Abstract
Peromyscus leucopus mice share physical similarities with laboratory mice Mus musculus (MM) but have higher agility and longer lifespan. We compared domesticated P. leucopus linville (PLL) and M. musculus C57BL/6 (MMB6) mice for cellular composition of peripheral blood (PB), bone marrow (BM) and spleen. PLL mice had significantly fewer platelets and significantly more monocytes in the blood, and notably fewer megakaryocytes in the BM. Spleens of PLL mice were significantly smaller, with 50% fewer cells and reduced ‘red pulp’. There was no obvious haematological change in PLL mice between 2–8 and 16–26 months of age, except for a significant increase in blood monocytes. Cellular reactive oxygen species (ROS) content showed no change with age but differed significantly between different cell types. Treating two to eight month-old PLL mice with antioxidant N-acetylcysteine in drinking water for three months did not affect cellular ROS content, but increased blood leucocytes especially the concentration of monocytes. The low platelets, low megakaryocytes, high monocytes and low splenic erythropoiesis in PLL mice resemble human measurements better than the values seen in MMB6.
Keywords: haematological measurements, monocytes, Mus musculus mice, Peromyscus leucopus mice, platelets, reactive oxygen species, megakaryocytes, spleen, disease modeling
Peromyscus leucopus linville (PLL) is a mouse strain of the species P. leucopus commonly referred to as ‘white-footed’ mice. These animals share many physical features with the popular laboratory mice Mus musculus (MM) and are the most populous rodent species in the north-eastern United States (Joyner et al. 1998). P. leucopus have been used for studies of social interactions and stress physiology (Southwick 1964), as well as for the investigation of photoperiodism and the action of melatonin on the regulation of hormone secretion and reproductive physiology (Glass & Lynch 1982; Glass & Knotts 1987; Glass & Dolan 1988; Weaver et al. 1990). These animals came to public attention as the primary reservoir host for Borrelia burgdorferi sensu stricto, the aetiological agent of lyme disease (Magnarelli et al. 1988, 1994; Hofmeister & Childs 1995; Hofmeister et al. 1999), and they may also be hosts for species of hantavirus (Hjelle et al. 1995; Lyubsky et al. 1996; Morzunov et al. 1998). More recent studies have focused on behavioural and reproductive physiology of P. leucopus mice using different breeding practices under long-term captivity, with data showing that adaptation can be rapid, affecting reproductive patterns and behaviour, even under breeding protocols designed to minimize the rate of genetic change due to random drift and inadvertent selection (Malo et al. 2010; Lacy et al. 2013).
Peromyscus leucopus have long lifespans that distinguish them from MM mice and from sister species within the genus Peromyscus (Sacher & Hart 1978; Steger et al. 1980; Cohen et al. 1987; Burger & Gochfeld 1992). Peromyscus show extended reproductive lifespan (Steger et al. 1980; Burger & Gochfeld 1992), accelerated wound repair (Cohen et al. 1987), reduced cellular reactive oxygen species (ROS) (Labinskyy et al. 2009; Shi et al. 2013) and increased vascular resistance to oxidative stress and inflammatory damages (Labinskyy et al. 2009). Despite their broad utilization in biomedical research, P. leucopus mice have not been well characterized. For example, haematological parameters are not fully available, because previous studies only provided partial measurements: one study reported haematocrit and total and differential WBC counts in a group of young P. leucopus mice, and another immunological and haematological values in pups born to normal dams or dams exposed to aroclor 1254 (Wu et al. 1999a,b). In the current work, we performed haematological measurements in PLL mice with reference to MM mice using C57BL/6 (B6) as representatives. We also attempted to test the effects of ageing and antioxidant N-acetylcysteine (NAC) treatment on haematological changes in P. leucopus animals. Here, we report that P. leucopus have lower platelet counts, fewer megakaryocytes and more monocytes than do MMB6 mice, with values closely resembling those of humans, suggesting that P. leucopus mice could potentially be a good model of some aspects of human haematology.
Materials and methods
Animals
Peromyscus leucopus linville mice were obtained from the Peromyscus Genetic Stock Center (PGSC) at the University of South Carolina (Columbia, SC, USA), after 28 years of domestication and restricted free mating (to avoid sister–brother mating). PLL mice at 16–26 months of age were obtained from PGSC directly, while 2- to 8-month-old PLL mice were produced at NIH from young breeders (sister–brother mating avoided). Normal C57BL/6 (MMB6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and were bred and maintained in NIH animal facilities with standard care and nutrition.
Ethical approval
All animal studies were approved by the Animal Care and Use Committee at the National Heart, Lung and Blood Institute.
Complete blood counts and cellular analyses
Blood was collected from the retro-orbital sinus into EDTA-added Eppendorf tubes, and complete blood counts (CBCs) were performed using a HemaVet 950 analyzer (Drew Scientific, Inc., Waterbury, CT, USA). Mice were euthanized by CO2 inhalation from a compressed source. Bone marrow (BM) cells were extracted from bilateral tibiae and femurs. The spleen of each animal was homogenized to obtain a single cell suspension. BM and spleen cells were filtered through 95-μm nylon mesh and counted using a Vi-Cell counter (Coulter Cooperation, Miami, FL, USA).
Flow cytometry
Blood, BM and spleen cells were first incubated with ACK solution twice for 10 minutes to lyse red blood cells (RBCs). Nucleated cells were stained with antibody mixtures on ice for 30 min. Anti-human CD3, CD4, CD8, CD14 and CD19 antibodies were all from BD Biosciences (San Diego, CA, USA). Anti-mouse CD3 (clone 145-2C11), CD4 (clone H129.19), CD8 (clone 53-6.7), CD41 (clone MW Reg30), CD44 (clone 1M7), CD45R (clone RA3-6B2), CD61 (clone 2C9.G2), CD62L (clone MEL-14), CD95 (clone Jo2), Gr1 (clone RB6-8C5) and Sca-1 (clone D7) antibodies were also from BD Biosciences. Anti-mouse CD11b (clone M1/70), CD34 (cloneMEC14.7) and CD117 (clone 2B8) antibodies were from Biolegend (San Diego, CA, USA). Anti-mouse CD25 (clone PC61.5) and CD150 (clone 9D1) antibodies were from eBiosciences (San Diego, CA, USA). Each antibody was conjugated to either fluorescein isothiocyanate, phycoerythrin (PE), PE-cyanin 5 (PE-Cy5), PE-cyanin 7 (PE-Cy7), allophycocyanin (APC) or allophycocyanin 7 (APC-Cy7). For the detection of cellular ROS, blood and BM cells were first stained with specific antibodies on ice for 30 min and then incubated with 20 μm fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCF-DA; Invitrogen Corporation, Camarillo, CA, USA) at 37 °C for 30 min.
Stained cells were acquired and analysed on a LSR II or Canto II flow cytometer using the FACSDiva software (Becton Dickson, San Diego, CA, USA). We gated cells to exclude residual RBCs and debris based on FSC-A/SSC-A display.
Cytology and histology
Blood smears were prepared from venous blood and were stained with Wright staining solution (Electron Microscopy Sciences, Hatfield, PA, USA) according to the manufacturer’s instructions. Spleen and sternums were preserved in 10% formalin, sectioned and Hematoxylin & eosin stained (Vitrovivo Biotechnology, Rockville, MD, USA). Slides from blood smears and spleen/sternum sections were examined under a Zeiss Axioskop2 plus microscope with images captured at 20 × by a Zeiss AxioCam HRC camera using the AxioVision 4.5 software (Carl Zeiss MicroImaging GmbH, Jena, Germany). For sternum samples, we counted the number of megakaryocytes–megakaryoblasts per field of view under 20× microscopic magnification based on their ‘mega cell’ characteristic. Average megakaryocytes–megakaryoblasts was calculated for each sample based on examination of three separate view fields.
N-acetylcysteine treatment
Chemical grade NAC (Sigma-Aldrich, St. Louis, MO, USA) of 16.3 g was dissolved in 2.5 l of de-ionized water to obtain 40 mm NAC water solution, adjusted to pH 3.0–4.0, filtered through 0.22 μm and then given to PLL mice as drinking water for 3 months. Control PLL mice were provided with normal water without NAC. Animals were bled at 2 and 3 months for CBC and flow cytometry analyses. BM cells were extracted after animal euthanasia at the third month and were analysed by flow cytometry to measure cellular ROS contents as described above.
Data analysis
Data were analysed by JMP statistical discovery software (SAS Institute Inc. 1998) through variance analysis and were shown as means with standard errors. Respective p values were given to indicate statistical significance (P < 0.05 being regarded as statistically significant).
Results
We first measured blood counts of two to eight month-old MMB6 and PLL mice. Haematologic parameters were relatively stable within the age range of mature young adult mice, and the majority of measurements were similar between the two rodent species, with modest levels of individual variation (Table 1). The most notable difference was a significantly lower (P < 0.0001) platelet count in PLL mice, <50% of that in MMB6 mice (Figure 1a). Platelets were also significantly smaller in PLL mice, with a 19% reduction (P < 0.0001) in mean platelet volume (MPV) relative to the size of MMB6 platelets (Figure 1a). Another notable difference was a 15% higher concentration (P < 0.0001) of circulating RBCs in PLL mice (Figure 1b). This RBC increase, however, was associated with a 13% reduction (P < 0.0001) in mean corpuscular volume (MCV) and a 16% reduction (P < 0.0001) in mean corpuscular haemoglobin (MCH) concentration (Table 1). A third difference was a significantly higher concentration of circulating monocytes (P < 0.0001) in PLL mice, a 79% increase compared to monocyte concentration in MMB6 animals (Figure 1b).
Table 1.
CBC in MMB6 and PLL mice
| Measurements | M. musculus B6 | P. leucopus linville | ||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| n | 32 | 27 | 45 | 29 |
| WBCs (×109/l) | 7.6 ± 0.5 | 9.9 ± 0.5 | 8.8 ± 0.4 | 8.2 ± 0.5 |
| Neutrophils (×109/l) | 1.4 ± 0.1 | 1.8 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.1 |
| Lymphocytes (×109/l) | 5.9 ± 0.4 | 7.9 ± 0.4 | 7.0 ± 0.3 | 6.5 ± 0.4 |
| Monocytes (×109/l) | 0.24 ± 0.03 | 0.19 ± 0.03 | 0.38 ± 0.02** | 0.38 ± 0.03** |
| RBCs (×1012/l) | 9.9 ± 0.3 | 9.7 ± 0.3 | 11.2 ± 0.3** | 11.4 ± 0.3** |
| Haemoglobin (g/dl) | 13.8 ± 0.4 | 13.1 ± 0.4 | 13.0 ± 0.3 | 13.2 ± 0.4 |
| Haematocrit (%) | 43.4 ± 1.3 | 42.3 ± 1.4 | 41.1 ± 1.1 | 43.0 ± 1.4 |
| MCV (fL) | 44.0 ± 0.5 | 43.4 ± 0.6 | 36.7 ± 0.4** | 37.8 ± 0.5** |
| MCH (pg) | 14.0 ± 0.1 | 13.5 ± 0.2 | 11.6 ± 0.1** | 11.6 ± 0.2** |
| RDW (%) | 18.4 ± 0.2 | 18.4 ± 0.2 | 18.4 ± 0.2 | 17.6 ± 0.2 |
| Platelets (×109/l) | 905 ± 40 | 827 ± 43 | 400 ± 33** | 421 ± 42** |
| MPV (fl) | 4.44 ± 0.04 | 4.36 ± 0.05 | 3.56 ± 0.04** | 3.54 ± 0.05** |
CBC, Complete blood count; MCH, Mean corpuscular haemoglobin; MPV, mean platelet volume; PLL, Peromyscus leucopus linville; RDW, RBC distribution width.
Strain difference P < 0.01.
Figure 1.
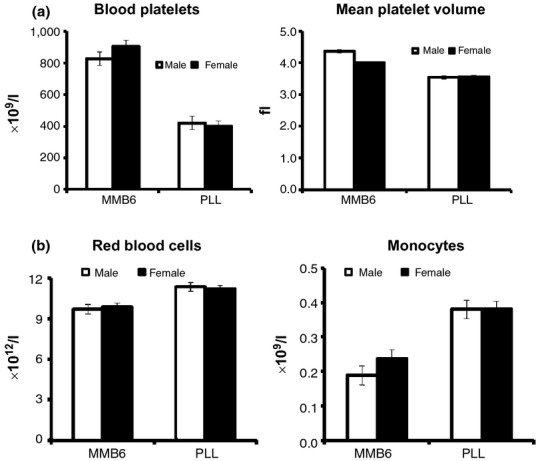
Cytological measurements in MMB6 and PLL mice. Orbital sinus blood from MMB6 female (n = 32), MMB6 male (n = 27), PLL female (n = 42) and PLL male (n = 29) mice was analysed at 2–8 months of age. Most blood measurements were relatively similar between MMB6 and PLL mice, as listed in Table 1. A major difference was a significantly lower (P < 0.0001) platelet concentration in PLL mice which is less than half of that in MMB6 mice (a). PLL mean platelet volume was also 19% smaller (P < 0.0001) than that of MMB6 platelets (a). There were significantly higher concentrations of red blood cells (P < 0.0001) and monocytes (P < 0.0007) in PLL mice relative to MMB6 animals (b). PLL, Peromyscus leucopus linville.
Microscopic examination of blood smears from two to eight month-old MMB6 and PLL mice revealed smaller RBCs in PLL than in MMB6 mice (Figure 2a), consistent with a 13% decline in MCV in PLL animals (Table 1). Spleens were significantly smaller in PLL mice, with an average 94 ± 13 × 106 cells/spleen, less than half (P < 0.0001) of the 195 ± 12 × 106 cells/spleen in MMB6 mice. Histologically, spleen sections from PLL mice had reduced red pulp areas in comparison with those of MMB6 mice (Figure 2b). Overall BM cellularity was similar between MMB6 and PLL mice; however, more megakaryocytes–megakaryoblasts were found in sections from MMB6 than from PLL mice (Figure 2c). The number of megakaryocytes–megakaryoblasts per view field was significantly higher (P < 0.0027) in MMB6 (16 ± 1) than in PLL (11 ± 1) BM (Figure 2d).
Figure 2.
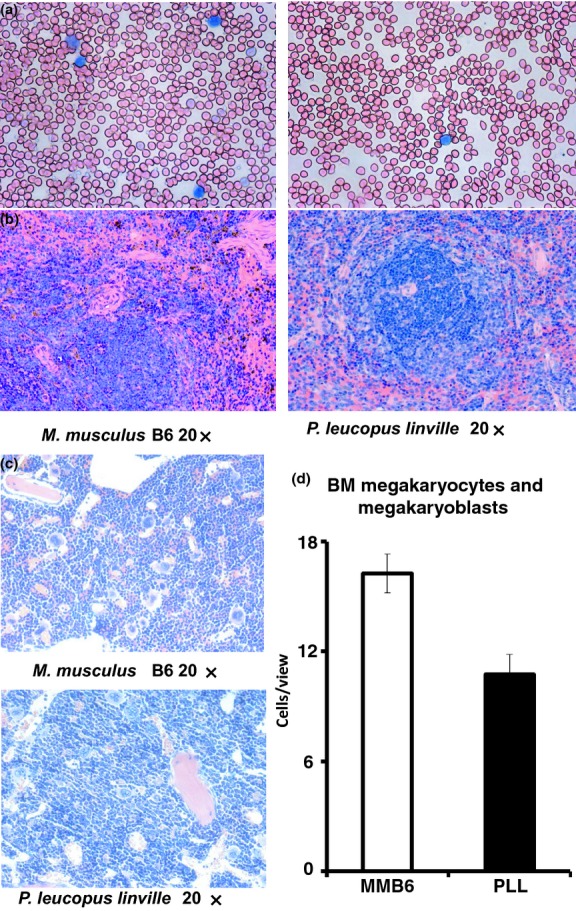
Histological comparisons between MMB6 and PLL mice. Blood smears prepared from young MMB6 (n = 8) and PLL (n = 8) mice were stained with Wright solution and examined under a Zeiss Axioskop2 plus microscope. Red blood cells from PLL mice were smaller than RBCs from MMB6 mice (a). Sections of spleens from PLL mice had much smaller red pulp areas of active erythropoiesis than those from MMB6 mice (b). sternum sections from PLL mice contained fewer megakaryocytes–megakaryoblasts relative to those from MMB6 mice (c). The number of megakaryocytes–megakaryoblasts per view field under 20× magnification was significantly lower in PLL (P < 0.0027) than in MMB6 mice when eight animals from each strain were examined (d). PLL, Peromyscus leucopus linville.
We attempted to define cellular differences between PLL and MMB6 mice in blood, BM and spleen by flow cytometry. No antibody specific for a Peromyscus antigen is commercially available, and we therefore tested an array of antibodies specific for human and MM antigens, with largely negative results. All five antibodies specific for human antigens we have tested showed no staining when incubated with PLL blood cells (Figure S1A). Ten antibodies specific for MM antigens showed very inconsistent staining patterns when incubated with peripheral blood leucocytes (Figure S1B, 1C). Thus, utilization of commercially available anti-mouse antibodies for the detection of specific cell populations in P. leucopus animals was not feasible.
Of the many anti-mouse antibodies we tested, we found that anti-mouse CD44 and anti-mouse CD45R antibodies produced consistent staining of distinctive cell populations in PLL mice. We used both antibodies to stain blood cells from two to eight month-old MMB6 and PLL mice in conjunction with the staining of DCF-DA to detect cellular ROS contents. Proportions of CD44 and CD45R cells were very similar between MMB6 and PLL mice, showing no significant difference (Figure 3a, Table 2). There were two populations of CD44-expressing cells in MMB6 mice: CD44high population comprising CD11b+ cells, and CD44 median population made of CD4 and CD8 T cells and some CD11b+ cells. The proportion of ROS− cells in blood leucocytes was 85 ± 2% for PLL mice, significantly lower (P < 0.0044) than that for MMB6 mice at 92 ± 1% (Figure 3b, Table 2). This strain difference was most obvious in CD44 cells: only 34 ± 3% CD44 cells from PLL mice were ROS−, while 66 ± 2% CD44 cells from MMB6 mice were ROS− (Figure 3b, Table 2). The ROS− cell proportion was extremely high, at 97–99%, in blood CD45R+ cells from both MMB6 and PLL mice (Figure 3b, Table 2).
Figure 3.
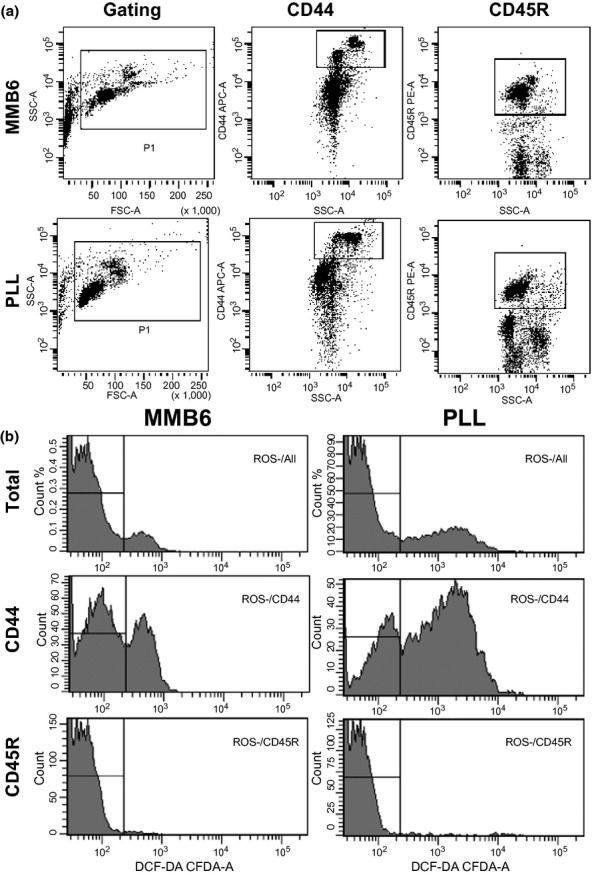
Cellular ROS in MMB6 and PLL mice. Blood cells from 2- to 8-month-old MMB6 (n = 12) and PLL (n = 8) mice were stained with anti-mouse CD44 and anti-mouse CD45R antibodies followed by incubation of samples with 20 μm DCF-DA at 37 °C for 30 min. PLL samples showed consistent staining of both antibodies, and no significant difference was detected between MMB6 and PLL mice in their blood CD44 and CD45R cell percentages (a). The proportion of DCF-DA− cells, reflecting the level of ROS− cells, was significantly lower (P < 0.0044) in PLL than in MMB6 mice, with the difference being greatest for CD44+ (P < 0.0001) cells and less obvious for CD45R+ (P < 0.0327) cells (b). In both MMB6 and PLL mice, the overall ROS− cell proportion was much higher in CD45R+ than in CD44+ cells (b). PLL, Peromyscus leucopus linville; ROS, reactive oxygen species.
Table 2.
Cellular ROS in blood leucocytes of MMB6 and PLL mice
| Animal type | MMB6 | PLL | Statistics |
|---|---|---|---|
| n | 12 | 8 | |
| CD44 (%) | 22.8 ± 2.1 | 21.9 ± 2.5 | P < 0.8025 |
| ROS−%CD44 | 66.3 ± 2.4 | 33.9 ± 2.9 | P < 0.0001 |
| CD45R (%) | 52.2 ± 2.2 | 48.3 ± 2.7 | P < 0.2816 |
| ROS−%CD45R | 98.9 ± 0.3 | 98.0 ± 0.3 | P < 0.0327 |
| ROS−% | 91.5 ± 1.3 | 84.6 ± 1.6 | P < 0.0044 |
ROS, Reactive oxygen species; PLL, Peromyscus leucopus linville.
Next, we stained peripheral blood leucocytes from two to eight and 16- to 26-month-old PLL mice using anti-mouse CD44, anti-mouse CD45R antibodies and DCF-DA dye. The proportions of CD44+ (48%±3.6% and 47%±3.6%) and CD45R+ (47%±3.2% and 44%±3.2%) cells did not differ between the two age groups. No age difference was detected on ROS− proportion, but the proportion of ROS− cells differed significantly between CD44+ and CD45R+ cells: 98–99% of CD45R+ cells were ROS−, and only 53–58% of CD44+ cells were ROS− (Figure 4a). There was a 5% decline in MCV (P < 0.0446), a 28% increase in platelets (P < 0.0246) and a 45% increase in monocytes in 16- to 26-month-old PLL animals relative to the younger two to eight month-old counterparts (Table 3).
Figure 4.
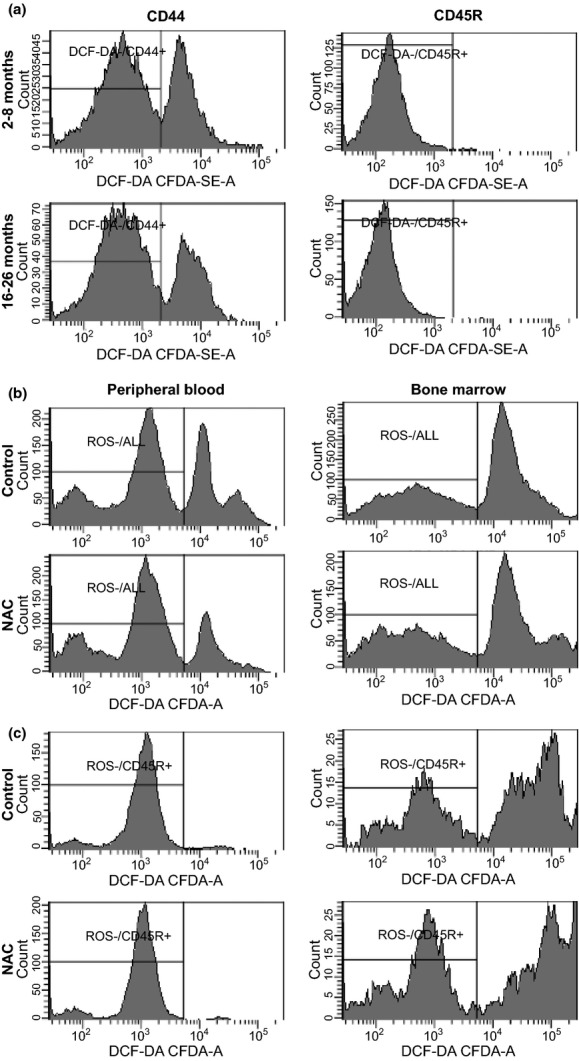
PLL cellular ROS unaffected by age or NAC treatment. We analysed the ROS− cell proportion in blood CD44+ and CD45R+ cells in PLL mice at 2–8 (n = 6) and 16–26 (n = 6) months respectively. There was no age difference in the proportion of ROS− cells (a). We treated PLL mice with 40 mm NAC water for 3 months and measured cellular ROS in blood and BM cells. ROS− cell proportions in blood and BM cells showed no change in response to NAC treatment (b). The proportion of ROS− cells was notably higher in blood CD45R+ cells than in BM CD45R+ cells without a significant effect of NAC treatment (c). PLL, Peromyscus leucopus linville; ROS, reactive oxygen species; NAC, n-acetylcysteine.
Table 3.
Age effects on CBC measurements in PLL mice
| Age (months) | 2–8 | 16–26 | Statistics |
|---|---|---|---|
| n | 74 | 12 | |
| WBCs (×109/l) | 8.6 ± 0.4 | 7.9 ± 0.9 | P < 0.4901 |
| Neutrophils (×109/l) | 1.3 ± 0.1 | 1.5 ± 0.2 | P < 0.3273 |
| Lymphocytes (×109/l) | 6.8 ± 0.3 | 5.8 ± 0.7 | P < 0.1973 |
| Monocytes (×109/l) | 0.38 ± 0.02 | 0.55 ± 0.04 | P < 0.0006 |
| RBCs (×1012/l) | 11.3 ± 0.2 | 10.9 ± 0.6 | P < 0.5587 |
| Haemoglobin (g/dl) | 13.1 ± 0.3 | 12.0 ± 0.7 | P < 0.1686 |
| Haematocrit (%) | 41.8 ± 1.0 | 38.6 ± 2.4 | P < 0.2119 |
| MCV (fL) | 37.1 ± 0.3 | 35.2 ± 0.9 | P < 0.0446 |
| RDW (%) | 18.1 ± 0.1 | 17.7 ± 0.4 | P < 0.3559 |
| Platelets (×109/l) | 409 ± 19 | 524 ± 47 | P < 0.0246 |
| MPV (fl) | 3.56 ± 0.03 | 3.60 ± 0.07 | P < 0.0122 |
CBC, Complete blood count; PLL, Peromyscus leucopus linville; MCV, mean corpuscular volume; MPV, mean platelet volume.
As the ROS− varied in different cell types but showed no change with age in PLL mice, we wondered whether antioxidant treatment would atter cellular ROS contents. We thus provided two to eight month-old PLL mice with 40 mm NAC in drinking water for three months. NAC treatment did not alter ROS− cell proportion in either peripheral blood (79 ± 1.6% vs. 78 ± 1.6%) or BM (52 ± 3.5% vs. 55 ± 3.5%) cells (Figure 4b). In CD45R+ cells, ROS− proportion differed significantly between blood (98 ± 0.3% and 98 ± 0.3%) and BM (48 ± 3.0% and 40 ± 3.0%) without significant NAC effect (Figure 4c). Analyses of CBC also found no NAC effect except that NAC-treated mice had a 71% increase (P < 0.0007) in blood monocytes (Table 4).
Table 4.
NAC treatment effect on CBC measurements in PLL mice
| Treatments | NAC | Control | Statistics |
|---|---|---|---|
| n | 17 | 17 | |
| WBCs (×109/l) | 6.7 ± 0.6 | 5.6 ± 0.6 | P < 0.1645 |
| Neutrophils (×109/l) | 0.8 ± 0.1 | 0.9 ± 0.1 | P < 0.5456 |
| Lymphocytes (×109/l) | 5.4 ± 0.5 | 4.3 ± 0.5 | P < 0.1350 |
| Monocytes (×109/l) | 0.53 ± 0.04 | 0.31 ± 0.04 | P < 0.0007 |
| RBCs (×1012/l) | 11.3 ± 0.3 | 11.1 ± 0.3 | P < 0.6701 |
| Haemoglobin (g/dl) | 13.2 ± 0.3 | 13.0 ± 0.3 | P < 0.7358 |
| Haematocrit (%) | 43.2 ± 1.1 | 42.5 ± 1.1 | P < 0.6125 |
| MCV (fL) | 38.4 ± 0.6 | 38.2 ± 0.6 | P < 0.8574 |
| RDW (%) | 19.4 ± 0.3 | 18.7 ± 0.3 | P < 0.1236 |
| Platelets (×109/l) | 441 ± 35 | 381 ± 35 | P < 0.2468 |
| MPV (fL) | 3.49 ± 0.04 | 3.49 ± 0.04 | P < 0.9264 |
NAC, N-acetylcysteine; CBC, complete blood count; PLL, Peromyscus leucopus linville; MCV, Mean corpuscular volume; MPV, mean platelet volume; RDW, RBC distribution width.
Discussion
The main finding from this study is a consistently lower concentration of circulating platelets in PLL mice in comparison with the more commonly used MMB6 mice. At 373–470 × 109/l, platelet counts for PLL mice are closer to those of humans at 161–385 × 109/l (Litchtman et al. 2010), much lower than the 780– 901 × 109/l platelets for MMB6 mice based on our current study, or the 1006–1050 × 109/l average platelet counts for 72 strains of MM animals reported (The Jackson Laboratory 2013). That average platelet size for PLL is significantly smaller than platelet size for MMB6 mice suggests that the lower platelet count in PLL mice is not compensated by larger platelets. Together with fewer megakaryocytes–megakaryoblasts in PLL BM, our observation suggests that PLL mice have a much more sensitive system than MMB6 mice in the regulation of platelet production as well as in the maintenance of haemostasis. Likely, genetic elements regulate the normal platelet range, as they do to CD8 and CD4 T-cell percentages (Chen & Harrison 2002) and the BM hematopoietic stem cell pool size (de Haan et al. 1997).
The much smaller spleens with significantly reduced erythropoietic centres are another feature of PLL mice indicating that the spleen may play less of a role in erythropoiesis and hematopoiesis in PLL than in MMB6 mice. On the other hand, the 15% increase in RBC counts in PLL animals may be regarded as a compensatory response to the smaller RBCs with reduced haemoglobin content per cell.
The long lifespan of P. leucopus mice offers an opportunity to examine ageing over an extended time period (Sacher & Duffy 1978; Sacher & Hart 1978; Peluso et al. 1980; Cohen et al. 1987; Burger & Gochfeld 1992; Labinskyy et al. 2009). In our study of PLL mice at two to eight months and 16–26 months of age, no significant difference was found in blood cell counts, different from observations in MMB6 mice, in which increasing age was associated with declines in RBCs, haemoglobin and the relative proportion of lymphocytes (Leuenberger & Kunstyr 1976). Notably, the age group 16–26 months, which can be regarded as old for MMB6 mice, may not represent old age in PLL mice because of their long lifespan. Even at very old ages, P. leucopus mice had the ability to maintain normal function of their hypothalamic–pituitary–ovarian axis to maintain fertility (Steger et al. 1980). Old P. leucopus mice also had rapid wound healing ability, perhaps related to their stress-related hormone levels (Cohen et al. 1987). The increased lifespan in P. leucopus mice could also be associated with decreased cellular ROS generation and increased resistance to pro-oxidant and pro-inflammatory effects of metabolic stress (Labinskyy et al. 2009). We found lower proportion of ROS− blood leucocytes in young PLL than in young MMB6 mice. Our observation of no age effect on cellular ROS in blood and BM cells is consistent with early reports suggesting that P. leucopus mice are apparently resistant to oxidative damage. Differences in ROS levels obtained from different cell types within the same PLL animals in the current study may represent variations in metabolic activities of each cell type. For example, BM cells are metabolically more active with lower proportions of ROS− cells than are peripheral blood leucocytes, as was the case for both MMB6 and PLL mice.
The highest blood monocyte count in PLL mice resembles the monocyte concentration in humans (Litchtman et al. 2010). Also, monocytes further increased in 16- to 26-month-old PLL mice, as in humans (Seidler et al. 2010).
In conclusion, P. leucopus mice have circulating platelets, BM megakaryocytes and circulating monocytes that resemble measurements in humans. The reduced splenic hematopoiesis and the extended lifespan add to these characteristic, making P. leucopus mice a possible model for the study of human haematology.
Funding source
This work was supported by National Heart, Lung and Blood Institute Intramural Research Program and by a scholarship from Soochow University to Dr. Yu Sun.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Ineffective staining of PLL leucocytes with antibodies specific for human and M. musculus antigens.
References
- Burger J. Gochfeld M. Survival and reproduction in Peromyscus leucopus in the laboratory: viable model for aging studies. Growth Dev. Aging. 1992;56:17–22. [PubMed] [Google Scholar]
- Chen J. Harrison DE. Quantitative trait loci regulating relative lymphocyte proportions in mouse peripheral blood. Blood. 2002;99:561–566. doi: 10.1182/blood.v99.2.561. [DOI] [PubMed] [Google Scholar]
- Cohen BJ, Cutler RG. Roth GS. Accelerated wound repair in old deer mice (Peromyscus maniculatus) and white-footed mice (Peromyscus leucopus. J. Gerontol. 1987;42:302–307. doi: 10.1093/geronj/42.3.302. [DOI] [PubMed] [Google Scholar]
- Glass JD. Dolan PL. Melatonin acts in the brain to mediate seasonal steroid inhibition of luteinizing hormone secretion in the white-footed mouse (Peromyscus leucopus. Proc. Soc. Exp. Biol. Med. 1988;188:375–380. doi: 10.3181/00379727-188-42750. [DOI] [PubMed] [Google Scholar]
- Glass JD. Knotts LK. A brain site for the antigonadal action of melatonin in the white-footed mouse (Peromyscus leucopus): involvement of the immunoreactive GnRH neuronal system. Neuroendocrinology. 1987;46:48–55. doi: 10.1159/000124795. [DOI] [PubMed] [Google Scholar]
- Glass JD. Lynch GR. Evidence for a brain site of melatonin action in the white-footed mouse, Peromyscus leucopus. Neuroendocrinology. 1982;34:1–6. doi: 10.1159/000123269. [DOI] [PubMed] [Google Scholar]
- de Haan G, Nijhof W. Van ZG. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89:1543–1550. [PubMed] [Google Scholar]
- Hjelle B, Lee SW, Song W, et al. Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: genetic characterization of the M genome of New York virus. J. Virol. 1995;69:8137–8141. doi: 10.1128/jvi.69.12.8137-8141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister EK. Childs JE. Analysis of Borrelia burgdorferi sequentially isolated from Peromyscus leucopus captured at a Lyme disease enzootic site. J. Infect. Dis. 1995;172:462–469. doi: 10.1093/infdis/172.2.462. [DOI] [PubMed] [Google Scholar]
- Hofmeister EK, Ellis BA, Glass GE. Childs JE. Longitudinal study of infection with Borrelia burgdorferi in a population of Peromyscus leucopus at a Lyme disease-enzootic site in Maryland. Am. J. Trop. Med. Hyg. 1999;60:598–609. doi: 10.4269/ajtmh.1999.60.598. [DOI] [PubMed] [Google Scholar]
- Joyner CP, Myrick LC, Crossland JP, Dawson WD. Deer mice as laboratory animals. ILAR J. 1998;39:322–330. doi: 10.1093/ilar.39.4.322. [DOI] [PubMed] [Google Scholar]
- Labinskyy N, Mukhopadhyay P, Toth J, et al. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am. J. Physiol Heart Circ. Physiol. 2009;296:H946–H956. doi: 10.1152/ajpheart.00693.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RC, Alaks G, Walsh A. Evolution of Peromyscus leucopus mice in response to a captive environment. PLoS One. 2013;8:e72452. doi: 10.1371/journal.pone.0072452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger HG. Kunstyr I. Gerontological data of C57BL/6J mice. II. Changes in blood cell counts in the course of natural aging. J. Gerontol. 1976;31:648–653. doi: 10.1093/geronj/31.6.648. [DOI] [PubMed] [Google Scholar]
- Litchtman MA, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT. Williams Hematology. 8th edn. New York: The McGraw-Hill Company Inc; 2010. [Google Scholar]
- Lyubsky S, Gavrilovskaya I, Luft B. Mackow E. Histopathology of Peromyscus leucopus naturally infected with pathogenic NY-1 hantaviruses: pathologic markers of HPS viral infection in mice. Lab. Invest. 1996;74:627–633. [PubMed] [Google Scholar]
- Magnarelli LA, Anderson JF, Hyland KE, Fish D. Mcaninch JB. Serologic analyses of Peromyscus leucopus, a rodent reservoir for Borrelia burgdorferi, in northeastern United States. J. Clin. Microbiol. 1988;26:1138–1141. doi: 10.1128/jcm.26.6.1138-1141.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli LA, Anderson JF. Stafford KC., III Detection of Borrelia burgdorferi in urine of Peromyscus leucopus by inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1994;32:777–782. doi: 10.1128/jcm.32.3.777-782.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo AF, Martinez-Pastor F, Alaks G, Dubach J. Lacy RC. Effects of genetic captive-breeding protocols on sperm quality and fertility in the white-footed mouse. Biol. Reprod. 2010;83:540–548. doi: 10.1095/biolreprod.110.085316. [DOI] [PubMed] [Google Scholar]
- Morzunov SP, Rowe JE, Ksiazek TG, Peters CJ, St Jeor SC. Nichol ST. Genetic analysis of the diversity and origin of hantaviruses in Peromyscus leucopus mice in North America. J. Virol. 1998;72:57–64. doi: 10.1128/jvi.72.1.57-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Montgomery MK, Steger RW, Meites J. Sacher G. Aging and ovarian function in the white-footed mouse (Peromyscus leucopus) with specific reference to the development of preovulatory follicles. Exp. Aging Res. 1980;6:317–328. doi: 10.1080/03610738008258367. [DOI] [PubMed] [Google Scholar]
- Sacher GA. Duffy PH. Age changes in rhythms of energy metabolism, activity, and body temperature in Mus and Peromyscus. Adv. Exp. Med. Biol. 1978;108:105–124. doi: 10.1007/978-1-4757-4460-6_6. [DOI] [PubMed] [Google Scholar]
- Sacher GA. Hart RW. Longevity, aging and comparative cellular and molecular biology of the house mouse, Mus musculus, and the white-footed mouse, Peromyscus leucopus. Birth Defects Orig. Artic. Ser. 1978;14:71–96. [PubMed] [Google Scholar]
- SAS Institute Inc. JMP Statistics and Graphics Guide. Cary, NC: USA; 1998. Version 3. [Google Scholar]
- Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Pulliam DA, Liu Y, et al. Reduced mitochondrial ROS, enhanced antioxidant defense, and distinct age-related changes in oxidative damage in muscles of long-lived Peromyscus leucopus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R343–R355. doi: 10.1152/ajpregu.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick CH. Peromyscus leucopus: an interesting subject for studies of socially induced stress responses. Science. 1964;143:55–56. doi: 10.1126/science.143.3601.55. [DOI] [PubMed] [Google Scholar]
- Steger RW, Peluso JJ, Huang HH, et al. Effects of advancing age on the hypothalamic-pituitary-ovarian axis of the female white-footed mouse (Peromyscus leucopus. Exp. Aging Res. 1980;6:329–339. doi: 10.1080/03610738008258368. [DOI] [PubMed] [Google Scholar]
- The Jackson Laboratory. Mouse Phenome Database. 2013. http://phenome.jax.org/ [Google Scholar]
- Weaver DR, Carlson LL. Reppert SM. Melatonin receptors and signal transduction in melatonin-sensitive and melatonin-insensitive populations of white-footed mice (Peromyscus leucopus. Brain Res. 1990;506:353–357. doi: 10.1016/0006-8993(90)91280-t. [DOI] [PubMed] [Google Scholar]
- Wu PJ, Greeley EH, Hansen LG. Segre M. Hematology values from clinically healthy Peromyscus leucopus. J. Zoo. Wildl. Med. 1999a;30:589–590. [PubMed] [Google Scholar]
- Wu PJ, Greeley EH, Hansen LG. Segre M. Immunological, hematological, and biochemical responses in immature white-footed mice following maternal Aroclor 1254 exposure: a possible bioindicator. Arch. Environ. Contam. Toxicol. 1999b;36:469–476. doi: 10.1007/pl00006620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Ineffective staining of PLL leucocytes with antibodies specific for human and M. musculus antigens.


