Abstract
Infection by Trypanosoma cruzi, the aetiological agent of Chagas disease, causes an intense inflammatory reaction in several tissues, including the myocardium. We have previously shown that transplantation of bone marrow cells (BMC) ameliorates the myocarditis in a mouse model of chronic Chagas disease. We investigated the participation of BMC in lesion repair in the heart and skeletal muscle, caused by T. cruzi infection in mice. Infection with a myotropic T. cruzi strain induced an increase in the percentage of stem cells and monocytes in the peripheral blood, as well as in gene expression of chemokines SDF-1, MCP1, 2, and 3 in the heart and skeletal muscle. To investigate the fate of BMC within the damaged tissue, chimeric mice were generated by syngeneic transplantation of green fluorescent protein (GFP+) BMC into lethally irradiated mice and infected with Trypanosoma cruzi. Migration of GFP+ BMC to the heart and skeletal muscle was observed during and after the acute phase of infection. GFP+ cardiomyocytes and endothelial cells were present in heart sections of chimeric chagasic mice. GFP+ myofibres were observed in the skeletal muscle of chimeric mice at different time points following infection. In conclusion, BMC migrate and contribute to the formation of new resident cells in the heart and skeletal muscle, which can be detected both during the acute and the chronic phase of infection. These findings reinforce the role of BMC in tissue regeneration.
Keywords: bone marrow cells, Chagas disease, chimeric mice, myocytes, tissue repair
Chagas disease is a zoonosis caused by the flagellate parasite T. cruzi. The disease is endemic in Latin American countries and continues to represent a major public health problem (Schofield et al. 2006). The prevalence of human T. cruzi infection is estimated at 15–16 million cases, with approximately 75–90 million people currently at risk of infection (Coura & Dias 2009).
The acute phase of the disease is transient and characterized by the presence of trypomastigote forms in the peripheral blood and amastigote proliferation within several host cell types (Koberle 1968). An intense inflammatory reaction is triggered by the presence of the parasite within tissues, resulting in a destruction of unparalleled proportions in the myocardium (Andrade 1983; Rassi et al. 2010), which, following the parasitaemia control, undergo a regenerative process. In humans, the acute phase regresses spontaneously after approximately 12 months (Andrade 1983; Rassi et al. 2010). About 30% of individuals infected by T. cruzi develop the symptomatic chronic cardiac form of the disease, for which there is no effective treatment (Andrade 1983). Thus, a more complete understanding of the cells and molecules that naturally participate in tissue repair in Chagas disease may open new avenues for the development of novel therapies.
Stem cell-based therapies represent a new frontier for the treatment of chronic degenerative diseases, including those affecting muscle and heart tissues. The bone marrow is an easily accessible source of stem cells, and its potential therapeutic applications have been intensely investigated. A number of studies have shown that bone marrow cells (BMC) migrate to injured organs, such as skeletal muscle and heart (Bianco et al. 2001; Goldenberg et al. 2008; Cao et al. 2009), leading to the formation of new specialized cells.
Specifically, we have observed that the transplantation of BMC obtained from both chagasic and naïve mice reduces the inflammatory infiltrates and fibrosis in the heart of chronically infected chagasic mice. Interestingly, BMC transplantation not only reduced inflammation and fibrosis, but also led to the formation of new cardiomyocytes (Soares et al. 2004, 2007). However, it is still not clear whether BMC are differentiating into GFP+ cardiomyocytes during different stages of the cardiomyopathy development and how BMC are specifically attracted to the sites of damaged tissue. Here, the mobilization and recruitment of bone marrow cells were studied in mice infected with T. cruzi. Secondly, the migration and fate of BMC during the development of Chagas disease were studied in infected BMC chimeras. By performing these experiments, we present evidence regarding the contribution of bone marrow-derived cells, as well as the role of inflammatory mediators, in lesions affecting the heart and skeletal muscle.
Materials and methods
Animals
Six- to eight-week-old female C57BL/6 mice were used as recipients for the production of chimeric animals. Four-week-old male C57BL/6 mice, transgenic for enhanced green fluorescent protein (GFP), were used as bone marrow cells donors for reconstitution of irradiated mice. All mice were raised and maintained in the animal facilities at the Gonçalo Moniz Research Center, FIOCRUZ/BA, and provided with rodent food and water ad libitum.
Ethical approval
Animals were handled according to the NIH guidelines for animal experimentation. All procedures described had prior approval from the local animal ethics committee.
Generation of chimeric mice
C57BL/6 female mice were irradiated with 6 Gy for bone marrow cell depletion in a 137Caesium source irradiator (CisBio International, Codolet, France). Bone marrow cells were obtained from femurs and tibiae from male GFP-transgenic mice and used to reconstitute irradiated mice. The mononuclear cells were purified by centrifugation in Ficoll gradient at 1000 g for 15 min (Histopaque 1119 and 1077, 1:1; Sigma-Aldrich, St. Louis, MO, USA). After two washings in DMEM medium (Sigma-Aldrich), the cells were filtered over nylon wool and resuspended in saline, and 200 μl was injected intravenously at 1 × 107 cells per mouse in all irradiated mice.
Parasites and infection
Trypomastigotes of the myotropic Colombian T. cruzi strain (Federici et al. 1964) were obtained from culture supernatants of infected LLC-MK2 cells. Infection of normal and chimeric mice was performed by intraperitoneal injection of 100 or 1000 T. cruzi trypomastigotes in saline respectively. Chimeric mice were infected 30 days after bone marrow transplantation. Parasitaemia of infected mice was evaluated at various time points following infection by counting the number of trypomastigotes in peripheral blood aliquots. Twenty-eight days after infection, chimeric animals were treated daily for 1 week with 40 mg/kg of benznidazole (Lafepe, Recife, Brazil) diluted in saline to control the parasitaemia.
Morphometric analysis
Groups of animals were euthanized 33, 66 and 192 days after infection, and different organs were removed and fixed in 10% buffered formalin. Tissue sections were analysed by light microscopy following paraffin embedding and then stained using a standard haematoxylin/eosin protocol. Inflammatory cells infiltrating heart and skeletal tissues were counted using a digital morphometric evaluation system. Images were digitalized using a colour digital video camera adapted to a microscope. The images were analysed using the Image Pro Plus Program (Media Cybernetics, San Diego, CA, USA), where inflammatory cells were counted and integrated with respect to area. Ten fields (100 μm2) per section were counted in one section per heart.
Sample preparation and real-time RT-PCR
The RNA was harvested from hearts and skeletal muscle and isolated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) using concentration determined by photometric measurement. The RNA quality was analysed in 1.2% agarose gel. High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used to synthesize cDNA from 2 μg of RNA following the manufacturer’s recommendations. Real-time RT-PCR assays were performed to detect the expression levels of different genes, using commercial probes, as follows: Sdf-1/CxCl12 (Mm 00443552_m1), Ccl2/MCP-1 (Mm 00441242_m1), Ccl8/MCP-2 (Mm 01297183_m1), Ccl7/MCP-3 (Mm 00443113_m1), IGF-1 (Mm00439561_m1) and VEGF (Mm00437304_m1). Amplification of qRT-PCR mixtures was performed with Universal Master Mix (Applied Biosystems) and the 7500 Real-Time PCR System (Applied Biosystems), under standard thermal cycling conditions, comprised of 10 min polymerase activation at 95 °C, 40 cycles at 95 °C for 15 s and 60 °C for 60 s. Experiments with coefficients of variation >5% were excluded. A no-template control and no-reverse transcription control (No-RT) were also included. The results are presented as the fold increase of expression from the individual mRNAs, with the target internal control GADPH, using the cycle threshold method.
Immunofluorescence analysis
Ten-micrometre frozen sections or 5-μm paraffin-embedded sections of hearts, livers, spleen and skeletal muscle were obtained and used for detection of GFP+ cells. The following primary antibodies were used: chicken anti-GFP (1:500; Aves Labs, Tigard, OR, USA), rabbit anti-myosin (1:200, Sigma-Aldrich), rabbit anti-von Willebrand Factor (1:50; Zymed Laboratories, San Francisco, CA, USA), mouse anti-PCNA (1:200; Dako Denmark A/S, Glostrup, Denmark) biotinylated with Dako Ark kit and mouse anti-Pax-7 (1:200; DSHB, Iowa city, IA, USA) stained using M.O.M. kit (Vector Labs, Burlingame, CA, USA). Secondary antibodies, anti-chicken Alexa Fluor 488 conjugated (1:200; Molecular Probes, Carlsbad, CA, USA) and anti-rabbit Alexa Fluor 568 conjugated (1:200; Molecular Probes), were used. For biotinylated antibodies stained sections, we used streptavidin Alexa Fluor 568 conjugated (1:200; Molecular Probes). Some heart sections were stained with phalloidin Alexa fluor 633 conjugated (1:100; Molecular Probes). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) (Vector Labs). Images were collected using the confocal microscope, FluoView 1000 (Olympus, Tokyo, Japan).
Flow cytometry analysis
Quantification of GFP+ cells was performed in blood samples obtained from naïve, bone marrow chimeric mice (one month after reconstitution) and GFP-transgenic control mice. Quantitative analysis of Sca-1+ and monocytes cells was performed in the blood of non-infected and acute chagasic mice, by flow cytometry. Cells were stained with labelled anti-mouse CD45 PE-Cy5.5, CD11b APC, CD34 PE, CD90 APC, SCA-1 FITC, F4/80 PerCP-Cy5 and Ly-6C/6G APC-Cy7 antibodies (BD Biosciences, San Diego, CA, USA) for 20 min, at room temperature. Red blood cells were lysed with lysis solution for 10 min at room temperature. Cells were washed twice with phosphate buffered saline (PBS), resuspended in 500 μl of PBS and then analysed using the cell analyser, LSRFortessa with FACSDiva software version 6.1.3 (BD Biosciences). To confirm the presence of GFP+ cells in chimeric mice, blood samples from irradiated and reconstituted mice were evaluated after 30 days of transplantation. Blood samples obtained from wild-type and GFP-transgenic C57BL/6 mice were used as negative and positive controls respectively. Acquisition and analysis were performed using a FACScalibur cytometer with the CellQuest software (BD Biosciences). At least 10,000 events were collected.
Statistical analyses
Statistical comparisons between groups were performed by Student’s t-test when comparing two groups and analysis of variance (anova) followed by Tukey’s test for multiple comparisons, using GraphPad Prism program (Software Inc., San Diego, CA, USA) version 5.0. Results were considered significant when P < 0.05.
Results
Infection with T. cruzi increases percentage of circulating stem cells and monocytes as well as chemokines expression in the heart and skeletal muscle
The mobilization of different cell subpopulations from the bone marrow was evaluated in the peripheral blood taken from C57BL/6 mice infected with Colombian T. cruzi strain. At the peak of infection (30 days after infection), a significant increase in the number of Sca-1+ cells and monocytes was observed in infected mice, when compared to naïve controls (Table 1). The Sca-1+ cells expressed either mesenchymal stem cell markers (CD90+ CD45−) or hematopoietic progenitor cell phenotype (CD34+ CD45+). Two monocyte subpopulations, expressing LY6Chi and LY6Clo, were also observed (Table 1).
Table 1.
Acute infection with T. cruzi induces the mobilization of Sca-1+ and monocytes to the peripheral blood
| Cell subpopulation | Uninfected | Infected | P value |
|---|---|---|---|
| MSC (Sca1+ CD90+ CD45−) | 0.23 ± 0.03 | 1.26 ± 0.18 | <0.01 |
| HSC (Sca1+ CD34+ CD45+) | 0.30 ± 0.06 | 1.71 ± 0.32 | <0.05 |
| MNC F4/80+ CD11b− LY6Clo | 2.17 ± 0.20 | 42.70 ± 1.96 | <0.0001 |
| MNC F4/80+ CD11b+ LY6Chi | 10.90 ± 0.95 | 54.95 ± 1.93 | <0.0001 |
Data represent the cell subpopulation% of naïve (n = 3) and infected (n = 8) mice and were expressed as means ± SEM. Statistical analysis was performed using Student’s t-test. MSC, mesenchymal stem cells; HSC, hematopoietic stem cells; MNC, monocytes.
The expression of chemokines participating in the recruitment of macrophages and stem cells was investigated at the transcriptional level by qRT-PCR in the heart and skeletal muscle. Gene expression of SDF-1 (CXCL12) in the heart was found to be similar between naïve and T. cruzi-infected mice. In contrast, SDF-1 expression in skeletal muscles increased in T. cruzi-infected mice in comparison with naïve mice (Figure 1). The expression of MCP1, 2 and 3 genes was significantly increased in both heart and muscle tissues of infected mice when compared to naïve mice (Figure 1).
Figure 1.
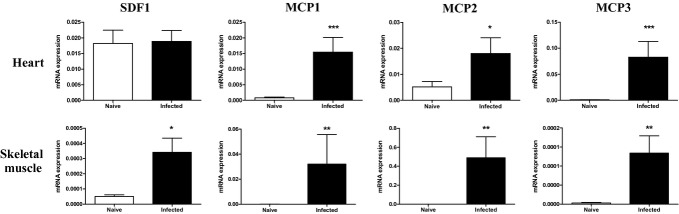
Gene expression of chemokines in the heart and skeletal muscle. Heart and skeletal muscle samples of uninfected or chagasic mice (33 days postinfection) were removed and analysed by qRT-PCR for the expression of SDF-1 and MCP 1, 2 and 3. Data represent the mean ± SEM of 5–8 mice per group. *P < 0.05; **P < 0.01; ***P < 0.001.
Presence of GFP+ cells in the heart and skeletal muscle of T. cruzi-infected chimeric mice
To investigate the fate of mobilized bone marrow cells following T. cruzi infection, we generated bone marrow chimeric mice by transplanting GFP+ bone marrow cells into lethally irradiated C57BL/6 recipients (Figure 2). Bone marrow reconstitution was confirmed by quantification of GFP+ cells by flow cytometry analysis (Figure 3 a–c). One month after reconstitution, chimeric mice were infected with 100 T. cruzi trypomastigotes and treated for 1 week with benznidazole, 28 days postinfection, to reduce mortality rate associated with the infection in chimeric mice. A decrease of parasitaemia levels in these animals was observed 30 days postinfection (Figure 3d). The numbers of inflammatory cells in the heart and skeletal muscle were quantified. As shown in Figure 3e, the number of inflammatory cells in the heart tissue was higher during the peak of parasitaemia (33 days after infection), decreasing after 192 days of infection. In contrast, the number of inflammatory cells remained elevated in skeletal muscle at all analysed time points (Figure 3f).
Figure 2.
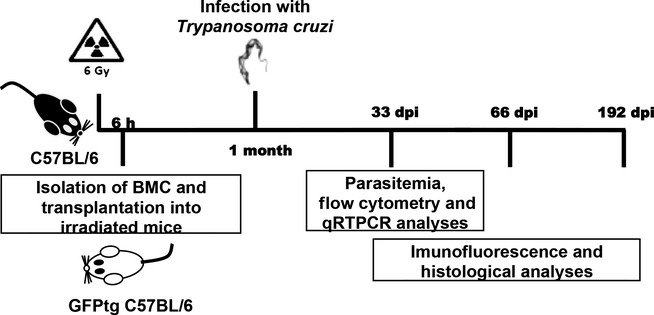
Generation of GFP+ bone marrow chimeric mice. Lethally irradiated wild-type C57BL/6 mice were transplanted with bone marrow obtained from C57BL/6 GFP transgenic mice.
Figure 3.
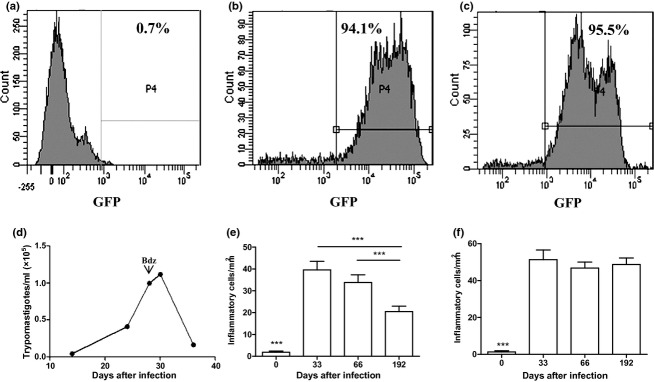
Infection of bone marrow chimeric mice with T. cruzi. Reconstitution of bone marrow chimeric mice was confirmed by flow cytometry analysis of GFP+ cells in the blood. (a), naïve mouse. (b), GFP-transgenic mouse. (c), chimeric mouse (one month after transplantation). One month after transplantation, chimeric mice were infected with 100 Colombian strain T. cruzi and treated with benznidazole (bdz) 28 days later. (d) Blood parasitaemia of T. cruzi-infected chimeric mice. Quantification of inflammation in the heart (e) and skeletal muscle (f) at various times after infection of chimeric mice. Data represent the mean ± SEM of 3–5 mice per group. ***P < 0.001.
Each time point analysed revealed the presence of GFP+ cardiomyofibres. The GFP+ cardiomyocytes were positively stained with an anti-myosin antibody or phalloidin (Figure 4a–c) and were visualized as a group of adjacent cells or individual cells within the myocardium. In contrast, heart sections of uninfected chimeric mice had sparse or no GFP+ cells present (Figure 4d). The GFP+ cells found in the hearts of uninfected chimeric mice did not present characteristic cardiomyocyte morphology, as well as a lack myosin expression.
Figure 4.
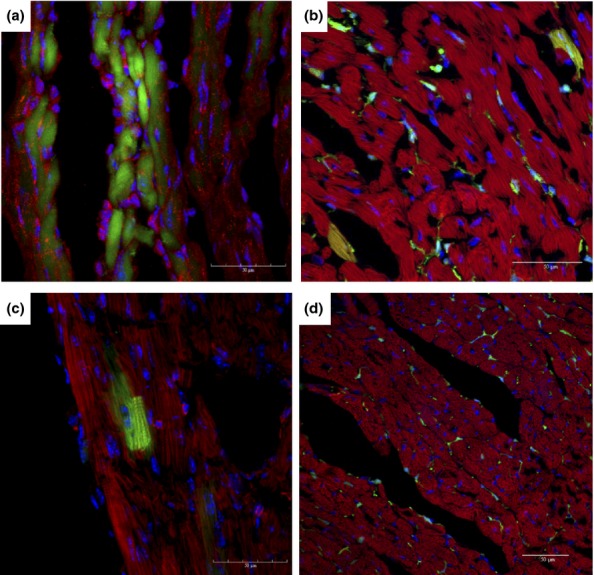
Presence of GFP+ cells in the hearts of chagasic chimeric mice. Hearts of chimeric mice euthanized at different time points after infection with T. cruzi were analysed by confocal microscopy. GFP+ (green) myosin+ (a) or F actin+ (b–d) (red) cardiomyocytes were found in heart sections of mice after 33 (a), 60 (b) and 192 (c) days of infection. (d) Heart section of an uninfected chimeric mouse. Nuclei were stained with DAPI (blue).
A significant number of GFP+ myofibres were found at all analysed time points (Figure 5a–c). The number of GFP+/myosin+ myofibres in skeletal muscle did not correlate with the severity of tissue inflammation. However, GFP+ myofibres were not observed in skeletal muscle sections obtained from uninfected chimeric mice, which included mice euthanized on day 192 (Figure 5d).
Figure 5.
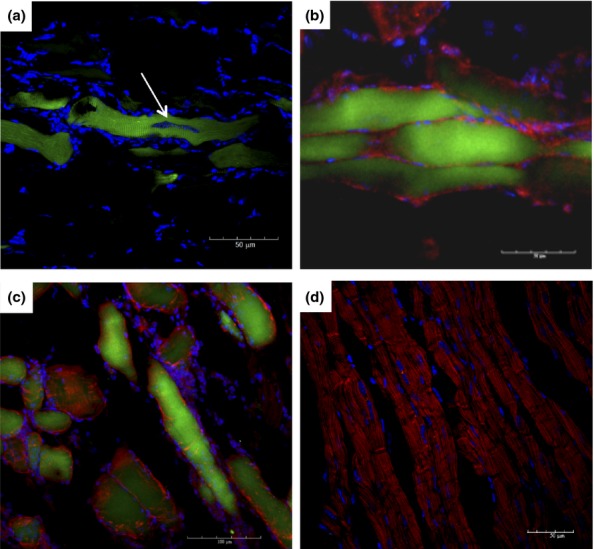
Presence of GFP+ cells in skeletal muscle of chagasic chimeric mice. Skeletal muscle of chimeric mice euthanized at different time points after infection with T. cruzi was analysed by immunofluorescence microscopy. (a) Parasite nest within a GFP+ myofibre (arrow) after 33 days of infection. (b and c) GFP+ (green) myosin+ (red) myofibres were found in skeletal muscle sections obtained from mice after 60 (B) and 192 (c) days of infection. (d) Skeletal muscle section obtained from an uninfected chimeric mouse. Sections were stained with anti-myosin antibody (red) and DAPI (blue).
In addition to myofibres, GFP+ cells were found within endothelial cells of chagasic heart blood vessels (Figure 6a), but were not observed in uninfected chimeric mice (Figure 6b). A subpopulation of these GFP+ cells was positive for von Willebrand factor, an endothelial cell marker. However, GFP+ satellites cells were not found in skeletal muscle of chimeric mice at any of the time points analysed, as shown by Pax7 stain (Figure 6c and d). GFP+ cells were also observed in other organs of chimeric chagasic mice, such as liver and spleen (data not shown).
Figure 6.
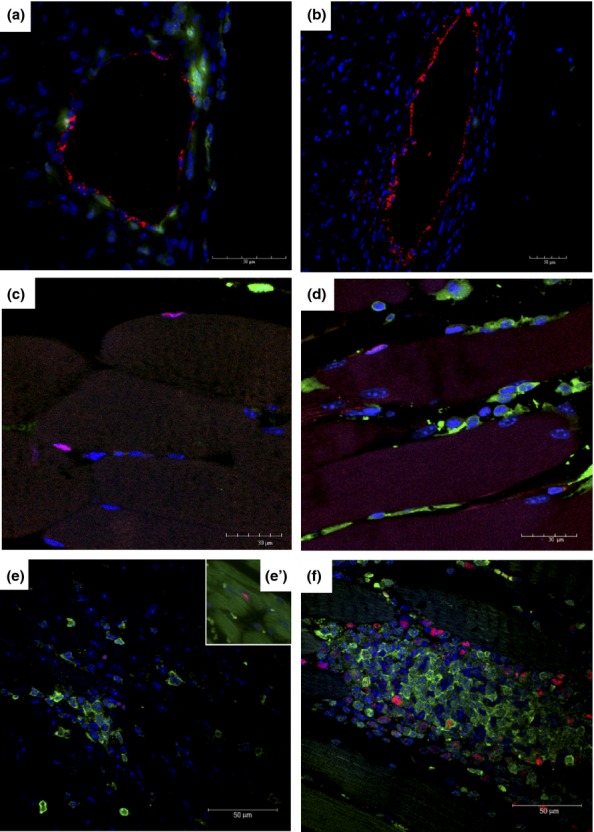
Characterization of GFP+ cells in different organs of T. cruzi-infected chimeric mice. GFP+ cells (green) were observed to be associated with blood vessels in the hearts of mice 33 days after infection (a), but not in uninfected chimeric mice (b). In red, staining for von Willebrand factor. Satellite cells Pax7+ in skeletal muscle sections of naïve (c) and T. cruzi-infected mice (d) 33 days after infection. Presence of GFP+ (green) proliferating PCNA+ cells (red) in the inflammatory infiltrates of the heart (e) and PCNA+GFP− cardiomyocytes stained with phalloidin (green; e’) and skeletal muscle (f) tissue, 33 days postinfection. Nuclei were stained with DAPI (blue).
Presence of proliferating GFP+ cells in inflammatory foci
Heart and skeletal muscle sections from chimeric mice euthanized at different time points following infection presented a massive influx of GFP+ inflammatory cells. Some GFP+ cells observed in inflammatory infiltrates of cardiac and skeletal muscle displayed positive nuclear staining for PCNA, a marker of cell proliferation (Figure 6e and f). Cardiomyocytes expressing PCNA were found in chimeric chagasic mice (Figure 6e’). However, myofibres expressing GFP and PCNA were not found in any heart or skeletal muscle sections analysed.
Acute T. cruzi infection upregulates the gene expression of IGF-1 and VEGF
The mRNA expression of IGF-1 and VEGF was investigated in the skeletal muscle and hearts of T. cruzi-infected mice by qRT-PCR. A significant increase in IGF-1 mRNA levels was observed in both heart and skeletal muscle samples of infected mice, when compared to normal mice (Figure 7). In contrast, VEGF mRNA levels were significantly increased in the skeletal muscle, but not in the hearts of infected mice, when compared to normal mice (Figure 7).
Figure 7.
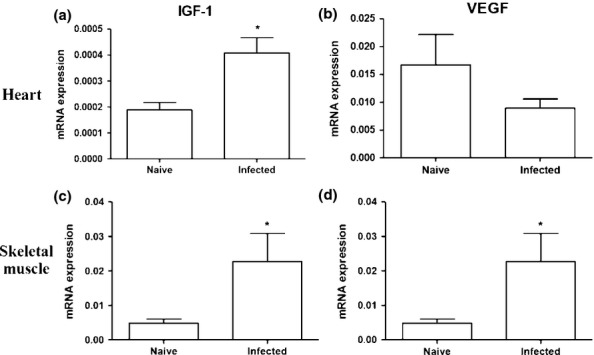
Expression of IGF-1 and VEGF in the heart and skeletal muscle. Naïve and T. cruzi-infected mice (33 days of infection) were euthanized to evaluate the gene expression of IGF-1 (a and c) and VEGF (b and d) by qRT-PCR. Data represent the mean ± SEM of 5–8 mice per group. *P < 0.05
Discussion
In the present study, we demonstrated that infection with T. cruzi can induce chemokine expression in inflamed tissues that continuously recruit bone marrow-derived cells to the peripheral blood and their contribution to the formation of resident cells in the heart and skeletal muscle tissues.
Chemokines MCP-1, -2, and -3 were also previously described to be highly expressed in the hearts of chagasic mice (Soares et al. 2010) and may induce the recruitment of monocytes and lymphocytes. It has been recently shown that some monocyte subpopulations can differentiate into specialized cell types, including endothelial cells and myocytes (Kodama et al. 2005; Kuwana et al. 2006), and may contribute to the GFP+ myocytes and endothelial cells observed in our study.
While MCPs play a role in the recruitment of immune cells, it is well known that SDF-1/CXCR4 is a major axis of stem cell chemotaxis. A recent study utilizing an alternative mouse model of Chagas disease also failed to detect a raise in the expression of SDF-1 in the heart and showed that hematopoietic stem cells (i.e. CD34+) were not recruited to the heart during infection (González et al. 2013). In our study, we observed an increased SDF-1 expression in the skeletal muscles and detected increased numbers of CD34+ cells in the peripheral blood, suggesting that CD34+ cells could be recruited to different inflamed tissues, as evidenced by the presence of GFP+ resident cells in the tissues analysed.
Post-natal cardiomyogenesis has been reported to occur in mice and humans, at a low frequency (about 1% per year), a process that declines considerably with age (Garbern & Lee 2013). New cardiomyocytes can be generated from stem cells (Laflamme et al. 2002; Agbulut et al. 2003; Deb et al. 2003; Fogt et al. 2003; Thiele et al. 2004) or by dividing mature cardiomyocytes (Senyo et al. 2013). Here, we detected the presence of bone marrow-derived cardiomyocytes, as well as the proliferation of pre-existing cardiomyocytes (GFP− PCNA+). Similarly, Arnaiz et al. (2002) observed proliferating cardiomyocytes (PCNA+) after different periods of infection by T. cruzi in rats (Arnaiz et al. 2002).
Previous studies have reported a low-frequency presence of bone marrow-derived skeletal myocytes following acute myotoxic injury (Corbel et al. 2003; Rudnick 2003). It has been suggested that these cells result from fusion between damaged myofibres and bone marrow-derived cells (Rudnick 2003). The frequency of bone marrow-derived myocytes was found to be higher in a Duchenne muscular dystrophy experimental model, which may result from a selective advantage (Dezawa et al. 2005). In the present study, we showed that persistent inflammation leads to an increased number of bone marrow-derived cells compared with those previously reported in acute injury experimental models.
Skeletal muscle regeneration is a dynamic process that occurs with the contribution of different stem cells sources, including skeletal muscle side population cells, bone marrow-derived cells, mesoangioblasts and pericytes (Otto et al. 2009). These cells can contribute to the satellite cell niche or to the generation of myofibres through other pathways (Xynos et al., 2010).
The fusion process is a physiological mechanism by which myoblasts form multinucleated muscle fibres, becoming a syncytium. A previous study demonstrated that macrophages play an important role in muscle regeneration (Arnold et al. 2007) by producing growth factors for myogenic progenitors that can also undergo fusion with myofibres (Camargo et al. 2003). Thus, as there is a potent mobilization of two different monocyte populations (Ly6Clo e Ly6Chi) and the presence of macrophages in the inflammatory foci, our data suggest that these cells are fusing with myocytes in our model of T. cruzi infection. Additionally, we found an increase in IGF-1 and VEGF gene expression upon T. cruzi infection. These factors have been implicated in the promotion of tissue repair by angiogenesis induction and promote muscle regeneration, two processes in which macrophages have been shown to play key roles (Lu et al. 2011; Santini & Rosenthal 2012).
Bone marrow-derived cells expressing satellite cell markers have been observed following bone marrow transplants in association with myofibres (Labarge & Blau 2002; Dreyfus et al. 2004). Camargo et al. (2003) also made this observation, although at a low frequency, following hematopoietic stem cell transplantation in a cardiotoxin-induced injury model (Camargo et al. 2003). In our study, we did not observe any bone marrow-derived cells (GFP+) expressing the satellite cell marker pax7, which seems to favour a fusion process rather than a transdifferentiation mechanism, in this model of T. cruzi infection.
In conclusion, we have demonstrated that bone marrow cells actively participate in the pathogenesis and regeneration process that occurs naturally in damaged skeletal muscles and hearts of an experimental model of Chagas disease. These observations support the potential benefits of bone marrow cell therapy during the chronic phase of Chagas disease, to increase a regeneration process that naturally occurs.
Acknowledgments
This work was supported by CNPq, FAPESB, FINEP and FIOCRUZ. The authors wish to thank Geraldo Pedral Sampaio for technical assistance in flow cytometry and Dr. Kyan James Allahdadi for careful revision of the manuscript.
References
- Agbulut O, Menot ML, Li Z, et al. Temporal patterns of bone marrow cell differentiation following transplantation in doxorubicin-induced cardiomyopathy. Cardiovasc. Res. 2003;58:451–459. doi: 10.1016/s0008-6363(03)00281-5. [DOI] [PubMed] [Google Scholar]
- Andrade ZA. Mechanisms of myocardial damage in Trypanosoma cruzi infection. Ciba Found. Symp. 1983;99:214–233. doi: 10.1002/9780470720806.ch12. [DOI] [PubMed] [Google Scholar]
- Arnaiz MR, Fichera LE. Postan M. Cardiac myocyte hypertrophy and proliferating cell nuclear antigen expression in Wistar rats infected with Trypanosoma cruzi. J. Parasitol. 2002;88:919–925. doi: 10.1645/0022-3395(2002)088[0919:CMHAPC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S. Robey PG. Bone marrow stromal cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Green R, Capetanaki Y, Jackson KA. Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat. Med. 2003;9:1520–1527. doi: 10.1038/nm963. [DOI] [PubMed] [Google Scholar]
- Cao F, Sun D, Li C, et al. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. Eur. Heart J. 2009;30:1986–1994. doi: 10.1093/eurheartj/ehp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel SY, Lee A, Lin Y, et al. Contribution of hematopoietic stem cells to skeletal muscle. Nat. Med. 2003;9:1528–1532. doi: 10.1038/nm959. [DOI] [PubMed] [Google Scholar]
- Coura JR, Dias JPC. Epidemiology, control and surveillance of Chagas disease 100 years after its discovery. Mem. Inst. Oswaldo Cruz. 2009;104(Suppl 1):31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- Deb A, Wang S, Skelding KA, Miller D, Simper D. Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: a study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Ishikawa H, Itokazu Y, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- Dreyfus PA, Chretien F, Chazaud B, et al. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am. J. Pathol. 2004;164:773–779. doi: 10.1016/S0002-9440(10)63165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici EE, Abelmann WH. Neva FA. Chronic and progressive myocarditis and myositis in C3H mice infected with Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 1964;13:272–280. doi: 10.4269/ajtmh.1964.13.272. [DOI] [PubMed] [Google Scholar]
- Fogt F, Beyser KH, Poremba C, Zimmerman RL. Ruschoff J. Evaluation of host stem cell-derived cardiac myocytes in consecutive biopsies in long-term cardiac transplant patients. J. Heart Lung Transplant. 2003;22:1314–1317. doi: 10.1016/s1053-2498(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Garbern JC. Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RC, Jelicks LA, Fortes FS, et al. Bone marrow cell therapy ameliorates and reverses chagasic cardiomyopathy in a mouse model. J. Infect. Dis. 2008;197:544–547. doi: 10.1086/526793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González MN, Dey N, Garg NJ. Postan M. Granulocyte coloning-stimulating factor partially repairs the damage provoked by Trypanosoma cruzi in murine myocardium. Int. J. Cardiol. 2013;168:2567–2574. doi: 10.1016/j.ijcard.2013.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koberle F. Chagas’ disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Adv. Parasitol. 1968;6:63–116. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- Kodama H, Inoue T, Watanabe R, et al. Cardiomyogenic potential of mesenchymal progenitors derived from human circulating CD14+ monocytes. Stem Cells Dev. 2005;14:676–686. doi: 10.1089/scd.2005.14.676. [DOI] [PubMed] [Google Scholar]
- Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y. Ikeda Y. Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells. 2006;24:2733–2743. doi: 10.1634/stemcells.2006-0026. [DOI] [PubMed] [Google Scholar]
- Labarge MA. Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Myerson D, Saffitz JE. Murry CE. Evidence for Cardiomyocyte Repopulation by Extracardiac Progenitors in Transplanted Human Hearts. Circ. Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- Lu H, Huang D, Ransohoff RM. Zhou L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011;25:3344–3355. doi: 10.1096/fj.10-178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto A, Collins-Hooper H. Patel K. The origin, molecular regulation and therapeutic potential of myogenic stem cell populations. J. Anat. 2009;215:477–497. doi: 10.1111/j.1469-7580.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassi A, Jr, Rassi A. Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Rudnick MA. Marrow to muscle, fusion versus fusion. Nat. Med. 2003;9:1461–1462. doi: 10.1038/nm1203-1461. [DOI] [PubMed] [Google Scholar]
- Santini MP. Rosenthal N. Myocardial regenerative properties of macrophage populations and stem cells. J. Cardiovasc. Transl. Res. 2012;5:700–712. doi: 10.1007/s12265-012-9383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CJ, Jannin J. Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MB, Lima RS, Rocha LL, et al. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. Am. J. Pathol. 2004;164:441–447. doi: 10.1016/s0002-9440(10)63134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MB, Garcia S, Campos de Carvalho AC. Ribeiro dos Santos R. Cellular therapy in Chagas’ disease: potential applications in patients with chronic cardiomyopathy. Regen. Med. 2007;2:257–264. doi: 10.2217/17460751.2.3.257. [DOI] [PubMed] [Google Scholar]
- Soares MB, de Lima RS, Rocha LL, et al. Gene expression changes associated with myocarditis and fibrosis in hearts of mice with chronic chagasic cardiomyopathy. J. Infect. Dis. 2010;202:416–426. doi: 10.1086/653481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele J, Varus E, Wickenhauser C, Kvasnicka HM, Metz KA. Beelen DW. Regeneration of heart muscle tissue: quantification of chimeric cardiomyocytes and endothelial cells following transplantation. Histol. Histopathol. 2004;19:201–209. doi: 10.14670/HH-19.201. [DOI] [PubMed] [Google Scholar]
- Xynos A, Corbella P, Belmonte N, Zini R, Manfredini R. Ferrari G. Bone marrow-derived hematopoietic cells undergo myogenic differentiation following a Pax-7 independent pathway. Stem Cells. 2010;28:965–973. doi: 10.1002/stem.418. [DOI] [PubMed] [Google Scholar]


