Abstract
Walking while simultaneously performing cognitively demanding tasks such as talking or texting are typical complex behaviors in our daily routines. Little is known about neural mechanisms underlying cortical resource allocation during such mobile actions, largely due to portability limitations of conventional neuroimaging technologies. We applied an EEG-based Mobile Brain-Body Imaging (MOBI) system that integrates high-density event-related potential (ERP) recordings with simultaneously acquired foot-force sensor data to monitor gait patterns and brain activity. We compared behavioral and ERP measures associated with performing a Go/NoGo response-inhibition task under conditions where participants (N=18) sat stationary, walked deliberately or walked briskly. This allowed for assessment of effects of increasing dual-task load (i.e. walking speed) on neural indices of inhibitory control. Stride time and variability were also measured during inhibitory task performance and compared to stride parameters without task performance, thereby assessing reciprocal dual-task effects on gait parameters. There were no task performance differences between sitting and either walking condition, indicating that participants could perform both tasks simultaneously without suffering dual-task costs. However, participants took longer strides under dual-task load, likely indicating an adaptive mechanism to reduce inter-task competition for cortical resources. We found robust differences in amplitude, latency and topography of ERP components (N2 and P3) associated with inhibitory control between the sitting and walking conditions. Considering that participants showed no dual-task performance costs, we suggest that observed neural alterations under increasing task-load represent adaptive recalibration of the inhibitory network towards a more controlled and effortful processing mode, thereby optimizing performance under dual-task situations.
Keywords: dual-task design, event-related potentials, gait, inhibitory control, EEG, Mobil Brain-Body Imaging
INTRODUCTION
Humans continuously process sensory and cognitive events while engaged in everyday activities such as walking. For example, we successfully navigate the aisles of a shopping center as we rehearse a shopping list and contemplate the necessary ingredients for that evening's dinner. Most everyday situations require this type of multitasking and brain processes have evolved to handle concurrent processing of cognitive and motor functions. However, research on multitask performance has provided clear evidence for costs, indicating that cognitive-motor interference (CMI) can compromise performance in one or both domains (Woollacott and Shumway-Cook, 2002, Al-Yahya et al., 2011). This is particularly the case for older individuals (Al-Yahya et al., 2011) where performing cognitively demanding tasks while walking greatly increases the risk of falling. As such, revealing the neural bases of CMI has important clinical implications and the development of objective brain measures of increased cognitive-motor interference could well provide biomarkers that predict increased risk of falls, allowing for early detection and intervention. In turn, these measures may serve as early risk indicators (i.e. endophenotypes) for those who will go on to develop clinically significant cognitive impairments such as Alzheimer's disease.
Behavioral dual-task studies have provided robust evidence for a mutual influence suggesting that motor and cognitive functions are supported in part by common neural processes (Woollacott and Shumway-Cook, 2002). Postural stability during walking varies depending on the complexity of the cognitive task (Woollacott and Shumway-Cook, 2002) and strong associations between age and speed reduction under dual-task conditions have been reported (Al-Yahya et al., 2011). At the same time, walking impinges on cognitive performance with studies showing impaired spatial memory capacity, and target detection time (Lajoie et al., 1993). More specifically, Lajoie and colleagues showed that detection time increased during the single-support phase (i.e. one foot in swing) of the gait cycle, suggesting that attentional demands co-vary with differences in balance requirements during the gait cycle (Lajoie et al., 1993).
While behavioral evidence suggests reliance on common brain processes, only a few studies have directly assessed cortical involvement during walking with (Holtzer et al., 2011, Uehara et al., 2011, Doi et al., 2012) and without (Suzuki et al., 2008, Harada et al., 2009, Gramann et al., 2011, Gwin et al., 2011, Kurz et al., 2012) engagement in a secondary task. Studies using functional magnetic resonance imaging (fMRI) have identified relevant cortical regions, showing activations during preparation and execution of rhythmic foot and leg movements in frontal and primary motor cortex (Sahyoun et al., 2004, Heuninckx et al., 2005, Heuninckx et al., 2008). Obviously these studies are limited by the lack of realistic mobility of the participants. Studies using functional near-infrared spectroscopy (fNIRS) assessing oxygenated hemoglobin (oxyHb) have reported increased oxyHb levels in the prefrontal and premotor cortices of participants who were preparing to walk (Suzuki et al., 2008), and that inter-individual variations in stride-time interval correlated positively with oxyHb response within the pre-central gyrus and supplementary motor area (Kurz et al., 2012). In a dual-task study by Holtzer and colleagues, oxyHb levels in prefrontal cortical (PFC) regions increased during a "walking and talking" dual-task scenario in contrast to a walking-alone condition, especially in young participants. An association between trunk stability under dual-task conditions and grey matter atrophy has been established (Doi et al., 2012), and a transcranial magnetic stimulation (TMS) study showed that the excitability of the primary motor cortex during a dual motor task varied as a function of gait speed (Uehara et al., 2011).
Neuroimaging studies have been successful in defining relevant cortical areas and related changes in activity under dual-task conditions (Holtzer et al., 2011). However, hemodynamic imaging methods lack the temporal resolution necessary to determine and dissociate the susceptibility of specific processing stages to CMI. Broader susceptibility with interference that affects multiple processing levels of the secondary task might be related to increased dual-task costs. Event-related potentials (ERP) provide temporally precise measures of information processing that are very well-suited to dissociate between sensory-perceptual, cognitive and motor processing stages. Work by our group (Nolan, 2009, Nolan et al., 2011, De Sanctis et al., 2012a, Nolan et al., 2012) and others (Gramann et al., 2010, Gwin et al., 2010, Gramann et al., 2011, Gwin et al., 2011, Bulea et al., 2013, Duvinage et al., 2013) has demonstrated the feasibility of acquiring high-density EEG to investigate evoked potentials related to perceptual and cognitive processes during active and passive self-motion. For example, participants standing or walking on a treadmill while performing a visual oddball task produced entirely typical ERP components with excellent signal-to-noise characteristics (Gramann et al., 2010).
Here, we deployed high-density scalp EEG recordings while participants performed a taxing visual Go/NoGo response inhibition task, which requires subjects to overcome a potent response tendency established by frequent Go stimuli to successfully inhibit response execution to NoGo stimuli. We also used force sensors attached to the sole of each foot to measure duration and variability of the gait cycle while participants walked on a treadmill. To assess the influence of walking load on response inhibition, we compared participants Go/NoGo task performance under three activity conditions: 1) sitting, 2) walking deliberately (2.4 km/hour) and 3) walking briskly (5.0 km/hour). We predicted that walking, particularly at higher speed, would compromise inhibitory control abilities. To assess the influence of response inhibition load on gait, we compared duration and variability of the gait cycle while participants walked on the treadmill with and without performance of the Go/NoGo inhibition task.
EEG based studies identifying specific phases of inhibitory network activity have distinguished relatively early automatic processes, as represented by the N2 component (250–350ms), from late controlled processes, as represented by the P3 component (400–550ms) (Eimer, 1993, Falkenstein et al., 1995, Falkenstein et al., 1999). We set out to investigate walking-related effects on the N2 and P3 components, allowing us to assess the susceptibility of different inhibitory processing stages to CMI. Furthermore, effects of CMI at the sensory-perceptual processing level were assessed by considering the visual-evoked potential (VEP) to the NoGo stimulus. Based on preliminary results by our group (De Sanctis et al., 2012a), which showed that CMI strongly modulated the N2 component, we predicted increased susceptibility of early and automatic processing stages of inhibitory control to motor load. Furthermore, we predicted an increase in stride-to-stride variability while participants perform the inhibitory task, indicative of dual-task costs in the form of less stable gait patterns.
METHODS
Participants
Eighteen neurologically healthy participants (10 male) with normal or corrected-to-normal vision participated in this experiment. The age range was 21.8 to 36.1 years (mean 27.2 years). Written informed consent was obtained from all participants according to a protocol approved by the institutional review board at The Albert Einstein College of Medicine. Participants were paid a modest fee of $12 per hour for participating in the study. All procedures employed were compliant with the tenets laid out in the Declaration of Helsinki for the responsible conduct of research.
Stimuli and Task
We used 168 pictures from the International Affective Picture System (IAPS), a set of normative photographs that includes content across a wide range of semantic categories(Lang et al., 2008). Only affectively neutral images were employed here and these were presented centrally for 600ms with a random stimulus-onset-asynchrony (SOA) ranging from 800 to 1000ms. On average, images subtended 28° horizontally by 28° vertically. Stimuli were presented using Presentation software version 14.4 (Neurobehavioral Systems, Albany, CA, USA). Participants performed a Go/NoGo task, responding quickly and accurately to every presentation of an image by clicking a computer mouse button, while withholding responses to the second instance of any stimulus repeated twice in a row. The probability of Go and NoGo trials was 0.85 and 0.15, respectively. Our choice of a Go/NoGo task as the cognitive challenge here was based on repeated findings that executive functioning, more than memory or verbal IQ, shares a common neural substrate with motor processing (Holtzer et al., 2006, Holtzer et al., 2012). Versions of this simple task have been widely employed in prior work (Garavan et al., 2002, Kelly et al., 2004), leading to entirely typical N2/P3 activation patterns (De Sanctis et al., 2012a, Morie et al., 2013) as well as hemodynamic activation within areas of the neural circuit typically associated with inhibitory control (Bell et al., 2013). The task also leads to relatively high rates of commission errors (i.e. erroneous button pushes to stimulus repeats) and is therefore very well-suited for the examination of the central hypotheses of the current study.
Data were acquired in five conditions: participants were asked to (1) sit and perform the response inhibition task, (2) walk deliberately (2.4 km/hr) and perform the response inhibition task, (3) walk briskly (5 km/hr) and perform the response inhibition task, (4) walk deliberately without performing the response inhibition task, or (5) walk briskly without performing the response inhibition task. Participants walked on a treadmill (LifeFitness TR-9000) approximately 1.5m from a black wall, onto which stimulus images were projected (InFocus XS1 DLP, 1400×1050 pxl). Participants completed 17 blocks (approximately 4 minutes apiece), consisting of a minimum of 4 blocks for each of the first 3 conditions. In addition, participants were asked to complete 2 blocks of walk deliberately and briskly without performing the cognitive task. Conditions were presented in a pseudorandom order. Subjects rested between blocks to prevent fatigue. Total testing time was between 75 and 105 minutes.
Gait Cycle Recording and Analysis
To quantify the gait cycle, we attached force sensors (Tekscan FlexiForce A201 transducers) to the sole of each foot. We placed transducers at the center of the back side of the heel, the big toe ball and midway along the outer longitudinal arch to detect changes in pressure force during different stance phases, including loading response, mid-stance, terminal stance, and pre-swing. The force signal was sampled at 512 Hz using an Analog Input Box (BioSemi), which was connected via optical fiber with the Biosemi ActiveTwo EEG system. The continuous data were butterworth low-pass filtered at 7Hz. Continuous data were epoched at 10 sec, and normalized against the standard deviation. To assess stride time, we measured peak-to-peak interval using the force signal derived from the right heel sensor (e.g. time of a complete cycle – heel contact to next heel contact). We used automatic peak detection software (MATLAB custom scripts) with one standard deviation as threshold to determine if each peak was significantly larger than the data around it. Peak-to-peak intervals (PPI) were excluded from the gait analysis if duration to complete a cycle was < 500 or > 1500 msec.
Event Related Potential Recording and Analysis
Brain activity was recorded using a 72-channel EEG system (Biosemi ActiveTwo EEG system). The data were recorded at 512 Hz and bandpass filtered from 0.05 to 100Hz (24 dB/octave). EEG data were re-referenced offline to an average reference. The data were analyzed offline using FieldTrip toolbox (Oostenveld et al., 2011) (see http://www.ru.nl/neuroimaging/ fieldtrip) and custom scripts for MATLAB (MathWorks, Natick, MA). The continuous data were bandpass filtered offline from 0.5–30Hz (12 dB/octave). We applied an automatic artifact rejection criterion of ±100 μV across all electrodes in the array. Trials with more than six artifact channels were rejected. On trials with less than six such channels, we interpolated any remaining bad channels using a nearest neighbor spline. Epochs of 1050ms, including a 50ms pre-stimulus baseline, were analyzed. Go trials on which the participants responded successfully were defined as hits. NoGo trials on which participants correctly withheld their response were defined as Correct Rejects (CR). Trials on which participants responded incorrectly were excluded from the analysis..
VEP amplitudes on NoGo-trials were computed using three electrode sites O1, Oz, and O2 over the occipital scalp region in a time window from 140 to 160ms. Walking-related differences in VEP latency and mean amplitude were assessed using two-way repeated-measures ANOVA with factors of condition (sitting/walking deliberately/walking briskly) and electrode site (O1/Oz/O2).
The N2 and P3 components on NoGo-trials were computed using three electrode sites: FCz, Cz, and CPz. Electrode sites FCz, Cz, and CPz were chosen based on previous studies by us and others indicating that NoGo N2/P3 amplitudes are generally maximal over front-central scalp regions (Eimer, 1993, Katz et al., 2010). We averaged across conditions and used the grand mean ERP peak latency to encapsulate a 100 ms time window for the N2 (peak latency N2CR-trials = 260ms, N2-diffCR minus Hit =290ms) and a 250 ms time window for the P3 (P3CR-trials = 475ms; P3CR minus Hit = 475ms) to compute the mean amplitude across the respective time windows. Walking-related differences in N2/P3 latency and mean amplitude were assessed using two-way repeated-measures ANOVA with factors of condition (sitting/walking deliberately/walking briskly) and electrode site (FCz/Cz/CPz). The latency on NoGo-trials was quantified using automatic peak-picking procedure (MATLAB custom scripts) which identified the maximal deflection within the latency period 210–310 for the N2 and 350–600 ms for the P3 component.
Statistical Cluster Plot
To provide a more general description of the spatio-temporal properties of dual-task ERP differences, we computed statistical cluster plots (SCP) for the CRERP between sitting versus walking deliberately, sitting versus walking briskly and walking deliberately versus walking briskly. This procedure has been used effectively in post hoc analyses as a means to more fully explore complex datasets and generate pointed follow-up hypotheses (Molholm et al., 2002, Murray et al., 2002). Point-wise two-tailed t-tests between a given pair of conditions were calculated at each time-point for all electrodes. The results of the point-wise t-tests from 64 electrodes are displayed as an intensity plot to efficiently summarize and facilitate the identification of the onset and general topographic distribution of walking-related modulation in ERP activity. The x-, y-, and z-axes, respectively, represent time, electrode location, and the t-test result (indicated by a color value) at each data point. For each scalp electrode, only the first time point where the t-test exceeded the 0.05 p-value criterion for at least 11 consecutive data points (>20 ms at a 512 Hz digitization rate) is considered significant (Guthrie and Buchwald, 1991, Foxe and Simpson, 2002). The resulting statistical cluster plots are a suitable alternative to Bonferroni correction for multiple comparisons, which would increase the likelihood of type II errors through overcompensation for type I errors (Snyder et al., 2012).
Topographical statistics (TANOVA)
To test for dual-task walking-related modulations in topography, we calculated the global dissimilarity (GD) (Lehmann and Skrandies, 1980) of the CRERP between sitting versus walking deliberately, sitting versus walking briskly and walking deliberately versus walking briskly. GD is a method to assess configuration differences between two scalp distributions, independent of their strength, as the data are normalized using the global field power. The GD equals the square root of the mean of the squared differences between the potentials measured at each of the 64-scalp electrodes. For each subject and time point, the GD indexes a single value, which varies between 0 and 2 (0 = homogeneity, 2 = inversion of topography). To create an empirical probability distribution against which the GD can be tested for statistical significance, the Monte Carlo MANOVA was applied. This is a nonparametric bootstrapping procedure, wherein each subject’s data from each time point was permutated such that they could ‘‘belong’’ to either condition. The dissimilarity was then calculated for each of 5000 such permutations for each time (Manly, 1997).
Topographical voltage maps
Scalp topographic maps represent interpolated voltage distributions, derived from 64-scalp measurements. These interpolated potential maps are displayed on the 3D reconstruction of a rendered scalp surface (derived from an anatomical MRI) as implemented in the BESA2000 (Ver. 5.0) multimodal neuroimaging analysis software package (MEGIS Software GmbH, Munich, Germany). The topographical mapping focused on the time period between 400 and 550 ms, during which TANOVA revealed most robust topographical differences between sitting and walking.
Signal-to-Noise Statistics
To test the signal to noise ratio (SNR) across the three conditions, we computed global field power (GFP) for hits and CR evoked potentials. The background noise was estimated from the pre-stimulus period (−100 to −40), and the signal was estimated from the first major peak (100–160ms). The squared signal was divided by squared noise and converted to decibels in order to be scale-invariant. The resulting SNRs were subjected to 3 (condition: sitting, walking-deliberately, walking briskly)×2 (trial: hits vs CR) repeated measures ANOVA. To confirm that there was minimal contamination of the broadband evoked response from muscles and eye movements which are commonly shown in frequencies of 8Hz or higher, we performed a Fast Fourier Transform on the epoched Go trials for each participant and computed the correlation coefficient matrix between conditions.
RESULTS
Behavioral Results
Table 1 shows reaction times (RT), hits and correct rejection (CR) rates for performing the Go/NoGo task during sitting, walking deliberately and walking briskly. Hit rates were higher for sitting compared to walking (hit: f2,34=8.8, p=.001), although this amounted to an extremely modest 0.2% performance difference in real terms. RT and CR rates did not differ between sitting and either walking speeds (RT: f2,34=1.9, p=.16; CR: f2,34=.73, p=.48).
Table 1.
Reaction time (RT) and percent hits on Go trial and correct withholds on NoGo trials as participants performing the Go/NoGo task were sitting, walking deliberately, and walking briskly. Standard errors are in parentheses.
| Sitting | Walking deliberately |
Walking briskly |
|
|---|---|---|---|
| RT in ms. | 364.6 (6.2) | 375.4 (8.2) | 369.4 (8.1) |
| Hit in % | 99.7 (0.1) | 99.5 (0.2) | 99.5 (0.2) |
| CR in % | 65.2 (3.3) | 66.2 (3.2) | 65.8 (3.3) |
Gait Analysis Results
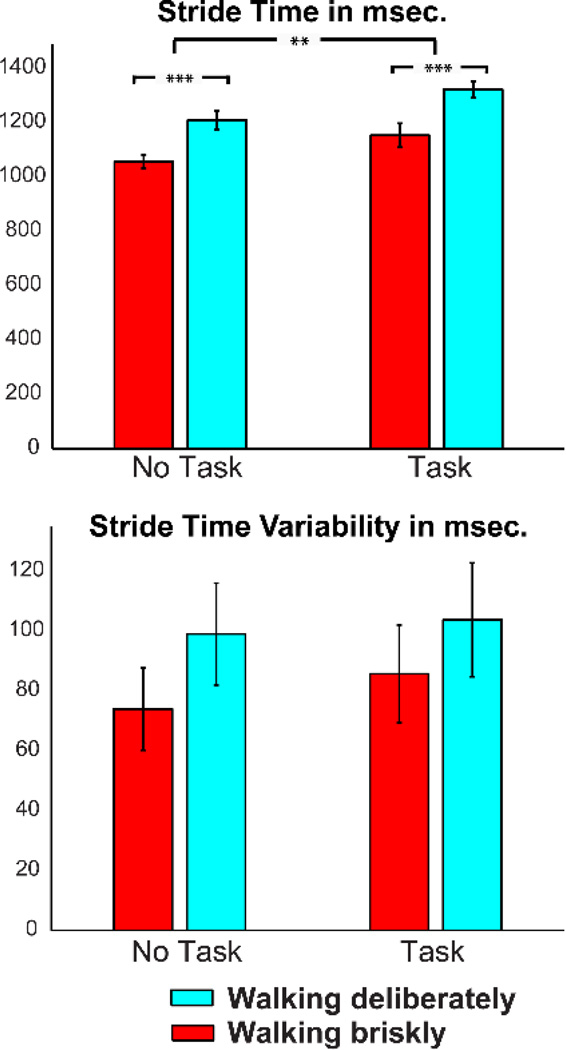
Data in figure 2 show the influence of performing an inhibitory task on stride time and variability for seventeen1 participants walking deliberately and briskly on the treadmill. Stride time increased while performing the inhibition task (f1,16 = 9.95, p<.006), indicating that participants increased stride length under dual-task load. Stride time decreased while walking briskly (f1,16=18.5, p<.001), indicating that participants required to walk faster decreased the time taken per stride. No effects of dual-task and walking speed on stride-to-stride variability were observed.
Figure 2.
Average stride time and variability (STD) in milliseconds for walking deliberate and brisk with and without engaging in a Go/NoGo inhibitory control task.
Electrophysiological Results
Feasibility of Recording
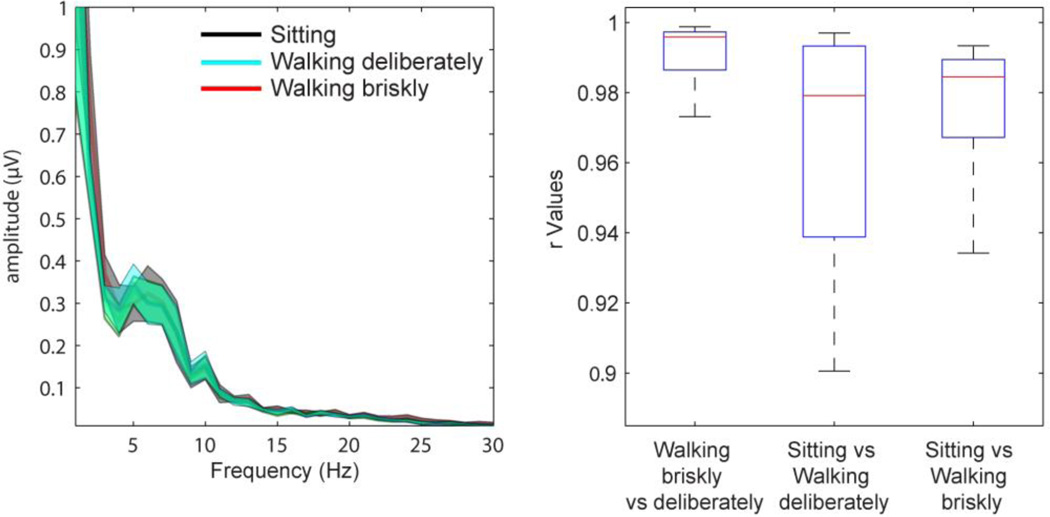
To illustrate the feasibility of recording ERPs with high SNR while participants walk on a treadmill, we compared the SNR for the hit and CR trials across all three conditions (see Table 2). The two way repeated-measures ANOVA with factors of condition (sitting/walking-deliberately/walking-briskly) and trial (hits/CR) revealed a main effect of trial (f1,17 = 63.7, p < .0001). The effect simply results from the differences in the probability of Go and NoGo trials. No other effects reached significance. All SNRs were extremely robust, pointing to the high sensitivity of the measures. These results concord with our previous findings where we showed no difference between sitting and walking conditions for the grand mean ERP and frequency spectrum (De Sanctis et al., 2012a). In addition, we compared the frequency spectra between sitting and walking using Fast Fourier Transform on the responses to the hit trials. The left panel of figure 3 shows the grand mean and standard deviation with largely overlapping spectra between conditions. The correlation coefficient values between conditions seen in the right panel of figure 3 were all > 0.95.
Table 2.
Mean and standard error signal-to-noise ratio (SNR) for hit and correct reject trials during sitting, walking deliberately, and walking briskly.
| Sitting | Walking deliberately |
Walking briskly |
|
|---|---|---|---|
| SNR Hit | 33.9 (2.3) | 37.1 (2.01) | 34.9 (1.8) |
| SNR CR | 23.4 (2.2) | 25.5 (2.2) | 26.3 (1.6) |
Figure 3.
Left panel; Grand mean and standard deviation spectra of participant’s visual evoked potential over central scalp regions for HIT trials during sitting, walking deliberately and walking briskly. Right panel; Box plot of the Pearson's correlation coefficient of the spectra between walking deliberately vs. walking briskly, sitting vs. walking deliberately, and sitting vs. walking briskly.
ERP Results
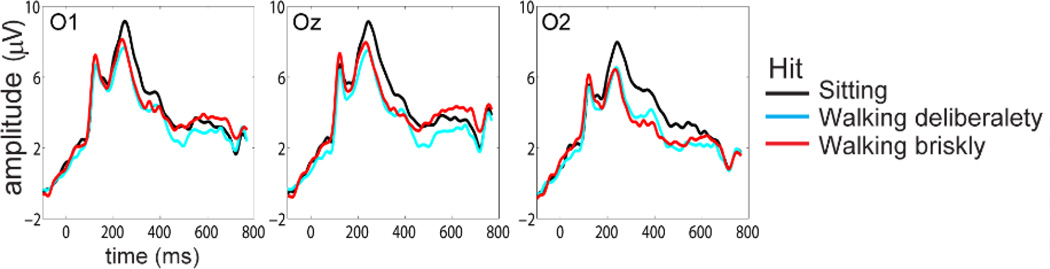
Figure 4 shows the waveforms of the VEP for CR trials at three occipital electrode sites while participants were sitting (black), walking deliberately (cyan) and walking briskly (red). Largely overlapping waveforms across all three electrode sites were observed. Statistical assessment of the first positive peak amplitude within the time period from 140 to 160ms using a two-way repeated-measures ANOVA with factors of condition (sitting/walking-deliberately/walking-briskly) and electrode location (O1/Oz/O2) revealed a significant main effect of electrode location (f2,34 = 3.8, p <0.031) reflecting enhanced amplitude over the left occipital scalp region. No further comparisons were significant.
Figure 4.
Grand mean (n=18) visual-evoked potentials (VEPs) over occipital scalp regions for Hit trials during sitting, walking deliberately, and walking briskly.
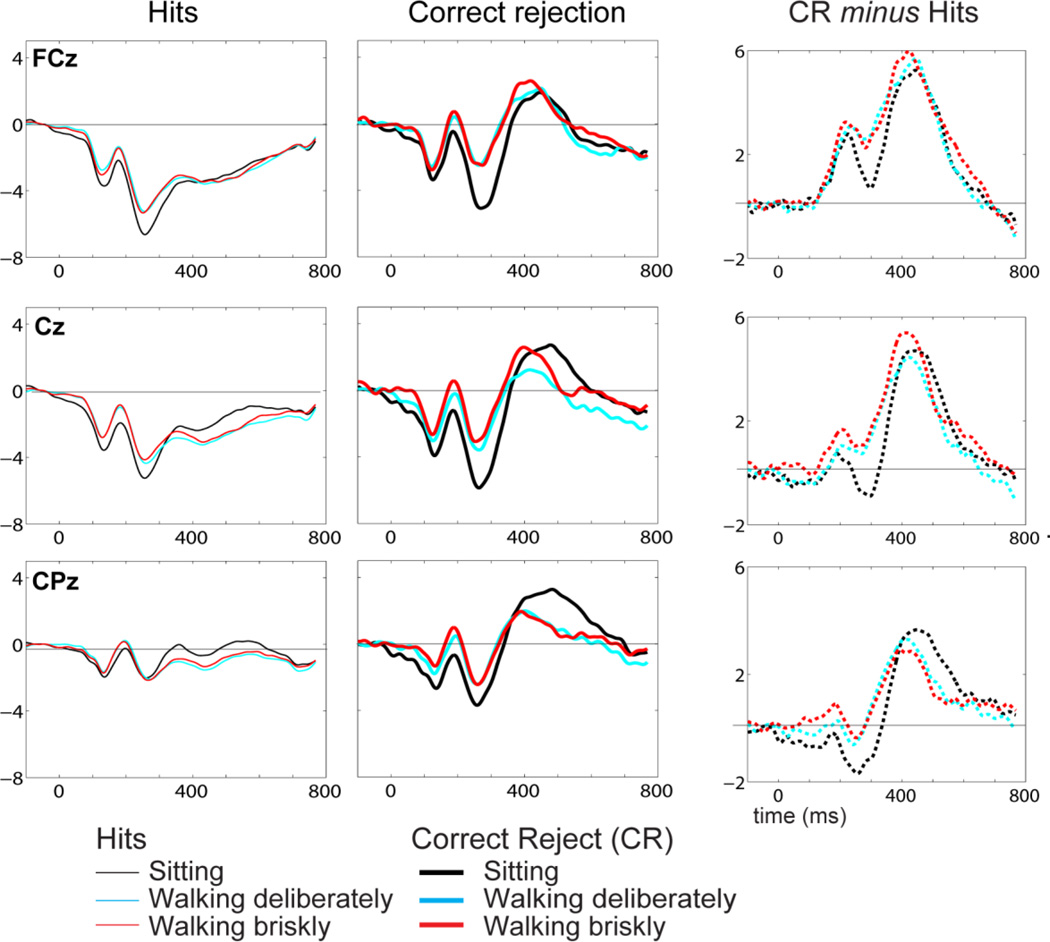
Figure 5 shows the N2/P3 component complex at three midline electrode locations (FCz, Cz, and CPz – average reference) for hits (thin lines) and correct rejections (thick lines) for sitting, walking deliberately, and walking briskly as well as the difference waves (CRERP minus HitERP; right column). As can be seen most clearly in the difference waves, a robust reduction in N2 amplitude was evident for both walking deliberately and briskly, compared to sitting. No differences in N2 peak latency were observed. During the P3 time period, amplitude reduction over centro-parietal region (i.e. CPz) was observed for walking deliberately and briskly, with the strongest decrease seen for walking briskly. In contrast, P3 amplitude increased over fronto-central regions (i.e. FCz/Cz) for walking briskly, compared to sitting.
Figure 5.
Grand mean ERPs for hits (left column) and correct rejections (middle column) as participants were sitting, walking deliberately, or walking briskly and performing the Go/NoGo task. The ERPs are shown at three midline electrode sites over frontal, central, and parietal scalp regions. Difference waves (ERPHits minus ERPCR) are displayed in the right column.
The two-way repeated-measures ANOVA with factors of condition (sitting/walking-deliberately/ walking-briskly) and electrode location (FCz, Cz, CPz) assessing N2 amplitude in correct rejection trials revealed a significant main effect of condition (f2,34 = 15.47, p <0.001), electrode (f2,34 = 5.8, p =0.006), and no condition by electrode interaction (p =0.48). Post hoc analysis confirmed that N2 reduction was reduced for walking at either speeds compared to sitting (p-values: 0.01 to 0.0001). The ANOVA assessing N2 difference waveforms revealed main effects for condition (f2,34 = 8.41, p <0.001) and electrode (f2,34 = 49.5, p <0.0001), and no condition by electrode interaction (p =0.52). Post hoc analysis confirmed N2 reduction for walking compared to sitting (p-values: 0.047 to 0.005).
The two-way repeated-measures ANOVA assessing P3 amplitude in correct rejection trials revealed a significant condition by electrode interaction (f4,68 = 6.7, p< 0.0001). Post hoc analysis confirmed that P3 reduction for walking at either speeds relative to sitting was only reduced over central and centro-parietal scalp (p-values: 0.01 to 0.001). No difference was observed between walking deliberately and walking briskly (p >0.15). The ANOVA assessing P3 difference waveforms revealed a significant effect of electrode (f2,34 = 20.49, p <0.0001) and a condition by electrode interaction (f4,68 = 2.7, p=0.035). Post hoc analysis did not reveal P3 differences between sitting and either walking speeds at any of the electrode sites (0.14 < p’s < 0.71).
For the P3 peak latency, a significant effect of condition (f2,34 = 6.01, p =0.006) and electrode (f2,34 = 3.97, p =0.028) was observed. Post hoc analysis confirmed that the P3 peaked earlier over centro-parietal regiosn for walking briskly (t17 = −2.4, p <0.027) and over fronto-central regions for walking deliberately (t17 = −2.8, p =0.012) compared to sitting. No difference was observed between walking deliberately and walking briskly (p=.17).
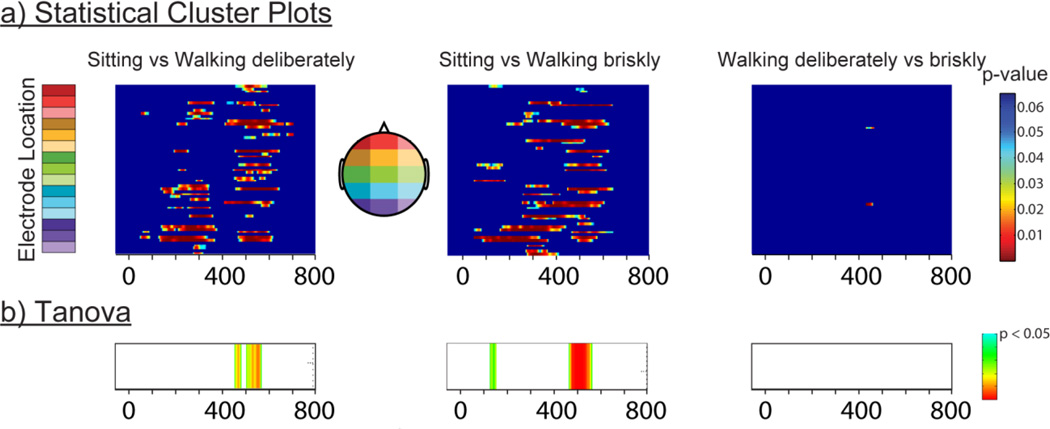
Figure 6a shows statistical cluster plots of differential activation in CR trials between sitting versus walking deliberately, sitting versus walking briskly and walking deliberately versus walking briskly. The results show robust clusters of differential activation between sitting and either walking speeds during the N2 and P3 latency period extending across fronto-parietal scalp regions. In contrast, no differential activation was observed between walking deliberately and walking briskly. Figure 6b presents the results of the TANOVAs assessing the dissimilarity of the topographic distribution between conditions across the 900-ms epoch. Topographical differences between sitting and both walking speeds were most robustly seen during the P3 latency period, while no difference was found for the comparison walking deliberately versus walking briskly.
Figure 6.
a) Statistical cluster plots (top row) assessing onset and distribution of differential ERP responses between sitting versus the two walking conditions (deliberately & briskly) and between walking deliberately versus walking briskly. Color values indicate the result of point-wise t-tests evaluating the differential activation across the 900ms time epoch (x-axis) and 64 electrode montage (y-axis; see Materials and Methods for details of electrode locations). For clarity, only p values < 0.05 are color-coded, and only then when fifteen consecutive data points (30 ms) exceeded this criterion. b) TANOVA (bottom row) assessing periods of statistically significant topographical differences between conditions.
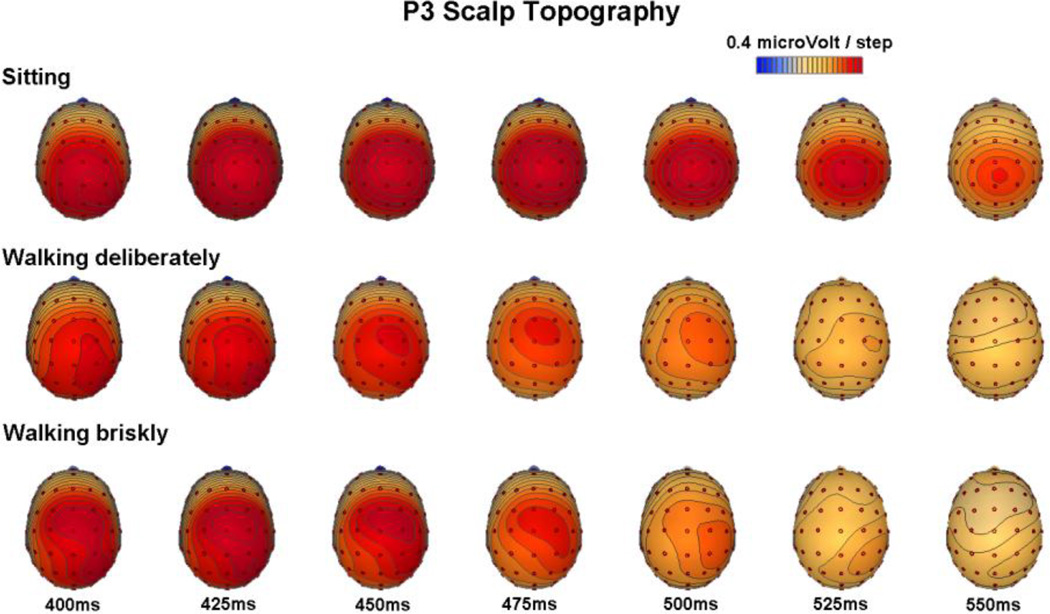
Figure 7 shows the scalp topographic maps during the P3 time period on correct rejection trials for sitting, walking deliberately and walking briskly. We singled out the P3 time period for topographic mapping based on TANOVA results indicating significant topographical differences during this timeframe, pointing to shifts in the underlying generator configuration. During sitting, as participants performed the Go/NoGo task under single-task load, P3 maps reveal the often replicated distribution with a maximum over central scalp (Polich, 2007). In contrast, during walking, as participants performed the Go/NoGo task under dual-task load, maps reveal a more anterior P3 distribution with a maximum over fronto-central scalp.
Figure 7.
Topographical distribution of voltage activity across the scalp depicting maps at 400, 425, 450, 475, 500, 525, and 550 ms. The interval from 400 to 550 ms was chosen based on the TANOVA results, which indicate significant differences of topographical distribution between sitting and walking conditions during this time period. Scalp maps spanning the P300 timeframe reveal a shift towards a more anterior distribution as participants perform the task while walking.
DISCUSSION
Dual-task designs, particularly when used in combination with EEG methods, have mostly deployed what could be described as a minimalistic behavioral approach, reducing behavior in response to task relevant stimuli to simple button presses (De Sanctis et al., 2012b). This minimalist approach allows for precise recording of stimulus- and response-evoked EEG activity and helps to limit the problems of separating neural from muscle-related activity, issues that can arise when participants engage in more complex real-world behaviors such as walking. However, and perhaps somewhat surprisingly, we found that walking-related muscle artifacts did not substantially compromise the recording of inhibition-related ERP components, and no differences in signal-to-noise ratio (SNR) and frequency spectra were evident in the ERPs recorded during sitting versus walking. Indeed, the standard signal-averaging approach appeared to be perfectly efficient in removing any walking-related artifacts, since the temporal dynamics of both the cognitive and walking tasks were unrelated to each other (see (Gwin et al., 2010, 2011) for an alternative approach). Here we built upon recent work demonstrating the feasibility of recording high quality task-related EEG activity while participants are asked to walk on a treadmill (Makeig et al., 2009, Gramann et al., 2010, Gwin et al., 2010, 2011). We compared behavioral and ERP measures associated with an inhibitory control task under conditions where participants were sitting, walking deliberately or walking briskly, to assess effects of increasingly higher dual-task load (i.e. walking speed) on the neuro-circuitry supporting sensory-perceptual and inhibitory processing. We also compared stride timing and variability between walking with and without performance of an inhibitory control task, to assess effects of dual-task load on gait parameters.
There were no differences in task performance between sitting and either walking speeds, suggesting that participants were perfectly well able to perform the inhibition task while walking without suffering behavioral costs. With regard to the gait results, we found no differences in stride-to-stride variability, which according to numerous dual-task studies has been found to increase under increasing load (Al-Yahya et al., 2011). However, significant differences in stride timing were observed, with an average increase in inter-stride timing of 44 msec under dual-task load. As walking pace was predetermined by the speed of the treadmill, it follows that participants increased their stride length, thereby making fewer, longer steps, while performing the response inhibition task. Considering that the center of gravity lies almost constantly outside the base of support during walking (except for during the relatively short phase of double-limb support), making longer steps should be associated with increased balance requirements. Previous work has suggested that longer steps are more challenging because the center of gravity is further from the moving base of support (Bhatt et al., 2005), and longer steps have been linked to higher slip probability (Moyer et al., 2006). On initial evaluation then, the current results seem rather counterintuitive. If one assumes that participants operate under limited resources, devoted and shared across both the walking and response inhibition task, making longer steps might appear a less efficient strategy. How then do the current results relate to the extant literature on walking-related dual-task costs?
Some studies found an increase of stride variability and a decrease in cognitive performance (Beauchet et al., 2005, Szturm et al., 2013) while others reported no differences under dual-task load (Li et al., 2012). In line with our results are findings by Loevden and colleagues (Lovden et al., 2008), who showed no differences in cognitive performance and an increase in stride time under dual-task load. These rather mixed results might be taken as indications that under certain circumstances young walkers are able to flexibly adapt performance so that costs in one or both tasks are prevented. With regard to our results, one could make the argument that the number of times one executes the walking task (i.e. takes a step) amounts to additional instances of inter-task competition. Thus, one strategy to improve performance might be to execute the walking task less often under dual-task load by increasing stride length. Similar results have been reported previously (Lovden et al., 2008, Li et al., 2012) and considered adaptive to reduce inter-task competition. In addition, one could make every effort to be out-of-synch with the competing task. Considering that the average inter-stimulus interval was 900 ms, a 44-msec increase in stride time from 1165 to 1209 ms might be suggestive of an effort to reduce CMI through increased de-synchronization of tasks. A far more consistent pattern of results can be found in studies with older individuals, where it is commonly reported that there is a reduction in walking speed and cognitive performance under dual-task load (for a review see (Al-Yahya et al., 2011)). Again, the fact that age-related studies show a far more consistent pattern with costs commonly seen in elderly speaks to the well-documented notion of higher processing resources in young walkers and their ability to adapt to dual-task load.
The absence of cognitive dual-task costs (even at increased walking speed) might indicate that walking is largely governed by automatic sub-cortical and spinal-based processes and no or minimal cognitive-motor interference (CMI) is therefore introduced. However, a growing number of electrophysiological and neuroimaging studies have produced evidence for cortical involvement in human locomotion (Suzuki et al., 2008, Harada et al., 2009, Gwin et al., 2011). Alternatively, cognitive-motor interference occurred, but the load introduced by walking was not sufficient to compromise inhibitory task performance. The present ERP results, however, clearly indicate differences in neural processing associated with inhibition between sitting and walking. The results also indicate that dual-task load targets specific processing stages of the inhibition task. That is, we found no VEP differences between sitting and walking, which indicates that sensory-perceptual processing stages of the inhibitory task are not affected by walking load. The most robust dual-task effects in terms of amplitude reduction were found for the N2, which was evident across all three midline electrode sites. No differences in N2 peak latency were observed under dual-task load. For the P3, amplitude reductions were only seen over centro-parietal scalp regions. Furthermore, the P3 peak latency was shortened by about 38 msec under dual-task load, indicating that the initiation of P3-related processing occurs earlier while walking. Finally, P3 scalp maps showed a shift from a parietal to a more central maximal distribution between single and dual-task load. Topographical analysis of the ERP revealed dissimilarity of scalp distributions between sitting and walking during a time period from ~475 to 550 ms. In summary, we found robust differences in amplitude, latency and scalp distribution of the ERP components associated with inhibitory functioning between sitting and walking, suggesting alteration of neuro-cognitive processing under increased task load.
There are numerous studies using the P300 amplitude as a measure of inter-task competition for processing resources (for a review see (Kok, 2001)). In a series of studies by Wickens and colleagues, the difficulty of tracking a visual stimulus using a control stick tracking device was manipulated in a ‘primary’ task, while participants were also required to detect infrequent auditory stimuli in a secondary oddball task (Wickens et al., 1977, Isreal et al., 1980a, Isreal et al., 1980b, Kramer et al., 1983). The underlying assumption was that by increasing the difficulty of the primary task one could use the P300 amplitude as a probe to measure the decrease in resources available to the secondary task. Reaction time for auditory oddball detection increased with increasing primary task load, indicating that task difficulty was indeed effectively manipulated. The effects of primary task load on the ‘secondary’ P3, however, yielded rather mixed results. In one of these studies, primary task was manipulated by varying the velocity and frequency of directional reversals of the visual stimulus making it increasingly difficult to (manually) track the stimulus (Isreal et al., 1980a). The auditory P3 amplitude decreased in the dual-task relative to single task conditions, but no further reduction of P3 amplitude was observed for increasing levels of difficulty in the control of the tracking device in the primary task. Results showing dual-task P3 reduction without further reduction by primary task load are difficult to reconcile with the notion that both tasks rely on common processes of limited capacity. In contrast, the same authors found that P3 amplitude to the auditory oddball was sensitive to the primary task difficulty, showing a further reduction for monitoring eight relative to four visually displayed elements in the primary task (Isreal et al., 1980b). Based on these and other results (Kramer et al., 1983, Kramer et al., 1987), Wickens and colleagues suggested that P3 amplitude is mainly sensitive to manipulations that tax perceptual and/or central processing as opposed to motor processing. Here, we found that walking lead to a reduction of the P3 amplitude on NoGo trials, but no further reduction was observed when load was increased (i.e. between deliberate and brisk walking speeds). And compellingly, there were no concomitant increases in behavioral costs in terms of reaction times to the Go stimuli or in the rates of commission errors to the NoGo stimuli as a function of walking speed, casting further doubt on the notion that P300 amplitude reduction under dual-task load reflects inter-task competition for limited processing resources.
A potentially more nuanced view emerges when one considers the fact that in addition to reduced P3 amplitude, we also observed a substantially different topographical distribution of the P3 here during the dual-task conditions relative to the single-task condition. That is, rather than a modulation of the same processing resources, the current results suggest that the processing strategy itself has been changed. That the P3 distribution we observe is more anterior during the dual-task walking conditions also suggests that additional prefrontal resources have been brought to bear and that perhaps a more cognitively demanding strategy has been engaged. Indeed, the fact that the earlier N2 is so greatly attenuated during the walking conditions and that the P3 is also found to peak earlier, also points to a significant change in processing strategy. We would propose that this pattern of effects, induced by the shift from single-task to dual-task performance, likely reflects a shift from a relatively automatic and early processing mode, as reflected by the strong induction of the N2, to a more cognitively effortful and somewhat later processing strategy that is reflected by engagement of more anterior frontal control regions during the P3 timeframe. It is of some interest in this context that more frontally distributed P300 responses with advanced age have been consistently reported in the aging literature (Friedman et al., 1993, Friedman and Simpson, 1994, Fabiani and Friedman, 1995, Anderer et al., 1996), with studies suggesting P300 anteriorization as a mechanism to engage additional resources to compensate for age-related decline, an account that accords well with our notion here of additional engagement of anterior regions under more effortful conditions.
The MOBI approach facilitates the integrated analysis of electro-cortical signals with precise measures of gait parameters while participants engage in demanding attentional tasks during active movement, providing a considerably more realistic assessment of performance under natural environmentally taxing situations. Evidence linking brain cortical markers to gait pattern and to failed adaption to multiple challenges, something we encounter constantly in our daily life, will greatly enhance our knowledge about cortical contributions to mobility issues in older adults. Results indicating a shift from automatic to more controlled processing of motor behavior in advanced age (Heuninckx et al., 2005, Heuninckx et al., 2010) would suggest that older individuals are further limited in adapting processing resources across dual-tasks. Assessing cortical flexibility in the face of these challenges allows us to identify individuals who fail to redeploy neural resources appropriately. Relating this inflexibility to fall risk will potentially lead to objective neural biomarkers of fall risk that can increase and accelerate our predictive capacity.
Study Limitations
Clearly, one of the express purposes of a study such as the current one is to move towards more naturalistic environmental conditions and to begin to assay human neural functioning while participants are actively exploring and physically engaged with their surroundings. Even so, it is evidently the case that walking on a treadmill, while going some way towards this goal, necessarily constrains the gait pattern, since the speed of walking is deterministic. Simply put, participants do not have the option of slowing down under dual-task situations. This is a clear limitation of our study, and a move to the use of portable, wearable high-density EEG systems will likely provide ever more realistic setups (Casson et al., 2008). Needless to say, as the participant begins to move more freely in the environment, the delivery of a tightly timed cognitive task becomes considerably more challenging, so there are inherent compromises to each new advance in the ability to record from moving humans. Despite the constraint of constant walking speed imposed by the treadmill, prior work using this method has shown robust dual-task costs on gait, including changes in step-width variability (Grabiner and Troy, 2005), cadence (Simoni et al., 2013), and gait variability (Li et al., 2012, Szturm et al., 2013). Thus, while slowing down may not be an option, it is clearly the case that meaningful adjustments of motoric behavior can be assayed. As such, this preparation does provide the opportunity to meaningfully assess the interplay between cognitive control systems and sensory-motor integration, albeit under not entirely naturalistic circumstances.
Figure 1.
Illustration of a participant walking on the treadmill wearing an EEG cap and foot pressure force sensors while performing the Go/NoGo-task.
Table 3.
Mean and standard error for N2/P3 amplitude and latency during sitting, walking deliberately, and walking briskly.
| Sitting | Walking deliberately | Walking briskly | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ampl. | FCz | Cz | CPz | FCz | Cz | CPz | Fz | FCz | CPz |
| N2 | −2.8(2.3) | −3.8(2.3) | −2.3(2.1) | −1.1(2.3) | 2.1(2.1) | 1.1(1.5) | −1.1(2.2) | −1.5(2.9) | −0.9(1.8) |
| N2 diff | 1.7(2.1) | −0.3(2.3) | −1.3(2.2) | 2.8(1.7) | 0.9(1.5) | 0.1(1.1) | 2.8(2.1) | 1.3(1.8) | 0.1(1.6) |
| P3 | 0.5(3.0) | 1.3(2.6) | 2.3(2.1) | 0.8(3.2) | 0.3(2.9) | 1.1(1.9) | 1.1(3.3) | 1.1(3.4) | 1.1(1.9) |
| P3 diff | 3.7(2.6) | 3.2(2.1) | 2.5(1.7) | 4.1(2.4) | 3.1(2.3) | 2.2(1.5) | 4.3(2.6) | 3.7(2.2) | 1.9(1.5) |
| Latency | FCz | Cz | CPz | FCz | Cz | CPz | Fz | FCz | CPz |
| N2 | 222(13.2) | 222(12.2) | 219(8.6) | 220(8.8) | 221(9.2) | 222(9.3) | 219(8.3) | 223(9.3) | 221(11.1) |
| N2 diff | 277(48.8) | 278(40.2) | 278(43.7) | 273(44.1) | 284(39.3) | 269(42.0) | 292(44.9) | 284(41.6) | 293(44.6) |
| P3 | 423(46.1) | 406(47.2) | 401(40.4) | 449(62.5) | 416(49.9) | 419(41.5) | 438(45.6) | 421(50.1) | 428(51.8) |
| P3 diff | 423(49.1) | 413(46.6) | 414(53.1) | 442(47.3) | 430(43.7) | 422(50.5) | 469(49.6) | 441(47.7) | 435(44.5) |
Acknowledgments
Sincere thanks go to Jason Green for help setting up the MOBI system and for data recording. Dr. Butler’s new address is: Trinity College Dublin, Center for Bioengineering, College Green, Dublin 2, Ireland. Drs. Foxe and De Sanctis take full responsibility for the integrity of the data and attests that all authors had full access to all the data in this study.
Contract grant sponsor: Support for this work was provided by a pilot grant from the Einstein-Montefiore Institute for Clinical and Translational Research (UL1-TR000086) and the Sheryl & Daniel R. Tishman Charitable Foundation. Participant recruitment and scheduling was performed by The Human Clinical Phenotyping Core at Einstein, a facility of the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (RFK-IDDRC) which is funded by a center grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD P30 HD071593).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Gait data from one participant were not obtained.
All authors declare no conflicts of interest, financial or otherwise.
REFERENCES
- Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: a systematic review and meta-analysis. Neuroscience and biobehavioral reviews. 2011;35:715–728. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Anderer P, Semlitsch HV, Saletu B. Multichannel auditory event-related brain potentials: effects of normal aging on the scalp distribution of N1, P2, N2 and P300 latencies and amplitudes. Electroencephalography and clinical neurophysiology. 1996;99:458–472. doi: 10.1016/s0013-4694(96)96518-9. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Dubost V, Herrmann FR, Kressig RW. Stride-to-stride variability while backward counting among healthy young adults. Journal of neuroengineering and rehabilitation. 2005;2:26. doi: 10.1186/1743-0003-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Ross LA, Garavan H. Intact inhibitory control processes in abstinent drug abusers (I): A functional neuroimaging study in former cocaine addicts. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt T, Wening JD, Pai YC. Influence of gait speed on stability: recovery from anterior slips and compensatory stepping. Gait & posture. 2005;21:146–156. doi: 10.1016/j.gaitpost.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Bulea TC, Kilicarslan A, Ozdemir R, Paloski WH, Contreras-Vidal JL. Simultaneous scalp electroencephalography (EEG), electromyography (EMG), and whole-body segmental inertial recording for multi-modal neural decoding. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/50602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson AJ, Smith S, Duncan JS, Rodriguez-Villegas E. Wearable EEG: what is it, why is it needed and what does it entail? Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2008;2008:5867–5870. doi: 10.1109/IEMBS.2008.4650549. [DOI] [PubMed] [Google Scholar]
- De Sanctis P, Butler JS, Green JM, Snyder AC, Foxe JJ. Mobile brain/body imaging (MoBI): High-density electrical mapping of inhibitory processes during walking. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2012a;2012:1542–1545. doi: 10.1109/EMBC.2012.6346236. [DOI] [PubMed] [Google Scholar]
- De Sanctis P, Foxe JJ, Czobor P, Wylie GR, Kamiel SM, Huening J, Nair-Collins M, Krakowski MI. Early sensory-perceptual processing deficits for affectively valenced inputs are more pronounced in schizophrenia patients with a history of violence than in their non-violent peers. Social cognitive and affective neuroscience. 2012b doi: 10.1093/scan/nss052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Makizako H, Shimada H, Yoshida D, Ito K, Kato T, Ando H, Suzuki T. Brain atrophy and trunk stability during dual-task walking among older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67:790–795. doi: 10.1093/gerona/glr214. [DOI] [PubMed] [Google Scholar]
- Duvinage M, Castermans T, Petieau M, Hoellinger T, Cheron G, Dutoit T. Performance of the Emotiv Epoc headset for P300-based applications. Biomedical engineering online. 2013;12:56. doi: 10.1186/1475-925X-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biological psychology. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32:579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Koshlykova NA, Kiroj VN, Hoormann J, Hohnsbein J. Late ERP components in visual and auditory Go/Nogo tasks. Electroencephalography and clinical neurophysiology. 1995;96:36–43. doi: 10.1016/0013-4694(94)00182-k. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining "early" visual processing. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Simpson G, Hamberger M. Age-related changes in scalp topography to novel and target stimuli. Psychophysiology. 1993;30:383–396. doi: 10.1111/j.1469-8986.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Simpson GV. ERP amplitude and scalp distribution to target and novel events: effects of temporal order in young, middle-aged and older adults. Brain research Cognitive brain research. 1994;2:49–63. doi: 10.1016/0926-6410(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Troy KL. Attention demanding tasks during treadmill walking reduce step width variability in young adults. Journal of neuroengineering and rehabilitation. 2005;2:25. doi: 10.1186/1743-0003-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramann K, Gwin JT, Bigdely-Shamlo N, Ferris DP, Makeig S. Visual evoked responses during standing and walking. Frontiers in human neuroscience. 2010;4:202. doi: 10.3389/fnhum.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramann K, Gwin JT, Ferris DP, Oie K, Jung TP, Lin CT, Liao LD, Makeig S. Cognition in action: imaging brain/body dynamics in mobile humans. Reviews in the neurosciences. 2011;22:593–608. doi: 10.1515/RNS.2011.047. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Gwin JT, Gramann K, Makeig S, Ferris DP. Removal of movement artifact from highdensity EEG recorded during walking and running. Journal of neurophysiology. 2010;103:3526–3534. doi: 10.1152/jn.00105.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwin JT, Gramann K, Makeig S, Ferris DP. Electrocortical activity is coupled to gait cycle phase during treadmill walking. NeuroImage. 2011;54:1289–1296. doi: 10.1016/j.neuroimage.2010.08.066. [DOI] [PubMed] [Google Scholar]
- Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2009;193:445–454. doi: 10.1007/s00221-008-1643-y. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Age-related reduction in the differential pathways involved in internal and external movement generation. Neurobiology of aging. 2010;31:301–314. doi: 10.1016/j.neurobiolaging.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66:879–887. doi: 10.1093/gerona/glr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor control. 2012;16:64–80. doi: 10.1123/mcj.16.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isreal JB, Chesney GL, Wickens CD, Donchin E. P300 and tracking difficulty: evidence for multiple resources in dual-task performance. Psychophysiology. 1980a;17:259–273. doi: 10.1111/j.1469-8986.1980.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Isreal JB, Wickens CD, Chesney GL, Donchin E. The event-related brain potential as an index of display-monitoring workload. Human factors. 1980b;22:211–224. doi: 10.1177/001872088002200210. [DOI] [PubMed] [Google Scholar]
- Katz R, De Sanctis P, Mahoney JR, Sehatpour P, Murphy CF, Gomez-Ramirez M, Alexopoulos GS, Foxe JJ. Cognitive control in late-life depression: response inhibition deficits and dysfunction of the anterior cingulate cortex. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2010;18:1017–1025. doi: 10.1097/JGP.0b013e3181d695f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. The European journal of neuroscience. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Sirevaag EJ, Braune R. A psychophysiological assessment of operator workload during simulated flight missions. Human factors. 1987;29:145–160. doi: 10.1177/001872088702900203. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Wickens CD, Donchin E. An analysis of the processing requirements of a complex perceptual-motor task. Human factors. 1983;25:597–621. doi: 10.1177/001872088302500601. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW, Arpin DJ. Stride-time variability and sensorimotor cortical activation during walking. NeuroImage. 2012;59:1602–1607. doi: 10.1016/j.neuroimage.2011.08.084. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Bard C, Fleury M. Attentional demands for static and dynamic equilibrium. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1993;97:139–144. doi: 10.1007/BF00228824. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instructional manual. Technical report A-8. gainesville, FL: University of Florida; 2008. 2008. [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboardevoked multichannel potential fields. Electroencephalography and clinical neurophysiology. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Li KZ, Abbud GA, Fraser SA, Demont RG. Successful adaptation of gait in healthy older adults during dual-task treadmill walking. Neuropsychology, development, and cognition Section B, Aging, neuropsychology and cognition. 2012;19:150–167. doi: 10.1080/13825585.2011.628375. [DOI] [PubMed] [Google Scholar]
- Lovden M, Schaefer S, Pohlmeyer AE, Lindenberger U. Walking variability and workingmemory load in aging: a dual-process account relating cognitive control to motor control performance. The journals of gerontology Series B, Psychological sciences and social sciences. 2008;63:121–128. doi: 10.1093/geronb/63.3.p121. [DOI] [PubMed] [Google Scholar]
- Makeig S, Gramann K, Jung TP, Sejnowski TJ, Poizner H. Linking brain, mind and behavior. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2009;73:95–100. doi: 10.1016/j.ijpsycho.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. London: Chapman and Hall; 1997. [Google Scholar]
- Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Brain research Cognitive brain research. 2002;14:115–128. doi: 10.1016/s0926-6410(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Morie KP, Garavan H, Bell RP, De Sanctis P, Krakowski MI, Foxe JJ. Intact inhibitory control processes in abstinent drug abusers (II): A high-density electrical mapping study in former cocaine and heroin addicts. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Moyer BE, Chambers AJ, Redfern MS, Cham R. Gait parameters as predictors of slip severity in younger and older adults. Ergonomics. 2006;49:329–343. doi: 10.1080/00140130500478553. [DOI] [PubMed] [Google Scholar]
- Murray MM, Wylie GR, Higgins BA, Javitt DC, Schroeder CE, Foxe JJ. The spatiotemporal dynamics of illusory contour processing: combined high-density electrical mapping, source analysis, and functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:5055–5073. doi: 10.1523/JNEUROSCI.22-12-05055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H, Butler JS, Whelan R, Foxe JJ, Bulthoff HH, Reilly RB. Motion P3 demonstrates neural nature of motion ERPs. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2011;2011:3884–3887. doi: 10.1109/IEMBS.2011.6090965. [DOI] [PubMed] [Google Scholar]
- Nolan H, Butler JS, Whelan R, Foxe JJ, Bulthoff HH, Reilly RB. Neural correlates of oddball detection in self-motion heading: a high-density event-related potential study of vestibular integration. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2012;219:1–11. doi: 10.1007/s00221-012-3059-y. [DOI] [PubMed] [Google Scholar]
- Nolan H, Whelan R, Reilly RB, Bulthoff HH, Butler JS. Acquisition of human EEG data during linear self-motion on a Stewart platform; 4th International IEEE/EMBS Conference on Neural Engineering; 2009. pp. 585–588. [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. NeuroImage. 2004;21:568–575. doi: 10.1016/j.neuroimage.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Simoni D, Rubbieri G, Baccini M, Rinaldi L, Becheri D, Forconi T, Mossello E, Zanieri S, Marchionni N, Di Bari M. Different motor tasks impact differently on cognitive performance of older persons during dual task tests. Clinical biomechanics. 2013;28:692–696. doi: 10.1016/j.clinbiomech.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Snyder AC, Shpaner M, Molholm S, Foxe JJ. Visual object processing as a function of stimulus energy, retinal eccentricity and Gestalt configuration: a high-density electrical mapping study. Neuroscience. 2012;221:1–11. doi: 10.1016/j.neuroscience.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Miyai I, Ono T, Kubota K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. NeuroImage. 2008;39:600–607. doi: 10.1016/j.neuroimage.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Szturm T, Maharjan P, Marotta JJ, Shay B, Shrestha S, Sakhalkar V. The interacting effect of cognitive and motor task demands on performance of gait, balance and cognition in young adults. Gait & posture. 2013 doi: 10.1016/j.gaitpost.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Uehara K, Higashi T, Tanabe S, Sugawara K. Alterations in human motor cortex during dual motor task by transcranial magnetic stimulation study. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2011;208:277–286. doi: 10.1007/s00221-010-2478-x. [DOI] [PubMed] [Google Scholar]
- Wickens CD, Israel JB, Donchin E. The event-related cortical potential as an index of task workload. In: Neal AS, Palasek RF, editors. Proceedings of the Human Factors Society 21st annual meeting. Santa Monica, CA: Human Factors Society; 1977. [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait & posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]