Abstract
Objective
Functional magnetic resonance imaging is sensitive to the variation in language network patterns. Large populations are needed to rigorously assess atypical patterns, which, even in neurological populations, are a minority.
Methods
We studied 220 patients with focal epilepsy and 118 healthy volunteers who performed an auditory description decision task. We compared a data-driven hierarchical clustering approach to the commonly used a priori laterality index (LI) threshold (LI < 0.20 as atypical) to classify language patterns within frontal and temporal regions of interest. We explored (n = 128) whether IQ varied with different language activation patterns.
Results
The rate of atypical language among healthy volunteers (2.5%) and patients (24.5%) agreed with previous studies; however, we found 6 patterns of atypical language: a symmetrically bilateral, 2 unilaterally crossed, and 3 right dominant patterns. There was high agreement between classification methods, yet the cluster analysis revealed novel correlations with clinical features. Beyond the established association of left-handedness, early seizure onset, and vascular pathology with atypical language, cluster analysis identified an association of handedness with frontal lateralization, early seizure onset with temporal lateralization, and left hemisphere focus with a unilateral right pattern. Intelligence quotient was not significantly different among patterns.
Interpretation
Language dominance is a continuum; however, our results demonstrate meaningful thresholds in classifying laterality. Atypical language patterns are less frequent but more variable than typical language patterns, posing challenges for accurate presurgical planning. Language dominance should be assessed on a regional rather than hemispheric basis, and clinical characteristics should inform evaluation of atypical language dominance. Reorganization of language is not uniformly detrimental to language functioning.
Functional magnetic resonance imaging (fMRI) is an established language lateralization method in epilepsy, rapidly supplanting the intracarotid amobarbital procedure (IAP) for presurgical evaluation.1 The higher incidence of bilateral and right lateralized language in epilepsy patients than healthy people2 is important for surgical planning.3–6 However, atypical language representation occurs in only 20 to 30% of epilepsy patients and about 5% of healthy volunteers; large study populations are necessary to accrue adequate numbers with atypical language for investigation.2,3,7–11
Methodological issues arise in determining language dominance. One is the lateralization index (LI). Although it is a continuous variable, a single value is used to categorize dominance (left, bilateral, or right). Even with bootstrapping techniques,12 0.20 is the most commonly used value (LI < 0.20 as atypical; see Seghier for review 13). However, language dominance thresholds differ across centers,1 ranging between |0.1| and |0.265|.4–17 LI values used to differentiate strong from weak language dominance also differ across centers.18,19 Although no specific values are recognized collectively as diagnostic, the degree of lateralization may have clinical implications.2,7,16,20–24
We report on 220 patients and 118 healthy volunteers over 9 years across a wide age range, allowing investigation of patterns difficult to interpret in smaller samples. We categorized language activation patterns based on regional LI of the Broca area and Wernicke area (WA) in children and adults with focal epilepsy using a word definition decision task activating the frontal–temporal language network.7,25,26 Based on our clinical experience and theoretical considerations using 2 regions of interest (ROIs) and an a priori threshold of LI < 0.20 for atypical activation, we predicted 15 possible patterns of language representation. In addition, we used a data-driven classification method—hierarchical clustering—to classify language localization patterns. We predicted that the data-driven method would reveal different LI thresholds for classification and highlight subject clinical characteristics not found using the a priori threshold.
Subjects and Methods
Participants
This cross-sectional, retrospective review of prospectively acquired data included 338 English-speaking participants (220 patients and 118 healthy volunteers, age range 4–57 years; Table). Patients were evaluated between 2003 and 2010 at a tertiary referral epilepsy center. We reported on 45 previously. 7,27 Clinical features, neurologic examination, ictal video electroencephalography, and high-resolution MRI were used to localize seizure foci. MRI was categorized as normal, mesial temporal sclerosis, lesional (tumors, focal cortical dysplasia), vascular (stroke, cavernomas, arteriovenous malformations), inflammatory (Rasmussen encephalitis), dual pathology, or other (encephalomalacia, traumatic brain injury, nonspecific MRI). Intelligence was assessed for a subset of patients (n = 128) by standardized administration with an age-appropriate measure yielding composite scores for overall (full-scale intelligence quotient [FSIQ]), verbal (verbal intelligence quotient [VIQ]), and nonverbal (performance intelligence quotient [PIQ]) intellectual skills (see Supplementary Materials). Overall, patients’ intellectual skills were average (mean FSIQ = 92), with a wide range from intellectually disabled (IQ = 42) to superior (IQ = 135).
TABLE 1.
Demographic and Seizure Characteristics for Categorical Language Activation Patterns of Patients
| Characteristic | Patients, n = 220 |
Controls, n = 118 |
|---|---|---|
| Gender | ||
| Male | 55%, n = 121 | 51%, n = 60 |
| Female | 45%, n = 99 | 49%, n = 58 |
| Mean age, Yr (SD) | 21.9 (12.2) | 17.8 (10.5) |
| Handedness | ||
| Right | 80%, n = 175 | 100%, n = 118 |
| Ambidextrous | 7%, n = 16 | 0%, n = 0 |
| Left | 13%, n = 29 | 0%, n = 0 |
| Full scale IQ | N/A | |
| Mean (SD) | 92 (16) | |
| Range | 42–135 | |
| Age of onset | N/A | |
| Early | 31%, n = 68 | |
| Late | 68%, n = 149 | |
| Missing | 1%, n = 3 | |
| Mean (SD) | 11.8 (9.2) | |
| Focus | N/A | |
| Left | 60%, n = 133 | |
| Right | 40%, n = 87 | |
| LT | 40%, n = 87 | |
| RT | 19%, n = 41 | |
| LF | 14%, n = 31 | |
| LP | 3%, n = 7 | |
| LO | 1%, n = 1 | |
| RF | 0%, n = 0 | |
| RP | 3%, n = 7 | |
| RO | 1%, n = 3 | |
| Multiple | 8%, n = 18 | |
| Missing | 11%, n = 25 | |
| MRI type | ||
| Normal | 30%, n = 66 | 100%, n = 118 |
| MTS | 20%, n = 43 | 0%, n = 0 |
| Lesion | 33%, n = 73 | 0%, n = 0 |
| Vascular | 10%, n = 22 | 0%, n = 0 |
| Inflammatory | 2%, n = 4 | 0%, n = 0 |
| Other | 3%, n = 7 | 0%, n = 0 |
| Dual pathology | 2%, n = 5 | 0%, n = 0 |
| Missing | 0%, n = 0 | 0%, n = 0 |
IQ = intelligence quotient; LF = left frontal; LO = left occipital; LP = left parietal; LT = left temporal; MRI = magnetic resonance imaging; MTS = mesial temporal sclerosis; N/A = not applicable; RF = right frontal; RO = right occipital; RP = right parietal; RT = right temporal; SD = standard deviation.
One hundred eighteen healthy volunteers were evaluated between 2002 and 2011. Twenty adult 25,26 and 57 pediatric volunteers were reported previously.10,28,29 Inclusion criteria were right-handedness on the Edinburgh Handedness Inventory (score ≥ 40) and normal MRI.
The study was approved by the National Institutes of Health Combined Neurosciences and Children’s National Medical Center institutional review boards. Adult patients and parents of pediatric patients provided informed consent; all minors provided assent.
fMRI Acquisition
All patients and 61 healthy volunteers were scanned on a 3.0T General Electric (Milwaukee, WI) scanner using echo planar imaging (EPI) blood oxygen level dependent (BOLD) techniques. Fifty-seven volunteers were scanned on a Siemens (Erlangen, Germany) 3.0T Magnetom Trio scanner using EPI BOLD. Acquisition methods were described previously7,26 (Supplementary Methods). Previous analyses showed no differences between scanners using these paradigms and acquisition parameters.10,29
Language Paradigm
Participants performed a semantic decision task based on aurally presented word definitions (eg, “A king’s hat is a crown”). Seventy percent of items were true, 30% false. The control condition was reverse speech with tone identification for 70% of the items. The task was presented in a block design of 5 cycles (30-second hemicycles; total duration = 5 minutes). This task reliably activates language-processing regions in temporal (WA) and frontal lobe (Broca area).7,10,25,26,29
Image Processing and ROIs
Language activation maps were processed in SPM2 (Wellcome Department of Imaging Neuroscience, University College, London, UK) using normalized Montreal Neurologic Institute space. Traditional language regions were used as ROIs: Broca area (inferior frontal gyrus [IFG]) and WA (Brodmann areas 21, 22, and 39) were defined using the Wake Forest PickAtlas (Wake Forest University School of Medicine, Winston-Salem, NC). We based ROIs on broad anatomic landmarks to avoid underestimating language-related activation. Both epilepsy and development can create variability in activation localization at normal functional group map margins.25,26
A Priori Laterality Categorization
We calculated LI for each subject’s IFG and WA and categorized language based on a commonly used a priori value of 0.20.13 Using the LI Toolbox bootstrap method (LI-Toolbox for SPM2),12 ROIs were individually categorized as left lateralized if LI ≥ 0.20, bilateral if LI < |0.20|, and right if LI ≤ −0.20.2,7,20,26,30–33
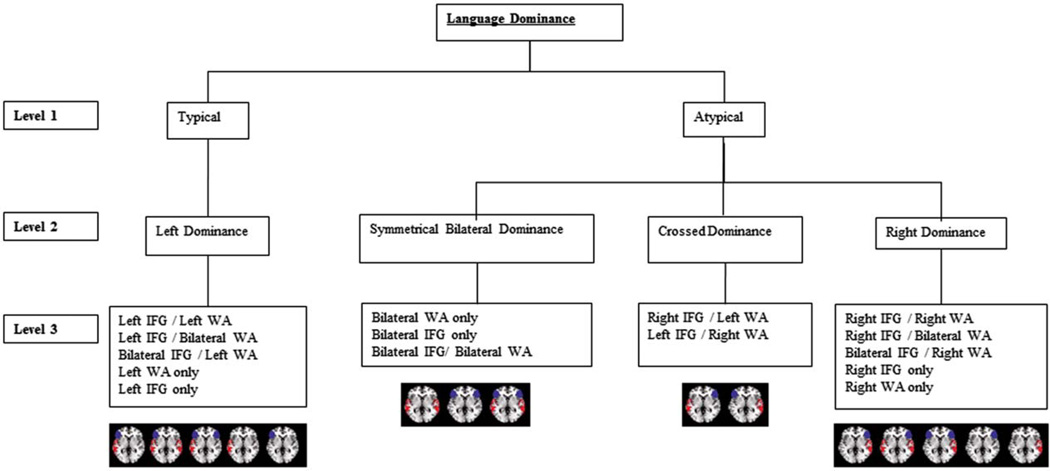
Fifteen different language activation patterns were possible in both patients and controls using all combinations of 3 lateralization categories (left, bilateral, right) and 2 ROIs (IFG and WA; Fig 1). We organized the 15 patterns into a 3-level hierarchy based on our clinical experience with language mapping using fMRI, IAP, and electrocortical stimulation. Our first level had 2 groups based on typical (left) and atypical (right=bilateral) language lateralization. The second level had 4 groups (left, symmetrically bilateral, crossed, right). Participants with bilateral activation in 1 region and unilateral activation in the other were considered dominant on the side of unilateral region activation.21,34,35 The third level included all 15 possible individual activation patterns.
FIGURE 1.
Hierarchy of 15 typical and atypical language patterns based on categorical criteria in both patients and controls. Language is classified into 3 levels: typical=atypical, language dominance (left, bilateral, crossed, or right), and individual activation patterns. Regions of interest are left lateralized if lateralization index (LI) ≥ 0.20, bilateral if LI < |0.20|, and right lateralized if LI ≤ −20.20. IFG = inferior frontal gyrus; WA = Wernicke area.[Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
Data-Driven Categorization
Hierarchical clustering can identify patient groups that are similar with respect to a given variable.36 We used hierarchical cluster analysis as a data-driven method of identifying language patterns and classifying patients into groups based on IFG and WA LI values. We used Ward’s method to measure similarity between cases, because this algorithm allows clusters with few members, which are likely for infrequent atypical language patterns. Distance coefficients (DCs) reflect mathematical similarity; high DC indicates that dissimilar clusters are joined, and that the previous step represented an appropriate cluster solution.
Statistical Analysis
Descriptive statistics were used to characterize laterality indices and clinical characteristics for each emerging pattern and cluster. For the cluster analysis, we report and compare findings for 3 solutions that mirror our 3 hierarchy levels: (1) a 2-cluster solution based on atypical/typical; (2) a 4-cluster solution based on the left, bilateral, crossed, and right divisions; and (3) a maximum of 9-cluster solution (see Results; not all 15 patterns were found). Concordances between our a priori and clustering models were analyzed.
Clinical characteristics were analyzed at the 3 categorical hierarchy levels. Nonparametric analyses (chi-square) were conducted for gender, age (<20 years), early seizure onset (<6 years old), handedness, focus, and underlying pathology. To reduce comparisons and determine whether the 2 classification methods had different sensitivity for clinical indicators, we targeted comparisons to groups with disagreement between the 2 methods that had at least 5 cases per cell. Group differences in age, epilepsy onset age, and IQ were analyzed using analysis of variance or 2-sample t tests.
Results
A Priori Categorical Patterns
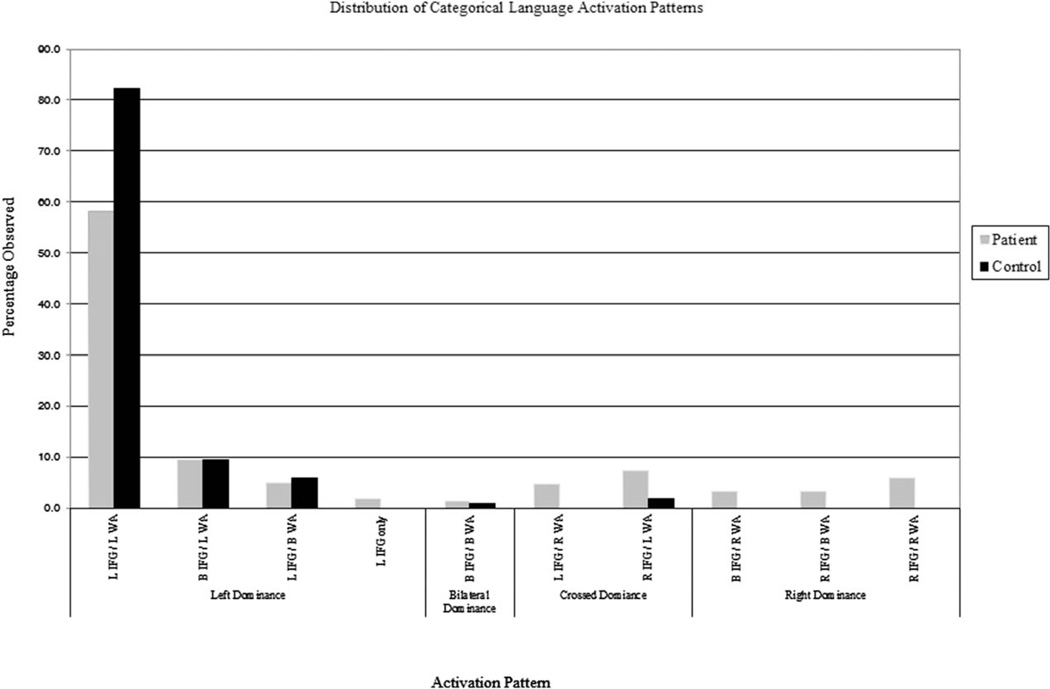
Patients fell into 10 of 15 possible patterns (Fig 2, Supplementary Table 1). Only 4 patients (1.8%) had a single ROI activated (all left [L] IFG only), indicating that the task, as designed, reliably activated frontal and temporal regions. Most (74.5%, n = 164) demonstrated typical left dominant language. The majority had both IFG and WA left lateralization. Fifty-six (25.5%) demonstrated atypical language dominance.
FIGURE 2.
Distribution of all language patterns for both patients and controls based on categorical criteria. B = bilateral; IFG = inferior frontal gyrus; L = left; R = right; WA = Wernicke area.
Healthy volunteers fell into 5 of 15 possible patterns (see Fig 2), with less atypical language classification than patients (2.5% vs 25.5%; chi-square = 27.983, df = 1, p < 0.001). Most healthy volunteers (n = 97, 82.2%) had the typical L IFG/L WA pattern.
Cluster Analysis—Comparison to A Priori Method
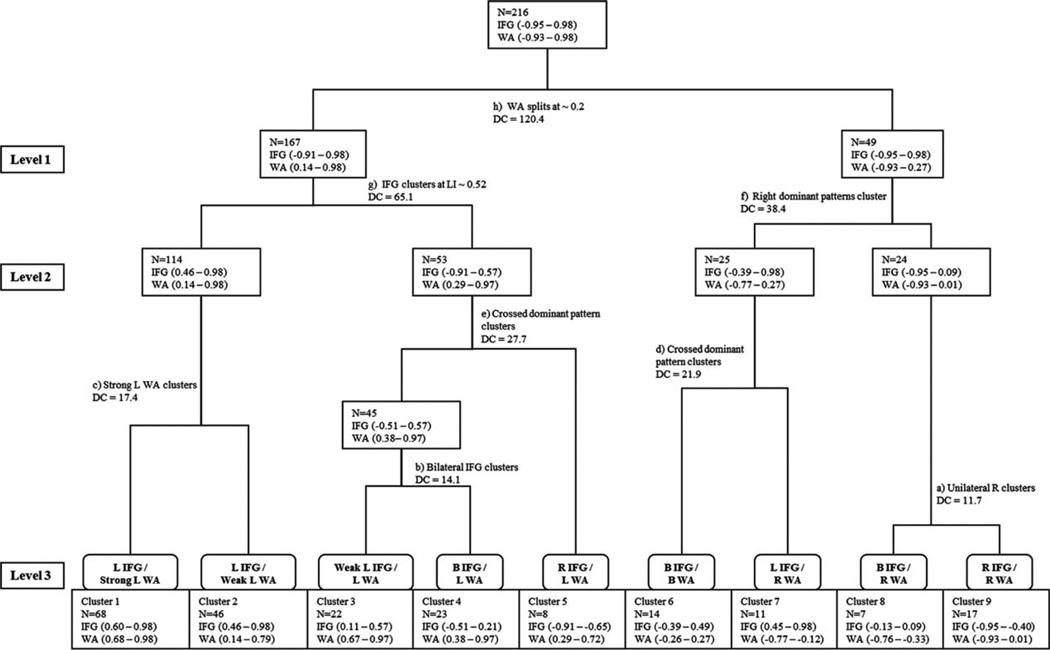
We first present the cluster analysis for patients, which yielded support for 3 possible solutions: 2, 4, and 9 clusters. The dendrogram represents the findings, including cluster number and distance metric; the LI range is also included for each ROI at each cluster (Fig 3). The 4 patients with L IFG activation alone were not included in the hierarchical clustering analysis, because the model required both IFG and WA LI data. Identifiable LI values separated clusters; the methods differed in how cases were classified.
FIGURE 3.
Hierarchical clustering of language patterns in patients based on inferior frontal gyrus (IFG) and Wernicke area (WA) lateralization index (LI). Branching point of region of interest (ROI) LI, distance coefficient (DC), and ROI LI range at each cluster is shown. Branches are in alphabetical order, with early letters (and lower DC) indicating more similarity between groups. B = bilateral; L = left; R = right.
TWO-CLUSTER SOLUTION
The language dominance groups were most dissimilar at this division point, clustering based on a WA LI of approximately 0.20 (see Fig 3, branch h). There was 88.4% agreement between both methods for case assignment for atypical or typical language dominance.
FOUR-CLUSTER SOLUTION
Similar to the a priori method, the 4-cluster solution included 1 cluster of typical and 3 of atypical language (see Fig 3, level 2). The 3 atypical clusters included 1 right dominant and 2 bilateral, rather than the a priori groups of 1 right, 1 bilateral, and 1 crossed. There was 100% agreement for typical left dominant (114 patients) and right dominant clusters (24 patients). The 2 bilateral clusters (n = 53 and n = 25) reflected a mixture of bilateral and crossed dominance patterns, with 72.7% agreement between methods. The clustering analysis did not distinguish crossed and bilateral dominance as unique clusters. The bilateral groups differed; 1 had lower WA LI and the other lower IFG LI.
NINE-CLUSTER SOLUTION
The maximum number of clusters considered was 9. Overall, there was 83.3% agreement between methods for the 9-cluster solution (Fig 4).
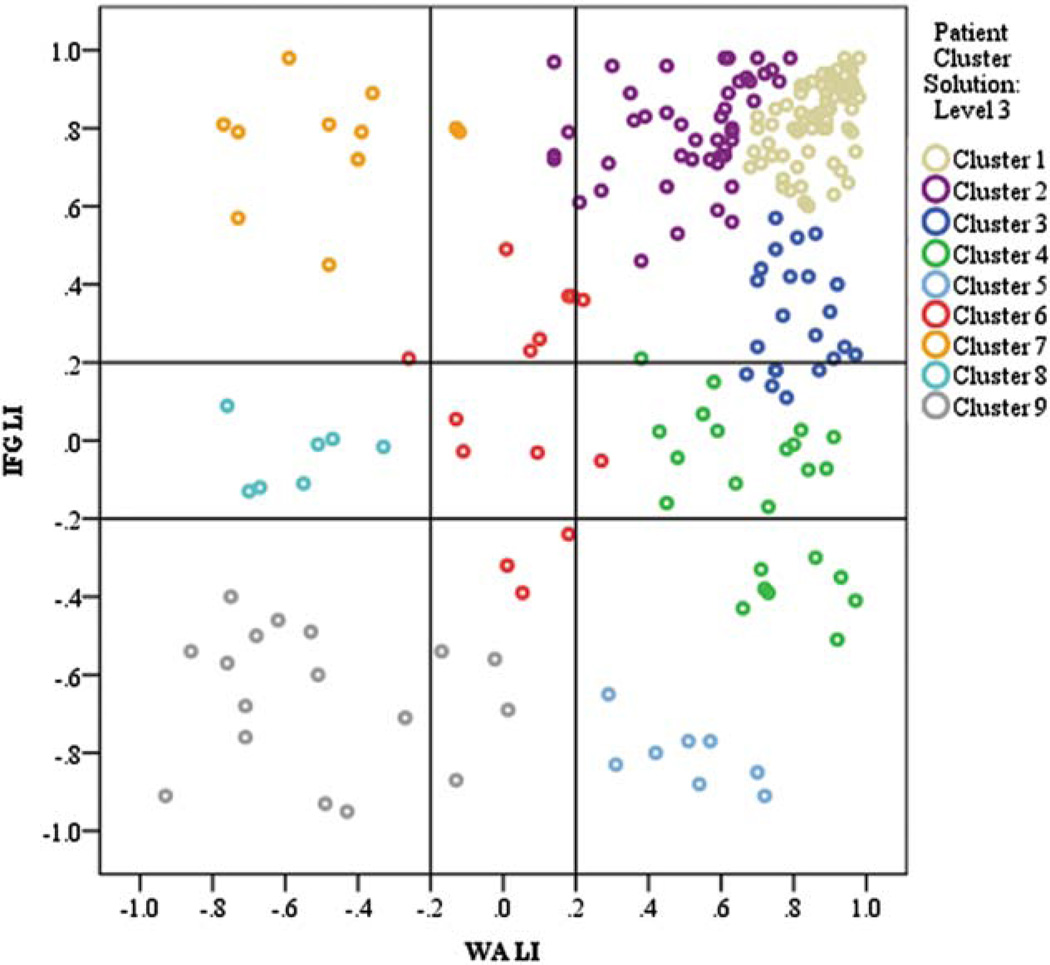
FIGURE 4.
Cluster analysis revealing similar categories of patterns and language dominance in epilepsy patients. Lines indicate thresholds for categorical thresholds of patterns. Colors indicate clusters. IFG = inferior frontal gyrus; LI = lateralization index; WA = Wernicke area. [Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
Four clusters were left dominant; 3 were subgroups of the L IFG/L WA category distinguished by IFG left lateralization strength. Patients with IFG LI greater than approximately 0.52 (clusters 1 and 2) were similar to each other, showing strong agreement with a priori grouping (cluster 1: 100%, cluster 2: 91.3% agreement with L IFG/L WA pattern). Patients with weaker IFG lateralization clustered into 2 groups (clusters 3 and 4) distinguished by an IFG threshold of approximately 0.20 (see Fig 3, branch b). Cluster 3 had 72.7% agreement with the a priori L IFG/L WA pattern, and cluster 4 had 60.9% agreement with the bilateral (B) IFG/L WA pattern.
The right dominant patterns (clusters 8 and 9) were distinguished by the smallest distance metric (see Fig 3, branch a). Cluster 8 included all 7 patients (100% agreement) in the B IFG/right (R) WA a priori pattern. Cluster 9 contained all (n = 13) patients in the R IFG/R WA a priori pattern and patients from the R IFG/B WA a priori pattern with 76.4% agreement between methods.
Two distinct crossed dominant cluster patterns emerged. The R IFG/L WA pattern (cluster 5) contained only patients in the a priori assignment (100% agreement), but had a more stringent R IFG threshold (< −0.65) than the a priori group, with fewer patients. Nine of 11 patients in the L IFG/R WA pattern (cluster 7) had the same a priori assignment (81.8% agreement).
Cluster 6 showed the greatest disagreement with the a priori method (21%) and reflected the symmetrically bilateral pattern. The 3 patients labeled as B IFG/B WA in the a priori model were included as well as 11 from 5 other activation patterns. No unilateral frontal (L or R IFG)/B WA patterns discretely clustered. The WA LI range for this cluster (−0.26 to 0.27) closely matched the 0.20 a priori value.
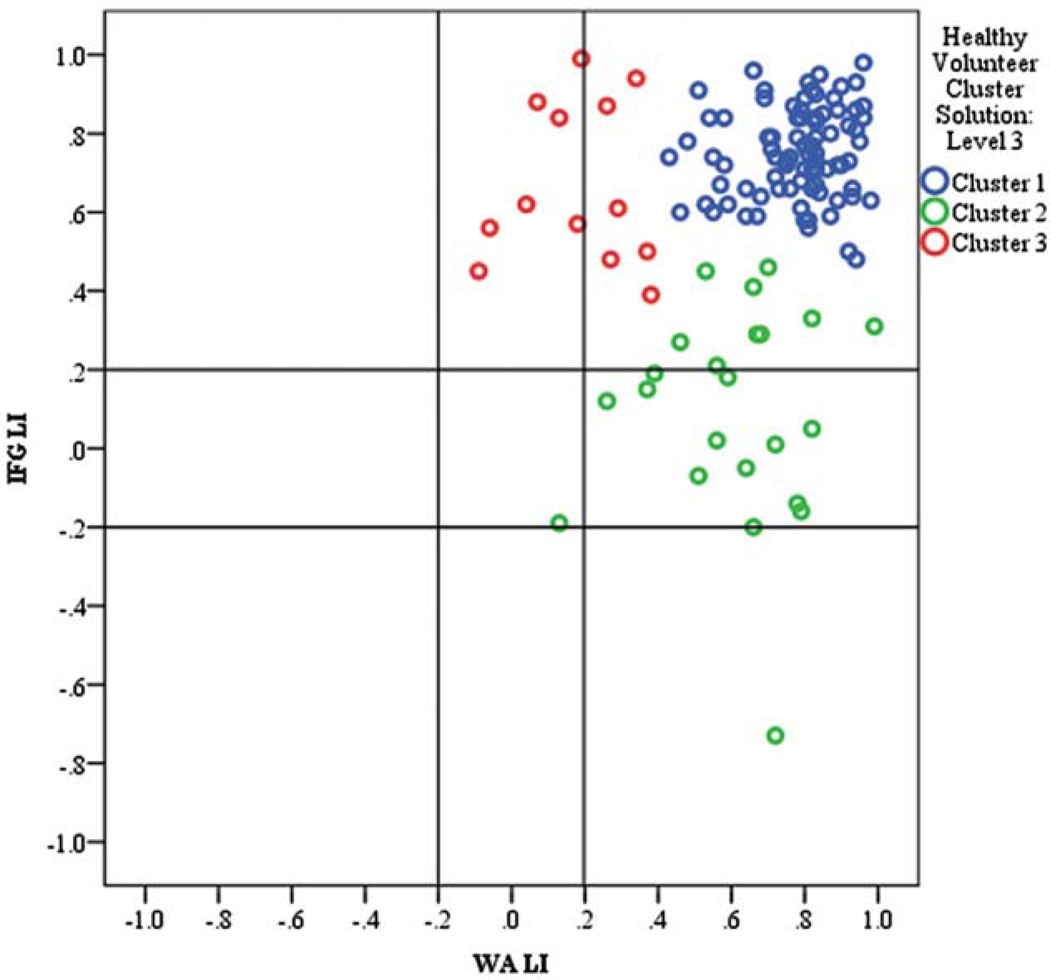
HEALTHY VOLUNTEERS
As with the categorical method, the clustering analysis revealed that healthy volunteers had more homogeneous language dominance than patients, with nearly all healthy volunteers being left hemisphere dominant (Fig 5; maximum DC 17.7 vs 120.4 for patients). Except for 1 outlying case of crossed dominance, atypically categorized volunteers were on or adjacent to the |0.20| LI threshold. Because healthy volunteers demonstrated less LI variance, cluster analysis does not yield results at the 4- and 9-cluster levels. We considered a 3-cluster solution based on the lowest DC value considered for patients (DC = 9.8 vs 11.7 for patients). The 2-cluster solution for healthy volunteers divided left-dominant clusters at IFG LI = 0.50, similar to the IFG LI = 0.52 that divided patient left dominant clusters 1 and 2.
FIGURE 5.
Cluster analysis of typically developing controls revealing 3 clusters of left dominance. Lines indicate thresholds for categorical thresholds of patterns. Colors indicate clusters. IFG = inferior frontal gyrus; LI = lateralization index; WA = Wernicke area. [Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
Patient Demographic and Seizure Characteristics
For hierarchy levels 1 and 2, seizure and demographic profiles were comparable between the 2 methods, agreeing with previous studies showing patients with atypical language dominance more likely to have atypical handedness (left and mixed dominance), early seizure onset, and abnormal MRI, particularly vascular pathology, but no differences in IQ. In contrast, important differences became apparent at the third level of the hierarchy, when all activation patterns are considered (Supplementary Tables 1 and 2).
HANDEDNESS
For level 3 comparisons, both methods found more frequent atypical handedness when IFG was right lateralized (a priori groups: chi-square = 23.788, df = 7, p = 0.001; cluster groups: chi-square = 38.007, df = 8, p < 0.001).
AGE OF SEIZURE ONSET
For both methods, patients with R IFG/R WA and L IFG/R WA had more frequent early seizure onset (a priori groups: chi-square = 20.793, df = 7, p < 0.01; cluster groups: chi-square = 14.969, df = 8, p = 0.06). Atypical WA lateralization was also associated with early seizure onset (chi-square = 4.630, df = 1, p < 0.05), but atypical IFG lateralization was not (p > 0.10).
MRI FINDINGS
For level 3 comparisons, patients with inflammatory, dual pathology, or MRI findings labeled as “other” were removed due to low cell count. Cluster analysis revealed that atypical language dominance, particularly in the temporal lobe, was associated with vascular pathology (a priori groups: chi-square = 36.06, df = 27, p > 0.05; cluster groups: chi-square = 43.89, df = 24, p < 0.01).
SEIZURE FOCUS LOCATION
There were no differences when side and lobar location were considered together; however, when only lateralization was considered, the cluster analysis at level 3 found a left focus more common in the R IFG/R WA cluster (chi-square = 15.379, df = 8, p = 0.05). Notably, this cluster did not exclusively have left foci; 3 patients had a right focus (17.6%).
AGE AND GENDER
No groups at any level differed significantly in age or gender (all p > 0.10).
IQ
No groups differed significantly for FSIQ, VIQ, or PIQ (all p > 0.10) for either method, likely due to small sample sizes per cell (smallest cell, n = 2); however, groups differed as much as 37 IQ points for categorical groups and 20 IQ points for cluster groups (see Supplementary Tables 1 and 2). Effect sizes for level 3 cluster analysis comparisons were large (Cohen d ranged from 0.92 to 1.14). Patients with strong lateralized patterns— either left or right dominance—had the highest IQs and were consistently strong in verbal and nonverbal skills. Patients with lower IQ skills had some degree of bilateral, weaker, or cross dominance. Findings were more striking and had less variability in cluster than categorical groups.
Discussion
Our study captured language dominance variance across a large sample. Although language dominance is a continuum, our results suggest meaningful distinctions in classifying laterality. We found greater sensitivity for differences and specificity for establishing LI thresholds using a data-driven, clustering approach than an a priori approach. Atypical language is not simply the right hemisphere mirror image of typical left language patterns; the range of language patterns—both typical and atypical— highlights the complexity of distributed language networks, especially associated with neurological disease or injury. Previous reports describing variability have not compared a large sample compared to healthy volunteers; we derived incidence estimates for specific patterns and the influence of clinical correlates. In addition to achieving greater understanding of language plasticity through determining the range of language patterns, our study may contribute to refining presurgical language mapping in epilepsy. Our results emphasize the importance of assessing the brain regionally, rather than on a hemispheric basis. We suggest that there may be clinical correlates of specific thresholds.
Clinical interpretation of language fMRI may be enhanced if there is greater specificity of outcome and/or function with LI values. Although the common a priori method largely agreed with the data-driven method and the a priori methods have high agreement with invasive procedures,37 the data-driven method provides potential new insights on defining language dominance and clinical factors associated with different patterns. One crucial issue is which LI value should be used to categorize language dominance. In patients, the data-driven method highlighted subdivisions within the typical L IFG/L WA pattern, suggesting that laterality strength at a LI of approximately 0.5 or stronger may distinguish groups. This finding is supported by 2 studies suggesting that greater left temporal lateralization predicts greater post-operative naming decline.16,24 Moreover, a comparison of fMRI and IAP for 229 patients found that bilateral language–on either mapping procedure–was the greatest predictor of disagreement in the 14% of cases where it occurred.37 Similarly, the greatest classification disagreement between our 2 approaches appeared as LI values approached zero. The data-driven model indicated a wider LI range (±0.4 vs ±0.2) for defining bilateral dominance. Care should be used in interpreting patient LI within this range. Although bilateral language is associated with more discrepancy, there remains the possibility—albeit very small6,37,38 —that even when a higher cutoff has been specified, fMRI and IAP will be discordant. Taken together, we suggest that strong LI values (>|0.5|) are clinically meaningful and that LI < |0.4| is a more comprehensive definition of bilateral language.
LI threshold specificity was helpful in defining crossed dominance. Reports of crossed language dominance are relatively uncommon (1% or n = 1–3), based on IAP cases3,39 and a few fMRI studies.39–41 We found the R IFG/L WA pattern had fewer members in the cluster analysis than in a priori assignment (8 [3%] vs 16 [7%]) because it contained only patients with a strongly right lateralized IFG (< −0.60). Although our sample may have a higher incidence of cross-dominance than previously reported, the data-driven method showed better agreement than the a priori method with estimated crossed dominance occurrence from historical IAP data.3 Our unexpectedly high number of patients with crossed dominance on a priori criteria may reflect methodological issues such as thresholding and task design or may be due to a referral bias of our center. Variability in clinical characteristics in this group implies influence of multiple factors on individual language expression.
Our results agree with the known relationship between atypical language and atypical dexterity,2,22,23 but extend previous findings by demonstrating that atypical dexterity is associated with shifted frontal rather than temporal activation. Right-lateralized IFG patients had the greatest proportion of atypical handedness. IFG lateralization, perhaps due to proximity to motor cortex, may be more related to atypical handedness than WA lateralization. Factors underlying motor dominance determination may underlie determination of frontal language dominance.23,41 This is the first fMRI study to demonstrate the relationship in epilepsy. A limitation of our data is that we excluded nondextral controls, which would clarify whether this relationship is unique to epilepsy.
Previous IAP and fMRI studies showed that early seizure onset age and brain injury are associated with atypical language representation.2,7,22,23 We found patients with early seizure onset likely to have both IFG and WA atypical language dominance. However, WA lateralization is more tightly linked than IFG to age of seizure onset. Patients with later onset are more likely to have strong left lateralization in both IFG and WA.42 WA likely plays a more important developmental role than IFG in establishing language dominance patterns, perhaps reflecting differences in regional brain structural and functional maturation trajectories.43–49
Among instances of bilateral dominance, there were differences between bilateral IFG and bilateral WA patterns. Fewer patients had bilateral WA, and all cases of bilateral WA represented a single cluster. In contrast, there were more instances of bilateral IFG that were dispersed across several cluster patterns. The single cluster of bilateral WA LI suggests that WA—more than IFG— may be a driving factor for differentiating groups. It is possible that because the majority of our group had a temporal focus, this may reflect epilepsy-specific factors. In contrast, the greater number of cases with bilateral IFG may reflect the effects of non–epilepsy-specific factors such as performance differences; frontal involvement in executive task processing control may evoke greater bilateral activation with increasing task difficulty.50,51 Bilateral frontal may reflect fundamentally different processes than bilateral temporal language dominance.
Side of seizure focus differed among groups identified by cluster analysis but not the a priori method. Some studies using similar a priori methods observed this finding.2,3,21 One might expect atypical language dominance to be more likely with a left seizure focus, whereas a right hemisphere seizure focus—or its cause— may reinforce typical left hemisphere language dominance. Our study, however, emphasizes that right focus patients may have atypical language dominance. Again, the nature of our referrals may have biased our sample toward a higher incidence of the rare right focus and atypical language cases.
The association of IQ with language patterns was not significant, suggesting that reorganization of language was not uniformly detrimental to cognitive functioning. However, the effect sizes and raw score differences in IQ were large for some atypical language groups. Thus, larger sample sizes—which are difficult to amass given the low incidence of these patterns—may reveal differential cognitive effects of cross-dominance or complete, mirror-image reorganization. The cluster analysis appeared to be more sensitive to these preliminary findings. We were not able to perform routine formal postoperative neuropsychological testing, but no patient had clinically evident postoperative aphasia. More specific study of cognitive outcomes would be an important next step for understanding the functional relevance of fMRI language patterns.
Data comparing intraoperative or subdural electrode language mapping with fMRI are limited.52–58 Overall discordance rates vary according to several factors, but reasonable estimates—including studies with large numbers and clear methodology—are from 14 to 24%.37 However, a large series comparing fMRI to IAP showed only 4 of 229 (<2%) patients with extreme cases of complete discordance,37 and fMRI predicted postsurgical naming outcome with relatively better accuracy than IAP.59 All 4 had left IAP, right fMRI, and a right seizure focus.37 The authors suggested that fMRI may reflect right hemisphere activation involved in but not essential to language processing. Although patients included within this study do not have IAP data available for comparison, our previous work with an earlier cohort of patients are included in the review of 24 IAP–fMRI comparison studies cited in the Janecek and colleagues article37 and is consistent with their reported rates of discordance. The current literature does not clearly show that 1 mapping procedure (fMRI vs IAP) is superior over the other.
Technical factors, such as impaired BOLD physiological response,60 poor overall fMRI activation,37 or the patient’s failure to understand task directions 6 might affect fMRI results. Varying cerebral perfusion patterns, drug response, or rate of injection may compromise the IAP. Confidence in the interpretation of fMRI studies can be increased. When a panel of fMRI tasks was used, as opposed to a single paradigm, there was no case of complete discordance, as at least 1 task agreed with IAP results.6 Formal conjunction analyses, and comparison with specific IAP test items, might improve language mapping.6,21
Our fMRI findings largely agree with large-scale studies on IAP language dominance patterns that found 4 patterns of language dominance (left, incomplete left, right, and bilateral [including crossed dominant cases]).3,8 These studies also reported a wide range of individual subpatterns within the bilateral group. Increased homologous fMRI activation may predict preserved function after lateralized injury or surgical resection. Further confirmation of the utility of specific thresholds and our proposed clusters would refine our ability to classify language by adding region and lateralization strength.
Conclusions
Our study used 338 subjects to highlight complexity and variability of language activation patterns in patients with focal epilepsy. Variation is present within both atypical and typical language lateralization. Our data-driven hierarchical clustering method verified rough classification of language lateralization based on left, right, and bilateral dominance. These language dominance categories (as well as more specific individual patterns) may reflect differing clinical factors, with implications for understanding their effect on language network reorganization and planning epilepsy surgery.
Supplementary Material
Acknowledgment
This work was supported by the NIH National Institutes of Neurological Disorders and Stroke (1ZIANS002858-18, W.H.T.; R01 NS44280, W.D.G.; K23NS065121-01A2, M.M.B.); NIH National Center for Research Resources (M01RR020359); Children’s Research Institute Avery Award (M.M.B.); NINDS Clinical Epilepsy Section Division of Intramural Research (W.H.T., W.D.G.); Intellectual and Developmental Disabilities Research Center; and Children’s National Medical Center (HD040677-07).
We thank Drs F. Ritter and P. Klein for referring patients, and the patients who participated.
Potential Conflicts of Interest
M.M.B.: employment, Epilepsy Foundation; grants/-grants pending, NIH K23. L.N.S.: grants/grants pending, Epilepsy Foundation of America. W.H.T.: GE stock dividends; Coeditor in Chief of Epilepsy Research until June 2012. W.D.G.: board membership, American Epilepsy Society, Editorial Board of Epilepsy Research; grants/grants pending, NSF, CDC, NIMH, NICHD, NINDS; paid educational presentations, ILAE for e-Education courses; stock/stock options, <$5,000 in Siemen’s stock.
Footnotes
Additional supporting information can be found in the online version of this article.
References
- 1.Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 2.Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(pt 11):2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 3.Kurthen M, Helmstaedter C, Linke DB, et al. Quantitative and qualitative evaluation of patterns of cerebral language dominance. An amobarbital study. Brain Lang. 1994;46:536–564. doi: 10.1006/brln.1994.1030. [DOI] [PubMed] [Google Scholar]
- 4.Wellmer J, Weber B, Weis S, et al. Strongly lateralized activation in language fMRI of atypical dominant patients—implications for presurgical work-up. Epilepsy Res. 2008;80:67–76. doi: 10.1016/j.eplepsyres.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Jansen A, Menke R, Sommer J, et al. The assessment of hemispheric lateralization in functional MRI—robustness and reproducibility. Neuroimage. 2006;33:204–217. doi: 10.1016/j.neuroimage.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Arora J, Pugh K, Westerveld M, et al. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50:2225–2241. doi: 10.1111/j.1528-1167.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaillard WD, Berl MM, Moore EN, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- 8.Loring DW, Meador KJ, Lee GP, et al. Cerebral language lateralization: evidence from intracarotid amobarbital testing. Neuropsychologia. 1990;28:831–838. doi: 10.1016/0028-3932(90)90007-b. [DOI] [PubMed] [Google Scholar]
- 9.Risse GL, Gates JR, Fangman MC. A reconsideration of bilateral language representation based on the intracarotid amobarbital procedure. Brain Cogn. 1997;33:118–132. doi: 10.1006/brcg.1997.0887. [DOI] [PubMed] [Google Scholar]
- 10.You X, Adjouadi M, Guillen MR, et al. Sub-patterns of language network reorganization in pediatric localization related epilepsy: a multisite study. Hum Brain Mapp. 2011;32:784–799. doi: 10.1002/hbm.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmstaedter C, Kurthen M, Linke DB, Elger CE. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age at onset of epilepsy. Brain Cogn. 1997;33:135–150. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- 12.Wilke M, Schmithorst VJ. A combined bootstrap=histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–530. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008;26:594–601. doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holland SK, Plante E, Weber Byars A, et al. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- 15.Liégeois F, Connelly A, Cross JH, et al. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127(pt 6):1229–1236. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- 16.Sabsevitz DS, Swanson SJ, Hammeke TA, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- 17.Adcock JE, Wise RG, Oxbury JM, et al. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 18.Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- 19.Anneken K, Konrad C, Dräger B, et al. Familial aggregation of strong hemispheric language lateralization. Neurology. 2004;63:2433–2435. doi: 10.1212/01.wnl.0000147265.71911.65. [DOI] [PubMed] [Google Scholar]
- 20.Berl MM, Balsamo LM, Xu B, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005;65:1604–1611. doi: 10.1212/01.wnl.0000184502.06647.28. [DOI] [PubMed] [Google Scholar]
- 21.Gaillard WD, Balsamo L, Xu B, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- 22.Woermann FG, Jokeit H, Luerding R, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- 24.Bonelli SB, Thompson PJ, Yogarajah M, et al. Imaging language networks before and after anterior temporal lobe resection: results of a longitudinal fMRI study. Epilepsia. 2012;53:639–650. doi: 10.1111/j.1528-1167.2012.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mbwana J, Berl MM, Ritzl EK, et al. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132(pt 2):347–356. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberger LR, Zeck J, Berl MM, et al. Interhemispheric and intrahemispheric language reorganization in complex partial epilepsy. Neurology. 2009;72:1830–1836. doi: 10.1212/WNL.0b013e3181a7114b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duke ES, Tesfaye M, Berl MM, et al. The effect of seizure focus on regional language processing areas. Epilepsia. 2012;53:1044–1050. doi: 10.1111/j.1528-1167.2012.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berl MM, Mayo J, Parks EN, et al. Regional differences in the developmental trajectory of lateralization of the language network. Hum Brain Mapp. 2012 Oct 3; doi: 10.1002/hbm.22179. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You X, Adjouadi M, Wang J, et al. A decisional space for fMRI pattern separation using the principal component analysis—a comparative study of language networks in pediatric epilepsy. Hum Brain Mapp. 2013;34:2330–2342. doi: 10.1002/hbm.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szaflarski JP, Binder JR, Possing ET, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 31.Balsamo LM, Xu B, Gaillard WD. Language lateralization and the role of the fusiform gyrus in semantic processing in young children. Neuroimage. 2006;31:1306–1314. doi: 10.1016/j.neuroimage.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Thivard L, Hombrouck J, du Montcel ST, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. 2005;24:841–851. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Sabbah P, Chassoux F, Leveque C, et al. Functional MR imaging in assessment of language dominance in epileptic patients. Neuroimage. 2003;18:460–467. doi: 10.1016/s1053-8119(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 34.Fernández G, de Greiff A, von Oertzen J, et al. Language mapping in less than 15 minutes: real-time functional MRI during routine clinical investigation. Neuroimage. 2001;14:585–594. doi: 10.1006/nimg.2001.0854. [DOI] [PubMed] [Google Scholar]
- 35.Gaillard WD, Balsamo L, Xu B, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–265. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- 36.Clatworthy J, Buick D, Hankins M, et al. The use and reporting of cluster analysis in health psychology: a review. Br J Health Psychol. 2005;10(pt 3):329–358. doi: 10.1348/135910705X25697. [DOI] [PubMed] [Google Scholar]
- 37.Janecek JK, Swanson SJ, Sabsevitz DS, et al. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: rates and predictors of discordance. Epilepsia. 2013;54:314–322. doi: 10.1111/epi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutten GJ, Ramsey NF, van Rijen PC, et al. FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. Neuroimage. 2002;17:447–460. doi: 10.1006/nimg.2002.1196. [DOI] [PubMed] [Google Scholar]
- 39.Ries ML, Boop FA, Griebel ML, et al. Functional MRI and Wada determination of language lateralization: a case of crossed dominance. Epilepsia. 2004;45:85–89. doi: 10.1111/j.0013-9580.2004.04403.x. [DOI] [PubMed] [Google Scholar]
- 40.Lehéricy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- 41.Staudt M, Grodd W, Niemann G, et al. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57:122–125. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- 42.Lee D, Swanson SJ, Sabsevitz DS, et al. Functional MRI and Wada studies in patients with interhemispheric dissociation of language functions. Epilepsy Behav. 2008;13:350–356. doi: 10.1016/j.yebeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sowell ER, Thompson PM, Leonard CM, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- 47.Lu L, Leonard C, Thompson P, et al. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- 48.Paus T, Zijdenbos A, Worsley K, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 49.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 50.Just MA, Carpenter PA, Keller TA, et al. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- 51.Gaillard WD, Pugliese M, Grandin CB, et al. Cortical localization of reading in normal children: an fMRI language study. Neurology. 2001;57:47–54. doi: 10.1212/wnl.57.1.47. [DOI] [PubMed] [Google Scholar]
- 52.FitzGerald DB, Cosgrove GR, Ronner S, et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR Am J Neuroradiol. 1997;18:1529–1539. [PMC free article] [PubMed] [Google Scholar]
- 53.Pouratian N, Bookheimer SY, Rubino G, et al. Category-specific naming deficit identified by intraoperative stimulation mapping and postoperative neuropsychological testing. Case report. J Neurosurg. 2003;99:170–176. doi: 10.3171/jns.2003.99.1.0170. [DOI] [PubMed] [Google Scholar]
- 54.Roux FE, Boulanouar K, Lotterie JA, et al. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52:1335–1345. doi: 10.1227/01.neu.0000064803.05077.40. discussion 1345–1347. [DOI] [PubMed] [Google Scholar]
- 55.Ruge MI, Victor J, Hosain S, et al. Concordance between functional magnetic resonance imaging and intraoperative language mapping. Stereotact Funct Neurosurg. 1999;72:95–102. doi: 10.1159/000029706. [DOI] [PubMed] [Google Scholar]
- 56.Carpentier A, Pugh KR, Westerveld M, et al. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42:1241–1254. doi: 10.1046/j.1528-1157.2001.35500.x. [DOI] [PubMed] [Google Scholar]
- 57.Kunii N, Kamada K, Ota T, et al. A detailed analysis of functional magnetic resonance imaging in the frontal language area: a comparative study with extraoperative electrocortical stimulation. Neurosurgery. 2011;69:590–596. doi: 10.1227/NEU.0b013e3182181be1. discussion 596–597. [DOI] [PubMed] [Google Scholar]
- 58.Brannen JH, Badie B, Moritz CH, et al. Reliability of functional MR imaging with word-generation tasks for mapping Broca's area. AJNR Am J Neuroradiol. 2001;22:1711–1718. [PMC free article] [PubMed] [Google Scholar]
- 59.Janecek JK, Swanson SJ, Sabsevitz DS, et al. Naming outcome prediction in patients with discordant Wada and fMRI language lateralization. Epilepsy Behav. 2013;27:399–403. doi: 10.1016/j.yebeh.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaillard WD, Berl MM. Functional magnetic resonance imaging: functional mapping. Handb Clin Neurol. 2012;107:387–398. doi: 10.1016/B978-0-444-52898-8.00024-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.