Abstract
Purpose
Robotic catheters have been proposed to increase the efficacy and safety of the radio-frequency ablation treatment. The robotized motion of current robotic catheters mimics the motion of manual ones—namely, deflection in one direction and rotation around the catheter. With the expectation that the higher dexterity may achieve further efficacy and safety of the robotically driven treatment, we prototyped a four-wire-driven robotic catheter with the ability to deflect in two- degree-of-freedom motions in addition to rotation.
Methods
A novel quad-directional structure with two wires was designed and developed to attain yaw and pitch motion in the robotic catheter. We performed a mechanical evaluation of the bendability and maneuverability of the robotic catheter and compared it with current manual catheters.
Results
We found that the four-wire-driven robotic catheter can achieve a pitching angle of 184.7° at a pulling distance of wire for 11mm, while the yawing angle was 170.4° at 11mm. The robotic catheter could attain the simultaneous two- degree-of-freedom motions in a simulated cardiac chamber.
Conclusion
The results indicate that the four-wire-driven robotic catheter may offer physicians the opportunity to intuitively control a catheter and smoothly approach the focus position that they aim to ablate.
Keywords: Flexible manipulator, Four-wire-driven, Medical robot, Quad-directional structure, Robotic catheter, Robotic surgery
Introduction
Catheter ablation is one of the methods of choice for the supraventricular and ventricular cardiac arrhythmias [1,2]. As the catheter ablation has been reported to be effective, we have witnessed a steady growth in the number interventions performed [3,4]. A recent study by Cosedis Nielsen et al. [5] suggested that atrial fibrillation (AF) ablation can be the first line of treatment, contrary to the prior notion that it should be performed only after drug therapy. In catheter ablations, the maneuverability of the catheter is a key to successful treatment as it helps catheters to accurately reach lesions and maintain optimal contact with the cardiac wall throughout the ablations [6]. In difficult cases, however, the lack of maneuverability of the catheter leads to complications, such as dissection or perforation of walls [7,8].
In an effort to increase the maneuverability of the catheters, researchers in industry and academia have investigated the use of medical robotics technology in enhancing catheters. Ganji et al. [9] reported a robotic catheter using motorized pull-wires for insertion and retraction, twisting, and deflecting motions. The details of the kinematic modeling of the Ganji et al.'s robot were reported in [10]. Xiao et al. [11] proposed a robotic catheter in master–slave fashion and attempted precise ablation using force feedback sensors to maintain optimal contact to the walls. Other improvements in robotic catheters include novel kinematics modeling or steering mechanisms [12–15].
Commercialized robotic catheters are now becoming available and have shown benefits over manual ones. The Sensei system (Hansen Medical, Mountain View, CA, USA), which employs pull-wire mechanisms in a double-sheath design [8,16–18], has been used in clinical trials. Di Biase et al. [18] have shown that robotic navigation and ablation of AF is safe and effective, and fluoroscopy time decreases with experience. A similar finding is reported in a multi-center study by Bai et al. [19]. Catheter Robotics Inc. produces a remote catheter system named Amigo™ (Catheter Robotics, Inc., Mount Olive, NJ) [20].
In all robotic catheters, deflection at the distal end of the catheters is achieved by single pitch motion to bend the catheter within a mechanically constrained plane, just as the manual approach does. To perform out-of-plane deflection, a roll motion is introduced through rotating the whole catheter by twisting the handle at the proximal end. Kinematically, the motion of such robotic catheters today has two independent motions—namely, bi-directional pitch motion and roll motion just like conventional manual catheters. The advantage of employing these two independent motions is that it allows physicians to continue using the maneuvering skills that they acquired from the manual approach; however, one may argue that the robotic approach does not fully offer highdexterity control of the catheter, thereby potentially limiting its impact.
The current state of the art in robotic catheters described above has led us to propose a novel mechanism that enables three-dimensional catheter control with pitch, yaw, and roll motion. This paper proposes a four-wire-driven mechanism, termed the ‘quad-directional structure’, combining two bidirectional bending mechanisms perpendicularly, which provides three-dimensional bending through a combination of yaw and pitch motions. We report our proof-of-concept prototype of such an intuitive yet advanced robotic catheter enabled by the newly proposed four-wire-driven mechanism.
Methods
Mechanical design
As shown in Fig. 1, we designed the distal end of the prototype robotic catheter having a 3.0mm outer diameter, 1.7mm central lumen diameter, and 65mm body length. We designed the catheter in CAD software (Inventor: Autodesk, Inc. Waltham, MA, USA). The catheter body was made of polypropylene produced by rapid prototyping. The catheter body was subdivided into segments and each segment has two pillars and a circular plate. Structural analysis was performed on this catheter body using a finite element analysis method using the CAD software by incorporating the physical properties of the polypropylene (Fig. 2). The physical properties of the polypropylene—Young's modulus: 1.1MPa, Poisson's Ration: 0.45, Density: 0.898–0.920g/cm3. In Fig. 2, displacement of the catheter is described by the color map. The distal end of the catheter was deflected by wires leading to the tip through the catheter's channels. The wire was assumed to be pulled from 0 to 13mm and fixed at the center of the channel. By elemental analysis, we confirmed that the proposed structure has enough stiffness and capability for deflection without breaking. Figure 3 shows the correlation between the pulled length of wire and bending angle. From the results of the structural analysis, wire-pull of 10.49mm was calculated to deliver 180° bending of the catheter body.
Fig. 1.
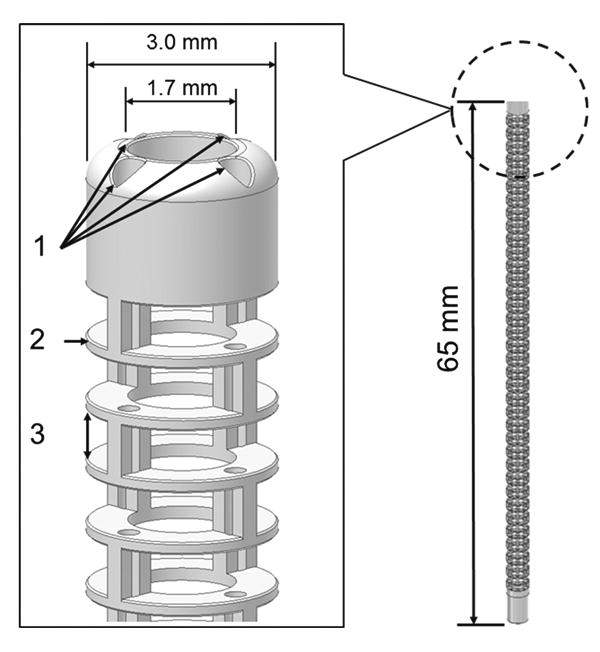
1 Four integrated channels for wire diameter of 0.35mm. The pitch circle diameter (PCD) of the channels is 2.35mm. 2 The thickness of circular plate is 0.2mm. 3 The 0.8mm height of the pillar is defined as the gap between circular plates. This catheter prototype was made of polypropylene using rapid prototyping
Fig. 2.
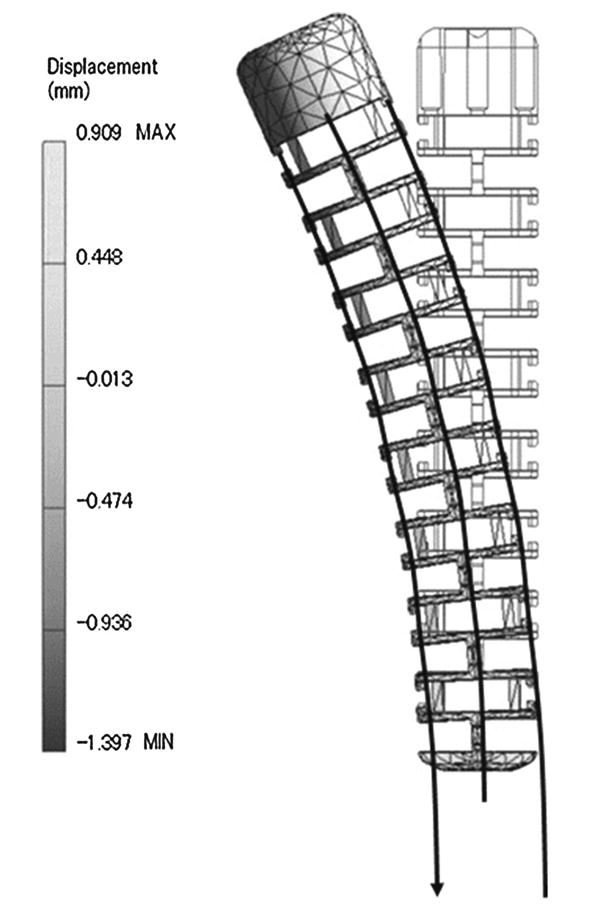
Screen capture during the structural analysis of the catheter CAD model. The displacement of the model is described as in the gray-scale shading map. The bending angle was calculated with the displacement values
Fig. 3.
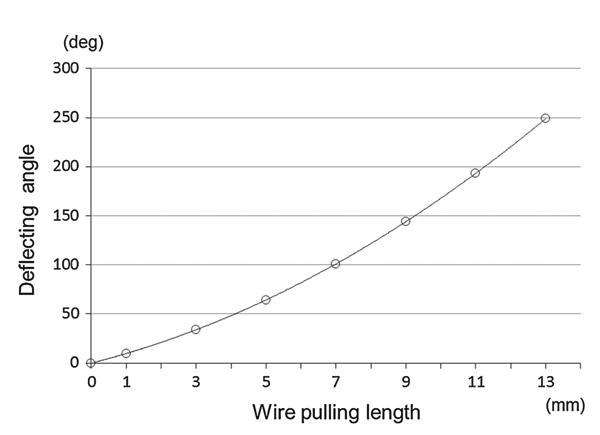
Relationship between bending angle and wire-pulled length on the catheter model. According to this analysis, actuators for pulling wires should have the ability to pull over 10.49mm
Channels for four wires with a diameter of 0.35mm and a pitch circle diameter (PCD) of 2.35mm were integrated through the catheter body. Four wires with a diameter of 0.21mm were led through the channels for bending the catheter body. Each structure has a pair of opposite channels; two of four wires enable the catheter to deflect pitch and yaw. When one of the wires is pulled, the corresponding side of the catheter body contracts and deflects (Fig. 4). The channels are spaced in a cyclic manner at equal intervals so that the catheter body can deflect in four directions (i.e., yaw and pitch motion). Moreover, through the combination of yawing and pitching, the tip of the distal end of the catheter is controlled three-dimensionally in any direction.
Fig. 4.
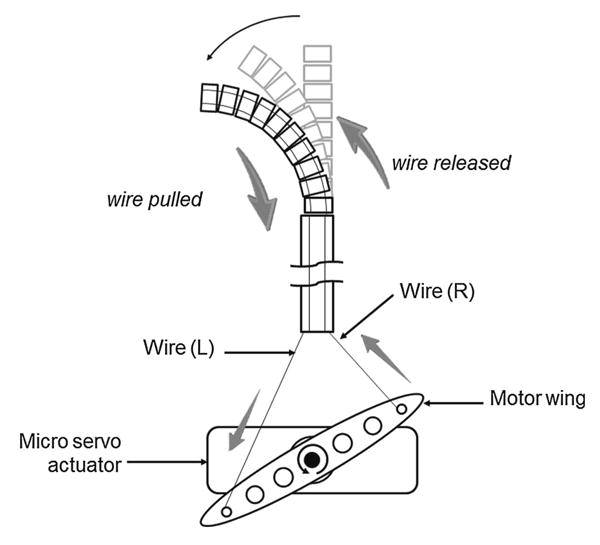
Mechanism that accomplishes deflecting. This deflection is achieved by pulling and releasing wires with a servo motor. This figure describes one example of deflection. When the motor rotates counterclockwise, wire (L) is pulled and wire (R) is released, deflecting the catheter tip leftward. When the motor rotates clockwise, wire (R) is pulled and wire (L) is released, deflecting the catheter tip rightward. Therefore, an actuator can make the tip of the catheter face two directions (bi-directional motion)
Figure 5 provides a whole view of the prototyped robotic catheter. Micro servo motors were located at the bottom of the robotic catheter (Micro Servo Module, SM-2309S, Arduino) to pull the wires, regulated by a joystick controller (TinkerKit Joystick, T000030, Arduino). One of the micro servo motors was used for yaw motion (rightward/leftward) and the other was used for pitch motion (forward/backward) swing. The four wires passed through a black sheath and were strung to a wire-pulling motor wing that was fixed at the rotator shaft of the micro servo motor. We defined the pulled length of wire as the input parameter to the micro servo motor. Thus, the deflection angle of the distal end of the catheter is provided by the pulling wire's length. The four wires were separated into two sets, each of which served as a structure for deflecting in one plane (yawing/pitching), and the deflecting angles were measured via the yaw and pitch motion of the catheter. The wires employed were SUS 304 straight wire with outer diameter of 0.21mm.
Fig. 5.
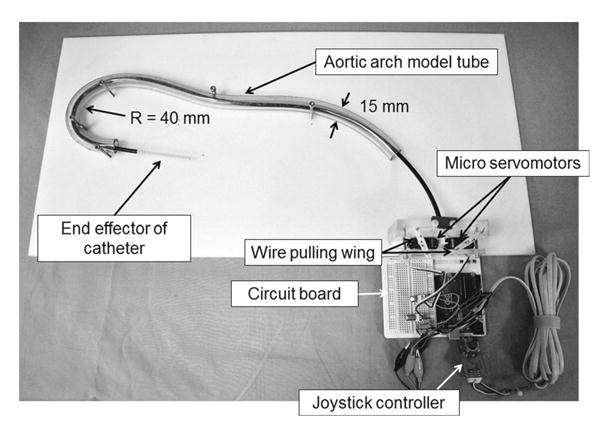
Full view of the prototype robotic catheter system. Based on the schematic shown in Fig. 4, two micro servo motors were fixed to the acrylic board side by side. The wires were tied at the hole farthest from the shaft on the wings. Output signals from a joystick controller were transmitted to a servo motor as input signals via the AVR microcomputer Arduino UNO
Performance analysis
We conducted a performance analysis by comparing the deflection angles (yawing and pitching) of the robotic catheter against the simulated (theoretical) angles to assess the bendability of the catheter body. This study took into account the fact that constant curvature deflection is commonly presumed for continuum manipulators in the absence of external constraints or disturbances. Since our robotic catheter concerned durability, external factors, and was designed as a continuum structure, a specific comparison with theoretical data was necessary.
Figure 6 describes the experimental apparatus used to substantiate our analytical predictions and to measure the tip position of the robotic catheter. The clear graduated acrylic boards were assembled with a square grid in order to measure the tip position of the deflected robotic catheter. The acrylic boards were set vertically to measure the three-dimensional position. One board is used for measuring yaw motion on the X–Y plane, PXY and the other is used for pitch measurement on the Y–Z plane, PY Z perpendicular to the PXY (Fig. 6). Thus, we measured and recorded the three-dimensional position of the catheter's distal end and length of pulled wire in each case. The independent variable in this study was defined as the length of pulled wires Lpull, the end of which is fixed at the motor wing of the actuator shaft. The dependent variables were defined as the measured tip position of the robotic catheter that made by a circular arch on the PXY , and on the PYZ. We continually recorded the tip coordinate position, over ten trials by varying the pulling length of wires every 2mm (Lpull = 1, 3, 5,…, 11). We also measured the yawing angle, θyaw pitching angle θpitch, and radius of each curve versus the length of pulled wire.
Fig. 6.
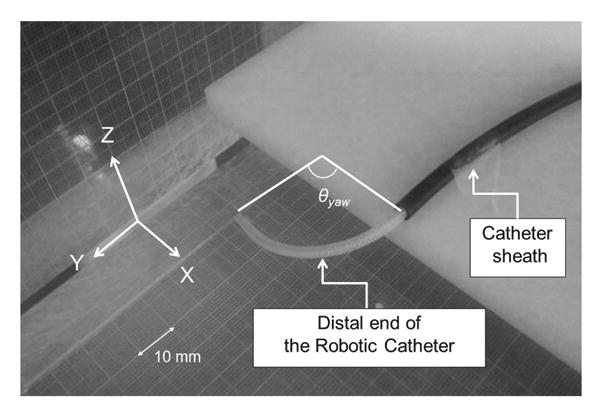
Experimental apparatus for measuring the bending angle and radius composed by the fabricated catheter. The bending motion to right-yawing was described. The angles θyaw,pitch created by the catheter curvature were measured and recorded
Maneuverability in Aortic arch model
This validation was conducted by comparing the quaddirectional robotic catheter and a clinically used manually controlled conventional cardiac catheter, which was used to represent a bi-directional catheter. This validation focused on differences in the rotating motion of the two catheters. To verify the maneuverability of each catheter through the curvature shape, we constructed two types of phantom model. The first was an aortic arch-shaped model, which is shown in Fig. 5. We utilized a clear vinyl tube with an inner diameter of 15mm to mimic a human aortic arch. The inner diameter of the human aortic arch is reported to be from 15 to 40mm with a radius arch of 40mm [21-23]. In the second model, the tube was fixed so as to be straight on a white acrylic board, and then each catheter was inserted. The values of the rotated angle were measured at the distal end of the proximal handle.
For the conventional cardiac catheter, we chose an electrode cardiac catheter with a 7 Fr (2.3mm) outer diameter (Livewire™ Electrophysiology Catheter: St. Jude Medical, Inc. St. Paul, MN, USA). The length of this catheter's distal end is 60mm, and it has the capacity to deflect 100° from right to left. This conventional cardiac catheter is operated by a proximal handle on the device to which narrow wires are connected. The independent variable was the input rotating angle at the proximal handle. In the case of the proposed catheter, data were obtained every 45° by varying the input angle from 0° to +/-90° using a joystick controller; this was repeated five times at each angle. The dependent variable was the rotated angle at the distal end.
We then compared the values of the rotated angle at the distal end with the angle at the proximal handle between the proposed robotic catheter and a conventional cardiac catheter.
Results
Performance analysis
Figure 7 plots the tip positions of the robotic catheter versus a conventional catheter. In Fig. 7, the robotic catheter and the conventional catheter were inserted from the middle of the PXZ. The result showed that the robotic catheter was able to three-dimensionally reach the area marked with black dots without twisting control, while the conventional catheter was moved only on the PXY vertically as drawn with gray dots. Figure 8 plots the bending degrees versus the wire pull length Lpull. The range of deviation of pitching θpitch was 2.2°–5.9° and of yawing angle θyaw was 1.4°–10.6°. The discrepancy of pitching and yawing angle at the same pulling lengths ranged from 3.7° to 21.2°. Note in Fig. 8 that the robotic catheter's curve approximately follows the inscribed circular arcs. The pattern of deflecting angles in the robotic catheter showed mostly same in both yawing and pitching although the displacement curves of the deflecting angle were not completely the same. The simulated values by pulling 9mm, however both of yaw and pitch deflected less than simulated angle pulling over 9mm. Discrepancy between simulated angle and pitching angle θpitch and yawing angle θyaw ranged from 1.0°–22.9° to 6.0°–24.9°, respectively. Figure 9 describes the length of the radius versus the wire's pulled length. The values of the radius for both yawing and pitching were measured. The range of deviation of the pitching radius was from 0.45 to 51.11mm, and of the yawing radius was from 0.79 to 33.52mm. The discrepancy between the pitching and yawing radius curves at the same pulling lengths ranged from 1.44 to 61.72mm.
Fig. 7.
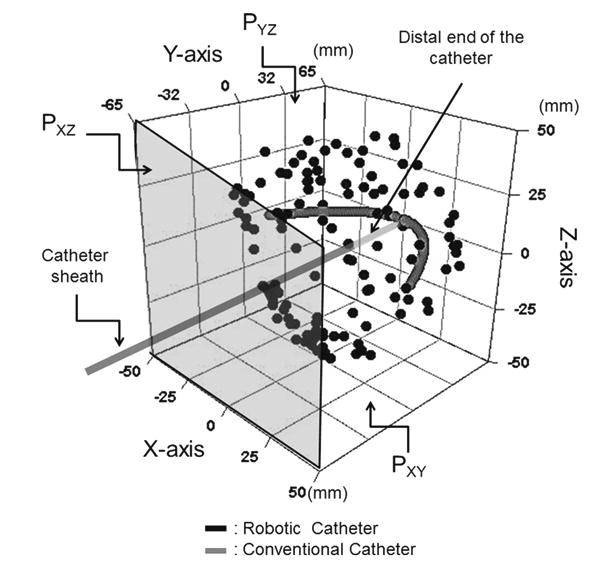
The measured distal end position of the robotic catheter versus the conventional catheter. The robotic catheter has ability to reach and face any direction without twisting motion as described by black dots. On the other hand, the conventional catheter was positioned only on the PXY
Fig. 8.
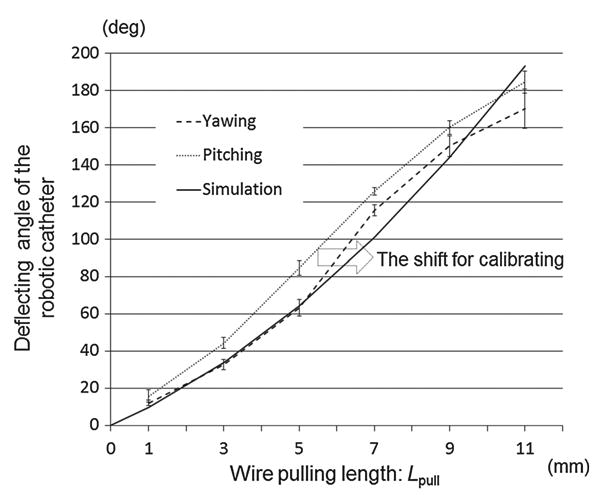
The measured curved angle versus wire's pulled length. The values of the bending curve both for yawing and pitching were measured and compared. Both yawing and pitching were stable as expected; however, some comparable differences arose among values due to the wire's pretension
Fig. 9.
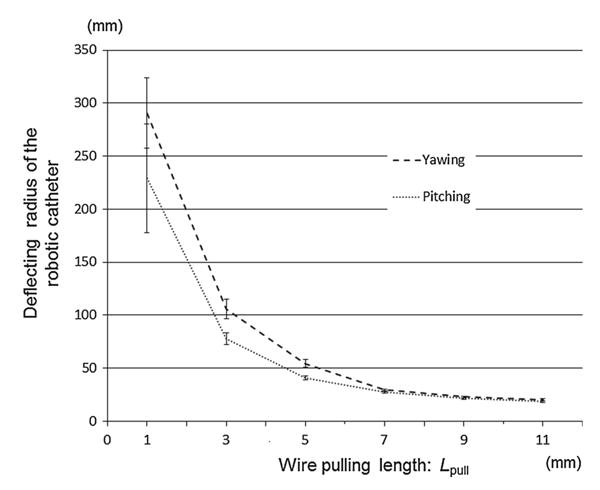
The measured radius curve versus wire's pulled length. The values of the radius curve both for yawing and pitching were measured and compared. The result showed both of values became stable when the pulled length went long, and the yawing radius was more stable than the pitching due to the proceeding error and the effects of the gravity
Maneuverability in Aortic arch model
Figure 10 describes the deviation of output rotating angle at the distal end versus the input rotating angle at the proximal handle. First of all, in the case of the straight-lined model, the mean range of rotation angle in the conventional catheter was 0.7°–9.5° and 0.4°–1.5° in the robotic catheter. Secondly, the robotic catheter performed produced relatively small errors with range of 0.78°–2.94° in the Aortic arch model. However, in the cases of the conventional catheter, the error of the tip rotation ranged 29.9°–81.9° in the case of the aortic arch model. The deviation of the conventional catheter became larger as the radius of the model became small. Finally, when the conventional catheter was placed through the tube simulating the aortic arch shape, the tip of the conventional catheter rotated only 8.1° per 90° rotation at the proximal handle. In contrast, no rotation of the robotic catheter was required for three-dimensional control in any case.
Fig. 10.
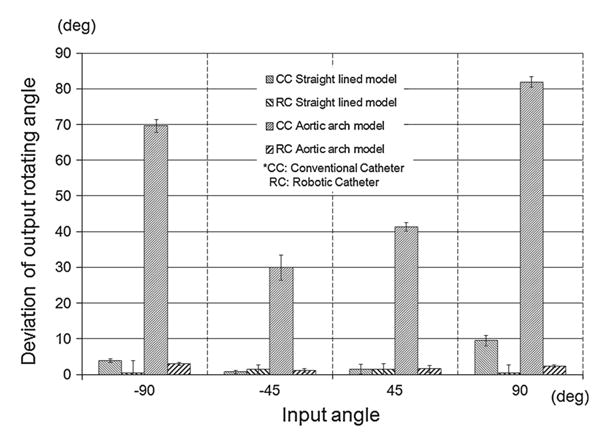
Relationship between input rotating angle and transmitted output rotating angle. Since the robotic catheter was not required to rotate in order to change facing direction, the deviation of the output angle was <10° and was superior to that of the conventional catheter. The output rotating angle of the conventional catheter in the aortic arch was smaller than that of the robotic catheter
Discussion
The aim of this study was to develop and implement the proof-of-concept prototype of a robotic catheter enabled by a newly proposed four-wire-driven mechanism that allows intuitive catheterization by steering the tip rightward, leftward, forward, or backward with its quad-directional structure.
We found that the four-wire-driven mechanism can achieve a pitching angle of 184.7°, while the yawing angle was 170.4° at maxim. The pattern of deflecting angles in the robotic catheter showed mostly the same pattern in both yawing and pitching although the displacement curves of the deflecting angle were not completely the same. As Fig. 9 shows, both radius values of yawing and pitching also showed a similar pattern. The values became smaller when the wire's pulled length went longer, while the pitching radius was more stable and less than the yawing. To correct and in order to cause both curves to match, the wires’ length were initially calibrated in advance as the pitching curve would be shifted in the right direction of the arrow in Fig. 8. The initial calibration aligns the catheter's stiffness by changing the length of slack. By this initial calibration, the slack that exists between the knob turning and the tip actuation would be equalized and removed for the catheter's control. Thus, the curve of radius for the pitching in Fig. 9 is expected to shift accordingly. Because the physician can crisscross the distal end of the catheter intuitively with the same operation, achieving this correction on a quad-directional structure significantly demonstrates its capability for intuitive maneuvering. The largest discrepancy between the measured angles and the simulated angles was 22.9° in yawing and 24.9° in pitching motion. The reason for this discrepancy was considered as fabricated error of the body structure. We recognized that the discrepancy became increasingly large as the pulled wire's length became longer. We confirmed that the distal end of the catheter was not deflected as was simulated because the sections of the catheter physically touched each other when it was deflected in a curve of a smaller radius.
The processing accuracy for this kind of catheter was significantly important. The robotic catheter could attain two degree-of-freedom motions in a simulated cardiac chamber, while the conventional catheter suffered from dragging force within the plane, which in turn led to an inconsistency between rotation angle at the proximal handle and the distal tip.
Our experimental setting was similar to the one presented in [13] studying the relationship between the pull-wire length and deflection angle of robotic catheters. The extended point of our verifications is that we evaluated simultaneous yaw and pitch motion by creating a four-wire-driven robotic catheter.
The quad-directional structure of our mechanism is expected to assist operating physicians by enabling more comfortable intuitive control when attempting to precisely approach. As shown in Fig. 10, the rotation at the proximal hand was transmitted to the distal tip of the catheter through the aortic arch-shaped model with an error of 0.4° – 2.94° From the results of this validation study, we concluded that the proposed catheter provides a capability to crisscross freely and intuitively. To validate the comfort and intuitiveness of this proposed catheter, further study regarding usability, time measurement, phantom study, and ergonomic study by physicians is necessary. The newly proposed model satisfies the requirements for an intuitive controllable structure with flexible bending and three-dimensional control. A similar notion was provide in [24]. Generally, the twisting operation interrupts precise control because of torsional loss during transmission that is caused by energy loss of the effect on performance due to friction between the surface of the wire and the guiding channels. In order to validate the proposed catheter and to prove its feasibility and clinical applicability, we need to further compare and challenge other procedures to confirm maneuverability in complicated approaches. For example, validation could be accomplished by applying the proposed catheter to the left atrium in order to treat preferentially complex atrial arrhythmias such as atrial fibrillation.
One of the limitations in catheters today is that rotation at the proximal end of the catheter may inaccurately reflect the rolling motion at the distal end. This misalignment happens when there is excessive contact friction between the catheter and the vessels [25]. Lack of alignment may confuse physicians, potentially leading to unexpected loss of contact between the tip and the endocardium. In fact, Mullins [26] argues that the response of a curve at the tip of the catheter becomes much more complex and slightly less predictable when loops are formed on catheters. Consequently, physicians need to make repetitive attempts in directing the catheter tip to reach arrhythmogenic zones before pacing or ablation can take place [10,27,28]. To compensate for this, in almost all catheterization cases, C-arm fluoroscopy and 3D mapping systems are used instead of 3D positioning sensors to confirm the location and position of the distal end through real-time imaging feedback. In lengthy operations, however, fluoroscopic imaging introduces harmful radiation exposure to patients as well as physicians [11,29]. The robotic catheter is reported to reduce imaging time and hence the radiation dose to patients and physicians. It is our expectation that the four-wire-driven approach newly proposed in this paper will further increase operating efficacy, thereby leading to less radiation exposure. To further increase operating efficiency, we need to prove that the proposed catheter was not affected by friction in the vessels and accomplishes smooth control to the target with the four-wire-driven structure. We need to confirm clinical efficiency by comparing the time needed to approach targets and inherent dose exposure with previous reports about robotic catheters.
Our robot used only a single type of wire, mainly chose for its ease of assembly into the lumen with a diameter of 0.35mm. The stiffness of the wire is considered to significantly affect the degree of bendability. Thus, other softer wires, such as wire rope or plastic nylon line, would contribute to more flexible bending. Additionally, we expect that the size of the outer diameter will be redesigned so as to be smaller. The extended future studies considering for clinical usage, when we compare the proposed prototype with other robotically assisted catheter, it ideally should be downsized to be at least the same size as a conventional catheter. In the production phase, perhaps rapid prototyping might not be appropriate for constructing this kind of long and a tiny part as a mono structure because the durability of the structure is still unconvincing. Alternatively, the continuum structure by a series of cell-structured units would be expected to be a more effective assembly.
Conclusions
We proposed a four-wire-driven robotic catheter that has ability to face in any direction without rotating motion from the proximal handle. This study investigated the feasibility of achieving bendability in three-dimensional control through a quad-directional structure. The results of the validation study of the bendability and maneuverability of the robotic catheter showed promising results and potential to be adapted for cardiac catheterization. Our approach may potentially overcome the limited utility of current conventional catheters and attain faster and intuitive control of bending motions.
Acknowledgments
Research reported in this publication was supported by The National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number P41EB015898. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. NH is a member of the Board of Directors of AZE Technology and has an equity interest in the company. AZE Technology develops and sells imaging technology and software. NH's interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. KY was in part supported by Global COE entitled “The Multidisciplinary Education and Research Center for the Establishment of Regenerative Medicine” at Tokyo Women's Medical University.
Contributor Information
Kitaro Yoshimitsu, Email: kitarof1@bwh.harvard.edu.
Takahisa Kato, Email: kato@bwh.harvard.edu.
Sang-Eun Song, Email: sam@bwh.harvard.edu.
Nobuhiko Hata, Email: hata@bwh.harvard.edu.
References
- 1.Haissaguerre M, Jais P, Hocini M, O’Neill MD, Sanders P. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias— reply. J Cardiovasc Electrophysiol. 2006;17(5):574–575. doi: 10.1111/j.1540-8167.2006.460_1.x. doi:10.1111/j. 1540-8167.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 2.Stabile G, Bertaglia E, Senatore G, De Simone A, Zoppo F, Donnici G, Turco P, Pascotto P, Fazzari M, Vitale DF. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation for the Cure of Atrial Fibrillation Study) Eur Heart J. 2006;27(2):216–221. doi: 10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 3.Lemola K, Desjardins B, Sneider M, Case I, Chugh A, Good E, Han J, Tamirisa K, Tsemo A, Reich S, Tschopp D, Igic P, Elmouchi D, Bogun F, Pelosi F, Kazerooni E, Morady F, Oral H. Effect of left atrial circumferential ablation for atrial fibrilation on left atrial transport function. Heart Rhythm. 2005;2(9):923–928. doi: 10.1016/j.hrthm.2005.06.026. doi:10.1016/ j.hrthm.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Taka-hashi Y, Rotter M, Pasquie J, Scavee C, Bordachar P, Clementy J, Haissaguerre M. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351(23):2373–2383. doi: 10.1056/NEJMoa041018. doi:10. 1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 5.Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367(17):1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 6.Criado FJ. Endovascular intervention: basic concepts and techniques. J Endovasc Ther. 2000;7(3):255. [Google Scholar]
- 7.Chun KRJ, Schmidt B, Koektuerk B, Tilz R, Fuernkranz A, Kon-stantinidou M, Wissner E, Metzner A, Ouyang F, Kuck KH. Catheter ablation—new developments in robotics. Herz. 2008;33(8):586–589. doi: 10.1007/s00059-008-3180-7. [DOI] [PubMed] [Google Scholar]
- 8.Ernst S. Robotic approach to catheter ablation. Curr Opin Cardiol. 2008;23(1):28–31. doi: 10.1097/HCO.0b013e3282f2c95c. [DOI] [PubMed] [Google Scholar]
- 9.Ganji Y, Janabi-Sharifi F, Cheema AN. Robot-assisted catheter manipulation for intracardiac navigation. Int J Comput Assist Radiol Surg. 2009;4(4):307–315. doi: 10.1007/s11548-009-0296-z. doi:10.1007/ s11548-009-0296-z. [DOI] [PubMed] [Google Scholar]
- 10.Ganji Y, Janabi-Sharifi F. Catheter kinematics for intracar-diac navigation. IEEE Trans Biomed Eng. 2009;56(3):621–632. doi: 10.1109/TBME.2009.2013134. doi:10. 1109/tbme.2009.2013134. [DOI] [PubMed] [Google Scholar]
- 11.Xiao N, Guo J, Guo S, Tamiya T. A robotic catheter system with real-time force feedback and monitor. Australas Phys Eng Sci Med. 2012;35(3):283–289. doi: 10.1007/s13246-012-0146-0. [DOI] [PubMed] [Google Scholar]
- 12.Camarillo DB, Carlson CR, Salisbury JK. Configuration tracking for continuum manipulators with coupled tendon drive. IEEE Trans Robotics. 2009;25(4):798–808. doi:10.1109/tro.2009. 2022426. [Google Scholar]
- 13.Camarillo DB, Milne CF, Carlson CR, Zinn MR, Salisbury JK. Mechanics modeling of tendon-driven continuum manipulators. IEEE Trans Robotics. 2008;24(6):1262–1273. doi:10.1109/tro. 2008.2002311. [Google Scholar]
- 14.Jayender J, Patel RV, Michaud GF, Hata N. Optimal transseptal puncture location for robot-assisted left atrial catheter ablation. Int J Med Robot Comput Assist Surg. 2011;7(2):193–201. doi: 10.1002/rcs.388. [DOI] [PubMed] [Google Scholar]
- 15.Tavallaei MA, Thakur Y, Haider S, Drangova M. Amagnetic-resonance-imaging-compatible remote catheter navigation system. IEEE Trans Biomed Eng. 2013;60(4):899–905. doi: 10.1109/TBME.2012.2229709. [DOI] [PubMed] [Google Scholar]
- 16.Riga CV, Bicknell CD, Hamady MS, Cheshire NJ. Evaluation of robotic endovascular catheters for arch vessel cannulation. J Vasc Surg. 2011;54(3):799–809. doi: 10.1016/j.jvs.2011.03.218. [DOI] [PubMed] [Google Scholar]
- 17.Saliba W, Reddy VY, Wazni O, Cummings JE, Burkhardt JD, Hais-saguerre M, Kautzner J, Peichl P, Neuzil P, Schibgilla V, Noelker G, Brachmann J, Di Biase L, Barrett C, Jais P, Natale A. Atrial fibrillation ablation using a robotic catheter remote control system–initial human experience and long-term follow-up results. J Am Coll Cardiol. 2008;51(25):2407–2411. doi: 10.1016/j.jacc.2008.03.027. doi:10.1016/j.jacc.2008.03. 027. [DOI] [PubMed] [Google Scholar]
- 18.Di Biase L, Wang Y, Horton R, Gallinghouse GJ, Mohanty P, Sanchez J, Patel D, Dare M, Canby R, Price LD, Zagrodzky JD, Bailey S, Burkhardt JD, Natale A. Ablation of atrial fibrillation utilizing robotic catheter navigation in comparison to manual navigation and ablation: single-center experience. J Cardiovasc Electrophysiol. 2009;20(12):1328–1335. doi: 10.1111/j.1540-8167.2009.01570.x. doi:10.1111/j.1540-8167. 2009.01570.x. [DOI] [PubMed] [Google Scholar]
- 19.Bai R, Di Biase L, Valderrabano M, Lorgat F, Mlcochova H, Tilz R, Meyerfeldt U, Hranitzky PM, Wazni O, Kanagaratnam P, Doshi RN, Gibson D, Pisapia A, Mohanty P, Saliba W, Ouyang F, Kautzner J, Gallinghouse GJ, Natale A. Worldwide experience with the robotic navigation system in catheter ablation of atrial fibrillation: methodology, efficacy and safety. J Cardiovasc Electrophysiol. 2012;23(8):820–826. doi: 10.1111/j.1540-8167.2012.02316.x. doi:10.1111/j.1540-8167.2012.02316. x. [DOI] [PubMed] [Google Scholar]
- 20.Khan EM, Frumkin W, Ng GA, Neelagaru S, Abi-Samra FM, Lee J, Giudici M, Gohn D, Winkle RA, Sussman J, Knight BP, Berman A, Calkins H. First experience with a novel robotic remote catheter system: Amigo mapping trial. J Interv Cardiac Electrophysiol Int J Arrhythm Pacing. 2013;37(2):121–129. doi: 10.1007/s10840-013-9791-9. doi:10.1007/ s10840-013-9791-9. [DOI] [PubMed] [Google Scholar]
- 21.Kahraman H, Ozaydin M, Varol E, Aslan SM, Dogan A, Altinbas A, Demir M, Gedikli O, Acar G, Ergene O. The diameters of the aorta and its major branches in patients with isolated coronary artery ectasia. Tex Heart Inst J. 2006;33(4):463–468. [PMC free article] [PubMed] [Google Scholar]
- 22.Mao SS, Ahmadi N, Shah B, Beckmann D, Chen A, Ngo L, Flores FR, Gao YL, Budoff MJ. Normal thoracic aorta diameter on cardiac computed tomography in healthy asymptomatic adults: impact of age and gender. Acad Radiol. 2008;15(7):827–834. doi: 10.1016/j.acra.2008.02.001. doi:10. 1016/j.acra.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litmanovich D, Bankier AA, Cantin L, Raptopoulos V, Boiselle PM. CT and MRI in diseases of the aorta. AJR Am J Roentgenol. 2009;193(4):928–940. doi: 10.2214/AJR.08.2166. [DOI] [PubMed] [Google Scholar]
- 24.Penning RS, Jung J, Ferrier NJ, Zinn MR. An evaluation of closed-loop control options for continuum manipulators. 2011 doi: 10.1109/icra.2012.6224735. [DOI] [Google Scholar]
- 25.Klemm HU, Steven D, Johnsen C, Ventura R, Rostock T, Lutomsky B, Risius T, Meinertz T, Willems S. Catheter motion during atrial ablation due to the beating heart and respiration: impact on accuracy and spatial referencing in three-dimensional mapping. Heart Rhythm. 2007;4(5):587–592. doi: 10.1016/j.hrthm.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Mullins DCE. Cardiac catheterization in congenital heart disease pediatric and adult. Wiley; Chichester: 2008. [Google Scholar]
- 27.Fu Y, Liu H, Huang W, Wang S, Liang Z. Steerable catheters in minimally invasive vascular surgery. Int J Med Robot. 2009;5(4):381–391. doi: 10.1002/rcs.282. [DOI] [PubMed] [Google Scholar]
- 28.Kandzari DE, Bhatt DL, Sobotka PA, O’Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol. 2012;35(9):528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bock M, Wacker FK. MR-guided intravascular interventions: techniques and applications. J Magn Reson Imaging. 2008;27(2):326–338. doi: 10.1002/jmri.21271. [DOI] [PubMed] [Google Scholar]


