Abstract
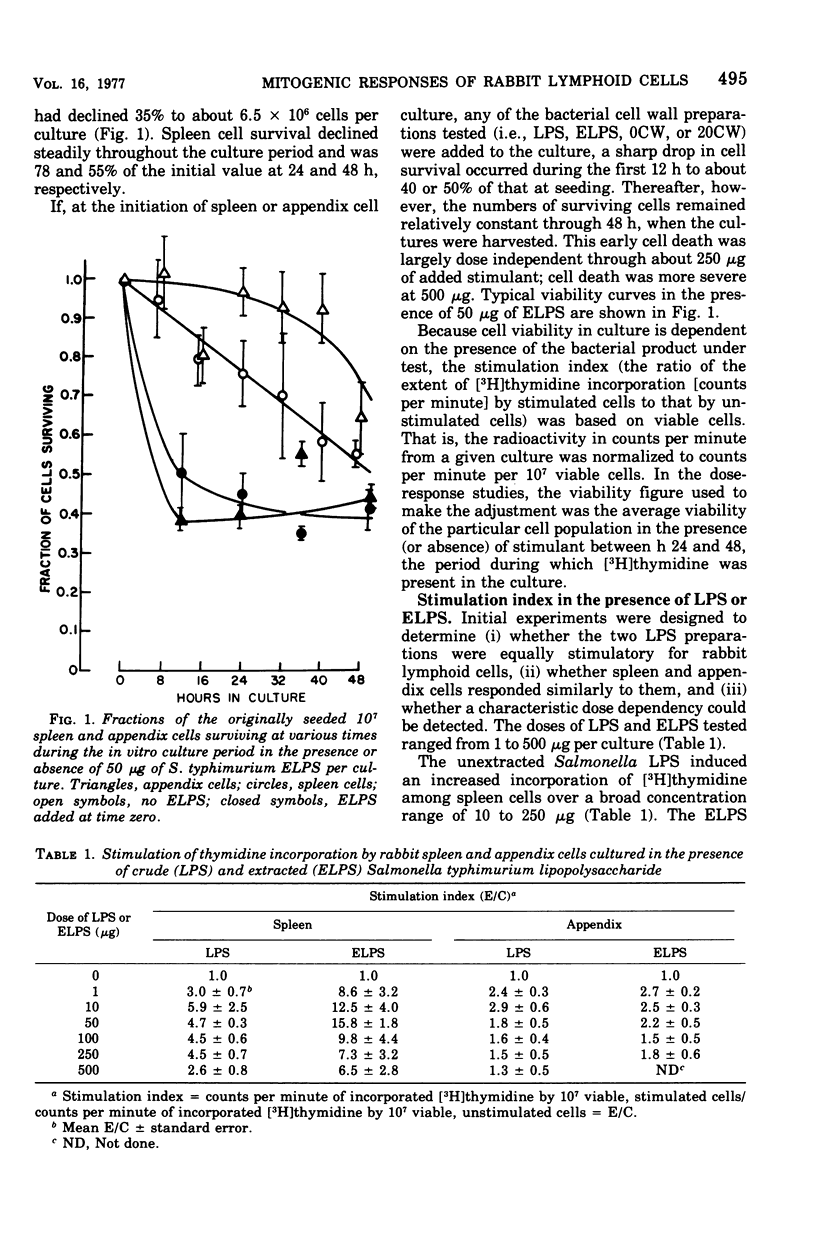
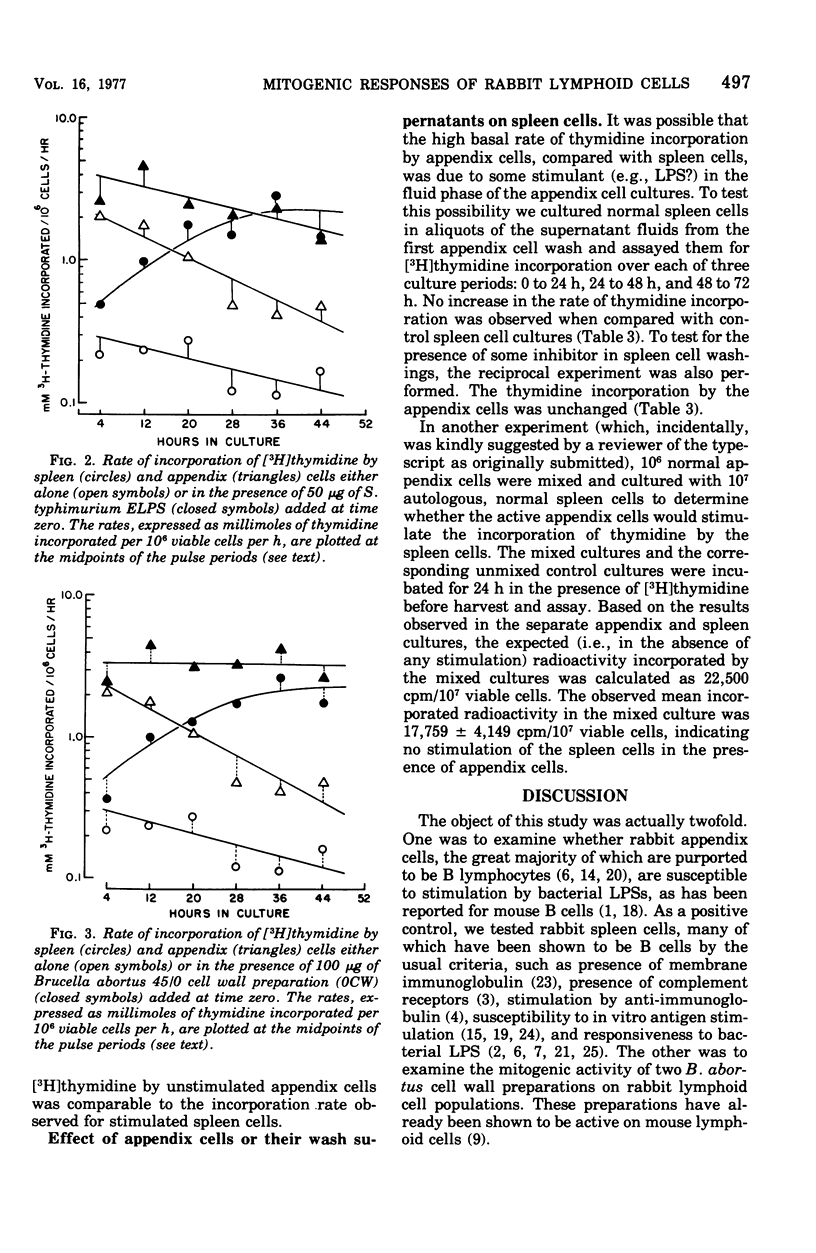
Rabbit spleen and appendix cells were used to test the mitogenic activity of a commercial lipopolysaccharide (LPS) preparation from Salmonella typhimurium (Difco), a preparation extracted from it, and cell wall preparations from smooth (45/0) and rough (45/20) strains of Brucella abortus. On the basis of [3H]thymidine incorporation ratios (E/C), that is, the incorporation rate among cells treated with the mitogen relative to that of untreated cells, the extracted LPS and both Brucella cell wall preparations, but not the commercial LPS were potent mitogens for rabbit spleen cells. By the same criterion, only the Brucella cell wall preparation produced a significant, but minimally so, response among appendix cells. The weak responsiveness of appendix cells may be more apparent than real, however, and may not imply a difference in intrinsic susceptibility to mitogens by these two populations, because unstimulated appendix cells incorporated thymidine at 10 times the rate of unstimulated spleen cells. Appendix cells, then, may not be susceptible to further stimulation, even by active mitogens. Therefore, the significance of E/C ratios may be equivocal when materials are assayed for mitogenic activity on lymphoid populations whose basal activity is relatively high.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Reed N. D. In vitro immune response to lipopolysaccharide: thymus-derived cells not required. Immunol Commun. 1973;2(3):335–340. doi: 10.3109/08820137309022804. [DOI] [PubMed] [Google Scholar]
- Bona C., Damais C., Dimitriu A., Chedid L., Ciorbaru R., Adam A., Petit J. F., Lederer E., Rosselet J. P. Mitogenic effect of a water-soluble extract of Nocardia opaca: a comparative study with some bacterial adjuvants on spleen and peripheral lymphocytes of four mammalian species. J Immunol. 1974 Jun;112(6):2028–2035. [PubMed] [Google Scholar]
- Calkins C. E., Carboni J. M., Waksman B. H. B cells in the appendix and other lymphoid organs of the rabbit: complement receptor lymphocytes. J Immunol. 1975 Nov;115(5):1339–1345. [PubMed] [Google Scholar]
- Calkins C. E., Ozer H., Waksman B. H. B Cells in the appendix and other lymphoid organs of the rabbit: stimulation of DNA synthesis by anti-immunoglobulin. Cell Immunol. 1975 Jul;18(1):187–198. doi: 10.1016/0008-8749(75)90047-7. [DOI] [PubMed] [Google Scholar]
- Elfenbein G. J., Harrison M. R., Green I. Demonstration of proliferation by bone marrow-derived lymphocytes of guinea pigs, mice and rabbits in response to mitogen stimulation in vitro. J Immunol. 1973 May;110(5):1334–1339. [PubMed] [Google Scholar]
- Elfenbein G. J., Harrison M. R., Mage R. G. Appearance of lymphocyte surface markers and functional responses in neonatal and young rabbit spleens. Cell Immunol. 1975 Feb;15(2):303–311. doi: 10.1016/0008-8749(75)90009-x. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Chin A. F., Abbott J. Ontogeny of murine B lymphocytes: sequence of B-cell differentiation from surface-immunoglobulin-negative precursors to plasma cells. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2008–2012. doi: 10.1073/pnas.73.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY C. D., LAYNE S. Bacteria found in the air over Canada and the American Arctic. Can J Microbiol. 1957 Apr;3(3):447–455. doi: 10.1139/m57-047. [DOI] [PubMed] [Google Scholar]
- Kreutzer D. L., Scheffel J. W., Draper L. R., Robertson D. C. Mitogenic activity of cell wall components from smooth and rough strains of Brucella abortus. Infect Immun. 1977 Mar;15(3):842–845. doi: 10.1128/iai.15.3.842-845.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ozer H., Jr, Waksman B. H. The response of rabbit lymphocytes to mitogens and alloantigens: evidence for T cell heterogeneity. J Immunol. 1974 Dec;113(6):1780–1792. [PubMed] [Google Scholar]
- Ozer H., Waksman B. H. Appendix and M antibody formation. V. Appendix and thymus cell synergism in the direct and indirect plaque-forming cell responses to sheep erythrocytes in the rabbit. J Immunol. 1972 Aug;109(2):410–412. [PubMed] [Google Scholar]
- Perey D. Y., Good R. A. Experimental arrest and induction of lymphoid development in intestinal lymphoepithelial tissues of rabbits. Lab Invest. 1968 Jan;18(1):15–26. [PubMed] [Google Scholar]
- Redelman D., Scott C. B., Sell S. In vitro studies of the rabbit immune system. I. Hemolytic plaque-forming response of dissociated spleen cells from normal and primed rabbits. Cell Immunol. 1975 Oct;19(2):230–244. doi: 10.1016/0008-8749(75)90206-3. [DOI] [PubMed] [Google Scholar]
- Robertson D. C., McCullough W. G. The glucose catabolism of the genus Brucella. I. Evaluation of pathways. Arch Biochem Biophys. 1968 Sep 20;127(1):263–273. doi: 10.1016/0003-9861(68)90225-7. [DOI] [PubMed] [Google Scholar]
- Robertson D. C., McCullough W. G. The glucose catabolism of the genus Brucella. II. Cell-free studies with B. abortus (S-19). Arch Biochem Biophys. 1968 Sep 20;127(1):445–456. doi: 10.1016/0003-9861(68)90249-x. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Nowotny A., Chused T., Mergenhagen S. E. In vitro transformation of mouse bone-marrow-derived (B) lymphocytes induced by the lipid component of endotoxin. Infect Immun. 1973 Sep;8(3):406–411. doi: 10.1128/iai.8.3.406-411.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffel J. W., Draper L. R. Spontaneous plaque-forming cells against autologous erythrocytes develop in cultures of normal rabbit appendix cells. Cell Immunol. 1976 Sep;26(1):54–67. doi: 10.1016/0008-8749(76)90347-6. [DOI] [PubMed] [Google Scholar]
- Sell S., Sheppard H. W., Jr Rabbit blood lymphocytes may be T cells with surface immunoglobulins. Science. 1973 Nov 9;182(4112):586–587. doi: 10.1126/science.182.4112.586. [DOI] [PubMed] [Google Scholar]
- Shek P. N., Chou C. T., Dubiski S., Cinader B. Mitogen stimulation of rabbit spleen cells before and after complement-mediated cell kill with an antiserum directed against the thymus antigen RTLA. Int Arch Allergy Appl Immunol. 1974;46(5):753–767. doi: 10.1159/000231175. [DOI] [PubMed] [Google Scholar]
- Sperry J. F., Robertson D. C. Erythritol catabolism by Brucella abortus. J Bacteriol. 1975 Feb;121(2):619–630. doi: 10.1128/jb.121.2.619-630.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorescu M., Mayer E. P., Reiter H., Dray S. Rabbit lymphocyte subpopulations. I. Separation of Ig+ and Ig- cells and their interaction in cultures stimulated by mitogens. Cell Immunol. 1976 Mar 1;22(1):66–75. doi: 10.1016/0008-8749(76)90007-1. [DOI] [PubMed] [Google Scholar]
- Theis G. A., Thorbecke G. J. The proliferative and anamnestic antibody response of rabbit lymphoid cells in vitro. II. Requirement for adherent and nonadherent cells of the responses to particulate antigens in spleen cell cultures. J Exp Med. 1970 May 1;131(5):970–980. doi: 10.1084/jem.131.5.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Waksman B. H., Ozer H., Blythman H. E. Appendix and M-antibody formation. VI. The functional anatomy of the rabbit appendix. Lab Invest. 1973 May;28(5):614–626. [PubMed] [Google Scholar]


