Abstract
Objective
While recent genomic studies have focused attention on triglyceride (TG) rich lipoproteins in cardiovascular disease (CVD), little is known of very low-density lipoprotein cholesterol (VLDL-C) relationship with atherosclerosis and CVD. We examined, in a high-risk type-2 diabetic population, the association of plasma VLDL-C with coronary artery calcification (CAC).
Methods
The Penn Diabetes Heart Study (PDHS) is a cross-sectional study of CVD risk factors in type-2 diabetics (n=2118, mean age 59.1 years, 36.5% female, 34.1% Black). Plasma lipids including VLDL-C were calculated (n=1879) after ultracentrifugation.
Results
In Tobit regression, VLDL-C levels were positively associated with increasing CAC after adjusting for age, race, gender, Framingham risk score, body mass index, C-reactive protein, exercise, medication and alcohol use, hemoglobin A1c, and diabetes duration [Tobit ratio (TR) and 95% confidence interval (CI) 0.38 (0.12–0.65), P=0.005] and even after inclusion of apolipoprotein B data [TR 0.31 (0.03–0.58), P=0.030]. Approximately 3-fold stronger effect was observed in women [TR 0.75 (0.16 – 1.34), P=0.013] than men [TR 0.20 (−0.10–0.50), P=0.189; gender interaction P=0.034]. Plasma VLDL-C was related more strongly to CAC scores than TG levels (e.g., Akaike information criteria of 7263.65 v. 7263.94) and had stronger CAC association in individuals with TGs >150mg/dl (TR 0.80, P=0.010) vs. those with TGs <150 mg/dl (TR 0.27, P=0.185).
Conclusions
In PDHS, VLDL-C is associated with CAC independent of established CVD risk factors, particularly in women, and may have value even beyond apolipoprotein B levels and in patients with elevated TGs.
Keywords: VLDL-C, triglycerides, subclinical atherosclerosis
INTRODUCTION
Serum triglyceride (TG) and TG-rich lipoproteins (TGRL) have emerged as a risk factor for cardiovascular disease (CVD) independent of low-density lipoprotein cholesterol (LDL-C).1–4 Many prospective epidemiological studies suggest that while elevated serum TGs are associated with coronary heart disease (CHD), this relationship is attenuated after adjusting for high-density lipoprotein cholesterol (HDL-C) and LDL-C.5 However, such adjustments may actually obscure true causal relationships particularly given our incomplete understanding of the biological pathways in metabolism of TGRL.6 Recent genomic studies provide additional evidence for a causal and independent association between loci related to TG pathways and CVD. Therefore, there is resurgent interest in TGRL as causal biomarkers of CVD risk and target for novel therapeutic agents.6–8
While TGRL may be causal in atherosclerosis and predictors of CVD, fasting serum TG levels, the most commonly measured biomarker of TGRL, may not capture the true extent of this risk. Practically, TG levels are subject to large physiologic and temporal variability resulting in heterogeneous values within the same patient. Indeed, postprandial or non-fasting TGs and lipids are now thought to be better predictors of vascular disease than fasting TGs.9 Serum TG levels also fail to fully reflect the cholesterol content of very low-density lipoprotein cholesterol (VLDL) and remnant lipoprotein particles (the metabolic products of VLDL and chylomicrons), which may be the most atherogenic TGRL particles. Thus, focus is shifting to alternative measures of TGRL metabolism as potential superior biomarkers of TGRL-related CVD risk.10 Such measures may be particularly informative in type 2 diabetes and metabolic syndrome where remnant particles may be elevated due to poor post-prandial clearance in insulin resistance. Remnant particles can be measured directly, but methods are not standardized and therefore challenging for reproducibility across large patient populations.11
VLDL-C measurement is relatively straightforward and captures a distinct aspect of TGRL than serum TG. For the current analysis, following serum ultracentrifugation, we estimated VLDL-C levels in a large sample (n=1879) of type 2 diabetic patients recruited to the Penn Diabetes Heart Study (PDHS). We determined the association of VLDL-C with coronary artery calcification (CAC), an independent predictor of CVD risk, and assessed whether VLDL-C had value in predicting CAC scores beyond standard risk factors, including levels of plasma apolipoprotein B (apoB) and TGs.
METHODS
Study Participants
Details of PDHS have been reported previously.12 Briefly, PDHS is a single center cross-sectional study of 2118 subjects with type 2 diabetes mellitus. PDHS was designed to identify novel clinical, biochemical, and genetic risk factors for coronary atherosclerosis in type 2 diabetes mellitus. Participants were recruited between 2001 and 2011 from primary care and endocrinology clinics associated with the Hospital of the University of Pennsylvania. The inclusion criteria were a clinical diagnosis of T2DM (defined as fasting blood glucose >126 mg/dl, 2-h post-prandial glucose >200 mg/dl, or use of oral hypoglycemic agents/insulin in a subject greater than age 40 years), age of 35–75 years, and negative pregnancy test (if female). The University of Pennsylvania (U.Penn) IRB approval was obtained and informed consent was obtained from all study subjects. Exclusion criteria included clinical coronary artery disease (defined as myocardial infarction, coronary revascularization, angiographic coronary disease or positive stress test), insulin use prior to age 35, a serum creatinine >2.5 mg/dl and a weight >300 lb (136.4 kg). Of 2118 subjects originally recruited, 239 were excluded due to incomplete lipid profile data, thus 1879 participants were included. The demographic and clinical characteristics of those excluded did not differ from the overall sample (not shown).
Study Parameters
Participants were evaluated at the Clinical and Translational Research Center at Penn after a 12-hour overnight fast. Clinical parameters, including blood pressure, body mass index, and waist circumference were assessed as previously reported.12 Serum lipid parameters (total TGs, total cholesterol (TC), HDL-C, LDL-C and VLDL-C) were determined after ultracentrifugation (β-quantification technique) at time of recruitment in Penn's Centers for Disease Control-certified lipid laboratory using enzymatic assays (Hitachi 912, Roche Diagnostic Systems Inc., NJ, USA).13 Specifically, VLDL-C was estimated as TC minus cholesterol in the bottom fraction i.e., cholesterol in the d>1.006 (bottom) fraction following ultracentrifugation.14 Plasma apoB and C-Reactive Protein (CRP) levels were batch-assayed using high-sensitivity latex turbidimetric immunoassay (Wako Ltd., Osaka Japan). Laboratory test results were generated by personnel blinded to the clinical characteristics and CAC scores of research subjects. Framingham risk scores were calculated using TC (similar results were obtained using LDL-C).15 Participants were classified as having the metabolic syndrome using the revised National Cholesterol Education Program definition; all PDHS participants were considered to have met glucose criteria.16 Cardiac electron beam CT studies were performed using an Imatron C-150 CT scanner (GE-Imatron, South San Francisco, CA). CAC scores were quantified by the Agatston method as previously published.17
Statistical Analyses
Data are reported as median (interquartile range, IQR) for continuous variables and as proportions for categorical variables. Our primary analysis was of the association of VLDL-C with CAC data; secondary and subgroup analyses were complementary. Therefore, we did not adjust for multiple testing. For univariate comparisons of cardiovascular risk factors between individuals in different VLDL-C quartile categories, chi-square tests, Student’s t-tests and Wilcox rank sum tests were used. Based on our and others experience, we applied Tobit conditional regression for multivariable analysis of CAC data.18 Briefly, Tobit regression combines a logistic regression of the presence of CAC (any CAC present vs. CAC zero score) with a linear regression (of log-transformed CAC) when CAC is present to produce a single estimate for the relation of risk factors with CAC data. Because of skewed distribution, VLDL-C was log-transformed for modeling. Multivariable associations of VLDL-C with CAC were assessed in incremental Tobit models: Model 1 was adjusted for age, gender and race; Model 2 was additionally adjusted for medications (statins/zetia, niacin/fibrates, insulin, metformin, thiazolidinediones, sulfonylureas, angiotensin converting enzyme inhibitor, calcium channel blocker, beta blocker, diuretic), alcohol use, exercise, TC-based Framingham risk score; Model 3 was further adjusted for duration of diabetes, hemoglobin A1c (HbA1c), body mass index (BMI) and CRP levels; Model 4 was adjusted in addition for plasma apoB levels. Because plasma TG and VLDL-C values were highly correlated (Spearman R=0.88), these lipid parameters were not included in models together. To explore whether VLDL-C was associated with CAC independent of plasma TGs, we assessed the relation of VLDL-C with CAC in adjusted models where participants were stratified into those with low (<150 mg/dl) and high TG (>150 mg/dl) values. We also compared plasma VLDL-C and TG association with CAC in non-nested models using Akaike information criteria (AIC) and Bayesian information criteria (BIC).19, 20 A priori we tested whether the association of VLDL-C with CAC was modified (interaction) by gender and race. All analyses were performed using STATA version 12.0 software (Statacorp, College Station, Texas).
RESULTS
Characteristics of the study sample by VLDL-C values
The PDHS study sample has been described previously.12 Briefly, median age of the full study sample (N= 2118 patients) was 59 (IQR 53–66), 36.5% were female and 60.3% were Caucasian with 34.1% black. Table 1 shows the characteristics of the VLDL-C study sub-sample (n=1879) across VLDL-C quartiles. Subjects with higher VLDL-C were more likely to be male, white, younger, more hypertensive and have longer duration of diabetes, higher HbA1c values and greater use of sulfonylureas. They were also more likely to smoke and drink alcohol but less likely to exercise and less frequently on a statin. Therefore, these potentially confounding variables were included in our multivariable modeling. Spearman correlations revealed modest association between VLDL-C and several lipid and metabolic parameters e.g., plasma TGs (R=0.88, P<0.0001), plasma apoB (R=0.45, P<0.0001), HDL-cholesterol (R=0.42, P<0.0001) and BMI (R=0.14, P<0.0001) in both men and women. In crude analyses, increasing VLDL-C quartiles were associated with higher CAC scores and with the prevalence of CAC scores >0 (Table 1). As expected CAC scores were higher in males than females and higher in whites and Hispanics than in Blacks. (Supplemental Table 1)
TABLE 1.
Characteristics of the study sample across VLDL-C Quartiles
| Variable | VLDL-C Quartile 1 (n=436) |
VLDL-C Quartile 2 (n=480) |
VLDL-C Quartile 3 (n=483) |
VLDL-C Quartile 4 (n=480) |
P value* |
|---|---|---|---|---|---|
| Age (years) | 60 (54–67) | 60 (54–66) | 60 (53–66) | 58 (53–64) | 0.012 |
| Female (%) | 43.6% | 39.8% | 32.1% | 28.5% | <0.001 |
| Race (%) | <0.001 | ||||
| White | 51.6% | 54.2% | 64.6% | 71.9% | |
| Black | 42.7% | 40.8% | 29.0% | 21.6% | |
| Hispanic | 28.6% | 21.4% | 28.6% | 21.4% | |
| Other | 18.8% | 21.7% | 27.5% | 31.9% | |
| Blood pressure (mmHg) | |||||
| Systolic | 129 (120–141) | 131 (121–141) | 131 (122–142) | 132 (122–142) | 0.008 |
| Diastolic | 74 (70–81) | 74 (71–82) | 77 (71–83) | 77 (72–83) | <0.001 |
| Total cholesterol (mg/dl) | 156.5 (137–179) | 163.5 (144–185) | 173 (153–196) | 197 (168–224) | <0.001 |
| HDL-cholesterol (mg/dl) | 52 (44–64) | 47 (41–56) | 43 (37–52) | 38 (33–46) | <0.001 |
| Triglycerides (mg/dl) | 68 (56–83) | 96.5 (82–113) | 133 (111–164) | 217 (179–276) | <0.001 |
| LDL-cholesterol (mg/dl) | 88 (74–108) | 93 (78–113) | 99 (81–119) | 102 (77–126) | <0.001 |
| Remnant Cholesterol (TC-LDLC-HDL) | 13.6 (11.2–16.6) | 19.3 (16-4–22.6) | 26.6 (22.2–32.8) | 43.4 (35.9–55.3) | <0.001 |
| Body mass index (kg/m2) | 30.8 (27.2–35.0) | 32.0 (28.2–36.1) | 32.9 (28.9–37.2) | 32.4 (29.6–36.7) | <0.001 |
| Waist circumference (cm) | 40.3 (36.5–44.0) | 41.0 (37.5–45.4) | 43.0 (39.9–46.5) | 42.5 (39.5–46.5) | <0.001 |
| Metabolic syndrome (%) | 64.2% | 71.0% | 83.0% | 94.8% | <0.001 |
| Duration of diabetes (years) | 6 (3–12) | 6 (2–11) | 6 (3–11) | 6 (3–10) | 0.128 |
| Hemoglobin A1c (%) | 6.6% (6.1–7.2) | 6.5 (6.0–7.4) | 6.7% (6.1–7.8) | 7.0% (6.4–8.1) | <0.001 |
| Glucose (mg/dl) | 107 (88–129) | 109 (90–137) | 120 (97–152) | 129 (105–164) | <0.001 |
| Current tobacco use (%) | 56.4% | 59.0% | 60.0% | 59.9% | 0.257 |
| Current alcohol use (%) | 52.1% | 50.4% | 53.4% | 49.9% | 0.763 |
| 10 year Framingham risk (%)‡ | 11% (7–16) | 13% (8–20) | 16% (10–25) | 20% (13–31) | <0.001 |
| C-reactive protein (mg/L) | 1.57 (0.73–3.52) | 1.58 (0.86–4.10) | 2.08 (0.96–4.56) | 2.10 (1.00–4.64) | <0.001 |
| Lipid lowering | |||||
| medications Statins | 59.4% | 61.9% | 55.3% | 47.2% | <0.001 |
| Niacin | 4.8% | 4.0% | 6.6% | 6.8% | 0.060 |
| Fibrates | 2.5% | 4.4% | 6.8% | 11.9% | <0.001 |
| Ezetimibe | 5.7% | 6.5% | 5.2% | 3.7% | 0.111 |
| Fish Oil | 11.0% | 13.1% | 17.8% | 12.9% | 0.161 |
| Diabetes medications Metformin | 62.6% | 61.0% | 65.6% | 61.1% | 0.992 |
| Sulfonylureas | 28.9% | 32.5% | 36.4% | 40.1% | <0.001 |
| Thiazolidinediones | 21.1% | 22.1% | 23.6% | 23.3% | 0.349 |
| Insulin | 24.3% | 20.40% | 22.40% | 18.9% | 0.104 |
| Serum creatinine (mg/dl) | 0.90 (0.76–1.00) | 0.90 (0.72–1.00) | 0.90 (0.80–1.04) | 0.90 (0.80–1.10) | <0.001 |
| CAC score | |||||
| Median (IQR) | 19.5 (0–252.5) | 19 (0–282.5) | 54 (0–283) | 63 (0–311) | 0.001 |
| >0 (%) | 59.4% | 62.3% | 67.7% | 70.9% | <0.000 |
Metabolic syndrome was defined as have 3 or more following: waist circumference ≥ 102 cm or 40 inches (male), ≥ 88 cm or 35 inches(female), dyslipidemia: TG ≥ 1.7 mmol/L (150 mg/dl), HDL-C < 40 mg/dl (male), < 50 mg/dl (female), blood pressure ≥ 130/85 mmHg (or treated for hypertension). Data are presented as median (IQR) or as percentages, except as indicated.
P-value is from nonparametric test for trend across ordered groups, an extension of the Wilcoxon rank-sum test. ‡Total cholesterol 10-year Framingham risk percentage.
Abbreviations:
CAC=coronary artery calcium
HDL= high density lipoprotein
LDL = low density lipoprotein
VLDL-C= very-low density lipoprotein cholesterol
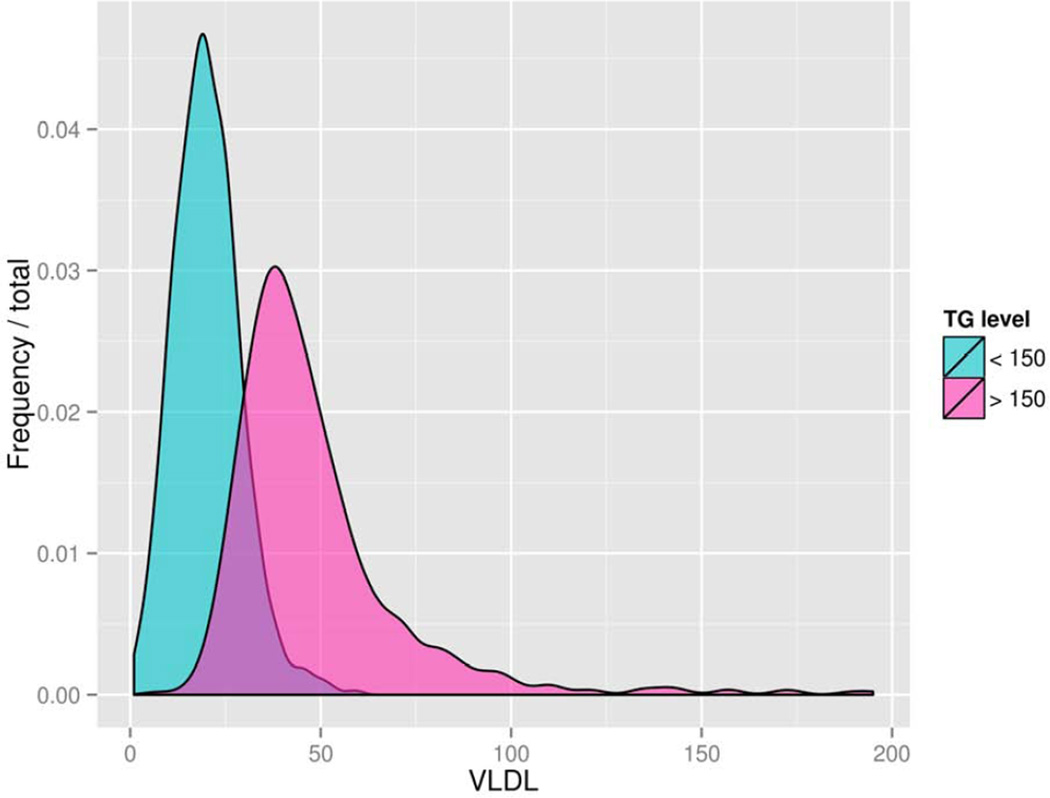
Figure 1 shows the distribution of VLDL-C values for PDHS participants with circulating TG values above and below 150 mg/dl, a clinically meaningful cut-point.16 Notably, there was substantial overlap in VLDL-C in these two groups with non-overlapping TG values suggesting that VLDL-C provides information distinct from total TGs regarding TGRL metabolism.
Figure 1.
Distribution of plasma VLDL-C across categories of low (<150 mg/dl) and high (>150 mg/dl) plasma triglyceride values.
Abbreviations:
TG=triglyceride
VLDL=very-low density lipoprotein
Association of VLDL-C with CAC in multivariable models
While there was no significant race interaction for VLDL-C association with CAC, there tended to be a gender difference, so results are presented stratified by gender as well as for the full sample. VLDL-C levels were positively associated with increasing CAC after adjusting for age, gender, and race, Framingham risk scores, exercise, HbA1c, medication and alcohol use, (Tobit Ratio (TR) 0.38 CI 0.12–0.65, P=0.005) and even after further adjusting for duration of diabetes, BMI, and CRP (TR 0.38 CI 0.12–0.65, P=0.005). Furthermore, addition of plasma apoB levels did not attenuate association of VLDL-C with CAC (TR 0.31 CI 0.03–0.58, p=0.030) (Table 2). Although the same trends were evident in both genders, there was approximately 3-fold stronger association of VLDL-C with CAC in women than in men (gender interaction P=0.034 in Model 1 and P=0.048 in Model 4), with statistically significant effects observed in women but not in men (Table 2). These finding persisted in subgroup analysis in patients not on lipid lowering therapy. (Supplemental Table 2)
TABLE 2.
Multivariate models of VLDL-C association with CAC
| Model | Full Sample Tobit Ratio (95% CI) P value |
Men Tobit Ratio (95% CI) P value |
Women Tobit Ratio (95% CI) P value |
|---|---|---|---|
| Model 1 | 0.40 (0.13–0.64) p=0.001 | 0.17 (−0.09–0.43) p=0.196 | 0.98 (0.41–1.54) p=0.001 |
| Model 2 | 0.38 (0.12–0.65) p=0.005 | 0.22 (−0.08–0.52) p=0.146 | 0.62 (0.04–1.22) p=0.035 |
| Model 3 | 0.38 (0.12–0.65) p=0.005 | 0.20 (−0.10–0.50) p=0.189 | 0.75 (0.16–1.34) p=0.013 |
| Model 4 | 0.31 (0.03–0.58) p=0.030 | 0.15 (–0.15–0.46) p=0.323 | 0.72 (0.09–1.36) p=0.026 |
Model 1: age gender, race.
Model 2: Model 1 + HbA1c, total cholesterol-based Framingham risk score, medications (zetia/statin, niacin/fibrate, ace inhibitor, calcium channel blocker, beta blocker, diuretic, metformin, sulfonylurea, thiazolidinediones, insulin), alcohol use, and exercise.
Model 3: Model 2 + BMI, duration of diabetes, and C-reactive protein.
Model 4: Model 3 + plasma apolipoprotein B.
Abbreviations:
BMI=body mass index
CAC=coronary artery calcium
CI=confidence interval.
HbA1c= hemoglobin A1c
VLDL-C= very-low density lipoprotein cholesterol
VLDL-C is associated with CAC in participants with elevated TG levels
VLDL-C may relate to CAC and CVD independent of TG levels because cholesterol-rich VLDL particles may impart greater atherogenic risk than total TGs which are distributed across all lipoprotein particles, not just VLDL. Because TG and VLDL-C values were highly correlated, however, their inclusion in models together can lead to excess colinearity and potential spurious findings. Therefore, we compared plasma VLDL-C and TG association with CAC in non-nested models using AIC and BIC scores and found that both AIC and BIC scores were slightly but consistently lower for VLDL-C (e.g., in fully adjusted Model 4; AIC 7263.65, BIC 7402.11) than TG (AIC 7263.94, BIC 7402.40). This suggests that VLDL-C performs better than TG in predicting CAC scores when all other confounders and covariates are held equal. Furthermore, we stratified PDHS participants into those with TG values below and above 150 mg/dl, a cut point used in clinical practice.16 In these stratified analyses, VLDL-C had stronger association with CAC in individuals with TGs >150mg/dl (TR 0.80, P=0.01) than those with TGs <150 mg/dl (TR 0.27, P=0.189) (Table 3) and followed the same gender patters as the primary analysis. Sensitivity analysis showed consistent results, TR 0.59 (P=0.013) and TR 0.19 (P=0.424) in individuals with TGs above or below the median value (116mg/dl) respectively.
TABLE 3.
Multivariate association of VLDL-C with CAC stratified by plasma TG levels
| Model | Full Sample Tobit Ratio (95% CI) P value |
Men Tobit Ratio (95% CI) P value |
Women Tobit Ratio (95% CI) P value |
|---|---|---|---|
| TG <150 (mg/dl) | 0.27 (−0.13–0.66) p=0.185 | 0.00 (−0.45–0.45) p=0.988 | 0.74 (−0.08–1.57) p=0.078 |
| TG >150 (mg/dl) | 0.80 (0.19–1.14) p=0.010 | 0.67 (0.00–1.34) p=0.051 | 1.31 (−0.13–2.76) p=0.074 |
Model includes age gender, race, HbA1c, total cholesterol-based Framingham risk score, medications (zetia/statin, niacin/fibrate, ace inhibitor, calcium channel blocker, beta blocker, diuretic, metformin, sulfonylurea, thiazolidinediones, insulin), alcohol use, exercise, BMI, duration of diabetes, and C-reactive protein.
Abbreviations:
CAC=coronary artery calcification
CI=confidence interval.
TG=triglycerides
VLDL-C=very-low density lipoprotein cholesterol
DISCUSSION
No prior studies have examined the relationship of CAC with circulating levels of VLDL-C, an alternative to serum TGs as a marker of TGRL. In patients with type 2 diabetes, a setting of abnormal TGRL metabolism and increased CVD risk, we found that VLDL-C levels were positively associated with higher CAC scores after controlling for numerous traditional CV risk factors, and even after inclusion of plasma apoB data. Consistent with published gender patterns for TGs, the association of VLDL-C with CAC was significantly stronger in women than men.2, 9 VLDL-C levels related to greater CAC scores in individuals with high TGs suggesting possible incremental value for VLDL-C in CVD risk prediction beyond serum TGs.
Renewed interest in TGRL as causal and therapeutic targets in heart disease has been driven by large epidemiological studies,1–4 trial failures of HDL-C therapies in CVD,21 and recent genomic work suggesting that TGRL may be causal in atherosclerotic CVD.6–8 The relationship between TGs and CVD has been the subject of much debate over the past several decades. On the one hand, many epidemiological studies have suggested that the relationship of TGs with CVD is lost after adjusting for both HDL-C and non-HDL-C, and that given knowledge of TC and HDL-C, assessment of TG provided little incremental information for CVD risk prediction.1, 5 This was particularly thought to be the case in diabetic patients, where high TG levels are often associated with low HDL-C levels and relative insulin resistance.22 The Framingham study showed than compared to healthy controls, diabetic patients were twice as likely to have low HDL-C levels, and accordingly, the independent effect of TGs on CVD was lost after adjusting for HDL-C.23 Yet other epidemiological studies and large meta-analyses support an independent association of TGs with CVD beyond LDL-C and HDL-C.1–4 PROCAM, a prospective study of 4849 middle aged men showed that fasting TGs were associated with future cardiovascular events, independent of LDL-C and HDL-C.24 In the Copenhagen Male Study of 2906 white men, rates of CVD were 50% higher in those with TGs in the middle tertile and 120% higher in the upper tertile as compared to those in the lowest TG tertile, after adjustment for conventional risk factors including LDL-C and HDL-C.3 A sub analysis of a post acute coronary syndrome population in PROVE IT-TIMI 22 showed that on treatment TG levels <150 mg/dl, independent of LDL-C, reduced risk of CVD, and that each 10mg/dl reduction in TG level was associated with a 1.6% reduction of primary endpoint.25 Finally, recent genetic data strongly support a causal role for TG associated loci in CAD.6–8 In large meta-analysis of genome wide association studies of plasma lipids, many TG-associated loci, unlike those for HDL-C, also relate to CVD. Indeed, Kathiresan and colleagues showed recently that even after accounting for effects on LDL-C or HDL-C, TG-associated loci still had a strong independent relation to CVD.8
There are several proposed mechanisms for why TGRL may have causal relations to CVD beyond more traditionally measured lipids, TC, LDL-C and HDL-C. TGRL particles are increased in excess calorie consumption particularly in insulin resistant states because of failure to clear post-prandial lipid excursions and due to increased hepatic VLDL production. VLDL particles from patients with hypertriglyceridemia may promote atherosclerosis. Each TGRL particle transports more cholesterol than each LDL particle, and therefore TGRLs are potentially more atherogenic on a per-particle basis.26 When TGRLs are elevated, as in subjects with high TG, large VLDL contain more cholesterol relative to these same particles from subjects with normal TG levels.27 VLDL sized lipoproteins are found in human intima and have been isolated from atherosclerotic lesions.28, 29 Furthermore VLDL and IDL levels are related to the progression of atherosclerosis in humans.30 Particular TGRLs are the only native, unmodified lipoproteins that can in vitro cause rapid, receptor-mediated macrophage lipid accumulation.26, 31 Additionally, VLDL from subjects with elevated TGs bind with high affinity both to LDL receptors and to a unique TGRL/apoB48 receptor expressed specifically by monocytes, macrophages, and endothelial cells.32 As a secondary consequence, increased TGRL levels also drive (via TG activation of cholesterol ester transfer protein (CETP) and exchange of TG for cholesterol) increased generation of small dense cholesterol-poor LDL particles that are atherogenic but do not result in increased circulating LDL-C levels. Similarly, TGRL drives remodeling of HDL-C to smaller cholesterol-poor particles that may lack atheroprotective functions and do not correlate with HDL-C levels.
Serum TGs are imperfect measures of atherogenic TGRL for several reasons. First, there is considerable inter-individual and intra-individual variation in plasma TG levels.33 Second, circulating TGRL are extremely heterogeneous particles both in terms of size and composition, and total serum TG levels are not sensitive to different types of particles carrying TG.34 Partially catabolized remnant particles are felt to be the most atherogenic.35 In this context, we hypothesized that cholesterol content of VLDL (VLDL-C) could be superior to total plasma TGs as an atherogenic marker because VLDL-C levels increase as VLDL is metabolized to smaller more atherogenic remnants.
TGRL are of particular importance in diabetic populations because insulin resistance increases hepatic VLDL production and decreases clearance of TGRLs. Studies of TGs in diabetic populations have provided mixed results. In a large study of 11,000 type 2 diabetics, TGs >150 were an independent risk factor for incident CVD in women but not men, yet non-HDL-C may be superior to TGs as a CVD risk factor in diabetes.36, 37 To our knowledge there are no published studies examining the relationship between VLDL-C and CAC, either in patients with diabetes or in the general population. In our type-2 diabetic PDHS sample, the association of VLDL-C with CAC persisted after adjusting for multiple traditional CVD risk factors, and more importantly, even beyond plasma apoB levels. Comparative non-nested models suggested that plasma VLDL-C has modestly stronger relation than TG levels to CAC. Further, the association of VLDL-C with CAC was strongest in patients with elevated TGs suggesting that VLDL-C may provide insight into atherosclerotic CVD beyond routine lipoprotein profiling. Our findings are supported by a prior small study of lipoprotein fractions in 313 diabetics patients followed for 7 years for CVD events in which higher VLDL-C was associated with increased risk of CVD, particularly in patients with low LDL-C and low HDL-C.38
In our study, there was a stronger association of VLDL-C with CAC in women than men. This may be explained by greater lipid abnormalities observed in post-menopausal diabetic women than men. In diabetic populations in particular, women have higher TG levels and have an overall more adverse lipid profile than men.39 Much of the atheroprotection conferred by female gender disappears after menopause.40 Indeed, postmenopausal state is associated with significantly higher postprandial TG levels despite similar fasting TGs.41 Of 672 women studied in PDHS, 83% were over 50 year old and 59% were over 55 years old, suggesting that most of the female PDHS sample was post-menopausal. Our finding is consistent with several studies showing a stronger relation of TGRL markers with CHD/CVD events in post-menopausal women, including diabetic patients, than in men.2, 42 For example, in a large meta-analysis of nearly 60,000 patients, relative risk for CVD with elevated TGs was 1.32 in men and 1.76 in women, with similar trends persisting after controlling for other risk factors.2
While we chose to estimate VLDL-C, alternative measures of TGRL can be performed. ApoB 100 is present on all apoB100-containing lipoproteins (one apoB per particle) including non-TGRL. Similarly, non HDL-C reflects cholesterol on all atherogenic particles including VLDL and TGRL. Several studies have demonstrated that both these measures are strong predictors of CVD and superior to LDL-C across a broad spectrum of risk including patients on statin therapy.43, 44 However, because plasma apoB and non HDL-C capture information regarding non TGRL, such as lipoprotein (a) and LDL, they are less specific tools when addressing the independent effects of TGRLs. Indeed, our data suggest that VLDL-C may provide utility beyond knowledge of plasma apoB.
Work by Nordestgaard suggested that a simple calculation (TC - LDL-C - HDL-C) could approximate remnant cholesterol, and that an increase of 1 mmol/l (39 mg/dl) in this estimate was associated with a 2.8-fold increased risk of CHD, independent of HDL-C levels.10 We calculated this estimate of remnant particles (TC - LDL-C - HDL-C) in our PDHS sample and found quite similar, but not identical, findings for the association with CAC compared to that for VLDL-C in our sample. (Supplemental Table 3) Direct measurement of remnant particles is also possible and these particles have been shown to strongly associate with CVD, especially in patients with normal TC levels.45 Yet accurate measurement of remnant particles is not straightforward. They are difficult to differentiate from their TG-rich precursors, are rapidly catabolized with low concentrations in plasma, and at any given time, are heterogeneous in size, density, and composition because of dynamic metabolism.11 Genes and their secreted products that regulate TGRL and relate to CVD (e.g., LPL and apoCIII) also provide biomarkers of TGRL and CVD risk that require further study.6–8, 46
Our work has several strengths and limitations. As noted, this is the first work to investigate the association of VLDL-C with CAC, a measure of subclinical atherosclerosis and a well-validated predictor of CVD risk including in type-2 diabetes.47–49 A large sample of almost 1900 type-2 diabetic patients including significant percentages of women and non-Caucasians were specifically recruited to be free of the confounding influence of chronic kidney disease or CVD at entry. Our analyses were robust to adjustment for multiple risk factors and potential confounders and pointed toward value for VLDL-C even beyond serum apoB and TG data. Limitations include cross-sectional design and lack of hard CVD outcomes or incident CVD events. However, CAC scores are strong independent predictors of CHD events in the general population as well as in prospective studies of patients with type 2 diabetes.47–50 Besides VLDL-C, additional markers of TGRL were not measured preventing us from making inference regarding the optimal measures for application in clinic. Future studies are needed to examine CVD outcomes for multiple measures of TGRL and their genetic predictors in large prospective studies of both diabetic and non-diabetic populations.
In conclusion, we show an association of VLDL-C with CAC, even after adjustment for traditional risk factors and beyond plasma apoB levels, and that such an association is stronger in females and when TG levels are elevated.
Supplementary Material
Highlights.
Association of VLDL-C with CAC after adjustment for traditional risk factors
Association of VLDL-C with CAC even beyond apoB levels
Association of VLDL-C with CAC is significantly stronger in women than men
Possible incremental value for VLDL-C in CVD risk prediction beyond serum TGs
ACKNOWLEDGEMENTS
This work was supported by a Clinical and Translational Science Award (UL1RR024134) from the National Center for Research Resources and a Diabetes and Endocrine Research Center award (P20-DK 019525), both from the NIH to the University of Pennsylvania, as well as research grants from GlaxoSmithKline and Merck Research Laboratories to M.P.R. M.P.R. is also supported by K24-HL107643.
S.B.P. contributed to data collection, conducted statistical analyses, interpreted findings, and had primary responsibility for writing the manuscript, C.K.M, J.F.F, K.R.R, A.B.B, contributed to data collection and management. M.R.R. provided endocrinology expertise for the analysis and interpretation of results and reviewed/edited the manuscript. M.P.R. was responsible for project conception, statistical analysis, interpretation of results, and manuscript writing with S.B.P. All authors have approved the manuscript for submission. M.P.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Parts of this study were presented in poster form at the 2013 American Heart Association Annual Meeting, Dallas, TX, 16-20 November 2013.
S.B.P, C.K.M, J.F.F, K.R.R, A.B.B, and M.P.R report no other potential conflicts of interest relevant to this article.
REFERENCES
- 1.Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 2.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. Journal of cardiovascular risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 3.Jeppesen J, Hein HO, Suadicani P, et al. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation. 1998;97:1029–1036. doi: 10.1161/01.cir.97.11.1029. [DOI] [PubMed] [Google Scholar]
- 4.Patel A, Barzi F, Jamrozik K, et al. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation. 2004;110:2678–2686. doi: 10.1161/01.CIR.0000145615.33955.83. [DOI] [PubMed] [Google Scholar]
- 5.Emerging Risk Factors, C. Di Angelantonio E, Sarwar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA : the journal of the American Medical Association. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triglyceride Coronary Disease Genetics, C, Emerging Risk Factors, C. Sarwar N, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Lipids Genetics, C. Willer CJ, Schmidt EM, et al. Discovery and refinement of loci associated with lipid levels. Nature genetics. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nature genetics. 2013;45:1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA : the journal of the American Medical Association. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 10.Varbo A, Benn M, Tybjaerg-Hansen A, et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. Journal of the American College of Cardiology. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 11.Jialal I, Devaraj S. Remnant lipoproteins: measurement and clinical significance. Clinical chemistry. 2002;48:217–219. [PubMed] [Google Scholar]
- 12.Martin SS, Qasim AN, Mehta NN, et al. Apolipoprotein B but not LDL cholesterol is associated with coronary artery calcification in type 2 diabetic whites. Diabetes. 2009;58:1887–1892. doi: 10.2337/db08-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirany S, Li D, Jialal I. A more valid measurement of low-density lipoprotein cholesterol in diabetic patients. The American journal of medicine. 1997;102:48–53. doi: 10.1016/s0002-9343(96)00375-0. [DOI] [PubMed] [Google Scholar]
- 14.Rifai N, Warnick GR, McNamara JR, et al. Measurement of low-density-lipoprotein cholesterol in serum: a status report. Clinical chemistry. 1992;38:150–160. [PubMed] [Google Scholar]
- 15.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy AE, Bielak LF, Kullo IJ, et al. Sex-specific associations of lipoprotein(a) with presence and quantity of coronary artery calcification in an asymptomatic population. Medical science monitor : international medical journal of experimental and clinical research. 2004;10:CR493–CR503. [PubMed] [Google Scholar]
- 19.Schwarz GE. Estimating the dimension of a model. Annals of statistics. 1978;6:461–464. [Google Scholar]
- 20.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 21.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. The New England journal of medicine. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 22.Harchaoui KE, Visser ME, Kastelein JJ, et al. Triglycerides and cardiovascular risk. Current cardiology reviews. 2009;5:216–222. doi: 10.2174/157340309788970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg A, Grundy SM. Management of dyslipidemia in NIDDM. Diabetes care. 1990;13:153–169. doi: 10.2337/diacare.13.2.153. [DOI] [PubMed] [Google Scholar]
- 24.Assmann G, Schulte H, Funke H, et al. The emergence of triglycerides as a significant independent risk factor in coronary artery disease. European heart journal. 1998;19(Suppl M):M8–M14. [PubMed] [Google Scholar]
- 25.Miller M, Cannon CP, Murphy SA, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. Journal of the American College of Cardiology. 2008;51:724–730. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 26.Gianturco SH, Bradley WA. Pathophysiology of triglyceride-rich lipoproteins in atherothrombosis: cellular aspects. Clinical cardiology. 1999;22:II7–II14. doi: 10.1002/clc.4960221403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenberg S, Gavish D, Oschry Y, et al. Abnormalities in very low, low and high density lipoproteins in hypertriglyceridemia. Reversal toward normal with bezafibrate treatment. The Journal of clinical investigation. 1984;74:470–482. doi: 10.1172/JCI111444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rapp JH, Lespine A, Hamilton RL, et al. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1994;14:1767–1774. doi: 10.1161/01.atv.14.11.1767. [DOI] [PubMed] [Google Scholar]
- 29.Proctor SD, Mamo JC. Retention of fluorescent-labelled chylomicron remnants within the intima of the arterial wall--evidence that plaque cholesterol may be derived from post-prandial lipoproteins. European journal of clinical investigation. 1998;28:497–503. doi: 10.1046/j.1365-2362.1998.00317.x. [DOI] [PubMed] [Google Scholar]
- 30.Phillips NR, Waters D, Havel RJ. Plasma lipoproteins and progression of coronary artery disease evaluated by angiography and clinical events. Circulation. 1993;88:2762–2770. doi: 10.1161/01.cir.88.6.2762. [DOI] [PubMed] [Google Scholar]
- 31.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annual review of biochemistry. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 32.Gianturco SH, Brown FB, Gotto AM, Jr, et al. Receptor-mediated uptake of hypertriglyceridemic very low density lipoproteins by normal human fibroblasts. Journal of lipid research. 1982;23:984–993. [PubMed] [Google Scholar]
- 33.Warnick GR, Albers JJ. Physiological and analytical variation in cholesterol and triglycerides. Lipids. 1976;11:203–208. doi: 10.1007/BF02532858. [DOI] [PubMed] [Google Scholar]
- 34.Fujioka Y, Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. Journal of atherosclerosis and thrombosis. 2009;16:145–154. doi: 10.5551/jat.e598. [DOI] [PubMed] [Google Scholar]
- 35.Zilversmit DB. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clinical chemistry. 1995;41:153–158. [PubMed] [Google Scholar]
- 36.Lu W, Resnick HE, Jablonski KA, et al. Non-HDL cholesterol as a predictor of cardiovascular disease in type 2 diabetes: the strong heart study. Diabetes care. 2003;26:16–23. doi: 10.2337/diacare.26.1.16. [DOI] [PubMed] [Google Scholar]
- 37.Avogaro A, Giorda C, Maggini M, et al. Incidence of coronary heart disease in type 2 diabetic men and women: impact of microvascular complications, treatment, and geographic location. Diabetes care. 2007;30:1241–1247. doi: 10.2337/dc06-2558. [DOI] [PubMed] [Google Scholar]
- 38.Laakso M, Lehto S, Penttila I, et al. Lipids and lipoproteins predicting coronary heart disease mortality and morbidity in patients with non-insulin-dependent diabetes. Circulation. 1993;88:1421–1430. doi: 10.1161/01.cir.88.4.1421. [DOI] [PubMed] [Google Scholar]
- 39.Walden CE, Knopp RH, Wahl PW, et al. Sex differences in the effect of diabetes mellitus on lipoprotein triglyceride and cholesterol concentrations. The New England journal of medicine. 1984;311:953–959. doi: 10.1056/NEJM198410113111505. [DOI] [PubMed] [Google Scholar]
- 40.Saha KR, Rahman MM, Paul AR, et al. Changes in lipid profile of postmenopausal women. Mymensingh medical journal : MMJ. 2013;22:706–711. [PubMed] [Google Scholar]
- 41.van Beek AP, de Ruijter-Heijstek FC, Erkelens DW, et al. Menopause is associated with reduced protection from postprandial lipemia. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2737–2741. doi: 10.1161/01.atv.19.11.2737. [DOI] [PubMed] [Google Scholar]
- 42.Stensvold I, Tverdal A, Urdal P, et al. Non-fasting serum triglyceride concentration and mortality from coronary heart disease and any cause in middle aged Norwegian women. Bmj. 1993;307:1318–1322. doi: 10.1136/bmj.307.6915.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA : the journal of the American Medical Association. 2012;307:1302–1309. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- 44.Barter PJ, Ballantyne CM, Carmena R, et al. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. Journal of internal medicine. 2006;259:247–258. doi: 10.1111/j.1365-2796.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 45.Masuoka H, Kamei S, Wagayama H, et al. Association of remnant-like particle cholesterol with coronary artery disease in patients with normal total cholesterol levels. American heart journal. 2000;139:305–310. doi: 10.1067/mhj.2000.100184. [DOI] [PubMed] [Google Scholar]
- 46.Musunuru K, Pirruccello JP, Do R, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. The New England journal of medicine. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raggi P, Shaw LJ, Berman DS, et al. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. Journal of the American College of Cardiology. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 48.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. The New England journal of medicine. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 49.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low-to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 50.Elkeles RS, Godsland IF, Feher MD, et al. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. European heart journal. 2008;29:2244–2251. doi: 10.1093/eurheartj/ehn279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.