Abstract
Introduction
Small cell, neuroendocrine tumors, and melanoma of the anus are rare. Limited data exists on incidence and management for these rare tumors. A large, prospective, population-based database was utilized to determine incidence and survival patterns of rare anal neoplasms.
Methods
The Surveillance Epidemiology and End Results (SEER) registry was queried to identify patients diagnosed between 1973 and 2005. Incidence and survival patterns were evaluated with respect to age, sex, race, histology, stage, and therapy.
Results
We identified 7,078 cases of anal canal neoplasms: melanoma (n=149), neuroendocrine (n=61), and small cell neuroendocrine (n=26). Squamous cell carcinoma (SCC) (n=6,842) served as the comparison group. Anal melanoma (AM) demonstrated lowest survival rate at 2.5%. Neuroendocrine tumors (NETs) demonstrated similar survival to SCC (10-year survival for regional disease of 25% and 22.3%, respectively). Ten-year survival of small cell NETs resembled AM (5.3% vs. 2.5%). Age≥60, sex, black race, stage, and surgery were independent predictors of survival.
Conclusions
This study presents the largest patient series of rare anal neoplasms. NETs of the anal canal demonstrate similar survival patterns to SCC while small cell NETs more closely resemble AM. Accurate histologic diagnosis is vital to determine treatment and surgical management as survival patterns can differ amongst rare anal neoplasms.
Keywords: anal, cancer, SEER, neuroendocrine tumor
INTRODUCTION
Case Presentation
A 64 year-old woman with multiple co-morbidities presented with worsening rectal pain and was found to have a thrombosed external hemorrhoid. She underwent local excision, histologically negative for malignancy. She later developed recurrent anal pain and underwent an elective hemorrhoidectomy for symptomatic hemorrhoidal disease. Pathologic evaluation of the left lateral hemorrhoidectomy specimen revealed an unsuspected finding of a poorly differentiated neuroendocrine carcinoma with lymphovascular invasion. The neoplasm extended to the margins of the excision. The tumor cells were positive for pankeratin, chromogranin, and synaptophysin. Immunohistochemical staining methods with K1-67/MIB1 markers were consistent with a high proliferation rate. The tumor cells were negative for pan-LCA. These findings were most consistent with poorly differentiated neuroendocrine carcinoma. This unexpected finding of poorly differentiated neuroendocrine carcinoma prompted a review of the literature, which revealed a persistent paucity of data for the diagnosis and management of neuroendocrine tumor (NET) of the anal canal.
Background
Neoplasms of the anal canal are rare malignancies that account for only 1.5% of digestive-system cancers in the United States.1 Approximately 6,200 cases of anal cancer are diagnosed annually in the United States, resulting in almost 800 deaths.2 Both tumor histopathology and anatomic location influence the diagnosis, treatment strategy and prognosis. A key distinction is drawn between tumors located in the anal canal and those arising from the anal margin. The surgical anal canal begins at the dentate line and extends proximally to the anorectal ring, while the anal margin is the perianal skin that extends from the anal verge to 5 cm outward on the perineum.3 There are a variety of lesions that comprise tumors of the anal canal, with squamous cell carcinoma (SCC) being the most frequent. Anal melanoma (AM) and neuroendocrine tumors (NETs) are significantly less common and frequently diagnosed incidentally.
Together, AM and NETs of the anal canal comprise less than 5% of all colorectal malignancies.3 Both are commonly diagnosed at an advanced stage, rendering poor overall prognoses.4–9 Only surgical resection offers patients with these histologic subtypes of anal cancer a chance for cure.10–13 However, the choice of operation and the degree of resection remains controversial due to the low overall survival rate associated with these rare neoplasms.
While surgery has been viewed as the mainstay treatment for AM due to its poor response to chemoradiation,14 there are, however, inconsistencies in the clinical management of patients with anal NETs, likely due to the poorly characterized diagnostic categorization of this histologic subtype.15 This inconsistency is largely due to the rarity of this anal canal neoplasm. It has been recognized in the literature, that NETs of the GI tract can have a spectrum of morphologic features that range from classic small cell carcinoma to large cell NET,4, 16–18 and it has been suggested that small cell carcinoma be separated from large cell NET due to differences in certain pathologic characteristics as well as clinical outcomes.15 However, patients diagnosed with anal canal neuroendocrine tumors have historically been grouped as one histologic subtype when considering prognosis and potential therapeutic options.
The incidental finding of an aggressive small cell NET in the case presentation prompted a literature search. Given the rarity of the disease, the literature search was limited and consisted of only small case series reports rendering it difficult to draw definitive conclusions about optimal treatment, management and prognostic information for this diagnosis. As a result, an investigation of the incidence and survival of rare anal canal tumors using a cancer registry was performed. This is the first comparative study examining the incidence and survival patterns of rare anal canal neoplasms using a national cancer registry.
METHODS
SEER Registry and Study Population
The Surveillance, Epidemiology, and End Results (SEER) project is a United States, population-based cancer registry started in 1973 and is supported by the National Cancer Institute and Centers for Disease Control and Prevention. SEER contains data on cancer incidence, prevalence, mortality, and population-based variables, representing now approximately 28% of the United States population sampled across multiple geographic regions. The SEER data set also contains information on the primary characteristics of the tumor, including site, spread, and histology when available, as well as limited information regarding treatment excluding chemotherapy.
Data Collection and Analysis
This study was reviewed and approved by the Institutional Review Board at the University of California, San Diego. We queried the SEER registry of the National Cancer Institute to identify patients diagnosed between 1973 and 2005 with anal canal neoplasm involving the following histologic subtypes: squamous cell carcinoma, anal melanoma, neuroendocrine, small cell neuroendocrine. Incidence and survival patterns were evaluated with respect to age, sex, race andethnicity, marital status, birthplace, state, country of residence, histologic diagnosis, stage, and therapy.
According to the SEER registry, staging is defined by presence of local, regional, or distant disease at the time of diagnosis (Table 1). The SEER extent of disease (EOD) coding scheme incorporates size of primary tumor, extension of tumor, and lymph node involvement. The code is based on clinical, operative, and pathologic diagnoses of the cancer. The size of the tumor is the size before systemic or radiation therapy. See Table 1 for definitions of stages. Categories of race/ethnicity as defined in SEER were Caucasian, Black, Asian, Hispanic, American Indian/Alaskan Native, and other/unknown. Marital status was defined as married, never married, divorced, separated or widowed.
Table 1.
Definition of Staging by SEER
| Stage | Definition |
|---|---|
| Localized Disease | Incidental finding of malignancy in hemorrhoids, invasive intraluminal extension of tumor confined to the layers of the mucosa, and localized NOS. |
| Regional Disease | Direct extension or nodal involvement; includes extension to ischiorectal fat, perianal skin, perineum, pelvic floor muscles and anorectal sphincters, and vulva, and/or anorectal hemorrhoidal, hypogastric, lateral sacral, perirectal, and superficial inguinal nodes. |
| Distant Disease | Involvement of distant lymph node and extension to bladder, broad ligaments, cervix uteri, corpus uteri, pelvic peritoneum, prostate, urethra, vagina, and metastasis. |
In univariate analyses, log-rank tests were used to compare survival functions, and Kaplan-Meier curves used to display these functions. Cox multivariate proportional hazard models were used to generate relative risk of death by any cause with 95% confidence intervals, controlling for stage, histology, surgical and chemoradiation treatment. Subset analyses explored the influence and interaction of other variables, including gender, ethnicity, marital status, stage at diagnosis, age at diagnosis, and treatment modality. Age at diagnosis was categorized into patients diagnosed younger than 30 years of age, and then increasing in 10-year intervals beginning at age 30.
Statistics
The data were extracted using the latest SEER*Stat software, version 6.6.2 (Stata Corp., College Stations, TX, USA). Statistical significance was defined as a Type I error probability of <0.05; all confidence intervals (CI) are reported as 95% CI.
RESULTS
Incidence
Using SEER cancer registry, we identified 7,078 neoplasms involving the anal canal, most of which were of epidermoid histology. There were 3,347 male and 3,731 female cases. Consistent with the existing body of literature, SCC comprised the majority of identified cases (97%, n=6,842) with a similar incidence occurring among men and women. The number of rare anal canal neoplasms was significantly lower with only 149 AM, 61 NET, and 26 small cell NET cases.
Age at diagnosis ranged from 27 to 90 years of age with a mean of 56.3 (SD: 21.7) and a median of 55 years of age. The bulk of cases occurred in the 4th to 7th decades of life. The majority of cases were diagnosed in non-Hispanic whites at 83.9% followed by blacks at 11.5%, with just a handful of cases diagnosed in Asians, Hispanics and Native Americans/Alaskans. (Table 2)
Table 2.
General patient characteristics
| Total N (%) |
Squamous Cell Carcinoma N (%) |
Anal Melanoma N (%) |
Neuroendocrine Tumor of Anal Canal N (%) |
Small Cell Neuroendocrine Tumor of Anal Canal N (%) |
|
|---|---|---|---|---|---|
| Gender | |||||
| Male | 3,347 (47.3) | 3,256 (97.3) | 50 (1.5) | 32 (1.0) | 9 (0.3) |
| Female | 3,731 (52.7) | 3,586 (96.1) | 99 (2.65) | 29 (0.8) | 17 (0.5) |
| Age at diagnosis | |||||
| <30 | 126 (1.78) | 125 (1.8) | 0 (0) | 1 (1.6) | 0 (0) |
| 30–39 | 824 (11.6) | 813 (11.9) | 4 (2.7) | 5 (8.2) | 2 (7.7) |
| 40–49 | 1,733 (24.5) | l,688 (24.7) | 18 (12.1) | 20 (32.8) | 7 (26.9) |
| 50–59 | 1,612 (22.8) | 1,573 (23.0) | 22 (14.8) | 11 (18.0) | 6 (13.1) |
| 60–69 | 1,286 (18.2) | 1,233 (18.0) | 37 (24.8) | 9 (14.8) | 7 (26.9) |
| 70–79 | 1,005 (14.2) | 954 (13.9) | 38 (25.5) | 10 (16.4) | 3 (11.5) |
| 80–89 | 427 (6.0) | 401 (5.9) | 22 (14.8) | 3 (4.9) | 1 (3.9) |
| ≥90 | 65 (0.9) | 55 (0.8) | 8 (5.4) | 2 (3.3) | 0 (0) |
| Ethnicity | |||||
| Non-Hispanic White | 5,485 (83.9) | 5.326 (84.2) | 104 (75.9) | 37 (67.3) | 18 (75.0) |
| Black | 752 (11.5) | 730 (11.5) | 9 (6.6) | 8 (14.6) | 5 (20.8) |
| Asian | 164 (2.5) | 137 (2.2) | 22 (16.1) | 5 (9.1) | 0 (0) |
| Hispanic | 96 (1.5) | 91 (1.4) | 2 (1.5) | 3 (5.5) | 0 (0) |
| Native American/Alaskan | 31 (0.5) | 29 (0.5) | 0 (0) | 2 (3.6) | 0 (0) |
| Unknown/other | 13 (0.2) | 12 (0.2) | 0 (0) | O (0) | 1 (4.2) |
| Treatment | |||||
| Surgery | 2,726 (69.7) | 2,623 (68.9) | 107 (95.5) | 28 (80.0) | 4 (36.4) |
| Radiation | 4,101 (58.8) | 4,052 (60.1) | 14 (9.6) | 18 (30.5) | 17 (65.4) |
| Both | 1,157 | 1,143 | 9 | 4 | 1 |
Treatment
Overall, the majority of patients diagnosed with carcinoma of the anal canal underwent surgical resection (69.7%) and radiation therapy (58.8%). Surgery was performed in the majority of patients diagnosed with SCC, AM and NETs of the anal canal. In contrast, only 36.4% of patients diagnosed with small cell NET underwent surgical resection. The majority of patients with NET (65.4%) received radiation treatment as well as patients diagnosed with squamous cell carcinoma of the anal canal (60.1%). Surgery was the mainstay treatment for patients with AM (95.5%) and NETs of the anal canal (80.0%). In contrast, radiation was rarely used to treat AM. (Table 2)
Stage at Diagnosis
Stage at time of diagnosis was significantly different amongst the various histologic subtypes (Table 3). Patients with SCC and NETs of the anal canal were more likely to be diagnosed at an early stage of the disease. Sixty-six percent of patients with SCC had localized disease at time of diagnosis and 72.1% of patients with NETs of the anal canal were diagnosed with local disease. Only 7% of patients with SCC had evidence of distant metastasis at the time of diagnosis. In contrast, small cell NETs of the anal canal were more likely to be diagnosed at later stages: 38.5% with regional disease and 46.2% with distant disease. Patients with AM had a fairly equal distribution across stages at time of diagnosis. The distribution of stage at diagnosis was not significantly different between races (p=0.281). However, women were found to present with regional (63.1% vs 36.9%) and distant (58.8% vs 41.2%) disease more frequently when compared to men (p<0.001).
Table 3.
Tumor staging characteristics
| Total N (%) |
Squamous Cell Carcinoma N (%) |
Anal Melanoma N (%) |
Neuroendocrine Tumor of Anal Canal N (%) |
Small Cell Neuroendocrine Tumor of Anal Canal N (%) |
|
|---|---|---|---|---|---|
| Local | 4.619 (65.3) | 4,512 (66.0) | 59 (39.6) | 44 (72.1) | 4 (15.4) |
| Regional | 1,925 (27.2) | 1,848 (27.0) | 60 (40.3) | 7 (11.5) | 10 (38.5) |
| Distant | 534 (7.5) | 482 (7.0) | 30 (20.1) | 10 (16.4) | 12 (46.2) |
| Total | 7,078 (100) | 6,842 (100) | 149 (100) | 61 (100) | 26 (100) |
Regardless of stage, patients were more likely to receive surgical resection while radiation was reserved more often for patients with advanced or late stage anal cancers (Table 4). The distribution of stage at time of diagnosis among the different histologic subtypes of anal cancer could explain differences in the surgical approach of these patients. Even though the frequency of surgical resection decreased with advancing stage, more than half of the patients in all staging categories underwent an operation to treat their anal cancer. There was, however, a significant increase in the frequency of radiation therapy as stage advanced.
Table 4.
Treatment by staging characteristics
| Total N (%) | Local N (%) | Regional N (%) | Distant N (%) | |
|---|---|---|---|---|
| Surgery | 2,762 (69.7) | 1,924 (73.7) | 703 (64.3) | 135 (52.5) |
| Radiation | 4,101 (58.8) | 2,176 (47.7) | 1,533 (81.3) | 392 (75.1) |
| Both | 1,157 | 644 | 439 | 74 |
Survival
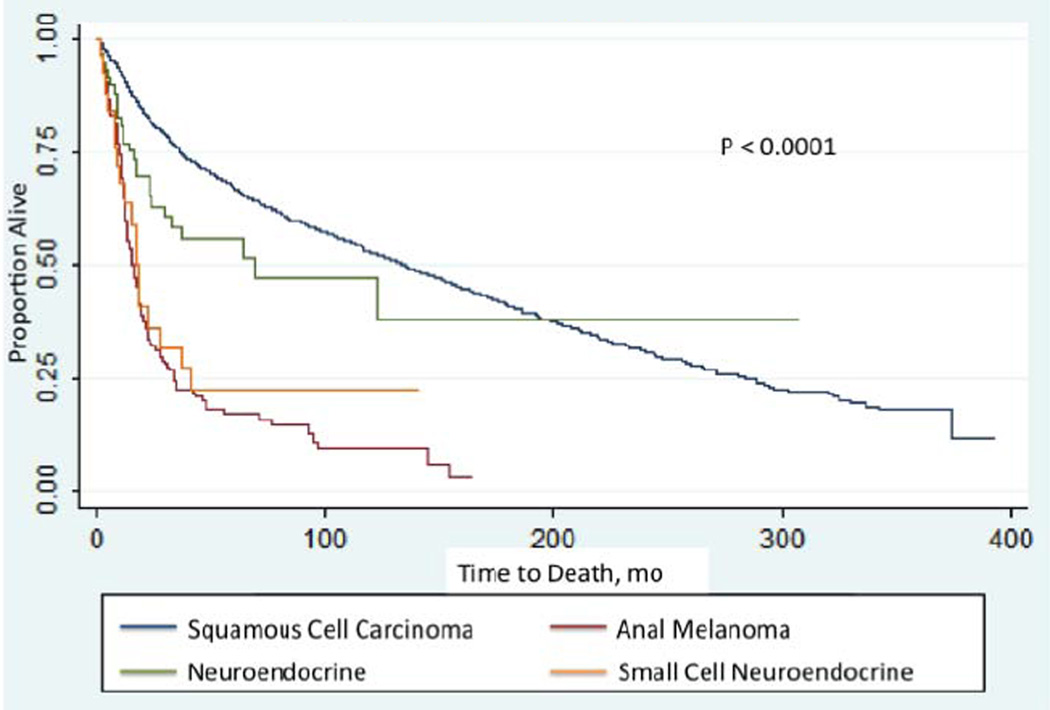
Survival analysis revealed significant differences in 10-year survival rates among the four histologic subtypes (Figure 1). SCC had the highest 10-year survival rates (27.8%) followed by NETs of the anal canal (16.7%). Small cell NET and AM demonstrated dismal 10-year survival rates at 5.3% and 2.5%, respectively. Kaplan-Meier analysis revealed similar survival trends between AM and small cell NETs. Conversely, NETs of the anal canal demonstrated survival trends that more closely resembled that of SCC. In Cox regression analysis, AM was associated with significantly worse prognosis compared to SCC (HR: 3, 95% CI 2.3–3.8). There was a trend to worsening prognosis of NETs and small cell NETs compared to SCC with small cell NETs demonstrating a slightly worse hazard ratio to NETs, although not statistically significant (Table 5). This divergence in 10-year survival by histologic subtype was more significant when reviewed by stage (Table 6). NETs of the anal canal followed a similar trend to that of SCC while small cell NETs more closely resembled AM.
Figure 1. Kaplan-Meier Survival Estimates.
Kaplan-Meier survival curves illustrate how overall mortality changes with histology. Overall survival of SCC was similar to that of NETs. However, AM demonstrated significantly worse overall survival compared to SCC. Small cell NETs demonstrated a similar survival trend to that of AM rather than with other NETs of the anal canal. Log-rank test, p<0.0001.
Table 5.
Survival Analysis by Histologic Subtype
| Histologic Subtype | Cases | 10-yr Survival |
Adjusted HR (95% CI) |
P |
|---|---|---|---|---|
| Squamous Cell Carcinoma | 6,842 | 27.8% | -- | -- |
| Neuroendocrine | 61 | 16.7% | 1.2 (0.7–2.1) | 0.457 |
| Small Cell Neuroendocrine | 26 | 5.3% | 1.5 (0.6–3.6) | 0.395 |
| Anal Melanoma | 149 | 2.5% | 3 (2.3–3.8) | <0.001 |
Table 6.
Ten-year Survival of Histologic Subtypes by Stage
| Histologic Subtype | Local Disease | Regional Disease | Distant Disease |
|---|---|---|---|
| Squamous Cell Carcinoma | 36.8% | 22.3% | 5.1 % |
| Neuroendocrine Tumor | 23.5% | 25% | 0% |
| Small Cell NET | 50% | 0% | 0% |
| Anal Melanoma | 4.7% | 2.0% | 0% |
In multivariate analysis, protective demographic factors included only female gender with an odds ratio (OR) of survival at 10 years of 2.0 (95% CI 1.5–2.7, p<0.001) compared to their counterparts (Table 7). However, age>60, black race and stage at diagnosis were all found to be poor prognostic factors in predicting 10-year survival. While surgery was a significant predictor of survival with an OR of 33.6 (95% CI 13.6–83.1, p<0.001), radiation therapy was not.
Table 7.
Independent Predictors of Survival
| Predictors | Adjusted R) (95% CI) | P-Value |
|---|---|---|
| Age≥60 | 0.4 (0.2–0.9) | 0.036 |
| Female | 2.0 (1.5–2.7) | <0.001 |
| Black Race | 0.6 (0.4–0.8) | 0.005 |
| Stage | 0.6 (0.4–0.7) | <0.001 |
| Surgery | 33.6 (13.6–83.1) | <0.001 |
| Radiation | 0.8 (0.6–0.97) | 0.029 |
DISCUSSION
Neoplasms of the anal canal are uncommon and infrequent neoplasms of the digestive tract. SCC, the most common lesion found in the anal canal, comprised 97% of the cases identified using the SEER cancer registry. Rare anal canal neoplasms such as AM, small cell NET, and NET comprised the remaining 3% of cases (AM 2%, NETs and small cell NETs 1%). Overall, the optimal treatment strategy and outcome are highly dependent on location and histopathology of the anal neoplasm. Due to the rare occurrence of AM, NETs and small cell NETS, limited data exists in the literature and reports consist mostly of small case series making it difficult for one to draw definitive conclusions about optimal treatment strategies and prognostic expectations.
Historically, SCCs of the anal canal were treated with abdominoperineal resection (APR) until treatment was revolutionized in the 1970s by Nigro and colleagues19, 20 who demonstrated that chemoradiation achieved survival and recurrence rates equivalent to those achieved with surgery and preserved sphincter function. For the approximately 30% of patients with persistent or recurrent disease after chemoradiation, APR is performed and achieves 5-year survival rates between 24% and 58%.21
Anal melanoma (AM) does not share the same outcome or prognosis with anal SCC. First reported by Moore22 in 1857, AM was found to be a rare and aggressive malignancy that accounts for 2% to 4% of all malignant neoplasms of the anus and rectum.23–25 Diagnosis is often delayed because of nonspecific presenting symptoms indistinguishable from other benign conditions (hemorrhoid, rectal polyp, or rectal prolapse) in this region, and is thus often misdiagnosed.26, 27 The correct diagnosis is usually established at a late stage, which results in a poor overall survival.7–9 Because AM responds poorly to chemoradiation, surgery is the mainstay treatment.10, 14 However, due to its poor prognosis, the debate continues as to the optimal surgical management for anorectal melanoma. Initially it was suggested that aggressive surgical intervention resulted in better survival, and local excision led to higher recurrence rates. However, more recent reports have concluded there is no difference in survival in patients with AM treated with wide local excision vs. APR.28, 29
Neuroendocrine tumors of the anal canal are exceedingly rare and its histogenesis is poorly understood. Traditionally, colorectal NETs were classified as low-grade or high-grade neuroendocrine tumors based on number of mitoses in HPF and degree of tumor necrosis.15 Current literature however suggests that high-grade neuroendocrine tumors follow a spectrum of morphologic features, ranging from classic small cell carcinoma to large cell neuroendocrine carcinoma. A retrospective review of the Memorial Sloan-Kettering Cancer Center pathology database by Shia, et al.15 revealed different pathologic and clinical characteristics of three subtypes (small cell, mixed-cell, and large-cell NETs), which led the authors to suggest separating small cell carcinoma from large cell and mixed NETs.
The outcome of the case presentation of the patient with an incidental finding of NET in a hemorrhoidectomy specimen was poor. Further medical evaluation with a positron emission tomography (PET) and computed tomography (CT) imaging study revealed evidence of widespread metastases. A biopsy of a suspicious left inguinal lymph node confirmed the presence of metastatic small cell NET. Chemotherapy and palliative options were reviewed with the patient in the setting of metastatic small cell NET and the patient elected to pursue hospice care and died within 4 months of the initial diagnosis of small cell NET.
Our review of the SEER database revealed similarly poor survival rates of AM and small cell NETs. Although not statistically significant, the differences in survival patterns supported the theory of histologically separating small cell from neuroendocrine tumors. Furthermore, these differences may also have a significant impact on the optimal management of patients diagnosed with these rare histologic subtypes. Another reason for the differences in outcome could be related to the late stage at presentation of patients with AM and small cell NETs, further illustrating the importance of early diagnosis and the diagnostic challenge of these rare cancers.
One limitation of this study is that the SEER database represents only 28% of the U.S. population. The National Health Institute collects information from specific geographic regions. Furthermore, changes in the diagnostic criteria in the past few decades may have resulted in under-reported anal canal cancer cases in the SEER registry. This small sample size of neuroendocrine tumors of the anal canal made it difficult through analysis to extrapolate true 10-year survival rates to draw definitive conclusions. There is also limited information on lymph node status as this information has only recently been collected as part of the SEER database within the past decade. Nevertheless, this is the largest study to analyze the incidence and survival patterns of patients with neoplasms of the anal canal.
Anal melanomas and neuroendocrine tumors of the anal canal continue to be diagnostic and therapeutic challenges. The prognostic considerations and surgical approaches for these tumors should be guided by age, stage, nodal involvement, and tumor histopathology. Early diagnosis and early surgical intervention are imperative. It is estimated that a large number of patients with small cell NET and AM will have evidence of metastatic disease at the time of diagnosis. NETs and AMs remain diagnostic challenges due to their nonspecific symptoms making early diagnosis extremely difficult. Further investigations are necessary to understand the differences in behavior based on tumor morphology as well as to develop novel target therapies to improve the outcomes of these rare, aggressive anal canal neoplasms.
REFERENCES
- 1.Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. New England Journal of Medicine. 2000;342(11):792–800. doi: 10.1056/NEJM200003163421107. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed Nov 7th, 2012];US National Institutes of Health. Available from: http://www.cancer.gov/cancertopics/types/anal.
- 3.Garrett K, Kalady MF. Anal neoplasms. Surg Clin North Am. 2010;90(1):147–161. doi: 10.1016/j.suc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Bernick PE, Klimstra DS, Shia J, et al. Neuroendocrine carcinomas of the colon and rectum. Diseases of the Colon & Rectum. 2004;47(2):163–169. doi: 10.1007/s10350-003-0038-1. [DOI] [PubMed] [Google Scholar]
- 5.Chung TP, Hunt SR. Carcinoid and neuroendocrine tumors of the colon and rectum. Clin Colon Rectal Surg. 2006;19(2):45–48. doi: 10.1055/s-2006-942343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saclarides TJ, Szeluga D, Staren ED. Neuroendocrine cancers of the colon and rectum. Results of a ten-year experience. Diseases of the Colon & Rectum. 1994 Jul;37(7):635–642. doi: 10.1007/BF02054405. [DOI] [PubMed] [Google Scholar]
- 7.Antoniuk PM, Tjandra JJ, Webb BW, Petras RE. Anorectal malignant melanoma has a poor prognosis. Int J Colorectal Di. 1993;8(2):81–86. doi: 10.1007/BF00299333. [DOI] [PubMed] [Google Scholar]
- 8.Goldman S, Glimelius B, Påhlman L. Anorectal malignant melanoma in Sweden. Diseases of the Colon & Rectum. 1990;33(10):874–877. doi: 10.1007/BF02051925. [DOI] [PubMed] [Google Scholar]
- 9.Quan SH. Anal cancers. Squamous and melanoma. Cancer. 1992;70(5 Suppl):1384–1389. doi: 10.1002/1097-0142(19920901)70:3+<1384::aid-cncr2820701528>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Gao F, Wan D. Abdominoperineal resection or local excision? A survival analysis of anorectal malignant melanoma with surgical management. Melanoma Res. 2010;20(4):338–341. doi: 10.1097/CMR.0b013e328339b159. [DOI] [PubMed] [Google Scholar]
- 11.Choi BM, Kim HR, Yun H-R, et al. Treatment outcomes of anorectal melanoma. J Korean Soc Coloproctol. 2011;27(1):27–30. doi: 10.3393/jksc.2011.27.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauven P, Ridge JA, Quan SH, Sigurdson ER. Anorectal carcinoid tumors. Is aggressive surgery warranted? Annals of Surgery. 1990;211(1):67–71. doi: 10.1097/00000658-199001000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peralta E. Rare Anorectal Neoplasms: Gastrointestinal Stromal Tumor, Carcinoid, and Lymphoma. Clin Colon Rectal Surg. 2009;22(02):107–114. doi: 10.1055/s-0029-1223842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KB, Sanguino AM, Hodges C, et al. Biochemotherapy in patients with metastatic anorectal mucosal melanoma. Cancer. 2004;100(7):1478–1483. doi: 10.1002/cncr.20113. [DOI] [PubMed] [Google Scholar]
- 15.Shia J, Tang LH, Weiser MR, et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am J Surg Pathol. 2008;32(5):719–731. doi: 10.1097/PAS.0b013e318159371c. [DOI] [PubMed] [Google Scholar]
- 16.Crafa P, Milione M, Azzoni C, et al. Pleomorph poorly differentiated endocrine carcinoma of the rectum. Virchows Arch. 2003;442(6):605–610. doi: 10.1007/s00428-003-0807-1. [DOI] [PubMed] [Google Scholar]
- 17.Gaffey MJ, Mills SE, Lack EE. Neuroendocrine carcinoma of the colon and rectum. A clinicopathologic, ultrastructural, and immunohistochemical study of 24 cases. Am J Surg Pathol. 1990;14(11):1010–1023. doi: 10.1097/00000478-199011000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kato T, Terashima T, Tomida S, et al. Cytokeratin 20-positive large cell neuroendocrine carcinoma of the colon. Pathol Int. 2005;55(8):524–529. doi: 10.1111/j.1440-1827.2005.01864.x. [DOI] [PubMed] [Google Scholar]
- 19.Nigro ND, Vaitkevicius VK, Buroker T. Combined therapy for cancer of the anal canal. Diseases of the Colon & Rectum. 1981;24(2):73–75. doi: 10.1007/BF02604287. [DOI] [PubMed] [Google Scholar]
- 20.Nigro ND. An evaluation of combined therapy for squamous cell cancer of the anal canal. Diseases of the Colon & Rectum. 1984;27(12):763–766. doi: 10.1007/BF02553933. [DOI] [PubMed] [Google Scholar]
- 21.Papaconstantinou HT, Bullard KM, Rothenberger DA, Madoff RD. Salvage abdominoperineal resection after failed Nigro protocol: modest success, major morbidity. Colorectal Dis. 2006;8(2):124–129. doi: 10.1111/j.1463-1318.2005.00911.x. [DOI] [PubMed] [Google Scholar]
- 22.Moore WD. Recurrent melanosis of the rectum after previous removal from the verge of the anus in a man aged 65. Lancet. 1857;1:290–294. [Google Scholar]
- 23.Longo WE, Vernava AM, 3rd, Wade TP. Rare anal canal cancers in the US veteran: patterns of disease and results of treatment. Am Surg. 1995;61(6):495–500. [PubMed] [Google Scholar]
- 24.Iversen K, Robins RE. Mucosal malignant melanomas. The American Journal of Surgery. 1980;139(5):660–664. doi: 10.1016/0002-9610(80)90358-x. [DOI] [PubMed] [Google Scholar]
- 25.Wanebo HJ, Woodruff JM, Farr GH, Quan SH. Anorectal melanoma. Cancer. 1981;47(7):1891–1900. doi: 10.1002/1097-0142(19810401)47:7<1891::aid-cncr2820470730>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Meguerditchian A-N, Meterissian SH, Dunn KB. Anorectal melanoma: diagnosis and treatment. Diseases of the Colon & Rectum. 2011;54(5):638–644. doi: 10.1007/DCR.0b013e31820c9b1b. [DOI] [PubMed] [Google Scholar]
- 27.van't Riet M, Giard RWM, de Wilt JHW, Vles W. Melanoma of the anus disguised as hemorrhoids: surgical management illustrated by a case report. Dig Dis Sci. 2007;52(7):1745–1747. doi: 10.1007/s10620-006-9485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross M, Pezzi C, Pezzi T, et al. Patterns of failure in anorectal melanoma. A guide to surgical therapy. Arch Surg. 1990;125(3):313–316. doi: 10.1001/archsurg.1990.01410150035007. [DOI] [PubMed] [Google Scholar]
- 29.Yeh JJ, Shia J, Hwu WJ, et al. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Annals of Surgery. 2006;244(6):1012–1107. doi: 10.1097/01.sla.0000225114.56565.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid Tumors. The Oncologist. 2008;13(12):1255–1269. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jetmore AB, Ray JE, Gathright JB, et al. Rectal carcinoids: the most frequent carcinoid tumor. Diseases of the Colon & Rectum. 1992;35(8):717–725. doi: 10.1007/BF02050318. [DOI] [PubMed] [Google Scholar]
- 32.Fahy BN, Tang LH, Klimstra D, et al. Carcinoid of the rectum risk stratification (CaRRS): a strategy for preoperative outcome assessment. Ann Surg Oncol. 2007;14(2):396–404. doi: 10.1245/s10434-006-9197-3. [DOI] [PubMed] [Google Scholar]
- 33.Kwaan MR, Goldberg JE, Bleday R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch Surg. 2008;143(5):471–475. doi: 10.1001/archsurg.143.5.471. [DOI] [PubMed] [Google Scholar]
- 34.Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54–63. doi: 10.1055/s-0031-1272824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer J, Balch G, Willett C, Czito B. Update on treatment advances in combined-modality therapy for anal and rectal carcinomas. Curr Oncol Rep. 2011;13(3):177–185. doi: 10.1007/s11912-011-0166-z. [DOI] [PubMed] [Google Scholar]