Abstract
Rapid cellular zinc influx regulates early mammalian development during the oocyte-to-egg transition through modulation of the meiotic cell cycle. Despite the physiological necessity of this zinc influx, the molecular mechanisms that govern such accumulation are unknown. Here we show that the fully grown mammalian oocyte does not employ a transcriptionally based mechanism of zinc regulation involving metal response element-binding transcription factor-1 (MTF-1), as demonstrated by a lack of MTF-1 responsiveness to environmental zinc manipulation. Instead, the mammalian oocyte controls zinc uptake through two maternally derived and cortically distributed zinc transporters, ZIP6 and ZIP10. Targeted disruption of these transporters using several approaches during meiotic maturation perturbs the intracellular zinc quota and results in a cell cycle arrest at a telophase I-like state. This arrest phenocopies established models of zinc insufficiency during the oocyte-to-egg transition, indicating the essential function of these maternally expressed transporters. Labile zinc localizes to punctate cytoplasmic structures in the human oocyte, and ZIP6 and ZIP10 are enriched in the cortex. Altogether, we demonstrate a mechanism of metal regulation required for female gamete development that may be evolutionarily conserved.
Keywords: oocyte, meiosis, human, zinc, zinc transporters
Introduction
Cell cycle control in the female germ cell requires massive intracellular fluxes of zinc for the oocyte to progress through meiosis and become fertilization-competent. The total intracellular zinc quota is traditionally divided into two distinct categories (Outten and O'Halloran, 2001). In the first category, a population of zinc ions forms tight covalent bonds to protein side-chains, where it mediates catalytic or structural roles. In the second category, zinc ions can be bound weakly to a number of biomolecules in a readily exchangeable form, and this is referred to as the free, or kinetically labile zinc ion pool (Dean et al., 2012). Under normal or resting conditions, most cells maintain cytosolic levels of labile zinc at low levels (Outten and O'Halloran, 2001; Bozym et al., 2006; Eide, 2006). In eukaryotic cells in a non-stimulated state, the free zinc in the cytosol is estimated to be <0.1 pM (Bozym et al., 2006; Qin et al., 2011). Several lines of evidence support the idea that temporal fluctuations in intracellular zinc availability, and the ensuing changes in zinc-binding to key receptor sites, may function in signaling pathways such as those that utilize calcium and cAMP (O'Halloran, 1993; Finney and O'Halloran, 2003; Yamasaki et al., 2007). Such examples can be found in neurons (Vogt et al., 2000; Baranano et al., 2001; Li et al., 2001; Ueno et al., 2002), immune cells (Kabu et al., 2006; Yamasaki et al., 2007; Haase et al., 2008; Nishida et al., 2009) and pancreatic β-cells (Dodson and Steiner, 1998; Kim et al., 2000; Ishihara et al., 2003; Mocchegiani et al., 2008). Recently, female germ cells were identified as another cell type in which fluctuations in cellular zinc levels and availability correlate with cell fate decisions.
In mammals, zinc flux drives a fundamental developmental event: the oocyte-to-egg transition (Kim et al., 2010, 2011; Suzuki et al., 2010b; Bernhardt et al., 2011; Tian and Diaz, 2011; Kong et al., 2012). This transition refers to the release of the oocyte from meiotic arrest in prophase I (PI), followed by progression through meiosis to a second arrest in metaphase II (MII), at which point the cell is referred to as a mature egg. Importantly, the entire process of meiotic maturation occurs in the absence of transcription (Medvedev et al., 2011). Single-cell metallomic analysis using synchrotron-based X-ray fluorescence microscopy (XFM) demonstrated that total intracellular zinc increases by ∼50% during meiotic maturation and decreases by ∼20% following fertilization (Kim et al., 2010). Zinc chelation causes premature arrest in a telophase I-like state demonstrating that this transition metal provides an elemental switch driving the oocyte-to-egg transition (Kim et al., 2010). One of the targets of zinc is Early Meiosis Inhibitor 2 (EMI2, official symbol FBXO43), whose activity is necessary to drive the cell cycle toward metaphase (Madgwick et al., 2006; Bernhardt et al., 2012). The activity of EMI2 depends on its zinc-binding region (ZBR), as mutations in the ZBR prevent cell cycle functions (Suzuki et al., 2010a; Bernhardt et al., 2012).
The precise mechanisms by which the oocyte controls such a prodigious influx of zinc are unknown, particularly in the absence of transcription during the oocyte-to-egg transition. In somatic cells, the availability of intracellular zinc is regulated by zinc finger transcription factors such as metal response element-binding transcription factor 1 (MTF-1), zinc-binding proteins called metallothioneins (MTs), and a number of integral membrane zinc transport proteins which move this divalent ion across lipid bilayers (Palmiter, 1998; Andrews, 2001; Palmiter, 2004; Hirano et al., 2008). Two families of zinc transporters are known in eukaryotes: the ZRT, IRT-like protein (ZIP) family, also known as solute carrier family 39 (SLC39), which modulate zinc uptake into the cytoplasm either from the extracellular environment or from storage compartments, and the zinc transporter (ZnT) family, also known as solute carrier family 30 (SLC30), which modulate zinc efflux from the cytoplasm. To date, 14 transporters of the ZIP family and 10 of the ZnT family have been identified in mammalian cells (Lichten and Cousins, 2009). In contrast, the metallothionein proteins are small (6 kDa) soluble proteins which contribute to intracellular zinc physiology by binding zinc and other heavy metals, and possibly acting as a zinc reservoir or a zinc-scavenging mechanism (Dalton et al., 1996; Suhy et al., 1999). The expression of several zinc transporters and MTs is under the transcriptional regulation of MTF-1, a transcription factor that modulates gene expression via activation or repression mechanisms in response to alterations in intracellular zinc levels (Heuchel et al., 1994; Andrews, 2001; Lichten et al., 2011).
In this study, we have identified the molecular mechanism by which the mammalian oocyte controls the zinc accrual required for meiotic maturation in the unique context of transcriptional quiescence (Bouniol-Baly et al., 1999). We found that MTF-1-based mechanisms of metal regulation are inactive in the fully grown mouse oocyte. Instead, zinc accrual and meiotic progression are dependent on the stable expression of ZIP6 and ZIP10, genes which we have identified as having a maternally derived expression pattern; these encode SLC39 family zinc transporters which import zinc ions across plasma or luminal membrane into the cytosol. Moreover, ZIP6 and ZIP10 protein expression is conserved in the human oocyte, suggesting an essential and evolutionarily conserved role of zinc in meiotic maturation, and perhaps a previously unrecognized role in human fertility and infertility.
Materials and Methods
Animals, cell collection and culture
All experiments were performed using CD-1 mice (Harlan Laboratories, Indianapolis, IN, USA) that were housed in a controlled barrier facility at Northwestern University's Center of Comparative Medicine under constant temperature, humidity and light (12 h light/12 h dark). Food and water were provided ad libitum. All animal use was approved under protocols submitted to the Northwestern University's Institutional Animal Care and Use Committee. Animals were maintained according to the National Institutes of Health's guidelines.
Meiotically incompetent PI-arrested oocytes were collected from the ovaries of 13-day-old female mice after enzymatic digestion with 0.1% collagenase and 0.01% DNase (Worthington Biochemical, Lakewood, NJ, USA) in Leibovitz's L-15 medium (L-15) (Sigma-Aldrich, St. Louis, MO, USA) as previously described (Xu et al., 2006). Fully grown, meiotically competent PI-arrested oocytes were collected from the ovaries of 21-day-old mice 44–46 h following injection with 5 IU equine chorionic gonadotrophin (eCG, EMD Biosciences, San Diego, CA, USA) into L-15 supplemented with 1% (v/v) fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA) and 10 μM milrinone (Sigma-Aldrich). MII eggs were collected from the oviducts of six- to ten-week-old female mice injected with 5 IU eCG and then 5 IU hCG (Sigma-Aldrich) 46–48 h later into L-15 supplemented with 1% FBS. For embryo collection, the same hormone stimulation protocol was used as for MII egg collection, and primed female mice were mated with CD-1 males. One-cell, 2-cell, 8-cell and blastocyst stage embryos were flushed from either the oviducts or the uteri at 20–21, 41–44, 68–70, 92–96 h post-hCG, respectively, into potassium simplex optimized medium with amino acids (KSOM+AA, Millipore, Billerica, MA, USA).
For metal responsiveness experiments, oocytes were cultured in in vitro maturation (IVM) medium comprised of minimum essential medium (MEM)-alpha GlutaMAX (Invitrogen) supplemented with 10% FBS for 14 h. 10 μM TPEN or 200 μM ZnSO4 (Sigma-Aldrich) was added to the base culture medium for select treatment groups. TPEN and ZnSO4 were prepared in Milli-Q water at stock concentrations of 1 and 10 mM, respectively. At the end of culture, samples were collected for MTF-1 staining or qRT–PCR, as described below.
Imaging of labile zinc
Labile zinc distribution was examined in live cells. Morpholino-injected oocytes were removed from culture at the defined time points. All cells were incubated in 50 nM ZincBY-1 followed by 10 μg/ml Hoechst 33342 (Invitrogen) for 5 min (unpublished data).
All samples were imaged in drops of IVM medium overlaid with embryo culture oil (Irvine Scientific, Santa Ana, CA, USA) in glass-bottom dishes (Bioptechs, Inc., Butler, PA, USA). Samples were imaged using a TCS SP5 confocal microscope, (Leica Microsystems, Heidelberg, Germany) equipped with a stage top incubator (Tokai Hit, Shizuoka, Japan), ×40 oil-immersion objective, and HeNe (543 nm), Ar (488 nm) and near-UV (405 nm) laser lines. Images were collected at 1 μm intervals along the Z-axis to span the entire volume of the cell. Following labile zinc imaging, selected oocytes were processed for immunofluorescence as described below.
Antibody production
All protein expression, purification and custom antibody synthesis for ZIP6 and ZIP10 were done by GenScript (Piscataway, NJ, USA). For ZIP10, a peptide was synthesized and used to produce a polyclonal antibody in rabbits. A cysteine was added to the C-terminus of the peptide to facilitate conjugation to keyhole limpet hemocyanin (KLH). For ZIP6, a portion of the protein corresponding to amino acids 455–666 was subcloned for bacterial expression. All antibodies were affinity purified and reconstituted to a final concentration of 5 mg/ml. The ZIP10 blocking peptide was also reconstituted to a final concentration of 5 mg/ml. All sequences are listed in Table I.
Table I.
Table of antibody and morpholino sequences.
| Antibodies | |
| ZIP6 Ab | KQFKDKKKKN QKKPENDEDV ESKKQLSKYD SQLSSNEEKV DPGERPESYL RADSQEPSPF DSQQPTMLEE EEVMIAHAHP QEVYNEYVPR GCKNKCHSHF HDTLGQSDDL IHHHHDYHHI LHHHHHQNHH PHSHSQRYSR EELKDAGIAT LAWMVIMGDG LHNFSDGLAI GAAFTEGLSS GLSTSVAVFC HELPHELGDF AVLLKAGMTV KQ |
| ZIP10 Ab | VTSKRNHKCDPEKEC |
| Morpholinos | |
| Zip6 MO | GGC CAT TGC ACC TTC CTC TCC TGG |
| Zip10 MO | TGC ACC TTC ATT TCT ACT TTT CCT C |
For function blocking with antibodies, oocytes were cultured in IVM medium containing rabbit IgG (GenScript), ZIP6, ZIP10 or a mixture of ZIP6 and ZIP10 antibodies for 14 h and subsequently processed for immunofluorescence. All antibodies were used at a final concentration of 50 μg/ml.
Immunofluorescence
For detection of MTF-1 (Santa Cruz Biotech, Santa Cruz, CA, USA), ZIP6 (GenScript Corporation) and ZIP10 (GenScript), oocytes and embryos were incubated briefly in acidic Tyrodes solution (Millipore) to remove the zona pellucida and fixed in 3.8% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in PBS for 1 h at room temperature. The cells were permeabilized in PBS containing 0.1% Triton X-100 and 0.3% bovine serum albumin (BSA) for 15 min at room temperature and rinsed twice through blocking solution of PBS containing 0.3% BSA and 0.01% Tween-20 (Sigma-Aldrich). The cells were then incubated in primary antibody overnight at 4°C. Dilutions used included: 1:10 MTF-1 (20 μg/ml), 1:200 ZIP6 (25 μg/ml) and 1:2000 ZIP10 (2.5 μg/ml). Samples were washed three times, incubated in secondary antibody with rhodamine-phalloidin (1:50, Invitrogen) for 1 h at room temperature and then washed again. Secondary antibody used: 1:100 Alexa Fluor 488-conjugated mouse anti-rabbit IgG (Invitrogen). Controls for ZIP6 included incubation with preimmune serum (1:200) and secondary antibody only (GenScript). Controls for ZIP10 included incubation with pre-absorbed antibody incubated with blocking peptide at a 1:5 ratio. After staining, all samples were mounted in Vectashield containing DAPI (Vector Laboratories, Burlingame, CA, USA), and imaged on an SP5 confocal microscope as described above for live imaging. For quantification of MTF-1 fluorescence intensity, regions of interest (ROIs) were designated for the entire cell and the nucleus using the ImageJ software (National Institutes of Health, Bethesda, MD, USA). The ratio of fluorescence intensity in the nucleus to the intensity in the cytoplasm was calculated based on pixel intensity.
For analysis of spindle morphology after microinjection, oocytes were fixed in 2% formaldehyde containing 0.1% Triton X-100 for 0.5 h at 37°C. Following fixation, samples were blocked at 4°C in phosphate-buffered saline (PBS) containing 0.01% sodium azide, 0.1% glycine, 3 mg/ml bovine serum albumin, 0.01% Tween 20, and 0.1% Triton X-100 (blocking solution). For staining, oocytes were incubated in a FITC-conjugated anti-α-tubulin antibody (1:100 dilution; Sigma-Aldrich) and rhodamine-phalloidin (1:50 dilution) in blocking solution for 1 h at 37°C followed by three washes.
Quantitative real-time PCR
Total RNA was isolated from sets of 50 oocytes or embryos of the indicated stages or treatments using the PicoPure RNA Isolation Kit (Invitrogen). Synthesis of cDNA was done using the SuperScript VILO cDNA synthesis kit with random hexamers (Invitrogen). Total RNA isolated was reverse transcribed in a 20 μl volume. Quantification was done by quantitative real-time PCR (qRT–PCR) using the ABI Taqman Assay-on-demand primer sets for the transporters indicated, and normalized to Gfp mRNA. One oocyte or embryo equivalent of cDNA was used for each real-time PCR reaction. Changes in expression were expressed as fold change using the comparative Ct method. PCR reactions were performed in duplicate for each sample, and each sample was collected from three independent experiments.
Morpholinos and microinjection
Morpholinos (MOs) were designed to target the 5′UTR of Zip6 and Zip10 (Genetools, Philomath, Oregon, sequences in Table I). All MOs were dissolved to 5 mM in molecular-grade water and stored at –80°C according to the manufacturer's instructions. Prior to injection, MOs were heated to 65°C for 10 min and centrifuged briefly to remove particulates. For microinjection, meiotically competent PI oocytes were collected and injected in L-15 medium containing 0.05% polyvinyl alcohol (Sigma-Aldrich), 0.5% penicillin-streptomycin (Invitrogen) and 10 μM milrinone (Sigma-Aldrich). Approximately 5–7 pl of MO was injected into the oocyte cytoplasm using an Eppendorf FemtoJet pressure microinjector with Femtotip injection capillaries (Eppendorf, Hauppauge, NY, USA). A cohort of injected oocytes were maintained in αMEM with 10% FBS and 10 μM milrinone, with or without 10 μM U0126, for 14–16 h. Another cohort of injected oocytes were transferred to in vitro maturation medium for 14 h. Uninjected oocytes served as controls. At the end of culture, the meiotic stage of these cells was scored morphologically by light microscopy. The cells were then imaged for labile zinc or fixed and imaged for spindle morphology as described above.
Human oocyte acquisition
Ovaries were surgically removed from females undergoing ovarian tissue cryopreservation for fertility preservation following informed consent under an Institutional Review Board-approved protocol at Northwestern University. The ovarian tissue was processed for cryopreservation using a standard technique in which the ovarian cortex was separated from the medulla (http://oncofertility.northwestern.edu/media/dissection-human-ovary-preparation-cryopreservation). As a consequence of this tissue processing, small antral follicles were disrupted causing the release of cumulus-oocyte-complexes (COCs) into the media. Up to 20% of this ovarian tissue, including the COCs, were designated for basic research. To obtain COCs, the media that remained post-tissue processing was passed through a 70 mm cell strainer (BD, Franklin Lakes, NJ, USA), and the COCs were collected manually. In some cases, the cumulus cells were removed from the oocyte by mechanical agitation. The denuded oocytes or COCs were then processed for labile zinc imaging or immunocytochemistry with ZIP6 and ZIP10 antibodies. In this study, we analyzed a total of 13 human oocytes from 6 participants ranging in age from 16 to 39 years (Table II).
Table II.
Table of human participant characteristics.
| ID | Age (years) | Medical diagnosis | Number of oocytes used for study | Fluozin- 3 AM staining | ZincBY-1 staining | α-ZIP6 staining | α-ZIP10 staining |
|---|---|---|---|---|---|---|---|
| A | 27 | Endometrial cancer | 3 | X | |||
| B | 33 | Endometrial cancer | 4 | X | |||
| C | 19 | Ewings sarcoma | 2 | X | |||
| D | 37 | Endometrial stromal sarcoma | 1 | X | X | ||
| E | 16 | Relapsed Pre-B ALL | 2 | X | |||
| F | 39 | Breast DCIS | 1 | X |
Statistical analysis
Data presented in all graphs represent the mean ± SEM. Significant changes between groups were analyzed by either one-way ANOVA followed by Bonferroni's post hoc test or by Student's t-test at a statistical significant of at least P < 0.05. All analysis was done using Prism 4 (GraphPad Software).
Results
Common zinc homeostasis mechanisms are inactive in fully grown mouse oocytes
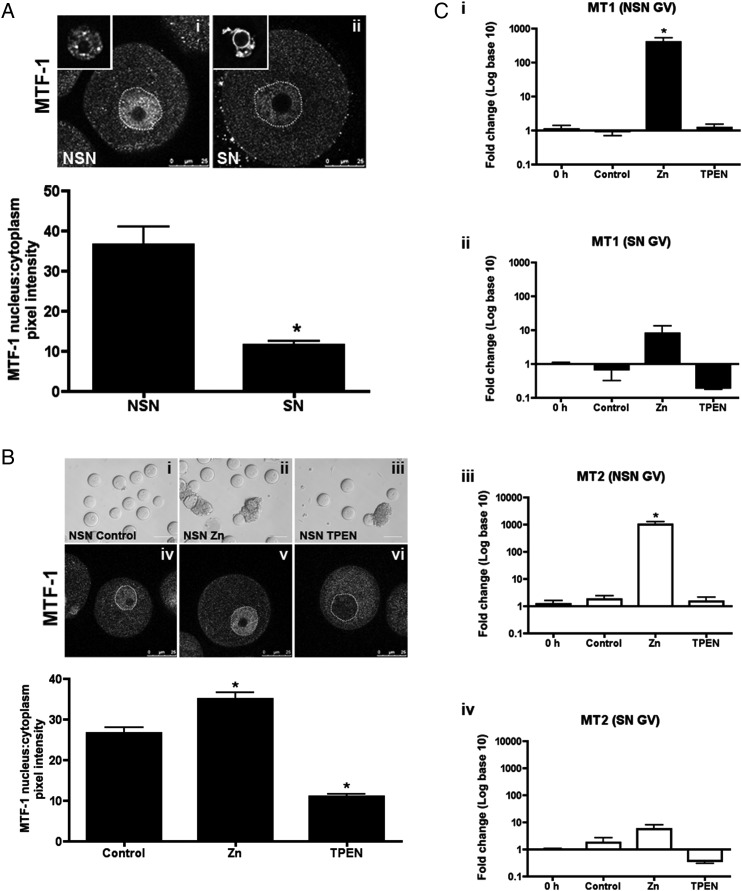
During meiotic maturation in mammalian oocytes, zinc levels rise significantly: over the course of 12 h, the fully grown oocyte accrues ∼20 billion zinc ions, an increase of over 50% (Kim et al., 2010). To determine how the oocyte manages such rapid changes in its intracellular zinc content, we examined known components of transition metal regulation in oocytes at different stages of development. The growing oocyte is immature and incapable of undergoing meiotic maturation (Zuccotti et al., 1995). These meiotically incompetent oocytes exhibit a high degree of transcriptional activity, which is tightly associated with a pattern of decondensed euchromatin known as the non-surrounded nucleolus (NSN) configuration (Debey et al., 1993) (Fig. 1Ai inset). Beginning at the antral follicle stage, the oocyte acquires meiotic competence and transcription ceases (Zuccotti et al., 2002; Miyara et al., 2003). Transcriptional quiescence in the fully grown oocyte is correlated with a transition of chromatin from the NSN configuration to the surrounded nucleolus (SN) configuration, in which the condensed heterochromatin tightly surrounds the nucleolus (Debey et al., 1993; Zuccotti et al., 1995) (Fig. 1Aii inset).
Figure 1.
Zinc responsiveness is suppressed in meiotically competent oocytes. (A) Meiotically incompetent oocytes (non-surrounded nucleolus configuration, NSN) and meiotically competent oocytes (surrounded nucleolus configuration, SN) were isolated and cultured in control media for 14 h, and immunofluorescence done for metal response element-binding transcription factor-1 (MTF-1). Representative optical confocal sections in NSN (Ai) and SN (Aii) oocytes are shown. Dotted lines outline the nuclei. Representative images of NSN and SN nuclear configurations are shown as insets. Graph represents quantification of MTF-1 immunofluorescence intensity as a ratio of nuclear:cytoplasmic fluorescence intensity. (B) NSN oocytes were cultured in control media or media supplemented with 200 μM ZnSO4 (Zn) or 10 μM TPEN (TPEN) for 14 h. Representative transmitted light images of oocytes at end of culture are shown in i-iii; images of MTF-1 staining are shown in iv-vi, dotted lines outline the nuclei. MTF-1 immunofluorescence intensity was quantified as in (A). In (A) and (B), scale bars in transmitted light images = 80 μm, in confocal images = 25 μm. At least 20 oocytes were measured from each treatment group from three independent experiments. Data are shown as the mean ± SEM. Asterisks represent statistical significance when compared with NSN (A) or NSN control (B) at P < 0.001 as calculated by student t-test. (C) Quantitative real-time PCR (qRT–PCR) of MT1 and MT2 in NSN and SN oocytes at time of isolation (0 h) and at the end of culture in control, Zn or TPEN media as in (B). RNA was isolated from 50 oocytes from each treatment group in three separate experiments. Data are shown as the mean ± SEM. Asterisks in (Di, iii) represent statistical significance when compared with 0 h oocytes at P < 0.001 by student t-test. See also Supplementary data, Fig. S1.
Given the well-established differences in transcriptional activity between oocytes with NSN and SN chromatin configurations, we hypothesized that differences may also exist in MTF-1 function between these two oocyte cohorts. Under standard cellular conditions, MTF-1 is a transcription factor that shuttles between the nucleus and cytoplasm. In the presence of zinc, MTF-1 rapidly translocates from the cytoplasm to the nucleus, where it binds to its cognate DNA motif, i.e. the metal response element (MRE), to regulate the expression of genes involved in maintaining zinc homeostasis (Radtke et al., 1993). Using an MTF-1-specific antibody, we detected MTF-1 staining in the nuclei of NSN oocytes, suggesting the presence of transcriptionally based mechanisms of zinc homeostasis during this stage of oocyte development (Fig. 1Ai, ii). Further, we found that the nuclear:cytoplasmic ratio of MTF-1 was significantly greater in oocytes with a NSN configuration compared with those with a SN configuration (Fig. 1A).
Given these differences in nuclear MTF-1 levels between oocytes that are transcriptionally inactive and fully grown (SN) versus those that are not (NSN), we next examined how these two oocyte cohorts responded to manipulations of environmental zinc levels. Extracellular zinc levels were increased or decreased by culturing oocytes in 200 μM ZnSO4 or 10 μM TPEN (tetrakis-(2-pyridylmethyl)ethylenediamine), a membrane-permeant zinc chelator for 14 h, respectively. All oocytes exposed to ZnSO4 or TPEN remained morphologically indistinguishable from control oocytes as visualized by light microscopy, suggesting that the treatments were not toxic to the cells (Fig. 1Bi–iii). In oocytes with a NSN configuration, clear changes in MTF-1 localization were apparent and these correlated with external zinc levels (Fig. 1Biv, v, vi). Exposure to excess zinc resulted in a significantly increased nuclear:cytoplasmic ratio of MTF-1 when compared with untreated oocytes, while zinc chelation resulted in a significant decrease (Fig. 1B). In contrast, no changes in MTF-1 localization were observed in oocytes with an SN configuration undergoing meiotic maturation regardless of external zinc levels (Supplementary data, Fig. S1).
To further examine responsiveness to zinc in these two oocyte cohorts, we assessed MTF-1 function by quantifying the transcript levels of the zinc-responsive genes Mt1 and Mt2 following exposure to ZnSO4 or TPEN. Excess zinc exposure in NSN oocytes caused a dramatic 400-fold increase in Mt1 and 1000-fold increase in Mt2 mRNA levels (Fig. 1Ci, iii), whereas the same exposure in SN oocytes had no effect on Mt expression (Fig. 1Cii, iv). In addition, no significant changes in Mt levels were detected with zinc deficiency induced by TPEN chelation in both NSN and SN oocytes. Taken together, these results suggest that the common MTF-1-mediated metal regulatory mechanisms present in most somatic cells are also present and functional in the growing, transcriptionally active oocyte of the NSN configuration, but are largely attenuated or absent in the fully grown SN oocyte.
ZIP6 and ZIP10 are highly abundant, maternally-derived zinc transporters in the oocyte
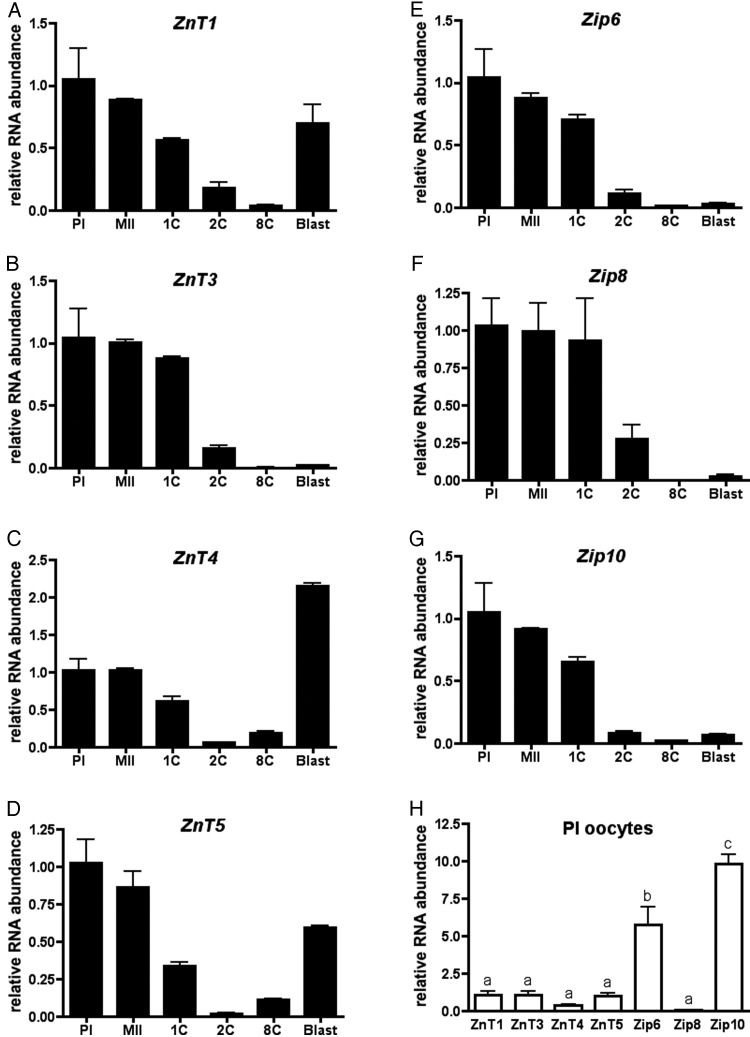
To determine how the oocyte acquires zinc during meiotic maturation in the unique environment of transcriptional quiescence, we broadly surveyed the expression patterns of all 24 zinc transporters using a combined approach of microarray data published on GEO Profiles and BioGPS (Su et al., 2004; Zeng et al., 2004) and semi-quantitative reverse-transcriptase PCR (data not shown). From this, we identified a cohort of seven transporters to examine further based upon their unique patterns of expression and/or their relative abundance, and profiled their expression in the oocyte, egg and early embryo using qRT–PCR (Fig. 2). Of these, ZnT1, ZnT4 and ZnT5 demonstrated a maternal-to-zygotic expression pattern, wherein expression was high in the oocyte, decreased during meiotic maturation and fertilization, and increased again during preimplantation embryo development (Fig. 2A, C and D). This expression profile of maternal transcripts being replaced by zygotic transcripts is common to many genes with basic cellular functions (Zeng et al., 2004). In contrast, ZnT3, Zip6, Zip8 and Zip10 demonstrated a maternal expression pattern, whereby expression was high in the oocyte and egg but decreased and remained low after fertilization (Fig. 2B and E–G). This limited expression pattern is characteristic of genes that are critical for meiotic maturation and early development prior to the activation of the zygotic genome (Zeng et al., 2004). Of note, Zip6 and Zip10 transcripts were approximately six- to ten-fold higher in relative abundance compared with all of the other zinc transporters examined in the oocyte (Fig. 2H). These expression profiles are consistent with results published in GEO Profiles and BioGPS (Su et al., 2004; Zeng et al., 2004).
Figure 2.
Zip6 and Zip10 are highly expressed in the mouse oocyte and display a maternal expression pattern. (A–G) Relative amounts of zinc transporter mRNAs were evaluated across meiotic maturation and early embryo development by qRT–PCR. In each experiment, 50 oocytes or embryos were used to isolate mRNA, and the experiment was performed in triplicate. Data represent the mean ± SEM relative to the value obtained for PI oocytes. PI, prophase I; MII, metaphase II; 1C, 1-cell embryo; 2C, 2-cell embryo; 8C, 8-cell embryo; Blast, blastocyst embryo. (H) The relative amounts of each zinc transporter in the PI oocyte were analyzed. The data represent the mean ± SEM relative to the value obtained for ZnT1. Letters denote statistically significant differences between relative transcript abundance for each transporter (P < 0.01).
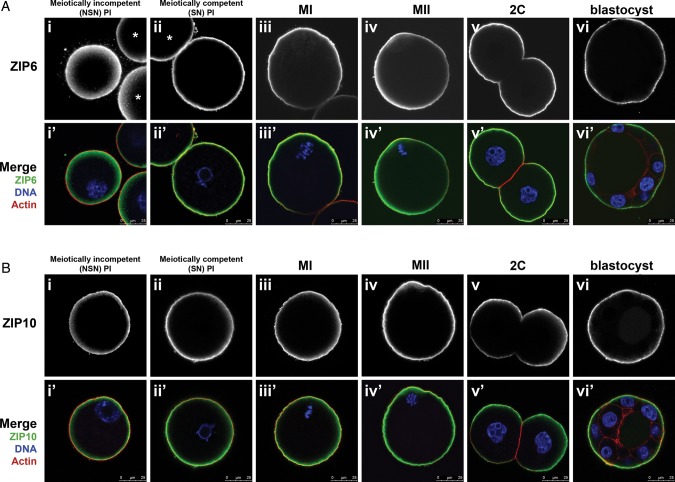
The maternal expression pattern, combined with the relative high abundance of Zip6 and Zip10 mRNA, suggested that these two zinc transporters may be key regulators of zinc accrual during meiotic maturation. To examine the role of these transporters during meiosis, we generated custom ZIP6- and ZIP10-specific antibodies. Immunocytochemistry of meiotically incompetent NSN oocytes, fully grown oocytes at different stages of meiosis, and preimplantation embryos revealed that both ZIP6 and ZIP10 localized to the cortex and overlapped with actin (Fig. 3). ZIP6 and ZIP10 localized at least in part to the cell surface, since non-permeabilized cells still displayed substantial cortical immunoreactivity (Supplementary data, Fig. S2Aii and Bii). However, this cortical staining was not uniform, indicating that antibody access to some fraction of the epitopes required membrane permeabilization. In the early embryo, ZIP6 and ZIP10 were excluded from regions of cell–cell apposition such that in blastocyst stage embryos, there was no ZIP6 or ZIP10 detectable in the inner mass cells (Fig. 3Av-vi, Bv-vi). Both of these antibodies were highly specific, as preimmune serum for ZIP6, peptide pre-absorption of the ZIP10 antibody, or omission of the primary antibody resulted in an absence of the immune-reactive signal (Supplementary data, Fig. S2Aiii–iv and Biii–iv). Thus, the cortical localization of ZIP6 and ZIP10 is consistent with a potential role of these transporters in regulating zinc accrual from the extracellular milieu during meiotic maturation.
Figure 3.
Temporal-spatial localization of ZIP6 and ZIP10 during meiotic maturation and preimplantation embryo development. (A) ZIP6 localization during meiotic maturation and early embryo development was determined by immunofluorescence using a custom synthesized antibody to ZIP6. Samples were collected at the indicated time points (NSN PI, SN PI, MI, MII, 2C, blastocyst) and processed for immunostaining. A minimum of 50 cells were examined in each group and representative images are shown. (B) ZIP10 localization was determined in a similar fashion as (A) with custom synthesis of an antibody to ZIP10. A minimum of 50 cells were examined in each group and representative images are shown. NSN, non-surrounded nucleolus; SN, surrounded nucleolus; PI, prophase I; MI, metaphase I; MII, metaphase II; 2C, 2-cell embryo. In merged images (Ai’-vi’, Bi’-iv’), ZIP6 (A) or ZIP10 (B) is green, F-actin is red and DNA is blue. Scale bars, 25 μm. At least 20 cells were visualized at each stage in three independent experiments. Asterisk represents an adjacent oocyte. See also Supplementary data, Fig. S2.
Disruption of Zip6 and Zip10 causes meiotic resumption in prophase I-arrested oocytes
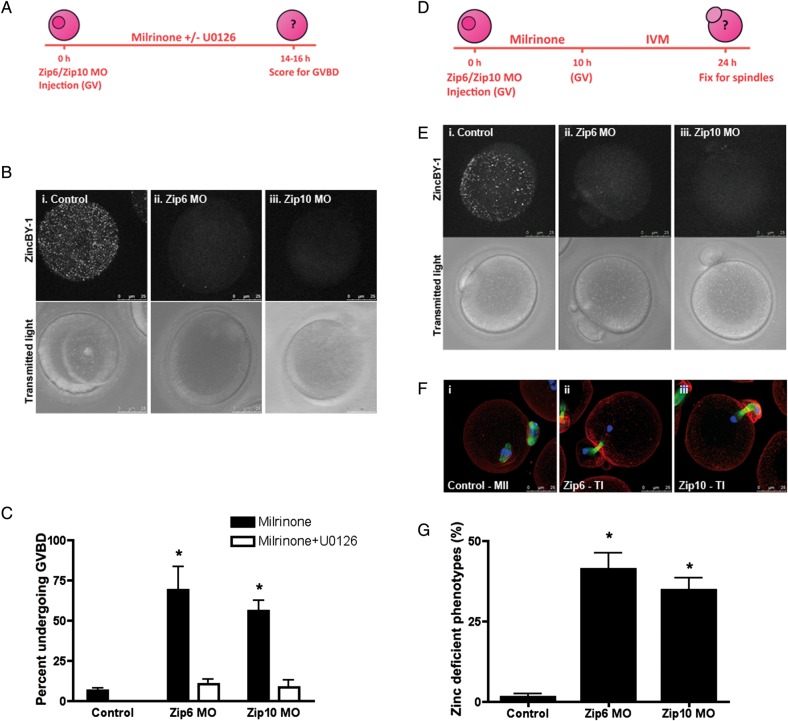
To determine if ZIP6 and ZIP10 are necessary for regulating zinc homeostasis during meiotic maturation, we functionally disrupted these transporters in oocytes using two independent approaches: microinjection of transporter-specific morpholinos and incubation with function-blocking antibodies. We hypothesized that if these methods were successful in perturbing intracellular zinc levels, then the oocytes should phenocopy the state of zinc insufficiency induced by zinc chelation with TPEN. These TPEN-induced phenotypes include the spontaneous resumption of meiosis in PI-arrested oocytes due to activation of the MOS-MAPK pathway (Kong et al., 2012) and premature meiotic arrest at telophase I during meiotic maturation (Kim et al., 2010; Bernhardt et al., 2011, 2012). To determine whether knockdown of the zinc transporters Zip6 and Zip10 could induce zinc insufficiency and cause the first of these phenotypes, i.e. meiotic resumption, we microinjected PI-arrested oocytes with Zip6 or Zip10 morpholinos (Fig. 4A). These morpholinos successfully disrupted zinc homeostasis, as the punctate fluorescence pattern detected in control oocytes with the labile zinc fluorophore ZincBY-1 (Que et al., manuscript under review) was ablated in morpholino-injected oocytes (Fig. 4B). These results suggest that disruption of zinc transporters indeed alters the labile zinc content of the cell.
Figure 4.
Disruption of Zip6 and Zip10 expression recapitulate phenotypes of TPEN-induced zinc insufficiency. (A) Oocytes were injected with 5 mM Zip6 or Zip10 morpholino (MO) and held in milrinone medium alone or milrinone medium supplemented with 10 μM U0126. Control oocytes were uninjected and held in milrinone medium. (B) Labile zinc distribution was visualized in injected oocytes by staining with 50 nM ZincBY-1. (C) At the end of culture, the percentage of oocytes undergoing germinal vesicle breakdown (GVBD) was calculated. Black bars represent oocytes held in milrinone, while white bars represent oocytes held in milrinone with U0126. (D–F) Oocytes were injected with 5 mM Zip6 or Zip10 MO, held for 10 h in milrinone medium and then transferred to in vitro maturation medium for 14 h before being imaged for labile zinc distribution (E) or fixed for immunostaining (F). (F) Spindle structure was interrogated by labeling oocytes with α-tubulin (green), actin (red) and chromatin (blue). Representative confocal images of MII and telophase I-like spindles are shown as Z-stack projections. (G) The percentage of oocytes arresting in a telophase I-like phenotype was calculated for control uninjected and Zip6 and Zip10 MO injected oocytes. Graphical data are presented as the mean ± SEM of three independent trials with at least 30 oocytes per trial. Statistical differences were calculated according to one-way ANOVA with Bonferroni post hoc test in comparison to the Control group (P < 0.001). Scale bar, 25 μm.
Phenotypically, >50% of Zip6 and Zip10 morpholino-injected PI-arrested oocytes spontaneously resumed meiosis as measured by the percent of cells that had undergone germinal vesicle breakdown (GVBD) 14–16 h post-injection (Fig. 4C). In comparison, only 6% of control oocytes resumed meiosis. To determine whether meiotic resumption induced by knockdown of Zip6 or Zip10 resulted from activation of the MOS-MAPK pathway, we cultured injected oocytes in medium containing the MEK1/2-specific inhibitor, U0126 (Favata et al., 1998). Indeed, U0126 rescued the ability of morpholino-injected oocytes to maintain meiotic arrest as demonstrated by rates of GVBD similar to control oocytes (Fig. 4C). These findings suggest that disruption of either Zip6 or Zip10 alters an essential zinc-dependent pathway in a manner that is analogous to treatment of the PI oocyte with the zinc chelator TPEN (Tian and Diaz, 2011; Kong et al., 2012).
Disruption of Zip6 or Zip10 induces a telophase I-like arrest during meiotic maturation
To determine if perturbation of ZIP6 and ZIP10 function could also impact zinc influx later on during meiotic maturation, we microinjected oocytes with Zip6 or Zip10 morpholinos and then allowed the cells to mature in vitro (Fig. 4D). Zinc insufficiency induced by TPEN during meiotic maturation prevents oocytes from reaching MII and instead causes arrest at a telophase I-like state with two separated chromatin masses (Kim et al., 2010; Bernhardt et al., 2012). Thus, to characterize the progression of meiosis in oocytes injected with Zip6 and Zip10 morpholinos, we examined their cytoskeletal morphology using immunocytochemistry. We found that 43 and 35% of the oocytes injected with Zip6 or Zip10 morpholinos, respectively, exhibited telophase I-like spindles compared with only 1% of control oocytes (Fig. 4F and G).
To further test whether these zinc transporters are required for proper meiotic progression, we disrupted ZIP6 and ZIP10 by maturing oocytes in the presence of specific antibodies designed to inhibit functional zinc transport. The ZIP6 and ZIP10 antibodies displayed a non-uniform cortical staining pattern in non-permeabilized oocytes, suggesting that these antibodies could likely block transporter function from the extracellular milieu (Supplementary data, Fig. S2Aii and Bii). Consistent with what was observed with morpholino injections, oocyte maturation in the presence of antibodies against either ZIP6 or ZIP10 resulted in a telophase I-like chromatin configuration in ∼60 and 90% of the oocytes, respectively (Fig. 5). In some oocytes, the chromatin masses were separated by a tubulin-enriched midbody, while in others, the midbody did not persist (Fig. 5Aii–iv). This phenotype was even more prominent when oocytes were matured in the presence of ZIP6 and ZIP10 antibodies together. In this case, 99% of the cells had decondensed chromatin masses (Fig. 5B). In contrast to oocytes matured in the presence of ZIP6 and ZIP10 antibodies, all of the control oocytes matured in an equivalent concentration of rabbit IgG progressed properly to MII, suggesting that the observed phenotype was due to specific disruption of ZIP6 and ZIP10 activity (Fig. 5B). Taken together, ZIP6- and ZIP10-specific morpholino injection or antibody incubation disrupted zinc homeostasis during meiotic maturation and phenocopied the state of zinc insufficiency induced by zinc chelation (Kim et al., 2010; Bernhardt et al., 2012). Based on these experiments, ZIP6 and ZIP10 regulate the zinc influx necessary for proper meiotic progression to MII.
Figure 5.
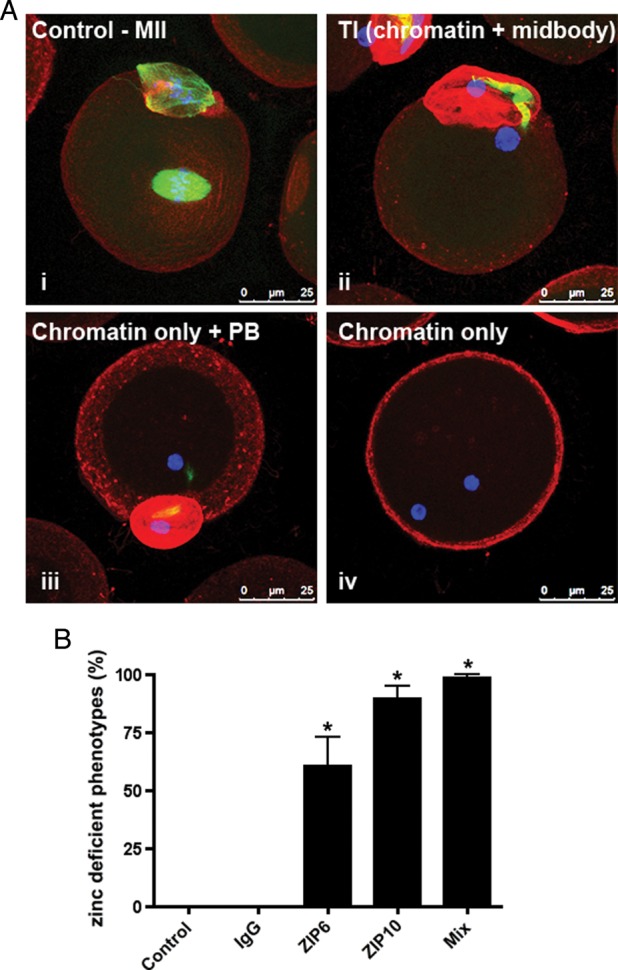
Neutralization of ZIP6 and ZIP10 function by antibody incubation during meiosis causes telophase I-like arrest. (A) Oocytes were cultured in in vitro maturation (IVM) medium with ZIP6 and/or ZIP10 antibody for 14 h. Base IVM medium and IVM medium with rabbit IgG antibody served as controls. At the end of culture, all samples were fixed and labeled for α-tubulin (green), F-actin (red) and chromatin (blue) to interrogate spindle morphology. Representative confocal images of control MII spindles (i) and telophase I-like spindles (two chromatin masses with midbody, ii; two chromatin masses without midbody, iii; two chromatin masses with cytokinesis failure, iv) are shown as Z-stack projections. Scale bars = 25 μm. (B) The percentage of oocytes arresting in a telophase I-like phenotype was calculated. Data are presented as the mean ± SEM of three independent trials with at least 30 oocytes per trial. Statistical differences were calculated according to one-way ANOVA with Bonferroni post hoc test in comparison to controls (P < 0.001).
Zinc-rch compartments and ZIP6 and ZIP10 expression are conserved in human oocytes
Although it is well-documented that zinc plays an essential role in regulating meiotic progression in the mouse oocyte, it is not known whether zinc and the key transporters identified in this study are conserved among mammalian species. To address this question, human PI oocytes were stained with two zinc-specific fluorophores that have structurally and spectroscopically different properties: ZincBY-1 (50 nM) and FluoZin-3-AM (10 µM) (Gee et al., 2002). This staining revealed punctate zinc localization enriched in the cortex of the human oocyte (Fig. 6A), similar to what has been previously described in the mouse oocyte (Kim et al., 2011). Moreover, both zinc transporters ZIP6 and ZIP10 are also present in the human oocyte and their cortical localization is conserved (Fig. 6B). ZIP10 appears to stain more uniformly throughout the cortex compared with ZIP6, which has a more punctate distribution. Taken together, these results illustrate that mechanisms for the regulation of zinc homeostasis in the egg are likely conserved and may contribute to the progression of human oocyte meiosis in a manner similar to that characterized in mouse oocytes.
Figure 6.
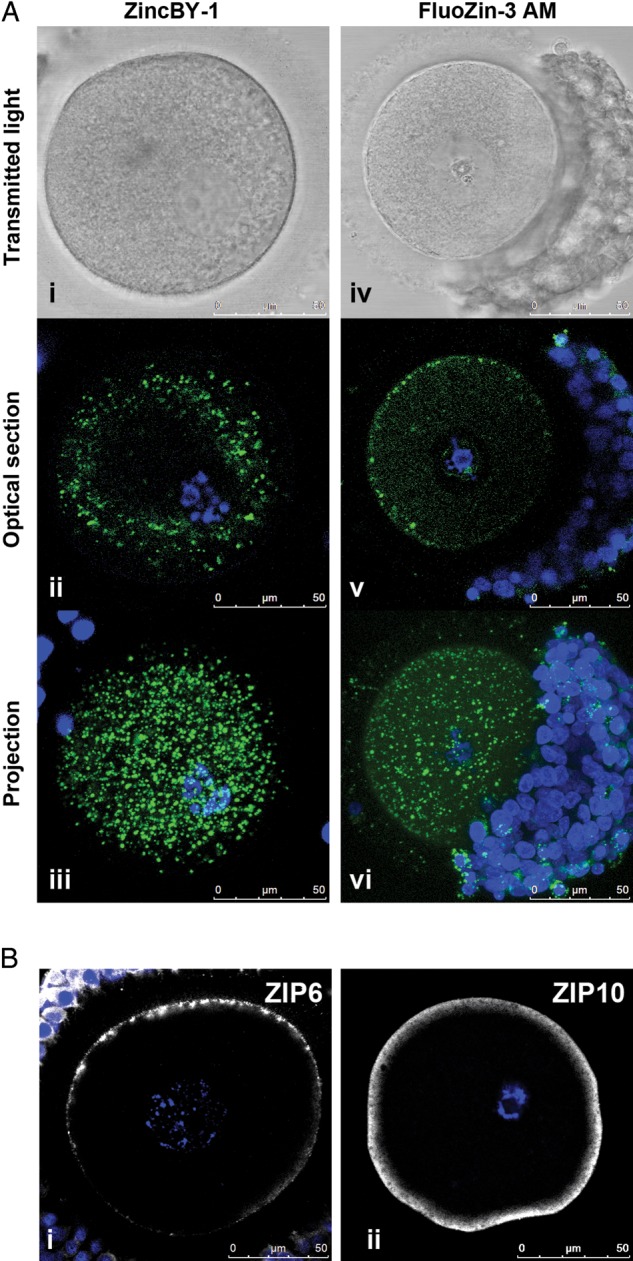
Labile zinc, ZIP6 and ZIP10 are conserved in the human oocyte. (A) Labile zinc was visualized in human oocytes using two zinc-specific fluorophores: ZincBY-1 (left) and FluoZin-3 AM (right). For each oocyte, a transmitted light image, an optical section and a projection are shown. (B) ZIP6 and ZIP10 were visualized using specific antibodies. A single optical section of each oocyte is shown. A total of 13 human oocytes from 6 individuals were used to perform these experiments as described in Table II. Representative images are shown. Labile zinc = green, DNA = blue, and zinc transporters = grayscale. Scale bars, 50 mm.
Discussion
In this study, we demonstrate that the mammalian oocyte does not exploit the standard mechanisms of zinc homeostatic regulation present in transcriptionally active cells. We instead demonstrate that large fluxes in intracellular zinc, which are required for the oocyte-to-egg transition, are controlled by newly described maternally derived zinc transporters. Specifically, we identified Zip6 and Zip10 as two of the most abundantly expressed transporters in the oocyte during the window of meiotic maturation. Disruption of these transporters by morpholino injection and incubation in function-blocking antibodies perturbed intracellular zinc and recapitulated all the phenotypes observed during zinc insufficiency induced by TPEN chelation. These results demonstrate the necessity of ZIP6 and ZIP10 in mediating the zinc-dependent events of meiosis.
Further, our studies suggest that these mechanisms are likely conserved in the human oocyte, as the distribution of ZIP6 and ZIP10 mirrored what was detected in mouse oocytes. This is consistent with another study demonstrating that the mRNAs encoding these transporters are highly expressed in the human oocyte (Ménézo et al., 2011). This result, in conjunction with the identification of zinc vesicular compartments in the human oocyte, suggests the conservation of a cytoplasmic control pathway that relies on transition metals. The study of oocyte physiology from the perspective of zinc not only expands our understanding of human gamete biology, but may also provide a novel marker for cytoplasmic maturation.
The discovery of dramatic zinc fluxes in total zinc content throughout oocyte development demonstrates the importance of zinc physiology in driving meiotic progression. However, the mechanism by which the oocyte acquires and regulates this zinc content was unknown until our studies were conducted. Transcriptionally active cells have been shown to respond quickly and effectively to zinc homeostasis perturbations by altering gene expression through the zinc sensing metalloregulatory protein MTF-1 (Radtke et al., 1993; Heuchel et al., 1994). When bound to zinc, MTF-1 associates with MRE elements in a variety of promoters, including those for MT1-4 and ZIP10 (Heuchel et al., 1994; Langmade et al., 2000; Andrews, 2001; Lichten et al., 2011). MTF-1 binding to MREs leads to transactivation at metallothionein promoters. However MTF-1 binding to the MREs in the ZIP10 promoter leads to transcriptional repression (Lichten et al., 2011). Ultimately, these changes in gene expression promote zinc scavenging by metallothioneins and reduce transporter-mediated zinc uptake.
During meiosis, the oocyte exceeds baseline zinc levels and accrues an additional 50% beyond the resting cell (mitotic cell) threshold in order to complete meiotic maturation (Kim et al. 2010). Although such dramatic fluxes in zinc ion concentration induce apoptosis in other cell types (Koh et al., 1996; Choi and Koh, 1998; Wätjen et al., 2002), the oocyte appears to not only tolerate such a wide range of intracellular zinc levels, but requires these rapid changes for meiotic progression. Hence, the utility of metal flux as a developmental signal may require active repression of the standard homeostatic mechanisms that regulate zinc transport. Indeed, a unique feature of mammalian oocytes is that transcription ceases prior to oocyte maturation and does not resume until after fertilization (Bachvarova, 1985). This transcriptional quiescence makes the fully grown oocyte uniquely poised to acquire the large zinc bolus. We show here that although MTF-1's transcriptionally-based mechanisms of zinc regulation are functional and active in growing oocytes, they are suppressed in the fully grown oocyte. These results are consistent with previous findings that MT genes were neither metal responsive nor constitutively expressed at high levels in mouse oocytes (Andrews et al., 1991).
We also demonstrate in the fully grown oocyte that zinc transporters function as key players in the mechanism of zinc accrual in the oocyte. In an initial screen of zinc transporter expression in the oocyte, we found that Zip6 and Zip10 were two of the most abundant transporters. Both of these transcripts had a maternal pattern of gene expression. These findings suggest that Zip6 and Zip10 may have specific functions in oogenesis, oocyte maturation, fertilization and/or the very early stages of preimplantation development. Interestingly, others have shown that Zip6 and Zip10 expression is significantly higher in the oocyte relative to the cumulus cells in both mouse and human samples, again supporting the notion that these transporters are specifically involved in zinc accumulation in the oocyte (Ménézo et al., 2011; Lisle et al., 2013). At the protein level, ZIP6 and ZIP10 localized to the cortex throughout maturation and early embryo development. Consistent with these findings are reports that describe the localization of these two transporters to the plasma membrane in other tissue types (Taylor and Nicholson, 2003; Pawan et al., 2007; Ryu et al., 2008; Croxford et al., 2011; Lichten et al., 2011). These independent studies showing localization to the plasma membrane corroborate our findings and support the idea that ZIP6 and ZIP10 directly mediate the acquisition of the large bolus of zinc from the extracellular environment during meiotic maturation.
Intriguingly, ZIP6 and ZIP10 are cortical in the oocyte, egg and early embryo, and they are excluded in regions of cell–cell apposition starting at the 2-cell embryo stage. Several maternal effect genes that comprise the subcortical maternal complex (SCMC) including FLOPED (gene name Ooep), MATER (gene name Nlrp5) and TLE6 display similar expression and localization (Li et al., 2010). Transcripts of these genes accumulate during oogenesis and become virtually undetectable in the early embryo, while the proteins persist on the apical cortex of outer cells through the blastocyst stage and are excluded from regions of cell–cell contact (Tong et al., 2000; Li et al., 2008). Maternal effect genes supply the crucial store of proteins that maintain the livelihood of the embryo during early development in the hiatus between maternal and zygotic transcription. The interaction of the proteins comprising the SCMC is important for early embryonic development, as ablation of any of the aforementioned maternal effect genes disrupts the formation of the SCMC and consequently causes developmental arrest at the 2-cell stage. Indeed, a recent study identified MATER as an important regulator of calcium transients at the time of fertilization likely via its lattice-mediated role in proper positioning of the endoplasmic reticulum, the source of calcium stores (Kim et al., 2014). Thus, the similarity in expression pattern between these known regulators of early development and the zinc transporters strongly corroborates our conclusion that zinc acquisition machinery has significant regulatory roles during this early developmental period. Moreover, the finding that zinc and calcium homeostatic mechanisms have similar spatial distributions within the cell is particularly intriguing given the close temporal association between intracellular calcium transients and extracellular zinc sparks that occur during egg activation (Kim et al., 2011).
Several papers in the last 3 years have described roles for zinc regulation in the maintenance of prophase I arrest, the progression to metaphase II arrest and the exit from MII during egg activation (Kim et al., 2010; Suzuki et al., 2010b; Tian and Diaz, 2011; Bernhardt et al., 2012; Kong et al., 2012). In all of these reports, pharmacological modulation of intracellular and extracellular zinc content by the heavy metal chelator TPEN has been utilized as a model of acute zinc insufficiency. In this manuscript, we establish the physiological mechanism for the regulation of zinc uptake within the oocyte by assaying for the function of the zinc transporters ZIP6 and ZIP10. We demonstrate that disruption of these transporters by morpholino injection or function-blocking antibodies results in striking similarities to phenotypes induced by TPEN-induced zinc insufficiency, including spontaneous resumption of meiosis from the PI arrest and premature arrest at a telophase I-like state. Further, the partially penetrate phenotype resultant from each morpholino and antibody individually supports the notion that a significant amount of redundancy exists within the zinc transporters themselves, a reflection of the evolutionary importance of zinc homeostasis within biology. These results suggest that proper regulation of the two meiotic arrest points require extraordinary transport of zinc into the cell. Thus, we present a model in which these zinc transporters facilitate the influx of zinc necessary for the oocyte to reach its key developmental milestone during meiotic maturation: the transition to a mature and fertilization-competent egg. Together this body of work presents a paradigm-shifting role for tightly controlled temporal fluctuations in zinc utilization during meiotic maturation.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors' roles
All authors contributed substantially to this manuscript. B.Y.K. and F.E.D. designed and executed all experiments, analyzed the data, and wrote the manuscript. E.L.Q. synthesized the fluorescent dye used to stain labile zinc. A.M.K, T.V.O. and, T.K.W. contributed intellectually and provided critical input on data interpretation and manuscript preparation. All authors reviewed, revised and approved the final manuscript.
Funding
This work was supported by the National Institutes of Health (P01 HD021921, U54 HD076188) and a Medical Research Award from the W. M. Keck Foundation. B.Y.K. was a fellow of the American Association of University Women.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We would like to acknowledge the Woodruff laboratory in particular Miranda Bernhardt for many insightful discussions. We also acknowledge the members of the Northwestern Center for Reproductive Science and Chemistry of Life Processes Institute for integral and informative discussions at the intersection of biology and chemistry. We acknowledge the members of the National Physicians Cooperative and Oncofertility Consortium for facilitating research with human gametes. We also thank the research study participants who have generously provided tissue for research to further our understanding of human gamete quality.
References
- Andrews GK. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals. 2001;14:223–237. doi: 10.1023/a:1012932712483. [DOI] [PubMed] [Google Scholar]
- Andrews GK, Huet-Hudson YM, Paria BC, McMaster MT, De SK, Dey SK. Metallothionein gene expression and metal regulation during preimplantation mouse embryo development (MT mRNA during early development) Dev Biol. 1991;145:13–27. doi: 10.1016/0012-1606(91)90209-l. [DOI] [PubMed] [Google Scholar]
- Bachvarova R. Gene expression during oogenesis and oocyte development in mammals. Dev Biol (NY 1985) 1985;1:453–524. doi: 10.1007/978-1-4615-6814-8_11. [DOI] [PubMed] [Google Scholar]
- Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Zinc requirement during meiosis I-meiosis II transition in mouse oocytes is independent of the MOS-MAPK pathway. Biol Reprod. 2011;84:526–536. doi: 10.1095/biolreprod.110.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Kong BY, Kim AM, O'Halloran TV, Woodruff TK. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod. 2012;86:1–10. doi: 10.1095/biolreprod.111.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szollosi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod. 1999;60:580–587. doi: 10.1095/biolreprod60.3.580. [DOI] [PubMed] [Google Scholar]
- Bozym RA, Thompson RB, Stoddard AK, Fierke CA. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem Biol. 2006;1:103–111. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- Croxford TP, McCormick NH, Kelleher SL. Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J Nutr. 2011;141:359–365. doi: 10.3945/jn.110.131318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton T, Fu K, Palmiter RD, Andrews GK. Transgenic mice that overexpress metallothionein-I resist dietary zinc deficiency. J Nutr. 1996;126:825–833. doi: 10.1093/jn/126.4.825. [DOI] [PubMed] [Google Scholar]
- Dean KM, Qin Y, Palmer AE. Visualizing metal ions in cells: an overview of analytical techniques, approaches, and probes. Biochim Biophys Acta. 2012;1823:1406–1415. doi: 10.1016/j.bbamcr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debey P, Szollosi MS, Szollosi D, Vautier D, Girousse A, Besombes D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol Reprod Dev. 1993;36:59–74. doi: 10.1002/mrd.1080360110. [DOI] [PubMed] [Google Scholar]
- Dodson G, Steiner D. The role of assembly in insulin's biosynthesis. Curr Opin Struct Biol. 1998;8:189–194. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Finney LA, O'Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J Am Chem Soc. 2002;124:776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- Haase H, Ober-Blobaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. Embo J. 1994;13:2870–2875. doi: 10.1002/j.1460-2075.1994.tb06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol. 2008;97:149–176. doi: 10.1016/S0065-2776(08)00003-5. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- Kabu K, Yamasaki S, Kamimura D, Ito Y, Hasegawa A, Sato E, Kitamura H, Nishida K, Hirano T. Zinc is required for Fc epsilon RI-mediated mast cell activation. J Immunol. 2006;177:1296–1305. doi: 10.4049/jimmunol.177.2.1296. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kim YH, Kim S, Kim JW, Koh JY, Oh SH, Lee MK, Kim KW, Lee MS. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes. 2000;49:367–372. doi: 10.2337/diabetes.49.3.367. [DOI] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O'Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Zhang X, Kan R, Cohen R, Mukai C, Travis AJ, Coonrod SA. The role of MATER in endoplasmic reticulum distribution and calcium homeostasis in mouse oocytes. Dev Biol. 2014;386:331–339. doi: 10.1016/j.ydbio.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kong BY, Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Zinc maintains prophase I arrest in mouse oocytes through regulation of the MOS-MAPK pathway. Biol Reprod. 2012;87:11. doi: 10.1095/biolreprod.112.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem. 2000;275:34803–9. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- Li Y, Hough CJ, Suh SW, Sarvey JM, Frederickson CJ. Rapid translocation of Zn(2+) from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J Neurophysiol. 2001;86:2597–2604. doi: 10.1152/jn.2001.86.5.2597. [DOI] [PubMed] [Google Scholar]
- Li L, Baibakov B, Dean J. A subcortical maternal complex essential for preimplantation mouse embryogenesis. Dev Cell. 2008;15:416–425. doi: 10.1016/j.devcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137:859–870. doi: 10.1242/dev.039487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- Lichten LA, Ryu M-S, Guo L, Embury J, Cousins RJ. MTF-1-mediated repression of the zinc transporter Zip10 is alleviated by zinc restriction. PLoS One. 2011;6:e21526. doi: 10.1371/journal.pone.0021526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisle RS, Anthony K, Randall MW, Diaz F. Oocyte-cumulus cell interactions regulate free intracellular zinc in mouse oocytes. Reproduction. 2013;145:381–390. doi: 10.1530/REP-12-0338. [DOI] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol. 2006;174:791–801. doi: 10.1083/jcb.200604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev S, Pan H, Schultz RM. Absence of MSY2 in mouse oocytes perturbs oocyte growth and maturation, RNA stability, and the transcriptome. Biol Reprod. 2011;85:575–583. doi: 10.1095/biolreprod.111.091710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménézo Y, Pluntz L, Chouteau J, Gurgan T, Demirol A, Dalleac A, Benkhalifa M. Zinc concentrations in serum and follicular fluid during ovarian stimulation and expression of Zn(2+) transporters in human oocytes and cumulus cells. Reprod Biomed Online. 2011;22:647–652. doi: 10.1016/j.rbmo.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Miyara F, Migne C, Dumont-Hassan M, Le Meur A, Cohen-Bacrie P, Aubriot FX, Glissant A, Nathan C, Douard S, Stanovici A, et al. Chromatin configuration and transcriptional control in human and mouse oocytes. Mol Reprod Dev. 2003;64:458–470. doi: 10.1002/mrd.10233. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Malavolta M. Zinc signalling and subcellular distribution: emerging targets in type 2 diabetes. Trends Mol Med. 2008;14:419–428. doi: 10.1016/j.molmed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Nishida K, Hasegawa A, Nakae S, Oboki K, Saito H, Yamasaki S, Hirano T. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J Exp Med. 2009;206:1351–1364. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran TV. Transition metals in control of gene expression. Science. 1993;261:715–725. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. The elusive function of metallothioneins. Proc Natl Acad Sci USA. 1998;95:8428–8430. doi: 10.1073/pnas.95.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc Natl Acad Sci USA. 2004;101:4918–4923. doi: 10.1073/pnas.0401022101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawan K, Neeraj S, Sandeep K, Kantaratho R, Rajendra P. Upregulation of Slc39a10 gene expression in response to thyroid hormones in intestine and kidney. Biochim Biophys Acta. 2007;1769:117–123. doi: 10.1016/j.bbaexp.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci USA. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Heuchel R, Georgiev O, Hergersberg M, Gariglio M, Dembic Z, Schaffner W. Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J. 1993;12:1355–1362. doi: 10.1002/j.1460-2075.1993.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu M-S, Lichten LA, Liuzzi JP, Cousins RJ. Zinc transporters ZnT1 (Slc30a1), Zip8 (Slc39a8), and Zip10 (Slc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J Nutr. 2008;138:2076–2083. doi: 10.3945/jn.108.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhy DA, Simon KD, Linzer DI, O'Halloran TV. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J Biol Chem. 1999;274:9183–9192. doi: 10.1074/jbc.274.14.9183. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Suzuki E, Yoshida N, Kubo A, Li H, Okuda E, Amanai M, Perry ACF. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development. 2010a;137:3281–3291. doi: 10.1242/dev.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry ACF. Full-term mouse development by abolishing Zn2+ dependent metaphase II arrest without Ca2+ release. Development. 2010b;137:2659–2669. doi: 10.1242/dev.049791. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim Biophys Acta. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology. 2011;153:873–886. doi: 10.1210/en.2011-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet. 2000;26:267–268. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, Nagano T, Matsuki N, Ikegaya Y. Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits. J Cell Biol. 2002;158:215–220. doi: 10.1083/jcb.200204066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Wätjen W, Haase H, Biagioli M, Beyersmann D. Induction of apoptosis in mammalian cells by cadmium and zinc. Environ Health Perspect. 2002;110(Suppl 5):865–867. doi: 10.1289/ehp.110-1241262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, et al. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol. 2004;272:483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Piccinelli A, Giorgi Rossi P, Garagna S, Redi CA. Chromatin organization during mouse oocyte growth. Mol Reprod Dev. 1995;41:479–485. doi: 10.1002/mrd.1080410410. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Ponce RH, Boiani M, Guizzardi S, Govoni P, Scandroglio R, Garagna S, Redi CA. The analysis of chromatin organisation allows selection of mouse antral oocytes competent for development to blastocyst. Zygote. 2002;10:73–78. doi: 10.1017/s0967199402002101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.