Abstract
Sperm capacitation involves an increase in intracellular Ca2+ concentration as well as in protein kinase A (PKA)-dependent protein tyrosine (Tyr) phosphorylation. Interestingly, in humans, a decrease in extracellular Ca2+ concentration ([Ca2+]e) during capacitation induces an increase in Tyr phosphorylation indicating the complexity of Ca2+ signaling during this process. In view of this, in the present study we further investigated the Ca2+-mediated signaling pathways implicated in Tyr phosphorylation during human sperm capacitation. Results revealed that sperm incubation in a medium without added Ca2+ (⊖ Ca2+) increased Tyr phosphorylation but did not modify PKA-mediated phosphorylation. Moreover, inhibition of either PKA or Src family kinase signaling cascades in ⊖ Ca2+ down-regulated both PKA substrate and Tyr phosphorylations, indicating that the [Ca2+]e effects on Tyr phosphorylation depend on PKA targets. Inhibition of calmodulin or Ser/Thr protein phosphatase 2B also increased Tyr phosphorylation without affecting PKA-mediated phosphorylation, supporting the potential role of these Ca2+ downstream effectors in the increase in Tyr phosphorylation observed in ⊖ Ca2+. Experiments aimed to identify the kinase responsible for these observations revealed the presence of proline-rich tyrosine kinase 2 (PYK2), a focal adhesion kinase (FAK) family member, in human sperm, and the use of PF431396, an FAK inhibitor, supported the involvement of PYK2 in Tyr phosphorylation downstream of PKA activation. Results also showed that PYK2 was activated in ⊖ Ca2+ as well as during capacitation and that PF431396 affected capacitated sperm motility, acrosome reaction and ability to penetrate both mouse cumulus matrix and zona-free hamster eggs. Together, our observations support PYK2 as an intermediary component of Ca2+ signaling between PKA-mediated and Tyr phosphorylations that is required for achieving functional human sperm capacitation.
Keywords: calcium, capacitation, human, sperm, tyrosine phosphorylation
Introduction
Sperm are highly specialized cells with only one commitment, that is to reach and fertilize the egg. To accomplish this, mammalian ejaculated sperm require first to undergo a process called capacitation which occurs during their transit through the female reproductive tract towards the site of fertilization. The capacitation process encompasses structural and functional changes in sperm that confer the ability to undergo both the acrosome reaction (AR) and hyperactivated motility, key events for penetration of the egg investments (i.e. Cumulus oophorus and zona pellucida) and gamete fusion (Chang, 1951; Austin, 1952). The bulk of evidence shows that Ca2+ signaling plays a major role in all these important events (Yanagimachi, 1994; Darszon et al., 2011). During capacitation, there is an increase in intracellular Ca2+ concentration ([Ca2+]i) (Ruknudin and Silver, 1990; Baldi et al., 1991) which is attained and decoded by a complex set of molecules present in mature sperm (Jimenez-Gonzalez et al., 2006; Publicover et al., 2007). Whereas Ca2+ influx is achieved via voltage-, stored-operated and ligand-gated channels (i.e. VOCCs, SOCs and CatSper), its clearance relies on ATP-driven Ca2+ pumps (i.e. plasma membrane Ca2+ ATPases) and Na+/Ca2+ exchangers. Intracellular Ca2+ is mobilized to or from internal stores present in the head (acrosome) as well as in the neck (redundant nuclear envelope), likely through inositol triphosphate and ryanodine receptors and the sarco/endoplasmic reticulum Ca2+-ATPase (Darszon et al., 2011). In addition, [Ca2+]i is sensed by calmodulin (CaM), an ubiquitous small protein which regulates a variety of Ca2+/CaM-dependent sperm proteins such as kinases (i.e. CaM kinases (CaMKs) and a Src family kinase (SFK) member (c-yes)), phosphodiesterases (PDE1) and phosphatases (i.e. protein phosphatase 2B (PP2B)) (Wasco and Orr, 1984; Tash et al., 1988; Carrera et al., 1996; Leclerc and Goupil, 2002; Marin-Briggiler et al., 2005). Interestingly, other proteins, such as the testis-specific soluble adenylyl cyclase (Adcy10, also known as sAC) (Chen et al., 2000), are Ca2+-dependent but CaM-independent (Jaiswal and Conti, 2003; Litvin et al., 2003), suggesting that Ca2+ affects capacitation via multiple pathways.
The molecular mechanisms involved in the Ca2+-mediated signaling underlying capacitation are known to some extent. Upon entry of both Ca2+ and into sperm, the levels of cAMP increase by the opposing activities of sAC and PDE, and activate a protein kinase A (PKA)-dependent phosphorylation of proteins in serine/threonine (Ser/Thr) residues (Visconti et al., 2011). In this regard, recent studies in mouse and humans revealed that PKA-dependent phosphorylation is regulated not only by the activation of the cAMP/PKA pathway but also by the SFK inactivation of Ser/Thr phosphatases (Krapf et al., 2010; Battistone et al., 2013). Phosphorylation of PKA substrates finally leads to protein phosphorylation in tyrosine (Tyr) residues in all the mammalian species studied so far (Visconti et al., 1995, 1999; Leclerc et al., 1996; Galantino-Homer et al., 1997; Osheroff et al., 1999; Da Ros et al., 2004). However, how PKA activation results in the increase in Tyr phosphorylation is still not well understood. Interestingly, in human sperm, a decrease in extracellular Ca2+ concentration ([Ca2+]e) during in vitro capacitation induces an increase in Tyr phosphorylation (Carrera et al., 1996; Luconi et al., 1996; Leclerc and Goupil, 2002; Marin-Briggiler et al., 2003; Baker et al., 2004), indicating the complexity of Ca2+ signaling during this process.
In view of this, in the present study we further explored the signaling pathways implicated in both PKA-dependent and Tyr phosphorylations in human sperm with the aim of identifying the Ca2+ signaling molecules involved in Tyr phosphorylation during capacitation. Our results support a role for proline-rich tyrosine kinase 2 (PYK2), a member of the focal adhesion kinase (FAK) family, as a Ca2+ signaling component implicated in Tyr phosphorylation but not in PKA substrate phosphorylation and required for achieving functional human sperm capacitation.
Materials and Methods
Materials
The following chemicals (catalog number in brackets) were used: bovine serum albumin (BSA) (A7906), cyclosporin A (CsA) (C-3662), igepal CA-630 (I-3021), ethylene glycol-bis(2-aminoethylether)-N,N,N,N’ tetraacetic acid (EGTA) (E-3889), N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (dbcAMP) (D-0627), 3-isobutyl-1-methylxanthine (IBMX) (I-5879), N-[2-[[[2-[(2,3-dihydro-2-oxo-1H-indol-5-yl)amino]-5-(trifluoromethyl)-4-pyrimidinyl]amino]methyl]phenyl]-N-methyl-methanesulfonamide hydrate (PF431396) (PZ0185), Pisum sativum agglutinin coupled to fluorescein isothiocyanate (PSA-FITC) (L0770), anti-β-tubulin antibodies (clone D66) and hCG (C610) from Sigma (St. Louis, MO, USA). N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W7) (681629), N-(6-aminohexyl)-1-naphthalenesulfonamide (W5) (681625) and N-[2-((p-bromocinnamyl)amino)ethyl]-5-isoquinolinesulfonamide (H89) (10010556) from Calbiochem (Billerica, MA, USA). 4-(2,4-dichloro-5-methoxyphenylamino)-6-methoxy-7-(3-(4-methylpiperazin-1yl)propoxy) quinoline-3-carbonitrile (SKI606) (S1014) from Selleck Chemical (Houston, TX, USA). 9,10-deepithio-9,10-didehydroacanthifolicin (okadaic acid, OA) (495604) from Cayman Chemical Company (Cayman, MI, USA). Equine chorionic gonadotrophin (eCG) (NV4811) from Syntex (Buenos Aires, Argentina). Anti-phosphotyrosine antibody (clone4G10) from Millipore (Billerica, MA, USA), anti-phospho-PKA substrates (clone100G7E), anti-PYK2 (3292s), anti-FAK (3285), anti-phospho-PYK2 (3291s) antibodies from Cell Signaling (Danvers, MA, USA) and anti-FAK (C-20, sc-558) antibody from Santa Cruz Biotechnology (Dallas, TX, USA).
Culture media
The modified Biggers, Whitten and Whittingham medium (BWW; Biggers et al., 1971) used in this study contained (in mM): NaHCO3 25, NaCl 94.5, KCl 4.8, CaCl2 1.7, KH2PO4 1.17, MgCl2 1.22, Na-Pyruvate 0.3, Na-lactate 25.7, glucose 5.5 and HEPES 10 supplemented with BSA (2.6% p/v). This medium has been proved to successfully support human sperm capacitation (Battistone et al., 2013). Because medium without Ca2+ still contains micromolar concentrations of the ion, it is considered a nominal zero Ca2+ medium, indicated here by ⊖ Ca2+. All media were adjusted to pH 7.4.
Ethical approval
Approval for the study protocol was obtained from the Bioethics Committee of the Institute of Biology and Experimental Medicine from the National Research Council (CONICET, Buenos Aires, Argentina), and the Institutional Review Board of the University of Massachusetts (Amherst, MA, USA). All human donors were provided with written information about the study prior to giving informed consent. Experiments involving animals were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health.
Sperm preparation and capacitation
All procedures involving human semen samples were conducted according to the World Health Organization recommendations (WHO, 2010). Ejaculated human semen were obtained by masturbation from 15 healthy donors (aged 20–35 years) with no known fertility problems, collected into a sterile plastic container and transported immediately to the laboratory. After liquefaction, motile sperm were recovered by standard swim-up technique in medium without , diluted to a final concentration of 1×107cells/ml and incubated for different periods (1–18 h) at 37°C as previously described (Battistone et al., 2013). Alternatively, incubation was carried out in media containing the following compounds: a CaM antagonist, W7 (0–100 µM); a PP2B inhibitor, CsA (0–20 µM); a cAMP analog, dbcAMP (5 mM) and an inhibitor of PDE, IBMX (0.2 mM); a PKA inhibitor, H89 (30 µM); a SFK inhibitor, SKI606 (30 µM); a Ser/Thr phosphatase inhibitor, OA (100 nM) or an FAK family inhibitor, PF431396 (0–30 µM). At the end of the incubation, aliquots were recovered and sperm were analyzed for the following end-points: (i) protein phosphorylation (both PKA substrate and Tyr phosphorylations), (ii) functional parameters (motility, hyperactivation and AR) and (iii) fertilization competence (ability to penetrate mouse cumulus-oocyte complex (COCs) and zona-free hamster oocytes) as described below.
Sperm viability was assessed by dye exclusion using 0.5% (v/v) eosin Y (Sigma) and the percentage of viable sperm was calculated as the number of sperm that did not incorporate the dye over the total number of sperm counted under the light microscope (×400).
Protein extracts
Sperm (1–2 × 106) were washed and proteins were solubilized in Laemmli sample buffer (Laemmli, 1970) and heated at 100°C for 5 min. For immunoprecipitation experiments, sperm (1 × 107) were lysed in a buffer containing (in mM) Tris–HCl 50 pH 7.5, EDTA 1, EGTA 1, NaCl 150, MgCl2 1 and glycerol 10% (v/v), Triton X-100 1% (v/v), then centrifuged at 13 400g for10 min, and the soluble and insoluble fractions were incubated with Laemmli sample buffer and boiled at 100°C for 5 min. As PYK2 was insoluble after this treatment, sperm were lysed in the same buffer but containing Igepal 1% (v/v) and sodium dodecyl sulfate (SDS) 0.1% (w/v) instead of Triton X-100, incubated at 100°C for 5 min and then subjected to the same procedures, as described above.
Western blotting analysis
The phosphorylation status of proteins was assessed by SDS–PAGE (7.5% polyacrylamide) and western blotting as previously described (Battistone et al., 2013) using anti-phospho-PKA substrate (1:1000) or anti-phosphoTyr (1:3000) antibodies. The specificity of these antibodies has been shown in previous work. For the detection of FAK family proteins, the following antibodies were used: anti-FAK (1:1000), anti-PYK2 (1:200) or anti-phospho-PYK2 (1:1000) antibodies. The membranes blotted with anti-PYK2 were then stripped and reprobed with anti-phospho-PYK2 (Tyr 402) or vice versa. Protein loading was analyzed by β-tubulin immunoblot (anti-β-tubulin antibody: α-tub; 1:5000). In all the cases, the reactive bands were detected by enhanced chemiluminescence (GE Healthcare, Piscataway, NJ, USA) and images captured with G:BOX GENI (Syngene, Synoptics, Ltd, Cambridge, England) according to the manufacturer's instructions. A representative blot from each set of experiments is shown.
Immunoprecipitation
Solubilized proteins were incubated with anti-PYK2 antibodies (2 μg) with constant agitation for 18 h at 4°C, and subsequently incubated with 20 µl of protein A-Agarose (Santa Cruz Biotechnology). The proteins bound to the agarose–antibody complexes were recovered by centrifugation at 13 400g for 10 min, washed three times and eluted with Laemmli sample buffer, boiled at 100°C for 5 min and subjected to SDS–PAGE and western blotting as described above.
Computer-assisted sperm analysis
Aliquots of sperm incubated under different conditions for 18 h were placed in a pre-warmed 20 µm chamber (Leja Slide, Spectrum Technologies, Healdsburg, CA, USA) and examined using the CEROS computer-assisted sperm analysis (CASA) system (Hamilton Thorne, Inc., Beverly, MA, USA) (Mortimer et al., 1998) at 37°C. For each sample, a minimum of 200 cells distributed in at least 20 different microscopy fields were scored (30 frames acquired at 60 Hz for each measurement). The following parameters were measured: average path velocity (VAP, µm/s), curvilinear velocity (VCL, µm/s), straight line velocity (VSL, µm/s), linearity (LIN, %), amplitude of lateral head displacement (ALH, µm) and straightness (STR, %) as previously described (Battistone at al., 2013). Sperm were considered progressively motile when presenting VAP > 25 and STR > 80%, and hyperactivated when exhibiting VCL ≥ 100, LIN < 60% and ALH ≥ 5 (Mortimer and Mortimer, 1990). A minimum of four independent experiments per treatment was carried out, using sperm from different donors each time.
AR assays
Sperm were incubated under different conditions for 18 h and then evaluated for both spontaneous and induced AR. For AR induction, sperm were exposed to 25 µM progesterone during the last 30 min of the incubation. Acrosomal integrity was evaluated as previously described (Battistone et al., 2013) using the acrosomal content marker PSA coupled to FITC. PSA labels acrosome-intact sperm and it is absent in acrosome-reacted sperm, as described elsewhere (Cross, 1996). Two hundred spermatozoa were evaluated in each treatment slide under a Nikon Optiphot microscope equipped with epifluorescence optics (×1250), and the percentage of spermatozoa without an intact acrosome was scored.
Cumulus penetration analysis
Adult (30–60 days old) female B6129F2 mice were maintained with food and water ad libitum in a temperature controlled room with light:dark (12:12) cycle. Females were superovulated by an i.p. injection of 5 IU of eCG, followed by i.p. administration of 5 IU of hCG 48 h later. The COCs recovered from the oviducts 14–16 h after hCG administration were washed in BWW and distributed in groups of 10–15 per treatment. An aliquot of human sperm incubated for 18 h in BWW either alone or containing PF431396 (10 or 30 µM) or in ⊖ Ca2+ was incubated with Hoechst 33342 (3 µg/ml final concentration, Sigma) for 10 min. After washing, sperm (1 × 104) were added to each group of COCs and co-incubation proceeded for 15 min (at 37°C and 5% CO2) in the same medium used for capacitation in each treatment group. In those cases in which sperm were capacitated in ⊖ Ca2+, co-incubation was carried out in complete BWW. Sperm exposed to PF431396 (30 µM) only during gamete co-incubation was used as a control. At the end of co-incubation, cells were washed and fixed in 2% (w/v) paraformaldehyde in phosphate-buffered saline for 10 min at room temperature. After washing, cells were mounted on slides and examined under a Nikon Optiphot microscope equipped with epifluorescence optics (×250) to score the number of fluorescent human sperm heads within the C. oophorus.
Zona-free hamster oocyte penetration assay
Analysis of zona-free hamster oocyte penetration was performed as previously described (Cohen et al., 2001). Briefly, zona-free oocytes were prepared from COCs recovered from superovulated immature hamsters (Mesocricetus aureatus) maintained with food and water ad libitum in a temperature controlled room with light:dark (14:10) cycle. An aliquot of motile human sperm (2 × 105 cells/20 µl) incubated for 18 h in BWW either alone or containing PF431396 (30 µM) or in ⊖ Ca2+ was added to 10–15 hamster oocytes in BWW (80 µl). Sperm exposed to 6 µM PF431396 (concentration calculated based on the residual amount of inhibitor present in the insemination drop) only during gamete co-incubation was used as a control. After 2.5 h of co-incubation, cells were fixed with 2.5% glutaraldehyde, stained with 1% aceto-carmine solution, observed under the microscope (×400) and the number of oocytes presenting either decondensing sperm heads or pronuclei and sperm tails in the ooplasm was recorded.
Calculations and statistical analysis
All the experiments were repeated at least three times and values are presented as mean ± SEM. Calculations were performed using the Prism 3.0 software (GraphPad Software, La Jolla, CA, USA). Statistical significance of the data was analyzed using the χ2 test for the percentages of acrosome-reacted sperm and penetrated oocytes, one-way analysis of variance for the CASA determinations and Student's t-test for number of sperm within the cumulus. In all cases, P-values <0.05 were considered statistically significant.
Results
Effect of [Ca2+]e on protein phosphorylation
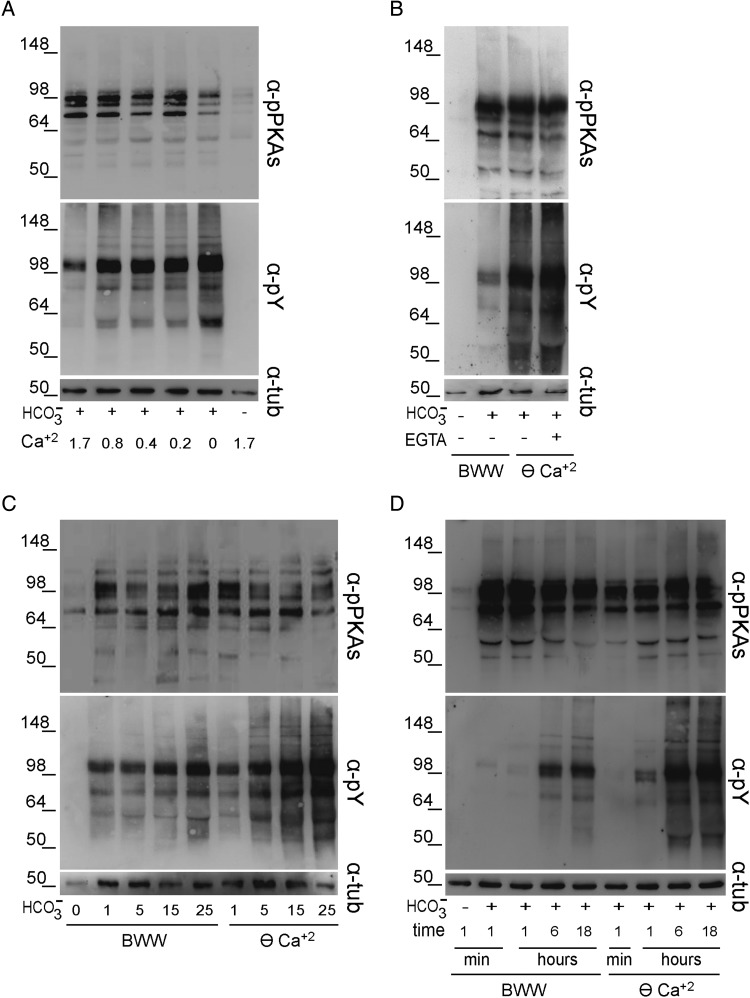
To study the effects exerted by Ca2+ during human sperm capacitation, different signaling events associated with this process were evaluated as a function of [Ca2+]e. After 6- h incubation, PKA substrate phosphorylation, which had not yet been analyzed in relation to [Ca2+]e, as well as Tyr phosphorylation were examined by western blotting using the corresponding specific antibodies. As previously reported (Osheroff et al., 1999; Battistone et al., 2013), incubation of human sperm in complete or -free BWW promoted or abrogated both PKA-dependent and Tyr phosphorylations, respectively (Fig. 1A). However, whereas similar patterns of PKA substrate phosphorylation were observed at all [Ca2+]e tested, Tyr phosphorylation levels increased as a function of decreasing [Ca2+]e (Fig. 1A). Similar results were obtained for sperm capacitated in BWW without added Ca2+ (nominal zero Ca2+ medium here indicated by ⊖ Ca2+) and containing EGTA to chelate residual Ca2+ (Fig. 1B). When sperm were incubated in ⊖ Ca2+ containing different concentrations to modulate the induction of capacitation, once again PKA-dependent phosphorylation did not show substantial modifications under any condition assayed, whereas Tyr phosphorylation positively correlated with concentrations showing bands more intensively stained than those corresponding to sperm incubated in regular BWW (Fig. 1C). Finally, when different incubation periods (1 min, 1 h, 6 h or 18 h) were evaluated, no significant modifications in PKA substrate phosphorylation were observed in either BWW or ⊖ Ca2+, whereas Tyr-phosphorylated proteins were detected earlier in ⊖ Ca2+ than in control medium (Fig. 1D). As no differences in phosphorylation levels were observed between 6 and 18 h of incubation, subsequent signaling experiments were performed using a 6-h incubation period. Together, these results indicate that [Ca2+]e modulates the level of Tyr phosphorylation without directly affecting PKA-dependent phosphorylation.
Figure 1.
Effect of [Ca2+]e on both PKA substrate and Tyr phosphorylations. (A) Human sperm were incubated for 6 h in BWW either lacking or containing different concentrations of Ca2+ (n = 4). (B) Sperm were incubated for 6 h in BWW (1.7 mM Ca2+) or in BWW without added Ca2+ (nominal zero Ca2+ medium indicated by ⊖ Ca2+) with or without EGTA (1 mM) (n = 4). (C) Sperm were incubated for 6 h in BWW or in ⊖ Ca2+ containing different concentration of (n = 3). (D) Sperm were incubated in BWW or in ⊖ Ca2+ for different time periods (n = 5). In all cases, protein extracts were analyzed for both PKA substrate and Tyr phosphorylations by western blotting using α-pPKAs or α-pY antibodies, respectively. β-Tubulin was used as the protein loading control.
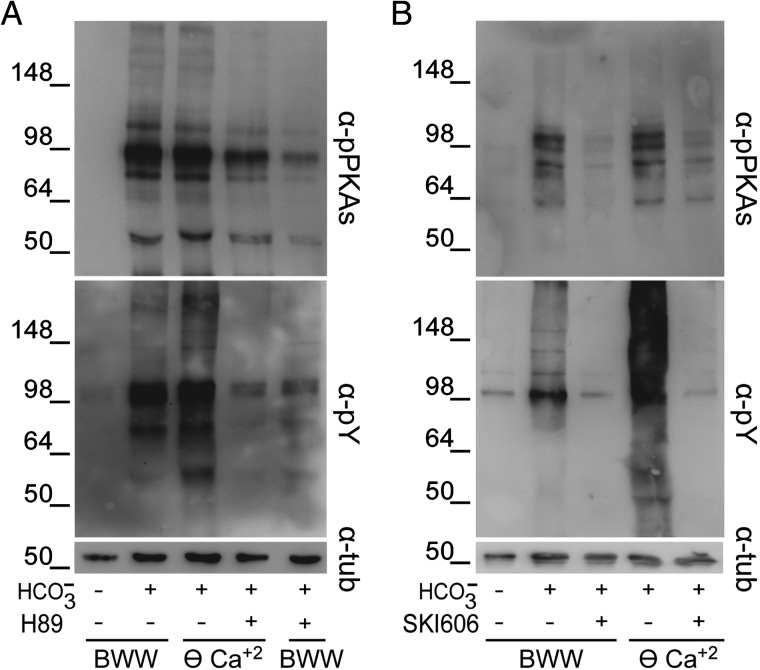
The increase in Tyr phosphorylation observed in the absence of Ca2+ might occur either independently of PKA activation or mediated by a Ca2+-dependent mechanism downstream of PKA. To evaluate these hypotheses, PKA activation was inhibited by two different approaches. First, PKA was directly inhibited by exposing sperm to H89 in either ⊖ Ca2+ or BWW. Results showed that H89 (30 µM) abolished both PKA substrate and Tyr phosphorylations in ⊖ Ca2+ as well as in complete media (Fig. 2A). As an alternative approach, sperm were incubated in ⊖ Ca2+ or BWW containing SKI606, an SFK inhibitor which affects PKA substrate phosphorylation (Krapf et al., 2010; Battistone et al., 2013). Similar to H89, SKI606 (30 µM) inhibited PKA substrate phosphorylation as well as the increase in Tyr phosphorylation in both media (Fig. 2B). Together, these results indicate that the increase in Tyr phosphorylation observed in ⊖ Ca2+ is downstream of PKA activation.
Figure 2.
Effect of the inhibition of cAMP/PKA or SFK/phosphatase signaling pathways on the [Ca2+]e-dependent increase in protein phosphorylation. Human sperm were incubated for 6 h in BWW or in ⊖ Ca2+containing either (A) the PKA inhibitor H89 (30 µM) or (B) the SFK inhibitor SKI606 (30 µM). In all cases, protein extracts were analyzed for both PKA substrate and Tyr phosphorylations by western blotting using α-pPKAs or α-pY antibodies, respectively (n = 3). β-Tubulin was used as the protein loading control.
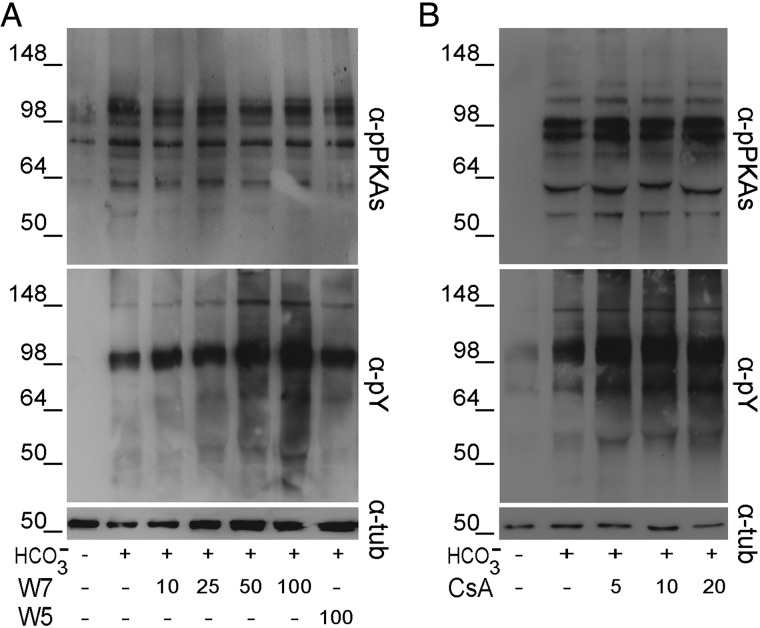
We next evaluated the potential involvement of Ca2+ downstream effectors such as CaM and one of its targets, PP2B, on both PKA-dependent and Tyr phosphorylations. For this purpose, sperm incubations were carried out in BWW containing the CaM antagonist W7 (using W5 as a control) (Fig. 3A) or the PP2B inhibitor CsA (Fig. 3B). Under these conditions, although no changes in PKA-dependent phosphorylation were observed, Tyr phosphorylation positively correlated with increasing concentrations of each inhibitor, supporting a role for both CaM and PP2B in a Ca2+-dependent signaling cascade leading to Tyr phosphorylation.
Figure 3.
Effect of CaM or PP2B inhibitors on protein phosphorylation. Human sperm were incubated for 6 h in BWW containing either (A) different concentrations of the CaM antagonist W7 or its inactive analog W5 or (B) different concentrations of the PP2B inhibitor CsA. In all cases, protein extracts were analyzed for both PKA substrate and Tyr phosphorylations by western blotting using α-pPKAs or α-pY antibodies, respectively (n = 5). β-Tubulin was used as the protein loading control.
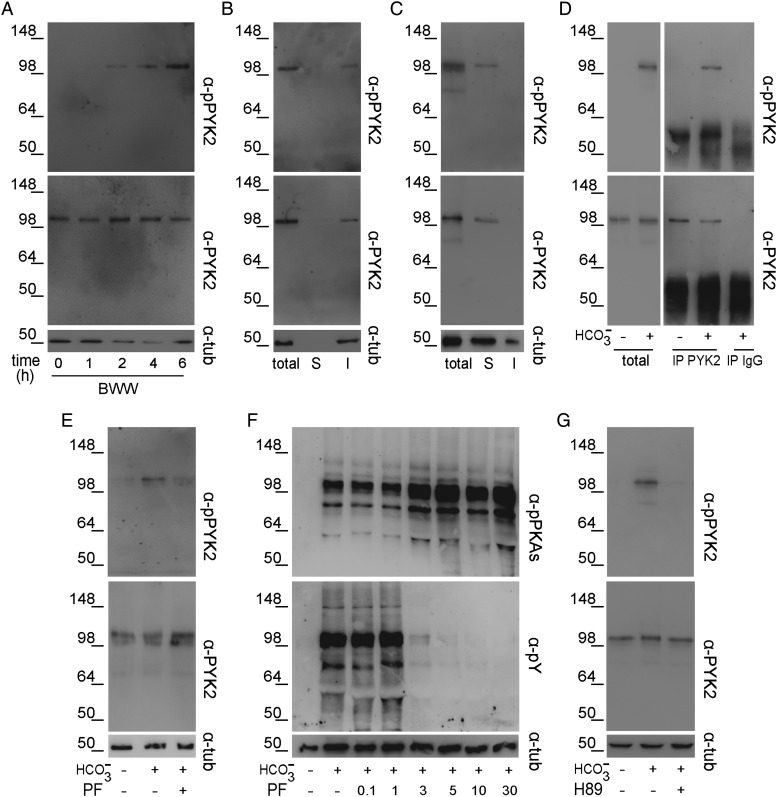
Involvement of PYK2 in protein phosphorylation
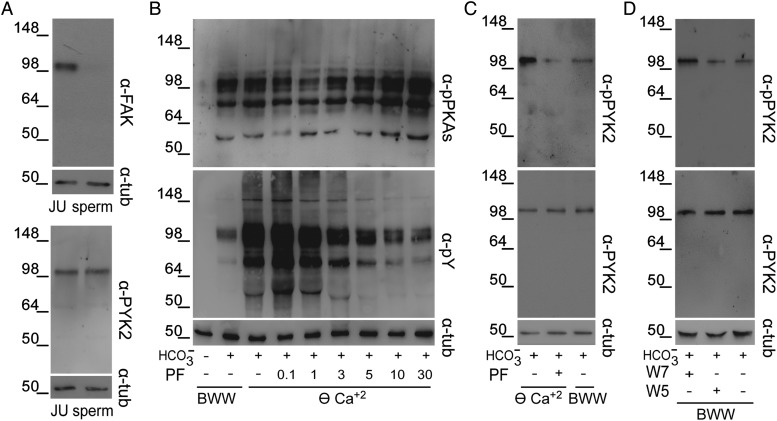
Activation of FAK proteins during stallion sperm incubation in ⊖ Ca2+ (Gonzalez-Fernandez et al., 2013) led us to investigate whether these proteins could be responsible for the Tyr phosphorylation increase observed in human sperm. Western blotting analysis using different specific antibodies against each of the two family members, i.e. FAK and PYK2 revealed the presence of PYK2 but not of FAK in human ejaculated sperm (Fig. 4A). Based on this, we next evaluated the effect of PF431396 (0–30 µM), a FAK/PYK2 inhibitor (Buckbinder et al., 2007; Han et al., 2009), on Tyr phosphorylation in ⊖ Ca2+ with the premise that PYK2 inhibition could prevent the increase in Tyr phosphorylation observed under this condition. Results showed that PF431396 concentrations >3 µM produced a slight increase in PKA-dependent phosphorylation, while blocking almost completely the increase in Tyr phosphorylation (Fig. 4B). To confirm that PF431396 was indeed inhibiting sperm PYK2, we evaluated the PYK2 activation state by determining the trans/autophosphorylation levels of this kinase (Avraham et al., 2000). Western blotting results using commercial antibodies against total PYK2 and phospho-PYK2 (Tyr 402) showed that when PF431396 was present in the medium, PYK2 levels remained constant while anti-phospho-PYK2 signal decreased (Fig. 4C). Interestingly, the intensity of the phospho-PYK2 band increased in ⊖ Ca2+ (Fig. 4C) as well as in BWW containing the CaM antagonist W7 (50 µM) (Fig. 4D). Altogether, these results support PYK2 as the candidate Tyr kinase responsible for the phosphorylation cascade observed in ⊖ Ca2+ in human sperm.
Figure 4.
Presence of PYK2 in human sperm and its activation in ⊖ Ca2+. (A) Jurkat cells (JU), as a positive control for FAK and PYK2 expression, or sperm protein extracts were analyzed by western blotting using α-FAK or α-PYK2 antibodies (n = 3). (B) Sperm were incubated for 6 h in ⊖ Ca2+ containing different concentrations of the FAK/PYK2 inhibitor PF431396, and protein extracts were analyzed for both PKA substrate and Tyr phosphorylations by western blotting using α-pPKAs or α-pY antibodies, respectively (n = 4). (C) Sperm were incubated for 6 h in BWW or in ⊖ Ca2+ with or without PF431396 (10 µM). Protein extracts were analyzed by western blotting using α-pPYK2 antibody and the membrane was then stripped and reprobed with α-PYK2 antibody (n = 4). (D) Sperm were incubated for 6 h in BWW with or without W7 (50 µM) or its analog W5 (50 µM) and protein extracts were analyzed by western blotting using α-PYK2 and then α-pPYK2 antibodies (n = 4). β-Tubulin was used as the protein loading control.
Based on these observations, we then explored the behavior of PYK2 during sperm capacitation in complete media. Western blotting results showed that phospho-PYK2 was not detected in fresh ejaculated sperm but increased in a time-dependent manner during capacitation, whereas total PYK2 levels remained constant (Fig. 5A). To confirm that the band detected was indeed PYK2, sperm extracts were immunoprecipitated with anti-PYK2 antibody and then analyzed by western blotting using the anti-phospho-PYK2 antibody. When Triton X-100 was used for sperm extract preparation, the bands corresponding to both total PYK2 and phospho-PYK2 were detected in the insoluble but not in the soluble fraction (Fig. 5B). Due to the insolubility of PYK2 in Triton X-100, sperm proteins were extracted using more stringent conditions (boiling of samples in 0.1% SDS and 1% Igepal), which allowed the solubilization of PYK2 as judged by its detection only in the soluble fraction (Fig. 5C). When this solubilized fraction was subjected to immunoprecipitation with anti-PYK2 and the immunoprecipitate analyzed by western blotting, a band corresponding to phospho-PYK2 was detected in capacitated but not in non-capacitated cells (Fig. 5D), confirming the previous direct western blot results indicating that PYK2 is indeed activated during sperm capacitation. This was further supported by the finding that PF431396 inhibited PYK2 phosphorylation without affecting total PYK2 (Fig. 5E).
Figure 5.
Activation of PYK2 during human sperm capacitation. (A) Sperm were incubated in BWW for different time periods. Protein extracts were analyzed by western blotting using α-pPYK2 antibody and the membrane was then stripped and reprobed with α-PYK2 antibody (n = 3). (B) Sperm were incubated in BWW for 6 h and proteins were extracted with 1% Triton X-100. The soluble (S) and insoluble (I) fractions were analyzed by western blotting using α-pPYK2 and then α-PYK2 antibodies (n = 3). (C) Sperm were incubated in BWW for 6 h and proteins were extracted with 0.1% SDS/1% Igepal. The soluble (S) and insoluble (I) fractions were analyzed by western blotting using α-pPYK2 and then α-PYK2 antibodies (n = 4). (D) Sperm were incubated for 6 h in complete or -free BWW, proteins were extracted with 0.1% SDS/1% Igepal and then subjected to immunoprecipitation with α-PYK2 antibody or rabbit IgG as a control. The immunoprecipitates (IP) were first analyzed using α-pPYK2 and then reblotted using α-PYK2 antibodies. (E) Sperm were incubated for 6 h in BWW with or without PF431396 (5 µM) and protein extracts were analyzed by Western blotting using α-PYK2 and then α-pPYK2 antibodies (n = 4). (F) Sperm were incubated for 6 h in BWW in the presence of PF431396, and protein extracts were analyzed for both PKA substrate and Tyr phosphorylations by western blotting using α-pPKAs or α-pY antibodies, respectively (n = 4). (G) Sperm were incubated for 6 h in BWW with or without H89 (30 µM) and protein extracts were analyzed by western blotting using α-PYK2 and then α-pPYK2 antibodies (n = 3). β-Tubulin was used as the protein loading control.
To study the involvement of PYK2 in capacitation-induced phosphorylation, sperm were incubated in BWW with or without PF431396 and both PKA-dependent and Tyr phosphorylations were evaluated. Results showed that although PKA substrate phosphorylation slightly increased as a function of the inhibitor concentration, Tyr phosphorylation decreased and was completely abolished at concentrations >5 µM (Fig. 5F), indicating a role for PYK2 downstream of PKA activation. Indeed, the inhibition of PKA activity by H89 reduced PYK2 phosphorylation, whereas total PYK2 levels remained constant (Fig. 5G).
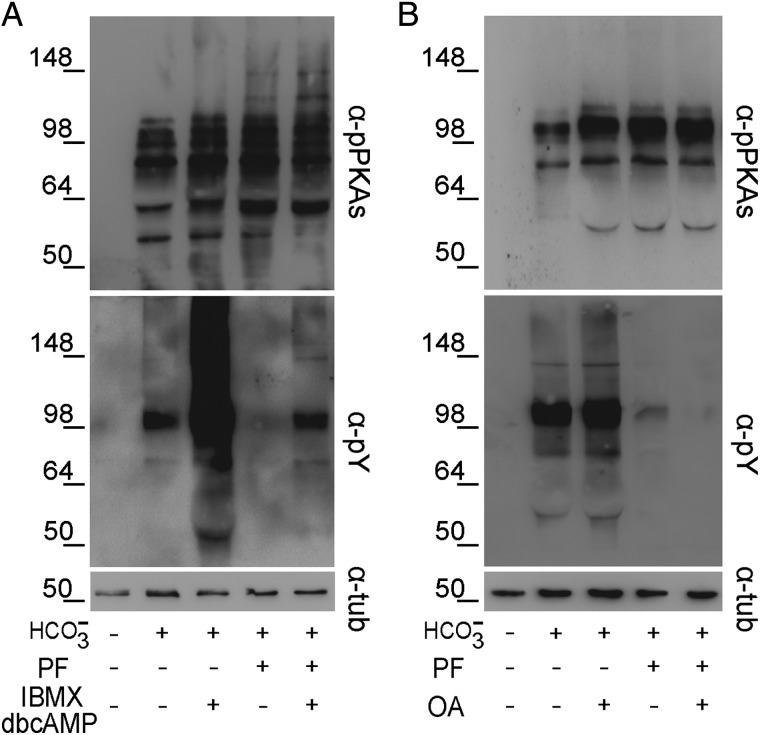
To gain insights into the mechanisms leading to PYK2 activation during capacitation, sperm were incubated under different conditions known to promote Tyr phosphorylation and the effect of PYK2 inhibition on both PKA-dependent and Tyr phosphorylations was evaluated. Results showed that exposure of sperm to dbcAMP and IBMX (5 mM and 200 µM, respectively), which stimulates the cAMP/PKA signaling cascade, produced an enhancement of both PKA-dependent and Tyr phosphorylations (Fig. 6A). However, whereas PF431396 did not modify the stimulated PKA substrate phosphorylation, it substantially reduced the increase in Tyr phosphorylation (Fig. 6A).
Figure 6.
Crosstalk between the PYK2 and cAMP/PKA or SFK/phosphatase signaling pathways. Human sperm were incubated for 6 h in BWW with or without PF431396 (10 µM) in the absence or presence of (A) the cAMP analog dbcAMP and the phosphodiesterase inhibitor IBMX (5/0.2 mM), or (B) the Ser/Thr phosphatase inhibitor OA (100 nM). In all cases, protein extracts were analyzed for both PKA substrate and Tyr phosphorylations by western blotting using α-pPKAs or α-pY antibodies, respectively (n = 4). β-Tubulin was used as the protein loading control.
To analyze whether the PF431396 effects could be overcome by exposure of sperm to OA, a Ser/Thr phosphatase inhibitor that increases Tyr phosphorylation (Krapf et al., 2010; Battistone et al., 2013), the effect of the simultaneous addition of both inhibitors on PKA-dependent and Tyr phosphorylations was evaluated. Contrary to our observations using the SFK inhibitor, PF431396 did not block PKA substrate phosphorylation and exposure to relatively high concentrations of OA (100 nM) could not overcome the inhibitory effect on Tyr phosphorylation (Fig. 6B). Altogether, these results indicate that the inhibition of PYK2 blocks the increase in Tyr phosphorylation downstream of PKA substrate phosphorylation.
Relevance of PYK2 for sperm function
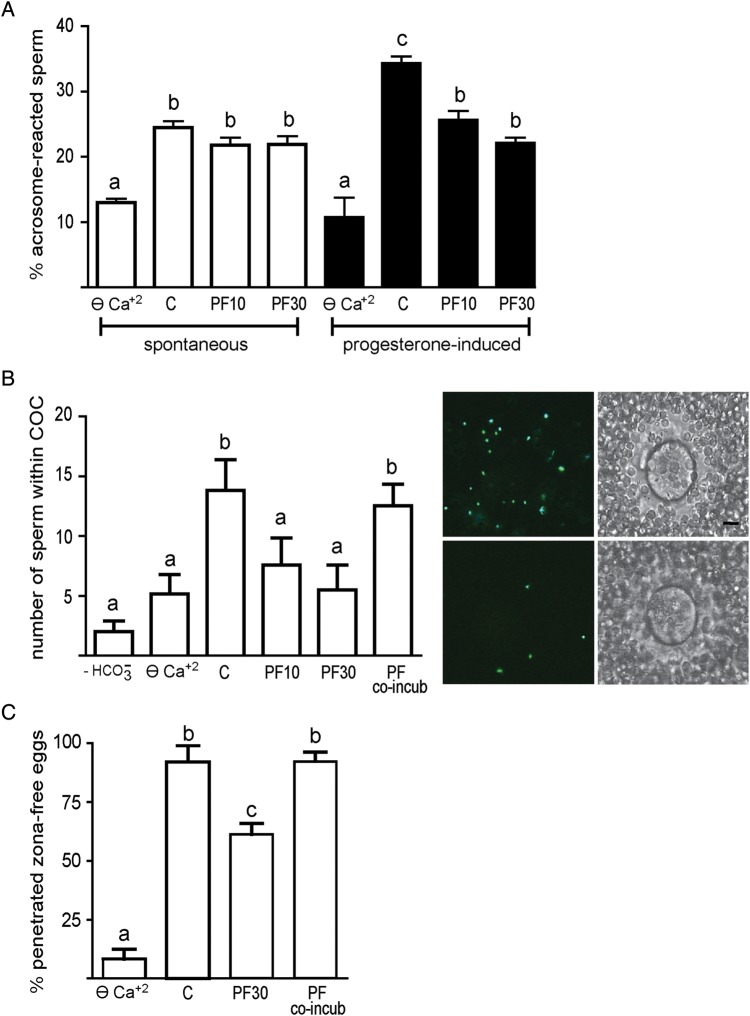
We next evaluated the functional relevance of PYK2 by testing the effect of its modulation on two well-known capacitation-associated events such as hyperactivation and AR. Results showed that similarly to what was observed in ⊖ Ca2+, exposure of sperm to PF431396 significantly reduced most (i.e. all except LIN and STR) (10 µM) or all (30 µM) of the motility parameters examined as well as the percentage of hyperactivation without modifying total motility (Table I). Regarding the occurrence of the AR, although both the spontaneous and the progesterone-induced AR rates were reduced in ⊖ Ca2+, addition of PF431396 (10 or 30 µM) during capacitation significantly inhibited the progesterone-induced AR without modifying the spontaneous AR rates (Fig. 7A). Evaluation of sperm viability at the end of the incubation with PF431396 30 µM confirmed the lack of inhibitor toxicity on sperm (inhibitor 92.0% ± 2.9 versus control 93.4% ±5.4, n = 4, NS).
Table I.
Relevance of PYK2 for human sperm motility during capacitation.
| VAP | VCL | VSL | ALH | LIN | STR | % Motility | % Progressive motility | % Hyperactivation | |
|---|---|---|---|---|---|---|---|---|---|
| C | 53.8 ± 2.4 | 85.2 ± 2.3 | 47.2 ± 2.5 | 4.1 ± 0.1 | 52.6 ± 1.8 | 82.8 ± 1.2 | 97.2±0.2 | 68.3±2.4 | 12.2±2.4 |
| ⊖ Ca2+ | 24.5 ± 1.3* | 53.2 ± 6.1* | 15 ± 1* | 2.9 ± 0.1* | 29.2 ± 1.6* | 58.9 ± 0.6* | 94.8±0.3 | 15.5±1.9* | 1.6±0.3* |
| PF 10 µM | 34.5 ± 3.4* | 59.2 ± 2.6* | 28.1 ± 2.8* | 3.1 ± 0.1* | 43.3 ± 3.9 | 73.9 ± 3.5 | 97.2±0.2 | 39.9±7.4* | 2.5±0.6* |
| PF 30 µM | 29.0 ± 2.5* | 54.9 ± 2.9* | 21.3 ± 2.5* | 3.2 ± 0.1* | 35.6 ± 1.8* | 66.8 ± 1.7* | 94.6±1.1 | 25±4.5* | 2.4±0.4* |
Computer-assisted sperm analysis was performed in sperm incubated for 18 h in BWW (C), or in BWW without added Ca2+ (⊖ Ca2+) or in BWW containing the FAK/PYK2 inhibitor PF431396 (PF 10 µM or 30 µM). VAP, average path velocity; VCL, curvilinear velocity; VSL, straight line velocity; ALH, amplitude of lateral head displacement; LIN, linearity; STR: straightness (n = 4). Statistical significance of the data was analyzed using one-way analysis of variance. *versus C, P < 0.05.
Figure 7.
Relevance of PYK2 for human sperm function. (A) Sperm were capacitated for 18 h in ⊖ Ca2+ or in BWW either alone (C) or containing PF431396 (10 or 30 µM) and both the spontaneous and progesterone (25 µM)-induced AR were evaluated by staining sperm with P. sativum agglutinin coupled to fluorescein isothiocyanate (n = 5). Statistical significance of the data was analyzed using the χ2 test. (B) Sperm were incubated for 18 h in ⊖ Ca2+ or in BWW either with (C) or without or containing PF431396 (10 or 30 µM), then co-incubated with mouse COCs for 15 min in BWW with or without PF431396, and the number of sperm within the COCs determined under microscope. Sperm exposed to PF431396 (30 μM) only during gamete co-incubation was used as a control (PF co-incub) (n = 4). Statistical significance of the data was analyzed using the Student's t-test. The images on the right show representative fluorescent (left) and phase-contrast (right) micrographs of COCs inseminated with either control (upper panel) or PF-treated (bottom panel) sperm (Hoechst-stained). The scale bar is 25 μm. (C) Sperm were incubated for 18 h in ⊖ Ca2+ or in BWW either alone (C) or containing PF431396 (30 µM). Sperm were then co-incubated with zona-free hamster eggs for 2.5 h in BWW, and the percentage of penetrated eggs determined. Sperm exposed to PF431396 only during gamete co-incubation was used as a control (PF co-incub) (n = 4). Statistical significance of the data was analyzed using the χ2 test. In all cases, bars with different letters are significantly different, P < 0.05.
To evaluate the relevance of PYK2 for sperm fertilizing ability, two different functional assays were used. First, we set up a heterologous penetration assay that examines the ability of human sperm to penetrate the mouse cumulus oophorus matrix. Results showed that sperm incubated in non-capacitating media (i.e. ⊖ Ca2+ or -free BWW) showed significantly lower penetration ability than those incubated under capacitating conditions, validating the use of this assay for the evaluation of the sperm functional capacitation state (Fig. 7B). When this assay was carried out using sperm exposed to PF431396 (10 or 30 µM) during the whole capacitation period, a clear reduction in the ability of sperm to penetrate the mouse cumulus matrix was observed compared with controls (Fig. 7B), whereas no differences were observed when the inhibitor (30 µM) was added only during the 15 min-gamete co-incubation period (Fig. 7B). As a second approach to analyze the functional state of sperm, we evaluated the ability of human sperm to penetrate zona-free hamster oocytes (WHO, 2010). As expected, sperm incubated in ⊖ Ca2+ exhibited a significant decrease in their ability to penetrate zona-free hamster eggs compared with controls. An inhibitory effect was also observed when sperm were capacitated in the presence of PF431396 (30 µM) but not when the inhibitor was present only during the gamete co-incubation period (Fig. 7C). Altogether, these results are consistent with a role of PYK2 in the acquisition of human sperm fertilizing ability during capacitation.
Discussion
Ca2+-mediated signaling has been extensively studied in relation to sperm physiology but only recently have these signaling components been associated with fertility problems (Khattri et al., 2012; Smith et al., 2013; Mohamed et al., 2013; Alasmari et al., 2013a; Tamburrino et al., 2014). In the present study, we explored the molecular mechanisms underlying the decodification of Ca2+ signals involved in human sperm capacitation which correlate with an increase in [Ca2+]i (Baldi et al., 1991; Wall et al., 1994; Perry et al., 1997) and Tyr phosphorylation (Leclerc et al., 1996; Osheroff et al., 1999). As an attempt to elucidate these mechanisms, we first evaluated the impact of different [Ca2+]e not only on Tyr phosphorylation but also on PKA-dependent phosphorylation which had not been previously analyzed. Our results show that PKA substrate phosphorylation did not exhibit major modifications in any of the assayed conditions which included media with different [Ca2+]e, concentrations or incubation periods. Previous reports have shown that incubation of human sperm in media without added Ca2+ produced a significant decrease in both sAC activity and cAMP levels (Jaiswal and Conti, 2003; Torres-Flores et al., 2011) with no changes in PDE1 activity (Lefievre et al., 2002). Although these results seem to be in conflict with the lack of changes in PKA substrate phosphorylation observed in our study, it is possible that low levels of cAMP are sufficient to activate PKA and maintain a sustained PKA-dependent phosphorylation as previously proposed (Battistone et al., 2013). Alternatively, the lack of changes in PKA-dependent phosphorylation levels could be due to compensatory effects of Ca2+ on enzymes that play a role in cAMP-dependent pathways and which finally lead to a steady state of PKA-mediated phosphorylation. In agreement with previous works (Luconi et al., 1996; Carrera et al., 1996; Leclerc and Goupil, 2002; Marin-Briggiler et al., 2003; Baker et al., 2004), Tyr phosphorylation increased as a function of decreasing [Ca2+]e. In addition, Tyr-phosphorylated proteins were detected either sooner or at lower concentrations when sperm were incubated in ⊖ Ca2+. A possible explanation for these observations is that the lack of added Ca2+ in the medium leads to an increase in Tyr kinase activity mediated by the higher levels of ATP observed in the absence of Ca2+ (Baker et al., 2004). This increase in ATP availability might result from a decrease in the activity of the ATP-consuming Ca2+-extruding mechanism that operates in the sperm to maintain resting [Ca2+]i, as well as from the reduced sperm vigorous motility observed in medium lacking Ca2+. Consistent with these hypotheses, exposure of sperm to thapsigargin, an endoplasmic Ca2+-ATPase inhibitor that produces an increase in [Ca2+]i (Dorval et al., 2003), generated a decrease in both ATP content (Baker et al., 2004) and Tyr phosphorylation (Luconi et al., 1996; Baker et al., 2004). Nevertheless, considering that the lack of Ca2+ in the medium is known to also produce a sodium-dependent plasma membrane depolarization as well as an increase in intracellular sodium concentration (Gonzalez-Martinez, 2003; Torres-Flores et al., 2008), we cannot exclude the possibility that other molecular mechanisms enhanced by low [Ca2+]e could negatively affect Tyr phosphorylation.
Interestingly, although [Ca2+]e does not directly affect PKA substrate phosphorylation levels, its effect on Tyr phosphorylation depends on PKA targets as judged by the fact that pharmacological inhibition of either PKA or SFK signaling pathways (which control PKA substrate phosphorylation status) abrogated both PKA-mediated and Tyr phosphorylations even in ⊖ Ca2+. These results suggest that Ca2+ target(s) are directly or indirectly linked to phosphorylation of PKA substrates. In order to gain further insights into the signaling pathway through which Ca2+ affects Tyr phosphorylation, we studied if CaM, a universal [Ca2+]i sensor, was required for Tyr phosphorylation in human sperm. Our results showed that inhibition of CaM by W7 maintained PKA substrate phosphorylation at a constant level but, in agreement with previous reports (Carrera et al., 1996: Leclerc et al., 1998), it produced a dose-dependent increase in Tyr phosphorylation. Based on the finding that direct inhibition of CaMK, one of the CaM targets, did not affect Tyr phosphorylation in either human (Marin-Briggiler et al., 2005) or stallion (Gonzalez-Fernandez et al., 2013) sperm, we tested the effect of pharmacologically inhibiting another CaM target, namely PP2B, detecting no modifications of PKA target phosphorylation and an increase in Tyr phosphorylation. The effect of PP2B inhibition on Tyr phosphorylation is in agreement with previous observations in human sperm (Carrera et al., 1996) but not with those recently reported in stallion (Gonzalez-Fernandez et al., 2013) probably due to inherent differences in species specificity. The fact that inhibition of both CaM and PP2B mimics the effects observed on both PKA-dependent and Tyr phosphorylations in ⊖ Ca2+ suggests that CaM and PP2B may be involved in the Ca2+ signaling pathway regulating Tyr phosphorylation. Moreover, the finding that similar results were obtained when inhibition of CaM and PP2B was carried out in ⊖ Ca2+ (data not shown) suggests that these Ca2+ downstream effectors could also mediate the increase in Tyr phosphorylation observed under this condition. In this regard, it is interesting to mention previous results postulating that CaM levels decrease during human sperm capacitation (Leclerc et al., 1998) which, according to our observations, could lead to an increase in Tyr phosphorylation.
Based on a recent report in stallion showing the activation of FAK family members (i.e. FAK and PYK2) in sperm incubated in a medium without added Ca2+ (Gonzalez-Fernandez et al., 2013), we investigated whether FAK proteins could mediate the phosphorylation cascade observed in our studies. In this regard, whereas the work in stallion involved only the use of antibodies against the phospho-FAK proteins, our studies were carried out using also the antibodies against the non-phosphorylated proteins. Results revealed, for the first time, the presence of PYK2 but not its homolog FAK in human sperm. Moreover, we observed that the addition of PF431396, a specific ATP-competitive inhibitor of FAK family proteins (Buckbinder et al., 2007; Han et al., 2009), prevented the increase in Tyr phosphorylation observed in ⊖ Ca2+, producing only a slight increase in PKA substrate phosphorylation. Accordingly, PF431396 inhibited the trans/autophosphorylation at Tyr402 of PYK2, which was enhanced in ⊖ Ca2+, arguing in favor of an increase in Tyr kinase activity in this medium as previously proposed (Luconi et al., 1996; Baker et al., 2004). As the SFK member c-yes is present in human sperm and is inhibited by Ca2+, it was postulated as the kinase involved in the Ca2+-mediated increase in Tyr phosphorylation (Leclerc and Goupil, 2002). However, the fact that PF431396 completely abolished the Tyr phosphorylation increase in ⊖ Ca2+ supports the role of a PF431396-sensitive Tyr kinase, such as PYK2, as the molecule responsible for inducing Tyr phosphorylation under this condition. In agreement with previous observations in both stallion sperm (Gonzalez-Fernandez et al., 2013) and cytotoxic T lymphocytes (Lysechko et al., 2010), our results showed that CaM inhibition by W7 enhanced phospho-PYK2. The finding that W7 produced an increase in both PYK2 activation and Tyr phosphorylation suggests a CaM-mediated increase in human sperm PYK2 phosphorylation in ⊖ Ca2+. In this regard, although a CaM-binding sequence has been identified in PYK2 (Kohno et al., 2008), it is likely that CaM indirectly regulates sperm PYK2 activation. In addition to this, we cannot exclude the possibility that PYK2 is activated in ⊖ Ca2+ by other mechanisms (i.e. an increase in ATP) as this kinase is known to respond to stress and extracellular ATP in other cell types (Block et al., 2011; Martel-Gallegos et al., 2013).
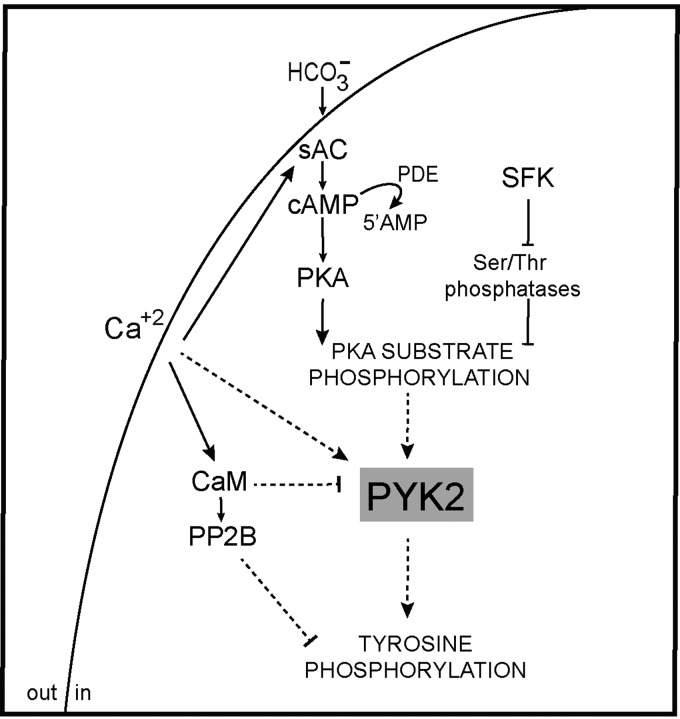
In light of our observations, we next performed a series of studies to investigate the potential involvement of PYK2 in sperm capacitation. Our results revealed that PYK2 is activated during capacitation as judged by the time-dependent increase in phospho-PYK2. As PYK2 has been described as a Ca2+-dependent kinase in other systems (Lev et al., 1995), its activation during capacitation might be a consequence of the increase in [Ca2+]i that occurs during this process (Baldi et al., 1991; Wall et al., 1994; Perry et al., 1997). PYK2 activation during capacitation was also indicated by the presence of the phosphorylated protein only in the immunoprecipitates obtained from capacitated cells. In this regard, it is interesting to note that phospho-PYK2 was detected in the insoluble fraction of Triton X-100-treated sperm consistent with the localization of the majority of the Tyr-phosphorylated proteins associated with the cytoskeleton (Visconti et al., 1997; Chung et al., 2014). The finding that PF431396 inhibited PYK2 phosphorylation and abrogated Tyr phosphorylation but not PKA substrate phosphorylation supports PYK2 as an intermediate component between both PKA-mediated and Tyr phosphorylations during human sperm capacitation (Fig. 8). This conclusion is further supported by the finding that H89 blocked PYK2 activation and by our observations that when the cAMP/PKA or SFK/phosphatase signaling pathways were stimulated by dbcAMP/IBMX or OA, respectively, the PYK2 inhibitor did not affect the induced PKA substrate phosphorylation but markedly inhibited Tyr phosphorylation. These results, together with the fact that both PYK2 and Tyr phosphorylations are downstream of PKA activation in all the conditions tested, indicate that PYK2 activity directly or indirectly depends on PKA-mediated phosphorylation. Moreover, the finding that blockage of both PYK2 and Tyr phosphorylations by PF431396 resulted in an enhancement of PKA target phosphorylation suggests the existence of a negative feedback mechanism that inhibits PKA-mediated phosphorylation during capacitation. In this regard, a recent article showing that PKA is Tyr-phosphorylated during capacitation (Chung et al., 2014) opens the possibility that this phosphorylation could play a role in the negative feedback suggested by our results.
Figure 8.
Working model for the signaling pathways proposed to be involved in Tyr phosphorylation during human sperm capacitation. In addition to the previously reported signaling pathways involved in Tyr phosphorylation (i.e. cAMP/PKA and SFK/phosphatase), we propose (dashed lines) PYK2 as a Ca2+ signaling component involved in Tyr phosphorylation downstream of PKA activation during human sperm capacitation.
The analysis of the functional relevance of our observations showed that PF431396 affected the development of hyperactivation during capacitation as well of the sperm ability to acrosome reaction in response to progesterone, similarly to what we observed by inhibiting the SFK signaling pathway (Battistone et al., 2013). In addition, exposure of human sperm to PF431396 during capacitation affected their ability to both penetrate mouse cumulus matrix and fertilize zona-free hamster eggs. Considering that sperm need to exhibit a vigorous motility to penetrate the viscous cumulus matrix and to undergo a functional AR to fuse with the plasma membrane, our results support the idea that PYK2 plays an important role in the acquisition of human sperm fertilizing ability during capacitation. In addition, our findings showing that the different functional parameters evaluated were also affected by incubation of sperm in ⊖ Ca2+ are consistent with previous evidence supporting the biological relevance of Ca2+ signaling for human fertilization (Ahmad et al., 1995; Marin-Briggiler et al., 2003; Shahar et al., 2011). In this regard, the fact that low [Ca2+]e also produces an increase in Tyr phosphorylation suggests that low resting [Ca2+]i could be sufficient for triggering Tyr phosphorylation but not for the acquisition of human sperm fertilizing ability. The latter might require a higher Ca2+ influx likely through CatSper channel which both produces a rapid transient [Ca2+]i increase followed by a slow sustained [Ca2+]i elevation after stimulation (Strunker et al., 2011), and may activate Ca2+ stores or store-operated channels (Lefievre et al., 2012). In this regard, a recent report proposed that human sperm penetration through a viscous medium (comparable to our cumulus matrix penetration assay) is enhanced by CatSper stimulation, whereas hyperactivated motility rather depends on the mobilization of intracellular stored Ca2+ (Alasmari et al., 2013b). All these observations reveal the spatio-temporal complexity of the Ca2+-mediating signaling that may lead to different sperm behaviors depending on the Ca2+ source (Alasmari et al., 2013b). In view of this, and considering the inhibitory effect of PF431396 on different sperm functional parameters, it is possible that PYK2 could be acting as a platform that integrates signals coming from different Ca2+ sources and leading to different downstream functional events.
In conclusion, in the present study we provide evidence that PYK2 is a Ca2+ signaling component involved in Tyr phosphorylation downstream of PKA activation and is required for functional human sperm capacitation. These results contribute to a better understanding of the molecular mechanisms involved not only in physiological human sperm capacitation but also in pathological conditions such as those associated with impaired sperm Ca2+ signaling.
Authors' roles
M.A.B., A.A., A.M.S. and V.G.D.R. carried out the experiments and analyzed the data. P.E.V., V.G.D.R. and P.S.C conceived the study, designed experiments, analyzed the data and wrote the article. M.A.B., A.A. and A.M.S. contributed in drafting the article.
Funding
This study was partially supported by the World Health Organization (H9/TSA/037), the National Research Council of Argentina (PIP 2009–290) and the National Agency for Scientific and Technological Promotion (PICT 2011, 2023) to P.S.C. and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH (RO1 HD38082 and HD44044) to P.E.V.
Conflict of interest
None declared.
Acknowledgements
The authors are grateful to members of Cuasnicu laboratory for their helpful comments.
References
- Ahmad K, Bracho GE, Wolf DP, Tash JS. Regulation of human sperm motility and hyperactivation components by calcium, calmodulin, and protein phosphatases. Arch Androl. 1995;35:187–208. doi: 10.3109/01485019508987871. [DOI] [PubMed] [Google Scholar]
- Alasmari W, Barratt CL, Publicover SJ, Whalley KM, Foster E, Kay V, Martins da Silva S, Oxenham SK. The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation. Hum Reprod. 2013a;28:866–876. doi: 10.1093/humrep/des467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari W, Costello S, Correia J, Oxenham SK, Morris J, Fernandes L, Kirkman-Brown J, Michelangeli M, Publicover S, Barratt CLR. Ca2+ signalling through CatSper and Ca2+ stores generate different behaviours in human sperm. J Biol Chem. 2013b;288:6248–6258. doi: 10.1074/jbc.M112.439356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Ecroyd H, Roman SD, Aitken RJ. Analysis of the mechanism by which calcium negatively regulates the tyrosine phosphorylation cascade associated with sperm capacitation. J Cell Sci. 2004;117:211–222. doi: 10.1242/jcs.00842. [DOI] [PubMed] [Google Scholar]
- Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl. 1991;12:323–330. [PubMed] [Google Scholar]
- Battistone MA, Da Ros VG, Salicioni AM, Navarrete FA, Krapf D, Visconti PE, Cuasnicu PS. Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol Hum Reprod. 2013;19:570–580. doi: 10.1093/molehr/gat033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers JD, Whitten WK, Whittingham DG. The culture of mouse embryos in vitro. In: Daniel JC, editor. Methods in Mammalian Embrology. San Francisco, CA: Freeman and Co. Press; 1971. pp. 86–116. [Google Scholar]
- Block ER, Tolino MA, Klarlund JK. Extracellular ATP stimulates epithelial cell motility through Pyk2-mediated activation of the EGF receptor. Cell Signal. 2011;23:2051–2055. doi: 10.1016/j.cellsig.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckbinder L, Crawford DT, Qi H, Ke HZ, Olson LM, Long KR, Bonnette PC, Baumann AP, Hambor JE, Grasser WA, 3rd, et al. Proline-rich tyrosine kinase 2 regulates osteoprogenitor cells and bone formation, and offers an anabolic treatment approach for osteoporosis. Proc Natl Acad Sci USA. 2007;104:10619–10624. doi: 10.1073/pnas.0701421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera A, Moos J, Ning XP, Gerton GL, Tesarik J, Kopf GS, Moss SB. Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin-dependent mechanism: identification of A kinase anchor proteins as major substrates for tyrosine phosphorylation. Dev Biol. 1996;180:284–296. doi: 10.1006/dbio.1996.0301. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Chung JJ, Shim SH, Everley RA, Gygi SP, Zhuang X, Clapham DE. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell. 2014;157:808–822. doi: 10.1016/j.cell.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DJ, Ellerman DA, Busso D, Morgenfeld MM, Piazza AD, Hayashi M, Young ET, Kasahara M, Cuasnicu PS. Evidence that human epididymal protein ARP plays a role in gamete fusion through complementary sites on the surface of the human egg. Biol Reprod. 2001;65:1000–1005. doi: 10.1095/biolreprod65.4.1000. [DOI] [PubMed] [Google Scholar]
- Cross NL. Effect of cholesterol and other sterols on human sperm acrosomal responsiveness. Mol Reprod Dev. 1996;45:212–217. doi: 10.1002/(SICI)1098-2795(199610)45:2<212::AID-MRD14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Da Ros VG, Munuce MJ, Cohen DJ, Marin-Briggiler CI, Busso D, Visconti PE, Cuasnicu PS. Bicarbonate is required for migration of sperm epididymal protein DE (CRISP-1) to the equatorial segment and expression of rat sperm fusion ability. Biol Reprod. 2004;70:1325–1332. doi: 10.1095/biolreprod.103.022822. [DOI] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Trevino CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev. 2011;91:1305–1355. doi: 10.1152/physrev.00028.2010. [DOI] [PubMed] [Google Scholar]
- Dorval V, Dufour M, Leclerc P. Role of protein tyrosine phosphorylation in the thapsigargin-induced intracellular Ca(2+) store depletion during human sperm acrosome reaction. Mol Hum Reprod. 2003;9:125–131. doi: 10.1093/molehr/gag017. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′5′-monophosphate-dependent pathway. Biol Reprod. 1997;56:707–719. doi: 10.1095/biolreprod56.3.707. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fernandez L, Macias-Garcia B, Loux SC, Varner DD, Hinrichs K. Focal adhesion kinases and calcium/calmodulin-dependent protein kinases regulate protein tyrosine phosphorylation in stallion sperm. Biol Reprod. 2013;88:138. doi: 10.1095/biolreprod.112.107078. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez MT. Induction of a sodium-dependent depolarization by external calcium removal in human sperm. J Biol Chem. 2003;278:36304–36310. doi: 10.1074/jbc.M304479200. [DOI] [PubMed] [Google Scholar]
- Han S, Mistry A, Chang JS, Cunningham D, Griffor M, Bonnette PC, Wang H, Chrunyk BA, Aspnes GE, Walker DP, et al. Structural characterization of proline-rich tyrosine kinase 2 (PYK2) reveals a unique (DFG-out) conformation and enables inhibitor design. J Biol Chem. 2009;284:13193–13201. doi: 10.1074/jbc.M809038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA. 2003;100:10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gonzalez C, Michelangeli F, Harper CV, Barratt CL, Publicover SJ. Calcium signalling in human spermatozoa: a specialized ‘toolkit’ of channels, transporters and stores. Hum Reprod Update. 2006;12:253–267. doi: 10.1093/humupd/dmi050. [DOI] [PubMed] [Google Scholar]
- Khattri A, Reddy VP, Pandey RK, Sudhakar DV, Gupta NJ, Chakravarty BN, Deenadayal M, Singh L, Thangaraj K. Novel mutations in calcium/calmodulin-dependent protein kinase IV (CAMK4) gene in infertile men. Int J Androl. 2012;35:810–818. doi: 10.1111/j.1365-2605.2012.01302.x. [DOI] [PubMed] [Google Scholar]
- Kohno T, Matsuda E, Sasaki H, Sasaki T. Protein-tyrosine kinase CAKbeta/PYK2 is activated by binding Ca2+/calmodulin to FERM F2 alpha2 helix and thus forming its dimer. Biochem J. 2008;410:513–523. doi: 10.1042/BJ20070665. [DOI] [PubMed] [Google Scholar]
- Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, Visconti PE. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem. 2010;285:7977–7985. doi: 10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leclerc P, Goupil S. Regulation of the human sperm tyrosine kinase c-yes. Activation by cyclic adenosine 3′,5′-monophosphate and inhibition by Ca(2+) Biol Reprod. 2002;67:301–307. doi: 10.1095/biolreprod67.1.301. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–692. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Interaction between Ca2+, cyclic 3′,5′ adenosine monophosphate, the superoxide anion, and tyrosine phosphorylation pathways in the regulation of human sperm capacitation. J Androl. 1998;19:434–443. [PubMed] [Google Scholar]
- Lefievre L, de Lamirande E, Gagnon C. Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3A in human spermatozoa. Biol Reprod. 2002;67:423–430. doi: 10.1095/biolreprod67.2.423. [DOI] [PubMed] [Google Scholar]
- Lefièvre L, Nash K, Mansell S, Costello S, Punt E, Correia J, Morris J, Kirkman-Brown J, Wilson SM, Barratt CL, et al. 2-APB-potentiated channels amplify CatSper-induced Ca2+ signals in human sperm. Biochem J. 2012;448:189–200. doi: 10.1042/BJ20120339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of ‘soluble’ adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–6. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- Luconi M, Krausz C, Forti G, Baldi E. Extracellular calcium negatively modulates tyrosine phosphorylation and tyrosine kinase activity during capacitation of human spermatozoa. Biol Reprod. 1996;55:207–216. doi: 10.1095/biolreprod55.1.207. [DOI] [PubMed] [Google Scholar]
- Lysechko TL, Cheung SM, Ostergaard HL. Regulation of the tyrosine kinase Pyk2 by calcium is through production of reactive oxygen species in cytotoxic T lymphocytes. J Biol Chem. 2010;285:31174–31184. doi: 10.1074/jbc.M110.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Briggiler CI, Gonzalez-Echeverría F, Buffone M, Calamera JC, Tezón JG, Vazquez-Levin MH. Calcium requirements for human sperm function in vitro. Fertil Steril. 2003;79:1396–1403. doi: 10.1016/s0015-0282(03)00267-x. [DOI] [PubMed] [Google Scholar]
- Marin-Briggiler CI, Jha KN, Chertihin O, Buffone MG, Herr JC, Vazquez-Levin MH, Visconti PE. Evidence of the presence of calcium/calmodulin-dependent protein kinase IV in human sperm and its involvement in motility regulation. J Cell Sci. 2005;118:2013–2022. doi: 10.1242/jcs.02326. [DOI] [PubMed] [Google Scholar]
- Martel-Gallegos G, Casas-Pruneda G, Ortega-Ortega F, Sanchez-Armass S, Olivares-Reyes JA, Diebold B, Perez-Cornejo P, Arreola J. Oxidative stress induced by P2X7 receptor stimulation in murine macrophages is mediated by c-Src/Pyk2 and ERK1/2. Biochim Biophys Acta. 2013;1830:4650–4659. doi: 10.1016/j.bbagen.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Mohamed TM, Zakeri SA, Baudoin F, Wolf M, Oceandy D, Cartwright EJ, Gul S, Neyses L. Optimisation and validation of a high throughput screening compatible assay to identify inhibitors of the plasma membrane calcium ATPase pump—a novel therapeutic target for contraception and malaria. J Pharm Pharm Sci. 2013;16:217–230. doi: 10.18433/j3pg68. [DOI] [PubMed] [Google Scholar]
- Mortimer ST, Mortimer D. Kinematics of human spermatozoa incubated under capacitating conditions. J Androl. 1990;11:195–203. [PubMed] [Google Scholar]
- Mortimer ST, Swan MA, Mortimer D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum Reprod. 1998;13:2139–2146. doi: 10.1093/humrep/13.8.2139. [DOI] [PubMed] [Google Scholar]
- Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, Kopf GS. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod. 1999;5:1017–1026. doi: 10.1093/molehr/5.11.1017. [DOI] [PubMed] [Google Scholar]
- Perry RL, Barratt CL, Warren MA, Cooke ID. Response of human spermatozoa to an internal calcium ATPase inhibitor, 2,5-di(tert-butyl) hydroquinone. J Exp Zool. 1997;279:284–290. doi: 10.1002/(sici)1097-010x(19971015)279:3<284::aid-jez9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm--making the most of what you've got. Nat Cell Biol. 2007;9:235–242. doi: 10.1038/ncb0307-235. [DOI] [PubMed] [Google Scholar]
- Ruknudin A, Silver IA. Ca2+ uptake during capacitation of mouse spermatozoa and the effect of an anion transport inhibitor on Ca2+ uptake. Mol Reprod Dev. 1990;26:63–68. doi: 10.1002/mrd.1080260110. [DOI] [PubMed] [Google Scholar]
- Shahar S, Wiser A, Ickowicz D, Lubart R, Shulman A, Breitbart H. Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum Reprod. 2011;26:2274–2282. doi: 10.1093/humrep/der232. [DOI] [PubMed] [Google Scholar]
- Smith JF, Syritsyna O, Fellous M, Serres C, Mannowetz N, Kirichok Y, Lishko PV. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc Natl Acad Sci USA. 2013;110:6823–6828. doi: 10.1073/pnas.1216588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- Tamburrino L, Marchiani S, Minetti F, Forti G, Muratori M, Baldi E. The CatSper calcium channel in human sperm: relation with motility and involvement in progesterone-induced acrosome reaction. Hum Reprod. 2014;29:418–428. doi: 10.1093/humrep/det454. [DOI] [PubMed] [Google Scholar]
- Tash JS, Krinks M, Patel J, Means RL, Klee CB, Means AR. Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J. Cell Biol. 1988;106:1625–1633. doi: 10.1083/jcb.106.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Flores V, García-Sánchez NL, González-Martínez MT. Intracellular sodium increase induced by external calcium removal in human sperm. J Androl. 2008;29:63–69. doi: 10.2164/jandrol.107.003368. [DOI] [PubMed] [Google Scholar]
- Torres-Flores V, Picazo-Juarez G, Hernandez-Rueda Y, Darszon A, Gonzalez-Martinez MT. Sodium influx induced by external calcium chelation decreases human sperm motility. Hum Reprod. 2011;26:2626–2635. doi: 10.1093/humrep/der237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Johnson LR, Oyaski M, Fornes M, Moss SB, Gerton GL, Kopf GS. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev Biol. 1997;192:351–363. doi: 10.1006/dbio.1997.8768. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. Beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999;274:3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Krapf D, de la Vega-Beltrán JL, Acevedo JJ, Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl. 2011;13:395–405. doi: 10.1038/aja.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M, O'Flaherty V, Ginty F, Houghton JA. Measurement of intracellular calcium levels of human spermatozoa acrosome reacted by electropermeabilization. Arch Androl. 1994;32:185–195. doi: 10.3109/01485019408987785. [DOI] [PubMed] [Google Scholar]
- Wasco WM, Orr GA. Function of calmodulin in mammalian sperm: presence of a calmodulin-dependent cyclic nucleotide phosphodiesterase associated with demembranated rat caudal epididymal sperm. Biochem Biophys Res Commun. 1984;118:636–642. doi: 10.1016/0006-291x(84)91350-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th edn. Geneva, Switzerland: World Health Organization Press; 2010. [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York, NY: Raven Press; 1994. pp. 189–317. [Google Scholar]