Abstract
Anxiety disorders pose one of the biggest threats to mental health in the world, and they predominantly emerge early in life. However, research of anxiety disorders and fear-related memories during development has been largely neglected, and existing treatments have been developed based on adult models of anxiety. The present review describes animal models of anxiety disorders across development and what is currently known of their pharmacology. To summarize, the underlying mechanisms of intrinsic ‘unlearned’ fear are poorly understood, especially beyond the period of infancy. Models using ‘learned’ fear reveal that through development, rats exhibit a stress hyporesponsive period before postnatal day 10, where they paradoxically form odour-shock preferences, and then switch to more adult-like conditioned fear responses. Juvenile rats appear to forget these aversive associations more easily, as is observed with the phenomenon of infantile amnesia. Juvenile rats also undergo more robust extinction, until adolescence where they display increased resistance to extinction. Maturation of brain structures, such as the amygdala, prefrontal cortex and hippocampus, along with the different temporal recruitment and involvement of various neurotransmitter systems (including NMDA, GABA, corticosterone and opioids) are responsible for these developmental changes. Taken together, the studies described in this review highlight that there is a period early in development where rats appear to be more robust in overcoming adverse early life experience. We need to understand the fundamental pharmacological processes underlying anxiety early in life in order to take advantage of this period for the treatment of anxiety disorders.
Linked Articles
This article is part of a themed section on Animal Models in Psychiatry Research. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-20
Introduction
Fear and anxiety have a primal role in ensuring survival; however, persistent fear can interfere with daily functioning and manifest as anxiety disorders. Anxiety disorders pose one of the biggest threats to mental health in the world (WHO, 2001). Although the causes of most anxiety disorders are unknown, data show that most adults with anxiety disorders manifest anxiety early in life (Pine, 2007). In fact, the median age of onset of anxiety disorders as revealed by the World Health Organization’s World Mental Health Survey is 7–14 years of age (Kessler et al., 2007). Furthermore, an epidemiological study showed that among adolescents with an anxiety disorder, ∼ 50% experienced onset by age 6 (Merikangas et al., 2010).
It is widely believed that childhood/adolescence onset of anxiety leads to an increased likelihood of anxiety disorders with more severe symptoms later in life, compared with if onset occurs during adulthood (Newman et al., 1996), thus posing major issues associated with lifetime prevalence and anxiety as an ongoing societal issue. Hence, early diagnosis, intervention and treatment of anxiety disorders may help to reduce the severity and persistence later in life and potentially prevent subsequent onset of secondary disorders associated with anxiety. Despite this body of evidence, existing behavioural and pharmacological therapies are largely developed to offer benefits to adult patients, and the study of anxiety disorders and fear-related memories during development has been largely neglected and is proposed as one of the important reasons for the lack of progress in identifying effective treatments (Insel, 2009).
The safety and ethical concerns make developmental studies of novel therapeutic agents difficult in humans (Fost, 2001), whereas preclinical animal studies provide great opportunities to develop potential treatments for paediatric anxiety (Gross and Hen, 2008; Pine, 2007; Pine et al., 2009; Pattwell et al., 2013). A large body of work has investigated anxiety and potential therapeutic treatments in adult rodent models, which have provided important preclinical information to guide treatment in adults (e.g. Rodgers, 2010; Cryan and Sweeney, 2011). Basic neuroscience research using animal models is especially informative because of the cross-species parallels in brain circuitry underlying anxiety and fear (Davis, 2000; LeDoux, 2000). Indeed, it has been noted that ‘cross-species preservation of neural circuitry in fear provides translational credibility for studying rodent models’ (Gottfried and Dolan, 2004). Also, many of the developmental milestones in human and rodent brain development have been conserved, so while the complexity of the developing human brain cannot be mimicked, rodents of different age groups can be used to represent these developmental periods in humans (see Semple et al., 2013 for review). Therefore, the purpose of the present article is to review and discuss animal models of anxiety disorders across development and what we currently understand of their underlying pharmacological mechanisms. The animal models generally belong to one of two categories: one category examining intrinsic, or ‘unlearned’ fear, and the second category studying acquired, or ‘learned’ fear. Both types of animal models have been instrumental in unravelling the mechanisms that underpin this primal behaviour, therefore, both will be discussed in detail in the present review.
Unlearned fear
The most influential theory behind the development of paediatric anxiety states that chronic anxiety critically depends on the intrinsic individual differences in threat perception (Pine et al., 2009). These intrinsic fear responses can be modelled in the laboratory using ‘unlearned’ fear, which refers to the innate fear that animals have to various stimuli without any previous learning experiences with these stimuli (see. Boulis and Davis, 1989; Walker and Davis, 1997a). Unlearned fear can be used to model some human psychiatric disorders including generalized anxiety disorder (GAD), separation anxiety disorder and many phobias, where there is no known ‘learned’ fear association precipitating the condition. These fear behaviours can become pathological when response to a potential threat is exaggerated and anxiety becomes abnormal (Lima, 1998; Nesse, 1999; Ohman and Mineka, 2001).
Ultrasonic vocalizations (USVs) induced by maternal separation
One of the major models of testing unlearned fear in developing rodents utilize USVs induced by maternal separation, this can be likened to a form of separation anxiety observed in children. Rodent pups produce vocalizations in the ultrasonic range when separated from their mother and littermates or when they are deprived of food or heat. The cries call the attention of the mother and have a characteristic developmental profile whereby, USV production in rat pups is low at birth, reaches a maximum at postnatal days (P) 6–8 and then progressively declines to low levels by P18 (Allin and Banks, 1971). Allin et al. assessed the functional importance of USVs and found that while adult male and virgin female rats oriented towards the general directions of the USVs, ∼ 50% of lactating female rats engaged in searching behaviour and left their nests at least once in response to these calls (Allin and Banks, 1972). Lactating female rats were also more accurate in localizing the sound source of the USVs, confirming that they are a unique anxiety response designed specifically to elicit the engagement of a lactating female rat which can provide what is necessary for the survival of the animal early in life (Allin and Banks, 1972).
These USVs, or ‘distress calls’, can be recorded and analysed both quantitatively and qualitatively in order to measure levels of anxiety-like behaviour in infant rodents (Groenink et al., 2008; Scattoni et al., 2009). Suppression of USV emission is believed to be an ethologically valid marker of anxiolytic drug efficacy (Gardner, 1985; Winslow and Insel, 1990; Kehne et al., 1991; Olivier et al., 1998; Iijima and Chaki, 2005). For example, the corticotropin-releasing factor receptor 1 antagonist CP 154 526 dose-dependently reduced separation-induced USVs in P9–11 pups (Kehne et al., 2000). The benzodiazepine diazepam, which increases GABA receptor signalling had a similar profile (Kehne et al., 2000). Antidepressant and antipsychotic therapeutics were also tested. Haloperidol had no effect on USV, which supports the conclusion that dopamine receptor antagonism does not affect this anxiolytic measure (Kehne et al., 2000). Clozapine (which binds to a number of receptors, including dopamine), decreased USVs, but impaired geotaxis which may be reflective of its sedative effects (Kehne et al., 2000). Separation-induced USVs were suppressed in rat pups by NMDA receptor antagonists (receptor nomenclature follows Alexander et al., 2013a) including those acting at the glutamate recognition site (D, L-amino-phosphonovaleric acid and MDL 100 453) or at the ion channel, MK-801 (Kehne et al., 1991). The tricyclic antidepressant, desipramine, which is a noradrenaline reuptake inhibitor, actually appeared anxiogenic in that it increased USVs, which is consistent with previous reports using catecholamine reuptake inhibitors (Winslow and Insel, 1990).
Additionally, there is strong evidence for an involvement of the serotonergic system in USV in rats (Winslow and Insel, 1991). For example, the 5-HT1A receptor agonist 8-OH-DPAT dose-dependently reduced the amount of USVs in maternally separated P10 rats (Hard and Engel, 1988; receptor nomenclature follows Alexander et al., 2013b). The selective serotonin reuptake inhibitor (SSRI; nomenclature follows Alexander et al., 2013c) zimelidine reduced USVs which is consistent with previous observations that other SSRIs (fluoxetine, citalopram and sertraline) also reduce USVs (Winslow and Insel, 1990). Further, buspirone (5-HT1A receptor partial agonist) also dose-dependently reduces USV in P9–11 rats (Kehne et al., 2000). Taken together, innate anxiety prompted by acute maternal separation appears to involve a variety of neurotransmitters such as GABA, glutamate, corticotropin and 5-HT. However, using USV as a model of unlearned fear across development is somewhat limited because the frequency of emitted USV is drastically reduced by P18 when rodents reach the late juvenile stage.
Predator/prey interactions
Predator/prey interactions model fear and anxiety depending on whether or not a threat is immediate or anticipated, and this threat level can be manipulated by changing the type of stimulus used (Blanchard and Blanchard, 1969). In humans, while we do not fear ‘predators’ per se, this rodent model is thought to be equivalent to the urgent desire to escape or flee a situation or place where extreme anxiety or panic occurs, which when maladaptive or excessive becomes panic disorder (Hohoff, 2009). Freezing, which is operationally defined as the absence of movement other than breathing is typically used as the measure of anxiety response to predator odours. Importantly, freezing is a species-specific defence response exhibited by rodents and is arguably the most widely used measure of fear in rodent research.
Fear to natural predator odours emerges around P10, which coincides with the functional emergence of the amygdala (Moriceau et al., 2004). This is consistent with how chemosensory information is relayed to the amygdala, which is critically involved in the detection and assessment of threatening stimuli in rats and humans (Davis, 2000; LeDoux, 2000; Fanselow and Gale, 2003). P10 is also the developmental stage where increasing corticosterone levels are observed and injection of corticosterone precipitates earlier expression of freezing to a predator odour to P8, as well as prematurely evoking amygdala activation, measured by immunostaining the immediate-early gene c-Fos (Moriceau et al., 2004). Conversely, adrenalectomy at P12 prevented both freezing behaviour and amygdala activation to a predator odour, which suggest that low neonatal corticosterone is protective against responding to naturally fearful stimuli (Moriceau et al., 2004).
Compared with maternal separation-induced USV, predator odours can induce fear responses in animals at various ages across development and well into adulthood. For example, in Kabitzke and Wiedenmayer (2011), P14 (juvenile), P26 (preadolescent), P35–45 (adolescent) and P90–100 (adult) rats were exposed to one of three odour stimuli; control odour, cat urine or cat fur, the cat urine being representative of the predator having been there and the fur representative of the more imminent threat of the presence of the cat (Kabitzke and Wiedenmayer, 2011). It was found that innate fear to predator odours was developmentally regulated. Specifically, while rats of all ages displayed significant freezing to cat urine and fur, juvenile and preadolescent rats froze significantly more to cat odour than the older rats, and freezing to cat fur decreased with increasing age (Kabitzke and Wiedenmayer, 2011). The reduction of freezing to cat fur with increasing age was explained to represent the vulnerability of the rats at each stage of development to predators. One study suggests that pharmacological mechanisms underlying fear to predator odours might be similar in preadolescent and adult rats. Specifically, a non-sedating dose of the benzodiazepine chlordiazepoxide injected in P25 rats decreased hiding and increased approach to the cat odour (Siviy et al., 2010), which is consistent with the anxiolytic effects of chlordiazepoxide observed in adult rats exposed to a cat odour (Zangrossi and File, 1992).
Although rats P10 and older exhibit fear responses to predator odours, the neural circuitry appears different across development. Chan et al. (2011) assessed the role of the medial prefrontal cortex (mPFC) in predator odour-induced freezing by temporarily inactivating either the infralimbic (IL) or prelimbic (PrL) subdivisions of the mPFC using the GABA receptor agonist, muscimol, prior to the odour exposure. Inactivation of the IL or PrL had no effects in P14 and P26 rats, indicating that the mPFC is not necessary for showing innate fear to predator odours in juvenile and preadolescent rats. While P14 rats also failed to show any c-Fos immunoreactivity in the mPFC, P26 rats did show c-Fos in the PrL, which suggest that while not necessary for the behaviour, it may still play a role in predator odour-induced freezing. Inactivation of the PrL significantly attenuated freezing in P38–42 rats (Chan et al., 2011). These findings are largely consistent with the emerging idea that the role of the mPFC in fear regulation changes across development (Bronson, 1968; Curio, 1993; Kim and Richardson, 2009a; Wiedenmayer, 2009; Li et al., 2012a). However, more studies are required to understand this neural circuitry because the PrL has been shown to be unnecessary for cat odour-induced freezing in adult rats (Corcoran and Quirk, 2007).
Unconditioned startle responses
Another measure of unlearned fear is the startle response, which is elicited by the presentation of an intense, unexpected stimulus (usually a noise, or flash of light or tactile stimulus), and it is a response conserved across many species (Davis, 2000). In humans, eyeblink is taken as the index of the startle response and is used as an indication of GAD, while in rodents the whole body jump is usually taken as the magnitude of startle (Davis et al., 1993). The startle reflex is increased by fear and aversive states in animals and humans (Davis, 1992; Grillon et al., 1993). Therefore, rats are nocturnal and they exhibit a potentiated acoustic startle response to a sharp noise when tested in a brightly illuminated environment compared with a dark or dim environment. The opposite is true for humans, who exhibit a larger startle response when tested in the dark (Grillon et al., 1997; 1999), reflecting species-specific differences in evolutionary history of potential danger.
P18 rats, but not P14 rats, show an elevated acoustic startle response when merely exposed to a bright light compared with when the light is absent, indicating that rats are capable of showing the startle response to unconditioned light by that age (Weber et al., 2003). Further, peripheral injection of the glycine receptor antagonist, strychnine hydrochloride, and intracerebroventricular infusions of corticotropin-releasing hormone both potentiated the acoustic startle response in P16–18 rats (Weber and Richardson, 2001). Light-potentiated startle appears to involve the serotonergic and the glutamatergic system in the bed nucleus of the stria terminalis in adult rats (Davis and Shi, 1999). However, none of those mechanisms have been assessed in developing rats, although such assessment would provide an important insight as to whether the neural and pharmacological basis of unlearned fear was maintained throughout development.
Approach-avoidance conflict
Lastly, behavioural paradigms based on the approach-avoidance conflict, where avoidance of exposed, brightly lit or elevated areas is measured, can be used to quantify trait anxiety. To our knowledge, little pharmacology has been assessed in rodents of various ages throughout development, however, the response of adolescent (P28–39), late adolescent (P51–55) and adult (P65–109) rodents for anxiety-like behaviour in the emergence test, large open field and elevated plus-maze has been examined in the past. Unfortunately, the results are largely inconsistent with studies reporting that adolescent animals are more anxious than older animals (e.g. Doremus et al., 2003; 2006; Lynn and Brown, 2010), whereas others have reported the opposite (e.g. Imhof et al., 1993; Macri et al., 2002; Andrade et al., 2003), or no age differences (e.g. Walker et al., 2004; Hefner and Holmes, 2007). Therefore, it has been suggested that trait anxiety measures are too sensitive to acute factors on the test day and the results need to be interpreted with caution (Doremus-Fitzwater et al., 2009).
It is clear that our understanding of the pharmacology involved in animal models of unlearned fear across development is still poor (Figure 1). It appears that while a substantial amount of pharmacological information is available on the maternal separation-induced USV, the developmental window during which USV is observed limits the ability to extend the pharmacological findings in the post-juvenile period. On the other hand, predator odour-induced freezing or startle behaviour appear more suitable for characterizing the pharmacological mechanisms underlying unlearned fear across development, and future studies will greatly benefit from using those animal models to understand anxiety early in life. For example, the unconditioned startle response is easily observed in both juvenile rats and humans – delineating the pharmacology and the neural circuitry behind such behaviour using rodent models will be directly translatable to the human population.
Figure 1.
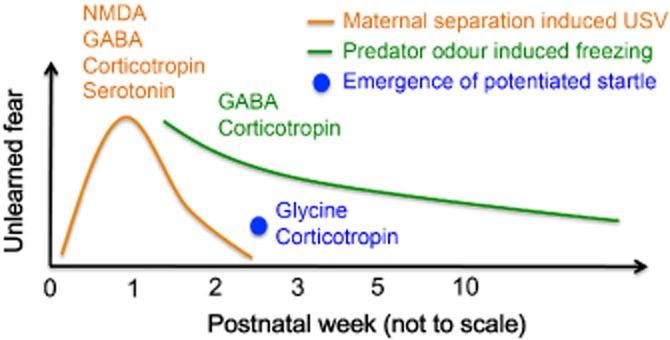
Developmental time course of different forms of unlearned fear and the neurotransmitter systems known to be involved. Maternal separation induced ultrasonic vocalizations (USVs; orange line) rise gradually from birth and peak between postnatal days (P) 6–8 then gradually declines, and are modulated by a number of neurotransmitters including NMDA, GABA, corticotropin and serotonin. Predator odour-induced freezing (green line) is developmentally regulated, with a reduction in freezing with increasing age. Increase in GABA receptor or reduction of corticotropin receptor signalling have been shown to reduce this type of unlearned fear. The emergence of light-potentiated startle occurs at P17 and is observed throughout life, although its intensity across development has not yet been characterized. Antagonism of the glycine receptor or the injection of corticotropin-releasing hormone can also potentiate startle at this age.
Finally, it should be noted that vocalization as a measure of anxiety appears to lack both face and construct validity in comparison with approach-avoidance conflict tests (Geyer and Markou, 2002; Cryan and Holmes, 2005; Markou et al., 2008), startle (Grillon et al., 1997; 1999) or responses to predator threat (Goswami et al., 2013). Furthermore, although all the mentioned models of unlearned fear do have a degree of predictive validity in terms of anxiolytic drug responses (Geyer and Markou, 2002; Cryan and Holmes, 2005; Markou et al., 2008), paediatric anxiety and fear in humans rarely prescribe anxiolytic drugs as a first-line treatment compared with cognitive-behavioural therapy. Therefore, it may be argued that while such models are useful to assess the neurobiological and pharmacological basis of anxiety symptoms early in life, it may not predict treatment success for paediatric anxiety disorders that do not necessarily rely on pharmacological interventions. It appears that rodent models in which behavioural reduction of fear can be examined may be more suitable in examining anxiety early in life.
Learned fear
One of the oldest ideas on the aetiology of anxiety disorders posits that memories, especially those that were formed early in life, are critically involved in the onset of anxiety. Specifically, Freud and Breuer (1893) suggested that ‘hysteria’ (the term they used to refer to an anxiety disorder) is a previously experienced fearful event that remains in memory and triggers anxious thoughts and maladaptive behaviours (Freud and Breuer, 1893). This general idea, that memories formed early in life are important in anxiety disorders, is still widely held (Jacobs and Nadel, 1985; 1999; Mineka and Zinbarg, 2006; Britton et al., 2011; Glenn et al., 2011). Consistent with this idea, alterations in fear learning and cognitive defects form an important facet of the clinical manifestation of anxiety disorders (American Psychiatric Association, 2000), including inappropriate processing of potentially threatening stimuli in GAD, panic disorder and phobias, as well as the long-term salience of traumatic memories seen in post-traumatic stress disorder and social anxiety disorder.
The development of fear memories can be modelled in rats using the processes of Pavlovian fear conditioning. Pavlovian fear conditioning involves pairings of a discrete conditioned stimulus (CS), such as a tone, with a biologically significant unconditioned stimulus (US), such as an electric foot shock. Initially, the CS is a neutral stimulus but when it is paired with the US, the CS starts to elicit autonomic and behavioural fear-conditioned responses (CR) on its own, such as freezing discussed earlier. Fear-potentiated startle is another commonly used index of fear, and is the increase in startle response when the fear-associated stimulus (e.g. CS associated with shock) is present compared with when it is absent. Other widely used measures include avoidance behaviour and changes in heart rate or arterial pressure.
Pharmacological studies of conditioned fear typically involve three distinctive phases (Figure 2A). Namely, the ‘acquisition’ phase refers to the actual associative phase during which paired presentations of the CS and the US are given to the animal. ‘Consolidation’ phase follows immediately after the acquisition phase and it is the time-window required for the working/short-term memory to become a stable, long-term memory. ‘Expression phase’ is when the animal is tested with CS-alone trials to examine whether the CS can elicit CR, which would indicate that CS-related memory is being retrieved.
Figure 2.
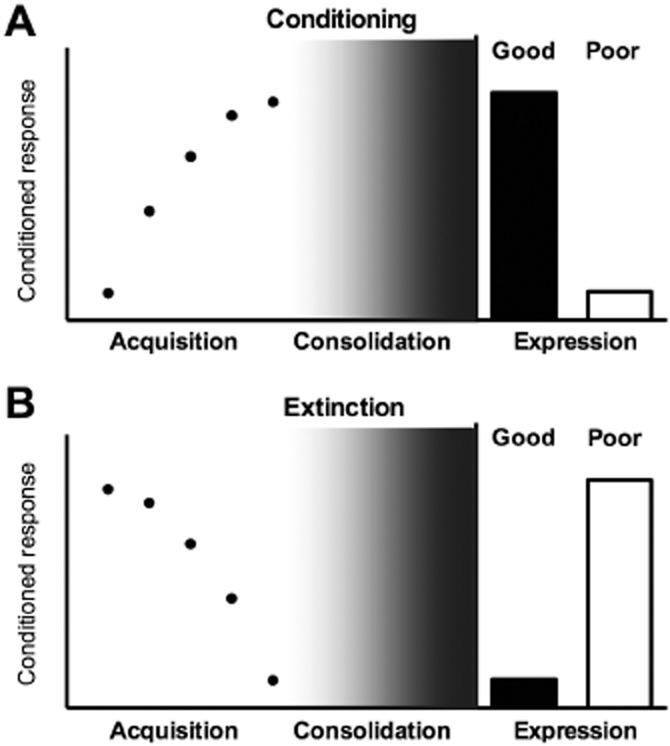
Phases of fear conditioning and extinction. (A) Acquisition of conditioned fear is the increase in fear-conditioned response (CR) at each conditioned stimulus (CS) – unconditioned stimulus (US) trial during the conditioning session. This learning is consolidated to form a CS–US association. Well-consolidated conditioning memory is expressed as high levels of CR to the CS when tested at least 1 day after acquisition. Poor acquisition, a failure in consolidation, or spontaneous forgetting will lead to low levels of expression of CR to the CS at test. (B) Acquisition of extinction is the decrease in fear CR at each CS-alone trial during the extinction session. This learning consolidates to form an extinction memory. Good extinction expression is the low levels of fear to the extinguished CS when tested later. Poor extinction expression (because of changes in the external, internal, or temporal context or a failure in consolidation) will lead to high levels of CS-elicited fear at test.
In adult animals, the amygdala is a central neural structure in which these different phases undergo pharmacologically dissociable processes. It is well established that the amygdala plays a critical role in fear conditioning, and synaptic plasticity of cortical and thalamic inputs to the basolateral nucleus of the amygdala appears necessary for normal fear conditioning (Blair et al., 2001; Maren, 2005). NMDA receptor signalling in the amygdala is particularly important, and it appears to be involved in all three phases (Fanselow and LeDoux, 1999; Maren, 2001; Johansen et al., 2011). Inhibitory GABA receptor neurotransmission in the amygdala also has a role in fear conditioning (see Paré et al., 2003; Ehrlich et al., 2009 for reviews), and its increase can impair whereas its decrease can facilitate acquisition and expression of conditioned fear (Guarraci et al., 1999; Bergado-Acosta et al., 2008; Wiltgen et al., 2009).
Central opioid receptors also play an important role in the acquisition and the consolidation of Pavlovian fear conditioning in the adult rodent (Fanselow and Bolles, 1979; Young and Fanselow, 1992; McNally et al., 2004a), and its increase can impair whereas its decrease can facilitate acquisition and consolidation of conditioned fear. Many other neurotransmitter systems such as dopamine, endocannabinoids and metabotropic glutamate receptors are also involved in conditioned fear (see Johansen et al., 2011), however, most of those have never been assessed in the context of developmental learning and will not be discussed in detail in the present review.
Paradoxical fear learning during infancy
The very early period of postnatal development is characterized by impaired aversion learning compared with their adult counterparts. A classic experiment by Hess (1962) demonstrated that when chicks were shocked in the presence of a surrogate mother, they showed a stronger following response to the surrogate mother than chicks that were not shocked; however, in chicks a few hours older, similar pairings resulted in an aversion to the surrogate. Harlow showed a similar phenomenon in baby monkeys (Harlow and Harlow, 1965) and this may also be the case for humans (Helfer et al., 1999). This ‘paradoxical learning’ is thought to be important in survival of the infant, as it promotes maintaining a mother-infant attachment necessary for survival despite potential adverse consequences during a critical developmental period.
Sullivan et al. are pioneers in studying the neural mechanisms underlying this paradoxical learning in the neonatal rat. Specifically, rat pups younger than P10 are said to be in a sensitive period where shock paired with an odour results in odour preference as measured by an approach behaviour, whereas rats older than P10 form an odour avoidance (Emerich et al., 1985; Barr, 1995; Sullivan et al., 2000; Fitzgerald and Beggs, 2001). This odour-preference learning in infant rats is characterized by the failure to engage the amygdala during conditioning (Moriceau et al., 2006). When the potential for synaptic plasticity of these amygdaloid neurons was assessed in vitro during the pre- and post-sensitive periods, distinct properties were observed between the two age groups. Tetanic stimulation of post-sensitive period (P12) brain slices containing the basolateral amygdala induced significant synaptic plasticity in vitro, but this did not occur in brain slices from P8 rats (Thompson et al., 2008). Interestingly, GABAA receptor blockade with rotoxin (10 μM) in post-sensitive period brain slices containing the amygdala reverts synaptic plasticity to sensitive period characteristics (Thompson et al., 2008). This may be indicative of a link between the developmental emergence of more ‘adult-like’ fear learning with GABAergic function and modulation of amygdala synaptic plasticity. It appears that the immature state of GABAergic inhibition and/or modulation in the neonatal amygdala may be responsible for the deficits in fear learning prior to P10 (Thompson et al., 2008). Interestingly, GABAA receptor function is excitatory at birth, because of high intracellular concentrations of chloride ions, until a developmental change to the chloride concentration gradient occurs to switch GABAA receptor function to inhibitory at approximately P10 (see Ben-Ari, 2002 for review). Such normal developmental change may be critical for the development of the ability to form CS-shock associations.
Shock-induced odour-preference learning during the sensitive period (<P10) in rat pups appears to also be facilitated by the endogenous opioid system. The opioid receptor antagonist naltrexone was delivered subcutaneously in rats either during (P8), or after (P12), the sensitive period to assess the three major phases of fear learning, acquisition, consolidation and expression/retrieval (Roth and Sullivan, 2001; 2003). Interestingly, naltrexone was found to disrupt all three phases of odour-preference learning following odour-shock training in P8 rats, whereas odour-aversion learning in P12 rats remained intact regardless of the timing of naltrexone injection (Roth and Sullivan, 2001; 2003). These results indicate that the endogenous opioid system appears necessary to acquire, consolidate and express odour-preference learning following a foot shock in P8 rats (Roth and Sullivan, 2003). Opioids can modulate memory consolidation through modulatory noradrenaline released from the locus coeruleus, which activate β-adrenoceptors in the amygdala (Gallagher and Kapp, 1978; McGaugh et al., 1988; Introini-Collison et al., 1989). Interestingly, β-adrenoceptor activation in the olfactory bulb and locus coeruleus are both necessary and sufficient for rat pup odour-preference learning (Langdon et al., 1997; Sullivan, 2001), which further support the opioid hypothesis of paradoxical learning early in life.
Additionally, the natural developmental profile of corticosterone is critically involved in the switch between attraction and fear via activity in the amygdala (Moriceau et al., 2006). Endogenous corticosterone release is attenuated and begins to increase in the rat at P10, which coincides with the time at which odour-shock preference turns to more ‘adult-like’ aversion (Rosenfeld et al., 1992; Grino et al., 1994; Sullivan et al., 2000; Levine, 2001). Systemic or intra-amygdala corticosterone administered either 24 h or 30 min before P8 rats received odour-shock conditioning, led to these rats exhibiting adult-like odour-avoidance at test (Moriceau et al., 2006). Interestingly, those rats that received corticosterone showed amygdala neural activity characteristic of that seen in older rats during conditioning (Moriceau et al., 2006). Conversely, when P12 rats receive intra-amygdala infusion of a corticosterone receptor antagonist (or corticosterone depletion via adrenalectomy), they exhibit odour-preference learning instead of an odour-aversion (Moriceau et al., 2006). Corticosterone appears to be important in the emergence of olfactory fear conditioning, acting via the amygdala as a switch between fear and attraction.
Taken together, it appears that rats are in a ‘stress hyporesponsive’ period prior to P10 where corticosterone levels are low but endogenous opioid signalling is high. During this time, fear learning in infants appears to be fundamentally different to adults with different neural circuitry underlying CS-shock learning (Figure 3). This may be a time during which infants are protected against the formation of pervasive negative learned associations; however, we still do not have a comprehensive understanding of what other neurotransmitters may be involved.
Figure 3.
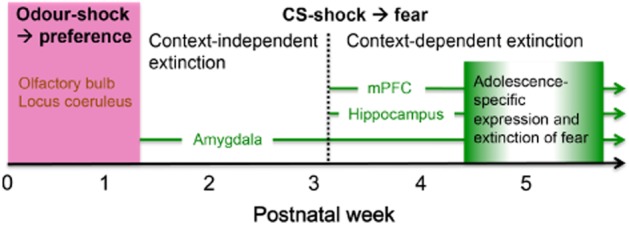
Developmental time course of fear learning and extinction, and the known brain regions involved. Prior to postnatal day (P) 10, odour-shock pairings induce an odour-preference learning in rodents. This paradoxical odour-shock learning depends on the olfactory bulb and the locus coeruleus. Rodents older than P10 can learn conditioned fear to a discrete cue, with amygdala being a critical structure for life. However, fear expression or extinction do not require the medial prefrontal cortex (mPFC) until after 3 weeks of age. Following 3 weeks of life, the hippocampus is also involved and rats can learn context-fear associations, and fear extinction becomes context- and mPFC-dependent. Early adolescence (4–6 weeks of age) appears to be a unique maturation period during which fear expression and extinction are both temporarily impaired before recovery during adulthood.
Learned fear to a discrete cue
As rodents continue to develop, learned behaviours become more complex as the infant’s behavioural repertoire incorporates more adult-like patterns of fear expression (Hunt and Campbell, 1997). The findings on the ontogeny of more adult-like conditioned fear in the rat clearly illustrate how the growth of the rat’s brain affects learning and memory. Specifically, fear conditioning to CS of different sensory modalities emerge in a similar sequence of basic sensory development. In basic sensory development, the functional onset of the tactile and chemical (e.g. olfactory) senses precedes that of the auditory system in the rat (see Alberts and Gubernick, 1984 for review). Further, the functional onset of the auditory system precedes that of the visual system across a wide range of species (Gottlieb and Klopfer, 1962; Hyson and Rudy, 1984; Hunt et al., 1998). Indeed, rats express a learned fear response to an odour CS as early as P12 before they express the same learned response to an auditory CS (Kucharski and Spear, 1984; Campbell and Ampuero, 1985), and express a learned fear response to an auditory CS before they express the same learned response to a visual CS (Hunt and Campbell, 1997). Similar to the sensory-specific emergence of associative learning, CR following fear conditioning also emerges in a response-specific sequence. For example, an auditory CS paired with an aversive US elicits freezing at P16, whereas it alters heart rate at P21; and it potentiates the startle response at P23 (see Hunt and Campbell, 1997 for review).
These studies on the ontogeny of conditioned fear provide converging evidence for the view that different neural structures mediate the different sensory modalities and responses involved in learned fear (Davis, 2000; LeDoux, 2000). The finding that these different fear responses emerge at different ages is usually interpreted as a result of the delayed development of the projections from the amygdala to the relevant downstream structures. Once these projections are developed, however, it appears that juvenile rats can acquire, consolidate and express learned fear.
The role of neurotransmitter systems important for conditioned fear in adulthood need to be tested during development to directly address such a hypothesis. For example, we have shown that NMDA receptor signalling is critical for conditioned fear learning to an auditory CS in juvenile and preadolescent rats (Langton et al., 2007). In that study, P17 and P24 rats were subcutaneously injected with the NMDA receptor antagonist MK-801 or saline, 10 min prior to fear conditioning. When rats were tested for CS-elicited freezing the next day, rats injected with MK-801 showed significantly lower levels of freezing than saline controls in both age groups, demonstrating that, as in adult rats (Rodrigues et al., 2001), acquisition and/or consolidation of learned fear is NDMA dependent in developing rats (Langton et al., 2007).
Although thus far it has been reviewed that fear to a discrete cue can be conditioned and expressed in rats older than P12, recent studies show that the neural circuitry underlying expression of CS-elicited freezing is still developmentally dissociated. Specifically, inactivation of the PrL using the GABA receptor agonist muscimol prior to test does not disrupt conditioned fear expression in P18 rats, whereas rats P25 and older show significant impairment (Corcoran and Quirk, 2007; Li et al., 2012a,b). That finding is suggestive of a simplified conditioned fear circuitry during the juvenile period of rodent development.
Learned fear to a context
An important trigger for fear not only includes discrete cues but also various different environments. These environments can alert animals to impending danger but provide imprecise information about timing compared with discrete cues. Context fear conditioning is modelled to such type of fear and it refers to learning that occurs when the US is presented in a distinctive environment without being signalled by a discrete cue. It is asserted that discrete cue conditioning might index fear/panic, whereas context conditioning might index anxiety (Walker and Davis, 1997b). For example, Grillon et al. propose that some anxiety disorders are caused by unpredictable aversive events that lead to hyperactive fear responses that are generalized across different situations and environments (Grillon et al., 2004). Specifically, if an aversive US (e.g. shock) is not signalled reliably by a CS (e.g. coloured shape), subjects show more fear to the training context compared with subjects that received the US signalled reliably by a CS (Grillon et al., 2004). Grillon et al. concluded that the elevated fear to the context exhibited by the unsignalled group represents generalized anxiety and related disorders.
Compared with cue conditioning, context conditioning involves different neural circuitry and requires the hippocampus as well as the amygdala (Kim and Fanselow, 1992; Phillips and LeDoux, 1992; but see Gewirtz et al., 2000). The hippocampus has long been known to be a late-maturing neural structure that undergoes major anatomical and physiological changes after birth in both rats and humans (Rudy, 1991; Bachevalier and Beauregard, 1993; Giedd et al., 1996). In rats, almost all the granule cells in the dentate gyrus are generated postnatally (Schlessinger et al., 1975). In addition, synaptogenesis, hormone and neurotransmitter receptor site development, and myelination continue throughout the hippocampal formation into the fourth postnatal week (Jacobson, 1963; Crain et al., 1973; Baudry et al., 1981).
Consistent with these findings on hippocampus immaturity, it has been observed that young rats are impaired in hippocampus-dependent tasks such as contextual conditioning (Rudy, 1993; Rudy and Morledge, 1994; Pugh and Rudy, 1996). Rudy (1993) was the first to examine conditioned fear to both a context and an auditory CS in P18 and P23 rats, using a clicker CS-foot shock US pairing. It was observed that both P23 and P18 rats exhibited robust freezing to the clicker CS, however, only the P23 rats displayed context-elicited freezing. That is, P18 rats failed to show any conditioning to the context. In a follow-up study, Rudy and Morledge (1994) demonstrated that P18 rats could show freezing to the conditioned context immediately after the conditioning episode but failed to show any freezing to the conditioned context when tested after a 24 h retention interval. They concluded that P18 rats may learn about the context but cannot consolidate a long-term contextual representation. The context fear learning failure in juvenile rats is also shown when a cat odour was used as a US (Kabitzke et al., 2011).
Interestingly, acute injection of the fibroblast growth factor 2 prior to fear conditioning can potentiate contextual fear learning in P16 rats (Graham and Richardson, 2009), which suggests that the consolidation failure proposed by Rudy and Morledge (1994) may be a quantitative impairment that may be overcome with a pharmacological treatment.
Additionally, post-training injection of the β-adrenoceptor antagonist propranolol inhibited contextual fear learning in P26 rats, which suggest that once contextual learning is possible, it may be subserved by similar pharmacological mechanisms as adult rats (Kabitzke et al., 2011). The expression of conditioned fear to a context, on the other hand, appears to change during early adolescence. Lee and Kim (1998) observed that P27 mice given three shocks in a distinctive context show high levels of context-elicited freezing when tested at next day and in adulthood, but significantly low levels of freezing when tested P28–P35 (Pattwell et al., 2011).
Taken together, research in learned fear to both discrete cues and contexts show that fear learning, consolidation and expression is a dynamic process that changes as a function of age. The recruitment of different neurotransmitter systems and the maturation of neural structures, such as the amygdala, begin to influence fear learning and the form of expression of those fears. This is most striking in the transition between the paradoxical aversive odour-preference learning that occurs very early in life, before the switch to the more adult-like CS–US association, with contextual learning occurring as the hippocampus matures (Figure 3). Pharmacological interventions targeting neurotransmitter systems involved in fear learning reveal that there can be a profound effect on acquisition, consolidation and expression of learned fear, but it is dependent on the stage of development of the rat.
Reducing learned fear by extinction
The current cognitive-behavioural treatments for anxiety disorders in humans largely involve some type of exposure therapies that rely on the process of ‘extinction’ (Hofmann, 2008). Extinction of fear is the decrease in fear responses expressed to a fearful stimulus because of the repeated exposure to the stimulus in a safe environment without any aversive outcome. Extinction has received considerable attention over the past decade because of its theoretical importance and its obvious clinical implications for the treatment of various anxiety disorders (Davis and Myers, 2002). Additionally, conditioned fear and extinction models are believed to have substantial face, construct and predictive validity (Hofmann, 2008; Goswami et al., 2013).
Early theoretical models of Pavlovian conditioning suggested that this decreased response to the CS after extinction was due to the ‘unlearning’ or ‘erasure’ of the original CS–US association (e.g. Rescorla and Wagner, 1972). That is, the association between the CS and US becomes broken so that the CS becomes neutral again. However, it is now widely believed that the decrease in CR after extinction is mainly due to new inhibitory learning (CS–no US association; or ‘safety’) (Bouton, 2002; Phelps et al., 2004). The evidence for this view comes from studies that show fear to an extinguished CS can relapse without retraining of the CS–US association, in reinstatement, renewal and spontaneous recovery. Reinstatement is the return of an extinguished CR following a pretest ‘reminder’ cue, such as a milder version of the shock used initially for conditioning (Rescorla and Heth, 1975). Renewal refers to the return of CR when tested in a different environment/context to where extinction occurred (Bouton and King, 1983). Spontaneous recovery is the return of CR simply because of time elapsing after extinction (Gogolla et al., 2009). These phenomena have been robustly observed in humans and animals. For example in humans, when exposure therapy is conducted in a safe environment such as a therapists’ office, patients may feel that their fear has subsided, however, the treated anxiety disorder can suddenly return when they return to an environment that is different from where therapy occurred, or after time passes, or when faced with an unrelated anxious situation. It appears that extinction training results in inhibition of fear that is fragile and easily reversed in adults.
Similar to fear conditioning, extinction appears to have three distinct phases of acquisition, consolidation and expression (Figure 1B). In fact, pharmacological studies indicate that extinction shares similar neural mechanisms with fear acquisition, further highlighting that extinction involves new learning rather than unlearning in adults. Indeed, blocking NMDA receptors systemically prevents the formation of long-term memory for extinction (e.g. Baker and Azorlosa, 1996; Santini et al., 2001). For example, it was found that systemic injection of the NMDA antagonist CPP (D(-)-3-(2-carboxy-piperazine-4-yl)-propyl-1-phosphonic acid) prior to extinction training impaired extinction expression at test the next day (Santini et al., 2001).
On the other hand, there are studies that show extinction encompasses mechanisms that are distinct from fear acquisition. As extinction leads to a decrease in fear, it is believed that extinction is likely to involve inhibitory mechanisms. Hence, GABA receptor signalling has been studied as the candidate underlying inhibition of fear following extinction. Indeed, pretest injections of the GABA receptor inverse agonist β-carboline-3-carboxylic acid N-methylamide (FG7142) have been shown to attenuate extinction (Harris and Westbrook, 1998). Harris and Westbrook (1998) concluded that FG7142 reduced the inhibitory activity of GABA, which led to the retrieval of the original fear memory. This result shows that extinction relies on inhibitory mechanisms that are distinct from acquisition of fear. Additionally, antagonism of the opioid receptor disrupts fear extinction, whereas fear learning is facilitated (Fanselow and Bolles, 1979; Young and Fanselow, 1992; McNally and Westbrook, 2003; McNally et al., 2004a,b; 2005). As with conditioned fear, many other receptor systems are involved in extinction of fear during adulthood (see Myers and Davis, 2006), however, most of them have not been assessed in the developmental framework.
The pharmacological and behavioural findings suggest the following three characteristics of extinction in adulthood. First, extinction largely reflects a formation of a new and separate memory from the initial fear acquisition memory. Second, extinction memory appears to be inhibitory. Third, extinction expression is contextually bound. Taken together, fear extinction appears to be a context-specific inhibitory learning that competes with the original fear memory expression.
There is now a powerful body of evidence that suggests extinction mechanisms are dynamic at different ages and has revealed that juvenile rats do not show renewal, reinstatement or spontaneous recovery of extinguished fear. That is, extinction early in life does not appear to involve new learning and may even be ‘erasure’ of the original CS–US association (Kim and Richardson, 2007a; Gogolla et al., 2009; see Kim and Richardson, 2010a for review). This means that extinction is more effective early in life and exposure therapy is likely to be most effective in children after the initial onset of the anxiety disorder compared with later in life. This research supports the idea that although fear is readily acquired early in development, fear may also be easily treated during this period, and a better understanding of the mechanisms underlying anxiety early in life is critical for the development of targeted treatments.
Neurotransmitter systems involved in extinction across development
NMDA receptor signalling is indicative of new learning and is a trademark of extinction in the adult rat; however, its role in extinction appears to be age-dependent. Pre-extinction injection of the NMDA receptor antagonist MK-801 has no effect on extinction of conditioned fear to an auditory CS in P17 rats, whereas it impairs long-term extinction in P24 rats (Langton et al., 2007; Kim and Richardson, 2010b). Regardless of age, all rats showed within-session extinction. Additionally, MK-801 has been observed to have an effect on conditioning in both age groups, so these behavioural differences are not due to differential activity of the drug between ages (Langton et al., 2007). Impaired retention of extinction in P24 rats and not juvenile rats (P17) suggests that extinction in juveniles is independent of NMDA receptors and new learning is not the dominant process.
The role of inhibitory GABAergic transmission in extinction also appears to be age-dependent. Pretest subcutaneous injection of FG7142, a partial inverse agonist of the GABAA receptor, causes a return of extinguished fear in P24 rats but not P17 rats (Kim and Richardson, 2007b). That is, reduction of GABAergic transmission facilitates retrieval of the original fear memory in P24 and older rats but not in P17 rats. Consequently, it does not appear that GABA is involved in expression of extinction in P17 rats, in the same way that it is for P24 and older rats.
The lack of GABA or NMDA receptor involvement in extinction in P17 rats suggest an interesting hypothesis – that extinction at this age may not involve the consolidation or expression of an extinction memory, and the decrease in fear during extinction may in fact reflect the erasure of the original CS–US association. In order to test this idea, we conducted an experiment to see whether an ‘extinction memory’ exists when extinction occurs at P17. While initial acquisition of memory is NMDA receptor-dependent, relearning of the same, or a related experience is NMDA receptor-independent (Bannerman et al., 1995; Saucier and Cain, 1995; Roesler et al., 1998; Sanders and Fanselow, 2003; Tayler et al., 2011; Wiltgen et al., 2011; but see Lee and Kim, 1998). This transition from NMDA receptor-dependence to independence has been largely viewed as evidence for the existence of the original memory. Therefore, one can test whether the original extinction memory is still retained following extinction by determining whether re-extinction requires NMDA receptor signalling. If extinction memory still exists, re-extinction should not require NMDA receptor signalling. In Kim and Richardson (2010a), we first conditioned all rats at P16. CS extinction was then given at either P17 or P24, and then all rats received reconditioning to the same CS at P25, and received re-extinction at P26. Prior to re-extinction, rats received systemic injection of saline or MK-801, and then were all tested for their re-extinction memory the next day. When rats received initial extinction at P24, re-extinction was not affected by MK-801, which suggests that the original extinction memory acquired at P24 exists. However, when rats received initial extinction at P17, re-extinction was disrupted by MK-801 and was NMDA receptor-dependent, which suggests that there is no original extinction memory at P17.
This finding was replicated using temporary inactivation of the amygdala. Similar to NMDA receptor signalling, the amygdala appears to be necessary when extinction happens for the first time, but not the second time in adult rats (Laurent et al., 2008). From this, it appears that the amygdala is involved in the initial learning of the CS–no US memory acquired in extinction training, but once that memory has been acquired, then the amygdala is no longer needed for subsequent extinction. We showed that the involvement of the amygdala in re-extinction was determined by the animal’s age at initial extinction. That is, when age was kept constant across these other stages, re-extinction was amygdala-independent if initial extinction occurred at P24 but amygdala-dependent if initial extinction occurred at P17 (Kim and Richardson, 2008).
The requirement of NMDA receptor signalling and the amygdala for re-extinction when extinction occurs at P17 strongly suggest that extinction at P17 may not exist as a new memory. Additionally, the neural circuitry underlying extinction is different at P17 compared with P24 and older rats, in that it does not require the mPFC (Kim et al., 2009). Specifically, dominant neural models propose that in adult rats extinction is expressed (i.e. fear is reduced) when the IL of the mPFC activates the inhibitory GABAergic interneurons and/or intercalated neurons in the amygdala, which then inhibit the output of the amygdala, thereby reducing the CR (Milad and Quirk, 2002; Likhtik et al., 2005; 2008; Milad et al., 2006; Quirk and Mueller, 2007; Peters et al., 2010). In a context that is different from where extinction had occurred, however, the hippocampus can excite the PrL or the amygdala, resulting in the expression of fear (Orsini et al., 2011; Orsini and Maren, 2012). Therefore, having the mPFC, amygdala and hippocampus involved in fear extinction allows the adult rat to flexibly express the CS–no US or the CS–US memory depending on its environment. However, the mechanisms underlying extinction in P17 rats are very different from those mediating extinction in P24 and older rats, with limited neural structures and receptors involved, which may lead to an inflexible learning system, during which extinction may lead to a permanent reduction of the conditioned fear memory.
Despite these studies demonstrating that extinction mechanisms changing across P17 to P24 is not due to the differences in the acquisition of conditioned fear, it has been demonstrated that conditioned fear in P16 mice may be more labile to change compared with older mice, because of the undeveloped perineuronal nets in the amygdala at P16 (Gogolla et al., 2009). Perineuronal nets are extracellular matrix structures surrounding neuronal cell bodies that can interfere with neuronal plasticity, and P16 rats show markedly less perineuronal nets compared with P23 and older rats. Degradation of these nets in the amygdala using chondroitinase ABC infusion prior to fear conditioning leads to erasure-prone extinction in adult rats (Gogolla et al., 2009).
It should be noted that as with adult rats, opioids are important for acquisition of extinction in P17 rats. Specifically, injection of naloxone before extinction training disrupts acquisition of extinction in both P17 and P24 rats (Kim and Richardson, 2009a). Additionally, neither pretest nor post-extinction injection of naloxone had any effects on extinction in either age group, suggesting opioids are not critically involved in retrieval or consolidation of the extinction memory (Kim and Richardson, 2009a), which is also the case in adult rats (McNally and Westbrook, 2003). Interestingly, a contemporary view of opioid signalling states that it is the neurochemical mechanism underlying ‘prediction error’ (McNally et al., 2004b; McNally, 2005; McNally and Cole, 2006; McNally and Westbrook, 2006), which is necessary for the erasure of the original CS–US association (Rescorla and Wagner, 1972). Consequently, opioid signalling during extinction at P17 may be indicative of an erasure mechanism. In contrast, extinction at P24 appears very similar to extinction during adulthood, relying on NMDA receptors, GABA receptors and opioids, as well as displaying a range of other pharmacology and behaviours similar to adult rats (Kim and Richardson, 2009b; Graham and Richardson, 2010; 2011a,b).
Consistent with the role of the mPFC as an important neural structure in extinction retention, recent studies have identified adolescence as a vulnerable period during which maintenance of extinction is impaired alongside disrupted plasticity in the mPFC (Kim et al., 2011; Pattwell et al., 2013). The developmental trajectory of the prefrontal cortex in both rats and humans is largely non-linear with a decline in volume during adolescence (Giedd, 2004; Steinberg, 2005; Paus et al., 2008; Shaw et al., 2008). Adolescent rats exhibit poorer extinction retention (greater fear relapse) when tested 24 h after extinction training compared with both younger and older rats (McCallum et al., 2010; Kim et al., 2011). This effect has been recently replicated in humans (Pattwell et al., 2012). However, adolescent rats can express facilitated extinction at test (to the level of P24 and adult rats) with immediate post-extinction administration of the partial NMDA receptor agonist, D-cycloserine, or by providing additional extinction training (McCallum et al., 2010). This blunted fear extinction during adolescence is associated with a lack of activity in the IL in rats (Kim et al., 2011) and this has also been observed in mice (Pattwell et al., 2012).
Spontaneous forgetting of learned fear
Fortunately, anxiety early in life is not necessarily chronic, and many children with anxiety will not manifest clinical conditions later in life (Pine et al., 2009). Neural processes that may be responsible for such a transition during development are poorly understood, however, it is hypothesized that a combination of brain maturation and changing cognitive processes play an important part (Campbell and Spear, 1972; Thomas et al., 2001; Craske et al., 2008; Mineka and Oehlberg, 2008). Understanding how memories are naturally forgotten, in particular, may provide clues as to how anxiety or fearful memories early in life can spontaneously disappear. Importantly, observing individual differences in fear and anxiety using the spontaneous forgetting model may have better face, construct and predictive validity in identifying vulnerability in humans that develop anxiety following stressful or traumatic events.
In fact, infant animals display a very rapid rate of spontaneous forgetting. This reduced ability to remember experiences that happened early in development is referred to as ‘infantile amnesia’. For example, a 20-year-old cannot remember events that occurred at 5 years of age as accurately as a 30-year-old can remember events that occurred at 15 years of age. This phenomenon received attention from scientists as early as 1895, as an important part of normal cognitive development (Henri and Henri, 1895). Interestingly, infantile amnesia is not a uniquely human phenomenon – most altricial animals demonstrate poor long-term retention early in development (for a review, see Campbell and Spear, 1972). Altricial animals are animals in which development occurs mostly after birth (e.g. humans), as opposed to precocial species in which development is virtually complete before birth (e.g. guinea pigs).
The rat was first used to study infantile amnesia in 1962, when Campbell and Campbell (1962) trained rats aged P18 to P100 on a passive avoidance procedure, in which rats were shocked while being confined in the black compartment of a black/white shuttle box. No shocks were given in the other, white compartment. Rats were then tested for retention of fear either immediately, or after a delay of 7, 21 or 42 days. Retention was measured by the avoidance of the black compartment, indicated by a longer latency to enter the normally preferred black side. There were no differences between the groups when tested immediately after training, with all age groups avoiding the black compartment. This finding indicates that animals of all ages were able to learn and express fear. However, when tested after a delay, retention increased dramatically with age. Eighteen-day-olds showed only moderate retention when tested after 7 days, which declined even more after 42 days. In contrast, 100-day-old rats showed nearly complete avoidance even at the 42-day interval.
Feigley and Spear (1970) also found that although the rate of acquisition of avoidance learning was similar across the different ages (as measured by the number of shock trials to express avoidance learning), infant rats showed a significant retention deficit compared with adult rats (Feigley and Spear, 1970). Furthermore, deliberately overtrained infant rats still exhibited markedly inferior memory retention to that of adult rats (Schulenburg et al., 1971). Schulenburg et al. (1971) concluded that the accelerated rate of forgetting by infant rats compared with adult rats is not a consequence of the degree of original learning.
Pharmacological studies further suggest that passive avoidance learning is acquired similarly across development. This learning depends upon normal cholinergic transmission in the limbic system in adults (Dumery and Blozovski, 1987). Its ontogenic development appears to parallel the maturation of muscarinic cholinergic synaptic elements located in the posteroventral hippocampo-subiculo-entorhinal area and in the basal lateral part of the posterior amygdala (Blozovski and Dumery, 1984). Microinjections of the muscarinic receptor antagonist atropine into the basal lateral part of the posterior amygdala induced dose-dependent deficits in acquisition from P13 to P20 in a passive avoidance task, although the sensitivity to atropine decreases at P17 and thereafter (Blozovski and Dumery, 1984).
Furthermore, infantile amnesia can at least be partly reversed by strengthening the consolidation phase following learning. For example, P16 rats that exhibit excellent retention in a passive avoidance task at 1 h but not at 24 h following training, display significantly improved performance at 24 h when given a post-training injection of adrenaline or noradrenaline, agents known to facilitate memory consolidation in adult rats (Gold et al., 1982). Glucose, which is an important memory modulator in adult mammals (Gold, 1986), can also improve memory retention in P18 rats when given immediately after passive avoidance training (Flint and Riccio, 1999). However, systemic injection of the μ-opioid receptor antagonist naloxone immediately after training does not alleviate infantile amnesia (Weber et al., 2006).
Because passive avoidance learning is a form of contextual learned fear in which a distinctive context (typically a black box) is associated with an aversive US (typically as a shock), it may not be surprising that juvenile rats show poor expression of avoidance behaviour compared with adults despite having comparable acquisition. However, that explanation alone is insufficient to explain why memory retention increases with age, because infantile amnesia has also been observed in hippocampus-independent tasks across a wide range of species (Campbell and Spear, 1972). For example, infant and juvenile rats show accelerated forgetting compared with older rats even following conditioned fear to a discrete auditory cue (Kim et al., 2006; Kim and Richardson, 2007c; Li and Richardson, 2013). Therefore, infantile amnesia appears to be an exaggerated version of natural forgetting, and any differences in consolidation appear to be quantitative rather than qualitative.
Further evidence against the argument that infantile amnesia is due to failure in consolidation comes from pharmacological studies that observed forgotten fear is retrieved through various pretest manipulations. That is, a memory that is consolidated well but not expressed should be accessible if given the correct treatment. For example, systemic injection of adrenaline in juvenile rats at test can reinstate the expression of passive avoidance memory compared with saline injection, without causing passive avoidance in non-trained rats (Haroutunian and Riccio, 1977; Flint et al., 2007). It was argued that adrenaline induces physiological arousal and thus may provide an internal cue that reminds the rat of the initial training episode. In any case, the recovery of the avoidance memory by adrenaline indicates that infantile amnesia involves a failure to retrieve the stored fear memory (Haroutunian and Riccio, 1977; Richardson et al., 1983).
The endogenous opioid system appears to be critical for such retrieval failure early in life. Systemic injection of the opioid antagonist naloxone prior to test alleviates forgotten fear to a discrete cue, an effect that was shown to be central not peripheral (Weber et al., 2006). GABA is also involved in the rapid forgetting shown in juvenile rats (Kim et al., 2006). We have shown that when P18 rats were conditioned to fear a white-noise CS, CS-elicited freezing that was very high 1 day after conditioning significantly decreased by 10 days (Kim et al., 2006). Pretest injection with FG7142, a GABAA receptor partial inverse agonist that reduces GABAergic transmission, however, restored CS-elicited freezing in those rats. Interestingly, GABA receptor signalling also plays an important role in behavioural manipulation to recover a forgotten fear. It has long been known that a pretest US reminder (at a low level that does not elicit the CR on its own) can retrieve a forgotten fear memory (Campbell and Jaynes, 1966; Spear and Parsons, 1976; Richardson et al., 1986; Kim and Richardson, 2007a). However, administration of the GABA receptor agonist midazolam immediately following a US reminder impaired the retrieval of a forgotten fear in the infant rat (Kim and Richardson, 2007c). It appears that behavioural reminder treatments that result in recovery of a forgotten memory, may work via alterations in GABA activity.
A recent study used a novel approach to examine whether the original conditioned fear memory was indeed intact in juvenile rats, despite the failure in expression (Li and Richardson, 2013). As mentioned previously, while initial acquisition of memory is NMDA receptor-dependent, relearning of the same, or a related experience is NMDA receptor-independent (Sanders and Fanselow, 2003; Tayler et al., 2011; Wiltgen et al., 2011). This transition from NMDA receptor-dependence to independence has been largely viewed as evidence for the existence of the original fear memory. Therefore, one can test whether the original fear memory is still retained following infantile amnesia by determining whether retraining the rat at the time of forgetting requires NMDA receptor signalling. Li and Richardson (2013) first gave P17 rats six CS–US pairings. These rats showed high levels of CS-elicited freezing when tested 1 day after training, but exhibited complete forgetting 14 days after training. Rats then received a systemic injection of the NMDA receptor antagonist MK-801 followed by a retraining of three CS–US pairings, and then were tested the next day. Rats that received MK-801 did not show any impairment in CS-elicited freezing compared with saline, which indicates that relearning is NMDA receptor-independent even after forgetting of the fear memory (Li and Richardson, 2013). This result is consistent with the finding that showed persistence of neural activity in the amygdala in infant rats that received CS–US pairings but did not show any CS-elicited freezing due to forgetting (Kim et al., 2012).
It is important to note that in Li and Richardson (2013), re-acquisition of forgotten fear remained NMDA receptor-independent even when rats were retrained 50 days following the initial fear conditioning at P17. This finding implies that the original fear memory is forgotten but not gone even in adulthood. In another study, however, it was demonstrated that when rats were initially fear-conditioned at P18, the forgotten fear was irretrievable via an injection of FG7142 at P78 (Kim et al., 2006). Such seemingly contradictory findings can be explained when the strength of original learning is considered. That is, the former study used six, whereas the latter study used three CS–US pairings during the initial learning. Therefore, the failure to recover a forgotten memory by a GABAergic manipulation at P78 is a possible, irrecoverable, memory decay of a weaker CS–US association.
Development of brain regions underlying rodent models of fear and anxiety
Surprisingly, little is known about the development and maturation of the brain regions associated with the rodent models discussed. Unlearned fear in adults is associated with the extended amygdala (Davis, 2000), while conditioned fear and extinction is dependent on communication between the amygdala, hippocampus and mPFC (Quirk and Mueller, 2007). Unfortunately, how the extended amygdala develops during the first few weeks of postnatal life is poorly understood. As mentioned previously, the hippocampus does not reach maturity until the end of the fourth postnatal week (Jacobson, 1963; Crain et al., 1973; Baudry et al., 1981). In other brain regions, such as the mPFC and amygdala, axons and synapses are overproduced during early puberty, followed by rapid pruning in later adolescence (Crews et al., 2007). Specifically, there is dendritic pruning in the amygdala, and basolateral amygdala volume increases between P20 and P35 and then decreases between P35 and P90 (Zehr et al., 2006; Pan et al., 2009; Rubinow and Juraska, 2009), with continual growth in the density of the fibres connecting the amygdala and the mPFC into early adulthood (Cunningham et al., 2002). The cortical layers of the mPFC are immature at P18, but attain their adult proportional width by P24 (Van Eden and Uylings, 1985). However, rapid changes occur in the mPFC during adolescence, which involve rapid myelinization, decreases in prefrontal gray matter, growth of afferent efferent axons and dendrites, maturation and reorganization of synapses (Casey et al., 2008; see review by Andersen, 2003). It is proposed that late maturation of amygdalo-cortical connectivity may provide an anatomical basis for the development and integration of normal and possibly abnormal emotional behaviour during periods of rapid growth. Not much is known about the growth of connectivity between the amygdala and the hippocampus between 1 and 5 weeks of postnatal rodent life. It has been shown that early maternal stress and juvenile emotional experiences produce enduring morphological changes in the hippocampus, including attenuation of synaptic development (Poeggel et al., 2003; Andersen and Teicher, 2004), which may affect connectivity between the amygdala and hippocampus.
Conclusions
Overall, studies on learned fear show that animals are particularly resilient against prolonged effects of fearful events early in life, an observation that is greatly associated with normal brain development (Figure 3). Initially, the amygdala is uninvolved in cue-shock learning, which leads to infant rats expressing preference to the cue. Once the amygdala is critically involved in conditioned fear, rodents do learn and express aversion, however, these young rodents forget such fear learning very rapidly compared with older rats. Also, prior to 3 weeks of postnatal life, rodents are impaired in contextual learning and exhibit relapse-resistant extinction that is expressed regardless of the context of test. In fact, extinction early in life may even erase the original fear association. However, it should be noted that our understanding is still poor and many open-ended questions remain. For example, rodent models with increasing face and construct validity are being developed in adult rodents (e.g. Siegmund and Wotjak, 2007; Goswami et al., 2013). Utilizing those models in developing rodents in future studies would be crucial to the advancement of this field.
Taken together, 3 weeks of life may represent the closing of a developmental window where extinction is more effective in rodents. It is difficult to pinpoint the human correlate of an equivalent age group, however, an influential study suggested that past 7 years of age is when children can acquire contextual/spatial memories reliably and may correspond to 3 weeks of age in rodents in terms of cognitive abilities (Overman et al., 1996). In any case, such a resilient period early in life may explain how some anxiety can disappear as children develop.
Regardless, the prevalence of anxiety disorders remains very high, emphasizing the importance of understanding forgetting and erasure in order to try and facilitate those processes. Recent animal studies strongly suggest that adverse early life stress can have such an effect on some individuals and may explain how anxiety disorders become chronic (see Callaghan and Richardson, 2013). For example, repeated maternal separation in infant rats (P2–P14) can lead to relapse-prone extinction as well as impaired infantile amnesia of fearful memories later in life (Callaghan and Richardson, 2011; 2012a,b). These observations have significant implications, especially considering the evidence that show stress early in life can cause epigenetic mechanisms that persist through adulthood and across generations (Gross and Hen, 2008; Franklin et al., 2010; Zhang et al., 2013). Importantly, future studies should address whether pharmacological interventions to treat anxiety early in life also cause positive or negative epigenetic changes.
Finally, it may also be the case that extinction (whether natural or in a clinic) of fearful associations does not actually occur in children. In fact, there are limited specialized therapeutics targeting children or adolescents with anxiety disorders, with cognitive-behavioural therapy and SSRIs being the two current treatments implemented for treating anxious children (Rapee et al., 2005; Bridge et al., 2007; Pine et al., 2009). It is also observed that fewer than one in five anxious children or adolescents are expected to receive treatment for their disorders (Merikangas et al., 2010). Cognitive therapies such as the FRIENDS programme are proving to be effective in early detection and treatment of childhood anxiety (Barrett et al., 2006), nevertheless, it is bleak that even developed countries such as the United Kingdom, United States and Australia have fewer than one counsellor per 1500 school children, with some countries such as South Korea reporting one counsellor per 138 183 school children (Jimerson et al., 2009), indicating that many schools do not have access to even one mental health specialist. Evolution may have given us a robust brain against anxiety early in life, but without changes in the present environment, anxiety disorders will remain a huge health issue.
Glossary
- CS
conditioned stimulus
- CPP
D(-)-3-(2-carboxy-piperazine-4-yl)-propyl-1-phosphonic acid
- CR
conditioned response
- FG7142
β-carboline-3-carboxylic acid N-methylamide
- GAD
generalized anxiety disorder
- IL
infralimbic cortex
- mPFC
medial prefrontal cortex
- P
postnatal day
- PrL
prelimbic
- SSRI
selective serotonin reuptake inhibitor
- US
unconditioned stimulus
- USV
ultrasonic vocalizations
Conflict of interest
None.
References
- Alberts JR, Gubernick DJ. Early learning as ontogenetic adaptation for ingestion by rats. Learn Motiv. 1984;15:334–359. [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013a;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013c;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allin JT, Banks EM. Effects of temperature on ultrasound production by infant albino rats. Dev Psychobiol. 1971;4:149–156. doi: 10.1002/dev.420040206. [DOI] [PubMed] [Google Scholar]
- Allin JT, Banks EM. Functional aspects of ultrasound production by infant albino rats (Rattus norvegicus) Anim Behav. 1972;20:175–185. doi: 10.1016/s0003-3472(72)80189-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th edn, text rev.) Washington: American Psychiatric Association; 2000. [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andrade M, Tomé MF, Santiago ES, Lúcia-Santos A, de Andrade T. Longitudinal study of daily variation of rats’ behavior in the elevated plus-maze. Physiol Behav. 2003;78:125–133. doi: 10.1016/s0031-9384(02)00941-1. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M. Maturation of medial temporal lobe memory functions in rodents, monkeys, and humans. Hippocampus. 1993;3:191–201. [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RGM. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Barr GA. Ontogeny of nociception and antinociception. NIDA Res Monogr. 1995;158:172–201. [PubMed] [Google Scholar]
- Barrett PM, Farrell LJ, Ollendick TH, Dadds M. Long-term outcomes of an Australian universal prevention trial of anxiety and depression symptoms in children and youth: an evaluation of the FRIENDS programs. J Clin Child Adolesc Psychol. 2006;35:403–411. doi: 10.1207/s15374424jccp3503_5. [DOI] [PubMed] [Google Scholar]
- Baudry M, Arst D, Oliver M, Lynch G. Development of glutamate binding sites and their regulation by calcium in rat hippocampus. Brain Res Dev Brain Res. 1981;1:37–48. doi: 10.1016/0165-3806(81)90092-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape H-C, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learn Mem. 2008;15:163–171. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Blozovski D, Dumery V. Implication of amygdaloid muscarinic cholinergic mechanisms in passive avoidance learning in the developing rat. Behav Brain Res. 1984;13:97–106. doi: 10.1016/0166-4328(84)90140-2. [DOI] [PubMed] [Google Scholar]
- Boulis NM, Davis M. Footshock-induced sensitization of electrically elicited startle reflexes. Behav Neurosci. 1989;103:504–508. doi: 10.1037//0735-7044.103.3.504. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process. 1983;9:248–265. [PubMed] [Google Scholar]
- Bridge JA, Iyengar S, Salary CB, Barbe R, Birmaher B, Pincus HA, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety. 2011;28:5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson GW. The development of fear in man and other animals. Child Dev. 1968;39:409–431. [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav Neurosci. 2011;125:20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Early-life stress affects extinction during critical periods of development: an analysis of the effects of maternal separation on extinction in adolescent rats. Stress. 2012a;15:671–679. doi: 10.3109/10253890.2012.667463. [DOI] [PubMed] [Google Scholar]
- Callaghan B, Richardson R. The effect of adverse rearing environments on persistent memories in young rats: removing the brakes on infant fear memories. Transl Psychiatry. 2012b;2:e138. doi: 10.1038/tp.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Early experiences and the development of emotional learning systems in rats. Biol Mood Anxiety Disord. 2013;3:8. doi: 10.1186/2045-5380-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BA, Ampuero MX. Dissociation of autonomic and behavioral components of conditioned fear during development in the rat. Behav Neurosci. 1985;99:1089–1102. doi: 10.1037//0735-7044.99.6.1089. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Campbell EH. Retention and extinction of learned fear in infant and adult rats. J Comp Physiol Psychol. 1962;55:1–8. doi: 10.1037/h0049182. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Jaynes J. Reinstatement. Psychol Rev. 1966;73:478–480. doi: 10.1037/h0023679. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Spear N. Ontogeny of memory. Psychol Rev. 1972;79:215–236. doi: 10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T, Kyere K, Davis BR, Shemyakin A, Kabitzke PA, Shair HN, et al. The role of the medial prefrontal cortex in innate fear regulation in infants, juveniles, and adolescents. J Neurosci. 2011;31:4991–4999. doi: 10.1523/JNEUROSCI.5216-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain B, Cotman C, Taylor D, Lynch G. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Res. 1973;63:195–204. doi: 10.1016/0006-8993(73)90088-7. [DOI] [PubMed] [Google Scholar]
- Craske MG, Waters AM, Lindsey Bergman R, Naliboff B, Lipp OV, Negoro H, et al. Is aversive learning a marker of risk for anxiety disorders in children? Behav Res Ther. 2008;46:954–967. doi: 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol. 2011;164:1129–1161. doi: 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Curio E. Proximate and developmental aspects of antipredator behavior. In: Slater P, Rosenblatt J, Snowdon C, Milinski M, editors. Advances in the study of behavior. San Diego: Academic; 1993. pp. 135–238. [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala. Vol. 2. Oxford: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- Davis M, Myers KM. The role of glutamate and Gamma-Aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:570–577. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Effects of pretest manipulation on elevated plus-maze behavior in adolescent and adult male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2009;92:413–423. doi: 10.1016/j.pbb.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumery V, Blozovski D. Development of amygdaloid cholinergic mediation of passive avoidance learning in the rat. Exp Brain Res. 1987;67:61–69. doi: 10.1007/BF00269453. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Scalzo FM, Enters EK, Spear NE, Spear LP. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Dev Psychobiol. 1985;18:215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Bolles RC. Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol. 1979;93:736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Feigley DA, Spear NE. Effect of age and punishment condition on long-term retention by the rat of active-and passive-avoidance learning. J Comp Physiol Psychol. 1970;73:515–526. doi: 10.1037/h0030234. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7:246–257. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- Flint RW, Bunsey MD, Riccio DC. Epinephrine-induced enhancement of memory retrieval for inhibitory avoidance conditioning in preweanling Sprague-Dawley rats. Dev Psychobiol. 2007;49:303–311. doi: 10.1002/dev.20212. [DOI] [PubMed] [Google Scholar]
- Flint RW, Jr, Riccio DC. Post-training glucose administration attenuates forgetting of passive-avoidance conditioning in 18-day-old rats. Neurobiol Learn Mem. 1999;72:62–67. doi: 10.1006/nlme.1998.3906. [DOI] [PubMed] [Google Scholar]
- Fost N. Ethical issues in research and innovative therapy in children with mood disorders. Biol Psychiatry. 2001;49:1015–1022. doi: 10.1016/s0006-3223(01)01181-7. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Freud S, Breuer J. On the psychical mechanism of hysterical phenomena: preliminary communications. In: Strachey J, Strachey A, editors. Studies in hysteria. London: Harmonsworth Penguin; 1893. pp. 53–69. [Google Scholar]
- Gallagher M, Kapp BS. Manipulation of opiate activity in the amygdala alters memory processes. Life Sci. 1978;23:1973–1977. doi: 10.1016/0024-3205(78)90565-9. [DOI] [PubMed] [Google Scholar]
- Gardner CR. Distress vocalization in rat pups a simple screening method for anxiolytic drugs. J Pharmacol Methods. 1985;14:181–187. doi: 10.1016/0160-5402(85)90031-2. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Is the hippocampus necessary for contextual fear conditioning? Behav Brain Res. 2000;110:83–95. doi: 10.1016/s0166-4328(99)00187-4. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A. 2002. pp. 445–455. The role of preclinical models in the development of psychotropic drugs. Neuropsychopharmacology: The Fifth Generation of Progress. Washington, DC: American College of Neuropsychopharmacology.
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G. The development of fear learning and generalization in 8–13 year-olds. Dev Psychobiol. 2011;54:675–684. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Lüthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Gold PE. Glucose modulation of memory storage processing. Behav Neural Biol. 1986;45:342–349. doi: 10.1016/s0163-1047(86)80022-x. [DOI] [PubMed] [Google Scholar]
- Gold PE, Murphy JM, Cooley S. Neuroendocrine modulation of memory during development. Behav Neural Biol. 1982;35:277–293. doi: 10.1016/s0163-1047(82)90713-0. [DOI] [PubMed] [Google Scholar]
- Goswami S, Rodríguez-Sierra O, Cascardi M, Parè D. Animal models of post-traumatic stress disorder: face validity. Front Neurosci. 2013;7:89. doi: 10.3389/fnins.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Gottlieb G, Klopfer PH. The relation of developmental age to auditory and visual imprinting. J Comp Physiol Psychol. 1962;55:821–826. doi: 10.1037/h0041161. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Acute systemic fibroblast growth factor-2 enhances long-term memory in developing rats. Neurobiol Learn Mem. 2009;91:424–430. doi: 10.1016/j.nlm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Fibroblast growth factor-2 enhances extinction and prevents renewal of conditioned fear. Neuropsychopharmacology. 2010;35:1348–1355. doi: 10.1038/npp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Fibroblast growth factor-2 alters the nature of extinction. Learn Mem. 2011a;18:80–84. doi: 10.1101/lm.2006511. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Intraamygdala infusion of fibroblast growth factor 2 enhances extinction and reduces renewal and reinstatement in adult rats. J Neurosci. 2011b;31:14151–14157. doi: 10.1523/JNEUROSCI.3014-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Foot M, Davis M. Fear-potentiated startle: relationship to the level of state/trait anxiety in healthy subjects. Biol Psychiatry. 1993;33:566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pellowski M, Merikangas KR, Davis M. Darkness facilitates the acoustic startle reflex in humans. Biol Psychiatry. 1997;42:453–460. doi: 10.1016/S0006-3223(96)00466-0. [DOI] [PubMed] [Google Scholar]
- Grillon C, Merikangas KR, Dierker L, Snidman N, Arriaga RI, Kagan J, et al. Startle potentiation by threat of aversive stimuli and darkness in adolescents: a multi-site study. Int J Psychophysiol. 1999;32:63–73. doi: 10.1016/s0167-8760(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. Blockade of alpha 2-adrenoceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology. 1994;135:2549–2557. doi: 10.1210/endo.135.6.7988443. [DOI] [PubMed] [Google Scholar]
- Groenink L, Verdouw PM, van Oorschot R, Olivier B. Models of anxiety: ultrasonic vocalizations of isolated rat pups. Curr Protoc Pharmacol. 2008;5 doi: 10.1002/0471141755.ph0518s43. DOI: 10.1002/0471141755.ph0518s43. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Kapp BS. Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Res. 1999;827:28–40. doi: 10.1016/s0006-8993(99)01291-3. [DOI] [PubMed] [Google Scholar]
- Hard E, Engel J. Effects of 8-OH-DPAT on ultrasonic vocalization and audiogenic immobility reaction in pre-weanling rats. Neuropharmacology. 1988;27:981–986. doi: 10.1016/0028-3908(88)90056-1. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The affectional systems. In: Schrier AM, Harlow HF, Stollnitz F, editors. Behavior of Non-human Primates, Volume 2. New York: Academic Press; 1965. pp. 287–334. [Google Scholar]
- Haroutunian V, Riccio DC. Effect of arousal conditions during reinstatement treatment upon learned fear in young rats. Dev Psychobiol. 1977;10:25–32. doi: 10.1002/dev.420100105. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology (Berl) 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer ME, Kempe RS, Krugman RD. The battered child. Chicago: University of Chicago Press; 1999. [Google Scholar]
- Henri V, Henri C. On our earliest recollections of childhood. Psychol Rev. 1895;2:215–216. [Google Scholar]
- Hess EH. Ethology: An approach toward the complete analysis of behavior. Austin: Holt, Rinehart and Winston; 1962. [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin Psychol Rev. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohoff C. Anxiety in mice and men: a comparison. J Neural Transm. 2009;116:679–687. doi: 10.1007/s00702-009-0215-z. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Campbell BA. Developmental dissociation of the components of conditioned fear. In: Bouton ME, Fanselow MS, editors. Learning, motivation and cognition: The functional behaviorism of Robert C. Bolles. Washington, DC: American Psychological Association; 1997. pp. 53–74. [Google Scholar]
- Hunt PS, Hess MF, Campbell BA. Inhibition of the expression of conditioned cardiac responses in the developing rat. Dev Psychobiol. 1998;33:221–233. doi: 10.1002/(sici)1098-2302(199811)33:3<221::aid-dev3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Hyson RL, Rudy JW. Ontogenesis of learning. II. Variation in the rat’s reflexive and learned responses to acoustic stimulation. Dev Psychobiol. 1984;17:263–283. doi: 10.1002/dev.420170307. [DOI] [PubMed] [Google Scholar]
- Iijima M, Chaki S. Separation-induced ultrasonic vocalization in rat pups: further pharmacological characterization. Pharmacol Biochem Behav. 2005;82:652–657. doi: 10.1016/j.pbb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Imhof JT, Coelho ZMI, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav Brain Res. 1993;56:177–180. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Insel TR. Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry. 2009;66:128–133. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Nagahara AH, McGaugh JL. Memory enhancement with intra-amygdala post-training naloxone is blocked by concurrent administration of propranolol. Brain Res. 1989;476:94–101. doi: 10.1016/0006-8993(89)91540-0. [DOI] [PubMed] [Google Scholar]
- Jacobs WJ, Nadel L. Stress-induced recovery of fears and phobias. Psychol Rev. 1985;92:512–531. [PubMed] [Google Scholar]
- Jacobs WJ, Nadel L. The first panic attack: a neurobiological theory. Can J Exp Psychol. 1999;53:92–107. doi: 10.1037/h0087302. [DOI] [PubMed] [Google Scholar]
- Jacobson S. Sequence of myelinization in the brain of the albino rat. A. Cerebral cortex, thalamus and related structures. J Comp Neurol. 1963;121:5–29. doi: 10.1002/cne.901210103. [DOI] [PubMed] [Google Scholar]
- Jimerson SR, Stewart K, Skokut M, Cardenas S, Malone H. How many school psychologists are there in each country of the world?: International estimates of school psychologists and school psychologists-to-student ratios. School Psychol Int. 2009;30:555–567. [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabitzke PA, Wiedenmayer CP. Effects of the stimulus and chamber size on unlearned fear across development. Behav Processes. 2011;86:257–262. doi: 10.1016/j.beproc.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabitzke PA, Silva L, Wiedenmayer C. Norepinephrine mediates contextual fear learning and hippocampal pCREB in juvenile rats exposed to predator odor. Neurobiol Learn Mem. 2011;96:166–172. doi: 10.1016/j.nlm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, McCloskey TC, Baron BM, Chi EM, Harrison BL, Whitten JP, et al. NMDA receptor complex antagonists have potential anxiolytic effects as measured with separation-induced ultrasonic vocalizations. Eur J Pharmacol. 1991;193:283–292. doi: 10.1016/0014-2999(91)90141-c. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Coverdale S, McCloskey TC, Hoffman DC, Cassella JV. Effects of the CRF1 receptor antagonist, CP 154 526, in the separation-induced vocalization anxiolytic test in rat pups. Neuropharmacology. 2000;39:1357–1367. doi: 10.1016/s0028-3908(00)00043-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Angermeyer M, Anthony JC, de Graaf R, Demyttenaere K, Gasquet I, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem. 2007a;88:48–57. doi: 10.1016/j.nlm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007b;121:131–139. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. Immediate post-reminder injection of gamma-amino butyric acid (GABA) agonist midazolam attenuates reactivation of forgotten fear in the infant rat. Behav Neurosci. 2007c;121:1328–1332. doi: 10.1037/0735-7044.121.6.1328. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. J Neurosci. 2008;28:1282–1290. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. The effect of the mu-opioid receptor antagonist naloxone on extinction of conditioned fear in the developing rat. Learn Mem. 2009a;16:161–166. doi: 10.1101/lm.1282309. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. Expression of renewal is dependent on the extinction-test interval rather than the acquisition-extinction interval. Behav Neurosci. 2009b;123:641–649. doi: 10.1037/a0015237. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol Psychiatry. 2010a;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. Extinction in preweanling rats does not involve NMDA receptors. Neurobiol Learn Mem. 2010b;94:176–182. doi: 10.1016/j.nlm.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Kim JH, McNally GP, Richardson R. Recovery of fear memories in rats: role of gamma-amino butyric acid (GABA) in infantile amnesia. Behav Neurosci. 2006;120:40–48. doi: 10.1037/0735-7044.120.1.40. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Li S, Richardson R. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb Cortex. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- Kim JH, Li S, Hamlin AS, McNally GP, Richardson R. Phosphorylation of mitogen-activated protein kinase in the medial prefrontal cortex and the amygdala following memory retrieval or forgetting in developing rats. Neurobiol Learn Mem. 2012;97:59–68. doi: 10.1016/j.nlm.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kucharski D, Spear NE. Conditioning of aversion to an odor paired with peripheral shock in the developing rat. Dev Psychobiol. 1984;17:465–479. doi: 10.1002/dev.420170505. [DOI] [PubMed] [Google Scholar]
- Langdon PE, Harley CW, McLean JH. Increased β adrenoceptor activation overcomes conditioned olfactory learning deficits induced by serotonin depletion. Brain Res Dev Brain Res. 1997;102:291–293. doi: 10.1016/s0165-3806(97)00090-4. [DOI] [PubMed] [Google Scholar]
- Langton JM, Kim JH, Nicholas J, Richardson R. The effect of the NMDA receptor antagonist MK-801 on the acquisition and extinction of learned fear in the developing rat. Learn Mem. 2007;14:665–668. doi: 10.1101/lm.692407. [DOI] [PubMed] [Google Scholar]
- Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 2008;15:304–314. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Li S, Richardson R. Traces of memory: reacquisition of fear following forgetting is NMDAr-independent. Learn Mem. 2013;20:174–182. doi: 10.1101/lm.029504.112. [DOI] [PubMed] [Google Scholar]
- Li S, Kim JH, Richardson R. Differential involvement of the medial prefrontal cortex in the expression of learned fear across development. Behav Neurosci. 2012a;126:217–225. doi: 10.1037/a0027151. [DOI] [PubMed] [Google Scholar]
- Li S, Kim JH, Richardson R. Updating memories: changing the involvement of the prelimbic cortex in the expression of an infant fear memory. Neuroscience. 2012b;222:316–325. doi: 10.1016/j.neuroscience.2012.06.038. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. In: Moller A, Milinski M, Slater P, editors. Advances in the study of behavior. San Diego: Academic Press; 1998. pp. 215–290. [Google Scholar]
- Lynn DA, Brown GR. The ontogeny of anxiety-like behavior in rats from adolescence to adulthood. Dev Psychobiol. 2010;52:731–739. doi: 10.1002/dev.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Adriani W, Chiarotti F, Laviola G. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Anim Behav. 2002;64:541–546. [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2008;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Introini-Collison IB, Nagahara AH. Memory-enhancing effects of posttraining naloxone: involvement of beta-noradrenergic influences in the amygdaloid complex. Brain Res. 1988;446:37–49. doi: 10.1016/0006-8993(88)91294-2. [DOI] [PubMed] [Google Scholar]
- McNally GP. Facilitation of fear extinction by midbrain periaqueductal gray infusions of rb101 (s), an inhibitor of enkephalin-degrading enzymes. Behav Neurosci. 2005;119:1672–1677. doi: 10.1037/0735-7044.119.6.1672. [DOI] [PubMed] [Google Scholar]
- McNally GP, Cole S. Opioid receptors in the midbrain periaqueductal gray regulate prediction errors during Pavlovian fear conditioning. Behav Neurosci. 2006;120:313–323. doi: 10.1037/0735-7044.120.2.313. [DOI] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behav Neurosci. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Predicting danger: the nature, consequences, and neural mechanisms of predictive fear learning. Learn Mem. 2006;13:245–253. doi: 10.1101/lm.196606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Blocking, unblocking, and overexpectation of fear: a role for opioid receptors in the regulation of Pavlovian association formation. Behav Neurosci. 2004a;118:111–120. doi: 10.1037/0735-7044.118.1.111. [DOI] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of Pavlovian fear conditioning. J Neurosci. 2004b;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Lee B-W, Chiem JY, Choi EA. The midbrain periaqueductal gray and fear extinction: opioid receptor subtype and roles of cyclic AMP, protein kinase A, and mitogen-activated protein kinase. Behav Neurosci. 2005;119:1023–1033. doi: 10.1037/0735-7044.119.4.1023. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He M, Burstein M, Swanson MSA, Avenevoli S, Cui ML, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Study-Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol (Amst) 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders. Am Psychol. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 2004;22:415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers K, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2006;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nesse R. Proximate and evolutionary studies of anxiety, stress and depression: synergy at the interface. Neurosci Biobehav Rev. 1999;23:895–903. doi: 10.1016/s0149-7634(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Newman DL, Moffitt TE, Caspi A, Magdol L, Silva PA, Stanton WR. Psychiatric disorder in a birth cohort of young adults: prevalence, comorbidity, clinical significance, and new case incidence from ages 11–21. J Consult Clin Psychol. 1996;64:552–562. [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Olivier B, Molewijk E, van Oorschot R, van der Heyden J, Ronken E, Mos J. Rat pup ultrasonic vocalization: effects of benzodiazepine receptor ligands. Eur J Pharmacol. 1998;358:117–128. doi: 10.1016/s0014-2999(98)00603-7. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and Prefrontal Projections to the Basal Amygdala Mediate Contextual Regulation of Fear after Extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH, Pate BJ, Moore K, Peuster A. Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behav Neurosci. 1996;110:1205–1228. doi: 10.1037//0735-7044.110.6.1205. [DOI] [PubMed] [Google Scholar]
- Pan B-X, Ito W, Morozov A. Divergence between thalamic and cortical inputs to lateral amygdala during juvenile-adult transition in mice. Biol Psychiatry. 2009;66:964–971. doi: 10.1016/j.biopsych.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. PNAS. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Lee FS, Casey BJ. Fear learning and memory across adolescent development: Hormones and Behavior Special Issue: Puberty and Adolescence. Horm Behav. 2013;64:380–389. doi: 10.1016/j.yhbeh.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus TÅ, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Helfinstein S, Bar Haim Y, Nelson E, Fox N. Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology. 2009;34:213–228. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P, Braun K. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. PNAS. 2003;100:16137–16142. doi: 10.1073/pnas.2434663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CR, Rudy JW. A developmental analysis of contextual fear conditioning. Dev Psychobiol. 1996;29:87–100. doi: 10.1002/(SICI)1098-2302(199603)29:2<87::AID-DEV1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2007;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, Kennedy S, Ingram M, Edwards S, Sweeney L. Prevention and early intervention of anxiety disorders in inhibited preschool children. J Consult Clin Psychol. 2005;73:488–497. doi: 10.1037/0022-006X.73.3.488. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process. 1975;1:88–96. [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and non-reinforcement. In: Prokasy AH, editor. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Croft; 1972. pp. 64–99. [Google Scholar]
- Richardson R, Riccio DC, Jonke T. Alleviation of infantile amnesia in rats by means of a pharmacological contextual state. Dev Psychobiol. 1983;16:511–518. doi: 10.1002/dev.420160607. [DOI] [PubMed] [Google Scholar]
- Richardson R, Riccio DC, Axiotis R. Alleviation of infantile amnesia in rats by internal and external contextual cues. Dev Psychobiol. 1986;19:453–462. doi: 10.1002/dev.420190506. [DOI] [PubMed] [Google Scholar]
- Rodgers R. Animal tests for anxiety. In: Koob GF, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Vol. 1. Oxford: Academic Press; 2010. pp. 90–100. [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler R, Vianna M, Sant’ Anna MK, Kuyven CR, Kruel AVS, Quevedo J, et al. Intrahippocampal infusion of the NMDA receptor antagonist AP5 impairs retention of an inhibitory avoidance task: protection from impairment by pretraining or preexposure to the task apparatus. Neurobiol Learn Mem. 1998;69:87–91. doi: 10.1006/nlme.1997.3810. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neurosci Biobehav Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Endogenous opioids and their role in odor preference acquisition and consolidation following odor–shock conditioning in infant rats. Dev Psychobiol. 2001;39:188–198. doi: 10.1002/dev.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Consolidation and expression of a shock-induced odor preference in rat pups is facilitated by opioids. Physiol Behav. 2003;78:135–142. doi: 10.1016/s0031-9384(02)00961-7. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Elemental and configural associations, the hippocampus and development. Dev Psychobiol. 1991;24:221–236. [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Fanselow MS. Pre-training prevents context fear conditioning deficits produced by hippocampal NMDA receptor blockade. Neurobiol Learn Mem. 2003;80:123–129. doi: 10.1016/s1074-7427(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier D, Cain DP. Spatial learning without NMDA receptor-dependent long-term potentiation. Nature. 1995;378:186–189. doi: 10.1038/378186a0. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Schulenburg CJ, Riccio DC, Stikes ER. Acquisition and retention of a passive-avoidance response as a function of age in rats. J Comp Physiol Psychol. 1971;74:75–83. doi: 10.1037/h0030350. [DOI] [PubMed] [Google Scholar]
- Semple Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Steets CL, DeBrouse LM. Effects of chlordiazepoxide on predator odor-induced reductions of playfulness in juvenile rats. Behav Brain Res. 2010;206:254–262. doi: 10.1016/j.bbr.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Spear NE, Parsons P. Analysis of a reactivation treatment: ontogenic determinants of alleviated forgetting. In: Davis RT, Medin L, Roberts WA, editors. Processes of Animal Memory. Hillsdale, NJ: Lawrence Erlbaum Associates; 1976. pp. 135–165. [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Unique characteristics of neonatal classical conditioning: the role of the amygdala and locus coeruleus. Integr Physiol Behav Sci. 2001;36:293–307. doi: 10.1007/bf02688797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Neurophysiology: good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler KK, Lowry E, Tanaka K, Levy B, Reijmers L, Mayford M, et al. Characterization of NMDAR-independent learning in the hippocampus. Front Behav Neurosci. 2011;5:28. doi: 10.3389/fnbeh.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, Wilson DA. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res. 2008;1200:58–65. doi: 10.1016/j.brainres.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HBM. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry. 1997a;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997b;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Weber M, Richardson R. Centrally administered corticotropin-releasing hormone and peripheral injections of strychnine hydrochloride potentiate the acoustic startle response in preweanling rats. Behav Neurosci. 2001;115:1273–1282. doi: 10.1037//0735-7044.115.6.1273. [DOI] [PubMed] [Google Scholar]
- Weber M, Watts N, Richardson R. High illumination levels potentiate the acoustic startle response in preweanling rats. Behav Neurosci. 2003;117:1458–1462. doi: 10.1037/0735-7044.117.6.1458. [DOI] [PubMed] [Google Scholar]
- Weber M, McNally GP, Richardson R. Opioid receptors regulate retrieval of infant fear memories: effects of naloxone on infantile amnesia. Behav Neurosci. 2006;120:702–709. doi: 10.1037/0735-7044.120.3.702. [DOI] [PubMed] [Google Scholar]
- WHO. 2001. World health report 2001: mental health: new understanding, new hope. World Health Organization. Report no. 9240681701.
- Wiedenmayer CP. Plasticity of defensive behavior and fear in early development. Neurosci Biobehav Rev. 2009;33:432–441. doi: 10.1016/j.neubiorev.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Godsil BP, Peng Z, Saab F, June HL, Van Linn ML, et al. The alpha-1 subunit of the GABA (A) receptor modulates fear learning and plasticity in the lateral amygdala. Front Behav Neurosci. 2009;3:37. doi: 10.3389/neuro.08.037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Wood AN, Levy B. The cellular mechanisms of memory are modified by experience. Learn Mem. 2011;18:747–750. doi: 10.1101/lm.024026.111. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic and catecholaminergic reuptake inhibitors have opposite effects on the ultrasonic isolation calls of rat pups. Neuropsychopharmacology. 1990;3:51–59. [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic modulation of the rat pup ultrasonic isolation call: studies with 5HT1 and 5HT2 subtype-selective agonists and antagonists. Psychopharmacology (Berl) 1991;105:513–520. doi: 10.1007/BF02244372. [DOI] [PubMed] [Google Scholar]
- Young SL, Fanselow MS. Associative regulation of Pavlovian fear conditioning: unconditioned stimulus intensity, incentive shifts, and latent inhibition. J Exp Psychol Anim Behav Process. 1992;18:400–413. doi: 10.1037//0097-7403.18.4.400. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Jr, File SE. Chlordiazepoxide reduces the generalised anxiety, but not the direct responses, of rats exposed to cat odor. Pharmacol Biochem Behav. 1992;43:1195–1200. doi: 10.1016/0091-3057(92)90502-7. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66:578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38:111–123. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]


