Abstract
Background and Purpose
Mice with functional ablation of substance P-preferring neurokinin-1 receptors (NK1R−/− mice) display behavioural abnormalities resembling those in attention deficit hyperactivity disorder (ADHD). Here, we investigated whether the ADHD treatment, guanfacine, alleviated the hyperactivity and impulsivity/inattention displayed by NK1R−/− mice in the light/dark exploration box (LDEB) and 5-choice serial reaction–time task (5-CSRTT), respectively. Following reports of co-morbid anxiety in ADHD, we also investigated effects of guanfacine on anxiety-like behaviour displayed by NK1R−/− and wild-type (WT) mice in the elevated plus maze (EPM).
Experimental Approach
Mice were treated with guanfacine (0.1, 0.3 or 1.0 mg·kg−1, i.p.), vehicle or no injection and tested in the 5-CSRTT or the LDEB. Only the lowest dose of guanfacine was used in the EPM assays.
Key Results
In the 5-CSRTT, a low dose of guanfacine (0.1 mg·kg−1) increased attention in NK1R−/− mice, but not in WT mice. This dose did not affect the total number of trials completed, latencies to respond or locomotor activity in the LDEB. Impulsivity was decreased by the high dose (1.0 mg·kg−1) of guanfacine, but this was evident in both genotypes and is likely to be secondary to a generalized blunting of behaviour. Although the NK1R−/− mice displayed marked anxiety-like behaviour, guanfacine did not affect the behaviour of either genotype in the EPM.
Conclusions and Implications
This evidence that guanfacine improves attention at a dose that did not affect arousal or emotionality supports our proposal that NK1R−/− mice express an attention deficit resembling that of ADHD patients.
Linked Articles
This article is part of a themed section on Animal Models in Psychiatry Research. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-20
Tables of Links
| TARGETS | LIGANDS | |
|---|---|---|
| α2A-adrenoceptors | d-Amphetamine | Methylphenidate |
| NK1 receptors | Guanfacine | RP 67580 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Mice from a 129/Sv × C57BL/6J genetic background (crossed with an outbred MF1 strain), with functional ablation of the substance P-preferring, neurokinin-1 (NK1) receptor gene (referred to as NK1R−/−mice) (de Felipe et al., 1998), express locomotor hyperactivity in an activity meter (Herpfer et al., 2005) and the light/dark exploration box (LDEB) (Herpfer et al., 2005; Fisher et al., 2007; Yan et al., 2010). They also display deficits in cognitive performance and response control when tested in the 5-choice serial reaction–time task (5-CSRTT). Specifically, when compared with their wild-type (WT) counterparts, they typically score greater %omissions (an index of inattentiveness) and increased %premature responses (an index of motor impulsivity) in this test (Yan et al., 2011; Dudley et al., 2013). Mechanism(s) that could explain how a lack of functional NK1 receptors could provoke locomotor hyperactivity, inattentiveness and impulsivity are reviewed in Yan et al. (2010) and Stanford (2014).
These abnormal behaviours (hyperactivity, inattention and impulsivity) resemble the core diagnostic features of attention deficit hyperactivity disorder (ADHD). Our proposal that NK1R−/− mice can be used to study the behavioural and cognitive abnormalities seen in ADHD patients is supported by evidence from translational studies, which discovered that an association between polymorphisms in, or near, the human equivalent of the NK1 receptor gene (TACR1) predicts increased vulnerability to ADHD (Sharp et al., 2009; 2014; Yan et al., 2010).
Psychomotor stimulants (d-amphetamine, methylphenidate and the prodrug, lisdexamfetamine) are the first-line treatments for ADHD. Only two other compounds are licensed for this clinical indication: the preferential noradrenaline reuptake inhibitor, atomoxetine (Preti, 2002), and the α2A-adrenoceptor agonist, guanfacine (Biederman et al., 2008; Sallee et al., 2009). An extended release formulation of guanfacine was approved for the treatment of ADHD in the United States in 2009.
We have reported previously that the hyperactivity of NK1R−/− mice is attenuated by the psychostimulants, d-amphetamine and methylphenidate, as in ADHD (Yan et al., 2009; 2010), but d-amphetamine did not prevent impulsivity or inattentiveness in the 5-CSRTT (Yan et al., 2011). This could be relevant to reports that d-amphetamine is ineffective in about 25% of ADHD patients (Heal et al., 2009). The first objective of this study was to investigate whether guanfacine ameliorates the deficits in cognitive performance or response control that are expressed by NK1R−/− mice in the 5-CSRTT, in line with its efficacy in treating ADHD.
A second objective was to establish whether or not guanfacine prevents the locomotor hyperactivity of NK1R−/− mice. It has already been reported that this drug reduces locomotor activity of the SHR (an established rodent model of ADHD) in the open field, but not that of their control strains (Wistar or Wistar Kyoto; Langen and Dost, 2011). However, we were mindful of the problem that animals’ emotional status (anxiety-like behaviour) can confound measures of locomotor activity in this test (see Stanford, 2007a,b; Wilcock and Broadhurst, 1967). This is especially important for studies of the effects of guanfacine on motor behaviour because this drug is used to treat anxiety, which is a common co-morbid disorder in ADHD (Sobanski, 2006). For that reason, we also compared the effects of guanfacine on the behaviour of NK1R−/− mice and WT in the elevated plus maze (EPM), an established screen for anxiolytic drugs.
A final caveat is that, although NK1R−/− mice display inattention and impulsivity when they derive from separate homozygous, inbred strains (‘homs’), the behavioural phenotype of homozygous progeny from heterozygote NK1R+/− (F1) breeding pairs (‘hets’) is as yet unknown. This, together with growing interest in epigenetic influences in ADHD, prompted us to investigate whether any effects of guanfacine on behaviour in the 5-CSRTT differ in WT and NK1R−/− mice derived from these two breeding methods.
The findings from these studies lead us to infer that a low dose of guanfacine (0.1 mg·kg−1) reduces inattentiveness of NK1R−/− mice in the 5-CSRTT, but that higher doses (10 mg·kg−1) reduce impulsivity in both genotypes. The latter response to guanfacine is likely to be a consequence of the sedative effects of this drug. Neither of these responses to guanfacine is dependent on the breeding method nor is likely to be explained by any change in animals’ emotional status.
Methods
Animals
All animal care and experimental procedures complied with the Animals (Scientific Procedures) Act, 1986 (UK) and were approved by the Ethical Review Panel at University College London. These studies comply with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 104 animals were used in these experiments (24 in the 5-CSRTT, 50 in the LDEB and 30 in the EPM).
Mouse colonies were bred at University College London in a facility held at 21 ± 2°C, 45 ± 5% humidity, with a 12:12 h light: dark cycle (lighting increased in steps from 07:00 to 08:00 h and reduced in steps from 19:00 to 20:00 h). The home cages incorporated environmental enrichment and were cleaned twice weekly (bedding obtained from Litaspen Premium, Lillico, Horley, Surrey, UK). Food supply was obtained from Harlan (Bicester, UK: 2018 global rodent diet).
All mice were bred from the same background strain (129/Sv × C57BL/6J crossed with an outbred MF1 strain, more than 10 generations ago) (de Felipe et al., 1998). Half of the mice used in the 5-CSRTT experiment comprised homozygous WT (NK1R+/+) and NK1R−/− mice from inbred homozygous parents (homs) (N = 6 per group). The subjects were the progeny from two breeding pairs for each genotype. The remainder, which were tested at the same time as the homs, comprised (F2) homozygous WT and NK1R−/− mice derived from three breeding pairs of heterozygous (NK1R+/−) parents, which were the (F1) progeny of inbred WT (NK1R+/+) mice crossed with inbred NK1R−/− mice (WT hets and NK1R−/− hets) (N = 6 per group).
Each home cage contained two to four mice. The wild-type and NK1R−/− homs were housed separately, but het mice were housed in cages that contained at least one WT and one NK1R−/− mouse. Only mice from homozygous parents (inbred strains) were used in the LDEB and EPM because there were no differences in the effects of guanfacine on the behaviour of the two colonies of mice in the 5-CSRTT.
5-CSRTT
Twelve WT male and 12 NK1R−/− male mice were used at 6–8 weeks of age (weighing WT hom: 30.5–35.6 g; NK1R−/− hom: 26.7–30.9 g; WT het: 31.1–41.1 g; NK1R−/− het: 30.5–34.7 g at the start of training). The mice were brought into the laboratory every day (Monday to Friday) between 09:00 and 09:30 h and weighed before training/testing in the 5-CSRTT, which took place between 10:00 and 12:00 h (AM session) or 13:00 and 15:00 h (PM session). The animals were returned to the holding room at 16:00 h and each cage supplied with a weighed quota of food, which was adjusted to stabilize subjects at 90% of their free-feeding body weight. Water was freely available at all times. One WT het and one NK1R−/− het failed to graduate through the training phase of the procedure and were withdrawn from the study.
The apparatus, supplied by Med Associates (St. Albans, VT, USA), was controlled by a Smart Ctrl Package 8IN/16OUT with an additional interface by MED-PC for Windows (Med Associates). This comprised four sound-attenuated operant chambers with five equally spaced apertures, incorporated into one wall of each chamber: these apertures could be illuminated independently. A nose-poke by the mice into each hole was scored following interruption of an infrared beam that spanned the hole. Interceptions of an infrared beam across a magazine in the opposite wall, which delivered the reward (0.01 mL of 30% condensed milk solution), were also scored: these occurred whenever a mouse collected the reward and initiated the next ‘trial’. Incorrect, omitted and premature responses were punished with a 5 s time out, during which the house light was extinguished and no new trials could be initiated. Perseverative responses were not punished.
The procedure was the same as that previously reported and is documented in full elsewhere (Yan et al., 2011). Briefly, mice were assigned to one of four test chambers in a fully counterbalanced design and were tested in the same chamber throughout. First, they were habituated to the apparatus for 3 days and then trained in the 5-CSRTT, graduating through increasingly challenging stages (1–6) after fulfilling the criteria for each stage (see Yan et al., 2011). The final stimulus duration (SD) at stage 6 was 1.8 s. After reaching the criteria for a stable baseline at stage 6 (total trials completed minus premature responses = 100; >75% accuracy; <25% omissions), for at least 3 consecutive days, the mice were eligible for testing with a variable inter-trial interval [VITI; 2, 5, 10 or 15 s (delivered on a random schedule) with an SD of 1.8 s]. Mice were tested with the VITI once weekly, on Fridays. A VITI test was used because it prevents the use of interval timing as a strategy for correct responding, thereby increasing cognitive load. The first week of testing was carried out with treatment–naïve mice [no injection (1): ‘NI-1′] only (reported elsewhere). In each of the following 5 weeks, mice were subject to a VITI test, 30 min after an i.p. injection of either vehicle (saline, 10 mL·kg−1) or guanfacine (0.1, 0.3 or 1.0 mg·kg−1), or a second untreated session [no injection (2): ‘NI-2′]. These drug doses were chosen because they modify relevant aspects of behaviour in mice: for example, working memory in a T-maze (Franowicz et al., 2002) and locomotor activity (Archer and Fredriksson, 2003). NI-2 (baseline behaviour of uninjected mice) was embedded within the sequence of vehicle/drug treatments to control for any systematic changes in behaviour arising from rehearsal of the test (see Weir et al., 2014). The vehicle/drug/NI-2 sessions were counterbalanced across subjects, using a pseudo William’s Latin square, such that each mouse received each treatment, or NI-2 session, once only.
The test sessions were terminated after either 45 min or after the mouse completed 100 trials, plus the number of premature responses, whichever occurred first. Performance scores in the 5-CSRTT were recorded and stored online (see Table 1). Omissions and premature responses were calculated per 100 trials to correct for any differences in the total number of trials completed by the mice.
Table 1.
Performance variables recorded in the testing phase of the 5-CSRTT
| Behavioural outcome | Method of calculation |
|---|---|
| Total number of trials completed | Total correct responses + total incorrect responses + total omissions |
| %Accuracy | [correct responses/(correct + incorrect responses)] × 100 |
| %Omissions | [total omissions/total number of trials] × 100 |
| %Premature responses | [premature responses/(total number of trials + prems)] × 100 |
| Latency to correct response | Duration between onset of stimulus and a nose-poke in the correct hole |
| Latency to collect the reward | Duration between a nose-poke in the correct hole and collection of reward from the magazine |
| Perseveration | Number of unnecessary responses into the correct hole after the initial correct response, before collection of reward, per 100 trials |
LDEB
A separate cohort of NK1R−/− and WT mice, from homozygous breeding pairs, was tested in the LDEB, which was dimly lit [dark zone (DZ): 4 lux, light zone (LZ): 20 lux]. One WT and one NK1R−/− mouse were tested simultaneously, with the same treatment and in adjacent LDEBs, to balance any nuisance factors across the two genotypes. The procedure is described fully in Fisher et al. (2007) and Herpfer et al. (2005). Briefly, mice were allowed to habituate to the test room between 10:00 and 13:00 h. At either 13:00 or 15:30 h, they were confined individually within the DZ of the LDEB for 60 min, after which they were injected with their allocated treatment, or left untreated (no injection, NI), and replaced in the DZ for a further 30 min. The treatments were either vehicle (0.9% saline, 10 mL·kg−1) or guanfacine (0.1, 0.3 or 1.0 mg·kg−1, i.p.) (N = 5 per group), which were given in a counterbalanced sequence; each mouse received only one treatment. After a total of 90 min in the DZ, the mice were transferred to the LZ and allowed to move freely between the two zones. Behaviour was recorded by a digital video camera for 30 min and scored later by an observer, unaware of the treatments. Because the activity of mice in the LDEB declined progressively to reach a ‘floor’ after approximately 15 min, only the first 10 min of activity after transfer to the LZ were used in the statistical analysis.
EPM
A third cohort of mice (from homozygous breeding pairs, only) was tested in the EPM. Mice were allowed to habituate to the test room between 10:00 and 14:00 h, and tested between 14:00 and 16:00 h on the EPM. They then received an i.p. injection of vehicle (saline) or guanfacine (0.1 mg·kg−1), 30 min before testing, or received no injection (N = 5 per group). Treatments were assigned in a counterbalanced order. The mice were tested individually, by placing them at the centre of the four arms, facing an open arm, after which they were allowed to explore the maze for 5 min. The maze was cleaned between each test with 70% ethanol. Behaviour was recorded by a video camera, positioned above the maze and was scored later by an observer, unaware of the treatments. The following behavioural measures were recorded:
%Time in open arms [time with all four paws in open arms/(time on open arms + time on closed arms)] × 100
%Time in centre; (time in centre/total time) × 100
Number of whole-body entries; all four paws enter the arm
Data analysis
Statistical analyses were performed using InVivoStat (Clark et al., 2012) and used raw or transformed data (arcsine, log10(score+1) or square root), according to whichever optimized the homogeneity of variance in the ‘predicted versus residuals’ plot in InVivoStat. The ‘normal probability plot’ in InVivoStat was used to examine whether or not the data were normally distributed. If not, a rank transformation was applied: that is, the data were assigned ranks, as for a non-parametric analysis, but the ranks were then subjected to parametric tests.
Repeated-measures analyses were used to examine data from the 5-CSRTT. The analyses used a mixed model approach: ‘within-animal’ correlations were modelled using a compound symmetric covariance structure (which assumes sphericity of the variance/covariance matrix). ‘Genotype’ and ‘colony’ were used as between-subject factors and ‘treatment’ was the within-subject factor. A fourth factor was ‘time of day’ (i.e. AM session/PM session). Our previous studies indicated that time of testing influences behaviour in the 5-CSRTT (Yan et al., 2011; Weir et al., 2014) and so this was used as a blocking factor, to account for any additional variance in the data. This factor was collapsed across all subjects if there was no effect of time of day on a given dependent variable. A main effect of genotype or treatment, or an interaction between them, was used as the criterion for carrying out post hoc pairwise comparisons.
In the LDEB and EPM, two-way anovas were performed on raw or transformed data, with the main factors genotype and treatment. First, the anova compared the factors across all groups (uninjected, vehicle and drug treated). If there was a main effect of either factor, or an interaction between them, further analyses were carried out using post hoc anova to compare vehicle controls with drug treatment (main effect of ‘drug’). Where there was a main effect of genotype or drug, or relevant interactions between the factors, post hoc LSD tests were performed to compare pairs of data.
Materials
Guanfacine hydrochloride was purchased from Tocris (Abingdon, UK), dissolved in 0.9% saline and injected i.p. in a volume of 10 mL·kg−1 throughout.
Results
Our previous experiments have confirmed that repeated experience of the VITI can influence behaviour in this test (see Weir et al., 2014). For this reason, the response to guanfacine in this study was compared with NI-2, which was the uninjected control embedded within the series of drug treatments. Findings from testing in NI-1 are to be reported elsewhere.
The 5-CSRTT experiment was carried out using mice derived from two colonies (homozygous breeders and heterozygous breeders). However, there was no statistically significant interaction between colony and the response to vehicle or drug treatment, for any of the dependent variables, and so the data were collapsed across colony.
As before (Yan et al., 2011; Weir et al., 2014), certain behaviours (specifically, %omissions and %premature responses) depended on time of day, which was used as a blocking factor in the analysis of these behaviours. Where there were no effects of time of day, data were collapsed across this factor.
Guanfacine has bidirectional effects on %omissions in NK1R−/− mice (Figure 1A)
Figure 1.
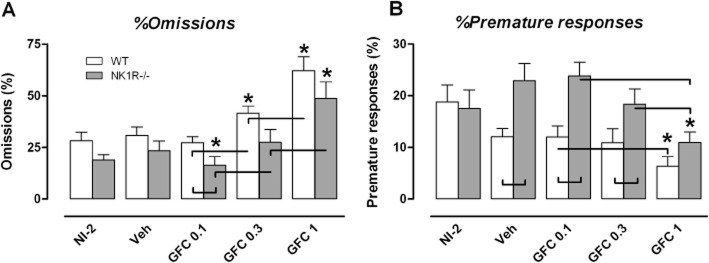
Effect of guanfacine (GFC; 0.1, 0.3 and 1.0 mg·kg−1, i.p.) on (A) %omissions and (B) %premature responses in the 5-CSRTT, compared with vehicle (saline), or NI-2 in wild-type (WT) and NK1R−/− mice. The lowest dose of guanfacine reduced %omissions in NK1R−/− mice, but not WTs. Guanfacine also decreased %premature responses, but to the same extent in both genotypes. Data show mean ± SEM. N = 9–10 per group. Lines linking bars indicate statistical significance of at least P < 0.05; * indicates P < 0.05 versus vehicle within genotype.
There were no overall differences in %omissions between the two genotypes [[sqrt, all groups] geno: F(1,18) = 3.40, P = 0.082] (Figure 1A). The effects of guanfacine across all doses also did not depend on genotype [[sqrt, veh vs. drug] drug*geno: F(3,49) = 1.33, P = 0.276]. However, this drug had bidirectional effects on this behaviour [[sqrt] drug: F(3,49) = 48.00, P < 0.001]. The lowest dose (0.1 mg·kg−1) reduced %omissions in NK1R−/− mice only, by comparison with either vehicle-treated NK1R−/− mice [[sqrt] veh vs. 0.1 mg·kg−1: P = 0.004] or drug-treated WTs [[sqrt] 0.1 mg·kg−1, WT vs. KO: P = 0.049]. The highest dose of guanfacine (1.0 mg·kg−1) increased %omissions in both genotypes to a similar extent [[sqrt] veh vs. 1.0 mg·kg−1, WT: P < 0.001, KO: P < 0.001].
Guanfacine attenuates premature responding in WT and NK1R−/− mice (Figure 1B)
Overall, NK1R−/− mice expressed more %premature responses than WTs [[sqrt, all groups] geno: F(1,18) = 8.39, P = 0.010] (Figure 1B). Guanfacine reduced the incidence of this behaviour, [[sqrt, veh vs. drug] drug: F(3,49) = 9.45, P < 0.001], especially at the highest dose [[sqrt] veh vs. 1.0 mg·kg−1, WT: P = 0.012, KO: P < 0.001]. However, the effect of the drug did not depend on genotype [[sqrt, veh vs. drug] drug*geno: F(3,49) = 0.39, P = 0.759].
Guanfacine blunts behaviour in measures of arousal and motivation (Table 2)
Table 2.
The effects of guanfacine on behaviour of NK1R−/− and wild-type (WT) mice in the 5-CSRTT
| Behaviour | WT | NK1R−/− | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NI-2 | Veh | 0.1 mg·kg−1 | 0.3 mg·kg−1 | 1.0 mg·kg−1 | NI-2 | Veh | 0.1 mg·kg−1 | 0.3 mg·kg−1 | 1.0 mg·kg−1 | |
| Total number of trials | 96.0 ± 3.5 | 99.8 ± 0.2 | 99.2 ± 0.8 | 99.0 ± 0.8 | 90.9 ± 5.0* | 99.3 ± 0.7 | 96.5 ± 1.8 | 100.0 ± 0 | 100.0 ± 0 | 89.1 ± 7.4 |
| %Accuracy | 96.5 ± 0.85 | 96.0 ± 1.41 | 96.1 ± 1.11 | 94.6 ± 1.82 | 92.2 ± 2.03 | 96.2 ± 0.82 | 92.6 ± 1.25 | 95.6 ± 0.91 | 94.0 ± 1.42 | 86.5 ± 4.20 |
| Latency to correct hole | 0.93 ± 0.06 | 1.01 ± 0.04 | 1.01 ± 0.06 | 1.12 ± 0.06 | 1.28 ± 0.11* | 0.96 ± 0.06 | 1.01 ± 0.06 | 1.04 ± 0.07 | 1.10 ± 0.05 | 1.61 ± 0.21* |
| Latency to magazine | 1.48 ± 0.08 | 1.52 ± 0.07 | 1.76 ± 0.07* | 2.64 ± 0.37* | 4.57 ± 1.01* | 1.44 ± 0.09 | 1.75 ± 0.19 | 1.90 ± 0.14 | 2.09 ± 0.26 | 4.11 ± 0.87* |
Values show mean ± SEM. N = 9–10 per group.
P < 0.05 versus vehicle control (highlighted in bold).
Accuracy, total trials, latency to correct response and latency to the magazine were all modified by guanfacine to the same extent in both genotypes (Table 2). Whereas guanfacine decreased %accuracy [[arcsine, veh vs. drug] drug: F(3,49) = 3.57, P = 0.020] and total trials [[arcsine, veh vs. drug] drug: F(3,49) = 3.84, P = 0.015], latency to correct [[log10, veh vs. drug] drug: F(3,49) = 16.47, P < 0.001] and latency to magazine [[rank, veh vs. drug] drug: F(3,49) = 39.42, P < 0.001] were both increased by the drug.
Guanfacine reduces activity in the light/dark exploration box (Figure 2)
Figure 2.
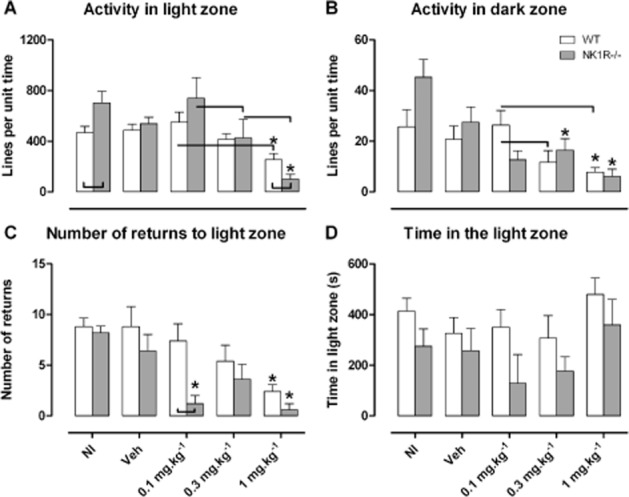
Guanfacine (0.1, 0.3 and 1.0 mg·kg−1, i.p.) reduced activity of wild-type (WT) and NK1R−/− mice in both the light zone and dark zone, and reduced crossings between the zones but had no effect on the time spent in either zone. (A) Activity per unit time in the light zone; (B) activity per unit time in the dark zone; (C) number of returns to the light zone; and (D) time in the light zone of a light–dark exploration box. NI, no injection; Veh, vehicle (saline). Data show mean ± SEM, N = 5 per group. Lines linking bars indicate statistical significance of at least P < 0.05 and * indicates P < 0.05 versus vehicle within genotype.
Compared with untreated WTs, NK1R−/− mice were hyperactive in the LZ of the LDEB [[sqrt, NI vs. veh] geno: F(1,16) = 4.75, P = 0.044; NI, WT vs. KO: P = 0.024, Figure 2A]. An apparent hyperactivity in the DZ just missed the criterion for statistical significance [[sqrt, NI vs. veh] geno: F(1,16) = 3.79, P = 0.069, Figure 2B].
Guanfacine reduced motor activity in the LZ [[rank, veh vs. drug] drug: F(3,32) = 15.71, P < 0.001]: at the highest dose, activity was decreased in both genotypes [[rank] veh vs. 1.0 mg·kg−1, WT: P = 0.007, NK1R−/−: P < 0.001, Figure 2A]. The same response was seen in the DZ [[sqrt, veh vs. drug] drug: F(3,30) = 5.07, P = 0.006]. However, an apparent reduction in locomotor activity in the DZ, after treatment with the 1.0 mg·kg−1 dose, was statistically significant in NK1R−/− mice only [[sqrt] veh vs. 1.0 mg·kg−1, WT: P = 0.068, KO: P = 0.002; Figure 2B]. Guanfacine also reduced the number of returns to the LZ in both genotypes [[sqrt, veh vs. drug] drug: F(3,32) = 4.99, P = 0.006; Figure 2C]. In NK1R−/− mice, this response was evident at 0.1 and 1.0 mg·kg−1 [[sqrt] veh vs. 0.1 mg·kg−1: P = 0.017, veh vs. 1.0 mg·kg−1: P = 0.005] but only the 1.0 mg·kg−1 dose caused such a reduction in WTs [[sqrt] veh vs. 1.0 mg·kg−1: P = 0.023]. Overall, NK1R−/− mice spent less time in the LZ than WTs [[rank, all groups] geno: F(1,32) = 4.75, P = 0.037] but this was unaffected by guanfacine [[rank, veh vs. drug]drug: F(3,32) = 2.05, P = 0.127; Figure 2D]. The effect of guanfacine did not depend on genotype in any measure.
NK1R−/− mice display increased anxiety-like behaviour in the EPM (Figure 3)
Figure 3.
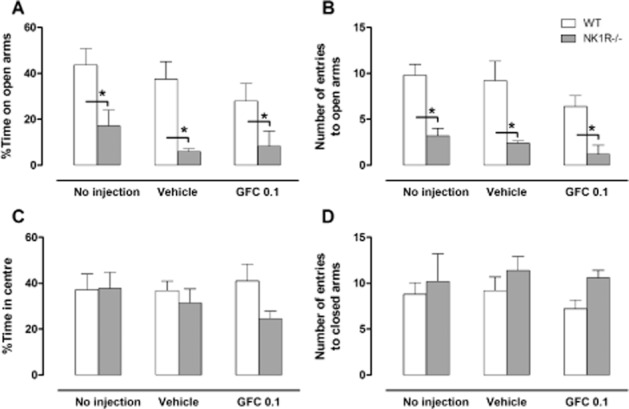
Guanfacine (GFC; 0.1 mg·kg−1) does not affect the behaviour of NK1R−/− mice in the EPM. (A) Time spent in the open arms; (B) number of entries to the open arms; (C) %time spent in the centre section number; and (D) number of entries to the closed arms of an EPM in wild-type (white bars) and NK1R−/− mice (grey bars). Data show mean ± SEM, N = 5. * P < 0.05.
There was a clear difference in the behaviour of NK1R−/− and WT mice in the EPM. Compared with WTs, NK1R−/− mice spent less %time on the open arms [[raw, all groups] geno: F(1,24) = 23.38, P < 0.001; Figure 3A] and made fewer entries into the open arms [[rank, all groups] geno: F(1,24) = 42.32, P < 0.001; Figure 3B]. There were no genotype differences in the %time spent in the centre square [[raw, all groups] geno: F(1,24) = 2.08, P = 0.1621; Figure 3C], or in the number of entries to the closed arms [[sqrt, all groups] geno: F(1,24) = 2.64 P = 0.1171; Figure 3D].
When compared with vehicle-treated mice, guanfacine had no overall effect on time in open arms [[raw, veh vs. drug] drug: F(1,16) = 0.32, P = 0.578; Figure 3A], or the number of entries to the open arms [[rank, veh vs. drug] drug: F(1,16) = 2.32, P = 0.147; Figure 3C]. The effect of guanfacine in the two genotypes did not differ for either measure.
Discussion
The findings from this study suggest that, regardless of colony, guanfacine improves attention and reduces impulsivity in NK1R−/− mice. A lack of any statistical interaction between the factors ‘drug treatment’ and ‘colony’ renders it unlikely that a difference in the response of the two colonies is masking any effects of guanfacine on the behaviours studied here.
Guanfacine improves ADHD-like behaviours in NK1R−/− mice
Attention
Unlike our previous studies (Yan et al., 2011; Weir et al., 2014), NK1R−/− mice did not display an inattentive phenotype at baseline (NI-2) in this study. This could be a result of improved performance after repeated testing (see Weir et al., 2014), or relate to reports that individuals with ADHD do not consistently present with the same behavioural subtype (Lahey et al., 2005; Todd et al., 2008). Another factor is that an increase in %omissions seems more robust when animals are tested with a prolonged, but fixed, inter-trial interval (LITI) than when using a variable ITI (unpublished observations). However, a previous study has confirmed that acute administration of an NK1 receptor antagonist (RP 67580) does aggravate inattentiveness in this test (Weir et al., 2014), suggesting that a lack of functional NK1 receptors could contribute to this behavioural abnormality.
Nevertheless, the lowest dose of guanfacine studied in the 5-CSRTT (0.1 mg·kg−1) caused a modest (−7%) reduction in %omissions (attention) in NK1R−/− mice, but not WTs. This beneficial response is consistent with the improvement in attention when spontaneously hypertensive rats (‘SHR’: a long-established rodent model of ADHD) are tested in an operant conditioning task (Sagvolden, 2006). This genotype-specific improvement in attention in NK1R−/− mice is unlikely to be explained by a change in animals’ state of arousal or motivation to respond because, at this dose, guanfacine did not affect the latency to correct response or the total number of trials completed by either genotype.
Unfortunately, it is difficult to make quantitative comparisons of the response to guanfacine in this study and in human trials because the scoring systems used in the two fields differ considerably. However, one human study has shown that when ADHD patients are tested in a choice reaction–time test after guanfacine extended release (GXR) treatment, they similarly show an improvement on ADHD rating scales, but no reduction in reaction speed compared with placebo (Kollins et al., 2011). An improved inattention subscale score has also been reported in ADHD patients after GXR treatment when given chronically (Biederman et al., 2008; Sallee et al., 2009; 2012; Newcorn et al., 2013) or in combination with a psychostimulant (Wilens et al., 2012).
This genotype-dependent response to guanfacine is interesting because it suggests that NK1R−/− mice are more sensitive to the effects of a low dose of this drug. Given the confirmed association of TACR1 gene polymorphism(s) and ADHD, it is reasonable to predict that guanfacine could also be more effective in ADHD patients with this genetic association. Whether or not this is the case, it is not necessary for guanfacine to be effective in only this genotype in order to be an effective treatment for ADHD.
By contrast, the highest dose of guanfacine (1.0 mg·kg−1) increased %omissions (i.e. reduced attention) in both genotypes. This impairment is most likely explained by the well-documented sedative effects of this drug (Van der Laan et al., 1985; Jakala et al., 1999). This is because both the latency to correct response and the latency to collect the reward were increased, whereas the total number of total trials completed by the mice was reduced at this dose. An alternative explanation is that guanfacine blunted animals’ motivation to carry out the task but, to the best of our knowledge, there is no evidence that guanfacine impairs motivation. In either case, the increase in %omissions is unlikely to be explained by a primary effect on cognition.
In tests of vigilance and working memory, activation of α2A-adrenoceptors enhances performance of both rats and monkeys in delayed alternation (Carlson et al., 1992) and delayed response tasks (Arnsten et al., 1988) respectively. Conversely, depletion of cortical noradrenaline impairs sustained attentional performance in the 5-CSRTT in rats (Carli et al., 1983). Low doses of α2A-adrenoceptor agonists improve performance, particularly in animals with either depletion of cortical noradrenaline (Milstein et al., 2007) or in older animals showing significant cortical loss of this neurotransmitter (Arnsten et al., 1988). However, deficits in working memory in mice with functional ablation of α2A-adrenoceptors are not relieved by guanfacine (Franowicz et al., 2002). Noradrenergic signalling strongly influences frontal cortical regions that mediate attention and working memory (see Arnsten and Li, 2005; Robbins and Roberts, 2007) and the classical bell-shaped treatment/response curve applies (Aston-Jones and Cohen, 2005; Arnsten, 2011).
The beneficial effects of guanfacine are thought to be mediated primarily by post-synaptic α2A-adrenoceptors located in the prefrontal cortex (PFC), which may become supersensitive as a consequence of a long-term deficit in noradrenergic transmission (Omiya et al., 2008). However, guanfacine could also reduce inattentiveness by activating somatodendritic α2A-adrenoceptors in the locus coeruleus (LC). This nucleus, which is the sole source of noradrenaline in the PFC (Loughlin et al., 1982), receives inputs from both GABAergic and glutamatergic projection neurones, from the nucleus prepositus hypoglossi and nucleus paragigantocellularis respectively (Ennis and Aston-Jones, 1989; Aston-Jones et al., 1991). Whereas GABAergic neurones tonically inhibit LC neurones, glutamate triggers their burst spiking in response to sensory stimuli (Foote et al., 1980; Ennis and Aston-Jones, 1989; Kawahara et al., 1999). Antagonism or functional ablation of NK1 receptors blunts this GABAergic inhibition (Maubach et al., 2002; Ebner and Singewald, 2007): such disinhibition would disrupt attention (Yan et al., 2009) but could be prevented by activation of somatodendritic α2A-adrenceptors, which suppresses LC excitation.
Accuracy is arguably an alternative index of attention (Robbins, 2002), but there are disparate reports on the effects of guanfacine on this measure. Whereas accuracy was increased in one preclinical study of aged macaques (O’Neill et al., 2000), there was no such response in a human study of cognitive performance (Jakala et al., 1999). Here, accuracy was not affected by any dose of guanfacine in either genotype, probably because the performance of the animals in the 5-CSRTT was already near maximum for this measure (∼96%) before drug treatment. One limitation of these findings is that such a high level of accuracy prevents this from being a useful index of attention when assessing the efficacy of the low dose of guanfacine. However, it is unlikely that the improvement in %omissions at the low dose is explained by increased motivation because neither measure of motivation (latency to correct response/reward) was affected.
Impulsivity
As in our previous studies, NK1R−/− mice were more impulsive than WTs (Yan et al., 2011; Dudley et al., 2013; Weir et al., 2014) but, here, this was evident only after they had experienced an i.p. injection. The reason for this is not clear but NK1 receptors are known to influence the noradrenergic stress response (for reviews, see Ebner and Singewald, 2006; Stanford, 2014). Guanfacine decreased impulsivity in both WT and NK1R−/− mice: evidently, this improvement (at 1.0 mg·kg−1) does not depend on functional NK1 receptors but could be secondary to non-specific inhibition of motor behaviour. Nevertheless, such a non-specific response could also explain the efficacy of this drug in the clinic. If so, it is possible that motor impulsivity is more likely to be attenuated than impulsive choice, such as the considered choosing of small, immediate rewards over larger, delayed rewards (Bari and Robbins, 2013).
Our findings are supported by evidence from other rodent studies: for instance, a high dose of guanfacine decreased impulsivity of both high-impulsive and low-impulsive rats in the 5-CSRTT, but also increased inattentiveness and response latencies (Fernando et al., 2012). Similarly, the response control of rats in the stop signal reaction–time task was improved by guanfacine, but this response was accompanied by a reduction in the speed of reaction in the task (Bari et al., 2009). In contrast, it was recently reported that local infusion of guanfacine into the ventral hippocampus of rats caused an improvement in impulsive choice, without affecting response latencies (Abela and Chudasama, 2014).
Hyperactivity
As before, uninjected NK1R−/− mice were hyperactive in the LDEB by comparison with WTs (Herpfer et al., 2005; Fisher et al., 2007; Yan et al., 2010). This is consistent with findings from our previous study in which acute administration of the NK1 receptor antagonists L 733060 or RP 67580 increased locomotor activity of wild-type mice (Yan et al., 2010). This latter finding points to a lack of functional NK1 receptors as a factor that underlies this behavioural abnormality.
Guanfacine reduced locomotor activity in both genotypes and so, like impulsivity, functional NK1 receptors are not necessary for this response, and both are likely attributed to the sedative effects of the drug (reviewed by Scheinin et al., 1989). However, the greater reduction in activity and number of returns to the LZ in NK1R−/− mice suggests that these mice could be more sensitive to the actions of guanfacine.
In the clinic, somnolence, sedation and fatigue are the most frequent reasons for discontinuing guanfacine therapy (Faraone et al., 2013; Hirota et al., 2014). Notwithstanding the confounding effects of sedation on measures of impulsivity, numerous preclinical studies have dissociated the sedative and cognitive effects of α2-adrenoceptor agonists (Arnsten et al., 1988; Franowicz and Arnsten, 1998; Jakala et al., 1999). For instance, the spatial working memory of rhesus monkeys was improved at a dose of guanfacine that had no sedative or hypotensive effects (Arnsten et al., 1988).
NK1R−/− mice display an anxiogenic phenotype
Anxiety is a common co-morbid problem for ADHD patients (Sobanski, 2006), and the two disorders may interact. For instance, Mancini et al. (1999) reported that the age of onset of anxiety is earlier, and its severity greater, in patients who had experienced childhood ADHD. Furthermore, ADHD patients with co-morbid anxiety have more pronounced attentional deficits (Sobanski, 2006).
Although the LZ of the LDEB was configured so as to render it ‘novel’, rather than aversive (i.e. low light intensity), these NK1R−/− mice (on a mixed background) displayed greater anxiety-like behaviour by comparison with their wild-types. This finding is consistent with our previous reports that NK1R−/− mice express greater active and passive avoidance of the LZ of the LDEB (Herpfer et al., 2005; Fisher et al., 2007). Nevertheless, the LDEB protocol used here has not been validated as a screen for anxiolytic drugs. For this reason, we also compared the behaviour of NK1R−/− and WT mice in the EPM. In this procedure, the NK1R−/− mice spent less time (∼17 vs. 44% in WTs) on the open arms and made fewer entries to the open arms, confirming that the NK1R−/− phenotype expresses more anxiety-like behaviour than WTs.
The effect of functional ablation of the NK1 receptor gene on anxiety-like behaviour and locomotor activity seems to depend on genetic background. This is inferred from evidence that mice lacking NK1 receptors on a 129/SvEv background spend a greater proportion of time on the open arms of an EPM than their WTs (i.e. they express less anxiety-like behaviour; Santarelli et al., 2001). By contrast, NK1R−/− mice on either a 129/Sv × C57BL/6 background (Murtra et al., 2000) or a J129/C57 hybrid background (Rupniak et al., 2001) do not. In fact, compared with WTs, NK1R−/− mice on a 129/Sv × C57BL/6 background spent less time on the EPM open arms (Gadd et al., 2003), an action that is consistent with increased anxiety-like behaviour. Furthermore, as we have reported previously (see above), mice from this colony display hyperactivity, compared with WTs, whereas for C57Bl6 mice, there is no such genotype difference (McCutcheon et al., 2008). It would be interesting to learn whether the abnormal locomotor activity and cognitive performance of NK1R−/− mice are similarly affected by background strain.
Guanfacine did not affect anxiety-like behaviour of either genotype in either the LDEB or the EPM test, at any dose. This finding contrasts with a report that this drug reduces anxiety-like behaviour of SHR, but not Wistar or WKY control rats, in the EPM (Langen and Dost, 2011). However, reports of the efficacy of guanfacine as an anxiolytic are inconsistent, despite its use for this clinical indication (Neylan et al., 2006; but see Connor et al., 2013). Nevertheless, the important finding here is that the effects of this drug on the behaviour expressed by NK1R−/− mice in the 5-CSRTT are unlikely to be secondary to a change in animals’ emotional status.
Conclusions
The effects of guanfacine on the behaviour of NK1R−/− mice in the 5-CSRTT closely resemble its clinical profile in ADHD patients. The finding that guanfacine can improve attention, without affecting impulsivity, supports our proposal that impulsivity and attention are underpinned by different neuronal networks (Dudley et al., 2013; Stanford, 2014). However, this distinctive response was evident only at the lowest dose of guanfacine, suggesting that, at higher drug doses, its influence on these networks is either less selective, or that the networks interact.
Because guanfacine did not affect anxiety-like behaviour at any dose, it is unlikely that the response to this drug is confounded by an underlying difference in co-morbid anxiety in NK1R−/− mice. Nevertheless, the influence of background strain on anxiety-like behaviour implies that modulation of this abnormal behaviour rests on an interaction between the NK1 receptor gene and other gene(s), or epigenetic factor(s), as yet unidentified. Such an influence is potentially clinically important because anxiety is a common co-morbid problem for ADHD patients (Sobanski, 2006). In light of these findings, the possibility that TACR1 polymorphism(s) contribute to co-morbid anxiety in ADHD patients merits further investigation.
Acknowledgments
This work was funded by an MRC-CASE award and the industrial sponsor, RenaSci Ltd. We wish to thank Simon Bate for his helpful advice on the statistical analysis of the data.
Glossary
- 5-CSRTT
5-choice serial reaction–time task
- ADHD
attention deficit hyperactivity disorder
- DZ
dark zone
- EPM
elevated plus maze
- KO
knockout
- LDEB
light/dark exploration box
- LZ
light zone
- VITI
variable inter-trial interval
- WT
wild-type
Author contributions
K. P. carried out testing in the 5-CSRTT, LDEB and EPM; analysis of results; and co-wrote the manuscript with S. C. S. A. J. P. contributed to training of mice in 5-CSRTT. J. A. D. contributed to training of mice in 5-CSRTT and genotyping of mice. Y. C. T. contributed to training of mice in 5-CSRTT. D. J. H. contributed to the study design and advised on translational aspects of experiments. S. C. S. supervised all experiments, advised on statistical analysis and co-wrote manuscript with K. P.
Conflict of interest
S. C. S. is a named inventor on an EU patent for the NK1R−/− mouse model of ADHD but declined the option to receive royalties.
References
- Abela AR, Chudasama Y. Noradrenergic alpha2A-receptor stimulation in the ventral hippocampus reduces impulsive decision-making. Psychopharmacology (Berl) 2014;231:521–531. doi: 10.1007/s00213-013-3262-y. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T, Fredriksson A. An antihypokinesic action of alpha2-adrenoceptors upon MPTP-induced behaviour deficits in mice. J Neural Transm. 2003;110:183–200. doi: 10.1007/s00702-002-0777-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:e89–e99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Carlson S, Tanila H, Rama P, Mecke E, Pertovaara A. Effects of medetomidine, an alpha-2 adrenoceptor agonist, and atipamezole, an alpha-2 antagonist, on spatial memory performance in adult and aged rats. Behav Neural Biol. 1992;58:113–119. doi: 10.1016/0163-1047(92)90327-z. [DOI] [PubMed] [Google Scholar]
- Clark RA, Shoaib M, Hewitt KN, Stanford SC, Bate ST. A comparison of InVivoStat with other statistical software packages for analysis of data generated from animal experiments. J Psychopharmacol. 2012;26:1136–1142. doi: 10.1177/0269881111420313. [DOI] [PubMed] [Google Scholar]
- Connor DF, Grasso DJ, Slivinsky MD, Pearson GS, Banga A. An open-label study of guanfacine extended release for traumatic stress related symptoms in children and adolescents. J Child Adolesc Psychopharmacol. 2013;23:244–251. doi: 10.1089/cap.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley JA, Weir RK, Yan TC, Grabowska EM, Grimme AJ, Amini S, et al. Antagonism of L-type Ca(v) channels with nifedipine differentially affects performance of wildtype and NK1R−/− mice in the 5-Choice Serial Reaction-Time Task. Neuropharmacology. 2013;64:329–336. doi: 10.1016/j.neuropharm.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31:251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N. Stress-induced release of substance P in the locus coeruleus modulates cortical noradrenaline release. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:73–82. doi: 10.1007/s00210-007-0185-3. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J Neurosci. 1988;8:3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. GABA-mediated inhibition of locus coeruleus from the dorsomedial rostral medulla. J Neurosci. 1989;9:2973–2981. doi: 10.1523/JNEUROSCI.09-08-02973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, McBurnett K, Sallee FR, Steeber J, Lopez FA. Guanfacine extended release: a novel treatment for attention-deficit/hyperactivity disorder in children and adolescents. Clin Ther. 2013;35:1778–1793. doi: 10.1016/j.clinthera.2013.09.005. [DOI] [PubMed] [Google Scholar]
- de Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, et al. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology (Berl) 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AS, Stewart RJ, Yan T, Hunt SP, Stanford SC. Disruption of noradrenergic transmission and the behavioural response to a novel environment in NK1R−/− mice. Eur J Neurosci. 2007;25:1195–1204. doi: 10.1111/j.1460-9568.2007.05369.x. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AF. The alpha-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology (Berl) 1998;136:8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd CA, Murtra P, De Felipe C, Hunt SP. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J Neurosci. 2003;23:8271–8280. doi: 10.1523/JNEUROSCI.23-23-08271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Herpfer I, Hunt SP, Stanford SC. A comparison of neurokinin 1 receptor knock-out (NK1–/–) and wildtype mice: exploratory behaviour and extracellular noradrenaline concentration in the cerebral cortex of anaesthetised subjects. Neuropharmacology. 2005;48:706–719. doi: 10.1016/j.neuropharm.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry. 2014;53:153–173. doi: 10.1016/j.jaac.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, Koivisto E, Riekkinen P., Jr Clonidine, but not guanfacine, impairs choice reaction time performance in young healthy volunteers. Neuropsychopharmacology. 1999;21:495–502. doi: 10.1016/S0893-133X(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Kawahara H, Westerink BH. Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studied by dual-probe microdialysis. Brain Res. 1999;823:42–48. doi: 10.1016/s0006-8993(99)01062-8. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, Lopez FA, Vince BD, Turnbow JM, Farrand K, Lyne A, et al. Psychomotor functioning and alertness with guanfacine extended release in subjects with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21:111–120. doi: 10.1089/cap.2010.0064. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Langen B, Dost R. Comparison of SHR, WKY and Wistar rats in different behavioural animal models: effect of dopamine D1 and alpha2 agonists. Atten Defic Hyperact Disord. 2011;3:1–12. doi: 10.1007/s12402-010-0034-y. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Fallon JH. Locus coeruleus projections to cortex: topography, morphology and collateralization. Brain Res Bull. 1982;9:287–294. doi: 10.1016/0361-9230(82)90142-3. [DOI] [PubMed] [Google Scholar]
- Mancini C, Van Ameringen M, Oakman JM, Figueiredo D. Childhood attention deficit/hyperactivity disorder in adults with anxiety disorders. Psychol Med. 1999;29:515–525. doi: 10.1017/s0033291798007697. [DOI] [PubMed] [Google Scholar]
- Maubach KA, Martin K, Chicchi G, Harrison T, Wheeldon A, Swain CJ, et al. Chronic substance P (NK1) receptor antagonist and conventional antidepressant treatment increases burst firing of monoamine neurones in the locus coeruleus. Neuroscience. 2002;109:609–617. doi: 10.1016/s0306-4522(01)00467-5. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Fisher AS, Guzdar E, Wood SA, Lightman SL, Hunt SP. Genetic background influences the behavioural and molecular consequences of neurokinin-1 receptor knockout. Eur J Neurosci. 2008;27:683–690. doi: 10.1111/j.1460-9568.2008.06043.x. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein JA, Lehmann O, Theobald DE, Dalley JW, Robbins TW. Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase-saporin impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology (Berl) 2007;190:51–63. doi: 10.1007/s00213-006-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405:180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Stein MA, Childress AC, Youcha S, White C, Enright G, et al. Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration. J Am Acad Child Adolesc Psychiatry. 2013;52:921–930. doi: 10.1016/j.jaac.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Lenoci M, Samuelson KW, Metzler TJ, Henn-Haase C, Hierholzer RW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry. 2006;163:2186–2188. doi: 10.1176/appi.ajp.163.12.2186. [DOI] [PubMed] [Google Scholar]
- Omiya Y, Yuzurihara M, Suzuki Y, Kase Y, Kono T. Role of alpha2-adrenoceptors in enhancement of antinociceptive effect in diabetic mice. Eur J Pharmacol. 2008;592:62–66. doi: 10.1016/j.ejphar.2008.06.087. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Fitten LJ, Siembieda DW, Ortiz F, Halgren E. Effects of guanfacine on three forms of distraction in the aging macaque. Life Sci. 2000;67:877–885. doi: 10.1016/s0024-3205(00)00681-0. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti A. Tomoxetine (Eli Lilly & Co) Curr Opin Investig Drugs. 2002;3:272–277. [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17(Suppl. 1):i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Carlson EJ, Webb JK, Harrison T, Porsolt RD, Roux S, et al. Comparison of the phenotype of NK1R−/− mice with pharmacological blockade of the substance P (NK1) receptor in assays for antidepressant and anxiolytic drugs. Behav Pharmacol. 2001;12:497–508. doi: 10.1097/00008877-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. The alpha-2A adrenoceptor agonist guanfacine improves sustained attention and reduces overactivity and impulsiveness in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD) Behav Brain Funct. 2006;2:41. doi: 10.1186/1744-9081-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, Lyne A, Wigal T, McGough JJ. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:215–226. doi: 10.1089/cap.2008.0080. [DOI] [PubMed] [Google Scholar]
- Sallee FR, Kollins SH, Wigal TL. Efficacy of guanfacine extended release in the treatment of combined and inattentive only subtypes of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22:206–214. doi: 10.1089/cap.2010.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Debs PC, Sibille ET, Blier P, Hen R, et al. Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc Natl Acad Sci U S A. 2001;98:1912–1917. doi: 10.1073/pnas.041596398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinin H, Virtanen R, MacDonald E, Lammintausta R, Scheinin M. Medetomidine – a novel alpha 2-adrenoceptor agonist: a review of its pharmacodynamic effects. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:635–651. doi: 10.1016/0278-5846(89)90051-1. [DOI] [PubMed] [Google Scholar]
- Sharp SI, McQuillin A, Gurling HM. Genetics of attention-deficit hyperactivity disorder (ADHD) Neuropharmacology. 2009;57:590–600. doi: 10.1016/j.neuropharm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Sharp SI, McQuillin A, Marks M, Hunt SP, Stanford SC, Lydall GJ, et al. Genetic association of the tachykinin receptor 1 TACR1 gene in bipolar disorder, attention deficit hyperactivity disorder and the alcohol dependence syndrome. Am J Med Genet B Neuropsychiatr Genet. 2014;165:373–380. doi: 10.1002/ajmg.b.32241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobanski E. Psychiatric comorbidity in adults with attention-deficit/hyperactivity disorder (ADHD) Eur Arch Psychiatry Clin Neurosci. 2006;256(Suppl. 1):i26–i31. doi: 10.1007/s00406-006-1004-4. [DOI] [PubMed] [Google Scholar]
- Stanford SC. The Open Field Test: reinventing the wheel. J Psychopharmacol. 2007a;21:134–135. doi: 10.1177/0269881107073199. [DOI] [PubMed] [Google Scholar]
- Stanford SC. Open fields (unlike wheels) can be any shape but still miss the target. J Psychopharmacol. 2007b;21:144. doi: 10.1177/0269881107074492. [DOI] [PubMed] [Google Scholar]
- Stanford SC. Psychostimulants, antidepressants and neurokinin-1 receptor antagonists (‘motor disinhibitors’) have overlapping, but distinct, effects on monoamine transmission: the involvement of L-type Ca channels and implications for the treatment of ADHD. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.03.021. DOI: 10.1016/j.neuropharm.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Todorov AA, Neuman RJ, Reiersen AM, Henderson CA, et al. Predictors of stability of attention-deficit/hyperactivity disorder subtypes from childhood to young adulthood. J Am Acad Child Adolesc Psychiatry. 2008;47:76–85. doi: 10.1097/chi.0b013e31815a6aca. [DOI] [PubMed] [Google Scholar]
- Van der Laan JW, Van Veenendaal W, Voorthuis P, Weick G, Hillen FC. The effects of centrally acting adrenergic agonists on temperature and on explorative and motor behaviour. Relation with effects on quasi-morphine withdrawal behaviour. Eur J Pharmacol. 1985;107:367–373. doi: 10.1016/0014-2999(85)90264-x. [DOI] [PubMed] [Google Scholar]
- Weir RK, Dudley JA, Yan TC, Grabowska EM, Peña-Oliver Y, Ripley T, et al. The influence of test experience and NK1 receptor antagonists on the performance of NK1R−/− and wild type mice in the 5-Choice Serial Reaction-Time Task. J Psychopharmacol. 2014;28:270–281. doi: 10.1177/0269881113495722. [DOI] [PubMed] [Google Scholar]
- Wilcock J, Broadhurst PL. Strain differences in emotionality: open-field and conditioned avoidance behavior in the rat. J Comp Physiol Psychol. 1967;63:335–338. doi: 10.1037/h0024363. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:74–85.e2. doi: 10.1016/j.jaac.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Yan TC, Hunt SP, Stanford SC. Behavioural and neurochemical abnormalities in mice lacking functional tachykinin-1 (NK1) receptors: a model of attention deficit hyperactivity disorder. Neuropharmacology. 2009;57:627–635. doi: 10.1016/j.neuropharm.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Yan TC, McQuillin A, Thapar A, Asherson P, Hunt SP, Stanford SC, et al. NK1 (TACR1) receptor gene ‘knockout’ mouse phenotype predicts genetic association with ADHD. J Psychopharmacol. 2010;24:27–38. doi: 10.1177/0269881108100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan TC, Dudley JA, Weir RK, Grabowska EM, Pena-Oliver Y, Ripley TL, et al. Performance deficits of NK1 receptor knockout mice in the 5-choice serial reaction-time task: effects of d-amphetamine, stress and time of day. PLoS ONE. 2011;6:e17586. doi: 10.1371/journal.pone.0017586. [DOI] [PMC free article] [PubMed] [Google Scholar]


