Abstract
Detection of latent Mycobacterium tuberculosis is a challenge in the diagnosis of asymptomatic, subclinical tuberculosis. We report the development of an immunofluorescence technique to visualize and enumerate M. tuberculosis in latently infected rabbit lungs where no acid-fast–stained organisms were seen and no cultivable bacilli were obtained by the agar-plating method.
Introduction
Latent Mycobacterium tuberculosis (Mtb) infection (LTBI) ensues in more than 90 % of immunocompetent individuals exposed to Mtb-containing aerosols (WHO, 2012). During LTBI, the infecting bacilli presumably exist in a slow or non-replicating state (O’Garra et al., 2013). However, the bacilli in LTBI cases can reactivate to cause symptomatic disease in individuals who become immunocompromised (Chao & Rubin, 2010; Gengenbacher & Kaufmann, 2012). Our understanding of the host response that drives the bacilli into latency is limited, and even less is known about the physiology of Mtb that survive in host tissues in a latent state.
To better understand LTBI, we developed a rabbit model of pulmonary tuberculosis (TB) in which infection with the virulent Mtb CDC1551 strain gave rise to transient bacillary growth in the lungs, followed by a gradual decrease in bacteria from about 106 c.f.u. at 4 weeks to no observable viable bacteria (i.e. 0 c.f.u.) and clearance of granulomas between 12 and 20 weeks post-infection, depending on the initial infectious inoculum (Flynn et al., 2008; Subbian et al., 2012). Importantly, in latently infected rabbits, immunosuppressive treatment, initiated after the complete clearance of bacteria from the lungs, resulted in resumed bacillary growth and reappearance of disease pathology. Thus, in the presence of an optimal host immune response, Mtb CDC1551 gradually transitioned from an actively replicating to non-replicating state, as determined by culture in liquid or solid medium. Since the enumeration of viable bacilli in infected tissue homogenates by the agar-plating method corresponds only to the number of actively replicating bacilli, this assay has limited sensitivity in detecting the total number of viable bacilli. In addition, latent bacilli are refractory to conventional microscopic detection by acid-fast staining such as the Ziehl–Neelsen (ZN) method (Seiler et al., 2003; Zhang, 2004).
Methods
Rabbit infection and lung bacterial load determination.
Mtb CDC1551 were grown in Middlebrook 7H9 medium (Difco) and used to infect New Zealand white rabbits through a ‘snout-only aerosol-exposure system’, as described previously (Subbian et al., 2011b). Four rabbits were killed at 3 h post-exposure (T = 0); the lungs were homogenized in sterile saline and serial dilutions were placed on Middlebrook 7H11 agar plates (Difco) and incubated for 4–5 weeks to determine the initial bacillary load by the agar plating method, as described previously (Subbian et al., 2011a). At 8, 16, 20, 24 and 26 weeks post-infection, lung homogenates were prepared from groups of three or four rabbits and used for further analysis. To reactivate LTBI, groups of rabbits (n = 3–4 rabbits per time point) were immunosuppressed with triamcinolone (Kenalog) treatment at 16 mg kg−1, administered through intramuscular injection for 4 weeks, starting at 20 weeks post-infection, when the animals had fully cleared the infection from the lungs (0 c.f.u. observed on agar plates) (Flynn et al., 2008; Subbian et al., 2012). Lungs from triamcinolone-treated and -untreated rabbits were harvested for the agar plating method and histological and immunohistological staining. The limit of detection for the assay was <10 bacilli. All rabbit procedures were approved by the Institutional Animal Care and Use Committee of Rutgers Biomedical and Health Sciences.
Histology.
Formalin-fixed lung sections from Mtb CDC1551-infected rabbits (n = 3–4 rabbits per time point) were paraffin embedded, cut into 5 μm sections and stained using the ZN method to visualize bacilli, as described previously (Kaplan et al., 2003; Subbian et al., 2011a). At least three stained lung sections from each rabbit per time point were analysed by a trained pathologist. Images were photographed using a Nikon Microphot-FX microscope.
Immunofluorescence staining of Mtb in rabbit lung tissue sections.
Paraffin-embedded rabbit lung tissue infected with Mtb CDC1551 was cut into 30–300 μm sections. Sections were dehydrated in a stepwise manner by passing them through an alcohol gradient in the following order: 30, 50, 60, 70, 90, 95, 100 and 100 %, and two passes in xylene for 5 min each. The sections were then rehydrated in the reverse order, followed by 15 min incubation in sterile 1× PBS. To improve the permeability of the antibodies, tissue sections were incubated in 0.1 % Triton X-100 for 1 min. The sections were boiled in citrate buffer (pH 6.0) for 20 min to retrieve Mtb antigens. Unlike standard 5 μm sections used for histological staining, thicker tissue sections allowed us to examine extensive X, Y and Z optical planes by confocal microscopy. This particular feature allowed us to identify infected host cell types and their location, as well as numbers of bacilli. Non-specific blocking was performed by incubating the sections in blocking solution containing 0.5 M EDTA, 1 % fish gelatin, 1 % IgG-free BSA and 1 % each of horse and human serum. Tissue sections were incubated overnight at 4 °C with a primary anti-Mtb–biotin antibody (GeneTex). This antibody was produced using Mtb purified protein derivative (a mixture of soluble proteins secreted by Mtb) and reacts with the Mtb antigens lipoarabinomannan, ESAT-6, CFP-10, 38 kDa protein, antigen 16 (HspX), Hsp65 (GroEL) and MoeX, as demonstrated by Western blot analysis (GeneTex).
Following incubation with the primary antibody, slides were washed three times with sterile 1× PBS and incubated with streptavidin conjugated to FITC (Invitrogen) at 1 : 1000 dilution for 3 h at room temperature, and again washed three times in sterile 1× PBS. Sections were mounted on glass slides using ProLong Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) and examined using an A1 confocal microscope equipped with a spectrum detection system (Nikon). Antibody specificity was confirmed by replacing the primary antibody with a non-specific myeloma antigen of the same isotype or with a non-immune serum. To assure objective quantification of Mtb in the stained sections, analysis was performed in a blinded manner. At least two stained lung sections from each rabbit per time point (n = 3–4 rabbits per time point) were stained and analysed.
Results
The agar plating method revealed a mean of 3.29 log10 Mtb CDC1551 implanted in the rabbit lungs at T = 0. At 8 and 16 weeks post-infection, the bacillary loads were 4.2 and 1.9 log10 c.f.u., respectively. No bacilli (i.e. 0 c.f.u.) were observed in the rabbit lungs at 20 weeks post-infection (Fig. 1). Importantly, immunosuppression of LTBI rabbits by triamcinolone treatment for 4 weeks, starting at 20 weeks post-infection, facilitated resumed bacillary growth to about 3.4 and 4.4 log10 c.f.u. at 24 and 26 weeks post-infection, respectively. At these time points, no viable bacilli were observed in the untreated, Mtb CDC1551-infected (control) rabbit lungs (Fig. 1). Thus, during LTBI, the infecting bacilli remained viable in a non-replicating state that was unable to form colonies on agar plates. Consistent with these results, acid-fast bacilli (AFB) were seen in ZN-stained lung sections at 4 weeks post-infection (Fig. 2a). However, no AFB were seen in lung sections after 16 weeks post-infection (Fig. 2b).
Fig. 1.
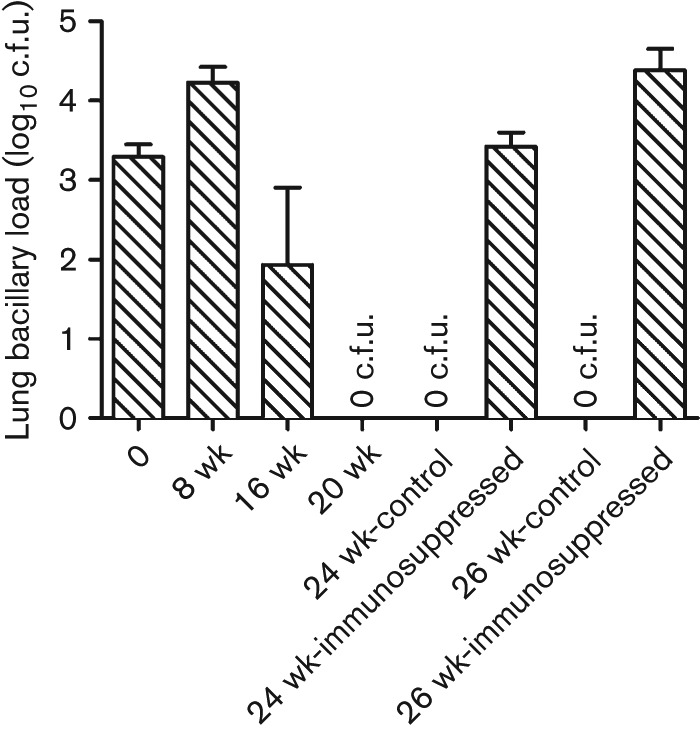
Bacillary load in the lungs of Mtb CDC1551-infected rabbits. The values plotted are means and sd of the number of c.f.u. in the lungs of Mtb CDC1551-infected rabbits (n = 3–4 rabbits per time point). No viable bacilli (i.e. 0 c.f.u.) were observed in the lungs of infected (control) rabbits at 20, 24 and 26 weeks (wk) post-infection. Starting at 20 weeks post-infection, a group of infected rabbits (n = 3–4 rabbits per time point) were treated with triamcinolone for 4 weeks. The number of viable bacilli was determined in these rabbits at the end of treatment (i.e. 24 weeks post-infection) or 2 weeks after the end of treatment (i.e. 26 weeks post-infection).
Fig. 2.
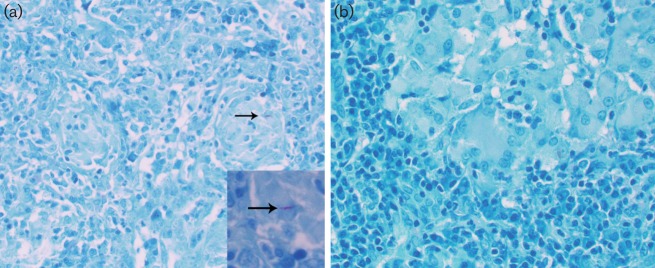
Acid-fast staining and visualization of Mtb CDC1551 in infected rabbit lungs. Representative image of Mtb-infected lung sections at 4 weeks (a) or 16 weeks (b) post-infection after staining by the ZN method and light microscopy analysis. The arrows indicate AFB; no AFB were detected in (b). Magnification, ×40 (a, b); ×100 (inset in a).
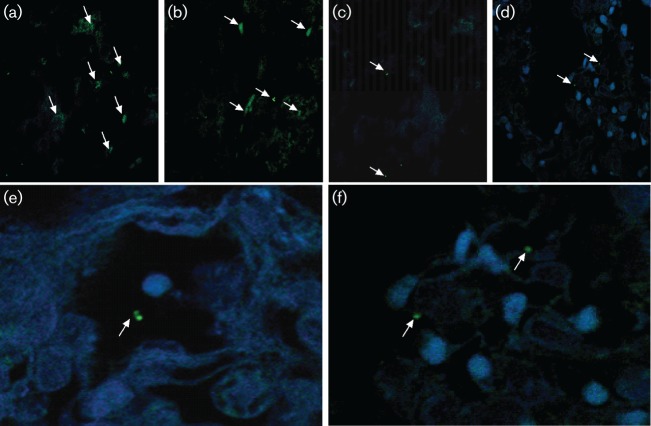
To determine whether non-cultivable and ZN stain-negative bacilli could be detected in the lungs, immunofluorescent anti-Mtb antibody-stained sections were analysed in three dimensions for bacilli size, length and fluorescent signature evaluation using NIS-Elements software (Nikon) (Fig. 3). First, we found that the three-dimensional size, length and medium fluorescence intensity of a full-body bacillus were 124 967±29 754 pixels (arbitrary units) for 145 bacilli analysed. Using this approach, we scanned the Mtb CDC1551-infected lung sections from three rabbits per time point and recorded the pixel values from 32 fields in multiple X-Y-Z sections (Fig. 3, Table 1). Small clumps of bacilli were seen in the lung sections at 8 weeks post-infection. Thereafter, individual bacilli were seen scattered in association with single or small clusters of host cell nuclei. Immunosuppression resulted in the reappearance of small clumps of bacilli (Fig. 3). Consistent with enumeration by the agar plating method, more bacteria were observed by immunofluorescent staining confocal microscopy at 8 weeks compared with 16 and 20 weeks, and in lung sections from immunosuppressed rabbits. Importantly, means of about 2.35, 2.27 and 2.2 log10 bacilli were found in the lungs of infected rabbits at 20, 24 and 26 weeks post-infection, respectively, when 0 c.f.u. was observed on agar plates (Table 1).
Fig. 3.
Confocal imaging of Mtb CDC1551 in infected rabbit lungs after immunofluorescent staining. (a–d) Representative images of rabbit lung sections at 8, 16, 20 and 24 weeks post-infection, respectively. (e, f) Representative images of lung sections from infected rabbits treated with triamcinolone at 24 (e) and 2 (f) weeks post-infection. Arrows indicate bacilli (green) in cells (nuclei stained blue).
Table 1. Quantification of fluorescence intensity and calibration of corresponding number of bacilli in Mtb CDC1551 infected rabbit lung sections.
| Time post-infection | Pixels (arbitrary units) | Number of bacteria* | ||
| Mean | sem | Mean | sd | |
| 8 weeks | 797 324.16 | 108 333.23 | 6.38 | 0.87 |
| 16 weeks | 574 820.59 | 51 193.07 | 4.59 | 0.41 |
| 20 weeks | 191 863.28 | 24 384.57 | 1.54 | 0.19 |
| 24 weeks: no treatment | 158 243.41 | 13 699.53 | 1.27 | 0.11 |
| 26 weeks: no treatment | 143 522.97 | 15 280.84 | 1.15 | 0.12 |
| 24 weeks: immunosuppressed | 3 609 472.74 | 737 211.79 | 18.44 | 28.53 |
| 26 weeks: immunosuppressed | 1 490 651.68 | 166 659.92 | 11.92 | 20.85 |
Calculated based on 32 X-Y-Z fields using 1.24±0.46 cm tissue sections.
Discussion
In this study, we determined, using immunofluorescence-based confocal microscopy imaging, the presence of intact Mtb that did not form colonies in the conventional agar-plating method and were not AFB-positive by ZN staining in the lungs of latently infected rabbits. The current sputum microscopy for TB diagnosis involves AFB staining (Garg et al., 2003; Norbis et al., 2013). However, several studies have reported the presence of dormant bacilli in human lung sections that were negative by ZN staining (Seiler et al., 2003). One factor that contributes to the inability of Mtb to form colonies on agar plates and to stain effectively by the ZN method is the perturbation of cell wall components, occurring as an adaptive response by the bacilli to survive in the hostile intracellular granulomatous environment (Wayne & Sohaskey, 2001; Zhang, 2004; Woolhiser et al., 2007). However, the exact mechanisms and the nature of the molecules impacted during reorganization of the Mtb cell wall during LTBI in vivo are not clearly understood (Ehlers, 2009; Mishra et al., 2011; Russell, 2013).
Visualization and enumeration of dormant Mtb in clinical samples can improve the sensitivity of TB diagnosis by microscopy and have implications for determining treatment outcomes (sputum AFB conversion). Identification of dormant Mtb in infected tissues may also help overcome our current inability to study the metabolism of this population of bacilli, which are difficult to detect with current methods. Finally, enumerating Mtb during LTBI would provide a potential tool for evaluating the ability of drug and vaccine candidates to eliminate such bacillary populations (Kim et al., 2013).
Acknowledgements
The authors acknowledge Paul O’Brien, Jennifer Holloway, Blas Peixoto and Liana Tsenova for their assistance with the rabbit experiments. This work was supported by a grant from NIH/NIAID to G. K. (AI054338).
Abbreviations:
- AFB
acid-fast bacilli
- LTBI
latent Mycobacterium tuberculosis infection
- Mtb
Mycobacterium tuberculosis
- TB
tuberculosis
- ZN
Ziehl–Neelsen
References
- Chao M. C., Rubin E. J. (2010). Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu Rev Microbiol 64, 293–311. 10.1146/annurev.micro.112408.134043 [DOI] [PubMed] [Google Scholar]
- Ehlers S. (2009). Lazy, dynamic or minimally recrudescent? On the elusive nature and location of the mycobacterium responsible for latent tuberculosis. Infection 37, 87–95. 10.1007/s15010-009-8450-7 [DOI] [PubMed] [Google Scholar]
- Flynn J. L., Izzo A., Tsenova L., Kaplan G. (2008). Experimental animal models of tuberculosis. In Handbook of Tuberculosis, pp. 389–426. Edited by Kaufmann S. H. E., Rubin E.. Weinheim: Wiley-VCH Verlag [Google Scholar]
- Garg S. K., Tiwari R. P., Tiwari D., Singh R., Malhotra D., Ramnani V. K., Prasad G. B., Chandra R., Fraziano M. & other authors (2003). Diagnosis of tuberculosis: available technologies, limitations, and possibilities. J Clin Lab Anal 17, 155–163. 10.1002/jcla.10086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbacher M., Kaufmann S. H. (2012). Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev 36, 514–532. 10.1111/j.1574-6976.2012.00331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Post F. A., Moreira A. L., Wainwright H., Kreiswirth B. N., Tanverdi M., Mathema B., Ramaswamy S. V., Walther G. & other authors (2003). Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun 71, 7099–7108. 10.1128/IAI.71.12.7099-7108.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., O’Brien K. M., Sharma R., Boshoff H. I., Rehren G., Chakraborty S., Wallach J. B., Monteleone M., Wilson D. J. & other authors (2013). A genetic strategy to identify targets for the development of drugs that prevent bacterial persistence. Proc Natl Acad Sci U S A 110, 19095–19100. 10.1073/pnas.1315860110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A. K., Driessen N. N., Appelmelk B. J., Besra G. S. (2011). Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host–pathogen interaction. FEMS Microbiol Rev 35, 1126–1157. 10.1111/j.1574-6976.2011.00276.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbis L., Miotto P., Alagna R., Cirillo D. M. (2013). Tuberculosis: lights and shadows in the current diagnostic landscape. New Microbiol 36, 111–120. [PubMed] [Google Scholar]
- O’Garra A., Redford P. S., McNab F. W., Bloom C. I., Wilkinson R. J., Berry M. P. (2013). The immune response in tuberculosis. Annu Rev Immunol 31, 475–527. 10.1146/annurev-immunol-032712-095939 [DOI] [PubMed] [Google Scholar]
- Russell D. G. (2013). The evolutionary pressures that have molded Mycobacterium tuberculosis into an infectious adjuvant. Curr Opin Microbiol 16, 78–84. 10.1016/j.mib.2012.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler P., Ulrichs T., Bandermann S., Pradl L., Jörg S., Krenn V., Morawietz L., Kaufmann S. H., Aichele P. (2003). Cell-wall alterations as an attribute of Mycobacterium tuberculosis in latent infection. J Infect Dis 188, 1326–1331. 10.1086/378563 [DOI] [PubMed] [Google Scholar]
- Subbian S., Tsenova L., Yang G., O’Brien P., Parsons S., Peixoto B., Taylor L., Fallows D., Kaplan G. (2011a). Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol 1, 110016. 10.1098/rsob.110016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbian S., Tsenova L., O’Brien P., Yang G., Koo M. S., Peixoto B., Fallows D., Dartois V., Muller G., Kaplan G. (2011b). Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium tuberculosis in rabbit lungs. PLoS Pathog 7, e1002262. 10.1371/journal.ppat.1002262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbian S., Tsenova L., O’Brien P., Yang G., Kushner N. L., Parsons S., Peixoto B., Fallows D., Kaplan G. (2012). Spontaneous latency in a rabbit model of pulmonary tuberculosis. Am J Pathol 181, 1711–1724. 10.1016/j.ajpath.2012.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Sohaskey C. D. (2001). Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol 55, 139–163. 10.1146/annurev.micro.55.1.139 [DOI] [PubMed] [Google Scholar]
- WHO (2012). Global Tuberculosis Report 2012. Geneva: World Health Organization. http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf [Google Scholar]
- Woolhiser L., Tamayo M. H., Wang B., Gruppo V., Belisle J. T., Lenaerts A. J., Basaraba R. J., Orme I. M. (2007). In vivo adaptation of the Wayne model of latent tuberculosis. Infect Immun 75, 2621–2625. 10.1128/IAI.00918-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. (2004). Persistent and dormant tubercle bacilli and latent tuberculosis. Front Biosci 9, 1136–1156. 10.2741/1291 [DOI] [PubMed] [Google Scholar]