Abstract
Similarities between laboratory animals and humans in anatomy and physiology of the cephalic nociceptive pathways have allowed scientists to create successful models that have significantly contributed to our understanding of headache. They have also been instrumental in the development of novel anti-migraine drugs different from classical pain killers. Nevertheless, modelling the mechanisms underlying primary headache disorders like migraine has been challenging due to limitations in testing the postulated hypotheses in humans. Recent developments in imaging techniques have begun to fill this translational gap. The unambiguous demonstration of cortical spreading depolarization (CSD) during migraine aura in patients has reawakened interest in studying CSD in animals as a noxious brain event that can activate the trigeminovascular system. CSD-based models, including transgenics and optogenetics, may more realistically simulate pain generation in migraine, which is thought to originate within the brain. The realization that behavioural correlates of headache and migrainous symptoms like photophobia can be assessed quantitatively in laboratory animals, has created an opportunity to directly study the headache in intact animals without the confounding effects of anaesthetics. Headache and migraine-like episodes induced by administration of glyceryltrinitrate and CGRP to humans and parallel behavioural and biological changes observed in rodents create interesting possibilities for translational research. Not unexpectedly, species differences and model-specific observations have also led to controversies as well as disappointments in clinical trials, which, in return, has helped us improve the models and advance our understanding of headache. Here, we review commonly used headache and migraine models with an emphasis on recent developments.
Linked Articles
This article is part of a themed section on Animal Models in Psychiatry Research. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-20
Introduction
About half of the adult population suffers from headache at least once annually according to the World Health Organization. The majority of these headaches are caused by primary headache disorders and have no serious underlying medical problem. Migraine is one of the most common primary headache disorders, affecting about 1/6 of the population (Headache Classification Committee of the International Headache Society, 2013). It is usually characterized by a unilateral, throbbing headache aggravated by exercise and accompanied by nausea, vomiting, sensitivity to light and sound (Headache Classification Committee of the International Headache Society, 2013). In approximately 20–30% of patients, a transient neurological dysfunction called aura precedes the headache by 20–60 min. With a complex and yet incompletely understood pathophysiology, migraine headache has been subject of extensive clinical and experimental research. Advances in the last decades have led to the development of effective migraine-specific novel drugs with the help of various experimental headache models. Although no model is able to simulate all aspects of this complex paradigm, they have been instrumental in the scientific progress achieved. The aim of this report is to review the currently available headache/migraine models by focusing particularly on recent developments.
Pathophysiology of migraine headache
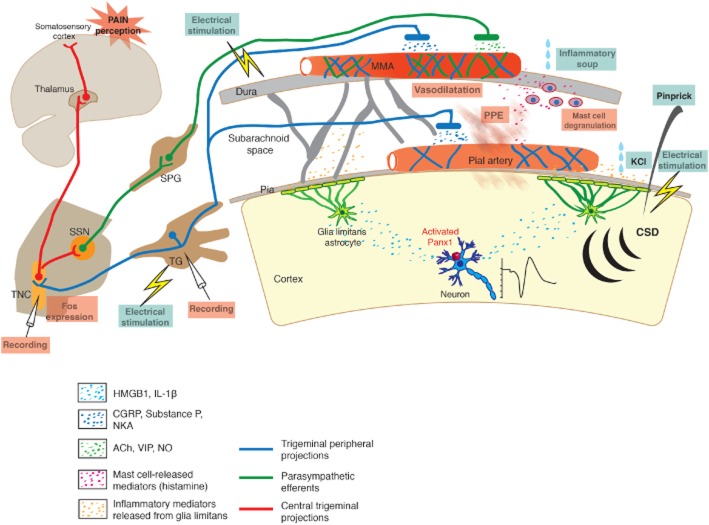
Pain-sensitive intracranial structures, such as dura mater, venous sinuses, meningeal and pial arteries are richly innervated by afferent sensory branches of the trigeminocervical nerves (Penfield, 1932; Ray and Wolff, 1940; Mayberg et al., 1984; Figure 1). This structure composed of blood vessels, trigeminal afferents and their central projections is called the trigeminovascular system (TVS; Andreou et al., 2010; Edvinsson, 2011; Noseda and Burstein, 2013). Cell bodies of trigeminal afferents lie in the trigeminal ganglion (TG). Mainly Aδ and C-type nociceptive fibres are responsible for conduction of pain signals to the brain. Central projections of the trigeminocervical neurons terminate on the second-order nociceptive neurons within the trigeminocervical complex (TCC; Kaube et al., 1993; Burstein et al., 1998; Strassman and Levy, 2006; Andreou et al., 2010). TCC extends from the rostral pons to upper cervical spinal cord levels. Nociceptive neurons in TCC cross to the other side, ascend rostrally and terminate on the third-order neurons in thalamic nuclei, mainly within the ventralposteromedial (also posterior and parafascicular) nucleus (Akerman et al., 2011; Noseda and Burstein, 2013). Thalamic neurons project to the primary somatosensory cortex, insular cortex, limbic structures and hypothalamus for conscious perception of the headache as well as to modify pain-related behaviours and autonomic responses (Noseda and Burstein, 2013; Pietrobon and Moskowitz, 2013).
Figure 1.
Schematic representation of the TVS mediating headache and some of the experimental methods used to activate it. Peripheral processes of the TG neurons terminate over the pial and dural vessels, whereas central processes terminate in TNC. Neurons in TNC project to thalamus and send collaterals to parasympathetic superior salivatory nucleus (SSN), which innervates dural vessels by way of spehenopalatine ganglion (SPG). Activation of TVS lead to release of several peptides and mediators from nerve endings around vessels, which induce dural vasodilatation, PPE and mast cell degranulation. TVS can be activated by electrical stimulation of the dura or TG, dural application of the inflammatory mediators (soup) as well as by CSD (evoked by pinprick, electrical stimulation or KCl). CSD stimulates trigeminal fibres around pial vessels by initiating a parenchymal inflammatory response triggered by the opening of neuronal pannexin-1 (Panx1) channels and release of HMGB1 and IL-1β. Activated nociceptors can be recorded from TG or TNC or detected by Fos immunolabelling in TNC. Dural vessel dilatation, PPE and mast cell degranulation can also be used to monitor TVS activation.
During migraine headache, the TVS becomes sensitized and remains overactive for an extended period of time. Activated perivascular nociceptive afferents release vasoactive mediators (mainly calcitonin gene-related peptide, substance P, neurokinin A and pituitary adenylate cyclase-activating polypeptide), trigger mast cell degranulation and cause sterile neurogenic inflammation in dura mater (Moskowitz, 1984; Markowitz et al., 1987; Levy et al., 2007). Additionally, a mono-synaptic reflex arc between the TCC and superior salivatory nucleus activates the parasympathetic nerve endings around dural blood vessels (which release ACh, NO and vasoactive intestinal polypeptide) and cause vasodilatation (Noseda and Burstein, 2013; Pietrobon and Moskowitz, 2013). The neurogenic inflammation probably sustains the nociceptive activity and sensitizes afferents (Burstein, 2001; Dalkara et al., 2006; Levy, 2012). Sensitization of perivascular afferents to mechanical stimuli is thought to account for the characteristic pulsatile, throbbing migraine headache although a recent work has challenged this view by showing that subjective report of the rhythm of throbbing sensation is distinct from the pulse rate but coincided with oscillations of the EEG α-power (Mo et al., 2013). However, sensitization of higher order central neurons leads to cutaneous allodynia and muscle tenderness, whereas convergence of retinal pathways with nociceptive pathways in the thalamus is believed to underly photophobia (Burstein et al., 2000; Dodick and Silberstein, 2006).
Although it is well established that the TVS mediates the migraine headache, the exact nature of the triggering stimuli responsible for this activation is currently unclear. The migraine generator theory proposes that migraine is a primary disorder caused by episodic activation of the TCC (Weiller et al., 1995; Akerman et al., 2011). Another theory proposes that the TCC activation is secondary to a noxious intracranial event such as cortical spreading depolarization (CSD), the putative cause of aura (Moskowitz et al., 1993; Pietrobon and Moskowitz, 2013; Figure 1). To improve our understanding of migraine pathophysiology and develop better therapeutics, we still need good experimental headache models, despite recent advances in imaging techniques for studying headache and CSD in patients. Striking similarities, despite expected differences, in the anatomy, physiology and pharmacology of headache between rodents, guinea pigs, cats and primates have encouraged the use of these animals to model headache and predict therapeutic potential of anti-migraine agents (Amenta et al., 1980; Edvinsson and Uddman, 1981; Keller et al., 1985; Uddman et al., 1989).
Electrophysiological recordings in TG and TCC
Neuronal activity in the TVS can be directly assessed with electrophysiological recordings from the TG or TCC (trigeminal nucleus caudalis and C1–2; Strassman et al., 1996; Messlinger and Ellrich, 2001; Strassman and Levy, 2006; Zhang et al., 2010; 2011a; Akerman et al., 2013; Noseda and Burstein, 2013; Figure 1). This technique allows detection of the corresponding dural and cutaneous receptive fields for each unit and, real-time monitoring of the nociceptive activity. TCC can be activated by electrical, chemical (e.g. 100 mM KCl, capsaicin, inflammatory molecules) or mechanical (e.g. calibrated von Frey monofilaments) stimulation of the ipsilateral dura, superior sagital sinus or TG and by stimulation of the facial skin (with brush, pressure or pinch). Studies on experimental animals have revealed that the majority of the dural nociceptors mediating headache are polymodal A∂ and C fibres, sharing several common features with peripheral nociceptors (Strassman et al., 1996; Strassman and Levy, 2006). They also provided insight into some features of migraine headache such as its throbbing character and aggravation on coughing or bending by demonstrating that dural nociceptors become rapidly sensitized to mechanical stimuli once activated, whereas sensitization of the second-order neurons in the TCC was shown to mediate cephalic allodynia and muscle tenderness (Burstein et al., 1998; Noseda and Burstein, 2013). Unambiguous identification of the nociceptive neurons in TG or TCC with the help of the above stimulation methods have revealed how they are activated by intrinsic noxious brain events such as CSD, providing strong evidence that CSD, the putative cause of migraine aura could also trigger headache (Zhang et al., 2010; 2011a). These studies also revealed that neuronal activation started with a mean delay of 14 and 25 min after CSD in the majority of the TG and TNC units, respectively, in accordance with the time gap between migraine aura and headache in patients. Moreover, electrophysiological recordings from the TCC combined with anterograde and retrograde tracing methods can be used to study modulation of the CSD-induced dural nociceptor activation by corticotrigeminal descending pathways (Noseda et al., 2010a) and to investigate the characteristics of third-order neurons in the thalamus, which has provided insights into migraine-associated symptoms such as photophobia (Noseda et al., 2010b). Neuronal activity in the ventroposteromedial nucleus in response to TVS stimulation is reportedly modulated by triptans (Shields and Goadsby, 2006) as well as drugs used in migraine prophylaxis such as propranolol (Shields and Goadsby, 2005), topiramate (Andreou and Goadsby, 2011) and valproate (Sokolov et al., 2013).
Increased metabolism in TCC
Increased neuronal activity in the TNC and upper cervical dorsal horn and related nociceptive structures results in increased blood flow. A laser Doppler probe positioned over these structures can demonstrate the activity-dependent blood flow changes, serving as an indirect marker of TCC activation (Goadsby and Zagami, 1991; Goadsby et al., 1997; Goadsby and Classey, 2000; Andreou et al., 2010). Similar results have also been obtained when the metabolic activity was measured by autoradiographic detection of glucose utilization (Goadsby and Zagami, 1991; Goadsby et al., 1997). PET and blood oxygen level-dependent functional MRI have been instrumental in showing the areas activated during induced or spontaneous migraine attacks and have allowed the investigation of migraine-associated phenomena such as allodynia in humans (Burstein et al., 2010; Denuelle et al., 2011; May, 2013).
Increased c-fos expression in TCC
The expression of Fos protein, the product of the c-fos gene is widely used as a marker of TCC activation (Morgan and Curran, 1989). Also, there is increasing evidence showing that phosphorylation of the extracellular signal-regulated kinase can be used as a marker of TG activation (Sixt et al., 2009; Iwashita et al., 2013). Activation of the c-fos gene begins within 5 min following stimulus and continues until the stimulus ceases. Fos protein becomes immunohistochemically detectable in neuronal nuclei approximately 30 min after the stimulus (Morgan and Curran, 1989). It has a half-life of 2 h after the end of stimulus (Svendsen and Lykkegaard, 2001). In addition to Fos immunoreactivity, c-fos mRNA can also be studied (Uhl et al., 1991; Nozaki et al., 1992; Ashmawi et al., 2003; Benjamin et al., 2004). Activation of the TCC by mechanical, chemical or electrical stimulation of the dura, superior sagital sinus or TG as well as by CSD results in immunoreactivity in nociception-related superficial laminae (I and II) of the C1–2 dorsal horns and TNC, especially in the ventrolateral segment of the region receiving inputs from the ophthalmic nerve (Uhl et al., 1991; Nozaki et al., 1992; Kaube et al., 1993; Moskowitz, 1993; Clayton et al., 1997; Mitsikostas et al., 1998; Mitsikostas and Sanchez del Rio, 2001) (Figure 2). Lamina V and other laminae may also be positively labelled for Fos but to a lesser extent. Depending on the stimulus used, the laminae involved may vary and c-fos expression in other brain regions such as periaqueductal grey matter, central nucleus of amygdala and hypothalamic nuclei is observed (Tassorelli and Joseph, 1995; Keay and Bandler, 1998; Mitsikostas and Sanchez del Rio, 2001). c-fos expression in TNC has been shown to be modulated by 5-HT1B/1D/1F/2B, neurokinin-1 (NK1), GABAA, NMDA, AMPA, metabotropic glutamate and μ-opioid receptors (for receptor nomenclature see Alexander et al., 2013a) and to be inhibited by triptans, calcitonin gene-related peptide (CGRP) antagonists and ergot alkaloids (Mitsikostas and Sanchez del Rio, 2001; Sixt et al., 2009).
Figure 2.
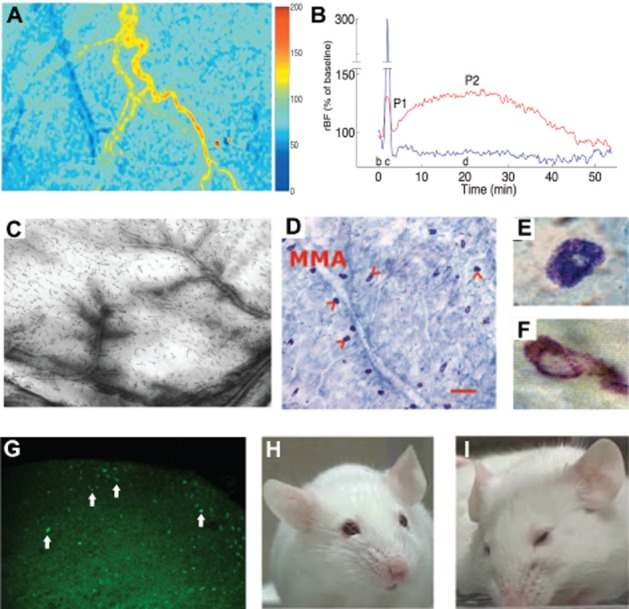
Activation of the TVS can be investigated with various techniques. (A) MMA blood flow: laser speckle images of the cortex, cortical blood vessels and MMA captured in a rat under anaesthesia illustrate the blood flow changes as two-dimensional relative flow maps. Cold colours indicate a decrease, whereas hot colours depict an increase in blood flow compared with baseline as shown in the scale bar on the right. Twenty minutes after induction of a single CSD with a pinprick, the cortex is hypoperfused (blue), whereas blood flow in the MMA is increased (yellow-red). (B) The MMA flow increase (red line) peaks around 20 min after CSD and lasts about 1 h during which the cortical blood (blue line) remains oligemic. (C) Plasma protein extravasation: leakage of i.v. administered HRP in a rat whole-mount dura preparation illustrates plasma protein extravasation from dural vessels after CSD. (D–F) Mast cell degranulation: incubation of dura with methylene blue reveals mast cells (arrowheads) along the course of MMA in a mouse; scale bar = 100 μm (D). Degranulated mast cells (F) can easily be distinguished from resting cells (E) with loss of blue cytoplasmic granules. (G) c-fos expression: Fos immunoreactivity appears in TNC 1 h after KCl-induced CSDs. Majority of the labelled cells (arrows) are located in the superficial layers (laminae I and II). (H, I) Pain-related behaviour: (H) normal mouse facial appearance, (I) facial expression of a mouse suffering from pain. A, B, C were reproduced from Bolay et al., 2002; D, E, F, from Karatas et al., 2013; and H, I; from Langford et al., 2010 with permission.
The number of Fos-positive nuclei within the superficial laminae can be used for quantification. Although it is a technically simple method to study neuronal activation, Fos is a rather difficult marker to evaluate. Its expression may be induced or inhibited by many confounding stimuli such as skin incisions, meningeal irritation, ear bars used to fix the head of the animal and hypotension; therefore, the changes detected may not be always specific to the stimulus used. A good experimental design controlling the confounding variables, adequate anaesthesia and maintenance of physiological conditions are essential. Measures such as topical application of lidocaine to skin incisions may be taken to increase specificity (Akerman et al., 2013). Additionally, to be able to unambiguously detect expression changes in Fos, repeated or intense stimulation may be required (Bullitt, 1989; Lima and Avelino, 1994). When evaluating Fos immunoreactivity, cell counting by at least two investigators blinded to the experimental groups is advisable.
Middle meningeal artery (MMA) dilatation
MMA dilatation can be induced by electrical stimulation of the MMA, dura mater or TG and, also by CSD (Williamson et al., 1997a,b; Bolay et al., 2002; Li et al., 2009; Karatas et al., 2013; Figure 1). The intravital microscopy is generally used to study dural blood vessel diameter changes through a closed cranial window (Williamson et al., 1997a). Although rats are commonly used for this model, guinea pigs and mice have also been used with some modifications (Williamson et al., 2001; Gupta et al., 2006). The skull of the animal is thinned with a micro drill so that dural and pial blood vessels under the parietal bone can be visualized. The window is covered with mineral oil preheated to 37°C. The image is displayed on a monitor and the diameters of the selected blood vessels are measured to determine their response to stimuli. In the past, video dimension analysers were used for this purpose, but today, many image-processing software can perform such actions. Usually, MMA, the largest artery supplying the dura mater is selected for analysis. It can easily be identified in rats with help of its convoluted course (Figure 2). In mice, the artery looks relatively straight and shows variable location, yet is still identifiable under a stereomicroscope as the artery with the largest diameter among the dural arteries, which are more superficially located and thinner compared with the underlying pial arteries.
The MMA dilatation triggered by TVS activation is mediated by parasympathetic nerves and the peptides released from nociceptive afferents. The arterial smooth muscle cells express CGRP receptor, vasoactive intestinal polypeptide (VIP) receptor 1 and 2 (VPAC1 and VPAC2) and pituitary adenylate cyclase-activating polypeptide (PACAP) receptor type 1 (PAC1), which can be stimulated with CGRP and PACAP released from trigeminal nociceptors and by VIP released from parasympathetic neurons (Oliver et al., 2002; Boni et al., 2009; Baun et al., 2011; Syed et al., 2012). These GPCRs are all vasodilatory (Edvinsson et al., 2002; Fizanne et al., 2004; Boni et al., 2009; Syed et al., 2012). The electrical stimulation-induced dilatation of dural blood vessels can be blocked with triptans by inhibiting CGRP release via presynaptic 5-HT1B/D receptors on nociceptive afferents and with CGRP receptor antagonists (Kurosawa et al., 1995; Messlinger et al., 1997; Petersen et al., 2004). PACAP-mediated vasodilatation can be blocked by VPAC1 or PAC1 receptor antagonists in experimental animals (Fizanne et al., 2004; Syed et al., 2012) and hence, targeting these receptors may have a therapeutic potential in migraine similar to CGRP antagonists and triptans. CGRP or PACAP infusion triggers migraine-like episodes in susceptible individuals, which can also be inhibited by triptans (Lassen et al., 2002; Schytz et al., 2009; Hansen et al., 2010). Interestingly, VIP does not cause attacks in migraineurs (Rahmann et al., 2008). Mast cells may play an important role in sustained activation of TVS as they express CGRP and VPAC1 receptors (Levy et al., 2007; Seeliger et al., 2010) and both CGRP and PACAP have been shown to degranulate dural mast cells (Levy et al., 2007; Levy, 2009; Baun et al., 2012). The dural stimulation induces NO release from the arterial endothelium, which also contributes to vasodilatation by facilitating CGRP release from nociceptors (Messlinger et al., 2000).
The time profile of the dural vascular response depends on the nature of the stimulus activating the TVS. Electrical stimulation of MMA or TG causes a rapid dilatation within 30 s that ends a few minutes after stimulation (Shepherd et al., 1997; Williamson et al., 1997a; Li et al., 2009). In contrast, a delayed and sustained dilation in MMA, beginning in 5–10 min and lasting an hour is observed after CSD (Bolay et al., 2002; Karatas et al., 2013). Perhaps this time-limited dilatation of the MMA could account for failure of some MR angiography studies to detect MMA dilatation during nitroglycerin-induced or spontaneous migraine attacks (Schoonman et al., 2008; Amin et al., 2013). Indeed, MMA dilatation was revealed when patients were scanned as soon as the delayed headache attack started about 5.5 h after CGRP infusion (Asghar et al., 2011). Controversies notwithstanding, these studies convincingly showed that MMA dilatation in humans was not always associated with headache (see also previous paragraph) despite the fact that the MMA dilatation, as a surrogate marker of dural neurogenic inflammation, has been instrumental in the development of triptans and CGRP antagonists using experimental models (see the ‘Neurogenic inflammation’ section below for details). This discrepancy is not surprising considering that several other successful drug screening tests (e.g. tests for antidepressant action) show quantitative and mechanistic differences compared with the disease mechanisms.
It should be noted that the diameter of the MMA is sensitive to hypocapnia or hypercapnia and the temperature of the animal; therefore, these physiological parameters should be monitored during the experiment (Petersen et al., 2005; Akerman et al., 2013). Unlike pial arteries, however, the MMA diameter does not significantly change in response to alterations in systemic arterial BP (Petersen et al., 2005). In mice, a methodological modification is necessary as the dura mater is very thin and more sensitive to manipulation compared with the other species. Dural blood vessels maximally dilate at the end of drilling process. Endothelin was used to preconstrict the arteries before activating TVS in the mouse (Gupta et al., 2006). As endothelin could exert confounding effects, a better alternative is to wait overnight after the surgery before starting MMA recordings, allowing time for the arteries to recover (Karatas et al., 2013).
An open cranial window can also be used after removing the skull in rats. This technique allows topical application of various chemicals and direct contact with dura for electrical stimulation; however, it may cause unwanted irritation of trigeminal afferents. Continuous application of artificial CSF at 37°C is necessary to prevent drying of dura and to mimic the physiological environment. The open window is nearly impossible in mice, in our experience, since dural blood vessels are thrombosed soon after the skull is removed.
MMA blood flow
In addition to measurement of the MMA diameter, the blood flow velocity may also be recorded as an indirect but more sensitive measure of the vessel calibre as the small diameter changes cannot be reliably assessed in video images, whereas the flow increases with the 4th power of the diameter. For laser Doppler flowmetry, a Doppler probe is placed over the MMA to record the blood flow changes. Precise positioning of the probe over the MMA is essential as displacements and small probe movements may corrupt the data. The laser speckle flowmetry is another technique introduced for studying dural and cortical blood flow by Dunn and his colleagues (Dunn et al., 2001). This technique allows construction of two-dimensional blood flow maps of an area of interest, and the temporal changes in the blood flow of a selected artery or tissue can be determined compared with baseline. Simultaneous evaluation of dural, pial and tissue blood flow changes is a major advantage. The target area is illuminated with a laser diode and raw speckle images are captured by a CCD camera. A special software then converts these images into speckle contrast and correlation time value images, the latter of which are inversely proportional to the blood flow velocity (Dunn et al., 2001). Laser speckle technique has successfully been used to test the activation of TVS in mice and rats (Bolay et al., 2002; Karatas et al., 2013; Figure 2). The same concerns described above for video imaging of the MMA through a closed cranial window also apply to the laser speckle monitoring.
Neurogenic inflammation
Sterile inflammation in the dura mater, initiated by peptides released from the trigeminal afferents is another measurable feature of the TVS activation. In addition to vasodilatation of dural vessels reviewed above, the neurogenic inflammation is characterized by increased vascular permeability, the resultant plasma protein extravasation (PPE) and mast cell degranulation (Figures 1 and 2). Rodents, including mice and guinea pigs, are suitable models for studying PPE (Markowitz et al., 1987; 1988; Yu et al., 1996). A radioisotope 125I linked with BSA is used to measure PPE (Markowitz et al., 1987), which is regarded as the most sensitive method (Bergerot et al., 2006). Five minutes after [125I]-BSA injection i.v., a stimulus is applied to activate the TVS. Dura is then bilaterally removed and the extravasated [125I]-BSA counts are compared between the stimulated and unstimulated sides. Additionally, detection of dural Evans blue leakage by fluorescence (Johnson and Phebus, 1998) or confocal microscopy (Schuh-Hofer et al., 2003) has been described. Evans blue (a dye binding to albumin) allows real-time monitoring of dural extravasation with intravital microscopy and quantification of the leakage by spectrophotometry at the end of the experiment (Johnson and Phebus, 1998). HRP administered i.v. has also been used to examine dural plasma extravasation ex vivo (Bolay et al., 2002; Figure 2).
The dural vasodilatation model was successfully used to predict the efficacy of antimigraine drugs like 5-HT1B/1D agonist triptans and dihydroergotamine (Kurosawa et al., 1995; Messlinger et al., 1997; Petersen et al., 2004). NK1 receptor antagonists on the other hand failed to inhibit dural vasodilatation and were also found to be clinically ineffective for migraine (Goldstein et al., 1997; Williamson et al., 1997b). Unlike dural vasodilatation, dural PPE is suppressed by clinically ineffective (neurokinin A and substance P inhibitors and endothelin blockers) as well as clinically effective (triptans and CGRP antagonists) agents (Moskowitz, 1992; 1993; O’Shaughnessy and Connor, 1994; Shepheard et al., 1995; May et al., 1996; Goldstein et al., 1997; Williamson et al., 1997c; Yu et al., 1997). These inconsistencies are further aggravated by species differences: for example, 5-HT1D receptor agonists more potently inhibit PPE in rats and guinea pigs (Shepherd et al., 1997), whereas 5-HT1B agonists are more potent in mice (Yu et al., 1996). Although neuromediators and their receptor subtypes involved in neurogenic inflammation vary between species, CGRP is reportedly a common mediator in most mammalian species (Geppetti et al., 2005). The above drawbacks led to concerns that the dural neurogenenic inflammation model could be more akin to meningitis than migraine. However, the rat experimental meningitis consistently causes gadolinium enhancement in meninges in MR, whereas dural neurogenic inflammation induced by TG stimulation does not, suggesting that the intensity of the inflammatory response is considerably different between the two models although some common mechanisms are shared (Hoffmann et al., 2002; Wiesmann et al., 2002).
To simulate neurogenic inflammation, the dura mater can be chemically stimulated by topical application of inflammatory substances. These can be applied through plastic cannulae that are fixed to the cranium without touching the dura (Wei et al., 2011). A few microliters of a mixture called ‘inflammatory soup’ (IS), composed of histamine, 5-HT, bradykinin and PGE2 can be used to activate the TVS (Strassman et al., 1996; Burstein et al., 1998; Figure 1). Capsaicin, IL-6, IL-1β, TNF-α, transient receptor potential channel-A1 (TRPA1) agonists and PGI2 are other inflammatory stimulants used (Mitsikostas et al., 1998; Zhang et al., 2007; 2011b; 2012; Materazzi et al., 2013).
TRPA1 channels (Alexander et al., 2013b) have recently emerged as a novel target based on the findings from experimental migraine models, underscoring the value of the animal models in identification of new pharmacological targets. These excitatory non-selective cation channels are expressed in a population of trigeminal afferent neurons innervating the dura (Edelmayer et al., 2012; Huang et al., 2012). They can be activated by various exogenous and endogenous stimuli such as cold temperatures, volatile irritants (e.g. formaldehyde, chlorine), cigarette smoke, isothiocyanates and PGs (Jordt et al., 2004; Bautista et al., 2005; 2006; McNamara et al., 2007; Andre et al., 2008; Bessac and Jordt, 2008; Materazzi et al., 2008; del Camino et al., 2010). Based on the observations that inhalation of TRPA1-activating irritants can trigger headaches in susceptible migraineurs, TRPA1 channels have been proposed to mediate irritant and odour hypersensitivity in migraine (Ziem and McTamney, 1997; Kelman, 2007; Lima et al., 2011). Indeed, TRPA1 agonists promote CGRP release from trigeminal nerve endings and increase meningeal blood flow (Kunkler et al., 2011; Nassini et al., 2012). TRPA1 activators like mustard oil and umbellulone, when topically applied to the dura in rats, cause cutaneous allodynia and headache-related behaviour such as decreased exploratory rearing activity in novel environments (Edelmayer et al., 2012). Selective TRPA1 antagonists like HC-030031 (McNamara et al., 2007) can inhibit CGRP release, meningeal vasodilatation and headache-related behaviour (Kunkler et al., 2011; Edelmayer et al., 2012).
Inflammatory substances can also be applied in awake animals for behavioural experiments and also for chronic dural stimulation with some modifications (Oshinsky and Gomonchareonsiri, 2007; Edelmayer et al., 2012; Yan et al., 2012; Melo-Carrillo and Lopez-Avila, 2013). For this, an indwelling supradural catheter is used to administer inflammatory mediators to awake and freely moving rats (Wieseler et al., 2012). Topical dural exposure to IS for 5 min results in short-term hyperactivity followed by long-term (approximately 2 h) hyper-responsiveness and increased receptive fields of the central trigeminal neurons (Jakubowski et al., 2007). This is inhibited by non-steroidal anti-inflammatory drugs (NSAIDs) with their direct action on central and peripheral neurons (Jakubowski et al., 2005; 2007; Levy et al., 2008). Unlike COX 1/2 inhibitors, triptans are unable to reverse central sensitization once it is formed in experimental animals. Similarly, triptans are not satisfactorily effective in patients who have already developed allodynia, unlike i.v. NSAIDs (Burstein and Jakubowski, 2004; Levy et al., 2004; Jakubowski et al., 2005).
Inflammatory mediators can alternatively be injected into cisterna magna through a catheter after making an incision extending from occipital protuberance to cervical area in rodents. It is suggested that the animals are held in reverse Trendelenburg position for 2 h following injection for effective distribution of the stimulants within subarachnoid space (Mitsikostas et al., 1998). Inflammatory substances activate meningeal sensory afferents and increase the jugular CGRP level within minutes (Hoffmann et al., 2009). This activation is blocked by either chronic administration of topiramate or acute i.v. triptan injection, simulating prophylactic and acute treatment of migraine.
Mast cell degranulation
Activation of the TVS results in degranulation of mast cells in the ipsilateral dura by way of the peptides released from trigeminal nerve endings (Dimitriadou et al., 1991; Keller et al., 1991; Levy et al., 2007; Figures 1 and 2). Mast cells release histamine and several other inflammatory mediators in their granules and add to the meningeal inflammatory changes, sensitization of afferent fibres and sustained nociceptive activity (Zhang and Levy, 2013b; Messlinger et al., 2012). Dura mater is heavily populated by mast cells in close proximity to meningeal nociceptors around vessels (Dimlich et al., 1991; Rozniecki et al., 1999; Artico and Cavallotti, 2001). Compound 48/80, a mast cell secretagogue agent, results in degranulation of dural mast cells, activation of meningeal nociceptors in electrophysiological recordings and increased c-fos expression in TCC (Shefler and Sagi-Eisenberg, 2001; Levy et al., 2007). Of note, a mast cell independent, direct excitatory action of compound 48/80 on enteric neurons and visceral afferents has also been reported (Schemann et al., 2012). The mast cell degranulation is also used as a TVS activation marker in headache models (Markowitz et al., 1989; Levy et al., 2007). For this, the dura is removed from mice and rats transcardially perfused with formaldehyde, then it is incubated with methylene blue. The degranulated mast cells can easily be detected by the absence of their typical blue, scattered cytoplasmic granules (Markowitz et al., 1989; Dimitriadou et al., 1991; Figure 2).
Neuropeptide changes in blood
Peptides released from activated trigeminal afferents can be measured in blood (Hoffmann et al., 2012). Jugular venous blood is preferred over the peripheral blood to avoid dilution of peptides in extracerebral circulation. Indeed, CGRP was found to increase during migraine attacks in the external jugular blood but did not change in the antecubital blood (Goadsby et al., 1990). Unlike migraine attacks, trigeminal nerve stimulation in humans results in increased substance P levels, in addition to CGRP, in jugular venous blood (Goadsby et al., 1988). CGRP and substance P were also shown to increase in the external jugular blood of experimental animals after trigeminal activation (Goadsby et al., 1988; Goadsby and Edvinsson, 1993). CGRP is rapidly degraded in plasma and has a short half-life of approximately 10 min (Deng and Li, 2005). Accordingly, the blood collected must be immediately centrifuged (Akerman et al., 2013). PACAP has recently been shown to be elevated ictally in patients’ cubital vein blood compared with interictal periods (Tuka et al., 2013). Similarly, in experimental rat models, increased levels of PACAP were demonstrated in blood following electrical TG stimulation or nitroglycerin injection (Tuka et al., 2012). Interestingly, neuropeptide Y, VIP as well as substance P levels in jugular blood were found to be unaltered during migraine attacks (Goadsby et al., 1990).
NO degradation products and pro-inflammatory cytokines have been found to be elevated in the internal jugular venous blood during migraine attacks (Sarchielli et al., 2000; 2004; 2006a,b). The internal jugular blood in humans carries less than 3% blood of extracerebral origin (Hodde and Sercombe, 1996), hence, mainly reflects the changes in brain parenchyma, rather than meninges, which drain into the external jugular vein. Therefore, it remains to be established whether or not these alterations in the internal jugular blood are caused by a parenchymal inflammatory response triggering the migraine headache as recently proposed (Karatas et al., 2013).
Pain-related behaviour
The very end point of nociceptive activation is the pain perception. Therefore, well-established models of pain-related behaviour in animals are badly needed in headache research. Various experimental approaches reported illustrate that both headache and associated migrainous symptoms such as allodynia, light and sound hypersensitivity can be modelled in mice and rats, which have recently been reviewed in detail (Romero-Reyes and Ye, 2013b). Spontaneous pain-related behaviours in rodents can be evaluated by video-taping a freely moving animal, and either still images or video fragments of these records can then be assessed off-line (Bennett, 1993; Akcali et al., 2010; Langford et al., 2010; Romero-Reyes et al., 2013a).
In a mouse model of trigeminal pain, masseter muscle was unilaterally injected with Freund’s adjuvant and facial grooming patterns such as forepaw rubbing and hind paw scratching directed to the injection area were analysed (Romero-Reyes et al., 2013a). Pain-related behaviours can also be elicited in rats with superior sagital sinus stimulation (Zhang et al., 2013a). With some modifications of the technique for use in conscious animals, the researchers detected a stimulation frequency-dependent alteration of grooming and head-flick activities, which were reversed by morphine and rizatriptan (Dong et al., 2011). Another approach called ‘Mouse Grimace Scale’ (MGS) was developed based on changes in facial expression like orbital tightening and nose bulging in response to painful stimuli (Langford et al., 2010) and has been successfully used to demonstrate the CSD-induced headache in wild type as well as CACNA1A transgenic mice that are prone to generate spontaneous CSDs (Langford et al., 2010; Karatas et al., 2013; Figure 2). More recently, a ‘rat grimace scale’ has been developed. This is largely similar with MGS apart from some differences in nose and cheek appearance (Sotocinal et al., 2011). Conditioned place preference in rats, an indicator of the motivated behaviour to seek relief, can also be used to evaluate potential headache therapeutics against various headache triggers such as dural inflammatory mediators (De Felice et al., 2013).
Periorbital cutaneous hypersensitivity and allodynia emerging during neurogenic dural inflammation (e.g. induced by topical application of inflammatory mediators) can be assessed in vivo by measuring head withdrawal latency in response to pressure applied by von Frey filament or to heat (Yamamura et al., 1999; Levy et al., 2007; Oshinsky and Gomonchareonsiri, 2007; Edelmayer et al., 2009; Bates et al., 2010; Maxwell et al., 2010; Elliott et al., 2012). An automatic system has been developed that increases the amount of force applied by the filament until head withdrawal occurs (Cunha et al., 2004). Head withdrawal can be accompanied by grooming behaviour directed to the stimulated area (Vos et al., 1998; Edelmayer et al., 2009). For these cutaneous hypersensitivity tests, the animal may need to be put in a restrainer to access the craniofacial region, creating a stressful situation for the animal. However, allodynia can also be evaluated in freely moving animals (Wieseler et al., 2010; 2012; Fioravanti et al., 2011) as well as in the periphery. Withdrawal latency of the hind paw after heat application or mechanical nociceptive thresholds with von Frey filaments confirmed development of thermal and mechanical allodynia 30–60 min after i.p. glyceryltrinitrate (GTN) application in mice (Bates et al., 2010) or dural application of mustard oil and environmental irritant umbellulone in rats (Edelmayer et al., 2012).
Photophobia can experimentally be studied by light-aversion behaviour in a chamber with two compartments, one is dark and closed and the other is light and open (Crawley and Goodwin, 1980). GTN, injected i.p., has been shown to trigger light-aversive behaviour in mice, 0–30 and 90–120 min after injection (Markovics et al., 2012). An interesting study in cats showed that superior sagittal sinus stimulation resulted in activation of cells in nucleus tractus solitarius, which is involved in regulating vomiting, and this connection is interrupted with triptans by their action on 5-HT1B/1D receptors (Hoskin et al., 2004).
CSD-mediated trigeminal activation
It is generally agreed that aura symptoms preceding headache by 20–60 min in migraineurs are mediated by CSD, a wave of neuronal and glial depolarization that spreads along the cortex at a speed about 2–6 mm·min−1 followed by depression of neuronal activity (Somjen, 2001). Although CSD is used as a model of migraine aura, recent experimental data suggest that CSD can also activate TVS (Bolay et al., 2002; Zhang et al., 2010; 2011a; Karatas et al., 2013). It has been shown that CSD leads to a delayed and sustained increase in MMA blood flow along with plasma protein extravasation in dura (Bolay et al., 2002). MMA dilatation and PPE were found to be mediated by trigeminal and parasympathetic nerves supplying the dura, and they were inhibited when CSDs were suppressed by an NMDA antagonist MK-801. It would be interesting to see whether or not a noxious brain event like CSD could more realistically model TVS activation than direct stimulation of the dura and TG (Dalkara et al., 2006), especially in transgenic or optogenetically engineered mice, in whom CSDs spontaneously emerge or could be evoked rather non-invasively by blue light respectively (Eikermann-Haerter et al., 2009; Charles and Baca, 2013)
CSD can be triggered in mammalian brains by electrical, mechanical or chemical stimuli. Agyrencephalic brains as in rodents are more susceptible to CSD generation compared with primate brains with sulci and gyri. Whatever the triggering stimulus is, the main requirement for CSD is collective depolarization of a critical mass of cortical tissue (Matsuura and Bures, 1971). Then, a depolarization wave starts to spread to the neighbouring areas. A basic mechanical method to trigger CSD is to swiftly prick the cortex by a needle, briefly known as the ‘pinprick’ method. Being relatively easy and highly reproducible, this technique also has the advantage of studying the effect of single CSDs that can be triggered at the exact time of interest. However, it is not suitable for triggering repeated CSDs, and it does not allow determination of a CSD threshold. Electrical stimulation of the cortex is another method for CSD induction; a series of square pulses is applied at varying current intensities to determine a threshold current value (Ayata et al., 2006; Ayata, 2013). Topical application over the dura or into the cortex of concentrated KCl (usually 0.5–1 M) is frequently used for initiating repeated CSDs. A threshold concentration can easily be detected (Reuter et al., 1998; Ayata et al., 2006). Other chemical stimuli that can be used to trigger CSD include glutamate, NMDA, sodium channel activators like aconitine and Na+/K+ ATPase inhibitor ouabain (Balestrino et al., 1999; Ayata, 2013). These chemicals can be applied topically over the dura or intraparenchymally by pressure injection or microdialysis (Obrenovitch et al., 2009).
CSD may be recorded by intracortical microelectrodes or by extracellular electrodes placed over the dura or cranium. A characteristic-negative slow potential shift can easily be recognized, and amplitude and duration of the wave can be measured. A simultaneous electrocorticogram recording can show suppression of the EEG activity for 15–30 min. When two or more electrodes are used, propagation speed of the depolarization wave can also be calculated. Although the electrophysiological recording is the standard method to detect CSDs, one can also monitor CSD-associated cortical blood flow changes by laser Doppler or laser speckle imaging techniques. In rats, cats and primates, a biphasic blood flow response consisting of an initial hyperaemia (corresponding to the depolarization phase) followed by a long-lasting oligaemia is recorded (Lauritzen et al., 1982; Wahl et al., 1987; Lacombe et al., 1992; Yokota et al., 2002). In mice, a triphasic blood flow response characterized by a brief hypoperfusion preceding the hyperperfusion and long-lasting hypoperfusion, is observed (Ayata et al., 2004; Brennan et al., 2007). An alternative method to detect CSD is to monitor the intrinsic optical signals in the brain tissue as light absorption characteristics of the tissue change due to shifts in haemoglobin concentration and water content (Ba et al., 2002; Brennan et al., 2007). A method combining voltage-sensitive dyes with the laser speckle technique was also proposed to study the relationship between depolarization and associated blood flow changes (Obrenovitch et al., 2009).
Monitoring physiological parameters of the animal is essential as the arterial hypotension and hypoxia can modify characteristics of CSD and accompanying blood flow changes (Sukhotinsky et al., 2008). CSD susceptibility may also vary depending on the body temperature, blood pH and pCO2 (Ayata, 2013; Hoffmann et al., 2013). The choice of anaesthetic is also critical, not only because anaesthesia may induce systemic hypotension but also because of the direct inhibitory effects of anaesthetics on cortical excitability. Urethane and α-chloralose have been found to have a much lower potential for suppressing CSD susceptibility when compared with ketamine, halothane, isoflurane and N2O (Saito et al., 1995; Kudo et al., 2008). Propofol also suppresses CSD (Sonn and Mayevsky, 2006; Dhir et al., 2012).
Evoking CSD in awake rodents induces anxiety, pain-related behaviour and allodynia (Akcali et al., 2010; Fioravanti et al., 2011; Karatas et al., 2013); however, it also poses some technical difficulties. A plastic tubing can be placed and fixed over a burr hole covering it without piercing the dura. Stimulating chemicals can be applied through the tubing by a microinjector (Akcali et al., 2010). In a recent study on freely moving mice, CSDs were triggered by a dry KCl pellet placed over the dura through a burr hole (Karatas et al., 2013).
CSD can also be employed to study the mechanisms of action of migraine prophylaxis and for development of prophylactic drugs. Indeed, more than 1 month (but not acute) administration of topiramate, valproate, propranolol, amitriptyline and methysergide have all been found to dose- dependently suppress CSD frequency and increase CSD induction threshold in parallel with the time required for effective prophylaxis in their clinical use (Ayata et al., 2006). This delayed action, which is also typical for several other CNS drugs like antidepressants, suggests drug-induced changes in gene expression and synaptic reorganization, reducing the susceptibility to CSD. However, these interesting possibilities still await clarification. Spreading depolarizations can also be triggered in the hippocampus in vivo by pressure microinjections of KCl, which also induce c-fos expression in TNC (Kunkler and Kraig, 2003). Hippocampal spreading depolarizations can bring insight to the mechanisms of memory impairment observed during migraine attacks. Moreover, hippocampal slices have been instrumental in studying neural mechanisms triggering spreading depolarization and to identify agents that can suppress susceptibility to spreading depolarization such as insulin-like growth factor-1 (Somjen, 2001; Grinberg et al., 2012).
Recently, CSD induced by pinprick or epidural KCl has been shown to open neuronal pannexin-1 channels and release high mobility group protein-B1 (HMGB1) and IL-1β, which then activate the NF-κB pathway in astrocytes (Karatas et al., 2013). NF-κB, by inducing expression of the inflammatory enzymes, triggered release of inflammatory mediators into subarachnoid space via glia limitans, and hence, activated the perivascular trigeminal afferents. In this model, TVS activation was monitored by MMA dilatation, dural mast cell degranulation and headache-related behaviours in mice. Trigeminal activation was blocked by knocking down or inhibiting pannexin-1 channels and HMGB1 as well as by NF-κB activation inhibitors and naproxen. These findings suggest that pannexin-1 channels may serve to detect neuronal stress and alarm the organism with headache by activating the parenchymal inflammatory pathways. Unlike transient release of potassium, NO and other vasoactive mediators to extracellular space during CSD, the above summarized inflammatory response better explains the delayed appearance of headache 20–60 min after aura (CSD). Activation of an inflammatory mechanism after CSD is also supported by PET findings in rats with 11C-PK11195, a ligand for peripheral-type benzodiazepine receptors showing microglial activation (Cui et al., 2009).
Mutant mouse models
It is now accepted that the susceptibility to migraine and CSD is, at least in part, genetically determined (van den Maagdenberg et al., 2007). Mutations affecting ion channels or transporters may modify the balance between the excitatory and inhibitory activity in cortex, making it more susceptible to spontaneous or induced CSD generation (Pietrobon and Moskowitz, 2013). Patients with familial hemiplegic migraine (FHM) and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) bear mutations leading to recurrent transient neurological symptoms and headache (Thomsen et al., 2002; Chabriat et al., 2009). The mutations identified in those patients have created an opportunity for building genetic models of CSD and migraine. However, it should be noted that mice carrying FHM mutations may also exhibit features more reminiscent of atypical rather than common forms of migraine (Schytz et al., 2010).
Vascular NOTCH3 knock-out or the NOTCH3 R90C knock-in mice have been developed for modelling CADASIL (Krebs et al., 2003; Ruchoux et al., 2003). Electrical and KCl stimulation showed decreased CSD thresholds in these mice, more prominently in knock-outs (Eikermann-Haerter et al., 2011). NOTCH3-associated vasculopathy may lead to brief ischaemic episodes in the brain and induce CSDs. However, the exact mechanism underlying the increased CSD susceptibility in CADASIL is unknown, and an impaired neurovascular coupling leading to disturbed energy homeostasis in cortex is also possible (Eikermann-Haerter et al., 2011).
Mouse knock-in FHM1 models bearing two different mutations (R192Q and S218L) in CACNA1A gene encoding the P/Q-type voltage-gated Ca2+ channel have been developed (van den Maagdenberg et al., 2004; 2010). Mutations in the presynaptic voltage-gated Ca channels result in their opening at more negative potentials, hence, in enhanced glutamate release (van den Maagdenberg et al., 2004; 2010; Tottene et al., 2009). In these mice, CSD threshold is decreased and propagation velocity is increased (van den Maagdenberg et al., 2004; 2010; Tottene et al., 2009). CSDs may lead to transient neurological deficits. The CSD susceptibility in FHM1 models is dependent on the allele dosage and type of mutation. Homozygous R192Q mice have no major phenotypic abnormalities; however, homozygous S218L mice exhibit persistent ataxia, seizures and spontaneous hemiparesis attacks, in line with the more severe clinical phenotype in S218L FHM1 (Kors et al., 2001; van den Maagdenberg et al., 2004; 2010). Female knock-in mice are more susceptible to CSD as seen in migraine (Eikermann-Haerter et al., 2009). FHM1 mutations, besides promoting CSD formation, also increase the tendency for inflammation in the TVS (Franceschini et al., 2013). Trigeminal ganglia from FHM1 mice display an enhanced neuroinflammatory reaction on exposure to lipopolysaccharide compared with wild-type mice. Behavioural studies also demonstrated that FHM1 mutations exibited behaviours that could be attributed to photophobia and spontaneous unilateral headache in mice (Chanda et al., 2013).
ATP1A2, associated with FHM2, is responsible for maintaining the resting membrane potential and, is found to be essential for clearance of released potassium and glutamate from synaptic clefts by astrocyte endfeet (Ikeda et al., 2003). Homozygous T2763C mutations are lethal in neonatal stage but heterozygous knock-in mutants are available. These mice have a lower CSD threshold and higher CSD velocity (Pietrobon, 2007; Leo et al., 2011). Haploinsufficiency of ATP1A2 leads to increased extracellular potassium and glutamate, promoting depolarization and therefore making the cortex more susceptible to CSD (Montagna, 2004; Pietrobon, 2007; Gritz and Radcliffe, 2013). These mice also show increased fear and anxiety behaviour (Gritz and Radcliffe, 2013).
Based on identification of a missense mutation in the casein kinase Iδ gene in two families with migraine, a mouse model carrying the T44A mutation has also been investigated. The T44A mutation causes reduced enzyme activity and results in lower CSD threshold, enhanced vascular response to CSD and a hyperalgesic phenotype after GTN administration (Brennan et al., 2013).
GTN infusion
An i.v. infusion of GTN, a NO donor, was shown to trigger headache attacks with a 4–6 h delay in migraine patients (Olesen et al., 1994; Iversen, 2001). These attacks can be aborted by triptans (Juhasz et al., 2005) and can be prevented by prophylactic use of propranolol and valproate (Tvedskov et al., 2004a,b). In rodent models, GTN can be applied either by i.v. infusion, s.c., i.p. or topically over the dura. Alternatively, infusion of sodium nitroprusside, another NO donor, can also be used (Galeotti and Ghelardini, 2013). GTN-induced changes vary with dose (e.g. Fos expression in TNC), and high doses lead to hypotension especially in anaesthetized animals (Tassorelli and Joseph, 1995; Ramachandran et al., 2012). It should be noted that GTN may be absorbed onto plastic or glass surfaces, causing the concentration to be lower than expected in injection solutions (Bergerot et al., 2006).
In contrast to the delayed onset of migraine-like headache after GTN, GTN induces dural and pial vasodilatation within minutes, making vasodilatation an unlikely mechanism for the attack (Akerman et al., 2002a; Strecker et al., 2002; Gozalov et al., 2007). However, GTN application also induces inflammatory changes in dura and mast cell degranulation (Reuter et al., 2001; Kim et al., 2008; Galeotti and Ghelardini, 2013). In cats (Lambert et al., 2000) and rodents, GTN infusion results in a delayed increase in mechanosensitivity of meningeal nociceptors (Zhang et al., 2013b) and in firing rate of individual TNC neurons (Koulchitsky et al., 2009). GTN application in rodents increases c-fos expression in TNC, periaqueductal grey matter, central nucleus of amygdala and hypothalamic nuclei (Tassorelli and Joseph, 1995; Greco et al., 2005). GTN also elicits pain-related behaviours in animal models. An i.p. application of 10 mg·kg−1 GTN in rats triggers hyperalgesic behaviour within 4 h as demonstrated by formalin and tail-flick tests (Greco et al., 2008). GTN also dose-dependently causes development of mechanical and thermal hind paw allodynia in mice, starting 30–60 min after its i.p. administration (Bates et al., 2010). Light-aversive behaviour in mice associated with an increase in meningeal blood flow was also observed with GTN and this response is thought to be mediated by PACAP (Markovics et al., 2012).
In human studies, GTN is usually infused for approximately 20–30 min at a dose of 0.125–0.5 μg·kg−1 min−1 (Thomsen et al., 1994; Christiansen et al., 2000; Bednarczyk et al., 2002; Tvedskov et al., 2004b). Equivalent doses of drugs are 12 times that of humans in mice and six times in rats, based on body-surface area (US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, 2005). Therefore, an approximate dose of 0.75–6 μg·kg−1·min−1 can be considered the human equivalent in rodent models. However, most of the studies in rats have employed i.p. application doses such as 10 mg·kg−1, which is a few orders higher compared with doses used in humans (Reuter et al., 2001; Gozalov et al., 2007; Varga et al., 2007; Greco et al., 2008; Markovics et al., 2012; Olesen and Jansen-Olesen, 2012). Such high doses can induce systemic hypotension especially in anaesthetized rats (Needleman, 1970), which can independently induce c-fos expression (Tassorelli and Joseph, 1995). Therefore, GTN dose should be carefully controlled in experimental models. A low-dose GTN model (4 μg·kg−1·min−1 infusion for 20 min) in awake animals was introduced to overcome the hypotensive effect, and an up-regulation of c-fos expression in TNC was still observed in the delayed phase (Ramachandran et al., 2012).
CGRP administration
Given the important role played by CGRP in migraine pathophysiology and effective treatment of migraine attacks with CGRP antagonists, exogenous application of CGRP has also been used to trigger migraine attacks (Williamson et al., 1997a; Cumberbatch et al., 1999; Akerman et al., 2002b; Gupta et al., 2010). CGRP, i.v., induces a delayed migraine-like episode in susceptible migraine patients and headache in non-migraineurs (Lassen et al., 2002). CGRP causes MMA dilatation in the rat accompanied by central sensitization of the TVS (Williamson et al., 1997a; Cumberbatch et al., 1999). 5-HT1B/1D agonists abolish this sensitization (Williamson et al., 1997a; Cumberbatch et al., 1999). Recordings from the meningeal nociceptors in TG showed that CGRP did not directly modify the activity or sensitivity of peripheral nociceptors (Levy et al., 2005). Instead, it promoted mast cell degranulation (Levy et al., 2005; Levy, 2009). However, CGRP has also central actions; i.c.v. administered CGRP triggers light-aversive behaviour in mice, which can be reduced by rizatriptan (Kaiser et al., 2012). This response is more prominent in transgenic mice sensitized to CGRP action by overexpressing RAMP1 protein, a regulatory subunit of the CGRP receptor involved in its trafficking and CGRP binding (Recober et al., 2010).
Chronic migraine models
Migraine becomes a chronic disorder in 3% of patients (Bigal and Lipton, 2008). Therefore, models involving chronic stimulation of trigeminal system have been developed to simulate the chronic nature of the pain. In a rodent model, repeated episodes of IS were applied to dura in awake rats for 4 weeks (Oshinsky and Gomonchareonsiri, 2007). This application increased the trigeminal sensitivity up to 3 weeks as tested by periorbital von Frey ligament thresholds (Burstein et al., 1998). In another study, dural IS was applied for 7 subsequent days and behavioural changes of animals were tested before and after. IS resulted in decreased exploratory and increased nociceptive behaviour and these behavioural changes were inhibited by NSAIDs as well as zolmitriptan (Melo-Carrillo and Lopez-Avila, 2013). However, chronic intermittent administration of GTN results in a progressive and sustained basal hyperalgesia along with acute hyperalgesia with each GTN dose (Pradhan et al., 2013). Acute hyperalgesia in this model is reversed by triptans while both acute and chronic hyperalgesia is diminished by prophylactic administration of topiramate. The medication overuse is a major risk factor for transformation from episodic to chronic migraine (Diener and Limmroth, 2004; D’Amico et al., 2005; Headache Classification et al., 2006). Several animal models of medication overuse headache have been developed. Continuous infusion or repeated administration of sumatriptan or naratriptan to rats elicits time-dependent sustained allodynia that can be reversed by CGRP antagonists or neuronal NOS inhibitors (De Felice et al., 2010a,b). These rats also show increased CGRP release to the circulation following administration of the NO donor, sodium nitroprusside (De Felice et al., 2010b) and an increased expression of NOS in trigeminal dural afferents (De Felice et al., 2010a), which may underlie the triptan-induced latent sensitization.
Conclusion
Headache models, like the models in other fields of science are merely an approximation of the reality based on the limited knowledge available. Accordingly, while they may provide significant insights into one aspect of headache, they may disappoint in other directions. Unlike cerebral ischaemia, for instance, in which the initial event starting the pathophysiological cascade is well known and common to other species, modelling becomes more complicated for disorders of unknown aetiology such as migraine. However, the limitations of the present animal models should not be considered discouraging as we always learn from our failures, and thus, can improve our models for deeper insight and better predictability.
At the present stage, models used for investigating the nociceptive mechanisms mediating the headache, which are in general similar to pain mechanisms in the periphery are thought to be satisfactory to a large extent. Nevertheless, the intrinsic brain events that trigger migraine headache are still an enigma. Despite much debate on the initiating mechanisms, however, there is agreement that they start within the brain. Therefore, current efforts should focus on models that activate the TVS by way of intrinsic brain events like CSD. Although CSD is the biological substrate of migraine with aura, as a model, it may also provide clues as to why and how TVS can be activated by episodic disorders that disturb the brain homeostasis in headache patients in general. Induction of CSD by non-invasive methods like optogenetics in awake animals holds promise in this regard. Optogenetics could also be instrumental for non-invasive stimulation of CNS cells and pathways as well as specific receptors selectively in brain areas of interest, providing unprecedented advantage over the conventional methods (Airan et al., 2009; Daou et al., 2013). Neurogenic inflammation induced by intrinsic brain events might more realistically simulate the meningeal events in migraine compared with the direct stimulation of meninges with chemical or electrical methods. Progress in monitoring pain-related behaviours in unanaesthetized animals along with human models and imaging studies (Wager et al., 2013) may also bring about exciting opportunities to investigate headache mechanisms and screen pharmacological agents.
Several technical aspects of the present models also need improvements. For example, paying attention to the use of human-equivalent doses and detection of the drug concentrations achieved at the site of action should be mandatory in every model used, as the observed changes, activated systems and receptors significantly vary with dose. Biodistribution and pharmocokinetics of the agents used should also be considered as effects in non-targeted tissues such as changes in BP and hormones may substantially modify CNS responses. Increasing collaboration between laboratories experienced on different techniques may allow improvements in the current models by bringing complementary expertise together. Proteomic and genomic studies may reveal species differences with regard to mediators involved in models and help predicting discrepancies.
Finally, improvements in the present models in parallel with the new discoveries in humans may increase the much desired translational potential of headache models. Ever expanding use of genetically engineered animals and innovative methods may lead to the creation of unprecedented novel models. In summary, the next decade holds great promise for the successful use of animal models in concert with human studies to understand headache and migraine, and develop new anti-migraine therapies
Acknowledgments
T. D.’s work is supported by the Turkish Academy of Sciences.
Glossary
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- CGRP
calcitonin gene-related peptide
- CSD
cortical spreading depolarization
- FHM
familial hemiplegic migraine
- GTN
glyceryltrinitrate
- HMGB1
high mobility group protein-B1
- IS
inflammatory soup
- MGS
Mouse Grimace Scale
- MMA
middle meningeal artery
- NK1
neurokinin-1
- NSAID
non-steroidal anti-inflammatory drug
- PAC1
PACAP receptor type 1
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PPE
plasma protein extravasation
- TCC
trigeminocervical complex
- TG
trigeminal ganglion
- TNC
trigeminal nucleus caudalis
- TRPA1
transient receptor potential channel-A1
- TVS
trigeminovascular system
- VIP
vasoactive intestinal polypeptide
- VPAC
VIP receptor
Conflict of interest
The authors declare no conflict of interest.
References
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Akcali D, Sayin A, Sara Y, Bolay H. Does single cortical spreading depression elicit pain behaviour in freely moving rats? Cephalalgia. 2010;30:1195–1206. doi: 10.1177/0333102409360828. [DOI] [PubMed] [Google Scholar]
- Akerman S, Williamson DJ, Kaube H, Goadsby PJ. The effect of anti-migraine compounds on nitric oxide-induced dilation of dural meningeal vessels. Eur J Pharmacol. 2002a;452:223–228. doi: 10.1016/s0014-2999(02)02307-5. [DOI] [PubMed] [Google Scholar]
- Akerman S, Williamson DJ, Kaube H, Goadsby PJ. Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br J Pharmacol. 2002b;137:62–68. doi: 10.1038/sj.bjp.0704842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011;12:570–584. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- Akerman S, Holland PR, Hoffmann J. Pearls and pitfalls in experimental in vivo models of migraine: dural trigeminovascular nociception. Cephalalgia. 2013;33:577–592. doi: 10.1177/0333102412472071. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14:G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta F, Sancesario G, Ferrante F, Cavallotti C. Acetylcholinesterase-containing nerve fibers in the dura mater of guinea pig, mouse, and rat. J Neural Transm. 1980;47:237–242. doi: 10.1007/BF01250604. [DOI] [PubMed] [Google Scholar]
- Amin FM, Asghar MS, Hougaard A, Hansen AE, Larsen VA, de Koning PJ, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 2013;12:454–461. doi: 10.1016/S1474-4422(13)70067-X. [DOI] [PubMed] [Google Scholar]
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou AP, Goadsby PJ. Topiramate in the treatment of migraine: a kainate (glutamate) receptor antagonist within the trigeminothalamic pathway. Cephalalgia. 2011;31:1343–1358. doi: 10.1177/0333102411418259. [DOI] [PubMed] [Google Scholar]
- Andreou AP, Summ O, Charbit AR, Romero-Reyes M, Goadsby PJ. Animal models of headache: from bedside to bench and back to bedside. Expert Rev Neurother. 2010;10:389–411. doi: 10.1586/ern.10.16. [DOI] [PubMed] [Google Scholar]
- Artico M, Cavallotti C. Catecholaminergic and acetylcholine esterase containing nerves of cranial and spinal dura mater in humans and rodents. Microsc Res Tech. 2001;53:212–220. doi: 10.1002/jemt.1085. [DOI] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, et al. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–645. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Ashmawi HA, Chambergo FS, Araujo Palmeira CC, de Paula Posso I. The effects of pyrilamine and cimetidine on mRNA C-fos expression and nociceptive flinching behavior in rats. Anesth Analg. 2003;97:541–546. doi: 10.1213/01.ANE.0000068883.63751.F8. [DOI] [PubMed] [Google Scholar]
- Ayata C. Pearls and pitfalls in experimental models of spreading depression. Cephalalgia. 2013;33:604–613. doi: 10.1177/0333102412470216. [DOI] [PubMed] [Google Scholar]
- Ayata C, Shin HK, Salomone S, Ozdemir-Gursoy Y, Boas DA, Dunn AK, et al. Pronounced hypoperfusion during spreading depression in mouse cortex. J Cereb Blood Flow Metab. 2004;24:1172–1182. doi: 10.1097/01.WCB.0000137057.92786.F3. [DOI] [PubMed] [Google Scholar]
- Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- Ba AM, Guiou M, Pouratian N, Muthialu A, Rex DE, Cannestra AF, et al. Multiwavelength optical intrinsic signal imaging of cortical spreading depression. J Neurophysiol. 2002;88:2726–2735. doi: 10.1152/jn.00729.2001. [DOI] [PubMed] [Google Scholar]
- Balestrino M, Young J, Aitken P. Block of (Na+,K+)ATPase with ouabain induces spreading depression-like depolarization in hippocampal slices. Brain Res. 1999;838:37–44. doi: 10.1016/s0006-8993(99)01674-1. [DOI] [PubMed] [Google Scholar]
- Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, et al. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2010;30:170–178. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baun M, Hay-Schmidt A, Edvinsson L, Olesen J, Jansen-Olesen I. Pharmacological characterization and expression of VIP and PACAP receptors in isolated cranial arteries of the rat. Eur J Pharmacol. 2011;670:186–194. doi: 10.1016/j.ejphar.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Baun M, Pedersen MH, Olesen J, Jansen-Olesen I. Dural mast cell degranulation is a putative mechanism for headache induced by PACAP-38. Cephalalgia. 2012;32:337–345. doi: 10.1177/0333102412439354. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bednarczyk EM, Wack DS, Kassab MY, Burch K, Trinidad K, Haka M, et al. Brain blood flow in the nitroglycerin (GTN) model of migraine: measurement using positron emission tomography and transcranial Doppler. Cephalalgia. 2002;22:749–757. doi: 10.1046/j.1468-2982.2002.00440.x. [DOI] [PubMed] [Google Scholar]
- Benjamin L, Levy MJ, Lasalandra MP, Knight YE, Akerman S, Classey JD, et al. Hypothalamic activation after stimulation of the superior sagittal sinus in the cat: a Fos study. Neurobiol Dis. 2004;16:500–505. doi: 10.1016/j.nbd.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Bennett GJ. An animal model of neuropathic pain: a review. Muscle Nerve. 1993;16:1040–1048. doi: 10.1002/mus.880161007. [DOI] [PubMed] [Google Scholar]
- Bergerot A, Holland PR, Akerman S, Bartsch T, Ahn AH, MaassenVanDenBrink A, et al. Animal models of migraine: looking at the component parts of a complex disorder. Eur J Neurosci. 2006;24:1517–1534. doi: 10.1111/j.1460-9568.2006.05036.x. [DOI] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology. 2008;23:360–370. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. The prognosis of migraine. Curr Opin Neurol. 2008;21:301–308. doi: 10.1097/WCO.0b013e328300c6f5. [DOI] [PubMed] [Google Scholar]
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- Boni LJ, Ploug KB, Olesen J, Jansen-Olesen I, Gupta S. The in vivo effect of VIP, PACAP-38 and PACAP-27 and mRNA expression of their receptors in rat middle meningeal artery. Cephalalgia. 2009;29:837–847. doi: 10.1111/j.1468-2982.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- Brennan KC, Beltran-Parrazal L, Lopez-Valdes HE, Theriot J, Toga AW, Charles AC. Distinct vascular conduction with cortical spreading depression. J Neurophysiol. 2007;97:4143–4151. doi: 10.1152/jn.00028.2007. [DOI] [PubMed] [Google Scholar]
- Brennan KC, Bates EA, Shapiro RE, Zyuzin J, Hallows WC, Huang Y, et al. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5:183ra156. doi: 10.1126/scitranslmed.3005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E. Induction of c-fos-like protein within the lumbar spinal cord and thalamus of the rat following peripheral stimulation. Brain Res. 1989;493:391–397. doi: 10.1016/0006-8993(89)91177-3. [DOI] [PubMed] [Google Scholar]
- Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–110. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(Pt 8):1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68:81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, et al. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabriat H, Joutel A, Dichgans M. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- Chanda ML, Tuttle AH, Baran I, Atlin C, Guindi D, Hathaway G, et al. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain. 2013;154:1254–1262. doi: 10.1016/j.pain.2013.03.038. [DOI] [PubMed] [Google Scholar]
- Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. 2013;9:637–644. doi: 10.1038/nrneurol.2013.192. [DOI] [PubMed] [Google Scholar]
- Christiansen I, Daugaard D, Lykke Thomsen L, Olesen J. Glyceryl trinitrate induced headache in migraineurs – relation to attack frequency. Eur J Neurol. 2000;7:405–411. doi: 10.1046/j.1468-1331.2000.00094.x. [DOI] [PubMed] [Google Scholar]
- Clayton JS, Gaskin PJ, Beattie DT. Attenuation of Fos-like immunoreactivity in the trigeminal nucleus caudalis following trigeminovascular activation in the anaesthetised guinea-pig. Brain Res. 1997;775:74–80. doi: 10.1016/s0006-8993(97)00930-x. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Cui Y, Takashima T, Takashima-Hirano M, Wada Y, Shukuri M, Tamura Y, et al. 11C-PK11195 PET for the in vivo evaluation of neuroinflammation in the rat brain after cortical spreading depression. J Nucl Med. 2009;50:1904–1911. doi: 10.2967/jnumed.109.066498. [DOI] [PubMed] [Google Scholar]
- Cumberbatch MJ, Williamson DJ, Mason GS, Hill RG, Hargreaves RJ. Dural vasodilation causes a sensitization of rat caudal trigeminal neurones in vivo that is blocked by a 5-HT1B/1D agonist. Br J Pharmacol. 1999;126:1478–1486. doi: 10.1038/sj.bjp.0702444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Vivancos GG, Moreira IF, Reis S, Parada CA, et al. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 2004;37:401–407. doi: 10.1590/s0100-879x2004000300018. [DOI] [PubMed] [Google Scholar]
- Dalkara T, Zervas NT, Moskowitz MA. From spreading depression to the trigeminovascular system. Neurol Sci. 2006;27(Suppl. 2):S86–S90. doi: 10.1007/s10072-006-0577-z. [DOI] [PubMed] [Google Scholar]
- Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, et al. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J Neurosci. 2013;33:18631–18640. doi: 10.1523/JNEUROSCI.2424-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico D, Grazzi L, Usai S, Rigamonti A, Curone M, Bussone G. Disability pattern in chronic migraine with medication overuse: a comparison with migraine without aura. Headache. 2005;45:553–560. doi: 10.1111/j.1526-4610.2005.05109.x. [DOI] [PubMed] [Google Scholar]
- De Felice M, Ossipov MH, Wang R, Dussor G, Lai J, Meng ID, et al. Triptan-induced enhancement of neuronal nitric oxide synthase in trigeminal ganglion dural afferents underlies increased responsiveness to potential migraine triggers. Brain. 2010a;133(Pt 8):2475–2488. doi: 10.1093/brain/awq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M, Ossipov MH, Wang R, Lai J, Chichorro J, Meng I, et al. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann Neurol. 2010b;67:325–337. doi: 10.1002/ana.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, et al. Capturing the aversive state of cephalic pain preclinically. Ann Neurol. 2013;76:257–265. doi: 10.1002/ana.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Li YJ. Calcitonin gene-related peptide and hypertension. Peptides. 2005;26:1676–1685. doi: 10.1016/j.peptides.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Denuelle M, Boulloche N, Payoux P, Fabre N, Trotter Y, Geraud G. A PET study of photophobia during spontaneous migraine attacks. Neurology. 2011;76:213–218. doi: 10.1212/WNL.0b013e3182074a57. [DOI] [PubMed] [Google Scholar]
- Dhir A, Lossin C, Rogawski MA. Propofol hemisuccinate suppresses cortical spreading depression. Neurosci Lett. 2012;514:67–70. doi: 10.1016/j.neulet.2012.02.058. [DOI] [PubMed] [Google Scholar]
- Diener HC, Limmroth V. Medication-overuse headache: a worldwide problem. Lancet Neurol. 2004;3:475–483. doi: 10.1016/S1474-4422(04)00824-5. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991;44:97–112. doi: 10.1016/0306-4522(91)90253-k. [DOI] [PubMed] [Google Scholar]
- Dimlich RV, Keller JT, Strauss TA, Fritts MJ. Linear arrays of homogeneous mast cells in the dura mater of the rat. J Neurocytol. 1991;20:485–503. doi: 10.1007/BF01252276. [DOI] [PubMed] [Google Scholar]
- Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46(Suppl. 4):S182–S191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Dong Z, Jiang L, Wang X, Yu S. Nociceptive behaviors were induced by electrical stimulation of the dura mater surrounding the superior sagittal sinus in conscious adult rats and reduced by morphine and rizatriptan benzoate. Brain Res. 2011;1368:151–158. doi: 10.1016/j.brainres.2010.10.059. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, et al. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153:1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia. 2011;31:737–747. doi: 10.1177/0333102411398152. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Uddman R. Adrenergic, cholinergic and peptidergic nerve fibres in dura mater – involvement in headache? Cephalalgia. 1981;1:175–179. doi: 10.1046/j.1468-2982.1981.0104175.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Alm R, Shaw D, Rutledge RZ, Koblan KS, Longmore J, et al. Effect of the CGRP receptor antagonist BIBN4096BS in human cerebral, coronary and omental arteries and in SK-N-MC cells. Eur J Pharmacol. 2002;434:49–53. doi: 10.1016/s0014-2999(01)01532-1. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Dilekoz E, Kudo C, Savitz SI, Waeber C, Baum MJ, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest. 2009;119:99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Yuzawa I, Dilekoz E, Joutel A, Moskowitz MA, Ayata C. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy syndrome mutations increase susceptibility to spreading depression. Ann Neurol. 2011;69:413–418. doi: 10.1002/ana.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MB, Oshinsky ML, Amenta PS, Awe OO, Jallo JI. Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury. Headache. 2012;52:966–984. doi: 10.1111/j.1526-4610.2012.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti B, Kasasbeh A, Edelmayer R, Skinner DP, Jr, Hartings JA, Burklund RD, et al. Evaluation of cutaneous allodynia following induction of cortical spreading depression in freely moving rats. Cephalalgia. 2011;31:1090–1100. doi: 10.1177/0333102411410609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizanne L, Sigaudo-Roussel D, Saumet JL, Fromy B. Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. J Physiol. 2004;554(Pt 2):519–528. doi: 10.1113/jphysiol.2003.053835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini A, Vilotti S, Ferrari MD, van den Maagdenberg AM, Nistri A, Fabbretti E. TNFalpha levels and macrophages expression reflect an inflammatory potential of trigeminal ganglia in a mouse model of familial hemiplegic migraine. PLoS ONE. 2013;8:e52394. doi: 10.1371/journal.pone.0052394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C. Inhibition of the PKCgamma-epsilon pathway relieves from meningeal nociception in an animal model: an innovative perspective for migraine therapy? Neurother. 2013;10:329–339. doi: 10.1007/s13311-012-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P, Capone JG, Trevisani M, Nicoletti P, Zagli G, Tola MR. CGRP and migraine: neurogenic inflammation revisited. J Headache Pain. 2005;6:61–70. doi: 10.1007/s10194-005-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Classey JD. Glutamatergic transmission in the trigeminal nucleus assessed with local blood flow. Brain Res. 2000;875:119–124. doi: 10.1016/s0006-8993(00)02630-5. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Zagami AS. Stimulation of the superior sagittal sinus increases metabolic activity and blood flow in certain regions of the brainstem and upper cervical spinal cord of the cat. Brain. 1991;114(Pt 2):1001–1011. doi: 10.1093/brain/114.2.1001. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Knight YE, Hoskin KL. Stimulation of the greater occipital nerve increases metabolic activity in the trigeminal nucleus caudalis and cervical dorsal horn of the cat. Pain. 1997;73:23–28. doi: 10.1016/s0304-3959(97)00074-2. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Wang O, Saper JR, Stoltz R, Silberstein SD, Mathew NT. Ineffectiveness of neurokinin-1 antagonist in acute migraine: a crossover study. Cephalalgia. 1997;17:785–790. doi: 10.1046/j.1468-2982.1997.1707785.x. [DOI] [PubMed] [Google Scholar]
- Gozalov A, Jansen-Olesen I, Klaerke D, Olesen J. Role of BK(Ca) channels in cephalic vasodilation induced by CGRP, NO and transcranial electrical stimulation in the rat. Cephalalgia. 2007;27:1120–1127. doi: 10.1111/j.1468-2982.2007.01409.x. [DOI] [PubMed] [Google Scholar]
- Greco R, Tassorelli C, Cappelletti D, Sandrini G, Nappi G. Activation of the transcription factor NF-kappaB in the nucleus trigeminalis caudalis in an animal model of migraine. Neurotoxicology. 2005;26:795–800. doi: 10.1016/j.neuro.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Greco R, Tassorelli C, Armentero MT, Sandrini G, Nappi G, Blandini F. Role of central dopaminergic circuitry in pain processing and nitroglycerin-induced hyperalgesia. Brain Res. 2008;1238:215–223. doi: 10.1016/j.brainres.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Grinberg YY, van Drongelen W, Kraig RP. Insulin-like growth factor-1 lowers spreading depression susceptibility and reduces oxidative stress. J Neurochem. 2012;122:221–229. doi: 10.1111/j.1471-4159.2012.07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz SM, Radcliffe RA. Genetic effects of ATP1A2 in familial hemiplegic migraine type II and animal models. Hum Genomics. 2013;7:8. doi: 10.1186/1479-7364-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Akerman S, van den Maagdenberg AM, Saxena PR, Goadsby PJ, van den Brink AM. Intravital microscopy on a closed cranial window in mice: a model to study trigeminovascular mechanisms involved in migraine. Cephalalgia. 2006;26:1294–1303. doi: 10.1111/j.1468-2982.2006.01219.x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Bhatt DK, Boni LJ, Olesen J. Improvement of the closed cranial window model in rats by intracarotid infusion of signalling molecules implicated in migraine. Cephalalgia. 2010;30:27–36. doi: 10.1111/j.1468-2982.2009.01888.x. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179–1186. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- Headache Classification C, Olesen J, Bousser MG, Diener HC, Dodick D, First M, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742–746. doi: 10.1111/j.1468-2982.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Hodde K, Sercombe R. The anatomy of the brain vasculature. In: Mraovitch S, Sercombe R, editors. Neurophysiological Basis of Cerebral Blood Flow Control: An Introduction. 1st edn. London: John Libbey&Company; 1996. pp. 111–166. [Google Scholar]
- Hoffmann J, Neeb L, Israel H, Dannenberg F, Triebe F, Dirnagl U, et al. Intracisternal injection of inflammatory soup activates the trigeminal nerve system. Cephalalgia. 2009;29:1212–1217. doi: 10.1111/j.1468-2982.2009.01858.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Wecker S, Neeb L, Dirnagl U, Reuter U. Primary trigeminal afferents are the main source for stimulus-induced CGRP release into jugular vein blood and CSF. Cephalalgia. 2012;32:659–667. doi: 10.1177/0333102412447701. [DOI] [PubMed] [Google Scholar]
- Hoffmann O, Keilwerth N, Bille MB, Reuter U, Angstwurm K, Schumann RR, et al. Triptans reduce the inflammatory response in bacterial meningitis. J Cereb Blood Flow Metab. 2002;22:988–996. doi: 10.1097/00004647-200208000-00010. [DOI] [PubMed] [Google Scholar]
- Hoffmann U, Sukhotinsky I, Eikermann-Haerter K, Ayata C. Glucose modulation of spreading depression susceptibility. J Cereb Blood Flow Metab. 2013;33:191–195. doi: 10.1038/jcbfm.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin KL, Lambert GA, Donaldson C, Zagami AS. The 5-hydroxytryptamine1B/1D/1F receptor agonists eletriptan and naratriptan inhibit trigeminovascular input to the nucleus tractus solitarius in the cat. Brain Res. 2004;998:91–99. doi: 10.1016/j.brainres.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Huang D, Li S, Dhaka A, Story GM, Cao YQ. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol Pain. 2012;8:66. doi: 10.1186/1744-8069-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Onaka T, Yamakado M, Nakai J, Ishikawa TO, Taketo MM, et al. Degeneration of the amygdala/piriform cortex and enhanced fear/anxiety behaviors in sodium pump alpha2 subunit (Atp1a2)-deficient mice. J Neurosci. 2003;23:4667–4676. doi: 10.1523/JNEUROSCI.23-11-04667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen HK. Human migraine models. Cephalalgia. 2001;21:781–785. doi: 10.1111/j.1468-2982.2001.00250.x. [DOI] [PubMed] [Google Scholar]
- Iwashita T, Shimizu T, Shibata M, Toriumi H, Ebine T, Funakubo M, et al. Activation of extracellular signal-regulated kinase in the trigeminal ganglion following both treatment of the dura mater with capsaicin and cortical spreading depression. Neurosci Res. 2013;77:110–119. doi: 10.1016/j.neures.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Jakubowski M, Levy D, Goor-Aryeh I, Collins B, Bajwa Z, Burstein R. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache. 2005;45:850–861. doi: 10.1111/j.1526-4610.2005.05153.x. [DOI] [PubMed] [Google Scholar]
- Jakubowski M, Levy D, Kainz V, Zhang XC, Kosaras B, Burstein R. Sensitization of central trigeminovascular neurons: blockade by intravenous naproxen infusion. Neuroscience. 2007;148:573–583. doi: 10.1016/j.neuroscience.2007.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KW, Phebus LA. A fluorescence-based method for assessing dural protein extravasation induced by trigeminal ganglion stimulation. J Neurosci Methods. 1998;81:19–24. doi: 10.1016/s0165-0270(98)00010-7. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25:179–183. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci. 2012;32:15439–15449. doi: 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339:1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- Kaube H, Keay KA, Hoskin KL, Bandler R, Goadsby PJ. Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical spinal cord following stimulation of the superior sagittal sinus in the cat. Brain Res. 1993;629:95–102. doi: 10.1016/0006-8993(93)90486-7. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Vascular head pain selectively activates ventrolateral periaqueductal gray in the cat. Neurosci Lett. 1998;245:58–60. doi: 10.1016/s0304-3940(98)00168-2. [DOI] [PubMed] [Google Scholar]
- Keller JT, Saunders MC, Beduk A, Jollis JG. Innervation of the posterior fossa dura of the cat. Brain Res Bull. 1985;14:97–102. doi: 10.1016/0361-9230(85)90181-9. [DOI] [PubMed] [Google Scholar]
- Keller JT, Dimlich RV, Zuccarello M, Lanker L, Strauss TA, Fritts MJ. Influence of the sympathetic nervous system as well as trigeminal sensory fibres on rat dural mast cells. Cephalalgia. 1991;11:215–221. doi: 10.1046/j.1468-2982.1991.1105215.x. [DOI] [PubMed] [Google Scholar]
- Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- Kim GM, Jin KS, Chung CS. Differential effects of corticosteroids on the expression of cyclooxygenase-2, tumour necrosis factor-alpha and matrix metalloproteinase-9 in an animal model of migraine. Cephalalgia. 2008;28:1179–1187. doi: 10.1111/j.1468-2982.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- Kors EE, Terwindt GM, Vermeulen FL, Fitzsimons RB, Jardine PE, Heywood P, et al. Delayed cerebral edema and fatal coma after minor head trauma: role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann Neurol. 2001;49:753–760. doi: 10.1002/ana.1031. [DOI] [PubMed] [Google Scholar]
- Koulchitsky S, Fischer MJ, Messlinger K. Calcitonin gene-related peptide receptor inhibition reduces neuronal activity induced by prolonged increase in nitric oxide in the rat spinal trigeminal nucleus. Cephalalgia. 2009;29:408–417. doi: 10.1111/j.1468-2982.2008.01745.x. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, et al. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis. 2003;37:139–143. doi: 10.1002/gene.10241. [DOI] [PubMed] [Google Scholar]
- Kudo C, Nozari A, Moskowitz MA, Ayata C. The impact of anesthetics and hyperoxia on cortical spreading depression. Exp Neurol. 2008;212:201–206. doi: 10.1016/j.expneurol.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Hippocampal spreading depression bilaterally activates the caudal trigeminal nucleus in rodents. Hippocampus. 2003;13:835–844. doi: 10.1002/hipo.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa M, Messlinger K, Pawlak M, Schmidt RF. Increase of meningeal blood flow after electrical stimulation of rat dura mater encephali: mediation by calcitonin gene-related peptide. Br J Pharmacol. 1995;114:1397–1402. doi: 10.1111/j.1476-5381.1995.tb13361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe P, Sercombe R, Correze JL, Springhetti V, Seylaz J. Spreading depression induces prolonged reduction of cortical blood flow reactivity in the rat. Exp Neurol. 1992;117:278–286. doi: 10.1016/0014-4886(92)90137-f. [DOI] [PubMed] [Google Scholar]
- Lambert GA, Donaldson C, Boers PM, Zagami AS. Activation of trigeminovascular neurons by glyceryl trinitrate. Brain Res. 2000;887:203–210. doi: 10.1016/s0006-8993(00)02919-x. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Jorgensen MB, Diemer NH, Gjedde A, Hansen AJ. Persistent oligemia of rat cerebral cortex in the wake of spreading depression. Ann Neurol. 1982;12:469–474. doi: 10.1002/ana.410120510. [DOI] [PubMed] [Google Scholar]
- Leo L, Gherardini L, Barone V, De Fusco M, Pietrobon D, Pizzorusso T, et al. Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 2011;7:e1002129. doi: 10.1371/journal.pgen.1002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep. 2009;13:237–240. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- Levy D. Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: the role of immuno-vascular interactions and cortical spreading depression. Curr Pain Headache Rep. 2012;16:270–277. doi: 10.1007/s11916-012-0255-1. [DOI] [PubMed] [Google Scholar]
- Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004;101:4274–4279. doi: 10.1073/pnas.0306147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol. 2005;58:698–705. doi: 10.1002/ana.20619. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Zhang XC, Jakubowski M, Burstein R. Sensitization of meningeal nociceptors: inhibition by naproxen. Eur J Neurosci. 2008;27:917–922. doi: 10.1111/j.1460-9568.2008.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Jia X, Murari K, Parlapalli R, Rege A, Thakor NV. High spatiotemporal resolution imaging of the neurovascular response to electrical stimulation of rat peripheral trigeminal nerve as revealed by in vivo temporal laser speckle contrast. J Neurosci Methods. 2009;176:230–236. doi: 10.1016/j.jneumeth.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Lima AM, Sapienza GB, Giraud Vde O, Fragoso YD. Odors as triggering and worsening factors for migraine in men. Arq Neuropsiquiatr. 2011;69(2B):324–327. doi: 10.1590/s0004-282x2011000300011. [DOI] [PubMed] [Google Scholar]
- Lima D, Avelino A. Spinal c-fos expression is differentially induced by brief or persistent noxious stimulation. Neuroreport. 1994;5:1853–1856. doi: 10.1097/00001756-199410000-00003. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–710. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Haan J, Terwindt GM, Ferrari MD. Migraine: gene mutations and functional consequences. Curr Opin Neurol. 2007;20:299–305. doi: 10.1097/WCO.0b013e3281338d1f. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Pizzorusso T, Kaja S, Terpolilli N, Shapovalova M, Hoebeek FE, et al. High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann Neurol. 2010;67:85–98. doi: 10.1002/ana.21815. [DOI] [PubMed] [Google Scholar]
- Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis. 2012;45:633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci. 1987;7:4129–4136. doi: 10.1523/JNEUROSCI.07-12-04129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated plasma extravasation in dura mater: effect of ergot alkaloids. A possible mechanism of action in vascular headache. Cephalalgia. 1988;8:83–91. doi: 10.1046/j.1468-2982.1988.0802083.x. [DOI] [PubMed] [Google Scholar]
- Markowitz S, Saito K, Buzzi MG, Moskowitz MA. The development of neurogenic plasma extravasation in the rat dura mater does not depend upon the degranulation of mast cells. Brain Res. 1989;477:157–165. doi: 10.1016/0006-8993(89)91403-0. [DOI] [PubMed] [Google Scholar]
- Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, et al. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materazzi S, Benemei S, Fusi C, Gualdani R, De Siena G, Vastani N, et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain. 2013;156:2750–2758. doi: 10.1016/j.pain.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura T, Bures J. The minimum volume of depolarized neural tissue required for triggering cortical spreading depression in rat. Exp Brain Res. 1971;12:238–249. doi: 10.1007/BF00237916. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Spangenberg RJ, Hoek JB, Silberstein SD, Oshinsky ML. Acetate causes alcohol hangover headache in rats. PLoS ONE. 2010;5:e15963. doi: 10.1371/journal.pone.0015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Pearls and pitfalls: neuroimaging in headache. Cephalalgia. 2013;33:554–565. doi: 10.1177/0333102412467513. [DOI] [PubMed] [Google Scholar]
- May A, Gijsman HJ, Wallnofer A, Jones R, Diener HC, Ferrari MD. Endothelin antagonist bosentan blocks neurogenic inflammation, but is not effective in aborting migraine attacks. Pain. 1996;67:375–378. doi: 10.1016/0304-3959(96)03137-5. [DOI] [PubMed] [Google Scholar]
- Mayberg MR, Zervas NT, Moskowitz MA. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984;223:46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Carrillo A, Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia. 2013;33:1096–1105. doi: 10.1177/0333102413486320. [DOI] [PubMed] [Google Scholar]
- Messlinger K, Ellrich J. Meningeal nociception: electrophysiological studies related to headache and referred pain. Microsc Res Tech. 2001;53:129–137. doi: 10.1002/jemt.1077. [DOI] [PubMed] [Google Scholar]
- Messlinger K, Hotta H, Pawlak M, Schmidt RF. Effects of the 5-HT1 receptor agonists, sumatriptan and CP 93 129, on dural arterial flow in the rat. Eur J Pharmacol. 1997;332:173–181. doi: 10.1016/s0014-2999(97)01072-8. [DOI] [PubMed] [Google Scholar]
- Messlinger K, Suzuki A, Pawlak M, Zehnter A, Schmidt RF. Involvement of nitric oxide in the modulation of dural arterial blood flow in the rat. Br J Pharmacol. 2000;129:1397–1404. doi: 10.1038/sj.bjp.0703220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ. CGRP and NO in the trigeminal system: mechanisms and role in headache generation. Headache. 2012;52:1411–1427. doi: 10.1111/j.1526-4610.2012.02212.x. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Sanchez del Rio M. Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine. Brain Res Brain Res Rev. 2001;35:20–35. doi: 10.1016/s0165-0173(00)00048-5. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Sanchez del Rio M, Waeber C, Moskowitz MA, Cutrer FM. The NMDA receptor antagonist MK-801 reduces capsaicin-induced c-fos expression within rat trigeminal nucleus caudalis. Pain. 1998;76:239–248. doi: 10.1016/s0304-3959(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Mo J, Maizels M, Ding M, Ahn AH. Does throbbing pain have a brain signature? Pain. 2013;154:1150–1155. doi: 10.1016/j.pain.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna P. The physiopathology of migraine: the contribution of genetics. Neurol Sci. 2004;25(Suppl. 3):S93–S96. doi: 10.1007/s10072-004-0261-0. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16:157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol Sci. 1992;13:307–311. doi: 10.1016/0165-6147(92)90097-p. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43(6 Suppl. 3):S16–S20. [PubMed] [Google Scholar]
- Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993;13:1167–1177. doi: 10.1523/JNEUROSCI.13-03-01167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012;135(Pt 2):376–390. doi: 10.1093/brain/awr272. [DOI] [PubMed] [Google Scholar]
- Needleman P. Tolerance to the vascular effects of glyceryl trinitrate. J Pharmacol Exp Ther. 1970;171:98–102. [PubMed] [Google Scholar]
- Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl. 1):S44–S53. doi: 10.1016/j.pain.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Noseda R, Constandil L, Bourgeais L, Chalus M, Villanueva L. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci. 2010a;30:14420–14429. doi: 10.1523/JNEUROSCI.3025-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010b;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki K, Moskowitz MA, Boccalini P. CP-93,129, sumatriptan, dihydroergotamine block c-fos expression within rat trigeminal nucleus caudalis caused by chemical stimulation of the meninges. Br J Pharmacol. 1992;106:409–415. doi: 10.1111/j.1476-5381.1992.tb14348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrenovitch TP, Chen S, Farkas E. Simultaneous, live imaging of cortical spreading depression and associated cerebral blood flow changes, by combining voltage-sensitive dye and laser speckle contrast methods. Neuroimage. 2009;45:68–74. doi: 10.1016/j.neuroimage.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Olesen J, Jansen-Olesen I. Towards a reliable animal model of migraine. Cephalalgia. 2012;32:578–580. doi: 10.1177/0333102412441719. [DOI] [PubMed] [Google Scholar]
- Olesen J, Thomsen LL, Iversen H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol Sci. 1994;15:149–153. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Wainwright A, Edvinsson L, Pickard JD, Hill RG. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J Cereb Blood Flow Metab. 2002;22:620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy CT, Connor HE. Investigation of the role of tachykinin NK1, NK2 receptors and CGRP receptors in neurogenic plasma protein extravasation in dura mater. Eur J Pharmacol. 1994;263:193–198. doi: 10.1016/0014-2999(94)90541-x. [DOI] [PubMed] [Google Scholar]
- Penfield W. Operative treatment of migraine and observations on the mechanisms of vascular pain. Tr Am Acad Ophth & Otolaryng. 1932;37:50–64. [Google Scholar]
- Petersen KA, Birk S, Doods H, Edvinsson L, Olesen J. Inhibitory effect of BIBN4096BS on cephalic vasodilatation induced by CGRP or transcranial electrical stimulation in the rat. Br J Pharmacol. 2004;143:697–704. doi: 10.1038/sj.bjp.0705966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KA, Dyrby L, Williamson D, Edvinsson L, Olesen J. Effect of hypotension and carbon dioxide changes in an improved genuine closed cranial window rat model. Cephalalgia. 2005;25:23–29. doi: 10.1111/j.1468-2982.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. Familial hemiplegic migraine. Neurother. 2007;4:274–284. doi: 10.1016/j.nurt.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2013;155:269–276. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–236. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Bhatt DK, Ploug KB, Olesen J, Jansen-Olesen I, Hay-Schmidt A, et al. A naturalistic glyceryl trinitrate infusion migraine model in the rat. Cephalalgia. 2012;32:73–84. doi: 10.1177/0333102411430855. [DOI] [PubMed] [Google Scholar]
- Ray BS, Wolff HG. Experimental studies on headache. Pain sensitive structures of the head and their significance in headache. Arch Surg. 1940;61:813–856. [Google Scholar]
- Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58:156–165. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter U, Weber JR, Gold L, Arnold G, Wolf T, Dreier J, et al. Perivascular nerves contribute to cortical spreading depression-associated hyperemia in rats. Am J Physiol. 1998;274(6 Pt 2):H1979–H1987. doi: 10.1152/ajpheart.1998.274.6.H1979. [DOI] [PubMed] [Google Scholar]
- Reuter U, Bolay H, Jansen-Olesen I, Chiarugi A, Sanchez del Rio M, Letourneau R, et al. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124(Pt 12):2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- Romero-Reyes M, Ye Y. Pearls and pitfalls in experimental in vivo models of headache: conscious behavioral research. Cephalalgia. 2013b;33:566–576. doi: 10.1177/0333102412472557. [DOI] [PubMed] [Google Scholar]
- Romero-Reyes M, Akerman S, Nguyen E, Vijjeswarapu A, Hom B, Dong HW, et al. Spontaneous behavioral responses in the orofacial region: a model of trigeminal pain in mouse. Headache. 2013a;53:137–151. doi: 10.1111/j.1526-4610.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC. Morphological and functional demonstration of rat dura mater mast cell-neuron interactions in vitro and in vivo. Brain Res. 1999;849:1–15. doi: 10.1016/s0006-8993(99)01855-7. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, Tournier-Lasserve E, et al. Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol. 2003;162:329–342. doi: 10.1016/S0002-9440(10)63824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R, Graf R, Hubel K, Taguchi J, Rosner G, Fujita T, et al. Halothane, but not alpha-chloralose, blocks potassium-evoked cortical spreading depression in cats. Brain Res. 1995;699:109–115. doi: 10.1016/0006-8993(95)00898-z. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20:907–918. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Vaianella L, Pierguidi L, Floridi A, Mazzotta G, et al. Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache. 2004;44:961–968. doi: 10.1111/j.1526-4610.2004.04189.x. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006a;46:200–207. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Floridi A, Mancini ML, Rossi C, Coppola F, Baldi A, et al. NF-kappaB activity and iNOS expression in monocytes from internal jugular blood of migraine without aura patients during attacks. Cephalalgia. 2006b;26:1071–1079. doi: 10.1111/j.1468-2982.2006.01164.x. [DOI] [PubMed] [Google Scholar]
- Schemann M, Kugler EM, Buhner S, Eastwood C, Donovan J, Jiang W, et al. The mast cell degranulator compound 48/80 directly activates neurons. PLoS ONE. 2012;7:e52104. doi: 10.1371/journal.pone.0052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonman GG, van der Grond J, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation – a 3T magnetic resonance angiography study. Brain. 2008;131(Pt 8):2192–2200. doi: 10.1093/brain/awn094. [DOI] [PubMed] [Google Scholar]
- Schuh-Hofer S, Boehnke C, Reuter U, Siekmann W, Lindauer U, Arnold G, et al. A fluorescence-based method to assess plasma protein extravasation in rat dura mater using confocal laser scanning microscopy. Brain Res Brain Res Protoc. 2003;12:77–82. doi: 10.1016/j.brainresprot.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132(Pt 1):16–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- Schytz HW, Schoonman GG, Ashina M. What have we learnt from triggering migraine? Curr Opin Neurol. 2010;23:259–265. doi: 10.1097/WCO.0b013e328337b884. [DOI] [PubMed] [Google Scholar]
- Seeliger S, Buddenkotte J, Schmidt-Choudhury A, Rosignoli C, Shpacovitch V, von Arnim U, et al. Pituitary adenylate cyclase activating polypeptide: an important vascular regulator in human skin in vivo. Am J Pathol. 2010;177:2563–2575. doi: 10.2353/ajpath.2010.090941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefler I, Sagi-Eisenberg R. Gi-mediated activation of the Syk kinase by the receptor mimetic basic secretagogues of mast cells: role in mediating arachidonic acid/metabolites release. J Immunol. 2001;167:475–481. doi: 10.4049/jimmunol.167.1.475. [DOI] [PubMed] [Google Scholar]
- Shepheard SL, Williamson DJ, Williams J, Hill RG, Hargreaves RJ. Comparison of the effects of sumatriptan and the NK1 antagonist CP-99 994 on plasma extravasation in Dura mater and c-fos mRNA expression in trigeminal nucleus caudalis of rats. Neuropharmacology. 1995;34:255–261. doi: 10.1016/0028-3908(94)00153-j. [DOI] [PubMed] [Google Scholar]
- Shepherd SL, Williamson DJ, Beer MS, Hill RG, Hargreaves RJ. Differential effects of 5-HT1B/1D receptor agonists on neurogenic dural plasma extravasation and vasodilation in anaesthetized rats. Neuropharmacology. 1997;36:525–533. doi: 10.1016/s0028-3908(97)00057-9. [DOI] [PubMed] [Google Scholar]
- Shields KG, Goadsby PJ. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine? Brain. 2005;128(Pt 1):86–97. doi: 10.1093/brain/awh298. [DOI] [PubMed] [Google Scholar]
- Shields KG, Goadsby PJ. Serotonin receptors modulate trigeminovascular responses in ventroposteromedial nucleus of thalamus: a migraine target? Neurobiol Dis. 2006;23:491–501. doi: 10.1016/j.nbd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Sixt ML, Messlinger K, Fischer MJ. Calcitonin gene-related peptide receptor antagonist olcegepant acts in the spinal trigeminal nucleus. Brain. 2009;132(Pt 11):3134–3141. doi: 10.1093/brain/awp168. [DOI] [PubMed] [Google Scholar]
- Sokolov AY, Lyubashina OA, Sivachenko IB, Berkovich RR, Panteleev SS. Intravenous valproate inhibits ongoing and evoked activity of dura-sensitive thalamic neurons in rats. Eur J Pharmacol. 2013;715:204–211. doi: 10.1016/j.ejphar.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Sonn J, Mayevsky A. Effects of anesthesia on the responses to cortical spreading depression in the rat brain in vivo. Neurol Res. 2006;28:206–219. doi: 10.1179/016164105X49445. [DOI] [PubMed] [Google Scholar]
- Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol. 2006;95:1298–1306. doi: 10.1152/jn.01293.2005. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384(6609):560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- Strecker T, Dux M, Messlinger K. Nitric oxide releases calcitonin-gene-related peptide from rat dura mater encephali promoting increases in meningeal blood flow. J Vasc Res. 2002;39:489–496. doi: 10.1159/000067206. [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I, Dilekoz E, Moskowitz MA, Ayata C. Hypoxia and hypotension transform the blood flow response to cortical spreading depression from hyperemia into hypoperfusion in the rat. J Cereb Blood Flow Metab. 2008;28:1369–1376. doi: 10.1038/jcbfm.2008.35. [DOI] [PubMed] [Google Scholar]
- Svendsen O, Lykkegaard K. Neuronal c-Fos immunoreactivity as a quantitative measure of stress or pain. Acta Agric Scand A Anim Sci. 2001;51:131–134. [Google Scholar]
- Syed AU, Koide M, Braas KM, May V, Wellman GC. Pituitary adenylate cyclase-activating polypeptide (PACAP) potently dilates middle meningeal arteries: implications for migraine. J Mol Neurosci. 2012;48:574–583. doi: 10.1007/s12031-012-9851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassorelli C, Joseph SA. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995;682:167–181. doi: 10.1016/0006-8993(95)00348-t. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Kruuse C, Iversen HK, Olesen J. A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol. 1994;1:73–80. doi: 10.1111/j.1468-1331.1994.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain. 2002;125:1379–1391. doi: 10.1093/brain/awf132. [DOI] [PubMed] [Google Scholar]
- Tottene A, Conti R, Fabbro A, Vecchia D, Shapovalova M, Santello M, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron. 2009;61:762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Tuka B, Helyes Z, Markovics A, Bagoly T, Nemeth J, Mark L, et al. Peripheral and central alterations of pituitary adenylate cyclase activating polypeptide-like immunoreactivity in the rat in response to activation of the trigeminovascular system. Peptides. 2012;33:307–316. doi: 10.1016/j.peptides.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Tuka B, Helyes Z, Markovics A, Bagoly T, Szolcsanyi J, Szabo N, et al. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia. 2013;33:1085–1095. doi: 10.1177/0333102413483931. [DOI] [PubMed] [Google Scholar]
- Tvedskov JF, Thomsen LL, Iversen HK, Gibson A, Wiliams P, Olesen J. The prophylactic effect of valproate on glyceryltrinitrate induced migraine. Cephalalgia. 2004a;24:576–585. doi: 10.1111/j.1468-2982.2003.00720.x. [DOI] [PubMed] [Google Scholar]
- Tvedskov JF, Thomsen LL, Iversen HK, Williams P, Gibson A, Jenkins K, et al. The effect of propranolol on glyceryltrinitrate-induced headache and arterial response. Cephalalgia. 2004b;24:1076–1087. doi: 10.1111/j.1468-2982.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- Uddman R, Hara H, Edvinsson L. Neuronal pathways to the rat middle meningeal artery revealed by retrograde tracing and immunocytochemistry. J Auton Nerv Syst. 1989;26:69–75. doi: 10.1016/0165-1838(89)90109-4. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Walther D, Nishimori T, Buzzi MG, Moskowitz MA. Jun B, c-jun, jun D and c-fos mRNAs in nucleus caudalis neurons: rapid selective enhancement by afferent stimulation. Brain Res Mol Brain Res. 1991;11:133–141. doi: 10.1016/0169-328x(91)90115-e. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. 2005. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Available at: http://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf (accessed 9/1/2014)
- Varga H, Pardutz A, Vamos E, Plangar I, Egyud E, Tajti J, et al. Cox-2 inhibitor attenuates NO-induced nNOS in rat caudal trigeminal nucleus. Headache. 2007;47:1319–1325. doi: 10.1111/j.1526-4610.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- Vos BP, Hans G, Adriaensen H. Behavioral assessment of facial pain in rats: face grooming patterns after painful and non-painful sensory disturbances in the territory of the rat’s infraorbital nerve. Pain. 1998;76:173–178. doi: 10.1016/s0304-3959(98)00039-6. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Lauritzen M, Schilling L. Change of cerebrovascular reactivity after cortical spreading depression in cats and rats. Brain Res. 1987;411:72–80. doi: 10.1016/0006-8993(87)90682-2. [DOI] [PubMed] [Google Scholar]
- Wei X, Edelmayer RM, Yan J, Dussor G. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia. 2011;31:1595–1600. doi: 10.1177/0333102411427600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- Wieseler J, Ellis A, Sprunger D, Brown K, McFadden A, Mahoney J, et al. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. J Neurosci Methods. 2010;185:236–245. doi: 10.1016/j.jneumeth.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieseler J, Sprunger D, Ellis A, Maier SF, Watkins LR. Indwelling supradural catheters for induction of facial allodynia: surgical procedures, application of inflammatory stimuli, and behavioral testing. Methods Mol Biol. 2012;851:99–107. doi: 10.1007/978-1-61779-561-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Koedel U, Bruckmann H, Pfister HW. Experimental bacterial meningitis in rats: demonstration of hydrocephalus and meningeal enhancement by magnetic resonance imaging. Neurol Res. 2002;24:307–310. doi: 10.1179/016164102101199792. [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural vessel diameter in the anaesthetized rat. Cephalalgia. 1997a;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat – intravital microscope studies. Cephalalgia. 1997b;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Shepheard SL, Hill RG, Hargreaves RJ. The novel anti-migraine agent rizatriptan inhibits neurogenic dural vasodilation and extravasation. Eur J Pharmacol. 1997c;328:61–64. doi: 10.1016/s0014-2999(97)83028-2. [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Hill RG, Shepheard SL, Hargreaves RJ. The anti-migraine 5-HT(1B/1D) agonist rizatriptan inhibits neurogenic dural vasodilation in anaesthetized guinea-pigs. Br J Pharmacol. 2001;133:1029–1034. doi: 10.1038/sj.bjp.0704162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura H, Malick A, Chamberlin NL, Burstein R. Cardiovascular and neuronal responses to head stimulation reflect central sensitization and cutaneous allodynia in a rat model of migraine. J Neurophysiol. 1999;81:479–493. doi: 10.1152/jn.1999.81.2.479. [DOI] [PubMed] [Google Scholar]
- Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6) Mol Pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota C, Kuge Y, Hasegawa Y, Tagaya M, Abumiya T, Ejima N, et al. Unique profile of spreading depression in a primate model. J Cereb Blood Flow Metab. 2002;22:835–842. doi: 10.1097/00004647-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Waeber C, Castanon N, Scearce K, Hen R, Macor JE, et al. 5-Carboxamido-tryptamine, CP-122 288 and dihydroergotamine but not sumatriptan, CP-93 129, and serotonin-5-O-carboxymethyl-glycyl-tyrosinamide block dural plasma protein extravasation in knockout mice that lack 5-hydroxytryptamine1B receptors. Mol Pharmacol. 1996;49:761–765. [PubMed] [Google Scholar]
- Yu XJ, Cutrer FM, Moskowitz MA, Waeber C. The 5-HT1D receptor antagonist GR-127,935 prevents inhibitory effects of sumatriptan but not CP-122 288 and 5-CT on neurogenic plasma extravasation within guinea pig dura mater. Neuropharmacology. 1997;36:83–91. doi: 10.1016/s0028-3908(96)00149-9. [DOI] [PubMed] [Google Scholar]
- Zhang M, Dai W, Liang J, Chen X, Hu Y, Chu B, et al. Effects of UCMS-induced depression on nociceptive behaviors induced by electrical stimulation of the dura mater. Neurosci Lett. 2013a;551:1–6. doi: 10.1016/j.neulet.2013.04.038. [DOI] [PubMed] [Google Scholar]
- Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010;30:8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011a;69:855–865. doi: 10.1002/ana.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Burstein R, Levy D. Local action of the proinflammatory cytokines IL-1beta and IL-6 on intracranial meningeal nociceptors. Cephalalgia. 2012;32:66–72. doi: 10.1177/0333102411430848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kainz V, Zhao J, Strassman AM, Levy D. Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors. Ann Neurol. 2013b;73:741–750. doi: 10.1002/ana.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Levy D. Modulation of meningeal nociceptors mechanosensitivity by peripheral proteinase-activated receptor-2: the role of mast cells. Cephalalgia. 2008;28:276–284. doi: 10.1111/j.1468-2982.2007.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Strassman AM, Burstein R, Levy D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]
- Zhang XC, Kainz V, Burstein R, Levy D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2011b;152:140–149. doi: 10.1016/j.pain.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziem G, McTamney J. Profile of patients with chemical injury and sensitivity. Environ Health Perspect. 1997;105(Suppl. 2):417–436. doi: 10.1289/ehp.97105s2417. [DOI] [PMC free article] [PubMed] [Google Scholar]