Abstract
Importance
Medication non-adherence, which has been estimated to affect 28-31% of US patients with hypertension, hyperlipidemia, and diabetes, may be improved by electronic medication packaging (EMP) devices.
Objective
To investigate whether EMP devices are associated with improved adherence and to identify and describe common features of EMP devices.
Evidence Acquisition
We systematically reviewed peer-reviewed studies testing the effectiveness of EMP systems in the MEDLINE, EMBASE, PsycINFO, CINAHL, and International Pharmaceutical Abstracts databases from searches conducted to June 13, 2014. We extracted the associations between the interventions and adherence, as well as other key findings. We assessed each study for bias using the Cochrane Handbook for Systematic Reviews of Interventions. We qualitatively assessed features of EMP devices and interventions.
Results
37 studies (32 randomized and 5 non-randomized) including 4,326 patients met review criteria: 10 patient-interface-only “simple” interventions and 29 “complex” interventions integrated into the health care system (2 qualified for both categories). Overall, the effect estimates for mean adherence ranged from -2.9 to 34.0% and the effect estimates for the proportion of patients defined as adherent ranged from -8.0 to 49.5%. We identified 5 common EMP characteristics: recording dosing events and storing a record of adherence, audiovisual reminders to cue dosing, digital displays, real-time monitoring, and providing patients with adherence performance feedback.
Conclusion and Relevance
Many varieties of EMP exist. However, data supporting their use are limited, with variability in the quality of studies testing EMP devices and evidence of reporting bias. Devices that are integrated into the care delivery system and that are designed to record dosing events are most frequently associated with improved adherence. Higher quality evidence is needed to determine the effect, if any, of these low cost interventions on medication nonadherence and to identify their most useful components.
Introduction
Medication nonadherence is a common and increasingly recognized problem in health care delivery.1,2 Medication non-adherence is common among US patients with hypertension (28% nonadherence), hyperlipidemia (28%), and diabetes (31%).3 Non-adherence has been linked to important adverse health effects including stroke in hypertensive patients, higher viral load in patients with HIV, and hospitalization and mortality in patients with heart failure. 4-6 Medication non-adherence is also common in resource limited settings. Incomplete adherence has been identified as a mechanism for the development of drug resistance in tuberculosis, malaria, and HIV.7 Improved adherence could improve mortality from chronic noninfectious conditions, like cardiovascular disease, that continue to increase their relative disease burden in resource limited settings.8 Thus, addressing non-adherence is a worldwide priority.9
Patient education, self-monitoring programs, family therapy, psychological therapy, telephone follow-up, and other supportive care measures have shown variable effectiveness in reducing medication nonadherence.10 However, many of these interventions are resource-intensive, and require well-integrated health care systems. Addressing adherence through health information technology (HIT) is an alternate approach. Electronic pill monitors can now greet patients and remind them to take their medications, and provide alerts to physicians or other caregivers when preprogrammed drug-use schedules are missed.11 Such tools may help overcome troublesome aspects of other adherence interventions, such as unspecified content, variable delivery methods, and impracticality for clinical practice settings.12 As a result, HIT could promote efficient and low-cost improvements in adherence.13
To study the application of one form of HIT to medication non-adherence, we conducted a systematic review of the association of electronic medication packaging (EMP) with medication adherence. We identify and describe common features of EMP devices. EMP encompasses electronic devices integrated into the containers in which pills, inhalers, or other products are dispensed. EMP is applicable in resource-poor settings because it requires little health care system infrastructure, although it can also be used as part of complex interventions.
Methods
Data Sources
We systematically searched the MEDLINE, EMBASE, International Pharmaceutical Abstracts (IPA), and PsycINFO databases using the OvidSP gateway, and CINAHL and Sociological Abstracts via their respective interfaces, through October 1 2013. Literature reviews in related subject areas and the literature cited in known studies aided in formulating the search strategy and identifying a comprehensive list of search terms. In the OvidSP gateway, we conducted the following Boolean search: ((medication adherence or patient compliance).sh. or adheren*.ti,ab. or non-adheren*.ti,ab. or nonadheren*.ti,ab. or non-complian*.ti,ab. or noncomplian*.ti,ab.) and (prescription drug.sh. or drug*.ti. or medicat*.ti,ab. or pharmacother*.ti,ab. or pill*.ti,ab. or prescri*.ti,ab.) and ((technology.sh. or alarm*.ti,ab. or device*.ti,ab. or digital*.ti,ab. or electronic.ti,ab. or monitor*.ti,ab. or remind*.ti,ab. or remote.ti,ab. or technolog*.ti,ab.)). We performed similar searches within the other databases. All searches were conducted in the English language. Search results were imported into a single grouping using EndNote X5, and screened for duplicate entries. A follow-up search using the same criteria conducted on June 13 2014 did not find any new studies after October 1, 2013, but revealed one publication of a previously-identified study that reported data from a later follow-up date, so we used these updated data in our analyses.
Study selection
Studies were included if: (1) they involved EMP, defined as electronic adherence-promoting devices integrated into the packaging of a prescription medication; (2) the medication at issue was a tablet, capsule, eye drop, topical cream, or inhaled agent prescribed on a routine ongoing schedule of administration; and (3) the authors reported results from a study testing the effect of the EMP on medication adherence. Both studies reporting the effect of the EMP on the proportion of adherent patients (typically defined as medication possession ratio ≥80%; with medication possession ratio defined as the number of days with medication supply/number of days in observation interval) and studies reporting the effect on the mean level of adherence were considered. Studies acceptable for inclusion were randomized, nonrandomized, controlled, prospective, or retrospective study designs. Case reports were excluded.
We excluded studies that reported on health outcomes without showing adherence data because they could not show that the effects on health outcomes were necessarily associated with differences in adherence. Our definition of EMP excluded other HIT adherence interventions, such as multi-pill dispensers and mobile phone-based interventions. We excluded studies of medications prescribed on as-needed bases. Studies of children or other patients whose adherence was mediated through another party were excluded.
To reach consensus on applying these criteria, two authors (KDC and ASK) reviewed a 10% sample of the search results independently and compared their results. One author (KDC) then screened the remaining abstracts and titles to identify studies for further review. Manual reference mining of studies and other reviews supplemented the search results.
Data extraction and analysis
Data were extracted (KDC) and checked (KFH, ASK), with disagreements resolved by consensus. Variables included: device name and major features; medication(s) studied; number of patients studied; study length; adherence outcomes; and supplemental findings including health outcomes, cost effectiveness results, and satisfaction surveys. The studies were separated into relevant categories to facilitate evaluation. We first divided studies into whether the EMP interfaced directly with the patient alone (“patient-interface only”), was part of a broader intervention in which a physician, pharmacist, or other health professional would engage the patient in a targeted intervention to increase adherence with or without using EMP derived data (“integrated”), or studied both patient-interface-only and integrated types of interventions. For example, in patient-interface-only interventions, patients would receive their prescriptions in EMP only; by contrast, in integrated interventions, patients would receive their prescriptions in EMP and also receive additional interventions from health professionals to improve adherence, which could be centered on the EMP adherence data.
We reviewed the methods of each study to understand which EMP elements each device used. The devices were categorized into the following mutually exclusive groups: adherence recorder only; adherence recorder and audiovisual alarm; adherence recorder and liquid crystal display; adherence recorder, audiovisual alarm, and liquid crystal display; and adherence recorder and real time monitoring. We also noted whether the adherence monitoring data collected by the EMP device was used to provide feedback on performance to the patient. This information enabled us to qualitatively identify and describe common features of EMP devices. We estimated the adherence effect in each study as the difference in the proportion of patients adherent or the difference in mean level of adherence with its accompanying 95% confidence interval, and present results graphically by means of a forest plot.
Following data extraction, one author (KDC), unblinded to the results, assessed the studies for bias using the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions.14 Studies were assessed for selection, detection, attrition, reporting, and other sources of bias. Selection bias was determined based on whether the method of sequence generation was a high-quality method such as random number generation, coin tossing, or minimization versus low-quality methods such as date of birth, or alternating order and whether the allocation order was adequately concealed from those recruiting participants. Detection bias was based on whether the participants and the personnel conducting the study were blinded to participants’ trial arm allocations. Attrition bias was determined by the magnitude of participants lost to follow-up, whether the dropout was even or uneven between trial arms, and whether any differences could be detected between those who completed the study and those lost to follow-up. Selective reporting was determined based on whether the study provided the expected measures of adherence based on the data available to the researchers, ideally but not necessarily based on a pre-registered trial protocol. We also included a domain that would capture any additional sources of bias other than those already specified. As an additional step, a funnel plot was created and examined for evidence of publication bias.
Results
Search results and study sample
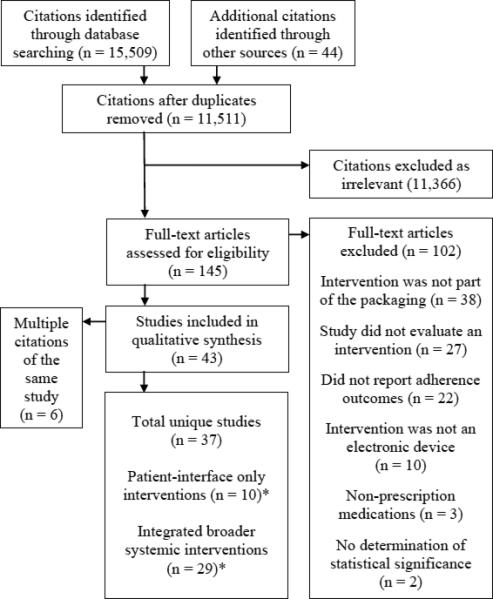
The search identified 11,511 publications, of which 11,366 were deemed irrelevant after reviewing the titles and abstracts. Among the remaining 145 articles, 102 were excluded after full-text review because they did not meet inclusion criteria. The 43 remaining articles described 37 unique studies including 4,326 patients, of which 10 were patient-interface only and 29 were more complex interventions integrated into broader systemic interventions (Figure 1).15-58 Two of the studies contained multiple intervention groups that qualified for both categories.18,21
Figure 1. Study flow diagram.
Flow diagram derived from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This diagram reports results of search through October 1 2013; a follow-up search through June 13 2014 revealed a publication reporting updated data from one of the publications identified here, so the updated data were used in our analysis.
* Of the 10 ‘patient only’ and 29 ‘integrated’ studies, 2 studies had one trial arm that fell into both categories, resulting in 37 studies in total.
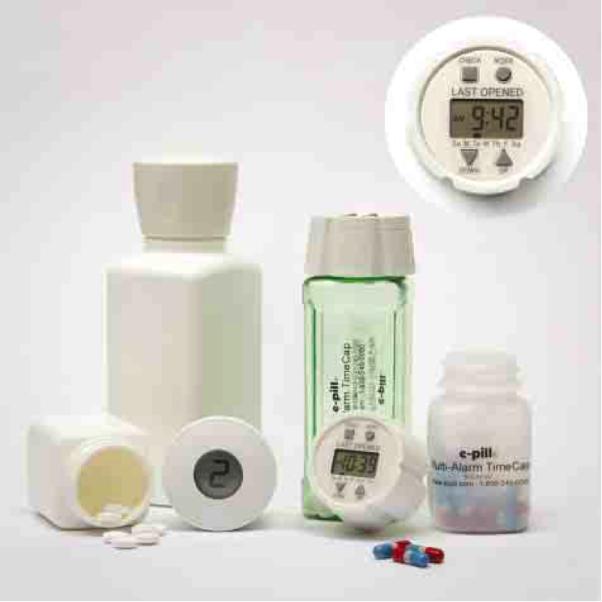
The final sample of studies (29 randomized controlled trials [RCTs], 3 crossover RCTs, 4 observational, 1 cohort) included experiences with 17 different devices and 14 different medical conditions: hypertension – 9; HIV – 6; psychiatric disorders – 4; diabetes/ hyperglycemia – 3; glaucoma – 3; asthma – 2; heart failure – 2; smoking cessation – 2; chronic obstructive pulmonary disease – 1; hyperlipidemia – 1; hyperparathyroidism – 1; inflammatory bowel disease – 1; platelet inhibition – 1; and renal transplant – 1. The number of patients ranged from 5 to 1,523 (median 50, interquartile range [IQR]: 25-133), and the interventions lasted from 1 to 24 months (median 5.5, IQR: 3-9). There were 22 studies of simple recorders, 6 of recorders with audio and or visual reminders, 5 of recorders with digital displays, 5 of recorders with audiovisual reminders and digital display, and 1 study of a device using real-time wireless monitoring. Examples of these features can be observed in Image 1, which contains pictures of representative devices.
Image 1. Examples of Electronic Medication Packaging.
Left, Medication Event Monitoring System 6 SmartCap (Aardex), which stores patients’ adherence record and, in this model, uses an LCD screen with a central number corresponding to vial openings since midnight and (not shown) indicators around the central number corresponding to hours since the last opening. Right and Inset, Prescript TimeCap (Wheaton Medical Technologies), which stores patients’ adherence records, uses an LCD screen with the time and day when the container was last opened and cues dosing with an audible beep and flashing LCD screen.
Patient-interface vs integrated interventions
Table 1 shows the studies of patient-interface-only devices. The majority of these studies did not find statistically significant improvements in adherence, when comparing the intervention to the control groups. For the effect of EMP on the proportion of patients who were adherent, the range of the increase was 1.0% to 49.5%. For the effect of EMP on mean levels of adherence, the range was from a decrease of 2.9% to an increase of 14.6%.
Table 1.
Characteristics of studies testing patient-interface-only electronic medication packaging
| Study | Device name (mfr) | Medical condition (medication) | Intervention group (n) | Control group (n) | Trial length (mos) | Study design | Details |
|---|---|---|---|---|---|---|---|
| ADHERENCE RECORDERS ONLY | |||||||
| Barrios, 200715 | Medication Event Monitoring System (MEMS) (Aprex) | Hypertension (lercanidipine) | 485 | 1,038 | 3 | RCT | 1:2 design MEMS vs actively monitored pill counts (usual-care). |
| Wagner, 200216 | Medication Event Monitoring System (Aardex) | HIV (various HAARTs) | 60 | 113 | 1 | RCT | Electronic monitoring caps vs medication diaries vs a no surveillance control group. |
| ADHERENCE RECORDER AND AUDIOVISUAL ALARM | |||||||
| Christensen, 20l017 | Helping Hand Data Capture (Bang & Olufsen Medicom) | Hypertension (telmisartan) | 219 | 179 | 12 | Crossover RCT | Comparing blister cards in a device with an auditory alarm against vs. standard blister packs. |
| Kooy, 201318† | Compliance Card (Service Apotheek) | Hyperlipidemia, statins (various) | 117 | 128 | 12 | RCT | Electronic reminder device delivered by mail vs usual care. |
| Charles, 200719 | Smartinhaler (Nexus6) | Asthma (fluticasone propionate) | 44 | 46 | 5.5 | RCT | Metered dose inhaler with vs without an audiovisual reminder function. |
| Hojlo, 200820 | Travalert Dosing Aid (Alcon) | Glaucoma (travoprost) | 34 | N/A, same patients | 6 | Observational | Patients received an electronic monitoring device. In Phase 1, the audible alarm was disabled and in Phase 2 it was activated. |
| ADHERENCE RECORDER, AUDIOVISUAL ALARM, AND LIQUID CRYSTAL DISPLAY | |||||||
| McKenney, 199 221† | Prescript TimeCap (Wheaton Medical Technologies) | Hypertension (various anti-hypertensives) | 17 | 16 | 5.5 | RCT | Vial equipped with a cap that displays the last time the cap was removed vs standard medication vials. |
| Santschi, 200722* | Intelligent Drug Administration System II (Bang & Olufsen Medicom) and Medication Event Monitoring System 6 SmartCap (Aardex) | Hypertension (irbesartan) | 25 | N/A, same patients | 2 | Crossover RCT | Blister packs showing the time since last opening and have an audible alarm (Intelligent Drug Administration System II) vs display the number of daily openings and hours since last opening (MEMS 6 SmartCap). |
| Laster, 199623 | Prescript TimeCap (Wheaton Medical Technologies) | Glaucoma (pilocarpine) | 13 | N/A, same patients | 2 | Crossover RCT | Cap that displays the last time the cap was removed vs standard caps. |
| ADHERENCE RECORDER AND REAL TIME MONITORING | |||||||
| Vervloet, 2010, 2011, & 201424,25,26 | Real Time Medication Monitoring (Evalan) | Diabetes / hyperglycemia (various antidiabetics) | 48 | 57 | 24 | RCT | Vials with Real Time Medication Monitoring (minus group) vs no intervention. |
RCT = randomized controlled trial; HIV = human immunodeficiency virus; MEMS = Medication Event Monitoring System, the trade name of the EMP device manufactured by Aardex/Aprex; HAART=highly active antiretroviral therapy; N/A = not applicable
Effect estimates could not be calculated either because the type of statistic reported or missing descriptive statistics.
Studies assess both patient-interface-only and integrated interventions and are therefore included in both Table 1 and Table 2.
A larger number of studies assessed integrated interventions (Table 2). For the effect of EMP on the proportion of patients who were adherent, the range was -8.0% to 33.5%. For the effect of EMP on mean levels of adherence, the range was from 1.0% to 14.6%.
Table 2.
Characteristics of studies testing integrated electronic medication packaging interventions*
| Study | Device name (mfr) | Adherence feedback (Y/N) |
Medical condition (medication) |
Intervention group (n) |
Control group (n) |
Trial length (mos) |
Study design |
Details |
|---|---|---|---|---|---|---|---|---|
| ADHERENCE RECORDERS ONLY | ||||||||
| van Onzenoort, 201127† | Medication Event Monitoring System V TrackCap (Aardex) | N | Hypertension (various anti-hypertensives) | 228 | 242 | 12 | RCT | MEMS and pill count vs pill count alone. |
| Murray, 2004 & 200728,29 | Medication Event Monitoring System V (Aardex) | Y | Heart failure (various) | 106 | 164 | 12 | RCT | Pharmacists received monitoring data and delivered education to the patients vs usual care. |
| Tashkin, 1991& Nides, 199330,31 | Nebulizer Chronolog (Forefront Engineering) | Y | COPD (ipratropium bromide) | 116 | 89 | 4 | RCT | Participants informed of the device and given feedback vs group that didn't know chronolog was recording actuations |
| De Bruin, 2010 a & b32,33 | Medication Event Monitoring System (Aardex) | Y | HIV (various HAARTs) | 66 | 67 | 9 | RCT | Minimal (for patients with >95% baseline adherence) and intensive intervention (for patients with <95%, adherence) vs usual care. |
| Wu, 201234 | Medication Event Monitoring System 6 (Aardex) | Y | Heart failure (various) | 54 | 28 | 9 | RCT | Education plus MEMS feedback vs theory-based education only vs usual care. |
| Elixhauser, 199035 | Medication Compliance Monitoring Device (US Patent Number 4,616,316) | Y | Psychiatric disorders (lithium) | 31 | 37 | 4 to 8 | RCT | Feedback using monitoring data and serum lithium levels vs serum lithium levels only. |
| Sabin, 201036 | Med-ic (Information Mediary Corp.) | Y | HIV (various HAARTs) | 31 | 33 | 12 | RCT | Electronically-monitored adherence and feedback vs standard of care. |
| Mooney, 200737 | Medication Event Monitoring System (Aardex) | Y | Smoking cessation (bupropion) | 27 | 28 | 1.5 | RCT | Therapy with 10 min of MEMS feedback vs therapy only. |
| Rigsby, 200038 | Medication Event Monitoring System (Aprex) | Y | HIV (various HAARTs) | 37 | 18 | 1 | RCT | Cue-dose training and feedback vs cue-dose training combined with cash reinforcement vs usual care. |
| Forni Ogna, 201239 | Medication Event Monitoring System (Aardex) | Y | Platelet inhibition (Clopidogrel) | 32 | 16 | 6 | RCT | Feedback to patients using MEMS adherence data vs simply registering adherence using MEMS. |
| Schmitz, 200540 | Medication Event Monitoring System IV (Aardex) | Y | Smoking cessation (bupropion-SR) | 22 | 24 | 1.5 | RCT | MEMS with feedback showing adherence vs control patients not informed about MEMS. |
| Cramer, 199941 | Medication Event Monitoring System (Aprex) | Y | Psychiatric disorders (various antipsychotics) | 25 | 20 | 6 | RCT | MEMS paired with special instruction and reinforcing techniques vs MEMS paired with usual care. |
| Matsuyama, 199342 | Medication Event Monitoring System III (Aprex Corp.) | Y | Diabetes / hyperglycemia (sulfonylureas) | 15 | 17 | 2 | RCT | Providers receiving input from lab and MEMs data vs providers receiving input from lab data and pill counts. |
| Kozuki, 200643 | electronic Drug Exposure Monitor (Aardex) | Y | Psychiatric disorders (various atypical antipsychotics) | 15 | 14 | 3 | RCT | Patients receiving special instruction from an electronic monitoring device at counseling sessions vs standard care. |
| Kruse, 199444 | Medication Event Monitoring System (Aprex) | Y | Hypertension (various anti-hypertensives) | 15 | 9 | 7 +/− 1 | Cohort study | Receiving compliance feedback from a physician vs not receiving this feedback based on the doctors' discretion. |
| KOzuki, 200545† | Medication Event Monitoring System (Aardex) | Y | Psychiatric disorders (various atypical antipsychotics) | 22 | N/A, same patients | 3 | Obs | Patients received performance feedback, attentive listening, teaching, and management strategies weekly for the first month and at 2 and 3 months. |
| Onyirimba, 200346 | Metered-Dose Inhaler Chronolog, MC-311 (Medtrac Technologies) | Y | Asthma (various inhaled steroids) | 10 | 9 | 2.5 | RCT | Feedback on inhaled steroid use from the clinician investigator vs usual care. |
| Russell, 2010 & 201147,48 | Medication Event Monitoring System V TrackCap (Aprex) | Y | Renal transplant (various immuno-suppressants) | 5 | 8 | 9 | RCT | Individual data evaluation and identification and implementation of personal system changes vs brochures. |
| Waeber, 199949 | Medication Event Monitoring System (unknown) | N | Hypertension (various anti-hypertensives) | 6 | N/A, same patients | 4.5 | Obs | Non-adherent patients during an observation period received an electronic medication monitoring device and instructions on how to use it. |
| Matteson, 201150† | Medication Event Monitoring System (Aardex) | Y | Inflammatory bowel disease (various) | 3 | 2 | 4 | RCT | Individual data evaluation and identification and implementation of personal system changes vs educational information. |
| ADHERENCE RECORDER AND AUDIOVISUAL ALARM | ||||||||
| K ioy, 201318‡ | Compliance Card (Service Apotheek) | Y | Hyperlipidemia (various statins) | 54 | 128 | 12 | RCT | Electronic reminder device with counseling vs usual care. |
| Okeke, 200951 | Dosing Aid (Alcon) | N | Glaucoma (travoprost) | 35 | 31 | 9 | RCT | Education, counseling, telephone reminders, and an adherence device vs usual care. |
| ADHERENCE RECORDER AND LIQUID CRYSTAL DISPLAY | ||||||||
| Davies, 20 1 052† | Medication Event Monitoring System 6 SmartCap (Aardex) | Y | HIV (various HAARTs) | 78 | 67 | 12 | RCT | Patient feedback using graphical readouts by clinical staff vs patients with no feedback or graphical output. |
| Mengden, 200653 | Medication Event Monitoring System (Aardex) | N | Hypertension (candersartan and hydro-chlorothiazide) | 44 | 18 | 3 | RCT | Hypertensive patients using MEMS with display (total doses and time since last dose) plus teaching program vs hypertensive patients using MEMS without display plus self BP checks vs well controlled patients using MEMS. |
| Forni Ogna, 201354† | Medication Event Monitoring System SmartCap (Aardex) | Y | Hyper-parathyroidism (cinacalcet) | 19 | 22 | 6 | RCT | Providing feedback to patients using MEMs adherence data at 2 month intervals vs simply registering MEMS adherence. |
| De Bruin, 200555 | Medication Event Monitoring System 6 SmartCap (Aardex) | Y | HIV (various HAARTs) | 19 | N/A, same patients | 3 | Obs | Adherence monitored for 2 months, then adherence data used to provide feedback to participants. |
| Ruppar, 2010 a & b57† | Medication Event Monitoring System SmartCap (Aprex) | Y | Hypertension (various anti-hypertensives) | 10 | 5 | 6 | RCT | Feedback, counseling, education, an instruction card, and an electronic cap with a digital display vs usual care. |
| ADHERENCE RECORDER, AUDIOVISUAL ALARM, AND LIQUID CRYSTAL DISPLAY | ||||||||
| McKenney, 199221‡ | Prescript TimeCap (Wheaton Medical Technologies) | N | Hypertension (various anti-hypertensives) | 34 | 16 | 5.5 | RCT | Vial with a display (time of last opening) plus cards to record BP values vs all plus a home BP cuff vs standard medication vials only. |
| Rosen, 200458 | Medication Event Monitoring System SmartCap (Aardex) | Y | Diabetes / hyperglycemia (metformin) | 16 | 17 | 3.5 | RCT | Caps with display (hours since last opening), cue-dose training, and adherence feedback vs display caps only. |
RCT = randomized controlled trial; MEMS = Medication Event Monitoring System, the trade name of the EMP device manufactured by Aardex/Aprex; Obs = observational; HIV = human immunodeficiency virus; HAART=highly active antiretroviral therapy; BP = blood pressure; COPD = chronic obstructive pulmonary disease; N/A = not applicable
In integrated interventions, health professionals delivered additional interventions including counseling, adherence interventions other than EMP, or feedback based on prior EMP adherence data.
Effect estimates could not be calculated either because the type of statistic reported or missing descriptive statistics.
Studies assess both patient-interface-only and integrated interventions and are therefore included in both Table 1 and Table 2.
Two studies had multiple trial groups that enabled direct comparison of patient-interface-only and integrated interventions.18,21 McKenney found that integrated interventions were more successful, and increasing the intensity of the interventions was associated with marginally higher improvements in adherence; that is, numerically increased levels of adherence were seen, but the 95% confidence intervals largely overlapped.21 Adherence among hypertensive patients receiving medication containers was 94% with a digital display, higher for those receiving a digital display along with cards to record home and office blood pressure values (99%), and highest for those receiving the digital display, card, and a blood pressure cuff (100%, p<0.01 for all three treatments compared to standard).21 Kooy et al. found adherence was lower in a group with EMP and counseling as compared to EMP alone; however, adherence in both intervention groups was above average (70.4% vs. 72.6%) and the difference was not statistically significant.18
Methodological quality and risk of bias assessments
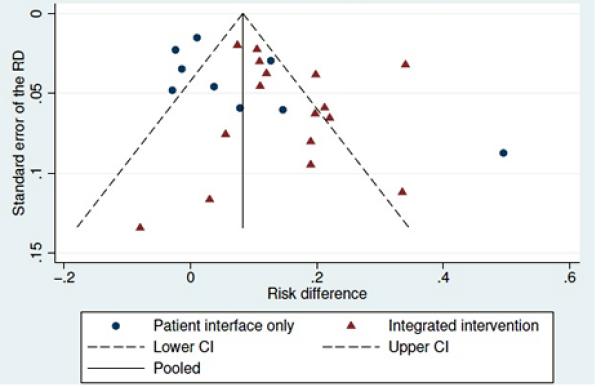
The methodological quality and risk of bias assessments are available in Table 3. Patient-interface-only interventions generally had lower risks of provider bias, which was more common in complex interventions that were characterized by providers delivering the active interventions. By electronically collecting the primary adherence outcome data, EMP naturally minimized the risk for assessor bias in studies whose main outcome was based on electronic adherence data. Other potential bias resulted from investigators’ using different measures of adherence for the intervention and control groups; however, this applied to only 3 studies. Visual inspection of the funnel plot reveals some asymmetry, suggesting that smaller studies with smaller effect sizes are under-represented in the literature (Figure 3). If present, such bias results in a more favorable assessment of the overall body of evidence.59
Table 3.
Methodological quality and risk of bias assessment summary
| Citation | Study | Design | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (detection bias) | Blinding of outcomes assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other (different outcomes for trial arms) |
|---|---|---|---|---|---|---|---|---|---|
| 15 | Barrios, 2007 | RCT | Unclear | Unclear | High | Unclear | Low | Low | High |
| 16 | Wagner, 2002 | RCT | Unclear | Unclear | High | Unclear | Low | Low | High |
| 17 | Christensen, 2010 | Crossover RCT | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| 18 | Kooy, 2013 | RCT | Low | High | High | Low | Unclear | Low | Low |
| 19 | Charles, 2007 | RCT | Low | Low | Low | Low | Low | Low | Low |
| 20 | Hollo, 2008 | Observational | N/A | N/A | Low | Low | Low | Low | Low |
| 21 | McKenney, 1992 | RCT | Unclear | Unclear | Low | Unclear | Low | Low | Low |
| 22 | Santschi, 2007 | Crossover RCT | Low | Unclear | High | Low | Low | Low | Low |
| 23 | Laster, 1996 | Crossover RCT | Unclear | Unclear | Low | Low | Low | Low | Low |
| 24,25,26 | Vervloet, 2010 & 2011 | RCT | Low | Low | High | Low | Low | Low | Low |
| 27 | van Onzenoort, 2011 | RCT | Unclear | Unclear | Low | Low | Low | Low | Low |
| 28,29 | Murray, 2004 & 2007 | RCT & CEA | Low | Low | High | Low | Low | Low | Low |
| 30,31 | Tashkin, 1991& Nides, 1993 | RCT | High | High | High | Low | Low | Low | Low |
| 32,33 | De Bruin, 2010 a & b | RCT | Unclear | High | High | Low | Low | Low | Low |
| 34 | Wu, 2012 | RCT | Unclear | Unclear | High | Low | Low | Low | Low |
| 35 | Elixhauser, 1990 | RCT | High | High | High | Low | Low | Low | Low |
| 36 | Sabin, 2010 | RCT | Low | High | High | Low | Low | Low | Low |
| 37 | Mooney, 2007 | RCT | Unclear | Unclear | High | Low | Unclear | Low | Low |
| 38 | Rigsby, 2000 | RCT | Low | Low | High | Low | Low | Low | Low |
| 39 | Forni Ogna, 2012 | RCT | Unclear | Unclear | High | Low | Unclear | Low | Low |
| 40 | Schmitz, 2005 | RCT | Unclear | Unclear | High | Low | Unclear | Low | Low |
| 41 | Cramer, 1999 | RCT | Unclear | Unclear | High | Low | Low | Low | Low |
| 42 | Matsuyama, 1993 | RCT | Unclear | Unclear | Low | Low | Low | Low | High |
| 43 | Kozuki, 2006 | RCT | Unclear | Unclear | High | Low | Low | Low | Low |
| 44 | Kruse, 1994 | Observational | N/A | N/A | High | Low | Low | Low | Low |
| 45 | Kozuki, 2005 | Observational | N/A | N/A | High | Low | Low | Low | Low |
| 46 | Onyirimba, 2003 | RCT | Unclear | Unclear | High | Low | Low | Low | Low |
| 47,48 | Russell, 2010, 2011 | RCT | Unclear | Unclear | High | Low | Low | Low | Low |
| 49 | Waeber, 1999 | Observational | N/A | N/A | High | Unclear | Low | Low | Low |
| 50 | Matteson, 2011 | RCT | Low | Low | High | Low | Low | Low | Low |
| 51 | Okeke, 2009 | RCT | Low | Low | High | Low | Low | Low | Low |
| 52 | Davies, 2010 | RCT | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| 53 | Mengden, 2006 | RCT | Unclear | Unclear | High | Low | Low | Low | Low |
| 54 | Forni Ogna, 2013 | RCT | Low | Unclear | High | Low | Low | Low | Low |
| 55 | De Bruin, 2005 | Observational | N/A | N/A | High | Low | Low | Low | Low |
| 56,57 | Ruppar, 2010 a & b | RCT | Low | Low | High | Low | Low | Low | Low |
| 58 | Rosen, 2004 | RCT | Unclear | Unclear | High | Low | Unclear | Low | Low |
RCT=Randomized controlled trial; CEA = cost effectiveness analysis; N/A=not applicable
Figure 3. Funnel plot with pseudo 95% confidence limits, grouped by type of intervention.
Subset of 20 studies (representing 25 effect estimates) for which the standard error of the difference in adherence could be estimated; includes both studies reporting the proportion of patients adherent and studies reporting the mean level of adherence. Nine effect estimates relate to patient interface only studies, and 16 effect estimates relate to integrated intervention studies.
Description of various EMP features
We identified 5 common features of EMP devices and interventions: recording dosing events and storing a record of adherence, audiovisual reminders to cue dosing, digital displays, real-time monitoring, and providing patients with adherence performance feedback (Figure 2).
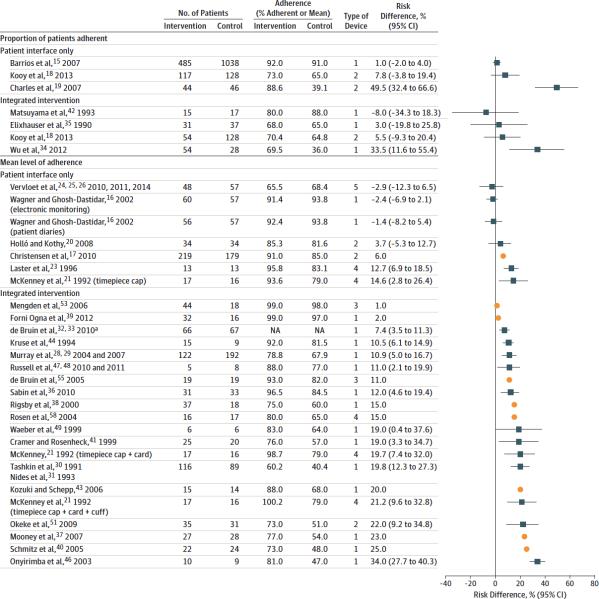
Figure 2. Difference in level of adherence at individual study level, grouped by type of intervention.
* Actual level of adherence by group not reported.
Studies are ordered by increasing effect size. Studies for which insufficient information was available to estimate 95% confidence intervals are displayed using squares. Type of device: 1=Adherence recorder only; 2=Adherence recorder and audiovisual alarm; 3=Adherence recorder and liquid crystal display; 4=Adherence recorder, audiovisual alarm, liquid crystal display; 5=Adherence recorder and real time monitoring. Certain studies from Tables 1 and 2 are not included in this Figure either because the type of statistic reported or missing descriptive statistics prevented inclusion. Two studies assess both patient-interface-only and integrated interventions and are therefore included in both categories.
Recorder and storage functions were included in every EMP device reviewed, making it the only feature present in all of the EMPs in our sample. While some devices recorded dosing events to provide accountability, the storage and export of adherence performance records also enabled complex interventions to be tailored to patient adherence patterns.
Digital displays and audiovisual reminders were the next most common feature of EMP devices. Digital displays were analyzed in 9 studies.21-23,52-55,57,58 Information provided by the digital displays included: the time the bottle was previously opened; the amount of elapsed time since the last opening, and/or the number of times the container had been opened on that day. Audio or visual reminders to cue dosing, which included auditory beeps or flashing lights to cue dosing, were also found in 9 studies in our sample.17-23,51,58
The only device that recorded adherence using real-time monitoring via an integrated antenna did not show a significant improvement in adherence, except when the real-time monitoring was combined with short messaging service (SMS) text reminders; however, the mobile phone component of these interventions of these studies was outside of the scope of this review.26
Providing patients with feedback based on their electronically monitored adherence patterns was a frequent component of the integrated EMP interventions. Examples of providing feedback to patient's included providing patients with graphical display of their adherence,52 providing adherence performance to clinicians for use in routine patient encounters,44 and having dedicated sessions apart from clinical patient encounters to discuss adherence performance and devise improvement strategies.48
We found that apart from the baseline monitoring and storing function present in all devices, the feature most frequently associated with improved adherence was combining digital displays that show the last time of container opening with audible reminder alarms and including EMP as a component of complex interventions.
Other findings
Ten studies reported noteworthy findings in addition to the association of the intervention on adherence. These fell into 3 categories: subjective patient perceptions (4 studies),22,48,55,58 factors other than EMP that affected adherence (3),44,52,53 and potential risks of EMP (3).16,35,45
In the studies we examined, patients asked about EMP tended to view their devices positively. 22,48,55,58 Surveyed patients preferred smaller devices and the ability to disable device alarms when in public.55 Additionally, all 3 studies that compared adherence on weekdays to weekends found that adherence was worse on weekends, with one study also finding that evening doses were omitted more frequently than morning doses.44,52,53
Three studies highlighted potential problems related to uptake of EMP. Wagner et al. conducted a study using multiple measures of adherence and found that the self-reported adherence of patients using electronic caps declined in four weeks, which raised “concerns about the potential harmful effects of restricting the use of common adherence strategies such as pill organizers and ‘pocketing’ doses, which are requirements associated with electronic monitoring.”16 Another concern emerged from studies investigating use of EMP for psychiatric drugs. One study found that psychiatric patients’ self-reported adherence to these drugs was actually lower than their electronic adherence record,45 while another discovered that patients in the monitored group ended the study with higher levels of anxiety, depression, and somatic complaints compared to the control group.35 Both studies concluded that patients with psychiatric disease may have difficulty adjusting to EMP.
Discussion
In this systematic review of studies evaluating EMP, we found that patient-interface-only and integrated interventions can be associated with increased medication adherence, although most studies evaluating EMP were short-term interventions with limited numbers of patients. Within the limited scope of these studies, simple devices that monitor and store adherence records and devices that combine digital displays with audible reminder alarms appeared to be the most useful characteristics of EMP devices at improving adherence.
Our review indicated that EMP interventions with greater complexity and integration into the health care system were frequently associated with improved adherence. The model of patient-centered care suggests that feedback to patients may also improve adherence,60 although the studies that we found did not clearly establish an added benefit of the practice. One explanation may be that feedback was tested in the context of other confounding interventions, such as patient education and providing patients with medical equipment like blood pressure cuffs. Additional studies will be needed to better determine whether feedback promotes adherence related to EMP, and if so, whether the type of mechanism of the interaction between feedback and the performance of EMP interventions may help develop better approaches to improving adherence more generally.
An unexpected qualitative observation was that EMP could be associated with adverse outcomes in some settings, such as in patients who use pill organizers and in patients with psychiatric illnesses. EMP may be most helpful when it augments an established organizational routine, or imposes a routine if one does not exist. In the limited circumstances in which EMP has paradoxical effects, it may interfere with an established medication adherence habit. In light of these observations, implementation of EMP could target patients who do not have effective medication adherence patterns already in place.
Numerous studies had methodological problems such as nonrandomized designs, and many showed improvements that did not reach statistical significance. Our inclusive definitions of EMP and study designs also led to substantial heterogeneity in our results and ruled out the possibility of conducting a meta-analysis. Publication bias is another consideration since the funnel plot is consistent with the possibility that smaller studies with less favorable effects of EMP interventions may not have been submitted to journals for publications. In analyzing the results, we relied to a greater extent on the larger prospective trials of EMP interventions that met conventional criteria of significance. In addition, most studies lasted less than 6 months. Longer-term evidence is needed regarding use of EMP in patients with chronic illness, since EMP, like some other adherence interventions, may lose its effect over time.10
This review was limited to English language articles and excluded studies of children; the global issue of medication adherence and the complex issue of medication adherence for children merit attention in subsequent analyses. This review also focused on studies that measured adherence and excluded studies that reported- health outcomes but did not include adherence. While secondary analyses indicated that studies that found improved adherence using EMP also had improved health outcomes, measuring the impact of EMP on health outcomes, and costs of care, should be a goal of future investigation.
Conclusion
We found that many varieties of EMP exist, although the data on any one of them are limited and there is variability in the quality of studies testing EMP devices with some evidence of reporting bias. Higher quality evidence is needed to determine the effect, if any, of these low cost interventions on the critical problem of medication nonadherence. Further study is also needed to identify the active intervention components of these complex interventions. Experiments in the US and around the world are currently underway to test integrated models of medical care delivery—using patient-centered homes, telemedicine, or other similar tools—and our review suggests that greater study is needed about the role for innovative EMP tools in these contexts to improve medication adherence.
Acknowledgements
Dr. Kesselheim's work is supported by a career development award from the Agency for Healthcare Research & Quality (K08HS18465-01), the Greenwall Faculty Scholars in Bioethics, an Ignition Award from the Harvard Program in Therapeutic Science, and a Robert Wood Johnson Foundation Investigator Award in Health Policy Research. Krista Huybrechts’ work is supported by a career development grant (K01MH099141) from the National Institute of Mental Health. These organizations played no role in: the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Mr. Checchi and Dr. Kesselheim had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Original systematic review protocol is on file with the authors and available to interested parties upon request.
References
- 1.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: Terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 2.Zullig LL, Peterson ED, Bosworth HB. Ingredients of successful interventions to improve medication adherence. JAMA. 2013;310(24):2611–2612. doi: 10.1001/jama.2013.282818. [DOI] [PubMed] [Google Scholar]
- 3.Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: Analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25(4):284–290. doi: 10.1007/s11606-010-1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muntner P, Halanych JH, Reynolds K, et al. Low medication adherence and the incidence of stroke symptoms among individuals with hypertension: The REGARDS study. J Clin Hypertens (Greenwich) 2011;13(7):479–486. doi: 10.1111/j.1751-7176.2011.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holstad MMD, DiIorio C, McCarty F. Adherence, sexual risk, and viral load in HIV-infected women prescribed antiretroviral therapy. AIDS Patient Care STDS. 2011;25(7):431–438. doi: 10.1089/apc.2010.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald AA, Powers JD, Ho PM, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail. 2011;17(8):664–669. doi: 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg DE, Siliciano RF, Jacobs WR., Jr Outwitting evolution: fighting drug-resistant TB, malaria, and HIV. Cell. 2012;148(6):1271–1283. doi: 10.1016/j.cell.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowry ADK, Shrank WH, Lee JL, et al. A systematic review of adherence to cardiovascular medications in resource-limited settings. Journal of General Internal Medicine. 2011;26(12):1479–1491. doi: 10.1007/s11606-011-1825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabaté E. Adherence to long-term therapies: Evidence for action. World Health Organization; Geneva: [January 25, 2014]. http://whqlibdoc.who.int/publications/2003/9241545992.pdf. Published January 2003. [Google Scholar]
- 10.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008:2. doi: 10.1002/14651858.CD000011.pub3. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Naditz A. Medication compliance—helping patients through technology: Modern “smart” pillboxes keep memory-short patients on their medical regimen. Telemed J E Health. 2008;14(9):875–880. doi: 10.1089/tmj.2008.8476. [DOI] [PubMed] [Google Scholar]
- 12.Granger BB, Bosworth HB. Medication adherence: Emerging use of technology. Curr Opin Cardiol. 2011;26(4):279–287. doi: 10.1097/HCO.0b013e328347c150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misono AS, Cutrona SL, Choudhry NK, et al. Healthcare information technology interventions to improve cardiovascular and diabetes medication adherence. Am J Manag Care. 2010;16(12 Suppl HIT):SP82–SP92. [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [January 25, 2014];The Cochrane Collaboration. 2011 http://www.cochrane-handbook.org.
- 15.Barrios V, Escobar C, Navarro Á , Calderón A, Ruilope LM. Antihypertensive effectiveness of lercanidipine administered using an electronic pillbox compared with usual care in a cohort of mild-to-moderately hypertensive patients: The ELECTRA study. Therapy. 2007;4(4):433–440. [Google Scholar]
- 16.Wagner G, Ghosh-Dastidar B. Electronic monitoring: Adherence assessment or intervention? HIV Clin Trials. 2002;3(1):45–51. doi: 10.1310/XGXU-FUDK-A9QT-MPTF. [DOI] [PubMed] [Google Scholar]
- 17.Christensen A, Christrup LL, Fabricius PE, et al. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: A randomized controlled trial. J Hypertens. 2010;28(1):194–200. doi: 10.1097/HJH.0b013e328331b718. [DOI] [PubMed] [Google Scholar]
- 18.Kooy MJ, van Wijk BLG, Heerdink ER, et al. Does the use of an electronic reminder device with or without counseling improve adherence to lipid-lowering treatment? The results of a randomized controlled trial. Front Pharmacol. 2013;4(69) doi: 10.3389/fphar.2013.00069. doi: 10.3389/fphar.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charles T, Quinn D, Weatherall M, Aldington S, Beasley R, Holt S. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. J Allergy Clin Immunol. 2007;119(4):811–816. doi: 10.1016/j.jaci.2006.11.700. [DOI] [PubMed] [Google Scholar]
- 20.Holló G, Kothy P. Can adherence to topical glaucoma medication be improved by using an audible alarm? A pilot study. Pharm Med. 2008;22(3):175–179. [Google Scholar]
- 21.McKenney J, Munroe W, Wright J. Impact of an electronic medication compliance aid on long-term blood pressure control. J Clin Pharmacol. 1992;32(3):277–283. doi: 10.1002/j.1552-4604.1992.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 22.Santschi V, Wuerzner G, Schneider MP, Bugnon O, Burnier M. Clinical evaluation of IDAS II, a new electronic device enabling drug adherence monitoring. Eur J Clin Pharmacol. 2007;63(12):1179–1184. doi: 10.1007/s00228-007-0364-7. [DOI] [PubMed] [Google Scholar]
- 23.Laster SF, Martin JL, Fleming JB. The effect of a medication alarm device on patient compliance with topical pilocarpine. J Am Optom Assoc. 1996;67(11):654–658. [PubMed] [Google Scholar]
- 24.Vervloet M, Santen-Reestman J, Vlijmen B, et al. Een oplettend medicijndoosje voor diabetes type 2 patiënten: Een onderzoek naar de effecten van real-time medication monitoring. Netherlands institute for health services research; [January 25, 2014]. http://www.nivel.nl/sites/default/files/bestanden/Rapport-oplettend-medicijndoosje.pdf. Published June 2010. [Google Scholar]
- 25.Vervloet M, van Dijk L, Santen-Reestman J, van Vlijmen B, Bouvy ML, de Bakker DH. Improving medication adherence in diabetes type 2 patients through real time medication monitoring: A randomised controlled trial to evaluate the effect of monitoring patients’ medication use combined with short message service (SMS) reminders. BMC health services research. 2011;11(5) doi: 10.1186/1472-6963-11-5. doi: 10.1186/1472-6963-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vervloet M, van Diik L, de Bakker DH. Short- and long-term effects of real-time medication monitoring with short message service (SMS) reminders for missed doses on the refill adherence of people with Type 2 diabetes: evidence from a randomized controlled trial. Diabet Med. 2014;31(7):821–8. doi: 10.1111/dme.12439. doi: 10.1111/dme.12439. [DOI] [PubMed] [Google Scholar]
- 27.van Onzenoort HAW, Verberk WJ, Kroon AA, et al. Electronic monitoring of adherence, treatment of hypertension, and blood pressure control. Am J Hypertens. 2011;25(1):54–59. doi: 10.1038/ajh.2011.153. [DOI] [PubMed] [Google Scholar]
- 28.Murray MD, Young JM, Morrow DG, et al. Methodology of an ongoing, randomized, controlled trial to improve drug use for elderly patients with chronic heart failure. Am J Geriatr Pharmacother. 2004;2(1):53–65. doi: 10.1016/s1543-5946(04)90007-4. [DOI] [PubMed] [Google Scholar]
- 29.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: A randomized trial. Ann Intern Med. 2007;146(10):714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 30.Tashkin DP, Rand C, Nides M, et al. A nebulizer chronolog to monitor compliance with inhaler use. Am J Med. 1991;91(4):S33–S36. doi: 10.1016/0002-9343(91)90260-5. [DOI] [PubMed] [Google Scholar]
- 31.Nides MA, Tashkin DP, Simmons MS, Wise RA, Li VC, Rand CS. Improving inhaler adherence in a clinical trial through the use of the nebulizer chronolog. Chest. 1993;104(2):501–507. doi: 10.1378/chest.104.2.501. [DOI] [PubMed] [Google Scholar]
- 32.de Bruin M. An electronic-monitoring based intervention improves HIV treatment adherence. J Int Assoc Physicians AIDS Care (Chic) 2010;9(4):255. [Google Scholar]
- 33.de Bruin M, Hospers HJ, van Breukelen GJP, Kok G, Koevoets WM, Prins JM. Electronic monitoring-based counseling to enhance adherence among HIV-infected patients: A randomized controlled trial. Health Psychol. 2010;29(4):421–428. doi: 10.1037/a0020335. [DOI] [PubMed] [Google Scholar]
- 34.Wu JR, Corley DJ, Lennie TA, Moser DK. Effect of a medication-taking behavior feedback Theory–Based intervention on outcomes in patients with heart failure. J Card Fail. 2012;18(1):1–9. doi: 10.1016/j.cardfail.2011.09.006. doi:10.1016/j.cardfail.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elixhauser A, Eisen SA, Romeis JC, Homan SM. The effects of monitoring and feedback on compliance. Med Care. 1990:882–893. doi: 10.1097/00005650-199010000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Sabin LL, DeSilva MB, Hamer DH, et al. Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in china. AIDS Behav. 2010;14(3):580–589. doi: 10.1007/s10461-009-9615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mooney ME, Sayre SL, Hokanson PS, Stotts AL, Schmitz JM. Adding MEMS feedback to behavioral smoking cessation therapy increases compliance with bupropion: A replication and extension study. Addict Behav. 2007;32(4):875–880. doi: 10.1016/j.addbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 38.Rigsby MO, Rosen MI, Beauvais JE, et al. Cue dose training with monetary reinforcement: Pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15(12):841–847. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forni Ogna V, Wuerzner G, Menetrey I, et al. Influence of Adherence Monitoring Program on Long-Term Platelet Inhibition by Clopidogrel: Results of a Randomized Controlled Trial. Circulation. 2012;126:A17116. [Google Scholar]
- 40.Schmitz JM, Sayre SL, Stotts AL, Rothfleisch J, Mooney ME. Medication compliance during a smoking cessation clinical trial: A brief intervention using MEMS feedback. J Behav Med. 2005;28(2):139–147. doi: 10.1007/s10865-005-3663-4. [DOI] [PubMed] [Google Scholar]
- 41.Cramer JA, Rosenheck R. Enhancing medication compliance for people with serious mental illness. J Nerv Ment Dis. 1999;187(1):53–55. doi: 10.1097/00005053-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Matsuyama JR, Mason BJ, Jue SG. Pharmacists’ interventions using an electronic medication-event monitoring device's adherence data versus pill counts. Ann Pharmacother. 1993;27(7-8):851–855. doi: 10.1177/106002809302700705. [DOI] [PubMed] [Google Scholar]
- 43.Kozuki Y, Schepp KG. Visual-feedback therapy for antipsychotic medication adherence. Int Clin Psychopharmacol. 2006;21(1):57–61. doi: 10.1097/01.yic.0000177016.59484.ce. [DOI] [PubMed] [Google Scholar]
- 44.Kruse W, Rampmaier J, Ullrich G, Weber E. Patterns of drug compliance with medications to be taken once and twice daily assessed by continuous electronic monitoring in primary care. Int J Clin Pharmacol Ther. 1994;32(9):452–457. [PubMed] [Google Scholar]
- 45.Kozuki Y, Poupore E, Schepp K. Visual feedback therapy to enhance medication adherence in psychosis. Arch Psychiatr Nurs. 2005;19(2):70–80. doi: 10.1016/j.apnu.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Onyirimba F, Apter A, Reisine S, et al. Direct clinician-to-patient feedback discussion of inhaled steroid use: Its effect on adherence. Ann Allergy Asthma Immunol. 2003;90(4):411–415. doi: 10.1016/S1081-1206(10)61825-X. [DOI] [PubMed] [Google Scholar]
- 47.Russell CL, Conn VS, Ashbaugh C, Coffey D, Wakefield M. A pilot RCT to increase medication adherence in transplantation. Am J Transplant. 2010;10(s4):376. doi: 10.1111/j.1600-6143.2010.03108.x. [Google Scholar]
- 48.Russell C, Conn V, Ashbaugh C, et al. Taking immunosuppressive medications effectively (TIMELink): A pilot randomized controlled trial in adult kidney transplant recipients. Clin Transplant. 2011;25(6):864–870. doi: 10.1111/j.1399-0012.2010.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waeber B, Vetter W, Darioli R, Keller U, Brunner H. Improved blood pressure control by monitoring compliance with antihypertensive therapy. Int J Clin Pract. 1999;53(1):37. [PubMed] [Google Scholar]
- 50.Matteson M. A pilot intervention to improve medication adherence in nonadherent inflammatory bowel disease patients [dissertation] University of Missouri; Columbia, MO: 2011. [Google Scholar]
- 51.Okeke CO, Quigley HA, Jampel HD, et al. Interventions improve poor adherence with once daily glaucoma medications in electronically monitored patients. Ophthalmology. 2009;116(12):2286–2293. doi: 10.1016/j.ophtha.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies S, Asghar S, Cooper V, et al. Does feedback of medication execution using MEMS caps aid adherence to HAART?: the MEMRI study (MEMS as realistic intervention). J Int AIDS Soc. 2010;13(Suppl 4):120. [Google Scholar]
- 53.Mengden T, Vetter H, Tousset E, Uen S. Management of patients with uncontrolled arterial hypertension–the role of electronic compliance monitoring, 24-h ambulatory blood pressure monitoring and Candesartan/HCT. BMC Cardiovasc Disord. 2006;6:36. doi: 10.1186/1471-2261-6-36. doi: 10.1186/1471-2261-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forni Ogna V, Pruijm M, Zweiacker C, et al. Clinical Benefits of an Adherence Monitoring Program in the Management of Secondary Hyperparathyroidism with Cinacalcet: Results of a Prospective Randomized Controlled Study. BioMed Research International. 2013 doi: 10.1155/2013/104892. 2013. doi:10.1155/2013/104892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Bruin M, Hospers H, Borne HWVD, Kok G, Prins J. Theory-and evidence-based intervention to improve adherence to antiretroviral therapy among HIV Infected patients in the Netherlands: A pilot study. AIDS Patient Care and STDsS. 2005;19(6):384–394. doi: 10.1089/apc.2005.19.384. [DOI] [PubMed] [Google Scholar]
- 56.Ruppar T. Pilot study of a feedback intervention to improve medication adherence in older adults with hypertension. Eur J Cardiovasc Nurs. 2010;9(Suppl. 1):S26. doi: 10.1097/JCN.0b013e3181d5f9c5. [DOI] [PubMed] [Google Scholar]
- 57.Ruppar TM. Randomized pilot study of a behavioral feedback intervention to improve medication adherence in older adults with hypertension. J Cardiovasc Nurs. 2010;25(6):470–479. doi: 10.1097/JCN.0b013e3181d5f9c5. [DOI] [PubMed] [Google Scholar]
- 58.Rosen MI, Rigsby MO, Salahi JT, Ryan CE, Cramer JA. Electronic monitoring and counseling to improve medication adherence. Behav Res Ther. 2004;42(4):409–422. doi: 10.1016/S0005-7967(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 59.Hedges L, Olkin I. Statistical Methods for Meta-analysis. Academic Press; Boston, Mass: 1985. [Google Scholar]
- 60.Mazor KM, Beard RL, Alexander GL, Arora NK, Firneno C, Gaglio B, Greene SM, Lemay CA, Robinson BE, Roblin DW, Walsh K, Street RL, Jr, Gallagher TH. Patients’ and family members’ views on patient-centered communication during cancer care. Psychooncology. 2013;22(11):2487–95. doi: 10.1002/pon.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]