Abstract
Background
Acute kidney injury (AKI) remains a deadly condition. Tissue inhibitor of metalloproteinases (TIMP)-2 and insulin-like growth factor binding protein (IGFBP)7 are two recently discovered urinary biomarkers for AKI. We now report on the development, and diagnostic accuracy of two clinical cutoffs for a test using these markers.
Methods
We derived cutoffs based on sensitivity and specificity for prediction of Kidney Disease: Improving Global Outcomes Stages 2–3 AKI within 12 h using data from a previously published multicenter cohort (Sapphire). Next, we verified these cutoffs in a new study (Opal) enrolling 154 critically ill adults from six sites in the USA.
Results
One hundred subjects (14%) in Sapphire and 27 (18%) in Opal met the primary end point. The results of the Opal study replicated those of Sapphire. Relative risk (95% CI) in both studies for subjects testing at ≤0.3 versus >0.3–2 were 4.7 (1.5–16) and 4.4 (2.5–8.7), or 12 (4.2–40) and 18 (10–37) for ≤0.3 versus >2. For the 0.3 cutoff, sensitivity was 89% in both studies, and specificity 50 and 53%. For 2.0, sensitivity was 42 and 44%, and specificity 95 and 90%.
Conclusions
Urinary [TIMP-2]•[IGFBP7] values of 0.3 or greater identify patients at high risk and those >2 at highest risk for AKI and provide new information to support clinical decision-making.
Clinical Trials Registration
Clintrials.gov # NCT01209169 (Sapphire) and NCT01846884 (Opal).
Keywords: acute kidney injury, acute renal failure, biomarkers, insulin-like growth factor binding protein (IGFBP)7 and tissue inhibitor of metalloproteinases (TIMP)-2, sensitivity and specificity (MeSH)
INTRODUCTION
Acute kidney injury (AKI) continues to be a challenging clinical problem for hospitalized patients, especially for those admitted to the intensive care unit (ICU). AKI is associated with a marked adverse impact on survival and significantly increases risk for subsequent complications, including chronic kidney disease (CKD) [1–6]. Although risk assessment for AKI is recommended by clinical practice guidelines, in practice this is difficult and no applicable standards have been described [4,7].
Recently, we reported two novel urinary biomarkers for AKI, tissue inhibitor of metalloproteinases (TIMP)-2 and insulin-like growth factor binding protein (IGFBP)7 [8]. We now report the development, diagnostic accuracy and verification of two cutoff values in order to guide clinicians in the clinical use of this test.
We prospectively set two clinical cutoff values for the urinary [TIMP-2]•[IGFBP7] panel, based on sensitivity, specificity and relative risk for predicting development of moderate or severe AKI within the subsequent 12 h in the Sapphire cohort. We then confirmed accuracy and clinical utility of these cutoff values in a new cohort of critically ill patients.
MATERIALS AND METHODS
Subjects
The Sapphire study has been reported in detail elsewhere [8]. Briefly, Sapphire enrolled 744 critically ill patients at 35 sites in North America and Europe from September 2010 through June 2012. The Opal study enrolled 154 critically ill subjects from six diverse academic and community hospital sites in the USA from November 2012 through April 2013 (see Supplementary Data for a list of the enrolling sites). Subjects were at least 21 years of age, admitted to ICU within 24 h of enrollment and expected to remain in the ICU with a urinary catheter for at least 48 h after enrollment.
Both Sapphire and Opal were reported according to the Standards for Reporting of Diagnostic Accuracy guidelines [9] and were approved by the Western Institutional Review Board (Olympia, WA, USA). In addition, the study protocols were approved by investigational review boards/ethics committees as required, by each participating institution. All subjects (or legally authorized representatives) provided written informed consent.
Sample and data collection
In the Opal study, a single urine sample was collected at enrollment by standard methods and centrifuged. The urine supernatant was frozen within 2 h of collection, stored at less than or equal to −70°C, and thawed immediately prior to analysis. Each study site measured the biomarkers in their samples and entered the biomarker data into electronic case-report forms. In both studies, clinical data including patient demographics, history of CKD, reason for ICU admission, weight, serum creatinine and hourly urine output were collected. Password-protected, anonymized clinical data collected with electronic case-report forms resided on servers at independent sites (Medidata Solutions, New York, NY for Sapphire and Document Solutions Group, Malvern, PA for Opal).
Clinical end points
AKI status was classified using the Kidney Disease: Improving Global Outcomes (KDIGO) guideline [7] based on the serum creatinine and urine output available in the hospital record. Baseline serum creatinine was assessed according to three methods: Method 1—median of at least five serum creatinine values in the time range starting 6 months and ending 6 days prior to enrollment; Method 2—if 1 unavailable—nadir value in the time range starting 5 days prior to enrollment and ending at enrollment if available; Method 3—if 1 and 2 unavailable—enrollment value [8].The primary end point for Opal (as in Sapphire) was the development of moderate or severe AKI (KDIGO Stages 2 or 3) within 12 h of sample collection. Because the frequency of serum creatinine testing available in the hospital record varied between patients, serum creatinine values were interpolated at hourly intervals (standard linear interpolation) to determine if patients met the end point by the serum creatinine criteria. The reference values for serum creatinine were obtained as previously described [8].We defined major adverse kidney events (MAKE30) as the composite of death, use of renal replacement therapy or persistence of renal dysfunction (defined by serum creatinine ≥200% of reference) at hospital discharge truncated at 30 days [10].
Clinical cutoff selection
The Sapphire investigators held a series of consensus conferences with the external statisticians and the sponsor and discussed the operating characteristics (Supplementary Data Tables S4 and S5) to arrive at cutoffs that were judged to be clinically appropriate for the intended clinical use [11]. A low cutoff was chosen that allows early recognition of the majority of patients who will develop AKI. This cutoff was selected to have high sensitivity for the primary end point and with the intent to be used in routine clinical practice to identify patients who are at high risk for AKI, and therefore candidates to receive kidney-sparing management strategies, such as those outlined in the KDIGO guideline for high-risk patients [7]. A high cutoff was also selected to allow identification of patients who will develop AKI with high specificity. This cutoff was selected to identify the subgroup of patients who are at the highest risk of AKI and can be considered for more active interventions when clinically appropriate.
Laboratory methods
We tested TIMP-2 and IGFBP7 using the NephroCheck® Test (Astute Medical, San Diego, CA, USA) at each Opal enrolling site. Further details of the test are provided in the supplement. All values for [TIMP-2]•[IGFBP7] are reported in units of (ng/mL)2/1000.
End point prevalence adjustment for positive and negative predictive value
To anticipate the prevalence expected in clinical practice [12], we used data from a previously published multicenter study of 14 356 patients from 303 ICUs examining the epidemiology of AKI in critically ill patients [13].
Statistical analysis
The primary analysis was based on the receiver operating characteristics (ROC) curves for [TIMP-2]•[IGFBP7] in the Sapphire and Opal cohorts for the development of the primary end point (KDIGO Stages 2–3 within 12 h of sample collection). The Sapphire ROC curve was used to identify two optimal cutoffs for [TIMP-2]•[IGFBP7]. At these cutoffs we determined sensitivity, specificity and relative risk for development of the primary end point and prospectively verified these results in the Opal cohort.
We examined the distributions of [TIMP-2]•[IGFBP7] as a function of AKI stage in the Sapphire and Opal cohorts. We calculated positive and negative predictive values (PPV and NPV) for the two cutoffs and adjusted these values for AKI prevalence using a bootstrap method to generate AKI prevalence that matched the AKI prevalence reported in Joannidis et al. [13] as described above. PPV and NPV were calculated for development of KDIGO Stages 2–3 within several time windows (12, 24, 36 and 48 h). Statistical analyses were performed using R 3.0.0 [14]. For all analyses, two-sided P-values <0.05 were considered statistically significant. For Sapphire, operating characteristics (sensitivity, specificity, PPV, NPV and relative risk) were calculated with bootstrap confidence intervals to account for clustered observations in subjects with >1 sample collected within 18 h of enrollment [15]. Relative risk was calculated for strata defined by cutoffs of 0.3 and 2.0; the reference stratum for relative risk comprised subjects with [TIMP-2]•[IGFBP7] values ≤0.3. Tests of trend in risk across strata used the Cochran–Armitage test [16].
Logistic regression models were built including clinical variables with or without [TIMP-2]•[IGFBP7]. All clinical covariates shown in Table 1 and Supplementary Data Table S2 of Kashani et al. [8] with P-value < 0.1 were included. Serum creatinine was included in the model as a dichotomous variable based on the KDIGO 1 staging criteria. CKD was also included because it is a known risk factor for AKI. Integrated discrimination improvement (IDI) and category-free net reclassification improvement (cfNRI) for [TIMP-2]•[IGFBP7] were calculated using methods described by Pencina et al. [17,18]. Variance in the area under the ROC curve (AUC) and the difference in AUC were calculated using the methods described by DeLong et al. [19,20].
Table 1.
Baseline patient characteristics. N (proportion) or median (IQR)
| End point positive | End point negative | |
|---|---|---|
| All subjects | 27 | 126 |
| Male | 12 (44%) | 75 (60%) |
| Age (years) | 64 (54–75) | 65 (54–78) |
| Enrollment serum creatinine (mg/dL) | 2.1 (1.2–2.5) | 1.1 (0.8–1.4) |
| Baseline serum creatinine (mg/dL) | 1.1 (0.9–2.1) | 1.0 (0.8–1.3) |
| Urine volume (mL) for 6 h period prior to enrollmenta | 214 (124–386) | 433 (240–650) |
| History of CKD | 4 (15%) | 9 (7%) |
| Race | ||
| Asian | 1 (4%) | 9 (7%) |
| Black or African American | 3 (11%) | 10 (8%) |
| Other/unknown | 1 (4%) | 10 (8%) |
| White or Caucasian | 22 (81%) | 97 (77%) |
| ICU type | ||
| Cardiac surgery | 0 (0%) | 1 (1%) |
| Combined ICU | 10 (37%) | 33 (26%) |
| Coronary care unit | 6 (22%) | 21 (17%) |
| Medical | 6 (22%) | 47 (37%) |
| Neurologic | 0 (0%) | 2 (2%) |
| Other/unknown | 2 (7%) | 3 (2%) |
| Surgical | 3 (11%) | 10 (8%) |
| Trauma | 0 (0%) | 9 (7%) |
| Reason for ICU admissionb | ||
| Respiratory | 15 (56%) | 66 (52%) |
| Surgery/post-operative | 2 (7%) | 21 (17%) |
| Cardiovascular | 16 (59%) | 48 (38%) |
| Sepsis | 5 (19%) | 24 (19%) |
| Cerebrovascular | 5 (19%) | 10 (8% |
| Trauma | 0 (0%) | 12 (10%) |
| Other | 14 (52%) | 43 (34%) |
aOne hundred and thirty-eight (24 end point positive and 114 end point negative) subjects had 6 h of urine output recorded prior to enrollment.
bSubjects may have multiple reasons for ICU admission.
RESULTS
Subject characteristics and event rates
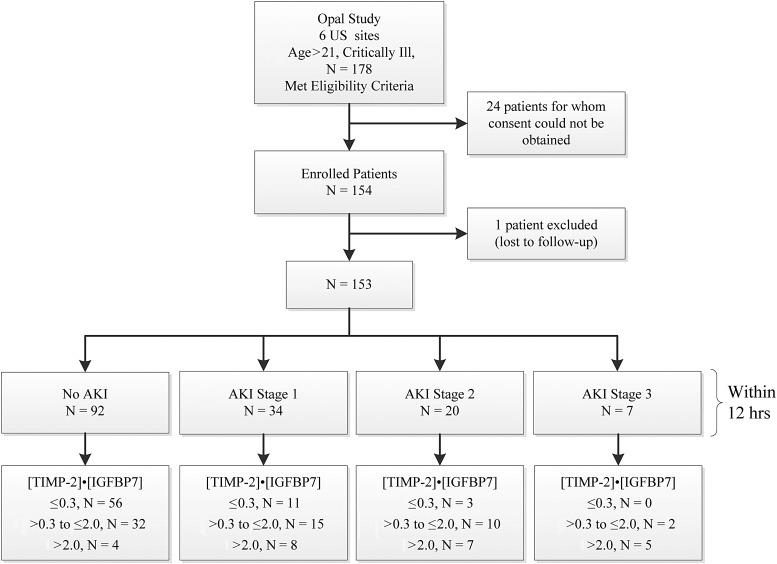
For Opal we enrolled 154 subjects; one subject was lost to follow-up and, therefore, excluded from the analysis (Figure 1). Demographic information is depicted in Table 1. Overall, 27 subjects (18%) in Opal met the primary end point of moderate or severe AKI within 12 h.
FIGURE 1:
Study design (Opal) and number of subjects.
Biomarker performance
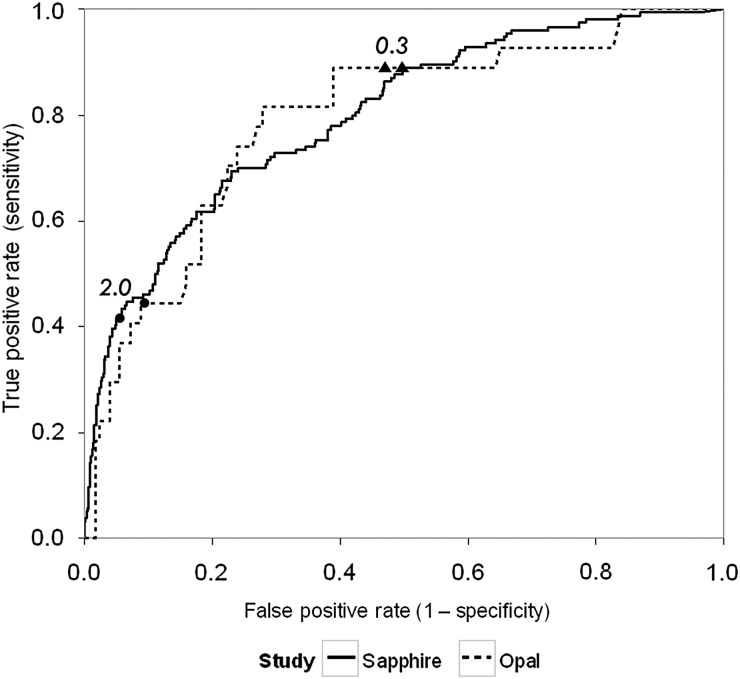
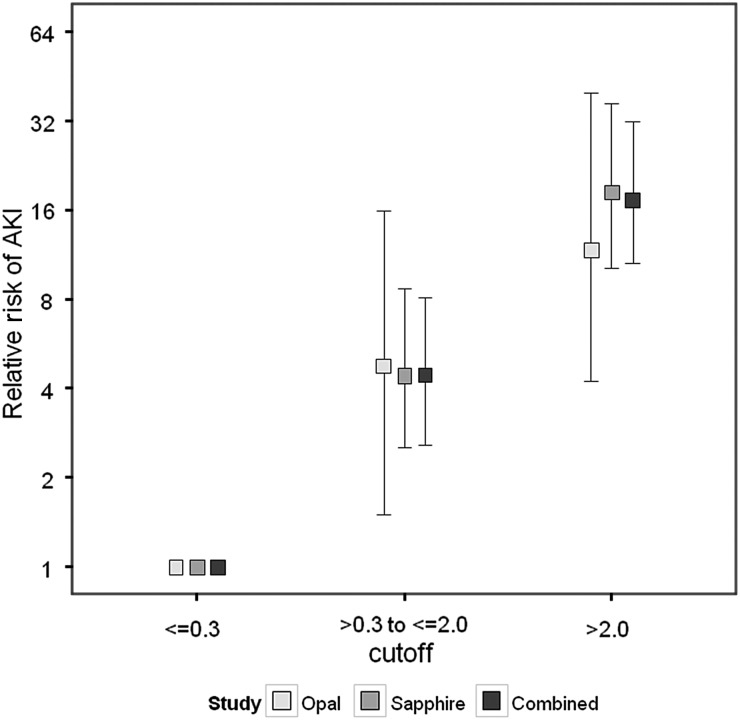
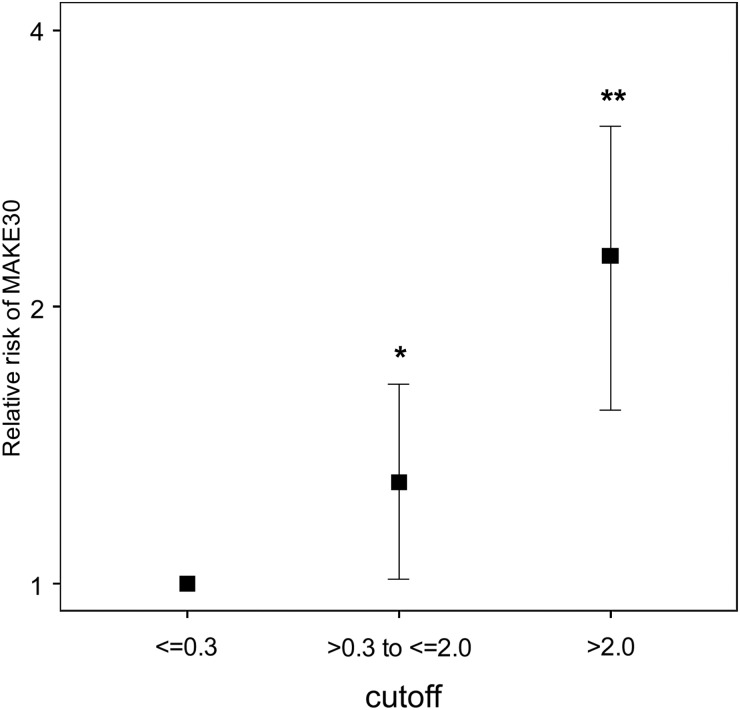
ROC curves and operating characteristics using the clinical cutoffs of 0.3 and 2.0 for urinary [TIMP-2]•[IGFBP7] from Sapphire and Opal are shown in Figure 2. Results from the two cohorts are similar showing that the performance of the two prospectively selected clinical cutoffs was reproduced in a new verification cohort (Opal). The cutoffs of 0.3 and 2.0 also showed comparable results for relative risk of AKI in both Sapphire and Opal cohorts (Figure 3). Specifically compared with baseline risk, subjects in the middle strata were >4-fold and those in the highest strata were >10-fold more likely to manifest moderate-to-severe AKI (KDIGO Stages 2–3) in the next 12 h. In Opal 46% of patients tested ≤0.3 while the upper two strata included 39 and 16% of patients. In order to evaluate the biomarker cutoffs in comparison with clinical variables we performed a multivariable logistic regression analysis with and without the biomarkers. The results are shown in Table 2 and demonstrate that the biomarkers continue to have significant explanatory value even after controlling for clinical variables. We further found that [TIMP-2]•[IGFBP7] (using these new cutoffs) added value to clinical variables using IDI and cfNRI based on data from the Sapphire Cohort. These results are presented in Supplementary Data Table S6 and are consistent with the regression analysis presented in Table 2. Note, that these analyses were performed using data from the Sapphire study but were not presented in Kashani et al. [8] because the cutoffs had not yet been derived. Finally, we show in Figure 4 that the cutoffs also stratify for risk of the composite outcome MAKE30 (death, use of renal replacement therapy or persistence of renal dysfunction at Day 30).
FIGURE 2:
[TIMP-2]•[IGFBP7] ROC curves and operating characteristics for the Sapphire (solid) and Opal (dash) cohorts. Closed triangles and circles indicate [TIMP-2]•[IGFBP7] cutoffs of 0.3 and 2.0, respectively. End point was AKI Stages 2 or 3 within 12 h of sample collection. Area under the ROC curve [95% confidence interval (CI)] = 0.80 (0.74–0.84) and 0.79 (0.69–0.88) for Sapphire and Opal, respectively. NPV and PPVs are presented in Supplementary Data Figure S4 of the supplement.
FIGURE 3:
Relative risk of AKI Stage 2 or 3 within 12 h in the Opal (light gray), Sapphire (medium gray) and combined Opal and Sapphire (dark gray) cohort. Samples were collected within 18 h of enrollment. Risk for each [TIMP-2]•[IGFBP7] range is shown relative to the lowest [TIMP-2]•[IGFBP7] range (≤0.3). Raw risk in lowest stratum = 4.3, 2.7 and 2.9%, respectively, for the Opal, Sapphire and combined cohorts. Error bars indicate the 95% CI. For both cohorts together 700 (46%) of patients had values ≤0.3; 675 (44%) had values between 0.3 and 2 (raw risk of AKI 12.6%); and 154 (10%) had values >2.0 (raw risk of AKI 49%). Cochran–Armitage test for significant trend: P < 0.001 for all three cohorts. Relative risk P < 0.001 for both the second and third stratum relative to the first stratum for all cohorts, except the second stratum of Opal for which relative risk P < 0.01.
Table 2.
Comparison of logistic regression models for risk of AKI in the sapphire cohort (i) using only clinical variables and (ii) using clinical variables plus [TIMP-2]•[IGFBP7]
| Variable | Clinical model |
Clinical model plus [TIMP-2]•[IGFBP7] |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Age | 1.02 (0.99–1.04) | 0.16 | 1.01 (0.99–1.04) | 0.27 |
| APACHE III (non-renal) score | 1.01 (1.00–1.02) | 0.070 | 1.01 (0.99–1.02) | 0.39 |
| Hypertension | 1.7 (0.8–3.4) | 0.14 | 1.9 (1.0–4.0) | 0.066 |
| Nephrotoxic drugs | 2.4 (1.2–4.7) | 0.011 | 2.2 (1.1–4.4) | 0.029 |
| Liver disease | 4.8 (1.9–12.1) | 0.001 | 4.7 (1.7–13.0) | 0.003 |
| Diabetes | 2.1 (1.1–3.8) | 0.02 | 1.9 (1.0–3.5) | 0.053 |
| Sepsis | 1.2 (0.6–2.4) | 0.56 | 1.0 (0.5–2.1) | 0.96 |
| History of CKD | 0.65 (0.26–1.62) | 0.35 | 0.86 (0.32–2.32) | 0.77 |
| KDIGO ≥ 1 by creatininea | 9.0 (5.0–16.3) | <0.001 | 5.7 (3.0–10.8) | <0.001 |
| [TIMP-2]•[IGFBP7] >0.3 to ≤2.0* | Not included in model | 3.5 (1.6–7.7) | 0.002 | |
| [TIMP-2]•[IGFBP7] >2.0* | Not included in model | 11.7 (4.6–29.4) | <0.001 | |
Models for risk of KDIGO 2 or 3 AKI within 12 h of the first sample collection. All patients with a [TIMP-2]•[IGFBP7] value and data for all clinical variables were included (N = 721). All clinical covariates shown in Table 1 and Supplementary Data Table S2 of Kashani et al. [8] with P-value <0.1 were included. CKD was also included because it is a known risk factor for AKI.
aKDIGO stage of 1 or greater by serum creatinine criteria at the time of sample collection.
*Overall P-value < 0.001 for [TIMP-2]•[IGFBP7] (likelihood ratio test).
FIGURE 4:
Relative risk of MAKE30 in the Sapphire cohort. Samples were collected within 18 h of enrollment. Risk for each [TIMP-2]•[IGFBP7] range is shown relative to the lowest [TIMP-2]•[IGFBP7] range (≤0.3). Raw risk in lowest stratum = 18%. Error bars indicate the 95% CI. Cochran–Armitage test for significant trend: P < 0.001. *P = 0.036; **P < 0.001.
Sensitivity analyses
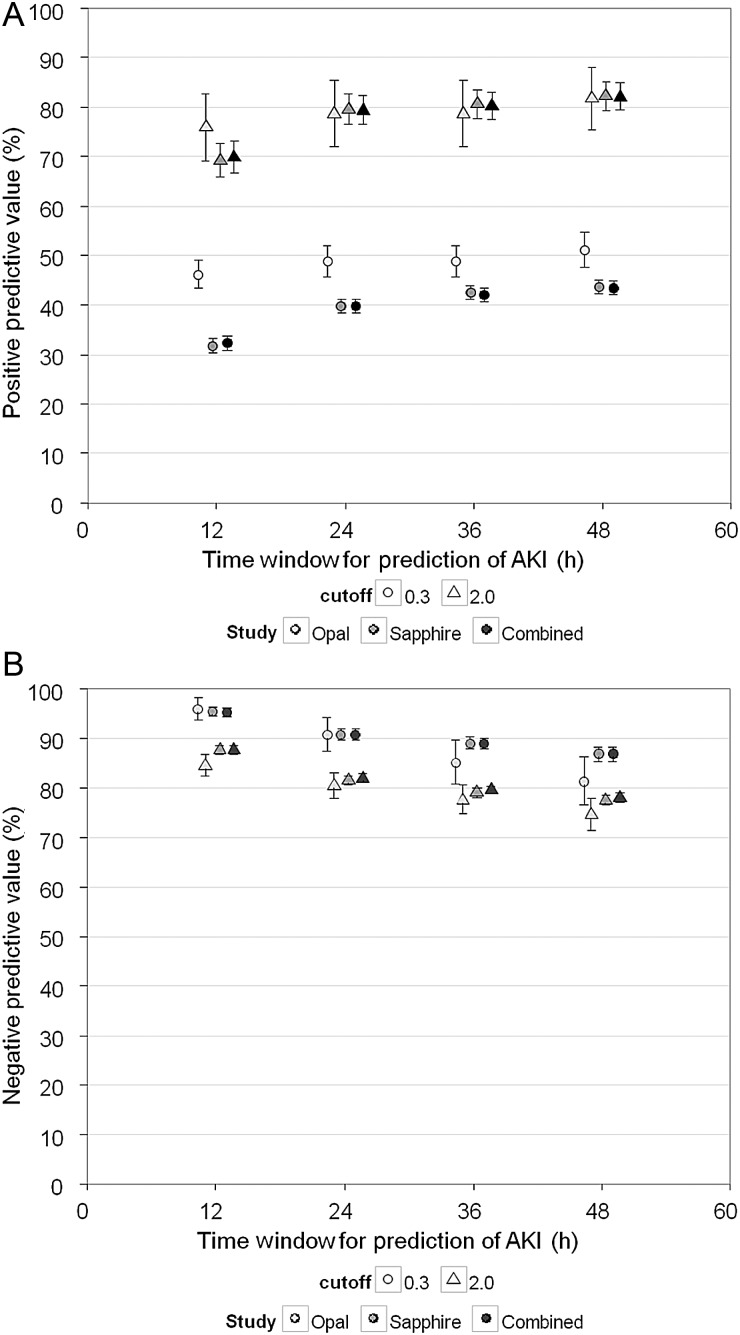
Supplementary Data Table S5 shows the NPV and PPV results for urinary [TIMP-2]•[IGFBP7] (Sapphire) adjusted by the prevalence of AKI reported in Joannidis et al. [13]. As expected, prevalence adjustment decreased NPV to 96% for the 0.3 cutoff (from 97% prior to adjustment); conversely PPV increased to 69% for the 2.0 cutoff (from 49%) for AKI within 12 h. We also varied the time window for detection of AKI out to 48 h (Sapphire and Opal and both studies combined; Figure 5). PPV increases modestly with increasing time window for prediction of AKI, because some patients manifest clinical AKI (KDIGO 2–3) >12 h after sample collection. Conversely, NPV decreases modestly with increasing prediction time. We also examined urinary [TIMP-2]•[IGFBP7] with respect to different severities of AKI (ranging from KDIGO Stages 1–3). These results are presented in Supplementary Data Figure S2.
FIGURE 5:
Prevalence adjusted PPV (A) and NPV (B) for [TIMP-2]•[IGFBP7] cutoff values of 0.3 and 2.0 in the Opal (light gray), Sapphire (medium gray) and combined Opal and Sapphire (dark gray) cohort. Samples were collected within 18 h of enrollment. End point is AKI Stage 2 or 3 within the time window for prediction of AKI indicated along the abscissa (zero time = time of sample collection). Prevalence was adjusted to match the AKI distribution from Joannidis et al. [13] as described in the text [13]. Error bars indicate the 95% CI. Median time from a positive test result to a positive end point was 12.5 h [interquartile range (IQR) 2.7–26] for the Sapphire study and 8 h (IQR 0–15.5) for Opal.
DISCUSSION
In this study we present the derivation and verification of clinical cutoffs for urinary [TIMP-2]•[IGFBP7] when used to assess the risk of AKI in highly susceptible (critically ill) patients. We set the clinical cutoffs based on operating characteristics (sensitivity, specificity and relative risk) by consensus of experts who judged them to be clinically appropriate based on the intended clinical use of the [TIMP-2]•[IGFBP7] test. Once these cutoffs were defined we verified them prospectively using an independent cohort of critically ill patients. Our results clearly show that a urinary [TIMP-2]•[IGFBP7] value can be used to identify patients at greater risk for AKI.
These results are noteworthy for several reasons. First, AKI can be difficult to identify early. An inpatient care audit in the UK revealed delays in diagnosis of AKI in 43% of cases and poor assessment of AKI risk [21]. Delay in diagnosis of AKI is thought to contribute significantly to ineffective care. Indeed, multiple studies have demonstrated that early nephrology consultation or early intervention for ICU patients reduces the number and severity of AKI cases [22,23]. The UK audit also found that 17% of cases had avoidable complications and another 22% were managed badly [21]. We tested the ability of urinary [TIMP-2]•[IGFBP7] to provide information not available from clinical variables alone first by multivariable regression and then by IDI and cfNRI. Both analyses showed considerable added information from the test (Supplementary Data Table S6).
Second, the very high (97%) NPV for the low 0.3 cutoff means that almost all patients testing negative for the test will be free of AKI for the next 12 h. Conversely, those testing positive are at much higher risk for AKI in the next 12 h. This risk stratification is precisely what is needed in order to implement the KDIGO guideline and to apply kidney-sparing therapies for those patients at highest risk—even though <50% of patients in this group will develop AKI [7]. The NPV remained virtually unchanged (96%) even after adjusting for the higher prevalence of AKI reported in observational studies, demonstrating that this cutoff can be used in the ‘real world’ environments where patients are cared for [13] (Figure 5).
Third, examining both cutoffs together reveals very similar results to those obtained with biomarkers used for management of other acute diseases such as acute decompensated heart failure and acute myocardial infarction [24–26]. For example, at 50 pg/mL, B-type-natriuretic peptide has an NPV of 96% and at 100 pg/mL, the PPV is 79% for acute heart failure [24]. Similarly, for acute myocardial infarction, high-sensitivity troponin I (>30 pg/mL) has an NPV of 95% (single measurement) in patients presenting with suspected acute coronary syndrome (PPV was 75%) [25]. For high-sensitivity troponin T a cutoff of 13 pg/mL yielded a PPV of 38% in ‘low-to-intermediate risk patients with chest pain’ (NPV was 96%) [26].The results of urinary [TIMP-2]•[IGFBP7] as a test for AKI in critically ill patients where the NPV for the 0.3 cutoff is 97% (96% after prevalence adjustment) and the PPV for the 2.0 cutoff is 49% (69% after prevalence adjustment) (Figure 5) are comparable with single measurements of B-type-naturetic peptide for acute heart failure and troponin for acute myocardial infarction. Finally, these same cutoffs identify differences in risk for long-term outcomes as shown by significant increased relative risk for MAKE30.
Recently, we reported a third study (Topaz) [27] where the cutoffs derived and validated in this paper were also tested using a clinical adjudication committee to ascertain the AKI end point. These results are complementary to the current study. Indeed the performance of urinary [TIMP-2]•[IGFBP7] has been very consistent across different cohorts. Including the initial discovery, the test has now been evaluated in three multicenter studies of heterogeneous ICU patients, including >1800 patients, and has proved reliable and reproducible, with nearly identical ROC characteristics across all three trials. Furthermore, beginning in the second cohort (Opal), the samples were collected and measured by hospital personnel using a clinical assay, as would occur in routine clinical use.
The opportunity to detect AKI early in its course has the potential to pay multiple clinical dividends for patient care. However, the open question remains as to exactly how clinicians might realize this potential in various clinical settings. We selected a 12 h window for developing AKI because it provides sufficient time to take various actions (e.g. stopping nephrotoxic drugs, assessing volume status and making admission decisions) while at the same time avoiding confounding from subsequent exposures (new AKI-causing events) that might take place during critical illness. Biomarkers are not ‘crystal balls’ and cannot forecast future events that have not yet taken place when the biomarkers were sampled. However, as can be seen in Figure 5, using the same cutoffs, PPV increases by >10% going out to 48 h since some AKI events will manifest after 12 h, while NPV (for both studies together) remains near 90% for the 0.3 cutoff.
Although there are no specific therapies for AKI, recent studies of protocolized supportive care in high-risk patients, including hemodynamic optimization [28,29] and avoidance of nephrotoxins [30,31], have shown the potential to reduce the incidence and severity of AKI [28–31] and improve long-term outcomes [29,31]. Importantly, previous clinical trials of AKI therapy have been hampered by late intervention [32]. Thus, it is hoped but not proven that biomarkers that can predict imminent AKI will facilitate delivery of existing interventions as well as facilitate future trials.
Our study has certain limitations, including those inherent when biomarkers are measured at a single time point in the course of complex critical illness. We recognize that subject enrollment was not synchronized with respect to the course of illness and in fact, while most subjects in the Opal study were recruited prior to clinical manifestations of AKI, some were enrolled soon after AKI was apparent. However, urine [TIMP-2]•[IGFBP7] clearly precedes the diagnosis of AKI based on either urine output or serum creatinine in the setting of early critical illness. Our decision to study patients at the time of ICU admission is based on the fact that patients are admitted to an ICU for a reason, whether it is planned (e.g. following major surgery) or unplanned. This new onset of critical illness represents an extraordinarily high-risk period for AKI—not unlike new onset of chest pain in the assessment of acute coronary syndrome. Given that AKI is usually asymptomatic, it is important to tie biomarker assessment to some clinically relevant time point. Future studies will be required to evaluate the role of other triggers for biomarker assessment (e.g. exposure to nephrotoxins). Finally, when baseline creatinine is not available and admission creatinine is used as baseline, AKI patients may be under-detected [13,33]. As this occurred in only two patients it probably had only limited effects on our results.
In conclusion, we have derived cutoffs for [TIMP-2]•[IGFBP7] that enable identification of patients at high risk for the development of moderate or severe AKI, and validated this in an independent cohort of patients. Given the unmet clinical need for risk assessment and early identification of AKI, the application of this test has the potential to improve care of patients who suffer from AKI.
AUTHORS’ CONTRIBUTIONS
M.G.W. and J.S. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study: J.A.K., L.S.C. and E.A.J.H. designed the study in conjunction with the sponsor. J.A.K. was principal investigator for both Sapphire and Opal studies. Data collection: T.F., E.A.J.H., M.J., K.K., P.A.M. and A.D.S. enrolled subjects, gathered and interpreted the data for the Sapphire study. D.L.U.G. and P.M. enrolled subjects, gathered and interpreted the data for the Opal study. Management: Study clinical data were managed by a contract research organization (Synteract, Carlsbad, CA for Sapphire and InVentiv Health Clinical, Princeton, NJ, for Opal) and could be entered or modified only from the collection site with the site investigator's permission. Statistical analysis: M.G.W. and J.S. performed the statistical analyses. Interpretation of the data: All authors reviewed the data and participated in discussions related to interpretation. Preparation, review or approval of the manuscript: J.A.K., E.A.J.H., L.S.C., A.D.S., J.S. and M.G.W. wrote the paper. All authors reviewed and edited the paper and have seen and approved the final draft.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the many staff, coordinators and investigators whose work was essential to completion of this study. A complete list of Sapphire and Opal Investigators and support personnel is provided in the online appendix and is available online. This study was funded by Astute Medical. Study sites were paid based on patient enrollment and data entry. [34].
CONFLICT OF INTEREST STATEMENT
K.K., P.A.M., P.M. and D.L.U.G. report no conflicts of interest. E.A.J.H. has consulting agreements with Astute Medical, Gambro and Pfizer. L.C. has consulting agreements with Abbott Medical, Affymax Medical, Alere Medical, AM Pharma, Astute Medical, Covidien Medical, Gambro Medical, Nxstage Medical, Sanofi and Bonner Kiernana Law Offices. L.C. has applied for research support from Eli Lilly and owns stock in MAKO Corporation for an orthopedic surgical robot. T.F. has received grant support from Astute Medical, BBraun, Fresenius and Roche. T.F. has received lecture fees from Alexion, Amgen, Astute Medical, BBraun, BMS, Cellpharm, Novartis, Otsuka, Roche and Teva. M.J. has received speaker fees from Astute Medical, Baxter, Fresenius and Gambro. M.J. has received consulting fees from Gambro, Baxter, Fresenius and AM Pharma. M.G.W. and J.S. have consulting agreements with Astute, and provide statistics consulting to pharma, device and diagnostic companies. A.D.S. has received fees for expert testimony from Abbott Laboratories and is a Medical Advisory Board member for FAST diagnostics for Optical GFR measurement and a Scientific Advisory Board member for NxStage Medical for CRRT in the ICU. J.A.K. has received consulting fees from Astute Medical, Alere, Opsona, Aethlon, AM Pharma, Cytosorbents, VenBio, Gambro, Baxter, Abbott, Roche, Spectral Diagnostics, Sangart and Siemens. J.A.K. has also received research grants from Astute Medical, Alere, Cytosorbents, Gambro, Baxter, Kaneka and Spectral Diagnostics, and has licensed unrelated technologies through the University of Pittsburgh to Astute Medical, Cytosorbents and Spectral Diagnostics.
ROLE OF THE SPONSOR
The study sponsor (Astute Medical) was primarily responsible for trial design and execution, with crucial assistance from the trial lead investigators (J.A.K., L.S.C.); data collection was performed by site investigator teams and data analysis was performed by the external biostatisticians (M.G.W., J.S.). All authors contributed to the interpretation of the results.
REFERENCES
- 1.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 4.Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 5.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 6.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 8.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Chem. 2003;49:1–6. doi: 10.1373/49.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Palevsky PM, Molitoris BA, Okusa MD, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol. 2012;7:844–850. doi: 10.2215/CJN.12791211. [DOI] [PubMed] [Google Scholar]
- 11.Cruz DN, Bagshaw SM, Maisel A, et al. Use of biomarkers to assess prognosis and guide management for patients with acute kidney injury. Contrib Nephrol. 2013;182:45–64. doi: 10.1159/000349965. [DOI] [PubMed] [Google Scholar]
- 12.Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford: Oxford University Press; 2003. [Google Scholar]
- 13.Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–1702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 14. The R Project for Statistical Computing www.R-project.org. (1 September 2004, date last accessed)
- 15.Zhou Xh, McClish DK, Obuchowski NA. Statistical Methods in Diagnostic Medicine. 2nd edn. Hoboken: Wiley; 2011. [Google Scholar]
- 16.Agresti A. Categorical Data Analysis. Hoben, NJ: Wiley; 2002. [Google Scholar]
- 17.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 18.Kundu S, Aulchenko YS, van Duijn CM, et al. PredictABEL: an R package for the assessment of risk prediction models. Eur J Epidemiol. 2011;26:261–264. doi: 10.1007/s10654-011-9567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 21.NCEPOD. Adding insult to injury 2009 http://www.ncepod.org.uk/2009aki.htm. (1 September 2004, date last accessed)
- 22.Balasubramanian G, Al-Aly Z, Moiz A, et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. Am J Kidney Dis. 2011;57:228–234. doi: 10.1053/j.ajkd.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Colpaert K, Hoste EA, Steurbaut K, et al. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med. 2012;40:1164–1170. doi: 10.1097/CCM.0b013e3182387a6b. [DOI] [PubMed] [Google Scholar]
- 24.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 25.Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 26.Januzzi JL, Jr, Bamberg F, Lee H, et al. High-sensitivity troponin T concentrations in acute chest pain patients evaluated with cardiac computed tomography. Circulation. 2010;121:1227–1234. doi: 10.1161/CIRCULATIONAHA.109.893826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 28.Brienza N, Giglio MT, Marucci M, et al. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079–2090. doi: 10.1097/CCM.0b013e3181a00a43. [DOI] [PubMed] [Google Scholar]
- 29.Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–1823. doi: 10.1016/S0140-6736(14)60689-9. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein SL, Kirkendall E, Nguyen H, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013;132:756–767. doi: 10.1542/peds.2013-0794. [DOI] [PubMed] [Google Scholar]
- 31.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 32.Hirschberg R, Kopple J, Lipsett P, et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patient with acute renal failure. Kidney Int. 1999;55:2423–2432. doi: 10.1046/j.1523-1755.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 33.Siew ED, Matheny ME, Ikizler TA, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77:536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sapphire investigators. http://www.ccm.pitt.edu/sapphire-investigators. (1 September 2004, date last accessed) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.