Abstract
Eating disorders, such as anorexia nervosa (AN), bulimia nervosa (BN) and binge eating disorders (BED), are described as abnormal eating habits that usually involve insufficient or excessive food intake. Animal models have been developed that provide insight into certain aspects of eating disorders. Several drugs have been found efficacious in these animal models and some of them have eventually proven useful in the treatment of eating disorders. This review will cover the role of monoaminergic neurotransmitters in eating disorders and their pharmacological manipulations in animal models and humans. Dopamine, 5-HT (serotonin) and noradrenaline in hypothalamic and striatal regions regulate food intake by affecting hunger and satiety and by affecting rewarding and motivational aspects of feeding. Reduced neurotransmission by dopamine, 5-HT and noradrenaline and compensatory changes, at least in dopamine D2 and 5-HT2C/2A receptors, have been related to the pathophysiology of AN in humans and animal models. Also, in disorders and animal models of BN and BED, monoaminergic neurotransmission is down-regulated but receptor level changes are different from those seen in AN. A hypofunctional dopamine system or overactive α2-adrenoceptors may contribute to an attenuated response to (palatable) food and result in hedonic binge eating. Evidence for the efficacy of monoaminergic treatments for AN is limited, while more support exists for the treatment of BN or BED with monoaminergic drugs.
Linked Articles
This article is part of a themed section on Animal Models in Psychiatry Research. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-20
Table of Links
| Targets | Ligands | |
|---|---|---|
| 5-HT1A receptor | 5-HT | Olanzapine |
| 5-HT1B receptor | Dopamine | Pimozide |
| 5-HT2A receptor | Noradrenaline | Quetiapine |
| 5-HT2C receptor | Amphetamine | Reboxetine |
| 5-HT3 receptor | α-MSH | Risperidone |
| 5-HT4 receptor | Aripiprazole | Sertraline |
| 5-HT6 receptor | Bupropion | Sibutramine |
| α1 adrenoceptor | meta-Chlorophenylpiperazine | Sulpiride |
| α2 adrenoceptor | Chlorpromazine | Δ9-tetrahydrocannabinol, THC |
| β2 adrenoceptor | Cyproheptadine | Topiramate |
| CRF1 receptor | DAMGO | |
| Dopamine D1 receptor | Desipramine | |
| Dopamine D2 receptor | Fenfluramine | |
| Dopamine D3 receptor | Fluoxetine | |
| Dopamine D4 receptor | cis-Flupenthixol | |
| Ghrelin receptor (GHS-R1a) | Fluvoxamine | |
| μ opioid receptor | Ghrelin | |
| Melanocortin MC1 receptor | Haloperidol | |
| NET, noradrenaline transporter | Leptin | |
| SERT, 5-HT transporter | Neuropeptide Y |
This Table lists the protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a–c).
Introduction
Eating disorders are conditions defined by significant disturbances in eating behaviour or behaviours intended to control body weight, which impair physical and mental health. Various types of eating disorders exist, among which are anorexia nervosa (AN), bulimia nervosa (BN) and binge eating disorders (BED). Some researchers suggest that AN and BN are two phases of the same disorder because majority of AN patients have episodes of bingeing and purging with a majority of BN patients starting with a short episode of starvation (Eddy et al., 2002; Södersten et al., 2006). Indeed, there is a high rate of crossover between the AN and BN diagnosis (Klump et al., 2001; Eddy et al., 2008; Peat et al., 2009). Nevertheless, the subdivision within eating disorders is clinically relevant as the subtypes differ in treatments, their effectiveness, medical complications and prognosis.
The progress in the treatment of eating disorders requires deepened understanding of their pathophysiology and symptoms. Although psychological and environmental factors play a pivotal role in the development of eating disorders, a better understanding of the biological basis that underlies the pathophysiology and symptomatology of eating disorders may help in the development of suitable pharmacological and psychological treatments. As the investigation of these aspects of eating disorders is difficult or impossible in humans, animal models are essential to study certain aspects of eating disorders in a controlled genetic and environmental manner. This review will cover animal models of eating disorders, their relation to humans and a description of pharmacological manipulations in animal models and humans.
Concepts of food intake
Richter’s classic experiments indicated that food and water intake in all mammals is episodic, not continuous (Richter, 1922). Episodes of feeding and drinking result from ongoing interactive mechanisms, which either stimulate these behaviours (hunger, thirst) or restrain them (satiety and satiation). In rats, daily food intake occurs in series of meals separated by intermeal intervals and overall intake consists of two components, meal size and meal frequency (Rosenwasser et al., 1981). These two components can be separately manipulated, suggesting regulation via separate physiological processes (Rosenwasser et al., 1981).
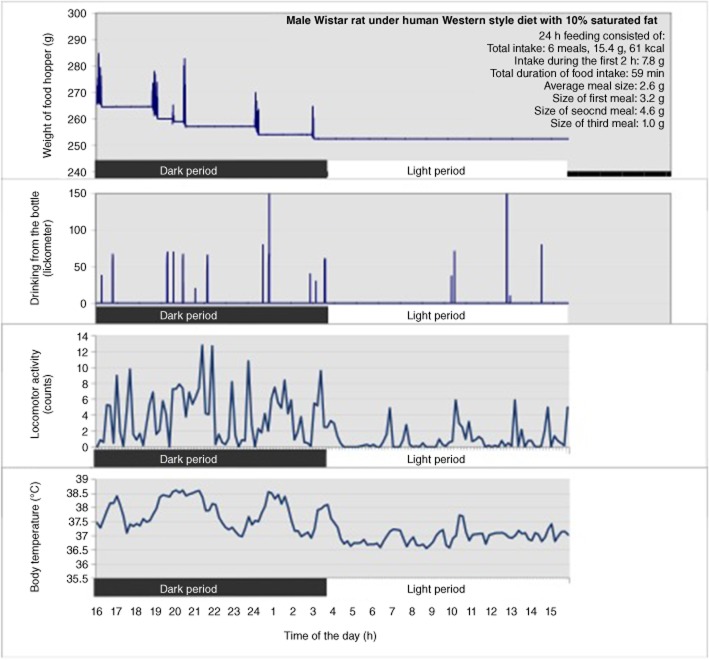
Rats display a circadian rhythm in active feeding behaviour and the majority of intake occurs during the dark period, after which food intake is minimal until occasional meals are again consumed during the latter part of the light phase (Figure 1) (Rosenwasser et al., 1981; Janhunen et al., 2011). Meal size is positively correlated with the duration of post-meal interval (Thomas and Mayer, 1968; Smith, 2000). This postprandial correlation is thought to reflect the action of meal-initiated metabolic events on the induction and maintenance of intermeal satiety.
Figure 1.
Example of 24 h behavioural data from one naïve male Wistar rat using automated monitoring system. Food intake was detected by automated weighing of the food hopper and water intake was measured by recording the animal’s contact with bottle nipple by lickometer. Each burst represents a meal or drinking session. Locomotor activity (counts) and core body temperature (°C) were measured by telemetric transmitters. All data were recorded to a computer. Dark bar represents the dark period (lights off, 16:00–04:00 h) and light bar represents the light period (lights on, 04:00–16:00 h).
Monitoring feeding behaviour in animals
Feeding behaviour can be monitored automatically and continuously every few seconds or minutes or by weighing food pellets or mash manually at selected timepoints once or twice per day. Automated monitoring improves throughput and accuracy of measurements and reduces manual work and stress caused by human interference. Large numbers of animals can be monitored over long periods of time under conditions supporting animals’ normal stress-free behaviour.
Unlike manual recording, automatic monitoring records meal patterning, consisting of meal size and meal frequency for each individual animal. Various automated monitoring methods have been used to study feeding behaviour in rodents. There are (i) operant methods, in which the animal presses a bar to obtain the food (Anliker and Mayer, 1956; Snowdon, 1969); (ii) devices which record the presence of either the whole animal inside a feeding chamber (Milner and De Caire, 1965; Madrid et al., 1989) or the animal’s head over the food cup (Madrid et al., 1993); (iii) devices which detect the animal’s contact with the food, commonly known as eatometers (Fallon, 1965; Kissileff, 1970; Madrid et al., 1995); (iv) pellet-detecting eatometers which deliver a pellet each time one is eaten (Kissileff, 1970; Madrid et al., 1995); and (v) electronic balances which continuously measure the weight of the food and relay the information to a computer (Pokrovsky and Le Magnen, 1963; Hulsey and Martin, 1991; Janhunen et al., 2011; 2013).
The automated electronic food weighing system in rats gives continuous data from an animal’s food intake and meal patterning (Tiesjema et al., 2007; de Backer et al., 2010; Janhunen et al., 2011; 2013; la Fleur et al., 2013; Merkestein et al., 2013). A meal is defined as a feeding episode, for example, with a minimal consumption of 0.5 g chow and a 5 min intermeal interval, information is sent every 12 s to a computer. Figure 1 shows an example of a 24 h data from a rat whose feeding, drinking and locomotor behaviour and core body temperature were automatically recorded. A lickometer, which monitors contact with the nipple of a water bottle, was combined with the food weighing system to investigate the animal’s drinking behaviour (Figure 1). Telemetric transmitters in the intraperitoneal cavity simultaneously monitored locomotor activity and core body temperature (Figure 1). Feeding behaviour consisted of meals of different sizes and meals were followed by varying intermeal intervals (Figure 1). Figure 1 shows that most feeding behaviour occurred during the dark period and drinking behaviour often followed a meal. The rat was most active during the dark period and particularly at the time when it consumed a meal. Core body temperature rose after these active feeding periods (Figure 1). Automated systems can be used for the monitoring of different components of energy expenditure after various acute or chronic drug treatments (van der Zwaal et al., 2008; Janhunen et al., 2011; 2013) or local injections of compounds in the activity-based anorexia (ABA) model (Verhagen et al., 2009).
Animal models of anorexia
There are at least four models of AN in rodents: (i) the dietary restriction model; (ii) the stress-induced appetite loss; (iii) the stress-induced hyperactivity (SIH) model; and (iv) the ABA model.
The dietary restriction model
There are two ways to achieve food restriction: (i) animals are given access to a fixed percentage of the food that they would normally consume during 24 h; or (ii) food is provided ad libitum for a fixed amount of time (Siegfried et al., 2003).
Stress-induced appetite loss
Various acute and chronic stressors may be used to evoke a loss of appetite in experimental animals, including tail pinch, cold swimming, direct brain stimulation or separation stress. Chronic stress results in overfeeding on palatable food; however, short-term stress in particular decreases food intake (Adam and Epel, 2007). Stress can also activate the immune system, which, in turn, can reduce food intake and appetite, a phenomenon commonly described with infections and sickness (Dantzer et al., 2008). Models classified in the category of stress-induced appetite loss are not suitable as models of AN because the limited food intake observed in AN patients does not result from a lack of appetite (Casper et al., 2008). AN patients were found to have no disturbances in appetite and their perception of hunger was similar to controls (Garfinkel, 1974).
The activity-based models target the combination of two AN symptoms: (i) restricted feeding and (ii) high levels of physical activity. These models promote symptoms similar to those in AN patients, including hypophagia, hyperactivity, progressive weight loss and disruptions of the ovarian reproductive cycle. The activity-based models are based on the observation that access to a running wheel causes a transient decrease in food intake in rats and mice (Routtenberg, 1968). Furthermore, the effect on energy expenditure is paradoxically potentiated when running wheel access is combined with dietary restriction, which leads to a further increase in activity levels (Finger, 1951; Hall and Hanford, 1954; Reid and Finger, 1955). Rodents exposed to this combination of factors are not able to compensate for their increased energy expenditure by increasing caloric intake and may eventually starve themselves to death (Routtenberg and Kuznesof, 1967). The phenotype in both activity-based models mimics symptoms of AN that are observed in starving patients. There are, however, also differences between the two models, which we will briefly explain in the succeeding paragraphs.
The SIH model
In this model, rodents have unlimited access to running wheels and receive a fixed percentage of the amount of food they would ingest per day under ad libitum conditions (Broocks et al., 1990). There is no time limit on the consumption of the fixed amount of food. Animals receive the same amount of food over the whole experimental period, which prevents the self-starvation observed in the ABA model. The primary focus of the SIH model is the increase of physical activity levels evoked by food restriction, whereas the ABA model emphasizes on the effect of increased activity in anticipation to meals, making the ABA model a more appropriate AN model than SIH (Gutierrez, 2013).
The ABA model
In this model, animals are given access to food for a limited amount of time per day (e.g. 2 h per day for mice and 1 h per day for rats) (Hall and Hanford, 1954; Routtenberg and Kuznesof, 1967; Dixon et al., 2003). This time is too short for animals to consume the amount of food they would ingest under ad libitum conditions. Moreover, in the ABA model, rats exposed to a running wheel will consume even less food than controls (rats without running wheel access) given an equal duration of food access. Interestingly, this is observed if food availability is limited to once per day and the difference in food intake disappears if the number of periods of food availability is increased, even when the total time of food availability remains the same (e.g. twice daily for 30 min in rats) (Routtenberg, 1968; Kanarek and Collier, 1983). One of the measures of increased running wheel activity (RWA) in the ABA model is the increase of activity in the hours preceding the period of food availability (Mistlberger, 1994; Richter, 1922). This phenomenon, known as food-anticipatory activity, is considered an equivalent to the search for food.
Binge models
Several animal models of binge eating have been developed, each of which addresses certain aspects of the disorder. Three models will be discussed with their pharmacological interventions.
The stress-induced hyperphagia model
This model evokes hyperphagia of palatable food as a result of alternating exposure to periods of food restriction and unlimited food access in combination with acute stress (Hagan et al., 2002; 2003; Artiga et al., 2007). The stress-induced hyperphagia model has been proposed to model the occurrence of binge eating because of negative emotional states and stress. Consistently, administration of a corticotropin-releasing factor CRF1 receptor antagonist resulted in an attenuation of stress-induced palatable food intake (Avena, 2013).
The sham-feeding model
This model aims to simulate the disturbances in satiety observed in BN patients (Davis and Campbell, 1973). Meals consisting of liquid food ingested by rats increase dramatically because of the draining of consumed food using a stomach cannula. Defective satiation caused by the cannula results in increased meal size. As a consequence, with time, rats learn to eat even more. This model is considered the most suitable of all available BN models to simultaneously mimic overeating, postprandial vomiting and impaired satiety (Casper et al., 2008).
Limited-access model
In this model, non-food-deprived rats develop binge-like behaviours after having limited access to an optional palatable fatty and/or sugary food (Corwin and Wojnicki, 2006; Wojnicki et al., 2007). Administration of 2-hydroxyestradiol, an estrogen metabolite, resulted in increased bingeing behaviour (Babbs et al., 2011; 2013). These results were especially driven by effects on male rats, while subtle effects were observed in female rats. This might be a novel mechanism for the sex differences in the risk of developing eating disorders.
Processes underlying eating disorders
Food intake and energy expenditure consisting of resting metabolism, physical activity and the thermogenic effect of food need to be in balance to maintain a stable and normal body weight. In eating disorders, this balance is shifted by an excessive or restricted food intake. Food intake is influenced by hunger, satiety and pleasure, which are regulated by a number of brain areas and neurotransmitters. Central biogenic amine systems that use dopamine, noradrenaline or 5-HT (serotonin) as neurotransmitters regulate many aspects of energy balance, such as feeding, motor activity, maintenance of body weight, mood, impulsivity and anxiety. Lesioning and electrical stimulation studies have indicated that separate, partly overlapping, neural circuits of the hypothalamus and brainstem regulate meal initiation and termination respectively (Hetherington and Ranson, 1940; Brobeck et al., 1943; Anand and Brobeck, 1951; Hoebel and Teitelbaum, 1966; Herberg and Blundell, 1967; Thomas and Mayer, 1968; Olney, 1969; Leibowitz et al., 1981; Seeley et al., 1994; Choi and Dallman, 1999).
Besides regulation by homeostatic processes, neuronal circuits involved in the reinforcing effects of reward also influence food intake. The nucleus accumbens, with connections to the amygdala, the lateral hypothalamus (LHA) and the ventral tegmental area, has been implicated in the hedonic control of energy consumption (Hernandez and Hoebel, 1988; Mendoza et al., 2005). In the next chapters, we first describe the general role of a neurotransmitter in feeding and then address its role in models of eating disorders. The effects of dopamine, 5-HT and noradrenaline transmission on feeding are summarized in Table 1.
Table 1.
The effects of different neurotransmitters during feeding
| Neurotransmitter | Brain region | Change in neurotransmitter during meal consumption | Effect on meal size and food intake | Mechanism |
|---|---|---|---|---|
| Dopamine | Striatum | Increased | Reduced | Reward and reinforcement |
| Lateral hypothalamus | Increased | Reduced | Regulation of meal size via initiation of meal termination | |
| Ventromedial hypothalamus | Decreased | Reduced | Regulation of meal size and compensatory change in meal frequency | |
| 5-HT (serotonin) | Hypothalamus | Increased | Reduced | Satiety and satiation |
| Nucleus accumbens | Increased | Reduced | Motivation to eat | |
| Noradrenaline | Anterior hypothalamus/ paraventricular nucleus | Increased | Activation of α1- or β2-adrenoceptors enhances food intake; activation of α2-adrenoceptors reduces meal size and food intake | Satiety |
Dopaminergic modulation of feeding
Dopamine is a neurotransmitter with a crucial role in eliciting reward-related behaviour, arousal, cognition and locomotor activity. This transmitter also plays a pivotal role in feeding behaviour and produces different actions at different brain regions, most importantly in striatal and hypothalamic nuclei. Striatal dopamine regulates food preference and the reinforcing effects of food, such that eating induces dopamine release in striatum, which correlates with palatability and rewarding aspects of the meal (Small et al., 2003; Cooper and Al-Naser, 2006).
Hypothalamic dopamine is required to initiate each meal and its release influences both meal duration and meal number (Meguid et al., 2000). The effects of hypothalamic dopamine depend on the actions of dopamine on selected nuclei and dopamine receptor subtypes, overall metabolic status and the route of administration of dopamine modulating signals (Ramos et al., 2005). The dopaminergic neurons from the ventral tegmental area and the substantia nigra to the hypothalamus influence feeding behaviour by dual action (Wellman, 2005). First, both transient and constant pharmacological increases of dopamine in the LHA decrease food intake (Vucetic and Reyes, 2010). Dopamine in the LHA increases in response to feeding, remains elevated during meal consumption, initiates meal termination by reaching a ‘threshold’ level and again normalizes after meal termination (Meguid et al., 2000). Meal size positively correlates with the amount of dopamine released in the LHA (Meguid et al., 1995). Further supporting the role of LHA dopamine in AN, a dopamine D2 receptor antagonist given into the LHA counteracted amphetamine-induced AN (Gilbert and Cooper, 1985).
Second, dopamine in the hypothalamic ventromedial nucleus (VMN) decreases meal size and food intake (Vucetic and Reyes, 2010). The dopamine levels in the VMN decrease during eating and return to baseline during fasting, such that a rat starts a new meal when food is again available (Meguid et al., 1997). Degree and duration of reduced dopamine levels in the VMN positively correlate with meal size and particularly with a compensatory decrease in meal frequency (intermeal interval) that is meal number (Meguid et al., 2000). Shortly, the net effect of dopamine on feeding is determined by changes in synaptic dopamine levels of striatal and hypothalamic nuclei, which vary during feeding and fasting (meal – interval) and depend upon brain region (Table 1).
Bridging AN to its animal models with dopamine
There is neurotransmitter, neuroimaging and genetic evidence that dopamine is altered in AN and reduced dopamine metabolism relates to the state of low weight. The levels of the dopamine metabolite, homovanillic acid (HVA), in CSF were reported to be reduced in AN (Gillberg, 1983; Barbarich et al., 2003). After weight recovery, the abnormal levels of HVA in CSF seem to normalize (Kaye et al., 1984).
Women who had recovered from AN did not seem to distinguish between positive (rewarding) and negative (anhedonic) events, shown by their responses in the anterior ventral striatum, an area strongly influenced by dopamine transmission (Wagner et al., 2007). On the other hand, activation in the ventral striatum was higher in AN during processing of underweight stimuli than when processing normal-weight stimuli, while the opposite was observed in healthy controls (Fladung et al., 2010). The AN patients may thus be able to experience reward and pleasure, but their appreciation of rewarding stimuli may be altered. Self-starvation may be driven by inappropriately assigned pleasure associated with food restriction through the ventral striatal dopamine reward system.
Women who recovered from AN showed higher D2/D3 receptor binding potential in the anteroventral striatum than controls and this positively correlated to harm avoidance in the dorsal caudate and dorsal putamen (Frank and Kaye, 2005; Frank et al., 2005). The genes for D2 and D4, but not D3 dopamine receptors were associated with AN, while findings from the dopamine-metabolizing enzyme, catecholamine-O-methyltransferase, gene were less conclusive (Rask-Andersen et al., 2010). Another study found up-regulation of the dopamine transporter (DAT) and down-regulation of D2 receptors in AN, but no association between D4 receptors and AN (Frieling et al., 2010). Thus, dopamine binding and genetic studies support an involvement of the dopaminergic system in AN.
Consistent with the individuals with AN, in the mouse ABA model, alterations in dopamine signalling have been linked to susceptibility to ABA. The A/J mice, that are more vulnerable to ABA than the C57BL/6J mice, show increased RWA, but lack food-anticipatory activity and motivation to eat when exposed to the ABA model (Gelegen et al., 2008). These were accompanied by increased D2 receptor expression in the caudate putamen. In ABA rats, dopamine release in the ventral striatum was normally increased during feeding behaviour, but not during the initiation of food-anticipatory behaviour (Verhagen et al., 2009). Treatment with cis-flupenthixol, a non-selective dopamine receptor antagonist, resulted in an attenuated body weight loss and increased food intake in ABA rats (Verhagen et al., 2009). After blocking dopamine receptors with pimozide in ABA rats, light phase activity decreased and survival increased (Lambert and Porter, 1992). Animal data support decreased synaptic dopamine or increased D2 receptor density in the ABA model, similar to those in the AN model. These changes may contribute to the characteristic harm avoidance, increased physical activity and disturbance of reward mechanisms contributing to anhedonia of feeding in AN.
Bridging binge eating to its animal models with dopamine
Like drug addiction, food overconsumption is characterized by lack of control over initiation and termination of consumption, compulsive behaviour, adaptation/sensitization to rewarding stimulus and inappropriate functioning of the brain dopamine reward system (Wang et al., 2004). Palatable food, such as sugar and fat, increase dopamine levels in the nucleus accumbens (Hoebel et al., 1989). Bulimic patients with a history of binge eating have lower levels of the dopamine metabolite HVA in CSF (Jimerson et al., 1992), suggesting overeating as a compensatory response to the hypofunctional striatal dopamine signalling.
Down-regulation of D2-type receptors and an attenuated response of striatal dopamine to (palatable) food and food cues are also associated with overweight (Volkow et al., 2002; Stice et al., 2010). Body mass index (BMI) and altered food intake because of attenuated dopamine signalling correlate with polymorphisms of the dopamine receptor genes DRD2, DRD4 and the dopamine-metabolizing enzyme MAO-A (Fuemmeler et al., 2008; Kaplan et al., 2008; Stice et al., 2008; 2010). An epistatic interaction between catecholamine-O-methyltransferase and DAT1 genes on eating psychopathology and BED was reported (Hersrud and Stoltenberg, 2009). Obese individuals with BED overrepresent alleles in two genes, DRD2 and the μ-opioid receptor, as compared with other obese patients without BED (Davis et al., 2009). Adaptations within the natural reward pathways, specifically the endogenous opioids and dopamine, have been implicated in the transition from overeating to (hedonic) binge eating (Mathes et al., 2009). Thus, there is genetic evidence for an involvement of both opioid and dopamine signalling in the susceptibility to develop BED.
Prolonged binge-like intake of sugar increases dopamine release and D1 receptor binding in the nucleus accumbens and reduces D2 receptor binding in striatum in rats (Colantuoni et al., 2001; Rada et al., 2005). After 36 h of deprivation from sugar and chow, these rats display anxiety and reduced accumbal dopamine release as compared with rats that had not binge eaten the sugar (Avena et al., 2008). The D2 receptor down-regulation and D1 receptor up-regulation in the VMN and down-regulation of D1 receptors in the LHA were related to behavioural sensitization for having larger but less frequent meals (Fetissov et al., 2002). Supporting the role for altered dopaminergic activity in consumption of larger meals, rodent obesity models, genetic or environmental, display brain region specific changes in basal dopamine levels and D2 receptor and DAT expression, as compared with lean animals (Figlewicz et al., 1998; Fetissov et al., 2002). Dopamine release in the LHA during eating is greater in obese than in lean rats, resulting in larger meal size (Yang and Meguid, 1995). In a sham-feeding model of bulimia, a D2 receptor agonist failed to reduce sham feeding of 5% sucrose in gastric fistulated rats, but reduced it in intact animals, suggesting an ingestive response to D2 receptor activation only when potent satiety stimuli are present (Cooper et al., 1989). Opiate receptor antagonist naloxone was shown to attenuate sham feeding of sucrose solution in gastric fistulated rats and the μ-opioid receptor agonist DAMGO, stimulated high-fat consumption, supporting the role of opioid and dopaminergic reward pathways in overeating disorders and their animal models (Rockwood and Reid, 1982; Kirkham and Cooper, 1988; Will et al., 2006).
Dopamine also interacts with several neuropeptides in the regulation of food intake (see Vucetic and Reyes, 2010) and these neuropeptides may open new ways to treat AN and are thus important to shortly mention. Melanocortin receptor activation results in decreased food intake, increased energy expenditure and stimulation of hypothalamic-pituitary-adrenal axis (reviewed by Hillebrand et al., 2002). As these symptoms are also observed in ABA, a hyperactive melanocortin system might underlie ABA. Indeed, melanocortin binding sites are increased in the VMN during ABA (Kas et al., 2003). Inhibition of the melanocortin pathway by chronic intracerebroventricular infusion of agouti-related protein(83-132) increased survival in ABA rats by increasing food intake, reducing physical activity and normalizing body temperature (Kas et al., 2003). Stimulation of melanocortinergic activity by chronic intracerebroventricular infusion of α-melanocyte stimulating hormone was found to enhance ABA (Hillebrand et al., 2005b). An increased RWA during the light phase decreased food intake and body weight and increased activity of the hypothalamic-pituitary-adrenal axis were observed. Conversely, treatment with neuropeptide Y in ABA rats increased running and decreased food intake (Nergårdh et al., 2007).
Leptin has been shown to reduce hyperactivity in rats exposed to the SIH model. Both pro-opiomelanocortin neurons and neuropeptide Y/agouti-related protein neurons are sensitive to leptin. Stimulation of these neurons by leptin decreased food intake (Schwartz et al., 1997; Friedman and Halaas, 1998; Cowley et al., 2001). Weight loss is further promoted by leptin as it also exhibits metabolic effects, for example increased energy expenditure and thermogenesis (van Dijk, 2001). Chronic intracerebroventricular administration of leptin decreased RWA and food intake and increased energy expenditure by thermogenesis in rats during exposure to the ABA model (Hillebrand et al., 2005c). This reduction in hyperactivity by leptin administration could not offset the decline in food intake and the increased thermogenesis, resulting in weight loss and increased mortality.
Another hormone of interest in the pathophysiology of AN that interacts with dopamine is ghrelin. Ghrelin GHS-R1a receptors in the ventromedial hypothalamus (VMH) and dorsomedial hypothalamus were shown to mediate food-anticipatory activity (Merkestein et al., 2013). A diminished food-anticipatory activity and unaltered food intake were observed after administration of a ghrelin receptor antagonist by an acute intracerebroventricular injection in rats or by chronic peripheral administration in mice (Verhagen et al., 2011).
Besides the leptin-melanocortin pathway, the potential role of reward pathways and the endocannabinoid system in AN has become of research interest. Although treatment of ABA mice with a cannabinoid agonist, Δ9-tetrahydrocannabinol (THC), or an endocannabinoid uptake inhibitor, OMDM-2, increased food intake, it was not sufficient to attenuate weight loss and treatment with the agonist even further decreased survival in ABA mice (Lewis and Brett, 2010). In rats, administration of THC did not result in an increased mortality rate, which might be explained by species differences. On the contrary, Verty et al. (2011) report increased food intake, reduced body weight loss and altered RWA following cannabinoid agonist administration in ABA rats (Verty et al., 2011).
5-HT modulation of feeding
Brain microdialysis shows an increase in the release of 5-HT in the rat hypothalamus during eating and pre-ingestive events (Schwartz et al., 1990). Hypothalamic 5-HT is involved in satiety and satiation processes and 5-HT release is increased following the ingestion of a meal to generate a satiety signal for the termination of the meal (Table 1) (Haleem, 1993b). Caudal 5-HT neurons control the excitability of the projection area of nucleus tractus solitarius, the parabrachial nucleus, and inhibit feeding likely via 5-HT3 receptors in the nucleus tractus solitarius because blocking these receptors protects against starvation when agouti-related protein neurons are ablated (Wu et al., 2012). Pharmacological agents that increase 5-HT neurotransmission by enhancing 5-HT release and inhibiting its reuptake, with d-fenfluramine (Gibson et al., 1993), or by selectively inhibiting 5-HT reuptake, with fluoxetine (Tao et al., 2002), reduce food intake in humans and experimental animals (Heisler et al., 1999; Halford et al., 2007). The inhibitory action of 5-HT agents on food intake is likely mediated via postsynaptic 5-HT1B and 5-HT2C receptors in the hypothalamus (Kennett and Curzon, 1988). In contrast, activation of 5-HT1A receptors stimulates feeding in normal-weight animals (Voigt et al., 2002).
In addition to the hypothalamus, 5-HT influences food intake through the nucleus accumbens (Table 1). This brain area is involved in food-related reward, modulation of the physiological drive to eat and appetite-suppressant 5-HT signalling to the hypothalamus (Georgescu et al., 2005). The nucleus accumbens contains a high density of 5-HT4 receptors and pharmacological or genetic inhibition of 5-HT4 receptors in mice reduced food intake in fed mice, but not in food-deprived mice (Jean et al., 2007). Deletion of 5-HT4 receptors also attenuated responses to stress- or ecstasy (MDMA)-induced hypophagia (Compan et al., 2004; Jean et al., 2007). These studies suggest a role of 5-HT4 receptors in the inhibition of the drive to eat, which can particularly be seen in fed animals. Thus, 5-HT release in hypothalamus strongly regulates feeding by enhancing satiety and satiation, while striatal 5-HT can modulate motivational aspects of food intake either directly or via interactions with for example, dopamine transmission (Table 1).
Bridging AN to its animal models with 5-HT
In addition to feeding, 5-HT has been associated with many behavioural changes in AN, such as refusal to eat, excessive exercise or hyperactivity, stress, depression/anxiety and impaired impulse control. Restricted feeding and malnutrition in AN reduce brain 5-HT content, the 5-HT metabolite 5-HIAA in CSF and tryptophan in plasma, but these return to normal after weight recovery (Schweiger et al., 1986; Kaye et al., 1988; Attia et al., 2005). Indeed, the reduced availability of tryptophan, the precursor of 5-HT, because of restricted diet can reduce the 5-HT synthesis and neurotransmission (Haleem and Haider, 1996). The diet-induced reduction of tryptophan was associated with decreased anxiety in AN, suggesting restricting dietary intake may represent a mechanism to counteract dysphoric mood in AN (Kaye et al., 2003).
Low 5-HT levels may be compensated by up-regulation of 5-HT2C receptor-mediated responses after food restriction (Cowen et al., 1996). Moderate dieting in healthy women reduces plasma tryptophan, but increases the prolactin response to 5-HT releaser d-fenfluramine or to a 5-HT2(C) agonist meta-chlorophenylpiperazine, suggesting a functional sensitivity of 5-HT2C receptors after reduced brain 5-HT levels (Cowen et al., 1996). One study suggested reduced 5-HT2A receptor binding in the mesial temporal lobe and in some other cortical regions in active and even recovered AN (Frank et al., 2002).
Polymorphism of 5-HT2A receptor and 5-HT transporter (SERT; SLC6A4) genes may increase the risk for AN (Hinney et al., 2010; Clarke et al., 2012). Weight loss in teenage girls was linked to a polymorphism of 5-HT2C receptors (Westberg et al., 2002). A meta-analysis suggested that the genetic variance of SERT-linked genes contributes towards the susceptibility to AN (Lee and Lin, 2010), while another study did not find an association between this genotype and recovery from AN (Castellini et al., 2012).
Long-term dietary restriction (several days or weeks) reduces plasma tryptophan levels, brain synthesis and content of 5-HT and density of in rats (Haleem and Haider, 1996; Huether et al., 1997). In ABA rats, the initiation of food-anticipatory behaviour failed to increase accumbal 5-HT release, the levels of 5-HT remained low and their circadian activity was blunted (Verhagen et al., 2009). In spite of low 5-HT levels, rats starved for 4 days showed post-fasting anorexia, suggesting an up-regulation of satiety signals (Duhault et al., 1993). The enhanced satiety and suppression of appetite may result from a compensatory up-regulation of 5-HT receptors that occurs after restricted diet-induced reduction of brain 5-HT content (Haleem and Haider, 1996). Similar to women on a diet, the prolactin response to meta-chlorophenylpiperazine was increased in rats after restricted diet, indicating a functional up-regulation of 5-HT2C receptors (Franklin et al., 1999). However, prolactin responses to 5-HT1A or 5-HT2A agonists were small and only transient, suggesting that only some, but not all, 5-HT receptor subtypes are up-regulated in response to low brain 5-HT (Franklin et al., 1999).
Dysregulation of 5-HT neurotransmission underlying behavioural changes in AN further supports the sex differences in AN. Central synthesis, metabolism and functional responses of 5-HT and the anorectic effect of 5-HT via the 5-HT2C receptor (located on the X-chromosome) are greater in female than in male rats (Haleem, 1993a). The 5-HT1A or 5-HT2C receptor activation or exposure to stress increases corticosterone in plasma more in female rats than male rats (Haleem et al., 1989; Haleem, 1993a). There is thus evidence to support a role for 5-HT in pathophysiology of AN. However, considering that 5-HT regulates several different behaviours, it cannot be ruled out that the other behavioural changes, such as stress, may contribute to 5-HT dysregulation and anorexia.
Bridging binge eating to its animal models with 5-HT
Findings from 5-HT dysfunction in BN or BED are complex and often overlap with findings from obese humans or animals, such that the 5-HT dysfunction in the two conditions cannot be completely separated. Bulimic patients with a history of binge eating have reduced levels of 5-HT metabolite 5-HIAA in CSF and the 5-HIAA level inversely correlates with binge frequency (Jimerson et al., 1992). Low central 5-HT activity may contribute to blunted satiety responses in BN. Several 5-HT receptor subtypes, namely 5-HT1B, 5-HT2A, 5-HT2C, 5-HT4 and 5-HT6, are implicated in the pathophysiology of eating disorders. The 5-HT2A receptor binding in the cerebral cortex positively correlates to BMI (Erritzoe et al., 2009) and 5-HT2A receptor binding in the midbrain was still altered in patients who had recovered from BN, suggesting altered 5-HT function even after recovery from BN (Kaye et al., 2001). There is a strong positive association between BMI and cerebral SERT binding or 5-HT4 receptor density in the nucleus accumbens, ventral pallidum, left hippocampal region and orbitofrontal cortex (Erritzoe et al., 2010; Haahr et al., 2012). Obese binge-eating women have decreased SERT binding in the midbrain compared with obese controls (Kuikka et al., 2001). Up-regulation of 5-HT2A receptors and down-regulation of SERT may be compensatory changes to lower brain 5-HT content in overweight individuals, leading to increased food intake (Erritzoe et al., 2009).
Changes in food intake and BMI correlate with polymorphism of 5-HT2A receptors and SERT (Fuemmeler et al., 2008; Sorlí et al., 2008). These may not directly be genetic risk factors for eating disorder, but play a role in determining BMI in obese subjects (Sorlí et al., 2008; Munn-Chernoff et al., 2012). In spite of being implicated in obesity, polymorphism of the human 5-HT2C receptor have not clearly been associated with BN or BED (Burnet et al., 1999; Nacmias et al., 1999).
Further evidence for the role of 5-HT in pathophysiology of BED originates from genetic and environmental animal models. The hypothalamic 5-HT release was decreased, SERT binding was lower and 5-HT2A and 5-HT4 receptor binding was higher, particularly in the reward-mediating nucleus accumbens shell in genetic or diet-induced obese rodents (De Fanti et al., 2000; Ratner et al., 2012). Chronic hyperphagia increased hypothalamic 5-HT2A, 5-HT2C and 5-HT1B gene expression, and on the contrary, inactivation of 5-HT2A receptors inhibited overfeeding and obesity in A(y) mice expressing the ectopic agouti protein (Nonogaki et al., 2006a,b). Moreover, 5-HT6 and 5-HT4 receptors have been suggested to play a role in overeating and obesity. Mice carrying a non-functional 5-HT6 receptor consumed less high-fat diet, gained less weight and had less fat accumulation (Frassetto et al., 2008). The 5-HT4 receptor is involved in food intake and pharmacological or genetic manipulation of 5-HT4 receptor in reward-related brain areas alters food intake (Jean et al., 2007).
Noradrenergic modulation of feeding
From the locus coeruleus noradrenergic fibres innervate the neocortex, thalamus, amygdala, hippocampus, hypothalamus and spinal cord. Noradrenaline is released during consumption of a meal within the anterior hypothalamus, but not within the lateral ventricles (Martin and Myers, 1975). The increase in noradrenaline in the paraventricular nucleus correlates with the size of a meal: when rats consumed large meals under satiated conditions at dark onset, levels of noradrenaline were higher than when rats consumed small meals (Table 1) (Paez et al., 1993). Noradrenaline can either elicit food intake or promote satiety depending on the site of application (Table 1). Low local concentration of exogenous noradrenaline in the hypothalamic paraventricular nucleus, innervated by noradrenergic fibres, elicited vigorous feeding (Grossman, 1975). Application of noradrenaline into the rat perifornical hypothalamus reduced eating, possibly by promoting satiety via α-adrenoceptors (Margules, 1970). Lesions positioned within the ascending ventral noradrenergic tracts, the paraventricular nucleus, the lateral border of VMN or the more caudal regions can result in depletion of noradrenaline and overeating to obesity (Ahlskog and Hoebel, 1973; Kirchgessner and Sclafani, 1988).
α-Adrenoceptor subtypes within the paraventricular nucleus exert antagonistic actions on feeding. Stimulation of α1- or β2-adrenoceptors activates descending feeding-inhibitory fibres in the paraventricular nucleus, which suppresses food intake. Activation of α2-adrenoceptors in the paraventricular nucleus induces inhibitory postsynaptic potentials, which results in disinhibition of the descending satiety cells, which, in turn, stimulates food intake (Wellman, 2000). Thus, pharmacological manipulations with direct adrenoceptor agonists or drugs that release noradrenaline or block transport of noradrenaline can increase or decrease food intake, depending on the site and type of noradrenaline manipulation. Many anti-obesity drugs, such as sibutramine and amphetamine, are suggested to reduce eating via release of noradrenaline and subsequent activation of α1-adrenoceptors (Ahlskog and Hoebel, 1973; Janhunen et al., 2011).
The paraventricular nucleus exhibits a reliable rhythm in the secretion of endogenous noradrenaline over the dark and light cycle. Microdialysis studies indicate that extracellular levels of noradrenaline within the paraventricular nucleus peak at the onset of the dark cycle, a period during which rats engage in eating (Figure 1) (Morien et al., 1995). Sensitivity to the stimulatory action of noradrenaline on feeding is higher at the onset of the dark, which may relate to increased numbers of α2-adrenoceptors within the paraventricular nucleus at the onset of dark (Jhanwar-Uniyal et al., 1986). The effects of noradrenaline on feeding and meal size can thus vary from hypophagia to hyperphagia, depending upon the subset of adrenoceptors and the brain region activated (Table 1).
Bridging AN to its animal models with noradrenaline
Noradrenaline is involved in arousal, alertness, anxiety, stress, mood and it affects activity of the reward system. Central and peripheral noradrenergic pathways are down-regulated in prolonged fasting and acute AN (Halmi et al., 1978). The noradrenaline levels in CSF are normal in underweight and weight-restored AN patients, but lower in long-term weight-recovered patients (Kaye et al., 1985), indicating a potential intrinsic disorder in central noradrenaline metabolism. Indeed, the noradrenaline response to a meal or exercise is reduced in AN patients (Pirke, 1996), but tends to normalize with weight gain and recovery (Kaye et al., 1985; Pirke, 1996).
As noradrenaline plays a role in stress behaviours, it is possible that stress contributes to altered noradrenaline transmission and AN. Noradrenaline also plays a role in anxiety, an important symptom of AN, and genes encoding proteins which remove noradrenaline from the synapse are good candidates for affecting susceptibility to AN. Polymorphism of the noradrenaline transporter protein, NET (SL6A2) were associated with AN (Urwin et al., 2002), however, another study did not confirm this association (Hu et al., 2007). A polymorphism of the noradrenaline-metabolizing enzyme, MAO-A, may affect susceptibility to AN, particularly the restricting AN (Urwin et al., 2003). MAO-A, NET and SERT gene variants appear to contribute additively to the risk of developing restricting AN (Urwin et al., 2003; Urwin and Nunn, 2005).
In the ABA model, the hypothalamic noradrenaline turnover, estimated by the concentration of its major metabolite, 3-methoxy-4-hydroxyphenylglycol (MHPG, MOPEG), was decreased in male rats with restricted feeding, but hyperactivity compensated this reduction (Broocks et al., 1990). Another study suggested that changes in food intake in ABA rats are due to increased activity of the hypothalamic-pituitary-adrenal axis rather than altered noradrenaline concentration (Burden et al., 1993). Interestingly, reduced noradrenaline turnover could underlie hyperactivity in AN and ABA because NET-deficient animals show enhanced responses to locomotor stimulation and because running activity of the semistarved rats can be blocked by α2-adrenoceptor agonists (Pirke et al., 1993). In addition to AN and hyperactivity, the low noradrenaline neurotransmission could underlie symptoms, such as low blood pressure, anxiety and mood disorder, found in AN patients. All in all, the role of noradrenaline in AN is complex and noradrenaline can contribute to several central and peripheral characteristics of AN.
Bridging binge eating to its animal models with noradrenaline
The noradrenergic system stimulates hunger and preferential consumption of carbohydrates in experimental animals and humans (Leibowitz, 1990; Paez et al., 1993). Impaired central noradrenaline function has been found in AN (described earlier), major depressive disorder, obsessive compulsive disorder and panic disorder. These pathologies are often associated with, or possibly concurring in, the development of BN (Kaye, 2008). However, the question whether central noradrenaline function is altered in BN needs more investigation. In the symptomatic phases of BN, noradrenaline levels in CSF are lower than in healthy controls, but return to normal baseline during recovery (Kaye et al., 1990a,b). Bingeing behaviour in BN was suggested to parallel with overactivity of hypothalamic α-adrenoceptors (Kaye and Weltzin, 1991). Basal levels of noradrenaline in plasma do not differ between BN and healthy individuals. A meal produces a higher increase of noradrenaline in BN, while the noradrenaline responses in plasma to pharmacological challenge with isoprenaline, exercise or stress are blunted compared with controls (George et al., 1990; Brambilla, 2001). Intake of highly caloric diets increases peripheral turnover and plasma levels of noradrenaline, which likely contributes to increased excretion of noradrenaline and hypertension (Kotsis et al., 2010).
There is a lack of evidence for the role of noradrenaline transmission in BN or BED in humans and animals. The few studies have investigated noradrenaline transmission in overeating or obese individuals. The thalamus, a part of the motivational neurocircuitry, becomes activated during pictures of high- versus low-calorie foods (Killgore et al., 2003). The NET binding potential was reduced in the thalamus in obese individuals (Li et al., 2013). Food craving positively correlates with activation of the thalamus and cortical-limbic-striatal areas during cues of favourite food and stress in obese, but not lean individuals (Jastreboff et al., 2013). Reduced NET activity in thalamic areas may underlie stress-induced overeating and obesity.
There is no clear evidence whether and how noradrenaline and adrenoceptors are altered in animals modelling BN or BED. Reduced brain noradrenaline metabolism, particularly in the hypothalamic VMN, LHA, paraventricular and dorsomedial nucleus, has been reported in genetic or diet-induced obese rodents (Johnston et al., 1986; Levin, 1995). Obese rats and mice exhibit increased number of α2-adrenoceptors located specifically in the paraventricular nucleus and in controlling energy intake and expenditure, in relation to high-fat diet. Furthermore, they display differences in genetic and hormonal factors that are known to contribute to the onset and maintenance of obesity (Jhanwar-Uniyal et al., 1988; 1991; Levin, 1996).
Treatment of AN with monoaminergic agents
Currently, the first line of treatment for underweight patients with AN, is feeding and weight restoration, combined with psychotherapy. In ABA rats, certain palatable diets have been found to affect the development of, and recovery from, ABA (Brown et al., 2008). Evidence for the efficacy of drug treatments for AN is limited, and currently there are no FDA or EMA approved drugs for the treatment of AN.
As described earlier, there is considerable evidence that patients with AN and BN have disturbances in dopamine, 5-HT and noradrenaline neurotransmission. Dopamine receptor agonists, including levodopa, have not been found to produce positive effects on weight gain in AN (Johnson et al., 1983). As antipsychotics affect dopamine, 5-HT and noradrenaline systems and affect feeding and body weight, it seems possible that they might also facilitate recovery from AN and BN. In the ABA animal model, the non-selective dopamine receptor antagonist, cis-flupenthixol, reduced activity levels, increased body weight and increased food intake (Verhagen et al., 2009). The atypical antipsychotic olanzapine reduced wheel running, starvation-induced hypothermia and activation of the hypothalamus-pituitary-adrenal axis, and thereby diminished development of ABA in rats (Hillebrand et al., 2005a). Olanzapine treatment also reduced physical activity in hyperactive AN patients in a subsequent small open-label study (Hillebrand et al., 2005a). One-week treatment with olanzapine increased survival and reduced food-anticipatory activity in ABA mice, but it did not alter food intake or RWA during ad libitum feeding or restriction conditions, or in mice housed without wheels (Klenotich et al., 2012). Possible sedative effects by high doses of olanzapine may have caused the different outcomes.
There are several case series reporting efficacy of antipsychotics in AN, including olanzapine, chlorpromazine, haloperidol, quetiapine and aripiprazole. Meta-analysis, however, failed to demonstrate efficacy for antipsychotics for body weight and related outcomes in females with AN (Kishi et al., 2012). There are nine randomized, controlled trials with contradictory findings from the efficacy of antipsychotics in AN. Olanzapine, studied in five trials, showed superiority to placebo in three studies, to chlorpromazine in one study and to aripiprazole in one study in terms of weight gain and/or reduction in obsessional symptoms (see Brewerton, 2012). Pimozide, but not risperidone or sulpiride, tended to facilitate weight gain as compared with placebo. Quetiapine had no efficacy in AN in a double-blind placebo-controlled trial (Powers et al., 2012). The use and efficacy of first-generation antipsychotics is often limited by adverse effects (Krüger and Kennedy, 2000). However, atypical antipsychotics, such as olanzapine, may be advantageous by reducing psychological symptoms that contribute to early relapse, including obsessions and depression.
The ABA rats treated with fenfluramine, a 5-HT releasing agent, prior to the daily 2 h food access displayed an accelerated rate of weight loss (Atchley and Eckel, 2005). However, another group reported only hypodipsia upon chronic treatment with fenfluramine in ABA rats, which may explain the loss of body weight (Hillebrand et al., 2006). The discrepancy between these findings may result from differences in methodology, animal species and strain and oestrous cycle (Hillebrand et al., 2006). Chronic administration of a selective 5-HT reuptake inhibitor (SSRI) has been shown to reduce ABA in rats by attenuation of weight loss (Altemus et al., 1996), decreased running activity (Altemus et al., 1996; Yokoyama et al., 2007) and increased food intake (Altemus et al., 1996). In ABA mice, 4-week treatment with the SSRI fluoxetine increased food intake and reduced food-anticipatory activity, but did not alter survival (Klenotich et al., 2012). Indeed, SSRI or other antidepressant medication seem to have limited effects in treating AN or preventing relapse (Walsh et al., 2006). The prevalence of non-responders (roughly one out of two) and the presence of a functional genetic polymorphism in the promotor region of the gene for SERT emphasize the potential utility of psychopharmacogenetics in prescribing SSRIs in the treatment of AN patients (Gorwood, 2004). Antidepressants might still treat the AN-associated anxiety and depression and are thus commonly described in AN.
Cyproheptadine is a 5-HT and histamine antagonist that was first observed to stimulate appetite in allergic patients, and some suggested it could stimulate appetite in the AN (Capasso et al., 2009). Systemic α2-adrenoceptor agonist clonidine increases feeding and wheel running but does not affect rate of weight loss in ABA rats (Rieg and Aravich, 1994). In AN patients, clonidine did not show advantages over placebo.
Treatment of binge eating disorder with monoaminergic agents
Monoaminergic drugs are so far the most widely and successfully used group of compounds in the treatment of overeating and obesity. The first major class was composed of amphetamine-like releasers of dopamine, 5-HT and noradrenaline, including phentermine and fenfluramine, alone and in combination. In spite of clear appetite suppressant and anorectic effects, their use was limited by adverse effects in cardiovascular system and the combination was withdrawn from clinical use in the late 90s (reviewed by Kintscher, 2012). These compounds were followed by the reuptake inhibitor of 5-HT and noradrenaline, sibutramine that reduced food intake by enhancing satiety and consequently reduced body weight. The satiety-enhancing effect of sibutramine is mainly mediated by α2B- and α1-adrenoceptors and 5-HT1B receptors (Janhunen et al., 2011). However, sibutramine’s clinical use was limited in 2010 after it was reported to cause cardiovascular adverse effects such as hypertension (Kintscher, 2012). There are open-label and randomized, double-blind, placebo-controlled studies reporting that sibutramine reduces binge episodes, body weight and depressive symptoms in obese patients with BED (Appolinario et al., 2002; 2003; Milano et al., 2005; Bauer et al., 2006).
There is also evidence that selective reuptake inhibition of either noradrenaline or particularly 5-HT has therapeutic efficacy in BN and BED. Antidepressants, especially SSRIs, are modestly effective in reducing binge eating over the short term in BN and BED and over the long term in BN (McElroy et al., 2012). Fluoxetine (60 mg) in obese patients with BED reduced weight and the number of binge attacks (Shapiro et al., 2007). Fluvoxamine and sertraline also appeared to reduce the frequency of eating, the quantity of food and the amounts of carbohydrates, as well as facilitate the induction satiety (Hay and Bacaltchuk, 2002). Case studies suggest that inhibitors of noradrenaline reuptake, such as reboxetine or desipramine, reduce binge episodes and depression in BN (El-Giamal et al., 2000). Topiramate has consistently been shown to decrease binge eating in BED and BN, but adverse effects limit its use (McElroy et al., 2012). In rodent models of obesity, topiramate reduced food intake and increased energy expenditure, resulting in reduced body weight (Richard et al., 2000). Also, tricyclic antidepressants, MAO inhibitors, mianserin, trazodone and bupropion have been shown to reduce binge episodes in humans (Bacaltchuk and Hay, 2003).
Similar to humans, in the animal model in which rats were submitted to caloric restriction and stress, sibutramine and fluoxetine inhibited (palatable) food intake in all conditions, topiramate selectively inhibited compulsive intake of highly palatable food and midazolam increased it, suggesting predictive validity for this preclinical model (Cifani et al., 2009; Avena, 2013).
The dopamine and noradrenaline reuptake inhibitor, bupropion, decreased food consumption by reducing meal size, postponing meal initiation and increased locomotor activity in rats (Zarrindast and Hosseini-Nia, 1988; Janhunen et al., 2013). Both effects likely contribute to bupropion’s weight reducing properties in obese individuals (Gadde et al., 2001). The effects of bupropion on meal size seem to be mediated via α1- and α2B-adrenoceptors and several dopamine receptor subtypes (Janhunen et al., 2013).
Concluding remarks
Eating disorders, such as AN, BN and binge eating disorder, display complex underlying mechanisms, which emphasize the importance of predictive and translational animal models in understanding these disorders and their treatment. This review discusses the most widely used and representative animal models of eating disorders and the related changes in monoaminergic neurotransmitters. Dopamine, 5-HT and noradrenaline in hypothalamic and striatal regions regulate food intake, satiety and rewarding and motivational aspects of feeding. Reduced neurotransmission in these systems has been implicated in the pathophysiology of different eating disorders in humans. Consistently, several monoaminergic drugs have been found efficacious in animal models and some of them have proven useful in the treatment of eating disorders, particularly those with overeating symptoms in humans.
Acknowledgments
This work was supported by TI Pharma, project T5-210-1.
Glossary
- SERT
5-HT (serotonin) transporter
- ABA
activity-based anorexia
- AN
anorexia nervosa
- BED
binge eating disorder
- BMI
body mass index
- BN
bulimia nervosa
- DAT
dopamine transporter
- HVA
homovanillic acid
- LHA
lateral hypothalamus
- NET
noradrenaline transporter
- RWA
running wheel activity
- SIH
stress-induced hyperactivity
- SSRI
selective 5-HT (serotonin) reuptake inhibitor
- THC
Δ9-tetrahydrocannabinol
- VMH
ventromedial hypothalamus
- VMN
ventromedial nucleus
Conflict of interest
SKJ is employed by Orion Pharma, which sells one or more of the drugs mentioned in this review.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, Hoebel BG. Overeating and obesity from damage to a noradrenergic system in the brain. Science. 1973;182:166–169. doi: 10.1126/science.182.4108.166. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. British Journal of Pharmacology. 2013c;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Glowa JR, Galliven E, Leong Y-M, Murphy DL. Effects of serotonergic agents on food-restriction-induced hyperactivity. Pharmacol Biochem Behav. 1996;53:123–131. doi: 10.1016/0091-3057(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- Anliker J, Mayer J. An operant conditioning technique for studying feeding-fasting patterns in normal and obese mice. J Appl Physiol. 1956;8:667–670. doi: 10.1152/jappl.1956.8.6.667. [DOI] [PubMed] [Google Scholar]
- Appolinario JC, Godoy-Matos A, Fontenelle LF, Carraro L, Cabral M, Vieira A, et al. An open-label trial of sibutramine in obese patients with binge-eating disorder. J Clin Psychiatry. 2002;63:28–30. doi: 10.4088/jcp.v63n0106. [DOI] [PubMed] [Google Scholar]
- Appolinario JC, Bacaltchuk J, Sichieri R, Claudino AM, Godoy-Matos A, Morgan C, et al. A randomized, double-blind, placebo-controlled study of sibutramine in the treatment of binge-eating disorder. Arch Gen Psychiatry. 2003;60:1109–1116. doi: 10.1001/archpsyc.60.11.1109. [DOI] [PubMed] [Google Scholar]
- Artiga AI, Viana JB, Maldonado CR, Chandler-Laney PC, Oswald KD, Boggiano MM. Body composition and endocrine status of long-term stress-induced binge-eating rats. Physiol Behav. 2007;91:424–431. doi: 10.1016/j.physbeh.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchley DPD, Eckel LA. Fenfluramine treatment in female rats accelerates the weight loss associated with activity-based anorexia. Pharmacol Biochem Behav. 2005;80:273–279. doi: 10.1016/j.pbb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Attia E, Wolk S, Cooper T, Glasofer D, Walsh BT. Plasma tryptophan during weight restoration in patients with anorexia nervosa. Biol Psychiatry. 2005;57:674–678. doi: 10.1016/j.biopsych.2004.11.045. [DOI] [PubMed] [Google Scholar]
- Avena NM. Animal Models of Eating Disorders. Totowa, NJ: Humana Press; 2013. [Google Scholar]
- Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94:309–315. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs RK, Wojnicki FHE, Corwin RLW. Effect of 2-hydroxyestradiol on binge intake in rats. Physiol Behav. 2011;103:508–512. doi: 10.1016/j.physbeh.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs RK, Unger EL, Corwin RLW. 2-Hydroxyestradiol enhances binge onset in female rats and reduces prefrontal cortical dopamine in male rats. Horm Behav. 2013;63:88–96. doi: 10.1016/j.yhbeh.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacaltchuk J, Hay P. Antidepressants versus placebo for people with bulimia nervosa. Cochrane database Syst Rev. 2003;(4) doi: 10.1002/14651858.CD003391. CD003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Backer MWA, Brans MAD, Luijendijk MCM, Garner KM, van den Heuvel DMA, Pasterkamp RJ, et al. Neuropeptide delivery to the brain: a von Willebrand factor signal peptide to direct neuropeptide secretion. BMC Neurosci. 2010;11:94. doi: 10.1186/1471-2202-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarich NC, Kaye WH, Jimerson D. Neurotransmitter and imaging studies in anorexia nervosa: new targets for treatment. Curr Drug Targets CNS Neurol Disord. 2003;2:61–72. doi: 10.2174/1568007033338779. [DOI] [PubMed] [Google Scholar]
- Bauer C, Fischer A, Keller U. Effect of sibutramine and of cognitive-behavioural weight loss therapy in obesity and subclinical binge eating disorder. Diabetes Obes Metab. 2006;8:289–295. doi: 10.1111/j.1463-1326.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- Brambilla F. Aetiopathogenesis and pathophysiology of bulimia nervosa: biological bases and implications for treatment. CNS Drugs. 2001;15:119–136. doi: 10.2165/00023210-200115020-00004. [DOI] [PubMed] [Google Scholar]
- Brewerton TD. Antipsychotic agents in the treatment of anorexia nervosa: neuropsychopharmacologic rationale and evidence from controlled trials. Curr Psychiatry Rep. 2012;14:398–405. doi: 10.1007/s11920-012-0287-6. [DOI] [PubMed] [Google Scholar]
- Brobeck JR, Tepperman J, Long CNH. Experimental hypothalamic hyperphagia in the albino rat. Yale J Biol Med. 1943;15:831–853. [PMC free article] [PubMed] [Google Scholar]
- Broocks A, Liu J, Pirke KM. Semistarvation-induced hyperactivity compensates for decreased norepinephrine and dopamine turnover in the mediobasal hypothalamus of the rat. J Neural Transm Gen Sect. 1990;79:113–124. doi: 10.1007/BF01251006. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Avena NM, Hoebel BG. A high-fat diet prevents and reverses the development of activity-based anorexia in rats. Int J Eat Disord. 2008;41:383–389. doi: 10.1002/eat.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden VR, White BD, Dean RG, Martin RJ. Activity of the hypothalamic-pituitary-adrenal axis is elevated in rats with activity-based anorexia. J Nutr. 1993;123:1217–1225. doi: 10.1093/jn/123.7.1217. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Smith KA, Cowen PJ, Fairburn CG, Harrison PJ. Allelic variation of the 5-HT2C receptor (HTR2C) in bulimia nervosa and binge eating disorder. Psychiatr Genet. 1999;9:101–104. doi: 10.1097/00041444-199906000-00009. [DOI] [PubMed] [Google Scholar]
- Capasso A, Putrella C, Milano W. Recent clinical aspects of eating disorders. Rev Recent Clin Trials. 2009;4:63–69. doi: 10.2174/157488709787047594. [DOI] [PubMed] [Google Scholar]
- Casper RC, Sullivan EL, Tecott L. Relevance of animal models to human eating disorders and obesity. Psychopharmacology (Berl) 2008;199:313–329. doi: 10.1007/s00213-008-1102-2. [DOI] [PubMed] [Google Scholar]
- Castellini G, Ricca V, Lelli L, Bagnoli S, Lucenteforte E, Faravelli C, et al. Association between serotonin transporter gene polymorphism and eating disorders outcome: a 6-year follow-up study. Am J Med Genet Part B Neuropsychiatr Genet. 2012;159B:491–500. doi: 10.1002/ajmg.b.32052. [DOI] [PubMed] [Google Scholar]
- Choi S, Dallman MF. Hypothalamic obesity: multiple routes mediated by loss of function in medial cell groups. Endocrinology. 1999;140:4081–4088. doi: 10.1210/endo.140.9.6964. [DOI] [PubMed] [Google Scholar]
- Cifani C, Polidori C, Melotto S, Ciccocioppo R, Massi M. A preclinical model of binge eating elicited by yo-yo dieting and stressful exposure to food: effect of sibutramine, fluoxetine, topiramate, and midazolam. Psychopharmacology (Berl) 2009;204:113–125. doi: 10.1007/s00213-008-1442-y. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Weiss ARD, Berrettini WH. The genetics of anorexia nervosa. Clin Pharmacol Ther. 2012;91:181–188. doi: 10.1038/clpt.2011.253. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Compan V, Charnay Y, Dusticier N, Daszuta A, Hen R, Bockaert J. [Feeding disorders in 5-HT4 receptor knockout mice] J Soc Biol. 2004;198:37–49. [PubMed] [Google Scholar]
- Cooper SJ, Al-Naser HA. Dopaminergic control of food choice: contrasting effects of SKF 38393 and quinpirole on high-palatability food preference in the rat. Neuropharmacology. 2006;50:953–963. doi: 10.1016/j.neuropharm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Rusk IN, Barber DJ. Sucrose sham-feeding in the rat after administration of the selective dopamine D2 receptor agonist N-0437, d-amphetamine or cocaine. Pharmacol Biochem Behav. 1989;32:447–452. doi: 10.1016/0091-3057(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FHE. Binge eating in rats with limited access to vegetable shortening. Curr Protoc Neurosci. 2006;9 doi: 10.1002/0471142301.ns0923bs36. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Clifford EM, Walsh AE, Williams C, Fairburn CG. Moderate dieting causes 5-HT2C receptor supersensitivity. Psychol Med. 1996;26:1155–1159. doi: 10.1017/s003329170003587x. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, et al. Dopamine for ‘wanting’ and opioids for ‘liking’: a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 2009;17:1220–1225. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- Davis JD, Campbell CS. Peripheral control of meal size in the rat: Effect of sham feeding on meal size and drinking rate. J Comp Physiol Psychol. 1973;83:379–387. doi: 10.1037/h0034667. [DOI] [PubMed] [Google Scholar]
- De Fanti BA, Gavel DA, Hamilton JS, Horwitz BA. Extracellular hypothalamic serotonin levels after dorsal raphe nuclei stimulation of lean (Fa/Fa) and obese (fa/fa) Zucker rats. Brain Res. 2000;869:6–14. doi: 10.1016/s0006-8993(00)02308-8. [DOI] [PubMed] [Google Scholar]
- van Dijk G. The role of leptin in the regulation of energy balance and adiposity. J Neuroendocrinol. 2001;13:913–921. doi: 10.1046/j.1365-2826.2001.00707.x. [DOI] [PubMed] [Google Scholar]
- Dixon DP, Ackert AM, Eckel LA. Development of, and recovery from, activity-based anorexia in female rats. Physiol Behav. 2003;80:273–279. doi: 10.1016/j.physbeh.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Duhault J, Lacour F, Espinal J, Rolland Y. Effect of activation of the serotoninergic system during prolonged starvation on subsequent caloric intake and macronutrient selection in the Zucker rat. Appetite. 1993;20:135–144. doi: 10.1006/appe.1993.1015. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Keel PK, Dorer DJ, Delinsky SS, Franko DL, Herzog DB. Longitudinal comparison of anorexia nervosa subtypes. Int J Eat Disord. 2002;31:191–201. doi: 10.1002/eat.10016. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165:245–250. doi: 10.1176/appi.ajp.2007.07060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Giamal N, de Zwaan M, Bailer U, Lennkh C, Schüssler P, Strnad A, et al. Reboxetine in the treatment of bulimia nervosa: a report of seven cases. Int Clin Psychopharmacol. 2000;15:351–356. doi: 10.1097/00004850-200015060-00006. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Frokjaer VG, Haugbol S, Marner L, Svarer C, Holst K, et al. Brain serotonin 2A receptor binding: relations to body mass index, tobacco and alcohol use. Neuroimage. 2009;46:23–30. doi: 10.1016/j.neuroimage.2009.01.050. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Frokjaer VG, Haahr MT, Kalbitzer J, Svarer C, Holst KK, et al. Cerebral serotonin transporter binding is inversely related to body mass index. Neuroimage. 2010;52:284–289. doi: 10.1016/j.neuroimage.2010.03.086. [DOI] [PubMed] [Google Scholar]
- Fallon D. Eatometer: a device for continuous recording of free-feeding behavior. Science. 1965;148:977–978. doi: 10.1126/science.148.3672.977. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Sato T, Zhang L-H. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am J Physiol Regul Integr Comp Physiol. 2002;283:R905–R910. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Patterson TA, Johnson LB, Zavosh A, Israel PA, Szot P. Dopamine transporter mRNA is increased in the CNS of Zucker fatty (fa/fa) rats. Brain Res Bull. 1998;46:199–202. doi: 10.1016/s0361-9230(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Finger FW. The effect of food deprivation and subsequent satiation upon general activity in the rat. J Comp Physiol Psychol. 1951;44:557–564. doi: 10.1037/h0055692. [DOI] [PubMed] [Google Scholar]
- Fladung A-K, Grön G, Grammer K, Herrnberger B, Schilly E, Grasteit S, et al. A neural signature of anorexia nervosa in the ventral striatal reward system. Am J Psychiatry. 2010;167:206–212. doi: 10.1176/appi.ajp.2009.09010071. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Luijendijk MCM, van der Zwaal EM, Brans MAD, Adan RAH. The snacking rat as model of human obesity: effects of a free-choice high-fat high-sugar diet on meal patterns. Int J Obes. 2013;2005:643–649. doi: 10.1038/ijo.2013.159. [DOI] [PubMed] [Google Scholar]
- Frank GK, Kaye WH. Positron emission tomography studies in eating disorders: multireceptor brain imaging, correlates with behavior and implications for pharmacotherapy. Nucl Med Biol. 2005;32:755–761. doi: 10.1016/j.nucmedbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Frank GK, Kaye WH, Meltzer CC, Price JC, Greer P, McConaha C, et al. Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biol Psychiatry. 2002;52:896–906. doi: 10.1016/s0006-3223(02)01378-1. [DOI] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58:908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Franklin M, Craven RD, Dowling B, Campling G, Elliott JM, Cowen PJ. Effect of a long-term low tryptophan diet on the prolactin responses to the 5-HT1A and 5-HT2C agonists, 8-OH-DPAT and mCPP in the male rat. J Psychopharmacol Oxf Engl. 1999;13:58–63. doi: 10.1177/026988119901300107. [DOI] [PubMed] [Google Scholar]
- Frassetto A, Zhang J, Lao JZ, White A, Metzger JM, Fong TM, et al. Reduced sensitivity to diet-induced obesity in mice carrying a mutant 5-HT6 receptor. Brain Res. 2008;1236:140–144. doi: 10.1016/j.brainres.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Frieling H, Römer KD, Scholz S, Mittelbach F, Wilhelm J, De Zwaan M, et al. Epigenetic dysregulation of dopaminergic genes in eating disorders. Int J Eat Disord. 2010;43:577–583. doi: 10.1002/eat.20745. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Agurs-Collins TD, McClernon FJ, Kollins SH, Kail ME, Bergen AW, et al. Genes implicated in serotonergic and dopaminergic functioning predict BMI categories. Obesity (Silver Spring) 2008;16:348–355. doi: 10.1038/oby.2007.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde KM, Parker CB, Maner LG, Wagner HR, 2nd, Logue EJ, Drezner MK, et al. Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese women. Obes Res. 2001;9:544–551. doi: 10.1038/oby.2001.71. [DOI] [PubMed] [Google Scholar]
- Garfinkel PE. Perception of hunger and satiety in anorexia nervosa. Psychol Med. 1974;4:309–315. doi: 10.1017/s0033291700042999. [DOI] [PubMed] [Google Scholar]
- Gelegen C, Heuvel J, van den Collier Campbell IC, Oppelaar H, Hessel E, et al. Dopaminergic and brain-derived neurotrophic factor signalling in inbred mice exposed to a restricted feeding schedule. Genes Brain Behav. 2008;7:552–559. doi: 10.1111/j.1601-183X.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- George DT, Kaye WH, Goldstein DS, Brewerton TD, Jimerson DC. Altered norepinephrine regulation in bulimia: effects of pharmacological challenge with isoproterenol. Psychiatry Res. 1990;33:1–10. doi: 10.1016/0165-1781(90)90143-s. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolaños CA, Marsh DJ, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EL, Kennedy AJ, Curzon G. d-Fenfluramine- and d-norfenfluramine-induced hypophagia: differential mechanisms and involvement of postsynaptic 5-HT receptors. Eur J Pharmacol. 1993;242:83–90. doi: 10.1016/0014-2999(93)90013-8. [DOI] [PubMed] [Google Scholar]
- Gilbert DB, Cooper SJ. Analysis of dopamine D1 and D2 receptor involvement in d- and l-amphetamine-induced anorexia in rats. Brain Res Bull. 1985;15:385–389. doi: 10.1016/0361-9230(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Low dopamine and serotonin levels in anorexia nervosa. Am J Psychiatry. 1983;140:948–949. doi: 10.1176/ajp.140.7.948c. [DOI] [PubMed] [Google Scholar]
- Gorwood P. Eating disorders, serotonin transporter polymorphisms and potential treatment response. Am J Pharmacogenomics. 2004;4:9–17. doi: 10.2165/00129785-200404010-00002. [DOI] [PubMed] [Google Scholar]
- Grossman SP. Role of the hypothalamus in the regulation of food and water intake. Psychol Rev. 1975;82:200–224. [PubMed] [Google Scholar]
- Gutierrez E. A rat in the labyrinth of anorexia nervosa: contributions of the activity-based anorexia rodent model to the understanding of anorexia nervosa. Int J Eat Disord. 2013;46:289–301. doi: 10.1002/eat.22095. [DOI] [PubMed] [Google Scholar]
- Haahr ME, Rasmussen PM, Madsen K, Marner L, Ratner C, Gillings N, et al. Obesity is associated with high serotonin 4 receptor availability in the brain reward circuitry. Neuroimage. 2012;61:884–888. doi: 10.1016/j.neuroimage.2012.03.050. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- Haleem DJ. Function specific supersensitivity of m-chlorophenyl piperazine-induced serotonergic neurotransmission in female compared to male rats. Life Sci. 1993a;52:PL279–PL284. doi: 10.1016/0024-3205(93)90693-w. [DOI] [PubMed] [Google Scholar]
- Haleem DJ. Serotonergic neurotransmission in the regulation of appetite: a receptor approach. Pak J Pharm Sci. 1993b;6:89–96. [PubMed] [Google Scholar]
- Haleem DJ, Haider S. Food restriction decreases serotonin and its synthesis rate in the hypothalamus. Neuroreport. 1996;7:1153–1156. doi: 10.1097/00001756-199604260-00011. [DOI] [PubMed] [Google Scholar]
- Haleem DJ, Kennett GA, Whitton PS, Curzon G. 8-OH-DPAT increases corticosterone but not other 5-HT1A receptor-dependent responses more in females. Eur J Pharmacol. 1989;164:435–443. doi: 10.1016/0014-2999(89)90251-3. [DOI] [PubMed] [Google Scholar]
- Halford JCG, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Hall JF, Hanford PV. Activity as a function of a restricted feeding schedule. J Comp Physiol Psychol. 1954;47:362–363. doi: 10.1037/h0060276. [DOI] [PubMed] [Google Scholar]
- Halmi KA, Dekirmenjian H, Davis JM, Casper R, Goldberg S. Catecholamine metabolism in anorexia nervosa. Arch Gen Psychiatry. 1978;35:458–460. doi: 10.1001/archpsyc.1978.01770280068007. [DOI] [PubMed] [Google Scholar]
- Hay P, Bacaltchuk J. Bulimia nervosa. Clin Evid. 2002;8:914–926. [PubMed] [Google Scholar]
- Heisler LK, Kanarek RB, Homoleski B. Reduction of fat and protein intakes but not carbohydrate intake following acute and chronic fluoxetine in female rats. Pharmacol Biochem Behav. 1999;63:377–385. doi: 10.1016/s0091-3057(99)00021-0. [DOI] [PubMed] [Google Scholar]
- Herberg LJ, Blundell JE. Lateral hypothalamus: hoarding behavior elicited by electrical stimulation. Science. 1967;155:349–350. doi: 10.1126/science.155.3760.349. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Hersrud SL, Stoltenberg SF. Epistatic interaction between COMT and DAT1 genes on eating behavior: a pilot study. Eat Behav. 2009;10:131–133. doi: 10.1016/j.eatbeh.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec. 1940;78:149–172. [Google Scholar]
- Hillebrand JJG, Wied D, de Adan RAH. Neuropeptides, food intake and body weight regulation: a hypothalamic focus. Peptides. 2002;23:2283–2306. doi: 10.1016/s0196-9781(02)00269-3. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJG, Elburg AA, van Kas MJH, Engeland H, van Adan RAH. Olanzapine reduces physical activity in rats exposed to activity-based anorexia: possible implications for treatment of anorexia nervosa? Biol Psychiatry. 2005a;58:651–657. doi: 10.1016/j.biopsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJG, Kas MJH, Adan RAH. a-MSH enhances activity-based anorexia. Peptides. 2005b;26:1690–1696. doi: 10.1016/j.peptides.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJG, Koeners MP, Rijke CE, de Kas MJH, Adan RAH. Leptin treatment in activity-based anorexia. Biol Psychiatry. 2005c;58:165–171. doi: 10.1016/j.biopsych.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJG, Heinsbroek ACM, Kas MJH, Adan RAH. The appetite suppressant d-fenfluramine reduces water intake, but not food intake, in activity-based anorexia. J Mol Endocrinol. 2006;36:153–162. doi: 10.1677/jme.1.01887. [DOI] [PubMed] [Google Scholar]
- Hinney A, Scherag S, Hebebrand J. Genetic findings in anorexia and bulimia nervosa. Prog Mol Biol Transl Sci. 2010;94:241–270. doi: 10.1016/B978-0-12-375003-7.00009-1. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Teitelbaum P. Weight regulation in normal and hypothalamic hyperphagic rats. J Comp Physiol Psychol. 1966;61:189–193. doi: 10.1037/h0023126. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Hernandez L, Schwartz DH, Mark GP, Hunter GA. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications. Ann N Y Acad Sci. 1989;575:171–191. doi: 10.1111/j.1749-6632.1989.tb53242.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Karwautz A, Wagner G, Holliday J, Li T, Treasure J, et al. No association between a promoter polymorphism in the noradrenaline transporter gene and anorexia nervosa. Psychiatr Genet. 2007;17:247–248. doi: 10.1097/YPG.0b013e3280ae6c2a. [DOI] [PubMed] [Google Scholar]
- Huether G, Zhou D, Schmidt S, Wiltfang J, Rüther E. Long-term food restriction down-regulates the density of serotonin transporters in the rat frontal cortex. Biol Psychiatry. 1997;41:1174–1180. doi: 10.1016/s0006-3223(96)00265-x. [DOI] [PubMed] [Google Scholar]
- Hulsey MG, Martin RJ. A system for automated recording and analysis of feeding behavior. Physiol Behav. 1991;50:403–408. doi: 10.1016/0031-9384(91)90086-4. [DOI] [PubMed] [Google Scholar]
- Janhunen SK, van der Zwaal EM, la Fleur SE, Adan RAH. Inverse agonism at α2A adrenoceptors augments the hypophagic effect of sibutramine in rats. Obesity (Silver Spring) 2011;19:1979–1986. doi: 10.1038/oby.2011.51. [DOI] [PubMed] [Google Scholar]
- Janhunen SK, la Fleur SE, Adan RAH. Blocking alpha2A adrenoceptors, but not dopamine receptors, augments bupropion-induced hypophagia in rats. Obesity (Silver Spring) 2013;21:700–708. doi: 10.1002/oby.20581. [DOI] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care. 2013;36:394–402. doi: 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, et al. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:16335–16340. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M, Roland CR, Leibowitz SF. Diurnal rhythm of alpha 2-noradrenergic receptors in the paraventricular nucleus and other brain areas: relation to circulating corticosterone and feeding behavior. Life Sci. 1986;38:473–482. doi: 10.1016/0024-3205(86)90073-1. [DOI] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M, Papamichael MJ, Leibowitz SF. Glucose-dependent changes in alpha 2-noradrenergic receptors in hypothalamic nuclei. Physiol Behav. 1988;44:611–617. doi: 10.1016/0031-9384(88)90326-5. [DOI] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M, Awad IR, Gearhart GM, Finkelstein JA, Leibowitz SF. Higher alpha-noradrenergic receptors in paraventricular nucleus of obese Zucker rats: decline after food deprivation. Pharmacol Biochem Behav. 1991;40:853–859. doi: 10.1016/0091-3057(91)90097-l. [DOI] [PubMed] [Google Scholar]
- Jimerson DC, Lesem MD, Kaye WH, Brewerton TD. Low serotonin and dopamine metabolite concentrations in cerebrospinal fluid from bulimic patients with frequent binge episodes. Arch Gen Psychiatry. 1992;49:132–138. doi: 10.1001/archpsyc.1992.01820020052007. [DOI] [PubMed] [Google Scholar]
- Johnson C, Stuckey M, Mitchell J. Psychopharmacological treatment of anorexia nervosa and bulimia. Review and synthesis. J Nerv Ment Dis. 1983;171:524–534. doi: 10.1097/00005053-198309000-00002. [DOI] [PubMed] [Google Scholar]
- Johnston JL, Romsos DR, Bergen WG. Reduced brain norepinephrine metabolism in obese (ob/ob) mice is not normalized by tyrosine supplementation. J Nutr. 1986;116:435–445. doi: 10.1093/jn/116.3.435. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Collier GH. Self-starvation: a problem of overriding the satiety signal? Physiol Behav. 1983;30:307–311. doi: 10.1016/0031-9384(83)90024-0. [DOI] [PubMed] [Google Scholar]
- Kaplan AS, Levitan RD, Yilmaz Z, Davis C, Tharmalingam S, Kennedy JL. A DRD4/BDNF gene-gene interaction associated with maximum BMI in women with bulimia nervosa. Int J Eat Disord. 2008;41:22–28. doi: 10.1002/eat.20474. [DOI] [PubMed] [Google Scholar]
- Kas MJH, Dijk G, van Scheurink AJW, Adan RAH. Agouti-related protein prevents self-starvation. Mol Psychiatry. 2003;8:235–240. doi: 10.1038/sj.mp.4001206. [DOI] [PubMed] [Google Scholar]
- Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Weltzin TE. Neurochemistry of bulimia nervosa. J Clin Psychiatry. 1991;52(Suppl):21–28. [PubMed] [Google Scholar]
- Kaye WH, Ebert MH, Gwirtsman HE, Weiss SR. Differences in brain serotonergic metabolism between nonbulimic and bulimic patients with anorexia nervosa. Am J Psychiatry. 1984;141:1598–1601. doi: 10.1176/ajp.141.12.1598. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Jimerson DC, Lake CR, Ebert MH. Altered norepinephrine metabolism following long-term weight recovery in patients with anorexia nervosa. Psychiatry Res. 1985;14:333–342. doi: 10.1016/0165-1781(85)90101-5. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Gwirtsman HE, George DT, Jimerson DC, Ebert MH. CSF 5-HIAA concentrations in anorexia nervosa: reduced values in underweight subjects normalize after weight gain. Biol Psychiatry. 1988;23:102–105. doi: 10.1016/0006-3223(88)90113-8. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Ballenger JC, Lydiard RB, Stuart GW, Laraia MT, O’Neil P, et al. CSF monoamine levels in normal-weight bulimia: evidence for abnormal noradrenergic activity. Am J Psychiatry. 1990a;147:225–229. doi: 10.1176/ajp.147.2.225. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Gwirtsman HE, George DT, Jimerson DC, Ebert MH, Lake CR. Disturbances of noradrenergic systems in normal weight bulimia: relationship to diet and menses. Biol Psychiatry. 1990b;27:4–21. doi: 10.1016/0006-3223(90)90015-t. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Frank GK, Meltzer CC, Price JC, McConaha CW, Crossan PJ, et al. Altered serotonin 2A receptor activity in women who have recovered from bulimia nervosa. Am J Psychiatry. 2001;158:1152–1155. doi: 10.1176/appi.ajp.158.7.1152. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Barbarich NC, Putnam K, Gendall KA, Fernstrom J, Fernstrom M, et al. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int J Eat Disord. 2003;33:257–267. doi: 10.1002/eat.10135. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology (Berl) 1988;96:93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Kintscher U. Reuptake inhibitors of dopamine, noradrenaline, and serotonin. Handb Exp Pharmacol. 2012:339–347. doi: 10.1007/978-3-642-24716-3_15. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Sclafani A. PVN-hindbrain pathway involved in the hypothalamic hyperphagia-obesity syndrome. Physiol Behav. 1988;42:517–528. doi: 10.1016/0031-9384(88)90153-9. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Cooper SJ. Naloxone attenuation of sham feeding is modified by manipulation of sucrose concentration. Physiol Behav. 1988;44:491–494. doi: 10.1016/0031-9384(88)90310-1. [DOI] [PubMed] [Google Scholar]
- Kishi T, Kafantaris V, Sunday S, Sheridan EM, Correll CU. Are antipsychotics effective for the treatment of anorexia nervosa? Results from a systematic review and meta-analysis. J Clin Psychiatry. 2012;73:e757–e766. doi: 10.4088/JCP.12r07691. [DOI] [PubMed] [Google Scholar]
- Kissileff HR. Free feeding in normal and ‘recovered lateral’ rats monitored by a pellet-detecting eatometer. Physiol Behav. 1970;5:163–173. doi: 10.1016/0031-9384(70)90060-0. [DOI] [PubMed] [Google Scholar]
- Klenotich SJ, Seiglie MP, McMurray MS, Roitman JD, Le Grange D, Dugad P, et al. Olanzapine, but not fluoxetine, treatment increases survival in activity-based anorexia in mice. Neuropsychopharmacology. 2012;37:1620–1631. doi: 10.1038/npp.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Kaye WH, Strober M. The evolving genetic foundations of eating disorders. Psychiatr Clin North Am. 2001;24:215–225. doi: 10.1016/s0193-953x(05)70218-5. [DOI] [PubMed] [Google Scholar]
- Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- Krüger S, Kennedy SH. Psychopharmacotherapy of anorexia nervosa, bulimia nervosa and binge-eating disorder. J Psychiatry Neurosci. 2000;25:497–508. [PMC free article] [PubMed] [Google Scholar]
- Kuikka JT, Tammela L, Karhunen L, Rissanen A, Bergström KA, Naukkarinen H, et al. Reduced serotonin transporter binding in binge eating women. Psychopharmacology (Berl) 2001;155:310–314. doi: 10.1007/s002130100716. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Porter JH. Pimozide mitigates excessive running in the activity-stress paradigm. Physiol Behav. 1992;52:299–304. doi: 10.1016/0031-9384(92)90275-7. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lin P-Y. Association between serotonin transporter gene polymorphism and eating disorders: a meta-analytic study. Int J Eat Disord. 2010;43:498–504. doi: 10.1002/eat.20732. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. The role of serotonin in eating disorders. Drugs. 1990;39(Suppl. 3):33–48. doi: 10.2165/00003495-199000393-00005. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27:1031–1040. doi: 10.1016/0031-9384(81)90366-8. [DOI] [PubMed] [Google Scholar]
- Levin BE. Reduced norepinephrine turnover in organs and brains of obesity-prone rats. Am J Physiol. 1995;268:R389–R394. doi: 10.1152/ajpregu.1995.268.2.R389. [DOI] [PubMed] [Google Scholar]
- Levin BE. Reduced paraventricular nucleus norepinephrine responsiveness in obesity-prone rats. Am J Physiol. 1996;270:R456–R461. doi: 10.1152/ajpregu.1996.270.2.R456. [DOI] [PubMed] [Google Scholar]
- Lewis DY, Brett RR. Activity-based anorexia in C57/BL6 mice: effects of the phytocannabinoid, Delta9-tetrahydrocannabinol (THC) and the anandamide analogue, OMDM-2. Eur Neuropsychopharmacol. 2010;20:622–631. doi: 10.1016/j.euroneuro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Potenza MN, Lee DE, Planeta B, Gallezot J-D, Labaree D, et al. Decreased norepinephrine transporter availability in obesity: positron Emission Tomography imaging with (S,S)-[(11)C]O-methylreboxetine. Neuroimage. 2013;86:306–310. doi: 10.1016/j.neuroimage.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid JA, Salido GM, Muñoz-Arrebola P, Martínez de Victoria E. Circadian rhythms of food intake in gastroduodenally-ulcerated rats: effects of three anti-ulcer drugs. Chronobiol Int. 1989;6:321–328. doi: 10.3109/07420528909056938. [DOI] [PubMed] [Google Scholar]
- Madrid JA, Lopez-Bote C, Martín E. Effect of neonatal androgenization on the circadian rhythm of feeding behavior in rats. Physiol Behav. 1993;53:329–335. doi: 10.1016/0031-9384(93)90213-y. [DOI] [PubMed] [Google Scholar]
- Madrid JA, Matas P, Sánchez-Vázquez FJ, Cuenca EM. A contact eatometer for automated continuous recording of feeding behavior in rats. Physiol Behav. 1995;57:129–134. doi: 10.1016/0031-9384(94)00214-p. [DOI] [PubMed] [Google Scholar]
- Margules DL. Alpha-adrenergic receptors in hypothalamus for the suppression of feeding behavior by satiety. J Comp Physiol Psychol. 1970;73:1–12. doi: 10.1037/h0030007. [DOI] [PubMed] [Google Scholar]
- Martin GE, Myers RD. Evoked release of [14C]norepinephrine from the rat hypothalamus during feeding. Am J Physiol. 1975;229:1547–1555. doi: 10.1152/ajplegacy.1975.229.6.1547. [DOI] [PubMed] [Google Scholar]
- Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite. 2009;52:545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova AI, Mori N, O’Melia AM. Current pharmacotherapy options for bulimia nervosa and binge eating disorder. Expert Opin Pharmacother. 2012;13:2015–2026. doi: 10.1517/14656566.2012.721781. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Yang ZJ, Koseki M. Eating induced rise in LHA-dopamine correlates with meal size in normal and bulbectomized rats. Brain Res Bull. 1995;36:487–490. doi: 10.1016/0361-9230(95)92128-3. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Yang ZJ, Laviano A. Meal size and number: relationship to dopamine levels in the ventromedial hypothalamic nucleus. Am J Physiol. 1997;272:R1925–R1930. doi: 10.1152/ajpregu.1997.272.6.R1925. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133:293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Merkestein M, van Gestel MA, van der Zwaal EM, Brans MA, Luijendijk MC, van Rozen AJ, et al. GHS-R1a signaling in the DMH and VMH contributes to food anticipatory activity. Int J Obes. 2013;2005:610–618. doi: 10.1038/ijo.2013.131. [DOI] [PubMed] [Google Scholar]
- Milano W, Petrella C, Casella A, Capasso A, Carrino S, Milano L. Use of sibutramine, an inhibitor of the reuptake of serotonin and noradrenaline, in the treatment of binge eating disorder: a placebo-controlled study. Adv Ther. 2005;22:25–31. doi: 10.1007/BF02850181. [DOI] [PubMed] [Google Scholar]
- Milner M, De Caire E. An improved photo-electric switching circuit for monitoring the feeding behaviour of rats which have the choice of several feeding troughs. S Afr J Med Sci. 1965;30:37–39. [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Morien A, Wellman PJ, Fojt J. Diurnal rhythms of paraventricular hypothalamic norepinephrine and food intake in rats. Pharmacol Biochem Behav. 1995;52:169–174. doi: 10.1016/0091-3057(95)00084-a. [DOI] [PubMed] [Google Scholar]
- Munn-Chernoff MA, McQueen MB, Stetler GL, Haberstick BC, Rhee SH, Sobik LE, et al. Examining associations between disordered eating and serotonin transporter gene polymorphisms. Int J Eat Disord. 2012;45:556–561. doi: 10.1002/eat.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacmias B, Ricca V, Tedde A, Mezzani B, Rotella CM, Sorbi S. 5-HT2A receptor gene polymorphisms in anorexia nervosa and bulimia nervosa. Neurosci Lett. 1999;277:134–136. doi: 10.1016/s0304-3940(99)00859-9. [DOI] [PubMed] [Google Scholar]
- Nergårdh R, Ammar A, Brodin U, Bergström J, Scheurink A, Södersten P. Neuropeptide Y facilitates activity-based-anorexia. Psychoneuroendocrinology. 2007;32:493–502. doi: 10.1016/j.psyneuen.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Nozue K, Oka Y. Hyperphagia alters expression of hypothalamic 5-HT2C and 5-HT1B receptor genes and plasma des-acyl ghrelin levels in Ay mice. Endocrinology. 2006a;147:5893–5900. doi: 10.1210/en.2006-0418. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Nozue K, Oka Y. Increased hypothalamic 5-HT2A receptor gene expression and effects of pharmacologic 5-HT2A receptor inactivation in obese Ay mice. Biochem Biophys Res Commun. 2006b;351:1078–1082. doi: 10.1016/j.bbrc.2006.10.173. [DOI] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Paez X, Stanley BG, Leibowitz SF. Microdialysis analysis of norepinephrine levels in the paraventricular nucleus in association with food intake at dark onset. Brain Res. 1993;606:167–170. doi: 10.1016/0006-8993(93)91586-h. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Research. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peat C, Mitchell JE, Hoek HW, Wonderlich SA. Validity and utility of subtyping anorexia nervosa. Int J Eat Disord. 2009;42:590–594. doi: 10.1002/eat.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirke KM. Central and peripheral noradrenalin regulation in eating disorders. Psychiatry Res. 1996;62:43–49. doi: 10.1016/0165-1781(96)02982-4. [DOI] [PubMed] [Google Scholar]
- Pirke KM, Broocks A, Wilckens T, Marquard R, Schweiger U. Starvation-induced hyperactivity in the rat: the role of endocrine and neurotransmitter changes. Neurosci Biobehav Rev. 1993;17:287–294. doi: 10.1016/s0149-7634(05)80012-0. [DOI] [PubMed] [Google Scholar]
- Pokrovsky V, Le Magnen J. [Design of a device for continuous and automatic graphic recording of food consumption by the white rat] J Physiol (Paris) 1963;55:318–319. [PubMed] [Google Scholar]
- Powers PS, Klabunde M, Kaye W. Double-blind placebo-controlled trial of quetiapine in anorexia nervosa. Eur Eat Disord Rev J Eat Disord. 2012;20:331–334. doi: 10.1002/erv.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. ily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Ramos EJB, Meguid MM, Campos ACL, Coelho JCU. Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition. 2005;21:269–279. doi: 10.1016/j.nut.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen M, Olszewski PK, Levine AS, Schiöth HB. Molecular mechanisms underlying anorexia nervosa: focus on human gene association studies and systems controlling food intake. Brain Res Rev. 2010;62:147–164. doi: 10.1016/j.brainresrev.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Ratner C, Ettrup A, Bueter M, Haahr ME, Compan V, le Roux CW, et al. Cerebral markers of the serotonergic system in rat models of obesity and after Roux-en-Y gastric bypass. Obesity (Silver Spring) 2012;20:2133–2141. doi: 10.1038/oby.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD, Finger FW. The rat’s adjustment to 23-hour food-deprivation cycles. J Comp Physiol Psychol. 1955;48:110–113. doi: 10.1037/h0042051. [DOI] [PubMed] [Google Scholar]
- Richard D, Ferland J, Lalonde J, Samson P, Deshaies Y. Influence of topiramate in the regulation of energy balance. Nutrition. 2000;16:961–966. doi: 10.1016/s0899-9007(00)00452-4. [DOI] [PubMed] [Google Scholar]
- Richter CP. A behavioristic study of the activity of the rat. Comp Psychol Monogr. 1922;1:1–55. [Google Scholar]
- Rieg TS, Aravich PF. Systemic clonidine increases feeding and wheel running but does not affect rate of weight loss in rats subjected to activity-based anorexia. Pharmacol Biochem Behav. 1994;47:215–218. doi: 10.1016/0091-3057(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Rockwood GA, Reid LD. Naloxone modifies sugar-water intake in rats drinking with open gastric fistulas. Physiol Behav. 1982;29:1175–1178. doi: 10.1016/0031-9384(82)90316-x. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Boulos Z, Terman M. Circadian organization of food intake and meal patterns in the rat. Physiol Behav. 1981;27:33–39. doi: 10.1016/0031-9384(81)90296-1. [DOI] [PubMed] [Google Scholar]
- Routtenberg A. Self-starvation’ of rats living in activity wheels: adaptation effects. J Comp Physiol Psychol. 1968;66:234–238. doi: 10.1037/h0025977. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol. 1967;64:414–421. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- Schwartz DH, Hernandez L, Hoebel BG. Serotonin release in lateral and medial hypothalamus during feeding and its anticipation. Brain Res Bull. 1990;25:797–802. doi: 10.1016/0361-9230(90)90173-w. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Schweiger U, Warnhoff M, Pahl J, Pirke KM. Effects of carbohydrate and protein meals on plasma large neutral amino acids, glucose, and insulin plasma levels of anorectic patients. Metabolism. 1986;35:938–943. doi: 10.1016/0026-0495(86)90058-2. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Grill HJ, Kaplan JM. Neurological dissociation of gastrointestinal and metabolic contributions to meal size control. Behav Neurosci. 1994;108:347–352. [PubMed] [Google Scholar]
- Shapiro JR, Berkman ND, Brownley KA, Sedway JA, Lohr KN, Bulik CM. Bulimia nervosa treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40:321–336. doi: 10.1002/eat.20372. [DOI] [PubMed] [Google Scholar]
- Siegfried Z, Berry EM, Hao S, Avraham Y. Animal models in the investigation of anorexia. Physiol Behav. 2003;79:39–45. doi: 10.1016/s0031-9384(03)00103-3. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M. gher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- Snowdon CT. Motivation, regulation, and the control of meal parameters with oral and intragastric feeding. J Comp Physiol Psychol. 1969;69:91–100. doi: 10.1037/h0027941. [DOI] [PubMed] [Google Scholar]
- Sorlí JV, Francés F, González JI, Guillén M, Portolés O, Sabater A, et al. Impact of the -1438G> a polymorphism in the serotonin 2A receptor gene on anthropometric profile and obesity risk: a case-control study in a Spanish Mediterranean population. Appetite. 2008;50:260–265. doi: 10.1016/j.appet.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Södersten P, Bergh C, Zandian M. Understanding eating disorders. Horm Behav. 2006;50:572–578. doi: 10.1016/j.yhbeh.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Fray A, Aspley S, Brammer R, Heal D, Auerbach S. Effects on serotonin in rat hypothalamus of D-fenfluramine, aminorex, phentermine and fluoxetine. Eur J Pharmacol. 2002;445:69–81. doi: 10.1016/s0014-2999(02)01751-x. [DOI] [PubMed] [Google Scholar]
- Thomas DW, Mayer J. Meal taking and regulation of food intake by normal and hypothalamic hyperphagic rats. J Comp Physiol Psychol. 1968;66:642–653. doi: 10.1037/h0026520. [DOI] [PubMed] [Google Scholar]
- Tiesjema B, Adan RAH, Luijendijk MCM, Kalsbeek A, la Fleur SE. Differential effects of recombinant adeno-associated virus-mediated neuropeptide Y overexpression in the hypothalamic paraventricular nucleus and lateral hypothalamus on feeding behavior. J Neurosci. 2007;27:14139–14146. doi: 10.1523/JNEUROSCI.3280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwin RE, Nunn KP. Epistatic interaction between the monoamine oxidase A and serotonin transporter genes in anorexia nervosa. Eur J Hum Genet. 2005;13:370–375. doi: 10.1038/sj.ejhg.5201328. [DOI] [PubMed] [Google Scholar]
- Urwin RE, Bennetts B, Wilcken B, Lampropoulos B, Beumont P, Clarke S, et al. Anorexia nervosa (restrictive subtype) is associated with a polymorphism in the novel norepinephrine transporter gene promoter polymorphic region. Mol Psychiatry. 2002;7:652–657. doi: 10.1038/sj.mp.4001080. [DOI] [PubMed] [Google Scholar]
- Urwin RE, Bennetts BH, Wilcken B, Lampropoulos B, Beumont PJV, Russell JD, et al. Gene-gene interaction between the monoamine oxidase A gene and solute carrier family 6 (neurotransmitter transporter, noradrenalin) member 2 gene in anorexia nervosa (restrictive subtype) Eur J Hum Genet. 2003;11:945–950. doi: 10.1038/sj.ejhg.5201077. [DOI] [PubMed] [Google Scholar]
- Verhagen LAW, Luijendijk MCM, Hillebrand JJG, Adan RAH. Dopamine antagonism inhibits anorectic behavior in an animal model for anorexia nervosa. Eur Neuropsychopharmacol. 2009;19:153–160. doi: 10.1016/j.euroneuro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Verhagen LAW, Egecioglu E, Luijendijk MCM, Hillebrand JJG, Adan RAH, Dickson SL. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur Neuropsychopharmacol. 2011;21:384–392. doi: 10.1016/j.euroneuro.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Verty ANA, Evetts MJ, Crouch GJ, McGregor IS, Stefanidis A, Oldfield BJ. The cannabinoid receptor agonist THC attenuates weight loss in a rodent model of activity-based anorexia. Neuropsychopharmacology. 2011;36:1349–1358. doi: 10.1038/npp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt J-P, Schade R, Fink H, Hörtnagl H. Role of 5-HT1A receptors in the control of food intake in obese Zucker rats of different ages. Pharmacol Biochem Behav. 2002;72:403–409. doi: 10.1016/s0091-3057(01)00763-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Logan J, Jayne M, Franceschi D, et al. ‘Nonhedonic’ food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synap. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter JC, et al. Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA. 2006;295:2605–2612. doi: 10.1001/jama.295.22.2605. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Norepinephrine and the control of food intake. Nutrition. 2000;16:837–842. doi: 10.1016/s0899-9007(00)00415-9. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Modulation of eating by central catecholamine systems. Curr Drug Targets. 2005;6:191–199. doi: 10.2174/1389450053174532. [DOI] [PubMed] [Google Scholar]
- Westberg L, Bah J, Råstam M, Gillberg C, Wentz E, Melke J, et al. Association between a polymorphism of the 5-HT2C receptor and weight loss in teenage girls. Neuropsychopharmacology. 2002;26:789–793. doi: 10.1016/S0893-133X(01)00417-1. [DOI] [PubMed] [Google Scholar]
- Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav. 2006;89:226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Wojnicki FHE, Stine JG, Corwin RLW. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007;92:566–574. doi: 10.1016/j.physbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Meguid MM. LHA dopaminergic activity in obese and lean Zucker rats. Neuroreport. 1995;6:1191–1194. doi: 10.1097/00001756-199505300-00029. [DOI] [PubMed] [Google Scholar]
- Yokoyama F, Onozawa K, Kakui N, Imanishi T. The selective serotonin reuptake inhibitor fluvoxamine suppresses post-feeding hyperactivity induced by food restriction in rats. Pharmacol Biochem Behav. 2007;87:98–103. doi: 10.1016/j.pbb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Hosseini-Nia T. Anorectic and behavioural effects of bupropion. Gen Pharmacol. 1988;19:201–204. doi: 10.1016/0306-3623(88)90061-4. [DOI] [PubMed] [Google Scholar]
- van der Zwaal EM, Luijendijk MCM, Adan RAH, la Fleur SE. Olanzapine-induced weight gain: chronic infusion using osmotic minipumps does not result in stable plasma levels due to degradation of olanzapine in solution. Eur J Pharmacol. 2008;585:130–136. doi: 10.1016/j.ejphar.2007.11.078. [DOI] [PubMed] [Google Scholar]