Abstract
p27Kip1 is a potent inhibitor of cyclin-dependent kinases that drive G1-to-S cell-cycle transition. Reduced p27Kip1 expression is prevalent in a wide range of human tumours; however, the exact mechanism(s) of p27Kip1-mediated tumour suppression remains obscure. In the present study, we identified a close inverse relationship between p27Kip1 and EGFR (epidermal growth factor receptor) expression: the parental T24 human bladder cancer cells had high p27Kip1 expression but low EGFR expression and, in striking contrast, the metastatic derivative of T24 (T24T) had low p27Kip1 expression but high EGFR expression. This relationship was also found in various human cancer tissues, and was not only just correlative but also causal; depletion of p27Kip1 in MEF (mouse embryonic fibroblast) cells resulted in markedly elevated EGFR expression, a result reproducible with an Egfr promoter-luciferase reporter in both T24 and MEF cells, suggesting transcriptional repression of EGFR by p27Kip1. Indeed, p27Kip1 was found to regulate EGFR expression via the JNK (c-Jun N-terminal kinase)/c-Jun transcription factor: p27Kip1 deficiency activated JNK/c-Jun, whereas inhibition of JNK/c-Jun by dominant-negative mutants dramatically repressed Egfr transcription. Furthermore, the proximal promoter of the Egfr gene was crucial for its transcription, where the recruiting activity of c-Jun was much greater in p27Kip1−/− cells than in p27Kip1+/+ cells. Introduction of GFP–p27Kip1 into T24T cells suppressed JNK/c-Jun activation, EGFR expression and anchorage-independent growth. The results of the present study demonstrate that p27Kip1 suppresses JNK/c-Jun activation and EGFR expression in MEFs and human bladder cancer cells, and the results obtained are consistent with those from human cancer specimens. The present study provides new insights into p27Kip1 suppression of cancer cell growth, migration and metastasis.
Keywords: bladder cancer, c-Jun N-terminal kinase (JNK)/c-Jun pathway, epidermal growth factor receptor (EGFR), p27Kip1, signal transduction pathway
Abbreviations: AP-1, activator protein 1; BME, basal medium Eagle; CDK, cyclin-dependent kinase; DMEM, Dulbecco’s modified Eagle’s medium; EGFR, epidermal growth factor receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HSF-1, heat-shock factor 1; Hsp, heat-shock protein; IHC, immunohistochemistry; JNK, c-Jun N-terminal kinase; MEF, mouse embryonic fibroblast; RT, reverse transcription; SP1, specificity protein 1
Short abstract
An inverse relationship between p27Kip1 and EGFR expression in parental T24 human bladder cancer cells and various human cancer tissues was found. Depletion of p27Kip1 in cells markedly elevated EGFR expression through transcriptional repression of Egfr by p27Kip1 via the JNK/c-Jun cascade.
INTRODUCTION
p27Kip1, encoded by the CDKN1B gene, is a strong inhibitor of the CDKs (cyclin-dependent kinases) that propel the progression of the cell cycle from G1- to S-phase [1]. When overexpressed in cultured cells, p27Kip1 causes G1 arrest, thereby inhibiting cell growth and, conversely, depletion of p27Kip1 accelerates G1 exit [2], promoting cell proliferation [3]. In accordance with these in vitro attributes, loss of p27Kip1, along with additional genetic alterations or carcinogen exposure, predisposes mice to a wide range of tumours of both epithelial [4] and mesenchymal [5] origins. Perhaps not surprisingly, reduced expression of p27Kip1, as a result of transcriptional down-regulation, post-translational phosphorylation, elevated ubiquitination or nuclear-to-cytoplasmic translocation, is found in many human malignancies [6]. A decreased p27Kip1 protein level, in particular the nuclear fraction, also correlates well with more advanced disease stages and poorer clinical outcomes than a normal level of p27Kip1 in various cancers [7]. Both mouse and human data therefore strongly support the notion that p27Kip1 acts as a tumour suppressor [8]. Nonetheless, there have been suggestions that p27Kip1 is an ‘unconventional’ tumour suppressor as mutations affecting the CDKN1B gene are rare in human cancers [9]. However, recent identification of p27Kip1 mutations in breast cancer [10] and multiple endocrine neoplastic syndromes [11] raises an interesting new possibility that mutational inactivation of p27Kip1 in other tumour types cannot be completely ruled out [12].
Although p27Kip1 is a tumour suppressor and its down-regulation in tumour cells takes place on multiple levels, much less is known about precisely how p27Kip1 deficiency leads to disturbances in downstream effectors that are pivotal for tumorigenesis [13]. Aside from its CDK-dependent functions in tumour suppression, p27Kip1 has been suggested to exert CDK-independent activities [14]. A notable example is the ability of this protein to modulate the actin cytoskeleton via the regulation of RhoA activation [15]. In so doing, p27Kip1 can affect, albeit indirectly, cancer cell motility and migration, and in turn their propensity for invasion and metastasis [15,16]. p27Kip1 has also been shown to be involved in apoptosis and autophagy, although whether it is stimulatory or inhibitory might be dependent on context and experimental conditions [17]. In spite of the long-held assumption of its role, robust evidence of CDK-independent activities of p27Kip1 in the context of tumour initiation and promotion remains scarce.
Our group has a longstanding interest in delineating the molecular signals and pathways that distinguish advanced tumours from an early stage [18,19]. It has been proven that p27Kip1 could suppress arsenite-induced Hsp (heat-shock protein) 27/Hsp70 expression through inhibiting JNK (c-Jun N-terminal kinase) 2/c-Jun- and HSF-1 (heat-shock factor 1)-dependent pathways [18]. By profiling gene and protein expression of the human T24 bladder cancer cell compared with its metastatic derivative T24T, we found a striking inverse relationship between p27Kip1 and EGFR (epidermal growth factor receptor) expression. The relationship between p27Kip1 and EGFR expression remains unclear. To the best of our knowledge, this is the first demonstration that p27Kip1 regulates EGFR expression and that it does so by influencing JNK/c-Jun transactivation. The results of the present study expand the existing repertoire of CDK-independent activities of p27Kip1, and suggest that the increased cell-cycle transition due to p27Kip1 deficiency can synergize with increased expression of growth-promoting signals (e.g. EGFR) to accelerate tumour progression.
EXPERIMENTAL
Plasmids, antibodies and other reagents
The dominant-negative mutant of c-Jun (TAM67) [19] and the dominant-negative mutant of JNK (DN-JNK) [20] have been described in our previous studies [19,20]. The PRL-TK-luciferase expression vector and pSUPER vector were purchased from Promega and have been used in our previous studies [21]. Adenovirus-driven GFP (Ad-GFP) and Adenovirus-driven-GFP–p27Kip1 (Ad-GFP–p27) plasmids were constructed and have been used in our previous studies [18]. Constructs expressing Egfr promoter-driven luciferase reporter and its various deletions as indicated in Figure 2(D) were a gift from Dr D.T. Smoot (Department of Medicine and Cancer Center, Howard University, Washington, DC, U.S.A.). Antibodies against c-Jun, EGFR, GAPDH (glyceraldehyde-3-phosphate dehydrogenase), histone H3, JNK1/2, p-c-Jun Ser63, p-c-Jun Ser73 and p-JNK were purchased from Cell Signaling Technology. Antibodies against c-Fos, Fra-1, SP1 (specificity protein 1) and c-Myc were from Santa Cruz Biotechnology. The antibody against p27Kip1 was obtained from Abcom Biochemicals.
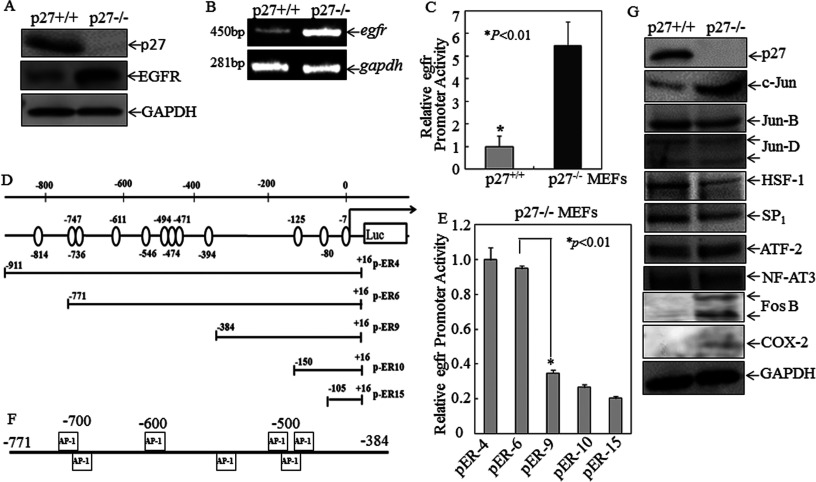
Figure 2. EGFR expression levels of protein, mRNA and transcription was significantly up-regulated in p27Kip1-knockout cells compared with wild-type counterparts, and mapping of a crucial Egfr promoter region for p27Kip1 suppression of Egfr transcription.
(A) Western blotting analysis of p27Kip1 and EGFR protein levels in p27Kip1+/+ compared with p27Kip1−/− MEFs. GAPDH was used as a protein loading control. (B) Egfr mRNA expression levels in p27Kip1+/+ compared with p27Kip1−/− MEFs was determined by RT–PCR. Gadph was used as a loading control. (C) Egfr promoter luciferase reporter plasmids were stably transfected into p27Kip1+/+and p27Kip1−/− MEFs in combination with pRL-TK used as an internal control. Results are presented as Egfr promoter activity of p27Kip1+/+ cells relative to p27Kip1−/− cells respectively. Values are means±S.D. for triplicate assay wells. (D) A schematic diagram of the various deletions of the Egfr promoter luciferase reporter with the open circles corresponding to AP-1-binding sites. The 5′ end maps to the following positions: pER4-luc (−911), pER6-luc (−771), pER9-luc (−384), pER10-luc (−150) and pER15-luc (−105). (E) A series of Egfr promoter-driven luciferase reporter stable transfectants were seeded into each well of 96-well plates. Cells were extracted with lysis buffer for a luciferase activity assay using the luciferase assay system (Promega). Results are presented as luciferase activity relative to the p27Kip1−/− MEFs transfected with pER4-luc only. Values are means±S.D. for triplicate assay wells. (F) Bioinformatics analysis indicating transcription factor AP-1-binding sites in the Egfr promoter area within −771 to −384. (G) Others transcription factors and c-Jun target protein including COX-2 and Fos B in p27Kip1+/+ and p27Kip1−/− MEFs were evaluated. GAPDH was used as a protein loading control.
Cell culture and transfection
Immortalized p27Kip1+/+ and p27Kip1−/− MEF (mouse embryonic fibroblast) cell lines have been described in our previous studies [19,22,23]. These cells and their stable transfectants were maintained at 37°C in a 5% CO2 incubator with DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% FBS, 2 μM L-glutamine and 25 μg/ml gentamycin. The monolayer growth of human bladder cancer T24 cells and its derived metastatic T24T cells were a gift from Dr Dan Theodorescu (University of Colorado, Denver, CO, U.S.A.) [24]. Both T24 and T24T cell lines were maintained in DMEM-F12 (1:1) (Invitrogen), supplemented with 5% heat-inactivated FBS, 2 μM L-glutamine and 25 μg/ml gentamycin. Stable cell transfectants were established by co-transfection of the indicated expression construct together with the pSUPER vector and/or the PRL-TK-luciferase expression vector using PolyJet™ DNA In Vitro Transfection Reagent (SignaGen Laboratories) by antibiotic selection, and surviving cells in each well were pooled as the stable mass transfectant as described in our previous studies [19,21,25,26].
Egfr promoter-driven luciferase reporter activity assay
The Egfr promoter-driven luciferase reporter stable transfectant was seeded into each well of 96-well plates. Cells were collected as indicated, and then extracted with lysis buffer [25 mmol/l Tris-phosphate (pH 7.8), 2 mmol/L-EDTA, 1% Triton X-100 and 10% glycerol] for the luciferase activity assay using the luciferase assay system (Promega) together with a microplate luminometer (Microplate Luminometer LB 96V, Berthold) as described in our previous studies [27].
IHC (immunohistochemistry) of human cancer specimens
A total of 120 patients with lung adenocarcinoma, colon cancer and bladder cancer (40 patients each) from Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, were included in the present study. All participants gave written informed consent and the study was approved by an Ethics Committee Institutional Review Board of the hospital. The mean age of the patients was 54.5 years (range from 32.5 to 72.3 years); 74 cases were male. Paraffin-embedded surgery specimens were sectioned at 5 μm thickness, and mounted on to Fisher brand Super frost plus slides (Fisher Scientific). After antigen retrieval, sections were incubated overnight at 4°C with monoclonal mouse antibodies against EGFR (Abcam) or p27Kip1 (Zymed). IHC was carried out by using the DAB (diaminobenzidine) Map Kit (Pierce), based on the avidin–biotin complex immunoperoxidase technique. Positively stained cells were evaluated using image analysis (Image-Pro Plus, version 4.5.1, Media Cybernetics) to reduce observer variation. The expression of EGFR and p27Kip1 was scored based on the intensity of immunostaining as follows: count 300 cells and score ‘0’ for negative staining of the considered cells, whereas scores 1–3 present <25%, 25–50% and >50% positive staining of the considered cells respectively.
Anchorage-independent growth assay
Anchorage-independent growth in soft agar (soft agar assay) was carried out as described in our previous studies [23]. Briefly, 1×104 cells mixed with or without the JNK inhibitor SP600125 (25 μmol/l) in 10% FBS BME (basal medium Eagle) containing 0.33% soft agar, was seeded over the bottom layer of 0.5% agar in 10% FBS BME in each well of six-well plates. The plates were incubated in a 5% CO2 incubator at 37°C for 21 days. Colonies were visualized under a microscope and photographed. Colonies consisting of over 32 cells were counted and presented [25,28].
Preparation of nuclear extracts
Nuclear extracts were prepared as previously described [21]. Briefly, p27Kip1+/+ and p27Kip1−/− MEFs were plated into 10-cm culture dishes and cultured until 80% confluence. The nuclear and cytosolic proteins were extracted according to the protocol of the Nuclear/Cytosol Fractionation kit (BioVison Technologies). Protein was quantified using a protein quantification assay kit (Bio-Rad Laboratories) and then subjected to Western blot analysis.
Western blot analysis
T24 cells, T24T cells, MEFs and their transfectants were seeded in six-well plates and cultured in normal medium containing 10% FBS until 70–80% confluence. Cells were extracted with cell lysis buffer [10 mM Tris/HCl (pH 7.4), 1% SDS and 1 mM sodium orthovanadate]. Cell extracts were subjected to Western blot analysis as described previously [18,21].
RT (reverse transcription)–PCR
Total RNA was extracted with TRIzol® reagent (Invitrogen) and cDNAs were synthesized with the Thermo-Script RT-PCR system (Invitrogen). The amount of mRNA present in the cells was determined by semi-quantitative RT–PCR. Primers used were: 5′-GAGAGGAGAACTGCCAGA-3′ and 5′-GTAGCATTTATGGAGAGTG-3′ for the human and mouse Egfr gene (450 bp), 5′-ATCAAGAAGGTGGTGAAGCAGGCA-3′ and 5′-TCTCTTGCTCAGTGTCCTTGCTGGG-3′ for the mouse Gapdh gene (281 bp), and 5′-AGAAGGCTGGGGCTCATTTG-3′ and 5′-AGGGGCCATCCACAGTCTTC-3′ for the human GAPDH gene (258 bp). PCR products were separated on 2% agarose gels and stained with ethidium bromide, and the results were imaged with the Alpha Innotech SP image system as described previously [29].
ChIP assay
The EZ-ChIP kit (Millipore Technologies) was used to carry out the ChIP assay according to the manufacturer's instructions and as described previously [30]. Briefly, p27Kip1+/+ and p27Kip1−/− MEFs were treated with 1% formaldehyde for 10 min at room temperature. Cells were then pelleted, resuspended in lysis buffer and sonicated to generate 200- to 500-bp chromatin DNA fragments. After centrifugation (13000 g for 10 min at 4°C), a 10-fold dilution of the supernatants were incubated with an anti-c-Jun antibody or the control rabbit IgG at 4°C overnight. The immune complex was captured with Protein G–agarose-saturated beads with salmon sperm DNA and then eluted with elution buffer. The reverse cross-linking of protein–DNA complexes to free DNA was conducted by incubating at 65°C overnight. The DNA was extracted and subjected to PCR analysis. To specifically amplify the region containing the AP-1 (activator protein 1)-binding sites on the mouse Egfr promoter, PCR was performed with the following pair of primers: 5′-CGTGAACAGCGTCCCCACCT-3′ (from −700 to −680 bp) and 5′-GCTGTGTGCAGCGGGTCAGT-3′ (from −474 to −455 bp). PCR products were separated on 2% agarose gels and stained with ethidium bromide. The images were scanned under UV light.
Statistical methods
Relationships between the expression of p27Kip1 and EGFR were analysed using Fisher's exact and Pearson's (χ2) tests. The Spearman test was performed to evaluate the relationship between EGFR and p27Kip1 expression in the IHC test. Student's t test was used to determine the significance of differences of Egfr promoter activities between groups. The differences were considered to be significant at P<0.05.
RESULTS
EGFR expression was inversely related to p27Kip1 expression in the T24 cell line compared with its metastatic derivative T24T cell line
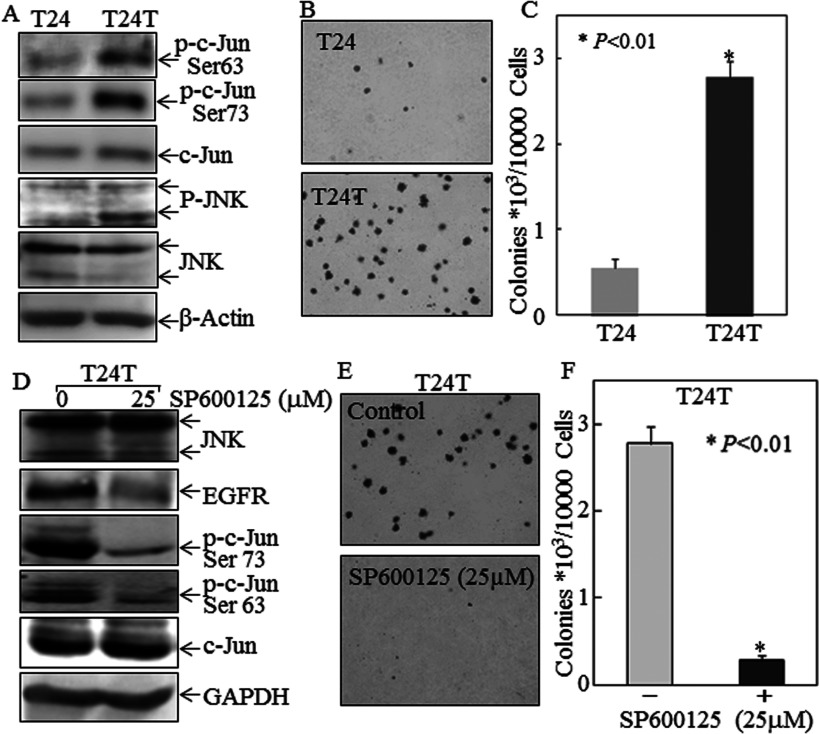
Although EGFR is overexpressed and p27Kip1 is down-regulated in many human cancer types [31], the molecular interplay between the growth promoter and the tumour suppressor had not been explored [7,32]. In an effort to identify new signalling pathways that were essential for tumour progression, we first analysed divergent signalling molecules between a pair of established cell lines, e.g. T24, originally from a high-grade human bladder cancer, and T24T, a T24 derivative that has acquired the ability to metastasize [24]. Of the differentially expressed proteins, we found EGFR and p27Kip1 to be among the most prominent. p27Kip1 expression was dramatically down-regulated in T24T cells compared with its parental T24 cells, whereas EGFR was exactly the opposite, i.e. low expression in T24 cells and high in T24T cells (Figure 1A). Overexpression of EGFR in T24T cells was found to occur at the mRNA level as analysed RT–PCR (Figure 1B), a result extended by the Egfr promoter-driven luciferase reporter assay (Figure 1C), indicating that the differential expression of EGFR between T24 and T24T cells was regulated at the transcriptional level.
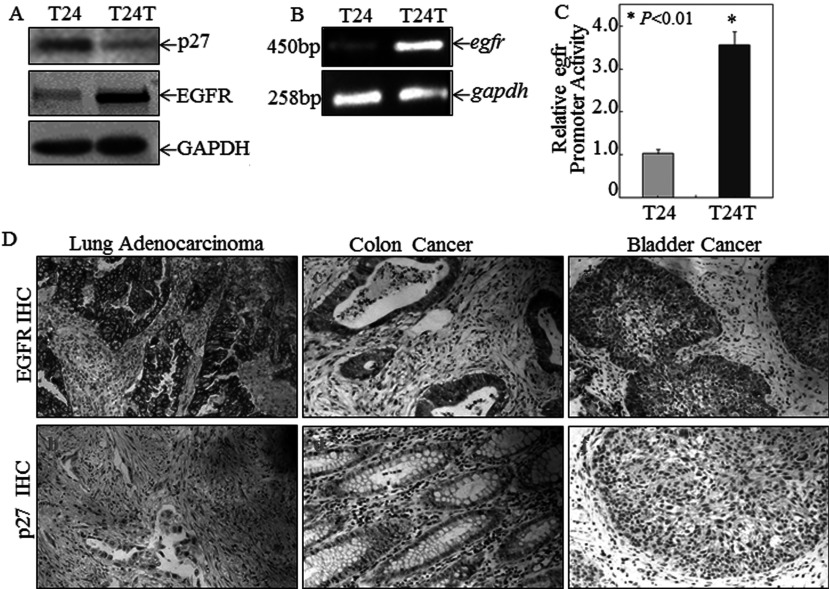
Figure 1. Inverse correlation of p27Kip1 and EGFR expression in human bladder cancer cell lines and human cancer tissues.
(A) Western blotting analysis of p27Kip1 and EGFR protein levels in T24 cells and their derived metastatic T24T human bladder cancer cells. GAPDH was used as loading control. (B) Egfr mRNA expression levels in T24 and T24T cells were determined by RT–PCR. Gadph was used as loading control. (C) Egfr promoter luciferase reporter was stably transfected into T24 and T24T bladder cancer in combination with pRL-TK used as an internal control. The results were presented as Egfr promoter activity in T24T cells relative to that in T24 cells. Values are means ±S.D. for triplicate assay wells. (D) Inverse correlation of p27Kip1 and EGFR expression in clinical tumour specimens detected with IHC, including in lung adenocarcinoma (n=40), colon cancer (n=40) and bladder cancer (n=40) (original magnification ×200).
Inverse correlation of p27Kip1 and EGFR expression in human cancer specimens
To further extend our findings to specimens obtained from human cancer patients, we evaluated the expression of both p27Kip1 and EGFR in human cancer specimens using IHC staining. As shown in Figure 1(D) and Table 1, p27Kip1 expression showed a significant inverse correlation with EGFR expression in lung adenocarcinoma specimens (Spearman correlation: rs=−0.360; P=0.019), colorectal cancer specimens (Spearman correlation: rs=−0.359; P=0.018) and bladder cancer specimens (Spearman correlation: rs=−0.337; P=0.039), strongly suggesting that p27Kip1 expression might be involved in the regulation of EGFR expression.
Table 1. The correlation between EGFR and p27 expression in human cancer specimens.
| EGFR protein expression | |||||||
|---|---|---|---|---|---|---|---|
| p27Kip1 protein expression | Cases | – | + | ++ | +++ | rs value | P value |
| Lung adenocarcinoma | 40 | −0.360 | 0.019 | ||||
| – | 23 | 5 | 6 | 6 | 6 | ||
| + | 7 | 4 | 1 | 1 | 1 | ||
| ++ | 6 | 2 | 2 | 1 | 1 | ||
| +++ | 4 | 3 | 1 | 0 | 0 | ||
| Colorectal adenocarcinoma | 40 | −0.359 | 0.018 | ||||
| – | 20 | 7 | 3 | 6 | 4 | ||
| + | 8 | 5 | 2 | 1 | 0 | ||
| ++ | 7 | 4 | 2 | 1 | 0 | ||
| +++ | 5 | 2 | 2 | 1 | 0 | ||
| Bladder cancer | 40 | −0.337 | 0.039 | ||||
| – | 26 | 6 | 5 | 6 | 9 | ||
| + | 6 | 3 | 1 | 1 | 1 | ||
| ++ | 5 | 2 | 2 | 1 | 0 | ||
| +++ | 3 | 1 | 1 | 1 | 0 | ||
EGFR expression was up-regulated in p27Kip1-knockout cells compared with their wild-type counterparts
The fact that p27Kip1 down-regulation in T24T cells led to EGFR overexpression strongly suggests that p27Kip1 plays an important role in negatively regulating EGFR expression. To establish this relationship more directly, we evaluated the effects of genetically disrupting p27Kip1 on EGFR expression in MEFs. As shown in Figures 2(A) and 2(B), knockout of p27Kip1 (p27Kip1−/−) resulted in increased EGFR expression at both the mRNA and protein level in comparison with p27Kip1+/+ cells. A similar effect occurred at the transcriptional level as evidenced by the Egfr promoter-driven luciferase reporter assay comparing p27Kip1+/+ with p27Kip1−/− MEFs (Figure 2C). It is therefore clear that p27Kip1 has a strong inhibitory effect on EGFR transcription.
The c-Jun-binding region in the Egfr promoter was crucial for p27Kip1 suppression of Egfr transcription
The fact that p27Kip1 inhibits Egfr gene transcription prompted us to perform bioinformatic profiling of the putative transcription-factor-binding motifs within the Egfr gene promoter using the TFSEARCH software (version 1.3). We found that the mouse Egfr proximal promoter contained putative DNA-binding sites for c-Jun, c-Fos, HSF-1, c-Myc, Fra-1 and SP1. To test the functional importance of these sites in p27Kip1-regulated Egfr gene transcription, we engineered truncated Egfr promoter regions to drive a luciferase reporter and then transfected these constructs (Figure 2D) into p27Kip1−/− cells. As shown in Figure 2(E), transcriptional activity with pER9, pER10 and pER15 was significantly impaired as compared with that with pER4 and pER6, whereas there was no significant difference among transfectants pER9, pER10 and pER15. These data suggest that the −771 to −384 Egfr promoter region contains transcription-factor-binding site(s) important for Egfr transcription. As shown in Figure 2(F), the Egfr promoter region between −771 and −384 contained exclusively the AP-1-binding sites, suggesting that AP-1 was an important transcription factor downstream of p27Kip1 for p27Kip1-mediated transcriptional inhibition of EGFR expression. Consistently, other transcription factors and c-Jun target proteins, including COX-2 and FosB, in p27Kip1+/+ and p27Kip1−/− MEFs were also significantly enhanced in p27Kip1−/− MEFs (Figure 2G). These data show that p27Kip1 inhibited EGFR expression by suppressing the activation of the JNK/c-Jun pathway.
p27Kip1 inhibited EGFR expression by suppressing activation of JNK/c-Jun
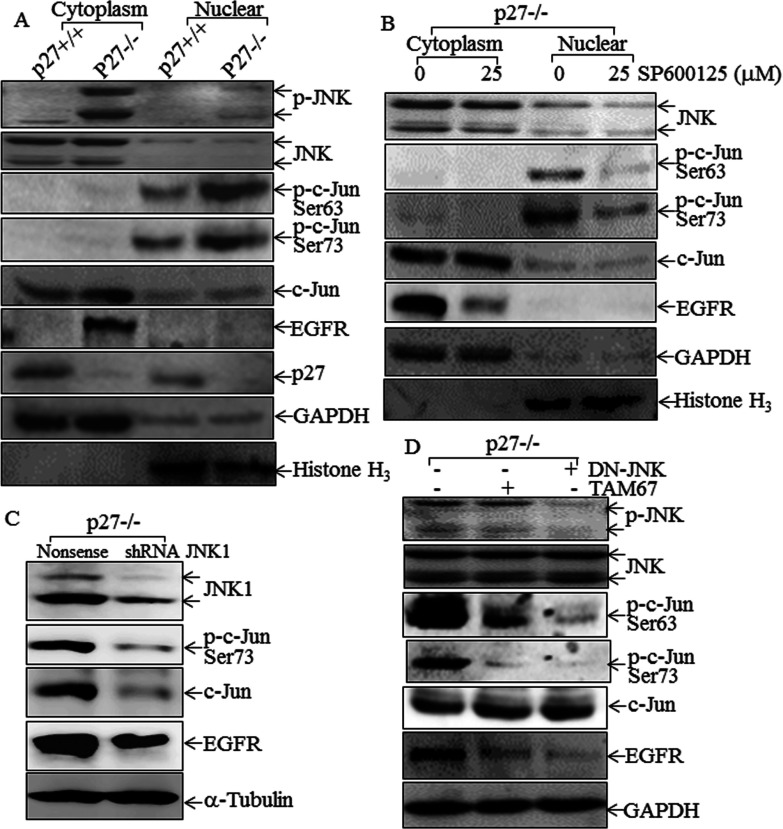
Since the Egfr proximal promoter contains an AP-1 site whose presence is indispensable for Egfr up-regulation in p27Kip1−/− cells, and since c-Jun is a major AP-1 component, we determined whether c-Jun and its upstream kinase JNK were regulated by p27Kip1. As shown in Figure 3(A), although there was no noticeable difference in JNK protein expression and its location between p27Kip1+/+ and p27Kip1−/− cells, JNK phosphorylation was markedly up-regulated in p27Kip1−/− cells, and all phosphorylated JNK was present in the cytosolic fraction. Both c-Jun phosphorylation and protein expression were elevated in p27Kip1−/− cells. Phosphorylated c-Jun was only present in the nuclear fraction, whereas non-phosphorylated c-Jun was mainly associated with the cytosolic fraction (Figure 3A). The phosphorylation of JNK and c-Jun therefore paralleled EGFR expression in p27Kip1+/+ compared with p27Kip1−/− cells, suggesting that the JNK/c-Jun pathway might participate in p27Kip1 regulation of EGFR expression. In contrast with JNK and c-Jun, we observed no difference between p27Kip1+/+ and p27Kip1−/− cells in the level, intracellular location or phosphorylation status of other transcription factors, including ATF-2 (activating transcription factor-2), HSF-1, Jun-B, Jun-D, NFAT3 (nuclear factor of activated T-cells 3) and SP1 (Figure 2G). To determine the role of JNK activation in EGFR expression in p27Kip1−/− cells, we then tested a chemical inhibitor (SP600125), a dominant-negative mutant for JNK (DN-JNK), shRNA JNK and TAM67. Inhibition of JNK activation by pretreatment of p27Kip1−/− cells with the JNK inhibitor SP600125 dramatically blocked both c-Jun phosphorylation and EGFR expression (Figure 3B). We further confirmed the implication of the JNK pathway in regulation of EGFR expression by using shRNA-JNK in p27Kip1−/− MEFs. The results showed that the EGFR protein level decreased markedly after being transfected with shRNA-JNK, with attenuated c-Jun and p-c-Jun levels (Figure 3C). Similarly, enforced expression of DN-JNK also impaired c-Jun phosphorylation and EGFR expression in p27Kip1−/− cells (Figure 3D). Furthermore, transfection with the dominant-negative mutant of c-Jun, TAM67, attenuated EGFR expression without affecting JNK phosphorylation (Figure 3D). In addition, we re-expressed p27Kip1 in the p27Kip1−/− MEFs to confirm that the increased JNK phosphorylation and the up-regulation of EGFR are direct consequences of the absence of p27Kip1. As shown in Figure 4(A), either GFP–p27 or GFP plasmid was transfected into p27Kip1−/− MEFs, and the results indicated that EGFR protein levels decreased greatly after being transfected with the GFP–p27 plasmid, accompanied by down-regulation of phosphorylation of p-JNK and p-c-Jun (Figure 4A). Our results clearly demonstrated that p27Kip1 inhibited EGFR expression by suppressing JNK/c-Jun activation.
Figure 3. p27Kip1 inhibited EGFR expression by targeting JNK/c-Jun activation.
(A) Cytosolic and nuclear proteins were isolated from p27Kip1+/+ and p27Kip1−/− MEFs with the Nuclear/Cytosol Fractionation kit. Western blotting assays were carried out to determine protein expression and phosphorylation as indicated. GAPDH and histone H3 were used as cytoplasmic and nuclear protein markers. (B) After pretreatment of cells with either control medium or 25 μM SP600125, cytosolic and nuclear proteins were isolated from p27Kip1−/− cells using the Nuclear/Cytosol Fractionation kit. The isolated proteins were subjected to Western blotting to determine protein expression and phosphorylation as indicated. GAPDH and histone H3 were used as cytoplasmic and nuclear protein markers. (C) Total cell lysates from p27Kip1−/− MEFs cells stably transfected with shRNA-JNK were subjected to Western blotting as indicated. α-Tubulin was used as a protein loading control. (D) Total cell lysates from p27Kip1−/− MEFs stably transfected with either DN-JNK or TAM67 were subjected to Western blotting as indicated. GAPDH was used as a protein loading control.
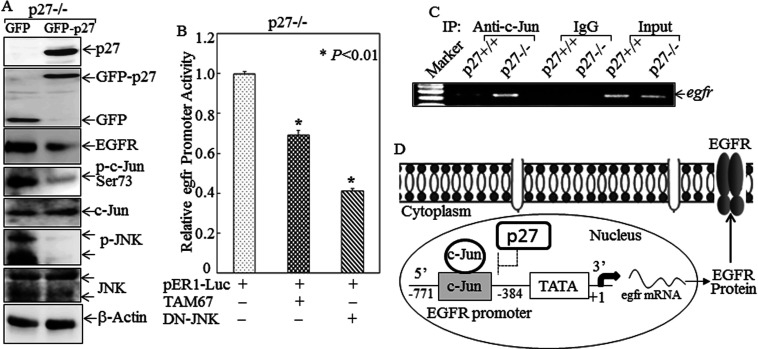
Figure 4. JNK/c-Jun played an essential role in Egfr promoter-dependent transcription activity.
(A) Either GFP or GFP–p27 plasmid was transfected into p27Kip1−/− MEFs. Cells were then extracted for determination of EGFR, JNK, p-JNK, p-c-Jun and c-Jun protein levels. (B) The pER-1 reporter plasmid was co-transfected with either DN-JNK or TAM67 into p27Kip1−/− MEFs. Cells were then extracted for determination of Egfr promoter-driven luciferase activity. (C) Soluble chromatin prepared from p27Kip1+/+ and p27Kip1−/− MEFs was subjected to a ChIP assay using an anti-c-Jun antibody. Immunoprecipitated chromatin DNA was amplified with the PCR primers that specifically annealed to the region flanking c-Jun-responsive elements within the Egfr promoter. (D) A proposed model for p27Kip1 suppression of EGFR expression.
Consistent with the effects on EGFR protein expression, enforced expression of either DN-JNK or TAM67 impaired Egfr promoter-driven luciferase reporter activity in p27Kip1−/− cells (Figure 4B). Finally, a ChIP assay showed that recruitment of c-Jun to the Egfr proximal promoter was much greater in p27Kip1−/−cells than in p27Kip1+/+ cells (Figure 4C). Since c-Jun is an obligate member of the AP-1 protein dimer that binds to this Egfr promoter region, our studies provide compelling evidence to indicate that activation of the JNK/c-Jun pathway increases c-Jun binding to the Egfr promoter and up-regulates EGFR transcription and expression in p27Kip1−/− cells. Our results suggest that under normal circumstances p27Kip1 inhibits EGFR transcription and expression via inhibition of the JNK/c-Jun axis as shown in schematic form in Figure 4(D).
Down-regulation of p27Kip1 induced JNK/c-Jun phosphorylation, EGFR up-regulation and anchorage-independent growth elevation
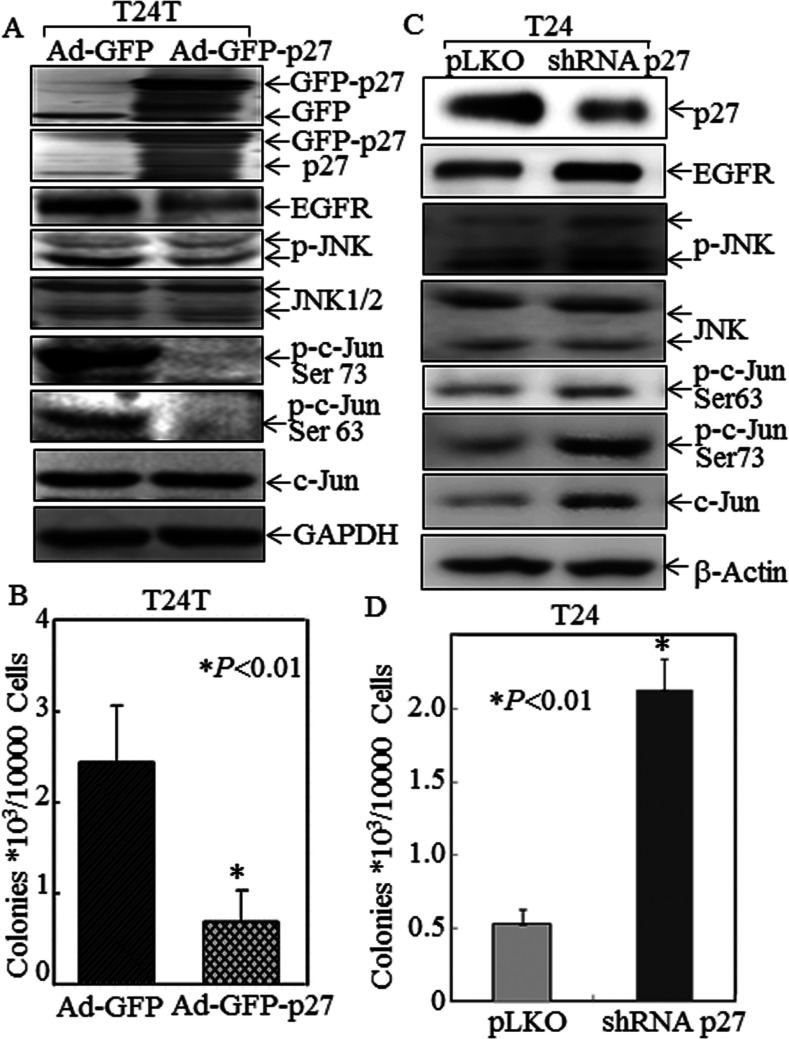
Compared with T24 cells, there was a reduction in p27Kip1 expression and up-regulation of EGFR expression in T24T cells (Figures 1A and 1B). To understand the biological consequences of p27Kip1-regulated EGFR expression, we also compared c-Jun and JNK activation as well as the anchorage-independent growth between T24 and T24T cells. As shown in Figure 5(A), the phosphorylation of JNK and c-Jun are both up-regulated in T24T cells. Consistently, an increase in anchorage-independent growth in T24T cells was also observed as compared with T24 cells (Figures 5B and 5C). Inhibition of JNK activation by pretreatment of T24T cells with the JNK inhibitor SP600125 blocked both c-Jun phosphorylation at Ser63/Ser73 and anchorage-independent growth of T24T cells (Figures 5D–5F). To provide direct evidence that p27Kip1-regulated EGFR expression via the JNK/c-Jun axis plays a role in cancer cell growth, we infected T24T cells with an adenovirus-driven-GFP–p27Kip1 and adenovirus-driven GFP as a negative control. Enforced expression of GFP–p27Kip1 in T24T cells dramatically inhibited the activation of JNK and c-Jun, expression of EGFR and anchorage-independent growth (Figures 6A and 6B). Moreover, knockdown of p27Kip1 in T24 cells led to an increased EGFR expression and anchorage-independent growth with induction of JNK and c-Jun activation (Figures 6C and 6D). Our results demonstrate that p27Kip1 down-regulation plays a key role in up-regulating the JNK/c-Jun pathway, leading to increased EGFR expression and anchorage-independent growth and endowing these cancer cells with a new metastatic potential.
Figure 5. Low p27Kip1 expression in T24T cells mediated JNK/c-Jun phosphorylation, EGFR up-regulation and increased anchorage-independent growth.
(A) Total cell lysates from T24 and T24T bladder cancer cells were subjected to Western blotting as indicated. β-Actin was used as a protein loading control. (B and C) Anchorage-independent growth of T24 and T24T cells was determined by soft agar assay. Colonies were photographed and counted, and values are means±S.D. for triplicate assays. (D) T24T cells were pretreated with or without SP600125, and then extracted for determination of protein expression by Western blotting. (E and F) T24T cells were pretreated with 25 μM SP600125 for 4 h and then subjected to anchorage-independent growth. Colonies were photographed and counted. The asterisk (*) indicates a significant decrease in anchorage-independent growth of T24T pretreated with SP600125 compared with T24T cells pretreated with 0.1% DMSO control (P<0.01).
Figure 6. Inhibitory effect of p27Kip1 on JNK/c-Jun phosphorylation, EGFR expression and anchorage-independent growth in T24 and T24T cells.
(A) T24T cells were infected with Ad-GFP or Ad-GFP–p27, and then extracted for determination of protein expression by Western blotting. (B) T24T cells were infected with Ad-GFP or Ad-GFP–p27, and then subjected to determination of anchorage-independent growth in soft agar assay. The asterisk (*) indicates a significant decrease in anchorage-independent growth of T24T cells infected with Ad-GFP–p27 compared with those infected with Ad-GFP (P<0.01). (C) T24 cells were stably transfected with shRNA p27 and the cell extracts were subjected to Western blotting for determination of p27 knockdown in regulating JNK, p-c-Jun and EGFR expression, as compared with those transfected with control vector pLKO. (D) T24 cells were stably transfected with shRNA p27 or pLKO and then subjected to anchorage-independent growth. The asterisk (*) indicates a significant increase in anchorage-independent growth of T24 cells transfected with shRNA p27 compared with T24 cells transfected with pLKO (P<0.01).
DISCUSSION
A principal finding we made in the present study is that p27Kip1 deficiency leads to a marked transcriptional up-regulation of EGFR expression. This finding is based on several independent lines of experimental evidence. First, an inverse relationship exists between p27Kip1 and EGFR levels, i.e. the T24 cell line that expresses a high level of p27Kip1 has a low level of EGFR, and the isogenic T24T cell that expresses a low level of p27Kip1 has a high level of EGFR. Secondly, an inverse correlation of p27 and EGFR expression was observed in clinical human cancer tissues. Thirdly, depletion of p27Kip1 in p27Kip1-expressing MEFs results in a dramatic up-regulation of EGFR expression, suggesting that p27Kip1-regulated EGFR expression is not only operative in bladder cancer cells, but also in non-bladder cancer and non-cancer cells. Fourthly, the levels of phosphorylation and nuclear translocation of the JNK and c-Jun transcriptional axis, whose interaction with the proximal promoter of the Egfr gene is essential for its gene transcription, are significantly higher in p27Kip1−/− cells than in p27Kip1+/+ cells. Chemical inhibitors and dominant-negative mutants of JNK and c-Jun specifically repress the transcription of the Egfr gene in p27Kip1-deficient cells, and the recruitment of c-Jun to the Egfr proximal promoter in the absence of these inhibitors is much greater in p27Kip1−/− cells than in p27Kip1+/+ cells. Finally, reintroduction of p27Kip1 into p27Kip1-deficient T24T cells markedly represses the activation of JNK and c-Jun and reduces EGFR expression. Taken together, these data firmly establish a signalling cascade linking p27Kip1 deficiency to EGFR overexpression through JNK/c-Jun transcriptional activation.
The results of the present study have important clinical implications on several fronts. The first relates to an improved understanding of the cellular functions of p27Kip1 in tumour suppression that are independent of its canonical role in CDK inhibition [33]. In normal resting cells, p27Kip1, expressed at high levels, hypo-phosphorylated and localized primarily in the nuclei, binds to CDK4–cyclin D and CDK2–cyclin E/A–CDK2 complexes, thereby functionally inactivating CDKs [34]. This is commensurate with the role of p27Kip1 in G1 arrest and tumour suppression [1,6,35]. Indeed, mice globally lacking p27Kip1 are significantly heavier than their wild-type counterparts because of multi-organ hyperplasia [35,36]. Although spontaneous tumours are uncommon in the p27Kip1−/− mice [36], they are highly prone to tumorigenesis when exposed to chemical carcinogens [37]. These and other data from mouse models are therefore strongly supportive of the CDK-dependent activities of p27Kip1 in suppressing tumour formation in vivo [38]. Whether p27Kip1 exerts other tumour suppressive effects that can account for the phenotypes observed in the knockout mice has not been carefully addressed. Interestingly, there was a report showing that ablating the CDKs in p27Kip1-deficient cells does not completely abolish the proliferative ability of the host cells [39], raising the possibility that p27Kip1 can regulate a broader spectrum of downstream targets beyond the CDKs. On the basis of the recent characterization of gene-knockout mice, c-Jun integrates signals of several developmental pathways, including EGFR/ERK (extracellular-signal-regulated kinase) [40] and EGFR/RhoA/ROCK (Rho-associated kinase). To our current knowledge, c-Jun remains at the centre stage of a complex molecular network, c-Jun interacting with many signalling pathways, some of which are yet to be discovered. As we have demonstrated in the present study, one such CDK-independent p27Kip1-regulated downstream target is EGFR.
EGFR is a key member of the receptor tyrosine kinase family, and is activated in response to growth stimulators such as growth factors, cytokines and growth hormones [41]. It plays a diverse role in cell proliferation, angiogenesis, motility, invasion and metastasis, and overexpression of EGFR is therefore considered oncogenic for many cell types [42]. Different deubiquitinating enzymes may be critical in balancing c-Cbl and SMURF2 activity [43], thus tightly regulating EGFR protein stability, alteration of which during oncogenesis leads to EGFR overexpression. However, there is no significant difference between c-Cbl and SMURF2 in p27Kip1+/+ and p27Kip1−/− MEFs (results not shown), suggesting that EGFR protein stability could be regulated independently of c-Cbl and SMURF2. Further to demonstrating the link between p27Kip1 and EGFR, we propose that the deficiency in p27Kip1 can lead to a synergistic effect on cell proliferation, one via the loss of CDK-dependent cell-cycle inhibition and another via EGFR overexpression. This is another example where a tumour suppressor can intersect with an oncogenic event to drive tumour formation although, in this case, the deficiency of one protein can lead to the dual effects. With respect to tumour progression, we wish to point out that EGFR overexpression only occurs in p27Kip1-deficient metastatic T24T cells, but not in the parental T24 cells which express p27Kip1 and are incapable of metastasizing. Although the role of EGFR overexpression in cancer progression is well established [44], the role of p27Kip1 is much less clear. In fact, increased cytoplasmic translocation of p27Kip1 has been associated with increased cell motility and invasion [45]. It has previously been reported that inhibiting Ser10 phosphorylation of p27 led to p27 accumulation in the nucleus and enhanced erlotinib-mediated cytotoxicity in breast cancer [46]. Therefore the net effect of p27Kip1 loss and EGFR overexpression on cancer cell metastasis needs to be further investigated [47].
Inhibition of JNK activation by pretreatment of T24T cells with the JNK inhibitor SP600125 blocked c-Jun phosphorylation at Ser63/Ser73 and anchorage-independent growth of T24T cells. This is consistent with the report by Cáceres et al. [48] that pretreatment with either SP600125 or the expression of a dominant-negative mutant JNK leads to down-regulation of EGFR phosphorylation and inhibits the invasive capacity of cancer cells. This notion is also supported by the results of Li et al. [49] which suggest that EGFR is involved in constitutive JNK activation in diffuse gliomas and that the ability to inhibit JNK activation might confer increased sensitivity to therapeutic modalities targeting this pathway.
A key point is how p27Kip1 might inhibit JNK activity. Our previous work indicated that p27Kip1 might target the Akt pathway which contributes to deactivation of the MKK7 (MAPK kinase 7)/JNK pathway [18]. It should be noted that overexpression of EGFR occurs in a wide range of human tumour tissues and, as with a reduced p27Kip1, is strongly associated with late-stage tumours and poor prognosis [5,50]. Thus far, few studies have examined the concordance between reduced p27Kip1 expression and increased EGFR expression, a topic that should be explored based on the information provided by present work [51]. Similarly, it will be interesting to revisit whether EGFR is overexpressed in p27Kip1-deficient mice and whether EGFR inhibition will render these mice resistant to tumorigenesis even after carcinogen treatment. Finally, the fact that p27Kip1-deficient cells overexpress EGFR raises the interesting possibility that tumours deficient in either or both tumour suppressors could benefit from a therapeutic approach combining chemotherapeutics with EGFR inhibitors [46]. The present study has demonstrated that p27Kip1 suppresses JNK/c-Jun activation and EGFR expression in mouse MEFs and human bladder cancer cells, and the results obtained are consistent with those from human cancer specimens. The results provide new insight into p27Kip1 suppressing cancer cell growth, migration and metastasis.
ACKNOWLEDGEMENTS
We thank Dr D.T. Smoot (Department of Medicine and Cancer Center, Howard University, Washington, DC, U.S.A.) for providing the Egfr promoter-driven luciferase reporter, and Dr Dan Theodorescu (University of Colorado, Denver, Colorado) for providing T24 and T24T cells.
AUTHOR CONTRIBUTION
Yong Fang and Chuanshu Huang conceived and designed the experiments. Yong Fang, Xue-Ru Wu and Chuanshu Huang wrote the paper. Yihong Wang performed pathology and IHC experiments. Yan Meng, Yulei Wang, Jingxia Li, Junlan Zhu, Honglei Jin, Dongyun Zhang and Yonghui Yu helped with molecular experiments and preparation of related samples.
FUNDING
This work was partially supported by the National Institutes of Health/National Cancer Institute [grant numbers CA165980, CA177665 and CA112557], and the Zhejiang Provincial Natural Science Foundation of China [grant number LY13H160013].
References
- 1.Bhatia B., Malik A., Fernandez-L A., Kenney A. M. p27(Kip1), a double-edged sword in Shh-mediated medulloblastoma: tumor accelerator and suppressor. Cell Cycle. 2010;9:4307–4314. doi: 10.4161/cc.9.21.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H., Kang M., Lee S. A., Kwak T. K., Jung O., Lee H. J., Kim S. H., Lee J. W. TM4SF5 accelerates G1/S phase progression via cytosolic p27Kip1 expression and RhoA activity. Biochim. Biophys. Acta. 2010;1803:975–982. doi: 10.1016/j.bbamcr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Pareja F., Ferraro D. A., Rubin C., Cohen-Dvashi H., Zhang F., Aulmann S., Ben-Chetrit N., Pines G., Navon R., Crosetto N. Deubiquitination of EGFR by Cezanne-1 contributes to cancer progression. Oncogene. 2012;31:4599–4608. doi: 10.1038/onc.2011.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler J. E., Spear B. T. Normal intestinal epithelial cell differentiation in the absence of p21 and p27: new insights from old knock-out mice. Cancer Biol. Ther. 2008;7:880–881. doi: 10.4161/cbt.7.6.6318. [DOI] [PubMed] [Google Scholar]
- 5.Berton S., Belletti B., Wolf K., Canzonieri V., Lovat F., Vecchione A., Colombatti A., Friedl P., Baldassarre G. The tumor suppressor functions of p27(kip1) include control of the mesenchymal/amoeboid transition. Mol. Cell. Biol. 2009;29:5031–5045. doi: 10.1128/MCB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan T. J., Al-Attar A., Rolland P., Harper S., Spendlove I., Durrant L. G. Cytoplasmic p27 expression is an independent prognostic factor in ovarian cancer. Int. J. Gynecol. Pathol. 2009;29:8–18. doi: 10.1097/PGP.0b013e3181b64ec3. [DOI] [PubMed] [Google Scholar]
- 7.Belletti B., Baldassarre G. New light on p27 (kip1) in breast cancer. Cell Cycle. 2012;11:3701–3702. doi: 10.4161/cc.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vervoorts J., Luscher B. Post-translational regulation of the tumor suppressor p27(KIP1) Cell. Mol. Life Sci. 2008;65:3255–3264. doi: 10.1007/s00018-008-8296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kossatz U., Malek N. P. p27: tumor suppressor and oncogene? Cell Res. 2007;17:832–833. doi: 10.1038/cr.2007.86. [DOI] [PubMed] [Google Scholar]
- 10.Spirin K. S., Simpson J. F., Takeuchi S., Kawamata N., Miller C. W., Koeffler H. P. p27/Kip1 mutation found in breast cancer. Cancer Res. 1996;56:2400–2404. [PubMed] [Google Scholar]
- 11.Ishida E., Yamada M., Horiguchi K., Taguchi R., Ozawa A., Shibusawa N., Hashimoto K., Satoh T., Yoshida S., Tanaka Y., et al. Attenuated expression of menin and p27 (Kip1) in an aggressive case of multiple endocrine neoplasia type 1 (MEN1) associated with an atypical prolactinoma and a malignant pancreatic endocrine tumor. Endocr. J. 2011;58:287–296. doi: 10.1507/endocrj.K10E-158. [DOI] [PubMed] [Google Scholar]
- 12.Timmerbeul I., Garrett-Engele C. M., Kossatz U., Chen X., Firpo E., Grünwald V., Kamino K., Wilkens L., Lehmann U., Buer J., et al. Testing the importance of p27 degradation by the SCFskp2 pathway in murine models of lung and colon cancer. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14009–14014. doi: 10.1073/pnas.0606316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.le Sage C., Nagel R., Egan D. A., Schrier M., Mesman E., Mangiola A., Anile C., Maira G., Mercatelli N., Ciafrè S. A., et al. Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007;26:3699–3708. doi: 10.1038/sj.emboj.7601790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen S., So Y., Singh K., Slingerland J. M., Resnick M. B., Zhang S., Ruiz V., Moss S. F. Promotion of cytoplasmic mislocalization of p27 by Helicobacter pylori in gastric cancer. Oncogene. 2013;31:1771–1780. doi: 10.1038/onc.2011.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshino D., Koshikawa N., Seiki M. A p27(kip1)-binding protein, p27RF-Rho, promotes cancer metastasis via activation of RhoA and RhoC. J. Biol. Chem. 2011;286:3139–3148. doi: 10.1074/jbc.M110.159715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo Y., Takata T., Ogawa I., Zhao M., Sato S., Takekoshi T., Miyauchi M., Nikai H. Reduced expression of p27(Kip1) correlates with an early stage of cancer invasion in oral squamous cell carcinoma. Cancer Lett. 2000;151:217–222. doi: 10.1016/S0304-3835(99)00419-X. [DOI] [PubMed] [Google Scholar]
- 17.Chen Q., Xie W., Kuhn D. J., Voorhees P. M., Lopez-Girona A., Mendy D., Corral L. G., Krenitsky V. P., Xu W., Moutouh-de Parseval L., et al. Targeting the p27 E3 ligase SCF (Skp2) results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood. 2008;111:4690–4699. doi: 10.1182/blood-2007-09-112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Zhang D., Mi X., Xia Q., Yu Y., Zuo Z., Guo W., Zhao X., Cao J., Yang Q., et al. p27 suppresses arsenite-induced Hsp27/Hsp70 expression through inhibiting JNK2/c-Jun- and HSF-1-dependent pathways. J. Biol. Chem. 2010;285:26058–26065. doi: 10.1074/jbc.M110.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding J., Huang Y., Ning B., Gong W., Li J., Wang H., Chen C. Y., Huang C. TNF-α induction by nickel compounds is specific through ERKs/AP-1-dependent pathway in human bronchial epithelial cells. Curr. Cancer Drug Targets. 2009;9:81–90. doi: 10.2174/156800909787313995. [DOI] [PubMed] [Google Scholar]
- 20.Huang C., Li J., Ma W. Y., Dong Z. JNK activation is required for JB6 cell transformation induced by tumor necrosis factor-α but not by 12-O-tetradecanoylphorbol-13-acetate. J. Biol. Chem. 1999;274:29672–29676. doi: 10.1074/jbc.274.42.29672. [DOI] [PubMed] [Google Scholar]
- 21.Fang Y., Yu Y., Hou Q., Zheng X., Zhang M., Zhang D., Li J., Wu X. R., Huang C. The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by downregulating overexpression of antiapoptotic protein XIAP. J. Biol. Chem. 2012;287:35234–35243. doi: 10.1074/jbc.M112.389494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C., Ma W. Y., Li J., Dong Z. Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res. 1999;59:3053–3058. [PubMed] [Google Scholar]
- 23.Zhang D., Li J., Costa M., Gao J., Huang C. JNK1 mediates degradation HIF-1α by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Res. 2010;70:813–822. doi: 10.1158/0008-5472.CAN-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y., McRoberts K., Berr S. S., Frierson H. F., Jr, Conaway M, Theodorescu D. Neuromedin U is regulated by the metastasis suppressor RhoGDI2 and is a novel promoter of tumor formation, lung metastasis and cancer cachexia. Oncogene. 2007;26:765–773. doi: 10.1038/sj.onc.1209835. [DOI] [PubMed] [Google Scholar]
- 25.Fang Y., Cao Z., Hou Q., Ma C., Yao C., Li J., Wu X. R., Huang C. Cyclin D1 downregulation contributes to anticancer effect of isorhapontigenin on human bladder cancer cells. Mol. Cancer Ther. 2013;12:1492–1503. doi: 10.1158/1535-7163.MCT-12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J., Zhang D., Liu J., Li J., Yu Y., Wu X. R., Huang C. RhoGDI SUMOylation at Lys-138 increases its binding activity to Rho GTPase and its inhibiting cancer cell motility. J. Biol. Chem. 2012;287:13752–13760. doi: 10.1074/jbc.M111.337469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouyang W., Zhang D., Li J., Verma U. N., Costa M., Huang C. Soluble and insoluble nickel compounds exert a differential inhibitory effect on cell growth through IKKalpha-dependent cyclin D1 down-regulation. J. Cell. Physiol. 2009;218:205–214. doi: 10.1002/jcp.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D., Li J., Gao J., Huang C. c-Jun/AP-1 pathway-mediated cyclin D1 expression participates in low dose arsenite-induced transformation in mouse epidermal JB6 Cl41 cells. Toxicol. Appl. Pharmacol. 2009;235:18–24. doi: 10.1016/j.taap.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang W., Ma Q., Li J., Zhang D., Liu Z. G., Rustgi A. K., Huang C. Cyclin D1 induction through IκB kinase β/nuclear factor-κB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytes. Cancer Res. 2005;65:9287–9293. doi: 10.1158/0008-5472.CAN-05-0469. [DOI] [PubMed] [Google Scholar]
- 30.Song L., Gao M., Dong W., Hu M., Li J., Shi X., Hao Y., Li Y., Huang C. p85α mediates p53 K370 acetylation by p300 and regulates its promoter-specific transactivity in the cellular UVB response. Oncogene. 2011;30:1360–1371. doi: 10.1038/onc.2010.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H., Stabile L. P., Gubish C. T., Gooding W. E., Grandis J. R., Siegfried J. M. Dual blockade of EGFR and c-Met abrogates redundant signaling and proliferation in head and neck carcinoma cells. Clin. Cancer Res. 2011;17:4425–4438. doi: 10.1158/1078-0432.CCR-10-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner A., Verdorfer I., Prelog M., Mayerl C., Mikuz G., Tzankov A. Large-scale analysis of cell cycle regulators in urothelial bladder cancer identifies p16 and p27 as potentially useful prognostic markers. Pathobiology. 2008;75:25–33. doi: 10.1159/000113792. [DOI] [PubMed] [Google Scholar]
- 33.Martín A., Odajima J., Hunt S. L., Dubus P., Ortega S., Malumbres M., Barbacid M. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27(Kip1) and p21(Cip1) Cancer Cell. 2005;7:591–598. doi: 10.1016/j.ccr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Itamochi H., Yoshida T., Walker C. L., Bartholomeusz C., Aoki D., Ishihara H., Suzuki N., Kigawa J., Terakawa N., Ueno N. T. Novel mechanism of reduced proliferation in ovarian clear cell carcinoma cells: cytoplasmic sequestration of CDK2 by p27. Gynecol. Oncol. 2011;122:641–647. doi: 10.1016/j.ygyno.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Guan X., Wang Y., Xie R., Chen L., Bai J., Lu J., Kuo M. T. p27(Kip1) as a prognostic factor in breast cancer: a systematic review and meta-analysis. J. Cell. Mol. Med. 2010;14:944–953. doi: 10.1111/j.1582-4934.2009.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakayama K., Ishida N., Shirane M., Inomata A., Inoue T., Shishido N., Horii I., Loh D. Y., Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/S0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 37.García-Fernández R. A., García-Palencia P., Sánchez M. Á., Gil-Gómez G., Sánchez B., Rollán E., Martín-Caballero J., Flores J. M. Combined loss of p21(waf1/cip1) and p27(kip1) enhances tumorigenesis in mice. Lab. Invest. 2011;91:1634–1642. doi: 10.1038/labinvest.2011.133. [DOI] [PubMed] [Google Scholar]
- 38.Molatore S., Kiermaier E., Jung C. B., Lee M., Pulz E., Höfler H., Atkinson M. J., Pellegata N. S. Characterization of a naturally-occurring p27 mutation predisposing to multiple endocrine tumors. Mol. Cancer. 2010;9:116–127. doi: 10.1186/1476-4598-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blain S. W. Switching cyclin D-Cdk4 kinase activity on and off. Cell Cycle. 2008;7:892–898. doi: 10.4161/cc.7.7.5637. [DOI] [PubMed] [Google Scholar]
- 40.Schnidar H., Eberl M., Klingler S., Mangelberger D., Kasper M., Hauser-Kronberger C., Regl G., Kroismayr R., Moriggl R., Sibilia M., Aberger F. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soria J. C., Mok T. S., Cappuzzo F., Jänne P. A. EGFR-mutated oncogene-addicted non-small cell lung cancer: current trends and future prospects. Cancer Treat. Rev. 2012;38:416–4130. doi: 10.1016/j.ctrv.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Jamal-Hanjani M., Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin. Cancer Res. 2012;18:938–944. doi: 10.1158/1078-0432.CCR-11-2529. [DOI] [PubMed] [Google Scholar]
- 43.Ray D., Ahsan A., Helman A., Chen G., Hegde A., Gurjar S. R., Zhao L., Kiyokawa H., Beer D. G., Lawrence T. S., Nyati M. K. Regulation of EGFR protein stability by the HECT-type ubiquitin ligase SMURF2. Neoplasia. 2011;13:570–578. doi: 10.1593/neo.11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandhare-Dash J., Mantri C. K., Gong Y., Chen Z., Dash C. XMRV accelerates cellular proliferation, transformational activity, and invasiveness of prostate cancer cells by downregulating p27(Kip1) Prostate. 2012;72:886–897. doi: 10.1002/pros.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baldassarre G., Belletti B., Nicoloso M. S., Schiappacassi M., Vecchione A., Spessotto P., Morrione A., Canzonieri V., Colombatti A. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 46.Zhang D., Tari A. M., Akar U., Arun B. K., LaFortune T. A., Nieves-Alicea R., Hortobagyi G. N., Ueno N. T. Silencing kinase-interacting stathmin gene enhances erlotinib sensitivity by inhibiting Ser10 p27 phosphorylation in epidermal growth factor receptor-expressing breast cancer. Mol. Cancer Ther. 2010;9:3090–3099. doi: 10.1158/1535-7163.MCT-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin X., Jin X., Sohn Y. W., Yin J., Kim S. H., Joshi K., Nam D. H., Nakano I., Kim H. Blockade of EGFR signaling promotes glioma stem-like cell invasiveness by abolishing ID3-mediated inhibition of p27(KIP1) and MMP3 expression. Cancer Lett. 2013;328:235–242. doi: 10.1016/j.canlet.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Cáceres M., Tobar N., Guerrero J., Smith P. C., Martinez J. c-jun-NH2 JNK mediates invasive potential and EGFR activation by regulating the expression of HB-EGF in a urokinase-stimulated pathway. J. Cell. Biochem. 2008;103:986–993. doi: 10.1002/jcb.21469. [DOI] [PubMed] [Google Scholar]
- 49.Li J. Y., Wang H., May S., Song X., Fueyo J., Fuller G. N., Wang H. Constitutive activation of c-Jun N-terminal kinase correlates with histologic grade and EGFR expression in diffuse gliomas. J. Neurooncol. 2008;88:11–17. doi: 10.1007/s11060-008-9529-1. [DOI] [PubMed] [Google Scholar]
- 50.Borriello A., Bencivenga D., Criscuolo M., Caldarelli I., Cucciolla V., Tramontano A., Borgia A., Spina A., Oliva A., Naviglio S., DellaRagione F. Targeting p27Kip1 protein: its relevance in the therapy of human cancer. Expert Opin. Ther. Targets. 2011;15:677–693. doi: 10.1517/14728222.2011.561318. [DOI] [PubMed] [Google Scholar]
- 51.Fiano V., Ghimenti C., Imarisio S., Silengo L., Schiffer D. PAkt, cyclin D1 and p27/Kip1 in glioblastomas with and without EGFR amplification and PTEN mutation. Anticancer Res. 2004;24:2643–2647. [PubMed] [Google Scholar]