Abstract
Angiogenesis is an important component of wound healing. Mobilization of circulating angiogenic cells (CACs) has been observed in patients with cutaneous burn wounds, but a systematic exploration of the phenomenon in an animal model has not been carried out. Using a murine model, in which burn depth can be varied precisely, and a validated culture method for quantifying circulating CACs, we found that increasing burn depth resulted in a progressive delay in the time to mobilization of circulating CACs. This delay in CAC mobilization was associated with a delay in perfusion and vascularization of the burn wound tissue. Analysis of CACs in the peripheral blood of human burn patients, using the same culture assay, confirmed results previously obtained by flow cytometry, that CAC levels peak early after the burn wound, and point to the clinical relevance of findings from the murine model.
INTRODUCTION
Burn injuries remain a major public health problem in the United States and around the world. It is estimated that more than one million Americans require medical attention each year for burn injuries (1–3). Superficial burns heal with minimal scarring. Deep third degree burns usually require skin grafting to achieve wound closure, but the cosmetic and functional result is less than optimal because the grafted skin is thin and vulnerable to re-injury. Burns heal with variable results and the factors that determine whether these burns will heal with skin regeneration rather than with a fibrous scar are currently unknown (4–6).
As classically described by Jackson, the angiogenic response to thermal injury appears to be a critical determinant of outcome for these wounds (7). The central region of vascular coagulation proceeds to full thickness tissue loss, and the outer region of hyperemia heals without scar. Between them is the zone of stasis where outcomes are variable and intervention might be beneficial. Injury and repair of the dermal vasculature seems to be a major determinant of whether burns heal promptly and primarily, or whether the healing is delayed leading to damaging scarring. Dermal blood flow is reduced in more severe burn wounds below levels seen in more superficial first-degree burns, as determined by laser Doppler perfusion imaging (LDPI), which utilizes a near-infrared laser diode to measure subcutaneous blood flow as a function of light scattering (Doppler shift) by moving red blood cells (5, 8–13).
A recent advance in the understanding of the cellular mechanisms controlling vascularization in response to tissue injury has been the discovery of endothelial progenitor cells (14) and other circulating angiogenic cells (CACs). (15). Angiogenic cytokines activate endogenous endothelial cells and also recruit circulating angiogenic cells (CACs), a term used here to denote a heterogeneous population of cells that have potential to participate in various aspects of angiogenesis. They include endothelial progenitor cells, which incorporate into the endothelium of new or remodeling vessels, as well as myeloid, mesenchymal, and hematopoietic stem cells, which promote vascular growth and remodeling through production of angiogenic cytokines. CACs are released from blood vessels, bone marrow, and other sites in response to the production of angiogenic cytokines at the site of tissue wounding. Following mobilization into the circulation, CACs home to the wound, where they participate in angiogenesis.
CACs in peripheral blood are quantified either by flow cytometric analysis, using antibodies against endothelial and progenitor cell surface proteins, or by culturing mononuclear cells (MNCs) in the presence of endothelial growth factors and scoring for cells with the ability to bind lectin and take up acetylated low density lipoprotein (LDL) (14–17). Prior studies in humans have identified CACs in peripheral blood of patients who have suffered thermal injury. VEGFR2+/CD15− cells appeared in the peripheral blood of individuals suffering burns within 12 hours after injury (18). CD45−/CD133+/CD144+/VEGFR2+ cells were significantly increased in the peripheral blood at 24 hours after burn wounding as compared to healthy control subjects(19).
A major limitation of the human studies is that an individual patient may manifest a wide range of tissue damage from superficial to severe, such that it is difficult to determine the precise relationship between the degree of tissue damage and the presence or absence of reparative responses. To address this issue, we have utilized a murine model in which uniform, graded severity of thermal injury could reliably be produced. The branding iron type burn (9, 20) was chosen over scald or flame burn (21–23). We prefer this model because it allowed us to produce burns of graded severity. We have used this model to investigate the relationship between burn severity and angiogenesis, as manifested by CAC mobilization, LDPI of wound tissue, and vascular morphometry.
MATERIALS AND METHODS
Burn wound protocol
Male 8-week-old 129S1/SvImJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All procedures were approved by The Johns Hopkins University Animal Care and Use Committee. Mice were anesthetized by intraperitoneal injection of ketamine and xylazine, shaved on the dorsum, and depilated with Nair cream (Church & Dwight Co., Princeton, NJ). A burn wound protocol previously established in rats (9, 20) was adapted for use in mice. A custom-made 220-g aluminum rod was heated in a 100°C water bath for 5 minutes. Two burns of 1.2-cm diameter each were produced on the dorsum of the animals. Contact time of 4, 6, or 8 seconds was measured with a standardized metronome. These contact times were chosen to produce burns of increasing severity. Fluid resuscitation was performed according to the Parkland formula (4 ml/kg × % body surface area wounded) by intraperitoneal injection of saline within one hour after burning (i.e., 2 ml of saline was administered to a 25-gram mouse with a burn covering 20% of body surface area). Buprenorphine was administered for analgesia. Blood samples for CAC analysis were obtained by cardiac puncture under ketamine-xylazine anesthesia.
Histology
Burn wounds were harvested with a rim of normal skin. Specimens from each site were bisected at the center and fixed in 10% buffered formalin solution overnight. Five-µm-thick paraffin-embedded sections were stained with hematoxylin and eosin and analyzed by light microscopy. Scar thickness was measured at its maximal dimension in the area laden with collagen fibers and granulation tissue immediately below the epithelium using an Olympus CK2 light microscope with a WHK 12.5XLH micrometer.
Analysis of CACs in mice
CACs were analyzed as previously described (24). Peripheral blood MNCs were isolated by Histopaque-1083 (Sigma-Aldrich, St. Louis, MO) density-gradient centrifugation. Residual red blood cells were lysed by addition of ammonium chloride solution (Stem Cell Technologies, Vancouver, Canada). MNCs were seeded on 96-well plates coated with rat vitronectin (Sigma-Aldrich) at 1.5 × 106 cells/cm2 and cultured in endothelial basal medium 2 supplemented with EGM-2-MV SingleQuots (Lonza, Allendale, NJ). After 4 days in culture, the cells were incubated with DiI-labeled acetylated LDL (Invitrogen, Carlsbad, CA) in the media at a concentration of 10 µg/ml at 37°C for 2 hours. The cells were then fixed with 4% paraformaldehyde (Sigma-Aldrich) for 10 minutes, and incubated with FITC-labeled lectin from Bandeira simplicifolia (Sigma-Aldrich) at a concentration of 10 µg/ml for 1 hour, counterstained with 4,6-diamidino-2-phenylindole dihydrochloride (Invitrogen), and DiI+/FITC+ cells were counted under fluorescence microscopy(17).
LDPI
Blood flow in wound areas was measured by 632.6-nm, He-Ne scanning laser Doppler imaging (Lisca Development AB, Linkoping, Sweden), which utilizes a near-infrared laser diode to measure subcutaneous blood flow as a function of light scattering by moving red blood cells. The imager uses a low power (1 mW) helium laser beam to sequentially scan the tissue through several thousand measurement points. A photodiode in the tile scanner head detects back-scattered light that has been frequency shifted by moving red blood cells according to the Doppler principle. For each measurement point, a signal is generated that scales linearly with tissue perfusion defined as the product of the blood cell velocity and concentration. This signal, termed the laser Doppler perfusion index was represented as a two-dimensional color image on a computer screen. The colors produced illustrate the spectrum of perfusion in the wound: dark blue depicts the lowest level of perfusion and red the highest. A photographic image was produced simultaneously, which allowed for direct anatomical comparison of corresponding areas of burn. For each burn, the wound site was selected by drawing freehand around an area of interest after exporting the image into the software package LDISOFT (Lisca Development AB). Then, the mean LDPI value within this area of interest was computed. The scanner was positioned 50 cm above each animal and scans were performed on days 3 and 7 to assess blood flow in the wound.
Immunohistochemistry
Mouse wound tissues were fixed with IHC zinc fixative (formalin-free; BD Pharmingen, San Diego, CA) for 24 hours and 5-µm-thick paraffin sections were prepared. To prevent non-specific binding, 100 µl of blocking solution containing 2% normal rabbit serum for CD31 immunohistochemistry or horse serum for α-smooth muscle actin (SMA) immunohistochemistru (Jackson ImmunoResearch) was applied for 30 minutes, and then 100 µl of primary anti-CD31 antibody (1:50 dilution; BD Pharmingen) or anti-α-smooth muscle actin antibody (1:200 dilution; Sigma-Aldrich) was applied to the sections for 1 hour at room temperature. The sections were further incubated with biotinylated secondary antibody (1:500 dilution; Vector Laboratories, Burlingame, CA). Streptavidin-biotin-horseradish peroxidase was used for signal amplification and diaminobenzidine was used for staining (Vector Laboratories). Counter-staining was performed with hematoxylin and nuclear fast red for 30 seconds respectively. 3% H2O2 (Fisher Scientific, Fair Lawn, NJ) was used for blocking endogenous peroxidase activity. All slides were examined in a blinded manner.
Wound area measurement
On days 0, 3, 7 and 14, the wound borders were traced in situ onto clear acetate paper. Images were digitized at 600 dpi (Paperport 6000, Visioneer, Fremont, CA). Wound areas (in pixels) were calculated using NIH Image software (Scion Image, Frederick, MD).
Human burn patients
Patients in the Johns Hopkins Burn Center were enrolled in the study using an IRB approved protocol. Inclusion criteria included burn of 1–70% body surface area, age 18–75 years, and evidence of hemodynamic stability with normal blood pressure, pulse and urine output. Written consent was obtained from each patient in accordance with the Declaration of Helsinki protocols. Clinical information related to the burn injury was collected. Venous blood was drawn at intervals after the burn injury with a maximum of two samples in a 7-day period.
Analysis of CACs in human burn patients
CACs were analyzed as previously described (16). MNCs were isolated from peripheral blood by Ficoll (Amersham Biosciences, Piscataway, NJ) density gradient centrifugation. Residual red blood cells were lysed by addition of ammonium chloride solution (Stem Cell Technologies). MNCs were seeded on 24-well plates coated with human fibronectin at 1.5×106 cells/well and cultured in endothelial basal medium 2 with EGM-2-MV SingleQuots (Lonza). After 4 days in culture, the cells were incubated with DiI-labeled acetylated LDL (Invitrogen), fixed with 4% paraformaldehyde (Sigma-Aldrich), incubated with FITC-labeled lectin from Ulex europaeus (Sigma-Aldrich) at a concentration of 10 µg/ml for 1 hour, counterstained with 4,6-diamidino-2-phenylindole dihydrochloride (Invitrogen), and DiI+/FITC+ cells were counted under fluorescence microscopy.
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). Differences in means between groups were analyzed for significance by Student’s t-test or ANOVA as appropriate using Sigma Stat (SYSTAT Software Inc., Point Richmond, CA).
RESULTS
Establishment of a murine burn model with uniform, graded wound severity
To produce burn injury, an aluminum rod was heated in a 100°C water bath for 5 minutes and two 1.2-cm-diameter cutaneous burns were produced on the dorsum of each mouse, representing approximately 20% of the body surface area. To ensure burn uniformity, only the weight of the aluminum rod itself provided pressure to the skin surface. To generate wounds of varying severity, the rod was placed in contact with the skin for 4, 6 or 8 seconds in different mice.
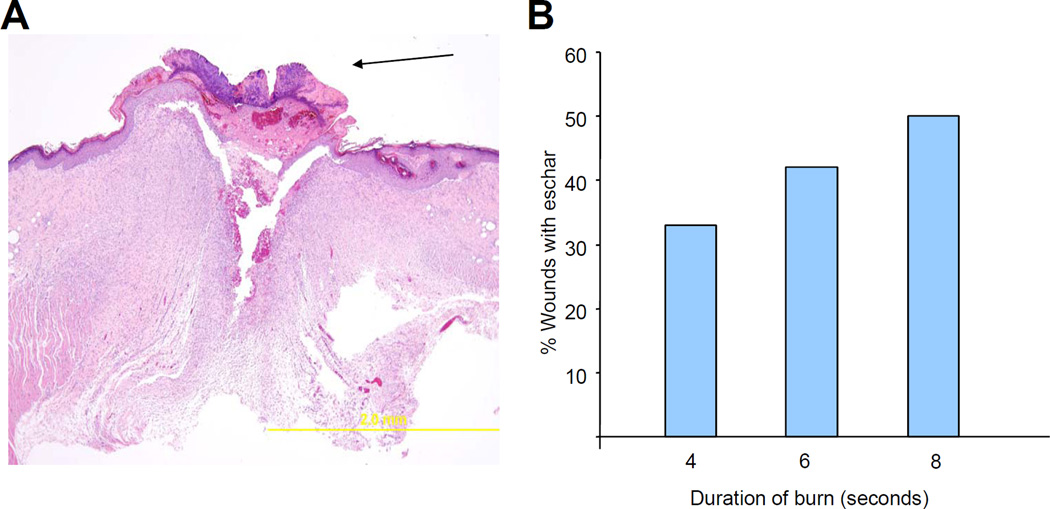
As burn contact time was increased from 4 to 8 seconds, the severity of burn injury increased, as determined by histological analysis on day 21. The presence of an eschar, composed of coagulated protein, indicates that the epithelial covering of the wound is not intact. The frequency with which an eschar was observed on the burn wound at day 21 tended to increase progressively as the burn contact time increased from 4 to 8 seconds. (Figure 1a)
Figure 1. Histological analysis.
(a) Eschar. Left panel: Photomicrograph shows an eschar (arrow) with inflammatory infiltrate at 21 days after burn wounding. Right panel: Bar graph shows the percentage of wounds with eschar on day 21. (b) Scar thickness. Left panel: Measurement of scar thickness in a hematoxylin- and eosin-stained section. Right panel: Bar graph shows mean (± SEM) scar thickness on day 21 (*P<0.05, ANOVA with Tukey test, n = 12).
A superficial burn heals without fibrous scarring, while deeper burn wounds heal with thick fibrous scars, which can contract and distort surrounding tissues, and result in a poor cosmetic and functional result. The thickness of the burn scar was measured in the center of the wound. With increasing burn duration, scar thickness on day 21 was found to increase significantly (P<0.05, one-way ANOVA with Tukey test; Figure 1b). Taken together, the results in Figure 1 demonstrate the graded severity of burn wounds as a function of contact time in this mouse model.
Effect of burn severity on CAC mobilization
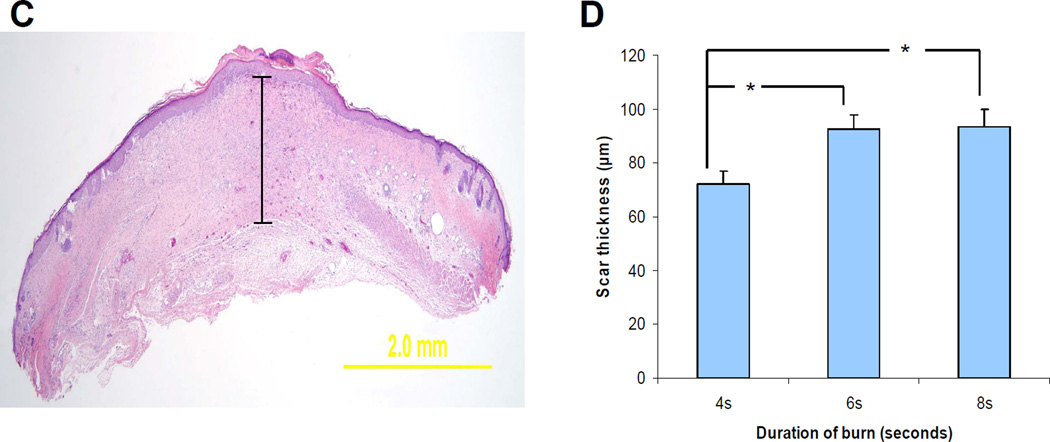
Having established the quantitative nature of the model, we investigated the effect of burn wound severity on CAC mobilization. MNCs were isolated from peripheral blood, cultured in the presence of endothelial growth factors for 4 days, and analyzed by fluorescence microscopy (Figure 2a) for the uptake of 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine (DiI)-labeled acetylated LDL (red fluorescence) and the binding of fluorescein isothiocyanate (FITC)-labeled lectin (green fluorescence). The double positive cells (yellow fluorescence) represent CACs (17). The number of CACs that were present in the peripheral blood increased in all burn groups, but the kinetics of mobilization was dependent on the burn duration. The peak mobilization of CACs was on day 3 after a burn of 4 seconds duration (Figure 2b), day 4 after a burn of 6 seconds (Figure 2c), and day 6 after a burn of 8 seconds (Figure 2d). In addition, the magnitude of the peak decreased with increasing burn duration. In all cases, the mobilization was remarkably transient.
Figure 2. Analysis of circulating angiogenic cells (CACs).
Analysis of CACs in the peripheral blood of mice subjected to burns of increasing duration. Mouse peripheral blood mononuclear cells cultured in the presence of endothelial growth factors were stained with FITC-lectin (green) and DiI-acetylated LDL (red). (a) Mice were subjected to 4-second (b), 6-second (c), and 8-second (d) burns and peripheral blood was analyzed for the presence of CACs on the indicated day after burn wounding (day 0, non-burned controls). *P<0.05 compared to day 0 (Student’s t test).
Effect of burn severity on tissue perfusion
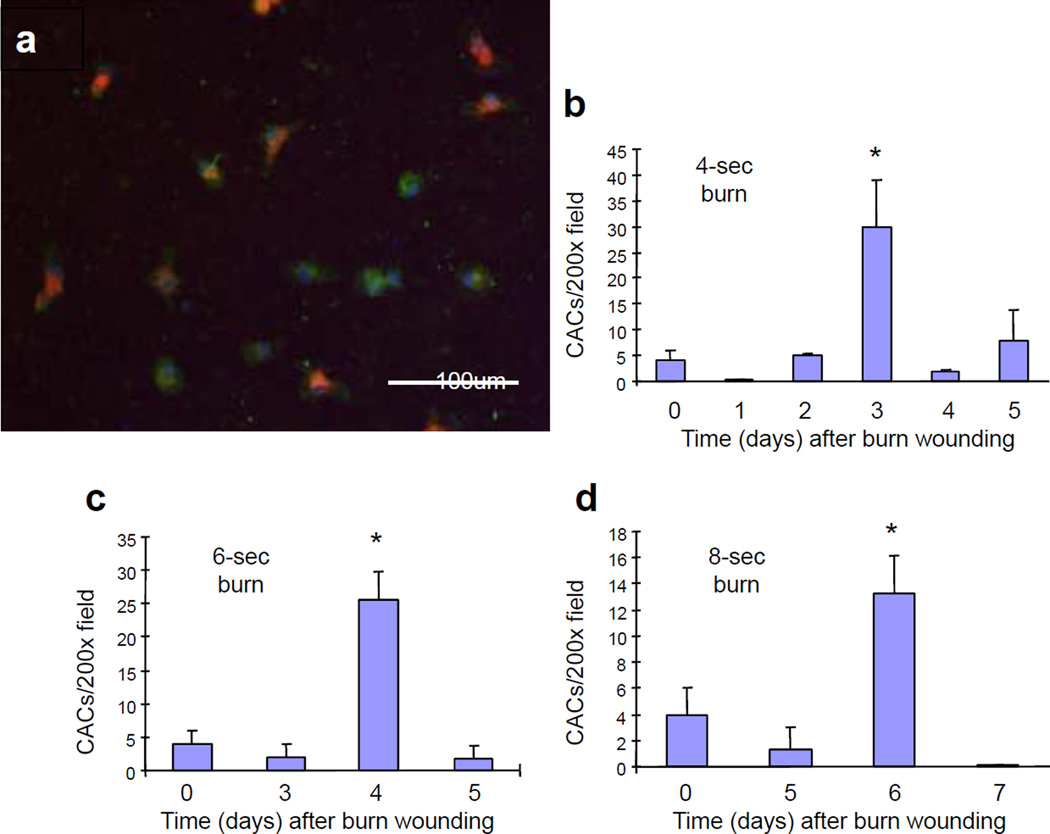
We reasoned that if the mobilization of CACs played an important role in the increased tissue perfusion that is required for effective wound healing, then the delay in CAC mobilization should result in a delay in tissue perfusion. To test this hypothesis, blood flow was measured by LDPI on days 3, 7, and 14 after burn wounding. The increase in wound perfusion on day 7 was progressively impaired as the severity of the burn increased (P<0.001, two-way ANOVA with Tukey test; Figure 3). By day 14, when the wound areas were approximately 30% of their original size, tissue perfusion was returning to normal levels and differences between groups were no longer observed. Thus, burn severity affected the magnitude of the increase in blood flow associated with wound healing.
Figure 3. Analysis of wound blood flow.
Laser Doppler perfusion imaging was performed on days 3, 7, and 14 after burning. Mean (± SEM) blood flow (*P<0.01, ANOVA with Tukey test, n = 8 for each group).
Effect of burn severity on wound vascularization
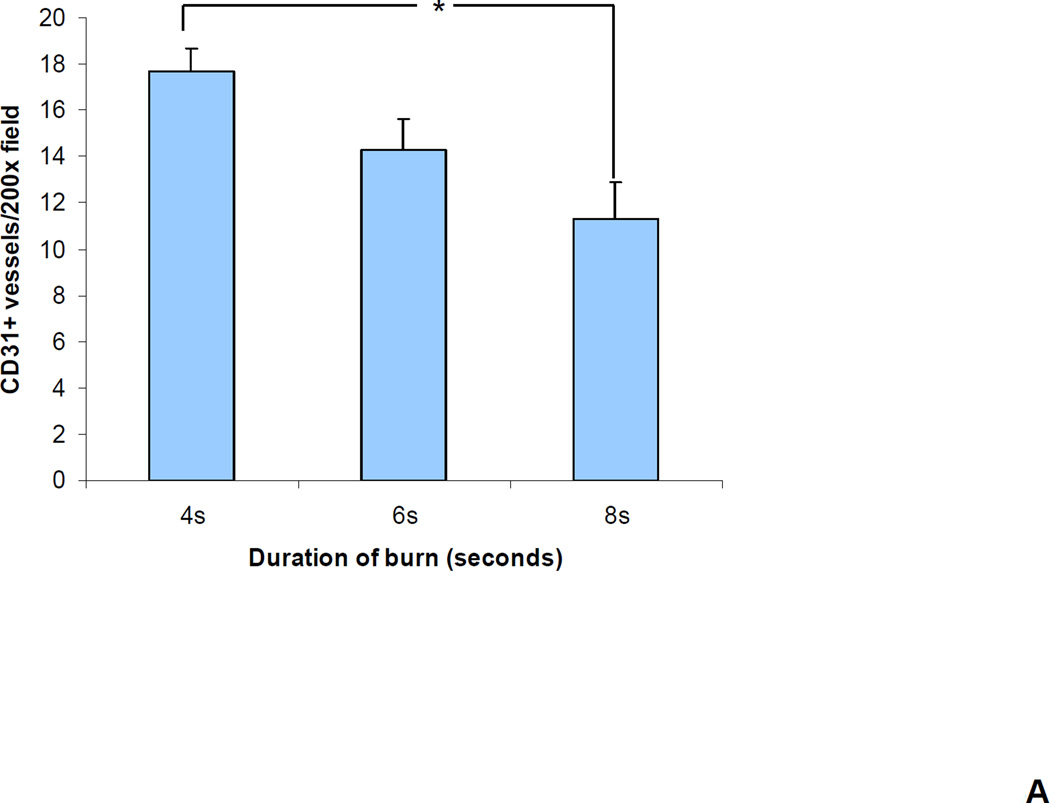
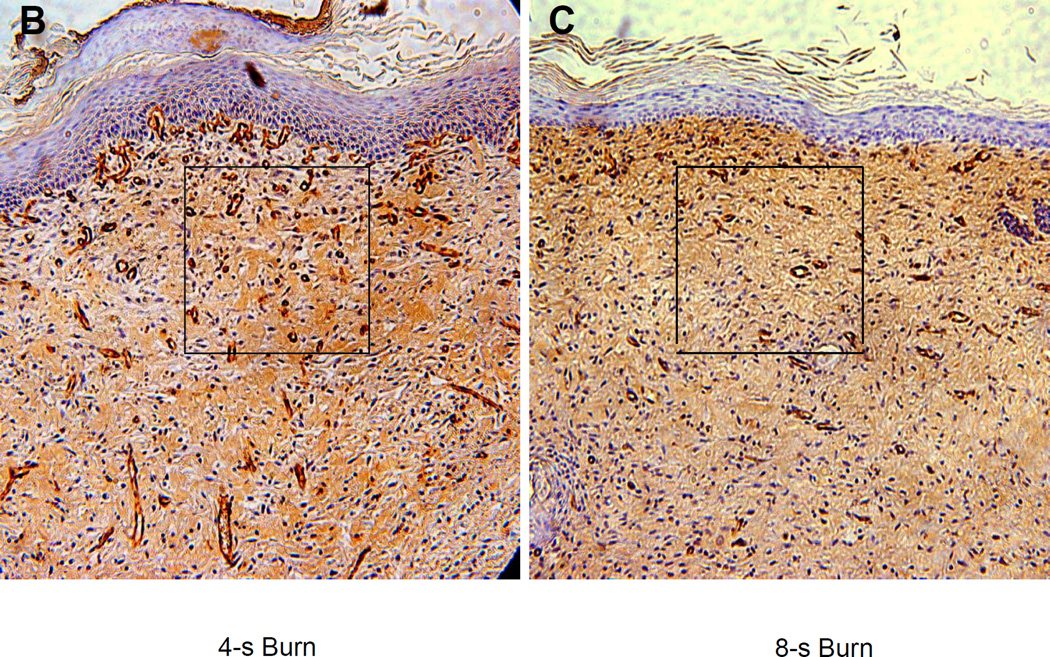
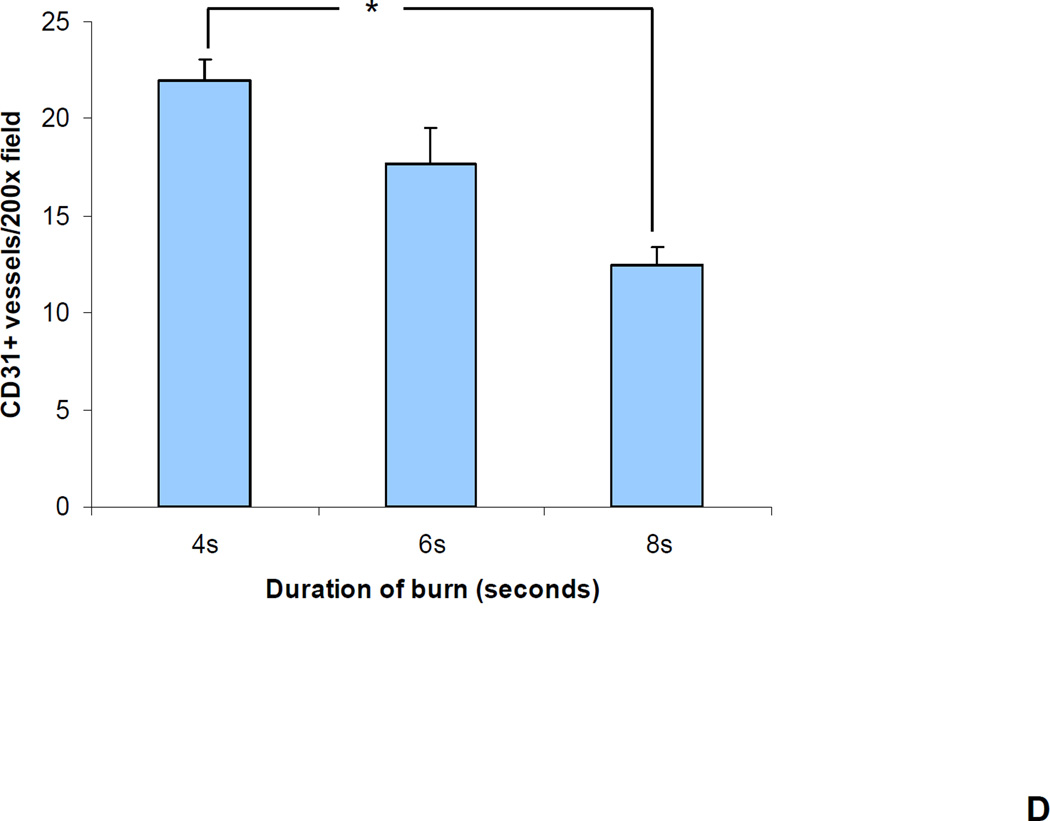
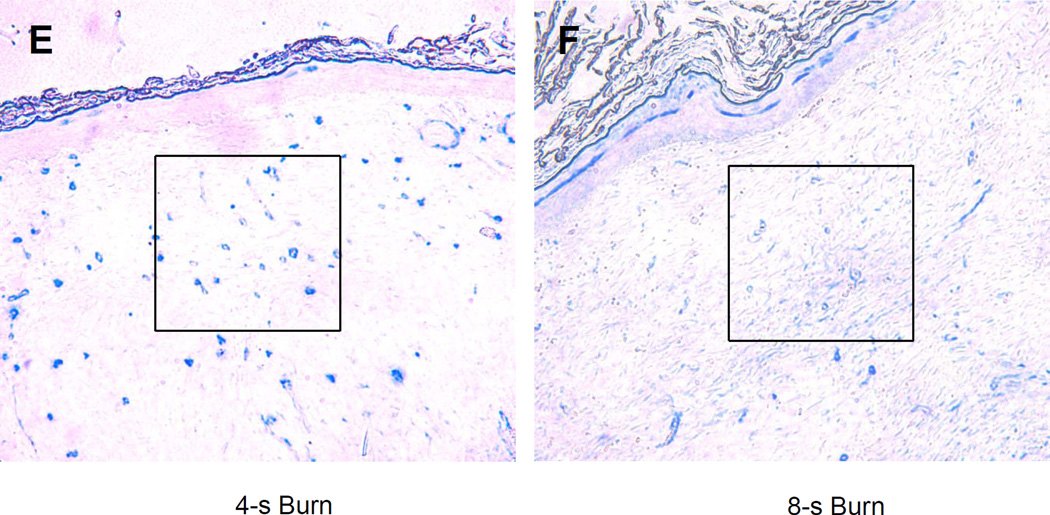
We reasoned that the differences in perfusion reflected differences in tissue vascularization. To test this hypothesis, vascular morphometry was performed on sections of the excised wounds on day 21 by immunohistochemical staining for CD31 and smooth muscle actin (SMA). The number of CD31+ or SMA+ vessels within a 200× field at the center of each wound was determined. There was a progressive decrease in the number of CD31+ (Figure 4a–b) and SMA+ (Figure 4c–d) vessels as burn duration increased from 4 to 8 seconds. (P<0.05, one-way ANOVA with Tukey test, for both CD31 and SMA).
Figure 4. Immunohistochemical analysis of wound vascularization.
(a–b) CD31 staining. (a) Bar graph shows the mean (± SEM) vessel counts per 200× field (*P<0.01, ANOVA with Tukey test, n = 8 for each group). (b) The central portion of a 4 and 8 second burn wound on day 21 stained with an antibody against CD31 (PECAM-1), which is expressed by vascular endothelial cells. (c–d) SMA staining. (c) Bar graph shows the mean (± SEM) number of vessels (*P<0.05, ANOVA with Tukey test, n = 8 for each group). (d) The central portion of a 4 and 8 second burn wound on day 21 stained with an antibody against smooth muscle actin (SMA), which is expressed by vascular pericytes and smooth muscle cells.
Effect of burn severity on wound closure
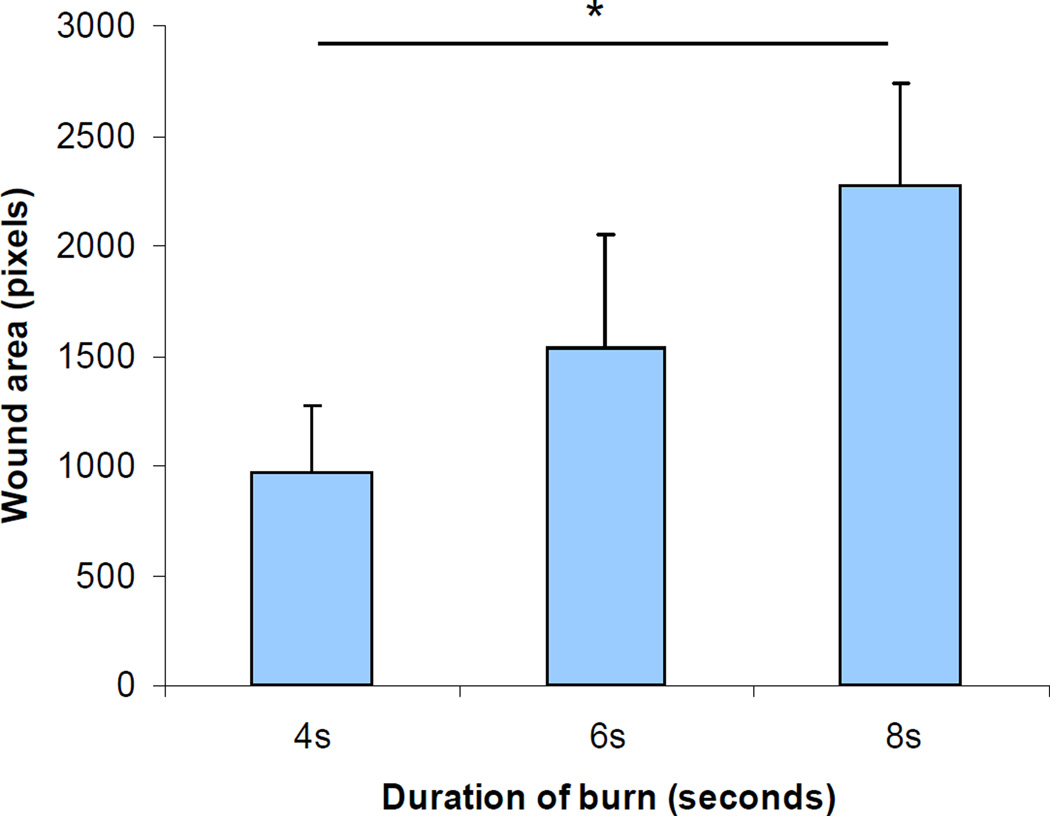
We reasoned that impaired vascularization would lead to impaired wound closure. To test this hypothesis, wound closure on day 21 was analyzed by computerized planimetry. As burn duration increased, wound closure was significantly delayed (P<0.05, one-way ANOVA with Tukey test; Figure 5).
Figure 5. Analysis of burn wound closure.
The wound area was measured by computer-assisted planimetry on day 21. The mean (± SEM) wound area is plotted in the bar graph (*P<0.001, ANOVA with Tukey test, n = 8 for each group).
CAC mobilization after thermal injury in humans
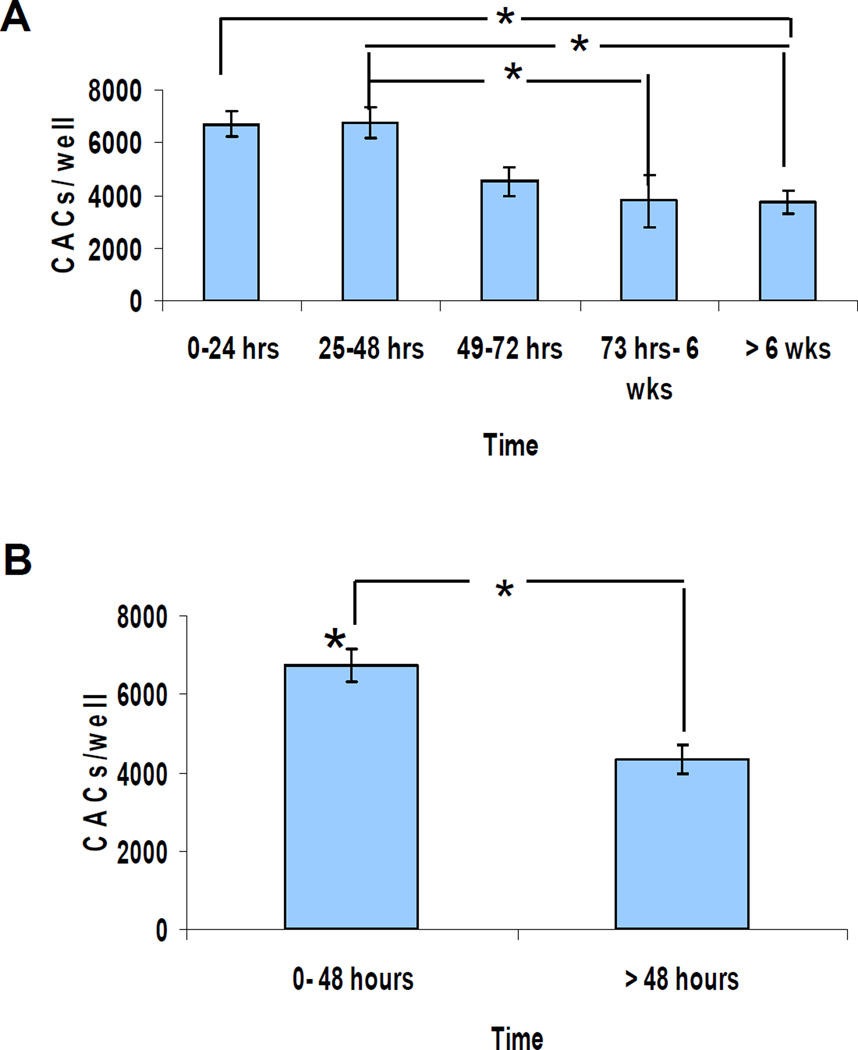
To investigate the clinical relevance of findings from the murine model, we analyzed CAC mobilization in burn wound patients using the same validated culture assay (16). Thirty one patients (24 males and 7 females) were enrolled for the study. The mean age was 39 years (range: 19–59). Mean burn surface area was 13% (range: 1–64%). One to three serial blood samples were obtained from each patient. Circulating CACs were increased at the earliest time point examined (between 12 and 24 hours) in all patients and remained elevated through 48 hours (Figure 6). On day 3, the number of circulating CACs started to decline, and they reached a plateau that extended out to greater than 6 weeks from the time of injury, suggesting a return to baseline levels.
Figure 6. CAC levels in burn patients at intervals after injury.
(a) Serial analysis of CACs in burn patients. CACs were quantified in blood samples (n = 7, 15, 7, 5, and 8 for the sequential time periods studied; P<0.05, ANOVA). (b) Comparison of samples drawn in the first 48 hours after injury with later samples. The data were pooled into two groups (*P<0.001, Student’s t test, n = 22 samples in the <48 hours group and n = 20 in the >48 hours group).
DISCUSSION
Previous clinical studies have revealed increased numbers of circulating CACs following burn wounding (18, 19). This study of the phenomenon in the murine model allows for a more systematic evaluation of the factors that influence the dynamics of mobilization of CACs than is possible within the constraints of a clinical study. Increased mobilization might result from increased tissue ischemia resulting in increased production of angiogenic factors that induce CAC mobilization, as is seen in limb and cardiac ischemia models (15, 24). Alternatively, tissue destruction might result in the loss of cells capable of producing these factors.
To answer this question, we have utilized a murine burn wound model, in which increasing duration of burn contact resulted in burn wounds of increasing severity. We have demonstrated that burns of increasing severity result in delayed and diminished mobilization of CACs. Impaired CAC mobilization and reduced blood flow were observed in wounds of increasing severity, which suggests that the progressive impairment of perfusion associated with increasing burn duration is caused not only by direct destruction of pre-existing vasculature with more severe burns, but also by impaired neovascularization resulting from the delay in CAC mobilization. Further studies are needed to test this hypothesis.
In contrast to the sharp peaks of CAC mobilization in the murine model, the clinical study revealed a sustained increase in CACs over the first 48 hours after burn wounding. Extensive human burns include areas of both superficial and deep wounding and thus the sustained increase in CACs may represent integration of the early and delayed responses that were observed in the murine model. However, there are likely to be many other factors that contribute to differences between the experimental and clinical data. The reduced magnitude of CAC mobilization in the patients may reflect the effect of aging or cardiovascular disease risk factors (16, 17, 24).
The mouse model will be useful for investigating the cellular and molecular mechanisms by which aging, genetic background, and chronic diseases such as diabetes influence burn wound healing. If CAC mobilization and wound perfusion are important determinants of clinical outcome, then strategies designed to augment angiogenic responses may improve outcome in patients with severe burn wounds. Further analysis of the molecular mechanisms regulating CAC mobilization and recruitment to burn wounds in the mouse model will provide a scientific foundation for the rational development of effective therapies.
ACKNOWLEDGEMENTS
Funding for this project was provided by Public Health Service grant P20-GM78494 from the National Institutes of Health, the Hendrix Burn Fund, Beijing Nova Star Plan/Grant No. 2004B29, and the Johns Hopkins Institute for Cell Engineering.
Abbreviations
- DiI
1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine
- CAC
circulating angiogenic cell
- FITC
fluorescein isothiocyanate
- LDL
low density lipoprotein
- LDPI
laser Doppler perfusion imaging
- VEGFR
vascular endothelial growth factor receptor
- MNC
mononuclear cell
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- 1.Brigham PA, McLoughlin E. Burn incidence and medical care use in the united states: Estimates, trends, and data sources. J Burn Care Rehabil. 1996 Mar-Apr;17(2):95–107. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kerby JD, McGwin G, Jr, George RL, Cross JA, Chaudry IH, Rue LW., 3rd Sex differences in mortality after burn injury: Results of analysis of the national burn repository of the american burn association. J Burn Care Res. 2006 Jul-Aug;27(4):452–456. doi: 10.1097/01.BCR.0000225957.01854.EE. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan RL. Burn care: Results of technical and organizational progress. JAMA. 2003 Aug 13;290(6):719–722. doi: 10.1001/jama.290.6.719. [DOI] [PubMed] [Google Scholar]
- 4.Druecke D, Lamme EN, Hermann S, Pieper J, May PS, Steinau HU, Steinstraesser L. Modulation of scar tissue formation using different dermal regeneration templates in the treatment of experimental full-thickness wounds. Wound Repair Regen. 2004 Sep-Oct;12(5):518–527. doi: 10.1111/j.1067-1927.2004.012504.x. [DOI] [PubMed] [Google Scholar]
- 5.Devgan L, Bhat S, Aylward S, Spence RJ. Modalities for the assessment of burn wound depth. J Burns Wounds. 2006 Feb 15;5:e2. [PMC free article] [PubMed] [Google Scholar]
- 6.Tredget EE. The basis of fibrosis and wound healing disorders following thermal injury. J Trauma. 2007 Jun;62(6 Suppl):S69. doi: 10.1097/TA.0b013e318065ae84. [DOI] [PubMed] [Google Scholar]
- 7.Jackson DM. The diagnosis of the depth of burning. Br J Surg. 1953 May;40(164):588–596. doi: 10.1002/bjs.18004016413. [DOI] [PubMed] [Google Scholar]
- 8.O'Reilly TJ, Spence RJ, Taylor RM, Scheulen JJ. Laser doppler flowmetry evaluation of burn wound depth. J Burn Care Rehabil. 1989 Jan-Feb;10(1):1–6. doi: 10.1097/00004630-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Kim DE, Phillips TM, Jeng JC, Rizzo AG, Roth RT, Stanford JL, Jablonski KA, Jordan MH. Microvascular assessment of burn depth conversion during varying resuscitation conditions. J Burn Care Rehabil. 2001 Nov-Dec;22(6):406–416. doi: 10.1097/00004630-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Holland AJ, Martin HC, Cass DT. Laser doppler imaging prediction of burn wound outcome in children. Burns. 2002 Feb;28(1):11–17. doi: 10.1016/s0305-4179(01)00064-x. [DOI] [PubMed] [Google Scholar]
- 11.Jeng JC, Bridgeman A, Shivnan L, Thornton PM, Alam H, Clarke TJ, Jablonski KA, Jordan MH. Laser doppler imaging determines need for excision and grafting in advance of clinical judgment: A prospective blinded trial. Burns. 2003 Nov;29(7):665–670. doi: 10.1016/s0305-4179(03)00078-0. [DOI] [PubMed] [Google Scholar]
- 12.Barachini P, Vezzoni GM, Palombo C, Franzoni F, Bigalli G. Skin blood flow pattern in burns outcomes. Burns. 2004 Jun;30(4):312–316. doi: 10.1016/j.burns.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Hemington-Gorse SJ. A comparison of laser doppler imaging with other measurement techniques to assess burn depth. J Wound Care. 2005 Apr;14(4):151–153. doi: 10.12968/jowc.2005.14.4.26757. [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997 Feb 14;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 15.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: Part II: Cell-based therapies. Circulation. 2004 Jun 8;109(22):2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 16.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001 Aug;108(3):391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001 Jul 6;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 18.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001 Feb 2;88(2):167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 19.Fox A, Smythe J, Fisher N, Tyler MP, McGrouther DA, Watt SM, Harris AL. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br J Surg. 2008 Feb;95(2):244–251. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 20.Light TD, Jeng JC, Jain AK, Jablonski KA, Kim DE, Phillips TM, Rizzo AG, Jordan MH. The 2003 carl A moyer award: Real-time metabolic monitors, ischemia-reperfusion, titration endpoints, and ultraprecise burn resuscitation. J Burn Care Rehabil. 2004 Jan-Feb;25(1):33–44. doi: 10.1097/01.BCR.0000105344.84628.C8. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch T, von Peter S, Dubin G, Mittler D, Jacobsen F, Lehnhardt M, Eriksson E, Steinau HU, Steinstraesser L. Adenoviral gene delivery to primary human cutaneous cells and burn wounds. Mol Med. 2006 Sep-Oct;12(9–10):199–207. doi: 10.2119/2006-00031.Hirsch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi M, Yoshida T, Takeuchi D, Jones VC, Shigematsu K, Herndon DN, Suzuki F. Gr-1+CD11b+ cells as an accelerator of sepsis stemming from pseudomonas aeruginosa wound infection in thermally injured mice. J Leukoc Biol. 2008 Mar 27; doi: 10.1189/jlb.0807541. [DOI] [PubMed] [Google Scholar]
- 23.Mizutani A, Enkhbaatar P, Esechie A, Traber LD, Cox RA, Hawkins HK, Deyo DJ, Murakami K, Noguchi T, Traber DL. Pulmonary changes in a mouse model of combined burn and smoke inhalation-induced injury. J Appl Physiol. 2008 Apr 24; doi: 10.1152/japplphysiol.00232.2007. [DOI] [PubMed] [Google Scholar]
- 24.Bosch-Marcé M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion following limb ischemia. Circ Res. 2007 Oct 11; doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]