Abstract
Background
Repetitive transcranial magnetic stimulation (TMS) of the dorsolateral prefrontal cortex (DLPFC) is an established treatment for depression, but its underlying mechanism of action remains unknown. Abnormalities in two large-scale neuronal networks—the frontoparietal central executive network (CEN) and the medial prefrontal-medial parietal default mode network (DMN)—are consistent findings in depression and potential therapeutic targets for TMS. Here, we assessed the impact of TMS on activity in these networks and their relation to treatment response.
Methods
We used resting state functional magnetic resonance imaging (rs-fMRI) to measure functional connectivity within and between the DMN and CEN in 17 depressed patients, before and after a five-week course of TMS. Motivated by prior reports, we focused on connectivity seeded from the DLPFC and the subgenual cingulate, a key region closely aligned with the DMN in depression. Connectivity was also compared to a cohort of 35 healthy controls.
Results
Prior to treatment, functional connectivity in depressed patients was abnormally elevated within the DMN and diminished within the CEN, and connectivity between these two networks was altered. TMS normalized depression-related subgenual hyperconnectivity in the DMN but did not alter connectivity in the CEN. TMS also induced anticorrelated connectivity between the DLPFC and medial prefrontal DMN nodes. Baseline subgenual connectivity predicted subsequent clinical improvement.
Conclusions
TMS selectively modulates functional connectivity both within and between the CEN and DMN, and modulation of subgenual cingulate connectivity may play an important mechanistic role in alleviating depression. The results also highlight potential neuroimaging biomarkers for predicting treatment response.
INTRODUCTION
Transcranial magnetic stimulation (TMS) has emerged as a promising tool in the psychiatric treatment arsenal with proven efficacy for the treatment of depression, including in patients otherwise resistant to antidepressant pharmacotherapy (1–3).
The current limitations of antidepressant medications highlight the clinical relevance of TMS: typically, only approximately one third of patients achieve full remission with medication during acute phase treatment, with less than half maintaining sustained remission after multiple medication trials, and side effects are a common obstacle to adherence (4–6). For patients who either fail to respond to an antidepressant or experience intolerable side effects, TMS is a potentially useful alternative that is both well-tolerated (2) and effective (7, 8). Still, most treatment-refractory patients will not achieve full remission (3), and recent metanalyses indicate that the effect size (Cohen’s d) lies in the 0.39–0.55 range (8, 9). For these reasons, there is a pressing clinical need to understand how and for whom TMS works. Studies suggest that the efficacy of TMS might also be improved by optimizing treatment protocols (2), particularly the neuroanatomical target for stimulation (10).
Efforts to predict treatment response and identify optimal stimulation sites will be facilitated by understanding the mechanisms that mediate clinical improvement with TMS, which are currently unknown. Acutely, TMS elicits transient current flow and neuronal depolarization in cortical tissue directly beneath the site of stimulation and in interconnected downstream circuits (11–13). In the longer term, repetitive TMS has more durable effects on neural function. In healthy human subjects, high-frequency TMS to the motor cortex causes long-lasting changes in electrophysiological measures of cortical excitability at the stimulation site (14). These effects depend on NMDA receptor signaling (15), which suggests that they involve long-term potentiation-like plasticity mechanisms. In addition to local effects at the stimulation site, TMS of the left dorsolateral prefrontal cortex (DLFPC) also modulates activity in more distant regions that function abnormally in depression (16, 17). These observations suggest that TMS may relieve depression by modulating synaptic strength both locally and at distant sites, thereby modulating functional connectivity in cortical networks.
Recent studies have shown that the human brain is intrinsically organized into spatially and temporally dissociable functional networks (18–21), and that neuronal activity patterns within at least two of these—the default mode network (DMN) and the central executive network (CEN)—are consistently abnormal in depression. The DMN has been implicated in rumination, self-referential processing, and episodic memory retrieval and includes areas of medial prefrontal cortex, posterior cingulate cortex, and multiple (mostly medial) areas of posterior parietal cortex (21–23). In depression, activity in the DMN is correlated with activity in the subgenual cingulate cortex and other limbic areas (24–26). The CEN plays a key role in regulating attention, working memory, and decision-making (27), and includes dorsolateral prefrontal cortex (DLPFC) and multiple (mostly lateral) areas of posterior parietal cortex. Early efforts to use TMS to treat depression focused on the left DLPFC (28, 29), a component of the CEN, because this region was consistently found to be hypoactive in depression (30–32). Likewise, hyperactivity (33, 34) and abnormal patterns of connectivity (24, 26, 33–36) between the subgenual cingulate (sgACC) and other default mode network structures are also consistent findings in depression. Whether TMS has any effect on functional connectivity within these two networks is unknown.
Importantly, activity in the CEN and DMN are also closely coupled. Anatomical tracer studies in non-human primates have identified strong, reciprocal connections between the DLPFC, sgACC, and medial prefrontal areas of the DMN (37–41). Activity in the CEN and DMN are anticorrelated in some contexts (18, 42), and DMN activity is suppressed during DLPFC-dependent cognitive control tasks (23, 43). Moreover, activation of the DLPFC by TMS stimulation concurrent with fMRI modulated DLPFC-DMN connectivity (44). In a recent cross-sectional resting state connectivity study, DLPFC target sites that yielded larger treatment effects in previous efficacy studies were more strongly coupled with the sgACC in healthy control subjects, and a similar pattern of DLPFC connectivity was observed in a separate cohort of patients with depression (10). Whether left dorsolateral prefrontal TMS modulates connectivity between these two networks in depression is unknown.
To answer this question, we used resting state functional magnetic resonance imaging (rs-fMRI) to measure functional connectivity in and between the DMN and CEN in depressed patients before and after a five-week course of dorsolateral prefrontal TMS. By first comparing patients with a cohort of healthy controls, we identified abnormal patterns of connectivity in the DMN and CEN prior to treatment and then tested for changes in connectivity after treatment. Our hypothesis was that TMS acts to relieve depression, at least in part, by normalizing patterns of connectivity both within and between these two networks. A corollary of this hypothesis is that abnormal patterns connectivity prior to treatment may be predictive of subsequent treatment response. Accordingly, we also tested whether baseline (pre-treatment) connectivity between DLPFC, sgACC, and other nodes of the CEN and DMN correlated with clinical improvements after TMS.
METHODS AND MATERIALS
Subjects
17 outpatients meeting DSM-IV TR criteria for a non-psychotic major depressive episode (mean age 42.3, SD = 17.3; 18% male) and 35 healthy control subjects (mean age 36.0, SD = 16.0; 34% male) participated in this study after providing informed consent. Patients were eligible for inclusion if they met DSM-IV-TR criteria for a major depressive episode with a diagnosis of major depressive disorder or bipolar II disorder, and if they also met criteria for treatment-resistance, including a failure to respond to at least two previous antidepressant trials at adequate doses for 8 weeks during the current episode. The recruitment procedure and other inclusion and exclusion criteria for patients and controls are described in the Supplemental Material. Diagnostic and medication history for the patient group are described in Supplemental Table S1. All aspects of our experimental protocol were approved by the Institutional Review Board of Weill Cornell Medical College, and conducted in accordance with institutional guidelines.
TMS protocol
All 17 patients completed 25 sessions of 10-Hz excitatory TMS (3)(Neuronetics, Inc: NeuroStar TMS Therapy System) over the left DLPFC during a 5-week period. We assessed treatment response using the 24-item Hamilton Rating Scale for Depression (HAM-D) at baseline and 1–3 days after completing treatment. Resting motor thresholds and stimulation intensity for each subject are listed in Supplemental Table S2, and other details of the TMS protocol are described in the Supplemental Material.
MRI data acquisition and analysis
MRI data (collected on a General Electric 3T scanner) were obtained from patients in two sessions that occurred before and after treatment, or in a single session for healthy controls. Each session included an rs-fMRI sequence (TR = 2.0s, 180 volumes) and a T1-weighted anatomical scan. Data preprocessing included motion correction, spatial smoothing, temporal band-pass filtering, detrending, and removal of nuisance signals by regression on six motion parameters (roll, pitch, yaw, and translation in three dimensions) and signal time courses for white matter and CSF regions-of-interest determined on an individual basis using an automated segmentation algorithm. One patient was excluded from further analysis due to loss-of-signal artifact (see Supplemental Figure S1). Additional preprocessing information is described in the Supplemental Material.
To test for functional connectivity differences in depression and for effects of TMS, we generated functional connectivity maps between seeds in the left DLPFC and subgenual cingulate cortex and targets in the CEN and DMN. ROIs comprising the DMN (Supplemental Fig. S2) and CEN (Supplemental Fig. S3) were defined a priori based on a previously published report (20), as were coordinates for the sgACC (Supplementary Table S3)(10, 24, 26, 33, 34, 45–50) and DLPFC seeds (10). We focused on seeds in the sgACC and DLPFC because functional connectivity between these structures has been implicated in the response to TMS (10). SgACC activity is increased in depression (24, 26, 33–36) and sensitive to treatment (33, 34, 45–49). Furthermore, although the sgACC does not lie within the DMN, sgACC and DMN activity are highly correlated, especially in depression (24, 26). In contrast, left DLPFC activity is consistently decreased in depression (30–32). Our DLPFC seed, which was predicted to lie within the stimulation field (see Supplemental Material), is adjacent to the CEN as defined in a prior report but not within it (20); however, activity in BA46 is highly correlated with activity throughout the CEN and plays a critical role in cognitive control processes (20, 24).
Correlating signal in these two seeds with targets in the DMN and CEN generated two within-network connectivity maps (DLPFC:CEN, sgACC:DMN) and two between-network connectivity maps (DLFPC:DMN, and sgACC:CEN). To test for functional connectivity differences in patients vs. controls, we used analysis of covariance (ANCOVA), with age, sex, and head motion as covariates. To test for effects of TMS on functional connectivity, we used repeated measures ANCOVA, co-varying for age, sex, baseline HAM-D score, TMS intensity (% of resting motor threshold), history of antipsychotic or mood stabilizer use, and lifetime number of antidepressant trials as a proxy for treatment resistance. Finally, to test whether baseline connectivity maps were related to subsequent treatment response, we divided patients into two groups based on a median split of the percent change in HAM-D, and tested for differences in their baseline functional connectivity maps (ANCOVA, covariates: age, sex, baseline HAM-D score, and lifetime number of antidepressant trials). In all analyses, significant effects were identified using a cluster threshold to correct for multiple comparisons (51). See Supplemental Material for additional details.
RESULTS
Subject characterization and effects of TMS on depression
17 patients with treatment-resistant depression were enrolled in this study, and all completed the 5-week course of TMS. Patients and healthy controls (N = 35) did not differ significantly in terms of age (t = 1.24, p = 0.21), sex (t = 1.21, p = 0.20), or head motion during fMRI scanning (t = 1.15, p = 0.25). Furthermore, patients’ head motion did not differ significantly in the pre-versus post-treatment fMRI scans (t = 1.22, p = 0.23).
On average, patients’ symptoms improved by 9.1 points on the HAM-D from the first to final session (SD 7.5; t = 4.92, p = 0.0002; Cohen’s d = 1.32). There were no significant differences between patients who showed a stronger response to treatment and those who did not (based on a median split of their percent change in HAM-D) in terms of age (t = 0.60, p = 0.56), sex (t = 1.89, p = 0.08), pre-treatment HAM-D (t = 1.48, p = 0.16), stimulation intensity (t = 0.01, p = 0.99), or lifetime number of failed antidepressant trials (t = 0.69, p = 0.50).
TMS effects on connectivity within the CEN and DMN
In order to identify likely targets for modulation by TMS, we began by assessing how functional connectivity was altered within the default mode network (DMN) and the central executive network (CEN) in patients with depression relative to a cohort of closely matched controls. To determine whether TMS modulates these connectivity patterns, we re-scanned the same patients shortly after completing the 5-week stimulation protocol and tested for differences in functional connectivity after treatment.
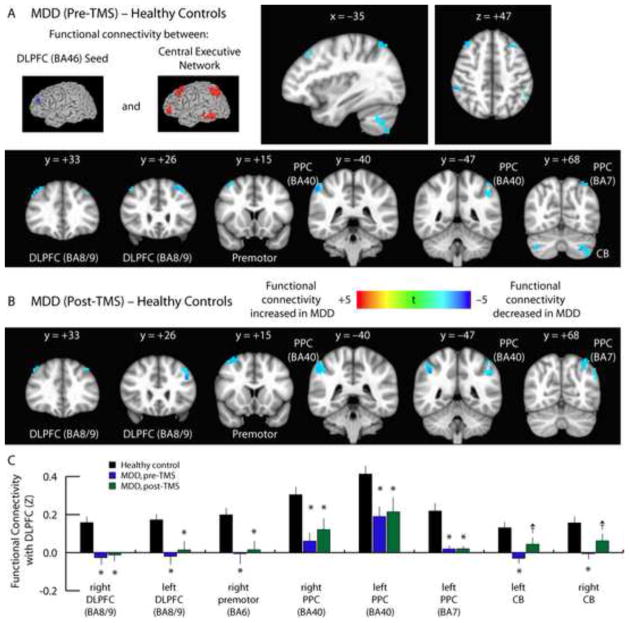
Within the CEN, we found that functional connectivity was significantly reduced in depressed patients relative to controls (Fig. 1A). We observed widespread reductions in functional connectivity between the left DLPFC and the premotor cortex (BA6), two posterior parietal areas (BA40, BA7), bilateral cerebellum, and other areas of the lateral PFC (BA8/9). Since TMS targets the left DLPFC for stimulation, we predicted that functional connectivity between this region and other areas of the central executive network would be altered, but this is not what we observed. Instead, there were no significant effects on functional connectivity within this network, and all areas of abnormal hypoconnectivity persisted after treatment (Fig. 1B–C). Similar results—widespread reductions in connectivity between the left DLPFC and targets in the CEN and other areas that persisted after treatment—were observed in unmasked whole brain analyses (Supplemental Figs. S4–S6). These findings indicate that it is unlikely that TMS acts by modulating functional connectivity within the central executive network.
Fig. 1. Persistence of depression-related hypoconnectivity in the CEN after TMS.
A. Compared to healthy control subjects, depressed patients exhibited decreased functional connectivity between the DLFPC and multiple nodes of the central executive network, including premotor cortex (BA6), two regions of posterior parietal cortex (PPC: BA40, BA7), bilateral cerebellum (CB), and other areas of lateral prefrontal cortex (DLPFC: BA8/9). Images depict t statistics for the contrast of patients pre-treatment versus healthy controls. These images and all subsequent images are presented in radiological convention and are labeled with the corresponding planar coordinate in MNI space.
B. These effects persisted when the same patients were scanned after completing a 5-week course of TMS.
C. Quantification of data extracted from the coordinates of the peak t statistic from each of the areas labeled in panels 1A and 1B. For coordinates and statistics, see Supplemental Table S4. Error bars = S.E.M. * = p < 0.05, corrected for multiple comparisons. † = p < 0.01, uncorrected, but not significant after correcting for multiple comparisons.
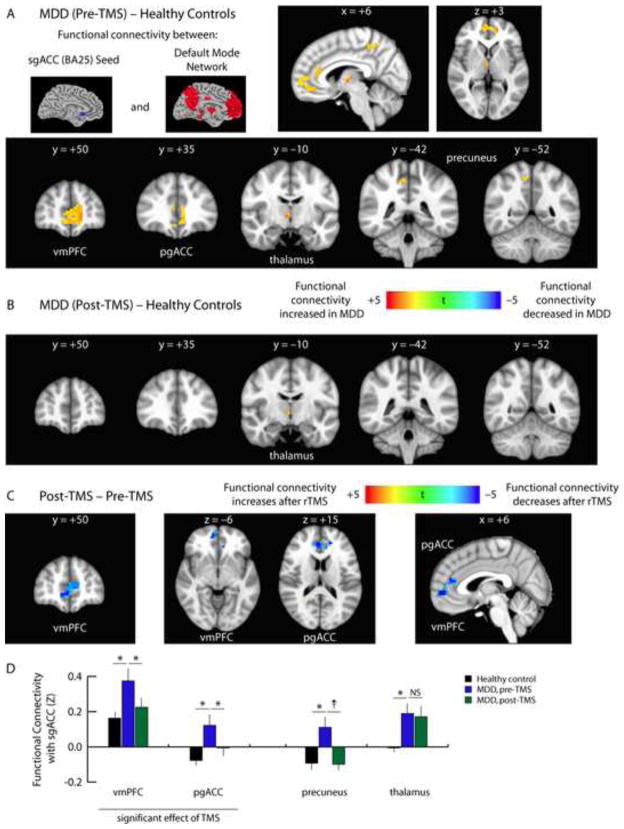
In contrast, within the default mode network, we found that functional connectivity was significantly elevated in depressed patients, and tended to decrease with treatment. Prior to treatment, we observed abnormally elevated functional connectivity between the subgenual anterior cingulate cortex (sgACC) and the ventromedial prefrontal cortex, pregenual anterior cingulate cortex, thalamus, and precuneus (Fig. 2A). Most of these abnormalities resolved after treatment (Fig. 2B). TMS significantly reduced patterns of hyperconnectivity in the ventromedial prefrontal cortex and pregenual anterior cingulate cortex, and connectivity with the precuneus was also statistically indistinguishable from controls after treatment (Fig. 2B–D). Only connectivity with the thalamus remained abnormally elevated (Fig. 2B). Again, similar results were observed in unmasked whole brain analyses (Supplemental Figs. S7–S9). Thus, depressed patients exhibited significant and contrasting abnormalities in within-network connectivity in the DMN and CEN, and TMS selectively attenuated abnormal hyperactivity only in the DMN.
Fig. 2. TMS attenuates depression-related hyperconnectivity within the DMN.
A. Compared to healthy control subjects, depressed patients exhibited increased functional connectivity between the sgACC and multiple nodes of the default mode network, including the ventromedial prefrontal cortex (vmPFC), pregenual anterior cingulate cortex (pgACC), thalamus, and precuneus. Images depict t statistics for the contrast of patients pre-treatment versus healthy controls.
B. All areas of sgACC hyperconnectivity normalized after TMS, except within the thalamus.
C. Repeated measures ANCOVA revealed significant effects of TMS on sgACC connectivity with the ventromedial prefrontal cortex (vmPFC) and pregenual anterior cingulate cortex (pgACC). Hyperconnectivity with the precuneus tended to normalize after treatment, but this effect did not reach significance after correcting for multiple comparisons.
D. Quantification of data extracted from the coordinates of the peak t statistic from each of the areas labeled in panels 2A–C. For coordinates and statistics, see Supplemental Table S5. Error bars = S.E.M. * = p < 0.05, corrected for multiple comparisons. † = p < 0.01, uncorrected, but not significant after correcting for multiple comparisons. NS = not significant.
TMS effects on connectivity between the CEN and DMN
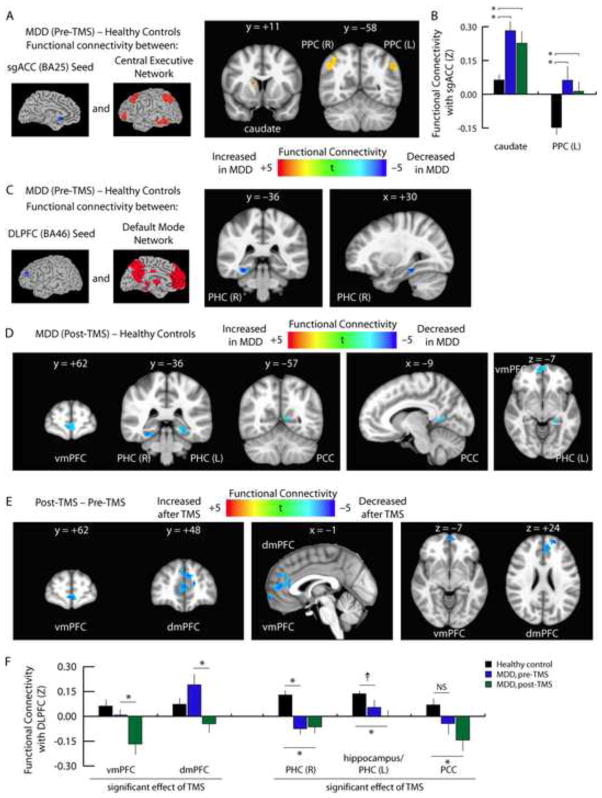
One plausible mechanism by which repetitive transcranial magnetic stimulation of the left DLPFC leads to connectivity changes in the default mode network is through modulating network-level interactions. Therefore, we tested for treatment effects on functional connectivity between these two networks. In our analysis of interactions between the sgACC and CEN, we found that depressed patients exhibited abnormally elevated sgACC connectivity with the caudate nucleus and bilateral posterior parietal areas prior to treatment (Fig. 3A). However, these effects persisted after treatment (Fig. 3B; Supplemental Fig. S10), and there were no significant effects of TMS.
Figure 3. TMS modulates interactions between the DMN and CEN.
A. Compared to healthy control subjects, functional connectivity between the sgACC and the CEN was abnormally elevated in depressed patients. Affected areas included the right caudate nucleus and bilateral posterior parietal cortex (PPC, BA40). Images depict t statistics for the contrast of patients prior to treatment versus healthy controls.
B. Quantification of the data depicted in panel 3A. Hyperconnectivity with the right caudate, left posterior parietal cortex, and right posterior parietal cortex (data not shown, see Supplemental Fig. S10) persisted after treatment, and there were no significant effects of TMS. Error bars = S.E.M. * = p < 0.05, corrected for multiple comparisons.
C. In contrast, depressed patients exhibited decreased functional connectivity between the DLPFC and a right parahippocampal area (PHC) of the DMN.
D. Hypoconnectivity between the DLPFC and the DMN either persisted or increased after TMS. Affected areas included ventromedial (vmPFC), bilateral parahippocampal cortex (PHC), and posterior cingulate cortex (PCC).
E. Repeated measures ANCOVA identified significant effects of TMS on functional connectivity between the DLPFC and two medial prefrontal areas of the DMN. The vmPFC cluster overlapped with the cluster in panel 3D. In both areas, connectivity was reduced, and neither area differed from controls prior to treatment.
F. Quantification of data extracted from the coordinates of the peak t statistic from each of the areas labeled in panels 3C–E. For coordinates and statistics, see Supplemental Table S6. Error bars = S.E.M. * = p < 0.05, corrected for multiple comparisons. † = p < 0.01, uncorrected, but not significant after correcting for multiple comparisons. NS = not significant.
In contrast, we found that TMS did significantly affect interactions between the DLPFC and DMN. In particular, we observed a pattern of reduced connectivity between the DLPFC and a right parahippocampal area of the DMN prior to treatment (Fig. 3C), and these differences tended to increase after TMS, expanding to include the ventromedial prefrontal cortex and posterior cingulate cortex (Fig. 3D–F). Notably, TMS tended to induce anticorrelations in functional coupling between the DLPFC and medial prefrontal areas of the DMN (Fig. 3E–F). In conjunction with the findings above, these results indicate that TMS acts not only by reducing subgenual cingulate hyperconnectivity within the DMN but also by modulating between-network interactions with central executive areas.
sgACC connectivity predicts antidepressant response to TMS
If TMS improves depressive symptoms by modulating functional connectivity within and between the DMN and CEN, then individual differences in functional connectivity at baseline may contribute to variability in the response to treatment. To evaluate this hypothesis, we tested for a relationship between patients’ pre-treatment functional connectivity maps (DLPFC:DMN, DLPFC:CEN, sgACC:DMN, and sgACC:CEN) and subsequent improvements in their depressive symptoms. In this analysis, we tested for functional connectivity differences between patients who showed a stronger response to treatment and those who did not, based on a median split of the percent change in HAM-D.
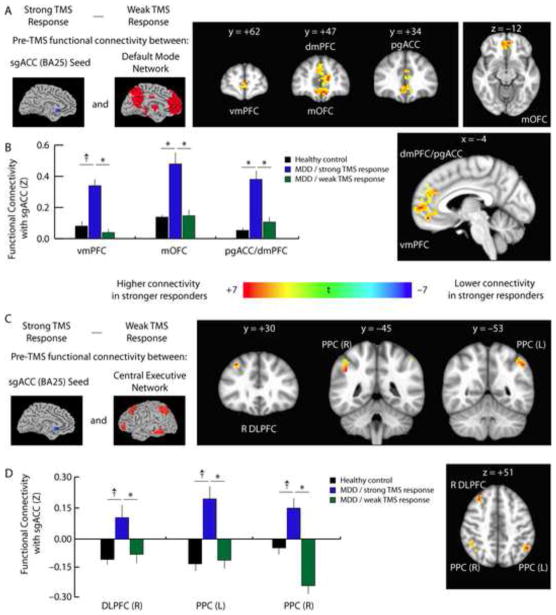
Unexpectedly, treatment response was unrelated to connectivity between the DLPFC and nodes of the CEN or DMN, in which we found no significant effects. By contrast, we found that sgACC hyperconnectivity at baseline strongly predicted larger improvements in HAM-D scores, even after controlling for clinical predictors of treatment response, including age, sex, depression severity, and history of treatment refractoriness. That is, in patients who showed a stronger response to TMS, sgACC connectivity prior to treatment was significantly higher in multiple nodes of the DMN (Fig. 4A–B), including ventromedial and dorsomedial prefrontal cortex, pregenual cingulate cortex, and posterior cingulate cortex. Similarly, higher connectivity between the sgACC and both prefrontal and posterior parietal areas of the CEN was associated with larger subsequent clinical improvements (Fig. 4C–D). Furthermore, patients who would subsequently show a stronger response to TMS were statistically indistinguishable from those who did not in terms of age (t = 0.60, p = 0.56), sex (t = 1.89, p = 0.08), depression severity as indexed by baseline HAM-D scores (t = 1.48, p = 0.16), or treatment refractoriness as indexed by lifetime number of failed antidepressant trials (t = 0.69, p = 0.50). Together, these findings indicate that baseline hyperconnectivity between the sgACC and multiple areas of the DMN and CEN are independently predictive of greater clinical improvements after TMS.
Fig. 4. Baseline subgenual cingulate connectivity predicts treatment response.
A. To test the hypothesis that individual differences in default mode and central executive network connectivity may influence patients’ response to TMS, we compared the pre-treatment functional connectivity maps for patients who subsequently showed a stronger to treatment versus those who showed a weaker response to treatment, based on a median split of patients’ percent change in HAM-D scores. Prior to treatment, patients who subsequently showed larger clinical benefits had higher subgenual cingulate connectivity with multiple nodes of the DMN, including the ventromedial (vmPFC) and dorsomedial prefrontal cortex (dmPFC), pregenual anterior cingulate (pgACC), and medial orbitofrontal cortex (mOFC).
B. Quantification of data extracted from the coordinates of the peak t statistic from each of the areas labeled in panel 4A.
C. Patients who showed a stronger response to TMS also exhibited higher functional connectivity between sgACC, right DLPFC, and bilateral posterior parietal (PPC) areas of the CEN.
D. Quantification of data extracted from the coordinates of the peak t statistic from each of the areas labeled in panel 4C. For coordinates and statistics, see Supplemental Table S7. Quantification of the data depicted in panel 4c. Error bars = S.E.M. * = p < 0.05, corrected for multiple comparisons. † = p < 0.01, uncorrected, but not significant after correcting for multiple comparisons.
DISCUSSION
This was the first study to investigate how left dorsolateral prefrontal TMS affects functional connectivity in patients undergoing treatment for depression using pre- and post-treatment rs-fMRI scans. We found that TMS selectively attenuates abnormal sgACC hyperconnectivity and modulates interactions between the DMN and CEN. The degree of sgACC hyperconnectivity at baseline was also predictive of subsequent clinical improvement after TMS. However, our results indicate that depression is also associated with widespread functional connectivity abnormalities that tend to persist after TMS, especially within the CEN. These findings have implications for our understanding of depression pathophysiology as well as for future efforts to optimize treatment protocols and enhance response rates.
By comparing healthy controls with depressed patients prior to initiating TMS, we identified contrasting patterns of abnormal connectivity in the DMN and CEN that have implications for understanding pathophysiological processes in depression. In the DMN, we observed a pattern of widespread hyperconnectivity with the subgenual cingulate cortex. This observation adds to a rapidly growing body of studies that have reported consistent differences in morphology, cerebral glucose metabolism, and neuronal activity level within the sgACC and other DMN structures (16, 17, 24, 26, 33–36), as well as several previous rs-fMRI functional connectivity studies highlighting hyperconnectivity within the DMN (24, 26, 33–36). It has been suggested that abnormalities in DMN connectivity may be related to rumination and deficits in emotion regulation (52).
In addition to these DMN findings, we also observed a pattern of widespread hypoconnectivity within the central executive network that has not, to our knowledge, been reported in prior rs-fMRI studies, but is consistent with previous findings of decreased activity during various cognitive tasks (53, 54). Altered functional connectivity in the CEN may contribute to deficits in memory and attention and other cognitive symptoms in depression. In a previous study (55), a remarkably similar pattern of deficits was identified in healthy human subjects that were exposed to chronic stress. However, in the chronic stress study, these deficits were reversible after cessation of the stressor, whereas in this study, most CEN abnormalities tended to persist after treatment. This finding raises the possibility that abnormal connectivity in the CEN may reflect a susceptibility to recurrent depression that persists independently of mood state. Future work tracking functional connectivity changes longitudinally for longer intervals and in larger cohorts of patients will be required to test this hypothesis.
Our study also has several implications for understanding the antidepressant mechanism of action of TMS. First, the results are consistent with our hypothesis that TMS acts by modulating functional connectivity within cortical networks. Second, they show that TMS effects are neuroanatomically specific: DMN hyperconnectivity was reduced but CEN hypoconnectivity was unaffected. The underlying mechanisms that generate these specific effects on connectivity are unclear. Prior studies have shown that high-frequency TMS enhances cortical excitability at the target site (14–15) and may modulate synaptic strength through LTP-like mechanisms (15, 56). PET studies indicate that TMS also modulates activity in remote cortical sites, inducing activity changes in the medial prefrontal cortex and other DMN targets after left DLPFC stimulation (17, 57–59). Accordingly, EEG studies have shown that TMS modulates connectivity between the target site and distal cortical areas (60–64). These neuroanatomically distributed effects may arise through direct projections to and from the stimulation target. Consistent with the effects on DMN connectivity that we observed here, retrograde tracer studies in non-human primates indicate that the DLPFC has dense, reciprocal connections with multiple nodes of the DMN, including the sgACC and medial prefrontal cortex (37–41). Thus, TMS may attenuate hyperconnectivity within medial prefrontal areas of the DMN through effects on direct projections to and from the stimulation site.
It is less clear why TMS does not also modulate hypoconnectivity within the CEN, as there are also dense reciprocal connections between the DLPFC and posterior parietal and lateral prefrontal nodes in this network (40, 65, 66). However, several other factors may explain this specific pattern of regional variability. First, projections from the DLPFC stimulation site may terminate on either excitatory pyramidal cells or inhibitory interneurons, and regional differences in these projections may lead to increases or decreases in synaptic strength (67). Second, EEG and fMRI studies have shown that TMS effects on a given region of cortex are modulated by its pre-existing activity state, and that TMS effects on connectivity vary with the pre-existing strength of the projection (60, 67–70). Third, therapeutic rTMS for depression may act primarily by reducing abnormally elevated connectivity but not by strengthening weak connections, which would explain the absence of an effect on hypoconnectivity in the CEN. In most EEG studies (61, 63, 64, 69) and at least one fMRI study (71), high-frequency TMS was found primarily to decrease functional connectivity, especially for oscillations in the low-frequency alpha band, to which rs-fMRI may be most sensitive. It is likely that a combination of these factors contributed to the specificity of the results we observed, which provide additional convergent evidence implicating the importance of subgenual cingulate hyperactivity and DMN hyperconnectivity in the pathophysiology of depression and the response to treatment (33, 34, 45–49).
Furthermore, our results indicate that TMS may act not only by normalizing connectivity within the DMN but also by modulating interactions between the DMN and CEN. Indeed, we found that TMS induced an anticorrelated pattern of connectivity between the DLPFC and medial prefrontal areas of the DMN that was absent prior to treatment (Fig. 3D–F). We observed similar results in a recent concurrent TMS/fMRI study, in which DLPFC stimulation induced anticorrelated functional connectivity with medial prefrontal areas acutely (44). Interactions between the DMN and CEN are thought to be critical for regulating internally oriented versus externally oriented processing and optimizing cognition (18, 19, 72, 73). Thus, the antidepressant mechanism of TMS may act in part at the level of network interactions. Optimizing TMS treatment through these neural mechanisms will require a more thorough understanding of the specific causal mechanisms that govern CEN/DMN interactions, by extending concurrent TMS/fMRI methods to patients undergoing treatment for depression.
The relatively modest patient sample size and the lack of a sham-treated control arm are limitations of our study, and we cannot rule out the possibility that some of the changes we observed after TMS were not causally related to the treatment. Some effects may be placebo-related or may result in part from a spontaneous regression to the mean. Arguing against this interpretation, however, is the fact that the effects of TMS were selective and specific: many abnormalities persisted (or even increased) after treatment, and TMS tended to modulate connectivity primarily in DMN (but not CEN) target areas.
Finally, our findings may inform future efforts to optimize TMS treatment protocols and enhance response rates. Hyperconnectivity between the sgACC and areas of the DMN and CEN was associated with a stronger response to treatment. This result is consistent with a recent rs-fMRI study that investigated whether mPFC connectivity was associated with response to an experimental TMS protocol targeting dorsomedial prefrontal cortex (74). Clinical improvements were associated with higher baseline connectivity between the DLPFC, sgACC, and other areas of the mPFC. Others have reported associations between baseline mPFC connectivity and response to psychopharmacological antidepressants (75, 76). Together, these results suggest that rs-fMRI scans obtained prior to treatment have the potential for applications in predicting treatment response. Our results may also inform investigations of alternative TMS target sites. In a prior report (10), dorsolateral prefrontal TMS targets that yielded larger clinical effects were associated with greater DLPFC-sgACC connectivity, suggesting that response rates could be enhanced by selecting targets based on their connectivity with sgACC. Our findings provide direct support for this hypothesis and for systematic efforts to investigate TMS targets based on rs-fMRI measures of functional connectivity.
Supplementary Material
Acknowledgments
This work was supported by grants from the Brain and Behavior Research Foundation (NARSAD Young Investigator Award) and Neuronetics, Inc., to M.J.D. and by funds from the Department of Psychiatry at Weill Cornell Medical College. C.L. was supported by grants from the National Institutes of Health (K99 MH097822) and the DeWitt Wallace Reader’s Digest Foundation at Weill Cornell. A.E. was supported by a grant from the Dana Foundation. B.J.C. was supported by a grant from the National Institutes of Health (P50-MH079513).
Footnotes
FINANCIAL DISCLOSURES. M.J.D. was supported by research grants from Neuronetics, Inc. All other authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.PascualLeone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 2.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder A Sham-Controlled Randomized Trial. Archives of General Psychiatry. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. American Journal of Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 5.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. American Journal of Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JC. The STAR*D study: A four-course meal that leaves us wanting more. American Journal of Psychiatry. 2006;163:1864–1866. doi: 10.1176/ajp.2006.163.11.1864. [DOI] [PubMed] [Google Scholar]
- 7.Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology. 2002;5:73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- 8.Schutter D. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychological Medicine. 2009;39:65–75. doi: 10.1017/S0033291708003462. [DOI] [PubMed] [Google Scholar]
- 9.Slotema C, Blom J, Hoek H, Sommer I. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. Journal of Clinical Psychiatry. 2010;71:874–885. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- 10.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of Transcranial Magnetic Stimulation Targets for Depression Is Related to Intrinsic Functional Connectivity with the Subgenual Cingulate. Biological Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 12.Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Current Biology. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 13.Driver J, Blankenburg F, Bestmann S, Vanduffel W, Ruff CC. Concurrent brain-stimulation and neuroimaging for studies of cognition. Trends in Cognitive Sciences. 2009;13:319–327. doi: 10.1016/j.tics.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y-Z, Rothwell JC, Edwards MJ, Chen R-S. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cerebral Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- 16.Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biological Psychiatry. 1999;46:1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- 17.Speer AM, Kimbrell TA, Wassermann EM, Repella JD, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biological Psychiatry. 2000;48:1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- 18.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nature Reviews Neuroscience. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- 20.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns. Cerebral Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 22.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheline YI, Wang S, Coalson R, Snyder AZ, Price JL, Mintun MA. Abnormalities in functional connectivity in depressed subjects identified using fMRI resting state correlations. Society for Neuroscience Abstract Viewer and Itinerary Planner. 2008:38. [Google Scholar]
- 26.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 28.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily Repetitive Transcranial Magnetic Stimulation (Rtms) Improves Mood in Depression. Neuroreport. 1995;6:1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 29.PascualLeone A, Catala MD, Pascual APL. Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology. 1996;46:499–502. doi: 10.1212/wnl.46.2.499. [DOI] [PubMed] [Google Scholar]
- 30.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 31.Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RSJ, Dolan RJ. The Anatomy of Melancholia - Focal Abnormalities of Cerebral Blood-Flow in Major Depression. Psychological Medicine. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 32.Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al. Reduction of Prefrontal Cortex Glucose-Metabolism Common to 3 Types of Depression. Archives of General Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 33.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biological Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 35.Anand A, Li Y, Wang Y, Wu JW, Gao SJ, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biological Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience and Biobehavioral Reviews. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Vogt BA, Pandya DN. Cingulate Cortex of the Rhesus-Monkey. 2. Cortical Afferents. Journal of Comparative Neurology. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 38.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 39.Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. Journal of Comparative Neurology. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- 40.Selemon LD, Goldmanrakic PS. Common Cortical and Subcortical Targets of the Dorsolateral Prefrontal and Posterior Parietal Cortices in the Rhesus-Monkey - Evidence for a Distributed Neural Network Subserving Spatially Guided Behavior. Journal of Neuroscience. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nature Reviews Neuroscience. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CE, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks. 2. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 44.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proceedings of the National Academy of Sciences of the United States of America; (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. European Neuropsychopharmacology. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 46.Kito S, Fujita K, Koga Y. Regional cerebral blood flow changes after low-frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment-resistant depression. Neuropsychobiology. 2008;58:29–36. doi: 10.1159/000154477. [DOI] [PubMed] [Google Scholar]
- 47.Nahas Z, Teneback C, Chae J-H, Mu Q, Molnar C, Kozel FA, et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. American Journal of Psychiatry. 1999;156:1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- 49.Kito S, Hasegawa T, Koga Y. Neuroanatomical correlates of therapeutic efficacy of low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry and Clinical Neurosciences. 2011;65:175–182. doi: 10.1111/j.1440-1819.2010.02183.x. [DOI] [PubMed] [Google Scholar]
- 50.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 51.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved Assessment of Significant Activation in Functional Magnetic-Resonance-Imaging (Fmri) - Use of a Cluster-Size Threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 52.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Research-Neuroimaging. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 55.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clinical Neurophysiology. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 57.Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. European Journal of Neuroscience. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- 58.Speer AM, Willis MW, Herscovitch P, Daube-Witherspoon M, Shelton JR, Benson BE, et al. Intensity-dependent regional cerebral blood flow during 1-Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with (H2O)-O-15 positron emission tomography: II. Effects of prefrontal cortex rTMS. Biological Psychiatry. 2003;54:826–832. doi: 10.1016/s0006-3223(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 59.Kimbrell TA, Dunn RT, George MS, Danielson AL, Willis MW, Repella JD, et al. Left prefrontal-repetitive transcranial magnetic stimulation (rTMS) and regional cerebral glucose metabolism in normal volunteers. Psychiatry Research-Neuroimaging. 2002;115:101–113. doi: 10.1016/s0925-4927(02)00041-0. [DOI] [PubMed] [Google Scholar]
- 60.Strens LHA, Oliviero A, Bloem BR, Gerschlager W, Rothwell JC, Brown P. The effects of subthreshold 1 Hz repetitive TMS on cortico-cortical and interhemispheric coherence. Clinical Neurophysiology. 2002;113:1279–1285. doi: 10.1016/s1388-2457(02)00151-7. [DOI] [PubMed] [Google Scholar]
- 61.Oliviero A, Strens LHA, Lazzaro V, Tonali PA, Brown P. Persistent effects of high frequency repetitive TMS on the coupling between motor areas in the human. Experimental Brain Research. 2003;149:107–113. doi: 10.1007/s00221-002-1344-x. [DOI] [PubMed] [Google Scholar]
- 62.Jing HK, Takigawa M. Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clinical Neurophysiology. 2000;111:1620–1631. doi: 10.1016/s1388-2457(00)00357-6. [DOI] [PubMed] [Google Scholar]
- 63.Serrien DJ, Strens LHA, Oliviero A, Brown P. Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neuroscience Letters. 2002;328:89–92. doi: 10.1016/s0304-3940(02)00499-8. [DOI] [PubMed] [Google Scholar]
- 64.Chen WH, Mima T, Siebner HR, Oga T, Hara H, Satow T, et al. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Journal of Neurophysiology. 2003;114:1628–1637. doi: 10.1016/s1388-2457(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 65.Cavada C, Goldmanrakic PS. Posterior Parietal Cortex in Rhesus-Monkey. 2. Evidence for Segregated Corticocortical Networks Linking Sensory and Limbic Areas with the Frontal-Lobe. Journal of Comparative Neurology. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- 66.Cavada C, Goldmanrakic PS. Posterior Parietal Cortex in Rhesus-Monkey. 1. Parcellation of Areas Based on Distinctive Limbic and Sensory Corticocortical Connections. Journal of Comparative Neurology. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 67.Bestmann S, Ruff CC, Blankenburg F, Weiskopf N, Driver J, Rothwell JC. Mapping causal interregional influences with concurrent TMS-fMRI. Experimental Brain Research. 2008;191:383–402. doi: 10.1007/s00221-008-1601-8. [DOI] [PubMed] [Google Scholar]
- 68.Bestmann S, Swayne O, Blankenburg F, Ruff CC, Haggard P, Weiskopf N, et al. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cerebral Cortex. 2008;18:1281–1291. doi: 10.1093/cercor/bhm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shafi MM, Westover MB, Oberman L, Cash SS, Pascual-Leone A. Modulation of EEG Functional Connectivity Networks in Subjects Undergoing Repetitive Transcranial Magnetic Stimulation. Brain Topography. doi: 10.1007/s10548-013-0277-y. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ridding MC, Taylor JL, Rothwell JC. The Effect of Voluntary Contraction on Corticocortical Inhibition in Human Motor Cortex. Journal of Physiology-London. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eldaief MC, Halko MA, Buckner RL, Pascua-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, et al. Resting-State Cortico-Thalamic-Striatal Connectivity Predicts Response to Dorsomedial Prefrontal rTMS in Major Depressive Disorder. Neuropsychopharmacology. doi: 10.1038/npp.2013.222. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kozel FA, Rao U, Lu H, Nakonezny PA, Grannemann B, McGregor T, et al. Functional connectivity of brain structures correlates with treatment outcome in major depressive disorder. Frontiers in Psychiatry. 2011;2:1–7. doi: 10.3389/fpsyt.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Wingen GA, Tendolkar I, Urner M, Van Marle H, Denys D, Verkes RJ, et al. Short-term antidepressant administration reduces default mode and task-positive network connectivity in healthy individuals during rest. Neuroimage. doi: 10.1016/j.neuroimage.2013.11.022. (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.