Abstract
Exposure to total-body radiation induces hematological changes, which can detriment one's immune response to wounds and infection. Here, the decreases in blood cell counts after acute radiation doses of γ-ray or proton radiation exposure, at the doses and dose-rates expected during a solar particle event (SPE), are reported in the ferret model system. Following the exposure to γ-ray or proton radiation, the ferret peripheral total white blood cell (WBC) and lymphocyte counts decreased whereas neutrophil count increased within 3 hours. At 48 hours after irradiation, the WBC, neutrophil, and lymphocyte counts decreased in a dose-dependent manner but were not significantly affected by the radiation type (γ-rays verses protons) or dose rate (0.5 Gy/minute verses 0.5 Gy/hour). The loss of these blood cells could accompany and contribute to the physiological symptoms of the acute radiation syndrome (ARS).
Keywords: Radiation, Hematopoietic Cells, Hematology
Introduction
The effects of exposure to ionizing radiation (IR) are of interest to the space exploration community as well as patients considering radiotherapy. IR has a sufficient amount of energy to induce physical symptomatology within minutes of exposure, appearing as the acute radiation syndrome (ARS). The prodromal phase of ARS includes nausea, vomiting, and fatigue. The quality of radiation, dose, and dose-rate are all contributing factors to the differential symptoms of ARS. These prodromal symptoms can be followed by a dramatic decrease in peripheral blood cell counts, as hematopoietic cells represent a renewal system consisting of cells with fast division rates that are known to be sensitive to IR.
Astronauts are exposed to chronic low-dose and low-dose rate IR during low-Earth orbit missions (Badhwar, 2002; Cucinotta et al., 2008). Contributing to low-Earth orbit radiation exposures are solar particle events (SPEs), which accelerate ions and release unpredictable doses of IR; therefore, SPE radiation exposure poses a threat to astronauts in a spacecraft where shielding is available, and especially during an extravehicular activity (EVA), in which shielding may only be provided by the space suit. SPE radiation consists predominantly of energetic proton particles with energies greater than 10 millielectron volts (MeV). The duration of radiation exposure from an SPE can last several days. It has been estimated that the largest dose of SPE radiation recorded from a historically large SPE (August 1972) was capable of delivering a 1.38 Gy dose (during an EVA) and a 0.46 Gy dose (in a spacecraft) to the blood forming organs to astronauts (Hu et al., 2009).
The Center of Acute Radiation Research (CARR) has been established to investigate the risks of ARS induced by SPE-like radiation, and develop countermeasures for those risks if appropriate. Thus, measuring the early effects of SPE radiation exposure in the hematopoietic system in response to space-relevant radiation is a focus. The hematological effects of space radiation contribute to the compromised immune defense in astronauts observed in the space environment as well as upon landing (Sonnenfeld et al., 2003; Sonnenfeld and Shearer, 2002; Crucian et al., 2008). The complications associated with the hematopoietic syndrome include infection and internal hemorrhage. The decrease in peripheral blood cell counts recorded within the first 48 hours of radiation exposure serve not only as a marker for the severity of the exposure, but also as a marker for treatment and prognosis.
As part of this work, we have previously evaluated the acute effects of gamma and proton radiation in the murine model (Maks et al., 2011; Ware et al., 2010). Recently, we reported increased blood clotting times in ferrets exposed to SPE-like proton radiation, which may contribute to radiation-induced coagulopathies (Krigsfeld et al., 2012). In the current report, we investigated the effects of SPE-like proton radiation on the hematopoietic system in the ferret model. The effects of proton radiation on peripheral blood cell counts were compared to the effects produced by conventional gamma-ray (γ-ray) radiation. Effective doses and relative biological effectiveness values were determined to compare the effects of the differing types of radiation.
Materials & Methods
Animals
Female descented ferrets (approximately 12-16 weeks of age) were obtained from Marshall Bioresources (North Rose, NY) and given an acclimation period of 7 days at the Loma Linda University Medical Center (LLUMC). Animals were group-housed and provided access to food and water ad libitum with a 12 hour light-dark cycle. The animals were maintained under standard husbandry conditions and all procedures for the animal care and treatment were approved by the Institutional Animal Care and Use Committees (IACUC) of the University of Pennsylvania and the LLUMC. Ferrets were randomly assigned to treatment groups, with each group consisting of 6–12 animals.
Physics and Dosimetry
γ-Ray radiation was chosen as the reference radiation for the determination of relative biological effectiveness (RBE) values and was delivered using a 60Co source (Eldorado Model ‘G’ machine, Atomic Energy of Canada Ltd., Commercial Products Division, Ottawa, Canada) at the LLUMC. For radiation exposures at a high dose rate (HDR) of 0.5 Gy/minute, the source to target distance was 150 cm, with a usable radiation field of 40 × 40 cm2 and a field flatness of 5.4% in the horizontal direction and 3.6% in the vertical direction. No additional material was placed between the source and target to modify the dose rate. For radiation exposure at a low-dose rate of 0.5 Gy/hour, the source to target distance was 195 cm with a useable field size of 40 × 40 cm2 and a field flatness of 4.0% in the horizontal direction and 2.3% in the vertical direction. To achieve the low dose rate (LDR), an additional 12.7 cm of steel plating was placed between the source and the target to attenuate the flux of the beam. Depth dose measurements were made using a calibrated PTW Markus ionization chamber for comparison with proton irradiation.
For the proton radiation exposures, experiments were performed in the LLUMC horizontal clinical beam-line using an incident beam of 155-MeV. The incident protons were scattered into a uniform field using the clinical 2-stage scattering system and modulated in depth using an 11-cm clinical modulator wheel. At the exit of the beam-line, the beam was degraded to the required energy using a pre-determined thickness of polystyrene. The proton experiments used two different apertures to target distances and two different polystyrene thicknesses to achieve the desired beam sizes and dose rates of 0.5 Gy/minute (HDR) or 0.5 Gy/hour (LDR), respectively. For the LDR proton experiments, the animal cages were placed 122 cm downstream of isocenter. The useable beam at this distance was measured as 22 × 22 cm2 while achieving a flatness of ±10%. This field size allowed only one ferret in the radiation chamber (length, width, and height was approximately 24 × 16 × 9 cm) to be irradiated. For the HDR proton experiments, the animal cages were placed at isocenter. The usable beam at this distance was experimentally verified and measured as 19 × 19 cm2, again allowing only one ferret in the chamber to be irradiated. The upstream polystyrene degrader was tuned to achieve a fully modulated proton beam of 110-MeV at the inside of the irradiation chamber. Depth dose profiles were measured for the optimized polystyrene degrader thickness using Gafchromic film, type MD-55, and verified with a PTW Markus ionization chamber.
To complete a more efficient proton radiation of multiple ferrets at the LDR, an alternate scattering system was developed and verified in the LLUMC Proton Research Room. The incident protons were scattered using a 2-stage scattering system to a useable radiation field of 50 cm diameter with flatness and depth dose profiles that were comparable to the clinical system described above. This system allowed for 12 ferrets to be irradiated at any one time.
γ-Ray or Proton Radiation Exposure
For the γ-ray and the proton radiation experiments, the animals were placed in Plexiglass radiation chambers measuring approximately 24 × 16 × 9 cm in length, width and height, respectively. The custom-made radiation chambers contained adequate holes for proper air circulation and animals were provided with NapaNectar hydrating gel (SE Lab Group Inc., Napa, CA). For the LDR experiments, the chambers were slightly modified to contain a watering system on the outside of each chamber that did not compromise the radiation dose delivered to each animal, so these animals were not provided NapaNectar gel. The LDR chambers also contained a thermistor which enabled real-time monitoring of temperature within the radiation chamber during the long LDR exposures. The average recorded temperature of an empty chamber prior to radiation was 70°C, and the average recorded temperature during/after the HDR and LDR radiation exposures (with an animal in the chamber) was 75°C; thus, these results indicated that the temperature during the containment remained adequate. The animals were not anesthetized during irradiation to total doses ranging from 0.75 Gy to 2.0 Gy and were able to express normal postural movements throughout. For the HDR experiments, although the actual radiation exposures were only minutes long (as opposed to hours in the case of the LDR experiments), the animals were restrained in the radiation chamber for the same amount of time as the LDR animals, corresponding to the appropriate dose.
Complete Blood Cell Count Analyses
Animals were anesthetized by isoflurane inhalation for blood collection. Blood samples were collected from the external jugular vein and immediately placed in tubes containing ethylenediaminetetraacetic acid (EDTA). Blood draws were performed on each animal at least 2 days prior to radiation exposure (Pre) and again at 3 hours (3 h) and 48 hours (48 h) post-irradiation. A complete blood count (CBC) with differential was performed using a Bayer Advia 120 Hematology Analyzer within 24 hours of blood collection (Antech Diagnostics, Irvine, CA).
Statistical Analyses
The average counts of white blood cells (WBC), neutrophils, lymphocytes, monocytes and eosinophils were determined prior to the radiation exposure as baseline values. The blood cell counts obtained in animals at different time points were divided by the respective baseline values and expressed as fractions of control for statistical analyses. The effects of experimental factors (Radiation Type, Dose Rate, and Dose) and their interactions on the blood cell counts were determined by variance analyses using a general linear model, which was performed using a Minitab statistical software, release 15 (Minitab Inc., State College, PA). The relationship between the dose and blood cell count was determined by fitting the data to a linear quadratic model, y = exp (-αD-βD2), where y is blood cell count expressed as fraction of pre-irradiation baseline value, D stands for radiation dose (Gray or Gy), and α and β are the fitted coefficients for the linear and quadratic components of the linear quadratic model. The linear quadratic curve fitting was performed using SigmaPlot graphics software (SPSS Inc., Chicago, IL). RBE values were calculated by first determining the dose of γ-ray radiation needed to produce the same blood cell counts as proton radiation, and secondly dividing the derived γ-ray radiation dose by the proton radiation dose. The RBE values were plotted against proton radiation dose and analyzed by a non-linear regression analysis to show the relationship between RBE value and proton radiation dose. The non-linear regression analysis showing the relationship between the RBE value and proton radiation dose and the associated 95% confidence interval (CI) was also performed using Minitab statistical software (release 15).
Results
There are no statistically significant changes in hematopoietic cell counts after confinement for 7 hours in the radiation chamber, without radiation exposure
The effect of confinement in the irradiation chamber was determined in a group of 5 animals that were placed in the irradiation chamber for up to 7 hours without radiation exposure. Using oneway ANOVA, no statistically significant differences are reported for each blood cell type (WBC, neutrophils, lymphocytes, monocytes, eosinophils, and platelets) when comparing the absolute cell count at each time point to the other time points (data not shown). Thus, the confinement up to 7 hours did not affect blood cell counts.
Radiation decreases white blood cell counts
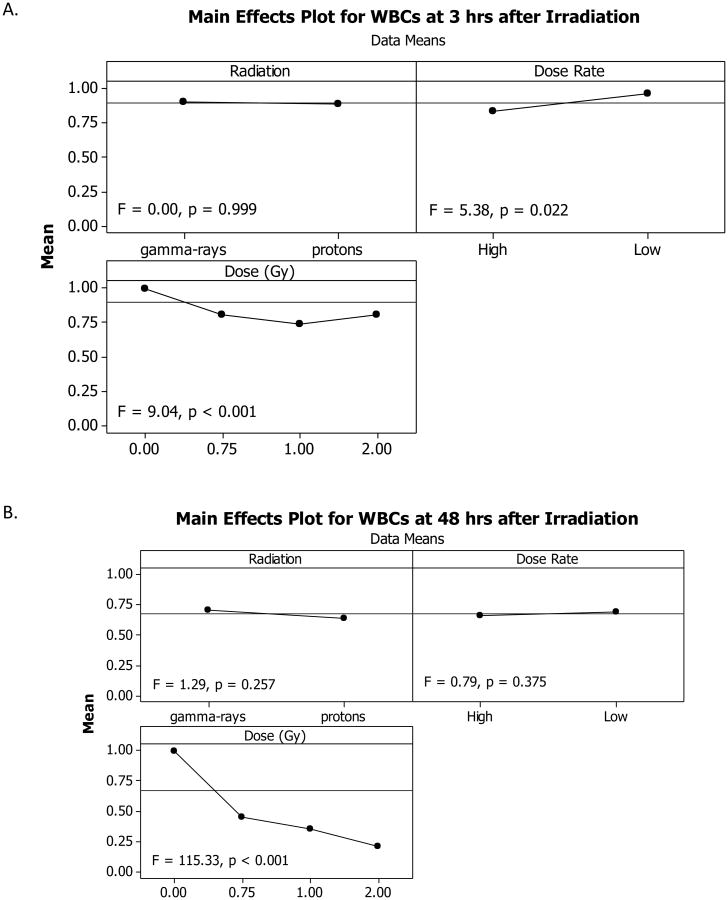
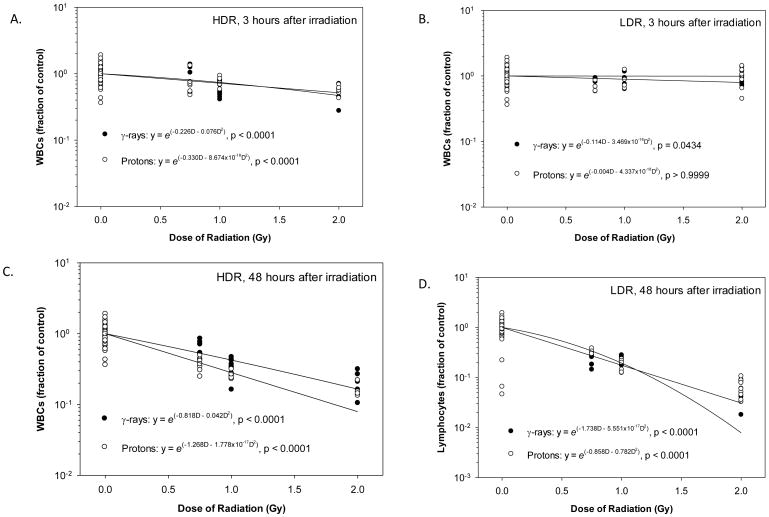
The effects of the experimental factors, which consisted of Radiation Type, Dose, and Dose Rate, were determined by variance analyses using the general linear model. The data obtained at 3 and 48 hours after irradiation were analyzed separately to compare the effects observed at these two time points. The results showed that the WBC count decreased within 3 hours after irradiation and the magnitude of the decrease observed at 48 hours after irradiation was approximately three times the decrease observed at 3 hours after irradiation (Figure 1). At 3 hours after irradiation, Dose was a significant influencing factor of the WBC count. The Dose Rate was also a significant influencing factor of the WBC count, which resulted in larger decreased counts after irradiation at HDR than at LDR. Radiation Type (γ-rays vs. protons) had almost no effect on the WBC count. At 48 hours after irradiation, the Dose was the only significant influencing factor of the WBC count, which decreased in a dose-dependent manner. As shown in the dose response curves in Figure 2, a significant and dose-dependent decrease in WBC count was observed in animals at 3 hours after irradiation with HDR γ-rays (Figure 2A), HDR protons (Figure 2A). and LDR γ-rays (Figure 2B), but not with LDR protons (Figure 2B). The coefficient for the linear component of the dose response curve slope was 0.266 and 0.333 for the animals irradiated with HDR γ-rays and protons, which were approximately twice or more of that for the animals irradiated with LDR γ-rays and protons. The coefficient for the quadratic component of the dose response curve slopes was negligible (≤ 3.469 × 10−18) except for the animals irradiated with HDR γ-rays. The effective dose (ED) ED10, ED50, and ED90 for the HDR γ-ray radiation were bracketed by the 95% confidence intervals of the ED10, ED50, and ED90 for the HDR proton radiation (Table 1), indicating that all three ED values were not significantly different between the HDR γ-ray and HDR proton radiation. The ED10, ED50, and ED90 values were not compared between the LDR γ-ray and proton radiation due to the absence of a significant dose response for the LDR proton irradiated animals. The fitted RBE values were 2.15 and 4.02 for HDR and LDR protons at 0.75 Gy, respectively (Table 2), which were significantly above 1.00. The RBE decreased with the increase of the proton radiation dose and the fitted RBE values for HDR and LDR protons at 1 and 2 Gy were not significantly different from 1.00, which was within the 95% confidence intervals for the respective RBE values.
Figure 1.
Variance analysis of white blood cell (WBC) results. Variance analysis was performed to determine which experimental factor(s) significantly affected the WBC counts in the animals 3 hours after irradiation (A) or 48 hours after radiation exposure (B). Radiation type (γ-rays vs. protons) did not significantly affect the overall WBC counts after radiation exposure whereas the dose rate did have an effect at 3 hour post-exposure, and total dose also contributed significantly to WBC count changes in the irradiated animals at both 3 hours and 48 hours after exposure. Note: the x-axes labels are included at the top of each panel, i.e., Radiation, Dose Rate, or Dose.
Figure 2.
Dose-response curves for WBC in animals after irradiation. The WBC count results obtained 3 hours after irradiation at HDR (A) or LDR (B) or 48 hours after irradiation at HDR (C) or LDR (D) were determined by a non-linear regression analysis using a linear quadratic model. Statistically significant dose responses were observed for HDR and LDR γ-rays and protons at both time points except for LDR protons at 3 hours after irradiation.
Table 1. Effective doses of γ-rays and protons to decrease blood cell count by 10% (ED10), 50% (ED50), and 90% (ED90).
| Cell Type | Dose Rate | Time after Irradiation | Effective dose (and 95% CI) for γ-rays | Effective dose (and 95% CI) for protons | ||||
|---|---|---|---|---|---|---|---|---|
| ED10 | ED50 | ED90 | ED10 | ED50 | ED90 | |||
| WBCs | High | 3 hours | 0.41 (0.14 – 0.67) | 1.87 (0.66 – 3.10) | 4.21 (1.48 – 6.95) | 0.32 (0.18 – 0.46) | 2.10 (1.17 – 3.03) | 6.96 (3.87 – 10.06) |
| Low | 0.91 (0.09 – 1.75) | 6.05 (0.59 – 11.50) | 20.09 (1.97 – 38.21) | NM | NM | NM | ||
| High | 48 hours | 0.13 (0.09 – 0.17) | 0.81 (0.55 – 1.08) | 2.50 (1.70 – 3.30) | 0.08 (0.06 – 0.11) | 0.55 (0.37 – 0.72) | 1.82 (1.24 – 2.39) | |
| Low | 0.11 (0.07 – 0.15) | 0.72 (0.47 – 0.96) | 2.38 (1.57 – 3.19) | 0.13 (0.09 – 0.18) | 0.88 (0.59 – 1.18) | 2.93 (1.94 – 3.91) | ||
| Neutrophils | High | 48 hours | 0.31 (0.11 – 0.52) | 2.04 (0.71 – 3.37) | 6.51 (2.25 – 10.76) | 0.18 (0.08 – 0.28) | 1.19 (0.55 – 1.84) | 3.95 (1.81 – 6.10) |
| Low | 0.27 (0.09 – 0.45) | 1.79 (0.61 – 2.98) | 5.96 (2.01 – 9.90) | 0.42 (0.05 – 0.79) | 2.12 (0.26 – 3.98) | 5.03 (0.61 – 9.45) | ||
| Lymphocytes | High | 3 hours | 0.05 (0.03 – 0.07) | 0.33 (0.18 – 0.49) | 1.11 (0.60 – 1.63) | 0.05 (0.03 – 0.07) | 0.32 (0.17 – 0.48) | 1.07 (0.56 – 1.58) |
| Low | 0.06 (0.03 – 0.09) | 0.41 (0.21 – 0.61) | 1.36 (0.71 – 2.01) | 0.06 (0.03 – 0.10) | 0.41 (0.18 – 0.64) | 1.35 (0.59 – 2.11) | ||
| High | 48 hours | 0.05 (0.02 – 0.07) | 0.33 (0.16 – 0.49) | 1.08 (0.55 – 1.62) | 0.08 (0.01 – 0.15) | 0.41 (0.06 – 0.75) | 0.98 (0.15 – 1.80) | |
| Low | 0.06 (0.03 – 0.09) | 0.40 (0.21 – 0.59) | 1.33 (0.69 – 1.96) | 0.11 (0.00 – 0.23) | 0.54 (0.00 – 1.11) | 1.25 (0.00 – 2.58) | ||
High dose rate: 0.5 Gy/minute; low dose rate: 0.5 Gy/hour. Neutrophil counts at 3 hours post-irradiation were not decreased and not included in this table.
Table 2.
Relationship between RBE and proton radiation dose for white blood cells (WBCs), neutrophils, lymphocytes.
| Cell Type | Dose Rate | Time after Irradiation | 0.75 Gy | 1 Gy | 2Gy | |||
|---|---|---|---|---|---|---|---|---|
| RBE | 95% CI | RBE | 95% CI | RBE | 95% CI | |||
| WBCs | High | 3 hours | 2.15 | 1.51 – 2.79 | 0.96 | 0.06 – 1.86 | 0.62 | 0.00a – 1.52 |
| Low | 4.02 | 2.25 – 5.79 | 1.76 | 0.39 – 3.13 | 0.59 | 0.00a – 1.56 | ||
| High | 48 hours | 1.60 | 1.42 – 1.77 | 1.59 | 1.34 – 1.84 | 1.04 | 0.79 – 1.29 | |
| Low | 1.19 | 0.92 – 1.45 | 0.80 | 0.59 – 1.00 | 0.77 | 0.62 – 0.92 | ||
| Neutrophils | High | 48 hours | 1.88 | 1.31 – 2.44 | 2.04 | 1.24 – 2.84 | 1.55 | 0.75 – 2.35 |
| Low | 2.11 | 1.32 – 2.91 | 0.62 | 0.00a – 1.23 | 0.90 | 0.46 – 1.34 | ||
| Lymphocytes | High | 3 hours | 1.10 | 0.99 – 1.21 | 1.00 | 0.84 – 1.15 | 0.67 | 0.52 – 0.83 |
| Low | 1.41 | 1.21 – 1.61 | 0.96 | 0.81 – 1.12 | 0.68 | 0.58 – 0.79 | ||
| High | 48 hours | 1.01 | 0.94 – 1.08 | 1.06 | 0.96 – 1.17 | 0.77 | 0.67 – 0.88 | |
| Low | 0.83 | 0.67 – 1.00 | 0.99 | 0.86 – 1.11 | 0.84 | 0.74 – 0.93 | ||
High dose rate: 0.5 Gy/minute; low dose rate: 0.5 Gy/hour. Neutrophil counts at 3 hours post-irradiation were not decreased and not included in this table.
At 48 hours after irradiation, a significant and dose-dependent decrease in WBC count was observed in animals of all groups with the coefficient for the linear component of the dose response curve slope ranging from 0.786 to 1.268 (Figures 2C and 2D). The coefficient for the quadratic component of the dose response curve slopes was again negligible (≤ 1.388 × 10−17) except for the animals irradiated with HDR γ-rays. The ED10, ED50, and ED90 for the HDR γ-ray radiation were greater than the upper limit of the 95% confidence intervals of the ED10, ED50, and ED90 for the HDR proton radiation (Table 1), indicating that HDR proton radiation was more effective than HDR γ-rays in reducing the number of WBCs. The ED10, ED50, and ED90 for the LDR γ-ray radiation were within the 95% confidence intervals of the three corresponding ED values for LDR proton radiation, suggesting that the LDR γ-rays and protons were not significantly different in the effectiveness of reducing the number of WBCs. The RBE values derived from results observed at 48 hours after irradiation also decreased with the increase of the proton radiation dose, but the downward trend was not as prominent as the trend observed at 3 hours after irradiation. The fitted RBE values ranged from 1.60 - 1.19 at 0.75 Gy and 1.04 - 0.77 at 2 Gy for HDR and LDR protons, respectively (Table 2), and were significantly above 1.00 for HDR protons at 0.75 and 1 Gy but significantly below 1.00 for LDR protons at 2 Gy.
Radiation induces changes in the average neutrophil count
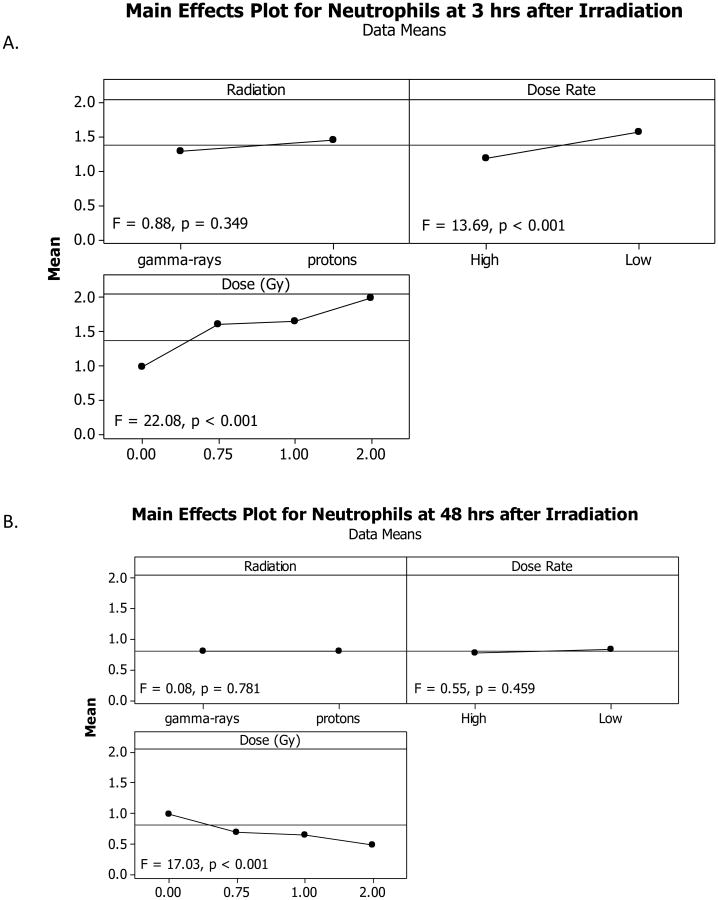
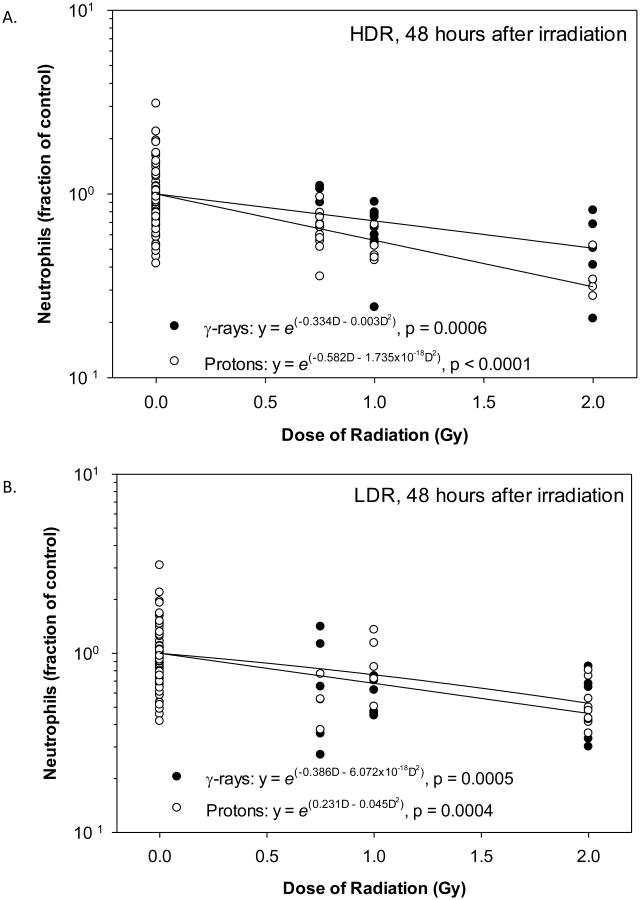
Of the three experimental factors evaluated, Radiation Dose was the most significant influencing factor of the neutrophil count, which increased at 3 hours after irradiation (Figure 3A) but decreased at 48 hours after irradiation (Figure 3B), both in a dose-dependent manner. Radiation Dose Rate significantly affected the neutrophil count at 3 hours after irradiation with a larger reduction after irradiation at HDR than at LDR. The Radiation Dose Rate did not significantly affect the neutrophil count at 48 hours after irradiation. The Radiation Type did not significantly affect the neutrophil count at either time points after irradiation. At 48 hours after irradiation with HDR γ-rays, HDR protons, LDR γ-rays, and LDR protons, the coefficient for the linear component of the dose response curve slope was 0.334, 0.582, 0.386, and 0.231, respectively (Figures 4A and 4B). The coefficient for the quadratic component of the dose response curve slopes was small (≤ 0.003) except for the animals irradiated with LDR protons. The ED10, ED50, and ED90 for the HDR γ-ray radiation were greater than the upper limit of the 95% confidence intervals of the ED10, ED50, and ED90 for the HDR proton radiation (Table 1), indicating that HDR protons were significantly more effective than HDR γ-ray radiation in reducing neutrophils. The ED10, ED50, and ED90 for the LDR γ-ray radiation were bracketed by the 95% confidence intervals of the ED10, ED50, and ED90 for the LDR proton radiation, suggesting that LDR γ-rays and protons were not significantly different in their effectiveness of killing neutrophils. The fitted RBE values for irradiation with HDR protons at 0.75 and 1 Gy and with LDR protons at 0.75 Gy were 1.88, 2.04, and 2.11, respectively (Table 2), which were significantly above 1.00. The fitted RBE values for irradiation with HDR protons at 2 Gy and with LDR protons at 1 and 2 Gy were 1.55, 0.62, and 0.90, respectively, and were not significantly different from 1.00.
Figure 3.
Variance analysis of neutrophil results. Variance analysis was performed to determine which experimental factor(s) significantly affected the neutrophil count in animals 3 hours after irradiation (A) or 48 hours after radiation exposure (B). Radiation type (γ-rays vs. protons) did not significantly affect the overall neutrophil counts after radiation exposure whereas the dose rate did have an effect at 3 hour post-exposure, but not 48 hours post-exposure. Lastly, the total dose did contribute significantly to neutrophil count changes in the irradiated animals at both 3 hours and 48 hours after exposure. Note: the x-axes labels are included at the top of each panel, i.e., Radiation, Dose Rate, or Dose.
Figure 4.
Dose-response curves for neutrophils in animals after irradiation. The neutrophil count results obtained 48 hours after irradiation at HDR (A) or LDR (B) were analyzed by a non-linear regression analysis using a linear quadratic model. Statistically significant dose responses were observed for HDR and LDR γ-rays and protons.
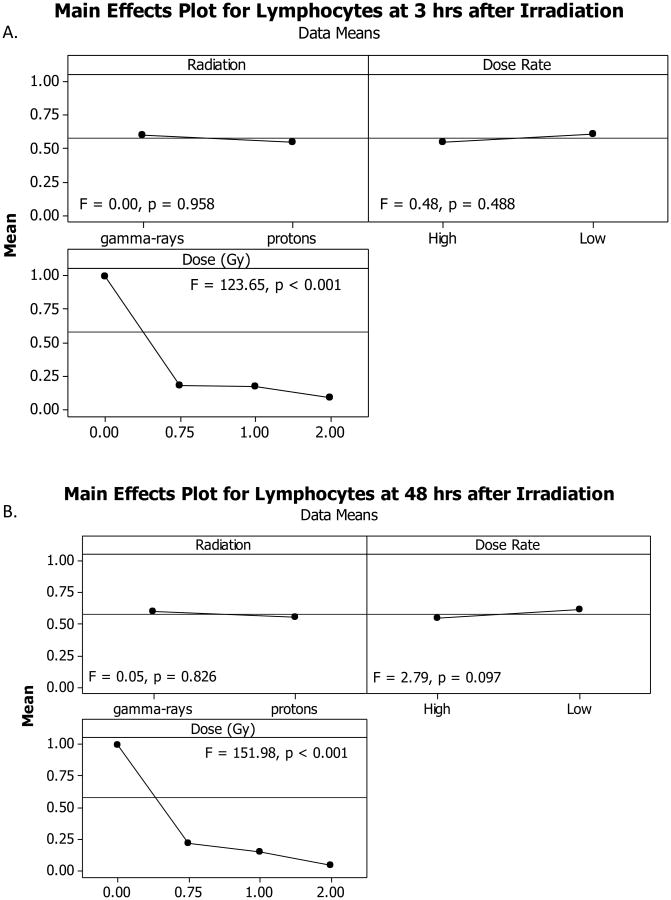
Radiation decreases lymphocyte counts as early as 3 hours after radiation exposure
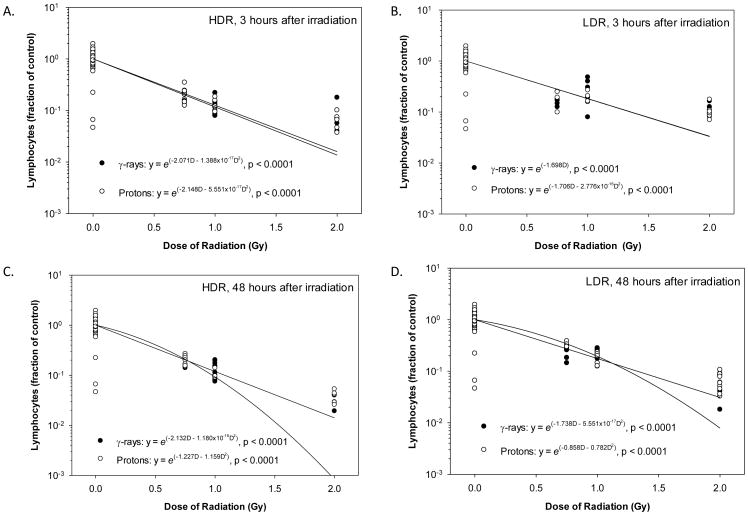
Of the three experimental factors evaluated, Radiation Dose was the only significant influencing factor of the lymphocyte count at 3 hours (Figure 5A) and 48 hours (Figure 5B) after irradiation. Neither the Radiation Type nor the Dose Rate significantly affected the lymphocyte count at either time points after irradiation. The dose response curves for the lymphocytes displayed a significant and dose-dependent decrease in lymphocyte count at 3 hours and 48 hours after irradiation with either γ-rays or protons at HDR or LDR (Figure 6). The coefficient for the linear component of the dose response curve slope ranged from 1.698 to 2.148 and the coefficient for the quadratic component of the dose response curve slopes was negligible (≤ 1.180 × 10−16) for all groups except the animals at 48 hours after irradiation with HDR protons and LDR protons, for which the coefficient for the linear component of the dose response curve slopes were 1.227 and 0.858 whereas the coefficient for the quadratic component of the dose response curve slopes were 1.159 and 0.782, respectively. The ED10, ED50, and ED90 for animals at 3 and 48 hours after irradiation with HDR and LDR γ-rays were within the 95% confidence intervals of the corresponding ED10, ED50, and ED90 for the HDR and LDR proton radiation (Table 1), indicating that γ-rays and protons were not significantly different in their effectiveness of reducing lymphocytes at HDR or LDR. The fitted RBE values for HDR and LDR protons at 0.75 Gy ranged from 0.83 to 1.41 at 3 and 48 hours after irradiation (Table 2). With the increase of proton radiation dose to 2 Gy, the fitted RBE values decreased to a range of 0.67 to 0.84 at 3 and 48 hours after irradiation at HDR or LDR. The RBE values were not significantly different from 1.00; however, the RBE was significantly higher than 1.00 for 0.75 Gy protons at LDR and significantly lower than 1.00 for 2 Gy HDR and LDR protons at 3 hours after irradiation and for 2 Gy LDR protons at 48 hours after irradiation.
Figure 5.
Variance analysis of lymphocyte results. Variance analysis was performed to determine which experimental factor(s) significantly affected the lymphocyte counts in irradiated animals 3 hours (A) or 48 hours (B) after exposure. Radiation type (γ-rays vs. protons) and dose rate did not significantly affect the overall lymphocyte count after radiation exposure, whereas the total dose contributed significantly to lymphocyte count changes in the irradiated animals. Note: the x-axes labels are included at the top of each panel, i.e., Radiation, Dose Rate, or Dose.
Figure 6.
Dose-response curves for lymphocytes in animals after irradiation. The lymphocyte count results obtained from animals at 3 hours after irradiation at HDR (A) or LDR (B) or 48 hours after irradiation at HDR (C) or LDR (D) were analyzed by a non-linear regression analysis using a linear quadratic model. Statistically significant dose responses were observed for HDR and LDR γ-rays and protons at both time points. It should be noted that a common log scale is used for y-axis, which has visually exaggerated the deviation of the dose response curves from the actual data points at the high end of the radiation dose range.
Monoctye, eosinophil, and platelet counts were statistically insignificant at both the 3 hour and 48 hour time points (data not shown).
Discussion
The effect of SPE-like proton radiation on hematopoietic cell counts in the ferret model was investigated in the present study. The energy of protons used to simulate SPE radiation was determined to ensure a homogenous dose distribution throughout the whole body. Hematopoietic cell loss was observed as early as 3 hours after the conclusion of radiation exposure, with a more profound decrease observed at the 48-hour time point. For the WBC and neutrophil counts determined at 3 hours after irradiation, a small but statistically significant dose rate effect was observed. Ferrets exposed to the HDR (0.5 Gy/min) radiation had lower average WBC and neutrophil counts than the animals exposed to the LDR (0.5/h) radiation. This result agrees with a similar experiment by Maks et al. (2011) in which mice were exposed to proton and γ-ray radiation at the same HDR or LDR dose rates. Although the LDR radiation exposures resulted in significant decreases in peripheral blood cell counts, the HDR radiation, for both the protons and γ-rays, resulted in even lower average blood cell counts compared to the LDR radiation. Interestingly, the dose rate effect was not observed at 48 hours after irradiation in the present study, suggesting that the higher dose rate might have only accelerated the onset of radiation effect but did not affect the overall magnitude of the radiation effect developed at the later time points. Given the fact that the dose rate effect was not observed at 48 hours after irradiation, a critical time with a pronounced reduction in circulating WBCs, neutrophils, and lymphocytes, the dose rate effect probably did not have a biologically meaningful impact on the blood cell counts in the irradiated animals.
In the present study, the time course for the changes in the neutrophil and lymphocyte counts were strikingly different. The neutrophil count displayed a dose-dependent increase at 3 hours after irradiation and a dose-dependent decrease at 48 hours after irradiation (Figure 3) whereas the dose response relationship for the lymphocytes were nearly identical between 3 hours and 48 hours after irradiation (Figure 5). It is conceivable that the increase in the neutrophil count might have partially canceled the decrease in the lymphocyte count when total WBC count was determined at 3 hours after irradiation, which might be the reason for the less pronounced dose response of the WBC count at 3 hours after irradiation. Since the neutrophils are an essential part of innate immunity, the early increase in their count could be at least partly due to rapid recruitment of these cells from the bone marrow (Christopher and Link, 2007). The different time courses for the neutrophil and lymphocyte counts observed after irradiation highlighted an importance to monitor the changes in the WBC differential in addition to the total WBC count since a change in one type of hematopoietic cells after radiation exposure may be masked by an opposite change in another type of hematopoietic cells. Based on the magnitude of the decrease and the time required to show a significant decrease in the blood cell count after irradiation, lymphocytes appeared to be the most sensitive to γ-ray and proton irradiation among the types of cells evaluated, which is consistent with the previous observation in a porcine model (Sanzari et al., 2013).
As expected, the dose of radiation was the most significant contributing factor in the observed radiation-induced cell loss. The dose-dependent decrease in peripheral WBC counts is consistent with previous findings in the mouse model after whole-body proton (Maks et al., 2011; Ware et al., 2010; Wambi et al., 2009) and photon irradiation (Wambi et al., 2008; Pecaut et al., 2001; Gridley et al., 2001) as well as high linear energy transfer (LET) iron ion exposure (Pecaut and Gridley, 2010). The dose response relationship of proton or γ-ray irradiation on hematopoietic cell loss observed here in the ferret model represents a genetically heterogenous, non-rodent test system. The present findings suggest that damage from IR causes a significant reduction in blood cell counts in a dose-dependent manner, which may be considered a potential health risk during space travel.
The hematologic profile of ferrets has been previously determined by standard methods (Lee et al., 1982). The average leukocyte count ranges from 2.5-8.6 × 103 cells/uL, compared to the human total leukocyte count range of 4.5-11 × 103 cells/uL. While the average number of circulating leukocytes may not differ between ferrets and humans, it is worthwhile to note that the percentage of each specific type of WBC may. Therefore, when translating the results from the ferret model to human consequence, the interspecies disparity merits careful attention.
We have observed increased clotting times and the consumption of coagulation factors in ferrets exposed to the doses of SPE-like proton radiation investigated in the current report and within the same time frame (Krigsfeld et al., 2012). The consumption of coagulation factors and platelets throughout the body's blood vessels, a condition known as disseminated intravascular coagulation (DIC), can result in internal and external hemorrhaging, and potentially death. Platelet numbers did not change in a significant manner within the first 48 hours of proton or gamma radiation exposure in this study. However, current studies underway suggest that radiation-induced coagulopathies and decreased platelet counts are present in animals exposed to the radiation doses reported in this study (up to 2 Gy) at time points later than 48 hours post-irradiation and with serious implications within weeks after radiation exposure (unpublished data). The observed coagulopathy changes in irradiated ferrets are accompanied by a severe loss of circulating white blood cells.
In conclusion, whole-body proton or γ-ray radiation results in a significant reduction in circulating WBCs in the ferret model. Using the spread-out Bragg peak to achieve a homogenous dose distribution, the proton doses used here (0.75-2.0 Gy) resulted in RBE values of 0.59 - 4.02 when evaluating radiation-induced reductions in WBC counts. Thus, whole body SPE-like radiation exposure poses threats to the hematopoietic system in an acute manner, which can result in increased susceptibility to infection, and an overall depressed immune response and function.
Acknowledgments
This work was supported by the Center of Acute Radiation Research (CARR) grant from the National Space Biomedical Research Institute (NSBRI) through NASA NCC 9-58 and NIH Training Grant 2T32CA00967. The authors would like to thank Mr. Celso Perez, Mr. Steven Rightnar, and the Accelerator Team at the Loma Linda University Medical Center for their assistance with the beam design and animal irradiations. The authors would also like to thank Drs. Gregory King and Alexandria Miller for collaborative tissue sharing.
References
- Badhwar GD. Shuttle radiation dose measurements in the International Space Station orbits. Radiation Research. 2002;157(1):69–75. doi: 10.1667/0033-7587(2002)157[0069:srdmit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Currrent Opinion in Hematology. 2007;14(1):3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- Crucian BE, Stowe RP, Pierson DL, Sams CF. Immune system dysregulation following short- vs long-duration spaceflight. Aviation, Space, and Environmental Medicine. 2008;79(9):835–843. doi: 10.3357/asem.2276.2008. [DOI] [PubMed] [Google Scholar]
- Cucinotta FA, Kim MH, Willingham V, George KA. Physical and biological organ dosimetry analysis for international space station astronauts. Radiation Research. 2008;170(1):127–138. doi: 10.1667/RR1330.1. [DOI] [PubMed] [Google Scholar]
- Gridley DS, Pecaut MJ, Miller GM, Moyers M, Nelson GA. Dose and dose rate effects of whole-body gamma-irradiation: II. Hematological variables and cytokines. In Vivo. 2001;15(3):209–216. [PubMed] [Google Scholar]
- Hu S, Kim MH, Mcclellan GE, Cucinotta FA. Modeling the acute health effects of astronauts from exposure to large solar particle events. Health Physics. 2009;96(4):465–476. doi: 10.1097/01.HP.0000339020.92837.61. [DOI] [PubMed] [Google Scholar]
- Krigsfeld GS, Sanzari JK, Kennedy AR. The effects of proton radiation on the prothrombin and partial thromboplastin times of irradiated ferrets. International Journal of Radiation Biology. 2012;88(4):327–334. doi: 10.3109/09553002.2012.652727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Moore WE, Fryer HC, Minocha HC. Haematological and serum chemistry profiles of ferrets (Mustela putorius furo) Laboratory Animals. 1982;16(2):133–137. doi: 10.1258/002367782781110241. [DOI] [PubMed] [Google Scholar]
- Maks CJ, Wan XS, Ware JH, Romero-Weaver AL, Sanzari JK, Wilson JM, Rightnar S, Wroe AJ, Koss P, Gridley DS, Slater JM, Kennedy AR. Analysis of white blood cell counts in mice after gamma- or proton-radiation exposure. Radiation Research. 2011;176(2):170–176. doi: 10.1667/RR2413.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecaut MJ, Gridley DS. The impact of mouse strain on iron ion radio-immune response of leukocyte populations. International Journal of Radiation Biology. 2010;86(5):409–419. doi: 10.3109/09553000903567995. [DOI] [PubMed] [Google Scholar]
- Pecaut MJ, Nelson GA, Gridley DS. Dose and dose rate effects of whole-body gamma-irradiation: I. Lymphocytes and lymphoid organs. In Vivo. 2001;15(3):195–208. [PubMed] [Google Scholar]
- Sanzari JK, Wan XS, Wroe AJ, Rightnar S, Cengel KA, Diffenderfer ES, Krigsfeld GS, Gridley DS, Slater JM, Kennedy AR. 2013. Acute hematological effects of solar particle event proton radiation in the porcine model. Radiation Research. 2013 May 14; doi: 10.1667/RR3027.1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenfeld G, Butel JS, Shearer WT. Effects of the space flight environment on the immune system. Reviews on Environmental Health. 2003;18(1):1–17. doi: 10.1515/reveh.2003.18.1.1. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G, Shearer WT. Immune function during space flight. Nutrition. 2002;18(10):899–903. doi: 10.1016/s0899-9007(02)00903-6. [DOI] [PubMed] [Google Scholar]
- Wambi C, Sanzari J, Wan XS, Nuth M, Davis J, Ko YH, Sayers CM, Baran M, Ware JH, Kennedy A. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiation Research. 2008;169(4):384–396. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambi CO, Sanzari JK, Sayers CM, Nuth M, Zhou Z, Davis J, Finnberg N, Lewis-Wambi JS, Ware JH, El-Deiry WS, Kennedy AR. Protective effects of dietary antioxidants on proton total-body irradiation-mediated hematopoietic cell and animal survival. Radiation Research. 2009;172(2):175–186. doi: 10.1667/RR1708.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JH, Sanzari J, Avery S, Sayers C, Krigsfeld G, Nuth M, Wan XS, Rusek A, Kennedy AR. Effects of proton radiation dose, dose rate and dose fractionation on hematopoietic cells in mice. Radiation Research. 2010;174(3):325–330. doi: 10.1667/RR1979.1. [DOI] [PMC free article] [PubMed] [Google Scholar]