists such as phencyclidine, (Mansbach, 1991) and serotonin agonists such as DOI (Sipes and Geyer, 1994). These PPI deficits are reversed by APDs, and thus PPI has been widely proposed as the basis of animal models with predictive validity for APDs (Geyer et al, 2001). PPI paradigms that are based on psychotomimetic drug-induced deficits have several significant limitations. First drug-induced PPI disruption is likely to be sensitive to reversal by other compounds that are active on the same neurotransmitter system. For example, dopamine agonist-induced disruption of PPI is sensitive to drugs that are dopamine antagonists, etc. In this way, the drug-induced model is likely to predict putative APDs that work via already established pharmacological mechanisms and are less likely to identify putative APDs that work via novel mechanisms. To address these issues, investigators have developed behavioral models based on non-pharmacologically induced PPI deficits.
Social isolation-rearing (Cilia et al, 2001; Geyer et al, 1993) and neonatal hippocampal lesions (Lipska et al, 1995) are two of the most investigated non-pharmacological models of PPI deficits. PPI deficits produced by isolation rearing can be attenuated by typical and atypical APDs (Cilia et al, 2001; Varty and Higgins, 1995). Neonatal hippocampal lesion-induced PPI deficits (Lipska et al, 1995) can be attenuated by atypical but not typical APDs (Le Pen et al, 2002).
Brattleboro (BRAT) rats have also been proposed as a non-pharmacological animal model. BRAT rats are Long Evans (LE) rats with a single base pair deletion in the gene coding for the neuropeptide/neurohormone vasopressin (AVP) resulting in the synthesis of an altered form of the AVP precursor peptide that cannot enter the secretory pathway (Birkett and Pickering, 1988).
BRAT rats display many behavioral and cognitive abnormalities. For example, they have been shown to have deficits in memory, emotional reactivity, social recognition, motivation, and attention (Bohus and de Wied, 1998; Williams et al, 1983). Abnormalities in these functions are exhibited by schizophrenia patients (Docherty and Gordinier, 1999; Malla, 1995; Phillips and David, 1995). In addition, BRAT rats exhibit an upregulation of D2 receptors (Shilling et al, 2006) consistent with reports of D2 receptor upregulation in brains of schizophrenia patients (Kestler et al, 2001).
BRAT rats also exhibit inherent PPI deficits compared to wild type Long Evans rats. In this regard, in our previous studies Long Evans rats exhibited approximately 59 % average PPI vs. 33 % for BRAT rats (Feifel et al, 2007; Feifel et al, 2004; Feifel and Priebe, 2001). These PPI deficits are reversed by APDs, and the putative APD PD149163, a neurotensin agonist (Cilia et al, 2010; Feifel et al, 2007; Feifel et al, 2004; Feifel et al, 2001). The social recognition deficits exhibited by BRAT rats are also reversed by clozapine and PD149163 (Feifel et al, 2009).
To exhibit optimal predictive validity for APD efficacy, an animal model should not only respond to established APDs (sensitivity) but also be unresponsive to non-APDs (specificity). The specificity of the reversal of BRAT rat PPI deficits to APDs has not yet been established. To address this issue, we tested the effects of three non-APDs, psychotropic drugs on BRAT rat PPI, imipramine, an antidepressant, diazepam, an anxiolytic, and valproic acid, a mood stabilizer. We also tested chlorpromazine, a typical APD, to further explore the sensitivity of BRAT rats to this family of drugs and to act as a comparator to the non-APDs. Furthermore, since PPI in female BRAT rats have not been studied, we also tested female BRAT rats to characterize their PPI response to psychotropic drugs.
Methods
One hundred twenty eight male and 128 female BRAT rats (n = 8), homozygous for the vasopressin mutation (170-350 grams at testing) were bred at UCSD (breeders from Harlan Laboratories, San Diego) and housed in groups of two in clear plastic chambers in a climate controlled room under a 12h/12h light/dark schedule (lights on/off - 7:00 A.M/7:00 P.M). They were allowed free access to food and water for the extent of the study. Behavioral testing was performed when rats were 8 – 10 weeks old, during the light phase of the rats' circadian illumination schedule as startle magnitude, PPI and drug effects on PPI are stable across the circadian cycle (Weiss et al, 1999). The rats were tested in startle chambers to characterize their baseline PPI and startle. Animals were assigned, based on their baseline PPI, to one of four groups matched so as to achieve comparable average PPI across groups. Drug treatment began a minimum of three days after baseline testing. All experimental procedures were conducted in accordance with the University of California, San Diego guidelines for animal care and experimentation.
In each experiment, 32 male and 32 female drug naive BRAT rats were used. In the first experiment, rats were administered subcutaneous (SC) injections of either 0 (saline), 1.0, 5.0, or 10 mg/kg chlorpromazine (Sigma Chemicals, St. Louis, MO). Doses were selected based on their demonstrated ability to reverse PPI deficits (Geyer et al, 2001). In the next experiment, rats were administered SC injection of either 0 (half volume 0.1 N HCl, half volume saline to neutral pH with 1 N NaOH) 3.0, 10.0, or 30.0 mg/kg imipramine (Sigma Chemicals, St. Louis, MO). In a third study, rats were administered SC injection of either 0 (Tween 80 and saline), 1.0, 3.0 or 10 mg/kg diazepam (Sigma Chemicals, St. Louis, MO). In the last study, animals were given either 0 (saline), 50, 100, 200 mg/kg valproic acid (Sigma Chemicals, St. Louis, MO). Volume injected for all 4 drugs was 1 ml/kg. Drug doses for diazepam, imipramine and valproic acid were chosen according to (Becker and Anton, 1990; Rigdon, 1991; Roche et al, 2008), respectively.
Animals were tested in startle chambers (San Diego Instruments, San Diego, CA) 30 minutes after drug administration. Once placed in startle chambers each rat had a 5-minute acclimation period. A 65-dB background noise was continuously present throughout the session. The acclimation was followed by a 15 minute PPI test session during which rats were presented with 40 msec 120 dB startle pulses without a prepulse, or pulses preceded 100 msec by a prepulse of either 4, 8 or 12 dB above background. These four types of active stimuli were presented in addition to a neutral (no sound) stimuli condition in pseudorandom order and repeated 10 times with an average of 15 seconds (7 - 23 seconds) between stimuli types.
A startle response was recorded for all stimuli presentations. PPI for each animal was calculated as a percentage of the pulse-alone startle magnitude using the following formula: [1- (startle magnitude after prepulse-pulse pair/startle magnitude after pulse only] ×100.
Statistical Analyses
To compare treatment groups, PPI data was subjected to a three-way ANOVA in which Prepulse Intensity was a within-subject factor, and Gender and Treatment (i.e., drug dose) were between-subject factors. There were no significant two-way interactions of prepulse intensity with any of the drugs tested. Therefore, this term was dropped from the model and the analysis reported is based upon a reduced model examining averaged PPI across the three prepulse intensities. Male and female data were analyzed by ANOVA followed by pair-wise comparisons using a Dunnett's two-tailed post hoc test to test the following specific hypothesis: Treatment with the test drugs facilitates PPI in both male and female BRAT rats. In addition, Pearson correlations were performed on PPI and startle data transformed into percent difference scores [(drug-induced PPI – baseline PPI)/baseline PPI] ×100, [(drug-induced startle magnitude – baseline startle magnitude)/baseline startle magnitude] ×100 to determine if there was a relationship between the effects of chlorpromazine on startle magnitude and PPI.
The acoustic startle response (ASR) to the startle stimuli presented without a prepulse data was subjected to analysis using two-way ANOVA with Gender and Treatment as between factors. Dunnett's post hoc tests were used to compare each drug group to saline treated male and female rats.
Results
Chlorpromazine
PPI (Figure 1, Main): There were main effects of Drug [F3,56) = 11.8 P < 0.001] and Prepulse Intensity as percent PPI increased with increasing prepulse intensity [F(2,112)= 10.5, P < 0.001]. There was a Prepulse Intensity X Gender interaction [F(2,112) -= 3.333, P < 0.05] reflected in a main effect of Prepulse Intensity in males [F(2,56)= 13.8, P < 0.001], but not females. In addition, there was a main effect of Drug in males [F(3,28) = 4.8, P < 0.01] and females [F(3,28) = 8.8, P < 0.001] as chlorpromazine increased percent PPI in BRAT rats in a dose-dependent manner (Figures 1A, 1B). For males, Dunnett's post hoc tests revealed that the highest dose significantly elevated percent PPI compared to saline treated rats (P < 0.01) and at the low and mid doses there were non significant trends in the same direction (P = 0.055 and P= 0.094, respectively). In females, both the mid and highest doses (P ≤ 0.001) significantly elevated percent PPI in BRAT rats. In addition, there were no significant Pearson correlations between percent change in PPI (baseline vs. drug-induced) among individual rats and their percent difference startle magnitude for either males or females at all three doses of chlorpromazine.
Figure 1.
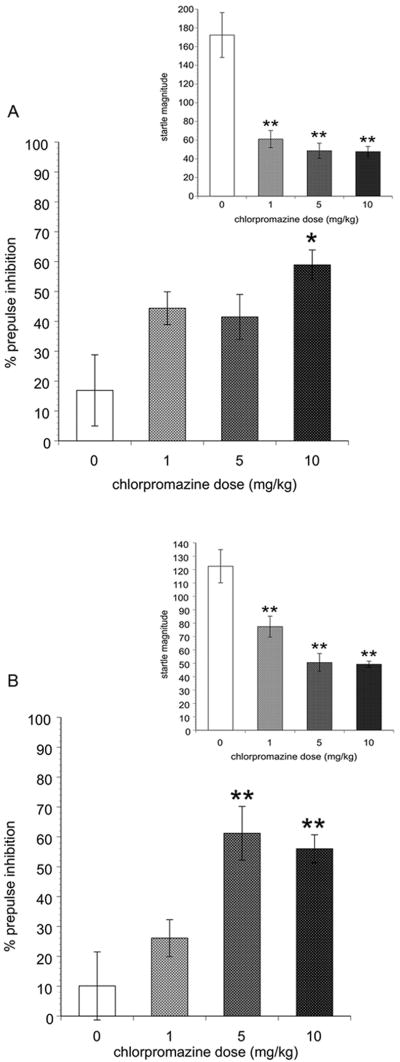
Prepulse inhibition (main) and acoustic startle response (inset) in male (A) and female (B) rats treated with chlorpromazine. Significantly different than vehicle-treated BRAT rats represented by * (P < 0.05) and ** (P < 0.01). Data represented as the mean ± SEM.
Startle (Figure 1A, 1B Insets): There was a main effect of Drug [F(3,63) = 34.3, P < 0.001] as startle magnitude decreased with increasing doses of chlorpromazine. There was also a significant Gender ×Drug interaction [3,63) = 3.3, P < 0.05]. For males and females Dunnett's post hoc tests revealed all three drug doses significantly attenuated startle magnitude compared to saline treated rats (P ≤ 0.001).
Imipramine
There was a significant main effect of Prepulse Intensity [F(2,112) = 10.8, P < 0.01] as percent PPI increased with increasing prepulse intensity. There were no other significant overall main effects or interactions. In addition, there were no significant effects of Drug on PPI or startle magnitude for males or females (Table 1).
Table 1.
Prepulse inhibition and acoustic startle response in rats treated with diazapam, imipramine or valproic acid. Data represented as the mean ± SEM (n=8).
| Avg % PPI (M) | Avg % PPI (F) | Pulse Alone (M) | Pulse Alone (F) | |
|---|---|---|---|---|
| Diazapam | ||||
| Vehicle | 23.3 ± 7.4 | 20.0 ± 7.5 | 365.6 ± 61.7 | 239.9 ± 66.4 |
| 1 mg/kg | 26.9 ± 7.5 | 15.0 ± 7.6 | 270.1 ± 50.3 | 175.8 ± 19.2 |
| 3 mg/kg | 28.9 ± 5.4 | 26.2 ± 6.2 | 255.1 ± 27.6 | 188.9 ± 39.9 |
| 10 mg/kg | 33.6 ± 3.5 | 27.8 ± 7.7 | 209.2 ± 23.4 | 144.3 ± 10.4 |
| Imipramine | ||||
| Vehicle | 23.8 ± 7.8 | 33.3 ± 7.6 | 360.0 ± 113.1 | 367.1 ± 92.9 |
| 1 mg/kg | 29.5 ± 2.3 | 32.8 ± 3.2 | 394.1 ± 128.2 | 347.6 ± 77.2 |
| 3 mg/kg | 28.0 ± 8.8 | 23.9 ± 4.6 | 197.5 ± 31.1 | 247.8 ± 50.8 |
| 10 mg/kg | 33.2 ± 4.4 | 43.7 ± 5.6 | 252.1 ± 37.6 | 329.0 ± 115.1 |
| Valproic Acid | ||||
| Vehicle | 26.2 ± 5.2 | 21.4 ± 3.1 | 99.8 ± 22.9 | 94.0 ± 11.1 |
| 50 mg/kg | 23.9 ± 5.3 | 21.8 ± 3.6 | 117.3 ± 11.1 | 101.2 ± 9.1 |
| 100 mg/kg | 33.1 ± 5.1 | 24.3 ± 5.0 | 143.9 ± 13.3 | 126.7 ± 24.8 |
| 200 mg/kg | 27.5 ± 2.4 | 24.6 ± 5.1 | 139.9 ± 24.3 | 91.4 ± 21.3 |
Abbreviations: Avg = average, M = male, F = female
Diazapam
There were no significant effects on PPI or startle magnitude (Insets) overall or for males and females analyzed separately. However, there was a strong trend towards a decrease in startle magnitude in Tween 80 and saline treated vs. high dose diazepam treated males (365.5 vs. 209.2) and females (239.9 vs.144.3) (Table 1).
Valproic Acid
There was a significant main effect of Prepulse Intensity [F(2,112) = 32.0, P < 0.001] as percent PPI increased with increasing prepulse intensity. There were no other significant overall main effects or interactions and no significant main effect of Drug in males or females on PPI. In addition, there were no significant effects of Gender or Drug on startle magnitude (Table 1).
Discussion
There were several notable findings emerging from this set of studies. First, baseline PPI in male BRATs was low (21.3 %) and startle magnitude high (299.3) consistent with previous findings (Cilia et al, 2010; Feifel et al, 2007; Feifel et al, 2004; Feifel et al, 2001). Second, PPI (21.1 %) and startle magnitude (243.2) were also low and high, respectively in females and this is the first report on PPI and startle in female BRAT rats. In these experiments, female BRATs exhibited baseline (saline) PPI that was comparable to males in magnitude and consistent across all four drug studies indicating that female BRAT rats may prove to be as useful as males as a model with predictive validity for the therapeutic effects of APDs.
In the third notable finding, chlorpromazine facilitated BRAT PPI and reduced startle magnitude in both male and female BRAT rats. The chlorpromazine-induced PPI facilitation was not correlated with its inhibitory effects on startle magnitude among rats and thus is not likely to be secondary to those startle effects.
The facilitation of PPI by chlorpromazine in male and female BRAT rats supports the notion that acute administration of typical APDs facilitates PPI in BRATS as has been demonstrated for atypical APDs. This is consistent with Cilia et al.'s finding (Cilia et al, 2010) that acute haloperidol also facilitated BRAT PPI. However, in a previous study we did not find that acute haloperidol facilitated BRAT PPI (Feifel et al, 2004). It is not clear why we did not detect an effect of haloperidol on BRAT PPI. One possibility is that the different findings of these two studies could be due to a difference in prepulse levels used in each laboratory or different housing conditions in the two colonies of BRAT rats.
The final important finding to emerge from this study is that neither the anxiolytic diazapam, antidepressant imipramine, nor the mood stabilizer/anticonvulsant, valproic acid had an effect on BRAT PPI, suggesting that PPI facilitation is not produced by psychotropic drugs that are not APDs. These data strengthen the specificity of the model for APDs. However, more typical and atypical APDs and psychotropic non-APDs will need to be tested to further confirm the overall specificity of this model while enhancing predictive validity. Interestingly, diazepam exhibited a strong trend to decrease startle magnitude without altering PPI, providing additional evidence to the large body of existing data that PPI and ASR exhibit reciprocal dissociation. Consistent with our findings, it has previously been reported that antidepressants such as impramine had no effect while anxiolytics such as diazapam (Rigdon, 1991) decreased startle.
Psychotropic drugs that lack APD efficacy have been previously tested in other rodent PPI deficit models with variable results. One study found that imipramine had no effects on apomorphine-induced PPI deficits in rats (Rigdon, 1991) but Duncan et al. (Duncan et al, 2006) found that imipramine increased PPI in mice and the antidepressant desipramine, an active metabolite of imipramine, has been reported to attenuate the PPI disruptive effects of both dopamine agonists (Pouzet et al, 2005) and PCP (Yamada et al, 1999). In contrast to rodent studies, imipramine and desipramine significantly decreased PPI in healthy human volunteers (Hammer et al, 2007; Oranje et al, 2004).
The benzodiazepine anxiolytic diazapam exhibited no effects on apomorphine-induced PPI deficits in rats (de Jong et al, 2006; Rigdon, 1991) but it has been found to increase PPI in C57BL6J, a mouse strain that has naturally low PPI (Ouagazzal et al, 2001). With regard to the anticonvulsant mood stabilizer valproic acid, Ong et al. (Ong et al, 2005) found that it had no effect on PPI in C57BL/6J mice, whereas Flood et al. (Flood et al, 2009) reported that it increased PPI in DBA.2 mice, another strain with naturally low PPI. Umeda et al. (Umeda et al, 2006) found that valproic acid reversed apomorphine-induced PPI deficits in mice. Based upon the above findings, there is mixed evidence for the specificity of psychostimulant-induced PPI deficits to respond to APDs. Similarly, mice strains with naturally low PPI that have been proposed as animal models with predictive validity for APDs (C57BL/6J, DBA.2) also exhibit some responsiveness to non-APD psychotropics, limiting their specificity. In this regard, the BRAT rat, the only genetic rat model of deficient PPI characterized to date, demonstrates the strongest specificity for APDs thus far.
In summary, consistent with previous studies, PPI in BRAT rats responds to psychotropic drugs with known APD effects in humans. BRAT rats do not respond to known psychotropic drugs that do not have any APD effects. In contrast to the DA agonist and the noncompetitive NMDA antagonist PPI models, and mouse models with naturally low PPI, BRAT rats, thus far, exhibit specificity for the effects of APDs on PPI. However, this will need to be further validated by testing additional non-APD drugs. Female BRATs also have inherent deficiencies in PPI, which seem to respond similarly to psychotropic drugs as males. Therefore, both male and female BRAT rats appear to be useful models for the preclinical evaluation of putative APDs.
Disclosures
In the past three years, Dr. Feifel has received funds in return for one or more of the following: conducting contracted research, conducting investigator-initiated research, advisory board participation, speaking from the following pharmaceutical companies: Abbott Labs, Astra Zeneca, Bristol Myers Squibb, Danipon-Sumitomo, Eli Lilly, Jansen, Shire, Wyeth, and Schering-Plough.
Acknowledgments
FUNDING ACKNOWLEDGMENTS: This work was funded by the National Institute of Mental Health [MH07070].
References
- Becker HC, Anton RF. Valproate potentiates and picrotoxin antagonizes the anxiolytic action of ethanol in a nonshock conflict task. Neuropharmacology. 1990;29(9):837–843. doi: 10.1016/0028-3908(90)90158-n. [DOI] [PubMed] [Google Scholar]
- Birkett SD, Pickering BT. The vasopressin precursor in the Brattleboro (di/di) rat. International Journal of Peptide and Protein Research. 1988;32(6):565–572. doi: 10.1111/j.1399-3011.1988.tb01388.x. [DOI] [PubMed] [Google Scholar]
- Bohus B, de Wied D. The vasopressin deficient Brattleboro rats: a natural knockout model used in the search for CNS effects of vasopressin. Prog Brain Res. 1998;119:555–573. doi: 10.1016/s0079-6123(08)61593-9. [DOI] [PubMed] [Google Scholar]
- Cilia J, Gartlon JE, Shilliam C, Dawson LA, Moore SH, Jones DN. Further neurochemical and behavioural investigation of Brattleboro rats as a putative model of schizophrenia. J Psychopharmacol. 2010;24(3):407–419. doi: 10.1177/0269881108098787. [DOI] [PubMed] [Google Scholar]
- Cilia J, Reavill C, Hagan JJ, Jones DN. Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats. Psychopharmacology (Berl) 2001;156(2-3):327–337. doi: 10.1007/s002130100786. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Snaphaan LJ, Pattij T, Veening JG, Waldinger MD, Cools AR, et al. Effects of chronic treatment with fluvoxamine and paroxetine during adolescence on serotonin-related behavior in adult male rats. Eur Neuropsychopharmacol. 2006;16(1):39–48. doi: 10.1016/j.euroneuro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Gordinier SW. Immediate memory, attention and communication disturbances in schizophrenia patients and their relatives. Psychological Medicine. 1999;29(1):189–197. doi: 10.1017/s0033291798007843. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH. Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology (Berl) 2006;184(2):190–200. doi: 10.1007/s00213-005-0214-1. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Priebe K, Shilling PD. The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res. 2007;181(2):278–286. doi: 10.1016/j.bbr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology. 2004;29(4):731–738. doi: 10.1038/sj.npp.1300378. [DOI] [PubMed] [Google Scholar]
- Feifel D, Mexal S, Melendez G, Liu PY, Goldenberg JR, Shilling PD. The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology. 2009;34(8):2011–2018. doi: 10.1038/npp.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Priebe K. Vasopressin-deficient rats exhibit sensorimotor gating deficits that are reversed by subchronic haloperidol. Biol Psychiatry. 2001;50(6):425–433. doi: 10.1016/s0006-3223(01)01100-3. [DOI] [PubMed] [Google Scholar]
- Flood DG, Choinski M, Marino MJ, Gasior M. Mood stabilizers increase prepulse inhibition in DBA/2NCrl mice. Psychopharmacology (Berl) 2009;205(3):369–377. doi: 10.1007/s00213-009-1547-y. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156(2-3):117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biological Psychiatry. 1993;34(6):361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Hammer TB, Oranje B, Glenthoj BY. The effects of imipramine on P50 suppression, prepulse inhibition and habituation of the startle response in humans. Int J Neuropsychopharmacol. 2007;10(6):787–795. doi: 10.1017/S1461145706007504. [DOI] [PubMed] [Google Scholar]
- Kestler LP, Walker E, Vega EM. Dopamine receptors in the brains of schizophrenia patients: a meta-analysis of the findings. Behav Pharmacol. 2001;12(5):355–371. doi: 10.1097/00008877-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Kumari V, Sharma T. Effects of typical and atypical antipsychotics on prepulse inhibition in schizophrenia: a critical evaluation of current evidence and directions for future research. Psychopharmacology (Berl) 2002;162(2):97–101. doi: 10.1007/s00213-002-1099-x. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology. 1995;122(1):35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Malla AK. Negative symptoms and affective disturbance in schizophrenia and related disorders. Canadian Journal of Psychiatry Revue Canadienne de Psychiatrie. 1995;40(7 Suppl 2):S55–59. doi: 10.1177/070674379504007s05. [DOI] [PubMed] [Google Scholar]
- Mansbach RS. Effects of NMDA receptor ligands on sensorimotor gating in the rat. Eur J Pharmacol. 1991;202(1):61–66. doi: 10.1016/0014-2999(91)90253-m. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94(4):507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Ong JC, Brody SA, Large CH, Geyer MA. An investigation of the efficacy of mood stabilizers in rodent models of prepulse inhibition. J Pharmacol Exp Ther. 2005;315(3):1163–1171. doi: 10.1124/jpet.105.090845. [DOI] [PubMed] [Google Scholar]
- Oranje B, Kahn RS, Kemner C, Verbaten MN. Modulating sensorimotor gating in healthy volunteers: the effects of desipramine and haloperidol. Psychiatry Res. 2004;127(3):195–205. doi: 10.1016/j.psychres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Ouagazzal AM, Jenck F, Moreau JL. Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity? Psychopharmacology (Berl) 2001;156(2-3):273–283. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- Phillips ML, David AS. Facial processing in schizophrenia and delusional misidentification: cognitive neuropsychiatric approaches. Schizophrenia Research. 1995;17(1):109–114. doi: 10.1016/0920-9964(95)00035-k. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Andersen MP, Hogg S. Effects of acute treatment with antidepressant drugs on sensorimotor gating deficits in rats. Psychopharmacology (Berl) 2005;178(1):9–16. doi: 10.1007/s00213-004-1976-6. [DOI] [PubMed] [Google Scholar]
- Rigdon GC, Viik K. Prepulse inhibition as a screening test for potential antipsychotics. Drug Development Research. 1991;23:91–99. [Google Scholar]
- Roche M, Shanahan E, Harkin A, Kelly JP. Trans-species assessment of antidepressant activity in a rodent model of depression. Pharmacol Rep. 2008;60(3):404–408. [PubMed] [Google Scholar]
- Shilling PD, Kinkead B, Murray T, Melendez G, Nemeroff CB, Feifel D. Upregulation of striatal dopamine-2 receptors in Brattleboro rats with prepulse inhibition deficits. Biol Psychiatry. 2006;60(11):1278–1281. doi: 10.1016/j.biopsych.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33(3-4):441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophrenia Bulletin. 1998;24(2):285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Umeda K, Suemaru K, Todo N, Egashira N, Mishima K, Iwasaki K, et al. Effects of mood stabilizers on the disruption of prepulse inhibition induced by apomorphine or dizocilpine in mice. Eur J Pharmacol. 2006;553(1-3):157–162. doi: 10.1016/j.ejphar.2006.09.050. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology. 1995;122(1):15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Feldon J, Domeney AM. Circadian time does not modify the prepulse inhibition response or its attenuation by apomorphine. Pharmacology, Biochemistry and Behavior. 1999;64(3):501–505. doi: 10.1016/s0091-3057(99)00100-8. [DOI] [PubMed] [Google Scholar]
- Williams AR, Carey RJ, Miller M. Behavioral differences between vasopressin-deficient (Brattleboro) and normal Long-Evans rats. Peptides. 1983;4(5):711–716. doi: 10.1016/0196-9781(83)90023-2. [DOI] [PubMed] [Google Scholar]
- Yamada S, Harano M, Annoh N, Nakamura K, Tanaka M. Involvement of serotonin 2A receptors in phencyclidine-induced disruption of prepulse inhibition of the acoustic startle in rats. Biological Psychiatry. 1999;46(6):832–838. doi: 10.1016/s0006-3223(98)00356-4. [DOI] [PubMed] [Google Scholar]


