Abstract
Background
The ARCHITECT HIV Ag/Ab Combo assay has a wide dynamic range for determining the sample-to-cutoff ratio (S/CO) values compared to other diagnostic HIV antibody assays.
Objectives
Determine the performance of an HIV testing algorithm that uses the ARCHITECT combo assay in the clinical setting and explore the utility of the signal-to-cutoff (S/CO) ratio to predict acute HIV-1 infection status.
Study design
A retrospective analysis of clinical samples from a hospital and referral population screened for HIV-1 infection between May 2011 and March 2013. Repeatedly reactive samples were tested using the Multispot HIV-1/HIV-2 rapid test and depending on that result, confirmatory orthogonal testing used the Western blot (WB) for HIV-1, Immunoblot for HIV-2 and nucleic acid amplification testing (NAAT) for HIV RNA.
Results
A total of 21,317 test results were evaluated of which 509 were ARCHITECT repeatedly reactive; of these, 422 were Multispot-reactive only for HIV-1 (413 WB-positive; 9 indeterminate), 4 were Multispot-reactive for both HIV-1 and HIV-2 (one HIV-2 immunoblot-positive with 17 HIV-2 RNA copies/mL) and 83 were Multispot-non-reactive of which 15 were HIV-1 RNA positive and represented acute HIV-1 infection. There was an association among the ARCHITECT S/CO (median; IQR) values for antibody-negative (0.14; 0.11–0.16), acute infection (33; 2.1–76) and established HIV-1 infection (794; 494–1,029) (Kruskal–Wallis, p < 0.0001).
Conclusions
The ARCHITECT combo assay with Multispot confirmation and reserved use of HIV-1 WB, HIV-2 Immunoblot and HIV NAAT for Multispot dual HIV-1/2 infection, and NAAT alone for Multispot-negative specimens, had a suitable test performance for detecting acute and established HIV infection.
Keywords: ARCHITECT HIV Ag/Ab Combo assay Sample-to-cutoff ratio Alternative HIV diagnostic algorithm
1. Background
The effective management of HIV infection requires the ability to distinguish and confirm primary (acute) from established infection quickly and efficiently, as acute HIV infection (AHI) is characterized by massive viral replication, which contributes disproportionally to onward HIV transmission [1–4]. In addition, HIV-1 must be distinguished from HIV-2 as therapeutic monitoring and treatment regimens differ between the two [5,6]. With an emphasis on these goals, the Centers for Disease Control and Prevention (CDC) has proposed an alternative two-step HIV diagnostic algorithm [7,8]. The first step uses a combined HIV-1 antigen and HIV-1/2 antibody fourth (4th)-generation assay for an HIV-1/2 screening test, which allows for identification of acute (HIV-1 p24 antigen) and established HIV infection (IgM and IgG HIV-1/2 antibodies) and importantly reduces the antibody-free “window” period by around one-week compare with 3rd-generation assays that rely on the detection of IgM and IgG antibodies only [9]. The second step uses a confirmatory orthogonal antibody test with the capacity to differentiate HIV-1 from HIV-2 infection. If the confirmatory test is negative, a nucleic acid amplification test (NAAT) is performed to resolve this discrepancy [10,11].
The two current FDA-approved 4th-generation HIV-1/2 combination antigen/antibody chemiluminescent, magnetic microparticle-based immunoassay (CMIA) and enzyme immunoassay (EIA) platform assays have significantly reduced the diagnostic seronegative window period but these assays are still insensitive to the three- to five-day window period when HIV-1 RNA can be detected in plasma but HIV-1 p24 antigen is not detected based on signal-to-cutoff ratio (S/CO) for a reactive test [1,12–14]. The lower sensitivity of the 4th-generation assays for HIV-1 p24 antigen is approximately 15 pg/mL of plasma on the French Agency for the Safety of Health Products (AFSSAPS) standard (0.65 IU/mL on the WHO standard) given for the GS HIV Combo Ag/Ab EIA assay (Genetic Systems, Redmond, WA), while the ARCHITECT HIV Ag/Ab Combo assay (Abbott Diagnostics, Chicago, IL) is approximately 18 pg/mL (kit insert). In the absence of immune-complexed antigen–antibody, 1 pg of p24 antigen is equivalent to approximately 40,000 HIV RNA copies/mL or 20,000 HIV virions/mL of plasma [15].
The ARCHITECT HIV Ag/Ab combo assay is a chemiluminescent, magnetic microparticle-based immunoassay (CMIA) with the ability to identify simultaneously HIV-1 p24 antigen, anti-HIV-1 gp41 antibody and anti-HIV-2 gp36 antibody; however, additional testing is needed to discriminate between the antigen and antibody assay targets. The only FDA-approved orthogonal supplemental test with the capability to differentiate HIV-1 from HIV-2 antibody is the Multispot HIV-1/HIV-2 (Multispot) rapid test (Bio-Rad Laboratories), which is a rapid immunoassay that detects and differentiates between HIV-1 and HIV-2 antibodies using an HIV-1 recombinant antigen and respective peptides for HIV-1 and HIV-2.
A S/CO ≥1 for the ARCHITECT assay is considered to be reactive. However, the broader dynamic readout range of the ARCHITECT assay compared to HIV-1/2 enzyme-linked immunosorbent assays (EIA), suggests that the magnitude of the ARCHITECT test S/CO value may be useful for determining the likelihood that a S/CO test result may represent acute or established HIV infection.
2. Objective
To determine the performance of an HIV testing algorithm that uses the ARCHITECT Combo assay in the clinical setting and to explore the utility of the S/CO ratio, particularly when less than 1.0, for guiding further HIV-1 RNA testing to identify acute HIV-1 infection in the seronegative window period.
3. Study design
A retrospective analysis was done using clinical HIV-1/2 test results obtained from a hospital and referral population between May 2011 and March 2013. All the specimens were submitted for standard HIV diagnostic testing at Harborview Medical Center and almost 99.7% of the specimens were plasma. The Clinical Laboratory Improvement Amendments (CLIA) of 1998 compliant, College of American Pathologists (CAP)-certified Clinical Retrovirology Laboratory was blinded to the specimen identify (and thus patient retests) or reason for testing, and the data analysis were conducted with de-identifiers for each test result in accordance with the Department of Laboratory Medicine’s University of Washington Human Subjects Review protocol for assay development and validation. The following ARCHITECT assay-based algorithm was used: All specimens that were repeatedly reactive using the ARCHITECT assay were tested with the orthogonal Multispot HIV-1/2 (Multispot) rapid test (Bio-Rad, Redmond, WA) for which a confirmatory Multispot rapid test result for HIV-1 antibody required reactivity for both the antigen and peptide spots. An HIV-1 confirmatory Western blot (WB) test was also done (Genetic System, Redmond, WA), further HIV-2 antibody testing was performed to confirm Multispot HIV-2 peptide reactivity (HIV-2 Immunoblot (IB), Focus Diagnostics), HIV-1 RNA was assessed using the Abbott m2000rt Real Time HIV-1 RNA (Abbott Molecular, Chicago, IL), and a quantitative CLIA-compliant HIV-2 RNA real time in-house PCR assay was available for HIV-2 confirmation when the HIV-2 peptide was reactive using Multispot [6]. We defined an HIV-1/2 ‘CMIA-reactive’ specimen strictly as a specimen that was repeatedly reactive with an S/CO ≥1 according to the manufacturer’s recommendations, as there is no recommended indeterminate S/CO range <1 for the assay that would trigger further testing. In addition, 635-blinded HIV-negative serum samples, screened using a 3rd-generation Genetic Systems HIV-1/HIV-2 Plus O EIA and pooled HIV NAAT [14], were obtained from men-who-have-sex with men seeking HIV testing through the Public Health - Seattle and King County HIV/STD Program and retested using the ARCHITECT assay in order to assess performance of the assay in a high-risk HIV-uninfected population.
All statistical analyses were performed using STATA 11.1 for Windows (StataCorp LP, College Station, TX, USA). The normality of the data distribution and variance were examined by Kolmogorov–Smirnov and Levene tests. Descriptive statistical analysis for the ARCHITECT assay S/CO and HIV-1 RNA were done and the Kruskal–Wallis test was used to evaluate the ARCHITECT S/CO relationship between negative, acute and established HIV-1 infection. Linear regression analysis used the HIV-1 RNA copies/mL and the ARCHITECT S/CO values from acute infection samples (all results were log10 transformed). The Receiver Operator Curve was derived from 718 samples (83 Architect positive, Multispot-negative and HIV-1 NAAT tested to confirm status and 635-blinded Architect and pooled HIV NAAT negative samples) to predict which positive ARCHITECT S/CO value is correlated with confirmatory HIV NAAT positive result to detect acute HIV-1 infection.
4. Results
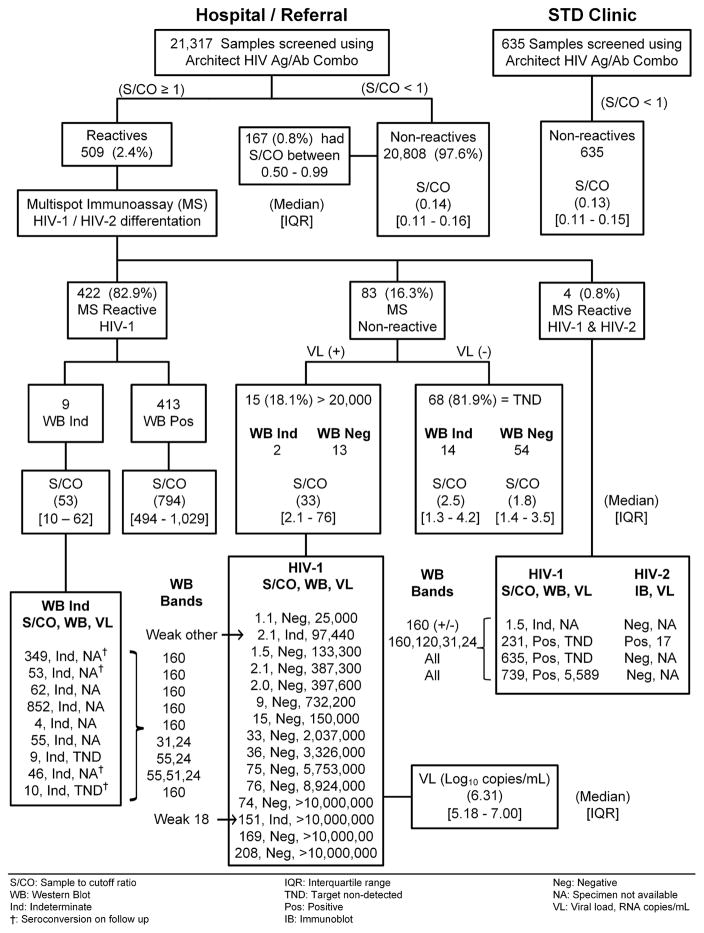
A total of 21,317 clinical samples were tested using the ARCHITECT assay of which 509 (2.4%) were repeatedly reactive with an S/CO ≥1 (Fig. 1). From among the CMIA-reactive samples, 422 (82.9%) were Multispot-reactive only for HIV-1, 4 (0.8%) were Multispot-reactive for both HIV-1 and HIV-2 and 83 (16.3%) were Multispot-non-reactive. Based on the Multispot HIV-1 reactive outcome, 422 samples were reported as “presumptive positive for HIV-1”; the WB resulted in 413 confirmed-positive and nine indeterminate results. From these nine WB-indeterminate specimens, only two had sufficient sample-volume for further NAAT and HIV-1 RNA was not detected; four of these nine persons were found to have seroconverted after subsequent testing. The ARCHITECT S/CO median value and interquartile range [IQR] was 794 [494–1,029] for the WB positives and 53 [10–62] for the WB indeterminate samples (Fig. 1).
Fig. 1.
Schematic flow-chart of the 4th-generation HIV 1/2 testing algorithm with HIV-1 Western blot bands, and a comparison of the ARCHITECT assay signal-to-cutoff (S/CO) median and interquartile range [IQR] among the different Multispot (MS) test results and between negative samples from high-risk STD clinic and lower-risk hospital/referral populations.
With regard to the testing algorithm, for the four specimens that were Multispot-reactive to both HIV-1 and HIV-2, one specimen was HIV-1 WB-indeterminate (weak gp160 band), HIV-2 IB-negative and was reported as HIV-1 infection not confirmed but with follow-up testing recommended. Two specimens were HIV-1 WB-positive (all bands present) and HIV-2 IB-negative, with high ARCHITECT assay S/CO values (635 and 739) and both were reported as HIV-1 infected. The last specimen was HIV-1 WB (four bands present) and HIV-2 IB-positive, negative for HIV-1 RNA, had 17 HIV-2 RNA copies/mL detected and was reported as HIV-2 infection.
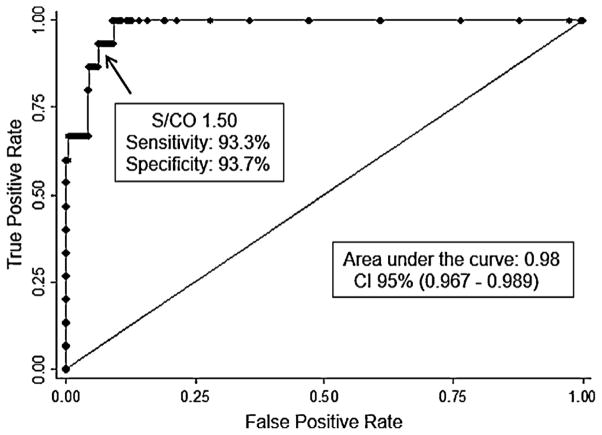
Finally, of 83 Multispot-non-reactive specimens that were evaluated for HIV-1 RNA, 15 (18.1%) specimens had a positive HIV-1 viral load (VL) >20,000 RNA copies/mL with a median log10 RNA copies/mL and IQR of 6.31 [5.18–7.0], and a median ARCHITECT assay S/CO value and IQR of 33 [2.1–76]. HIV-1 RNA was not detected in the remaining 68 specimens (81.9%); of these, 54 specimens were WB-negative and 14 were WB-indeterminate with an ARCHITECT assay S/CO median value and IQR of 1.8 [1.4–3.5] and 2.5 [1.3–4.2], respectively. The WB-negative group was reported as “HIV-1 negative,” while the WB-indeterminate group was reported as “HIV-1 infection not confirmed” with follow-up testing recommended. Based on the 15 samples with detectable VL as a truly acute HIV-1 infection only, 92% (57/62) of the samples with ARCHITECT assay S/CO values between 1–5 and 92% (11/12) of the samples with ARCHITECT assay S/CO values between 5 and 15 were false acute HIV-1 infection, whereas all samples above ARCHITECT assay S/CO value of 15 were 100% (9/9) correlated with acute HIV-1 infection. Thus, the Receiver Operator Curve (ROC curve) using the ARCHITECT S/CO to predict acute HIV-1 infection results showed an area under the curve of 0.98 [95% confidence interval (CI), 0.97–0.99] with a sensitivity and specificity for an ARCHITECT assay S/CO = 1.5 of 93.3% and 93.7% respectively (Fig. 2). For an ARCHITECT assay S/CO = 1 (manufacture insert) the sensitivity and specificity were 100% and 90.3% respectively.
Fig. 2.
Receiver Operator Curve for signal-to-cutoff ratio (S/CO) and viral HIV-1 RNA detection from 718 samples; 83 Architect positive and Multispot-negative with 15 confirmed acute HIV-1 infection through HIV-1 NAAT positive test results and 635 Architect and pooled HIV NAAT negative samples.
A total of 20,808 (97.6%) hospital/referral and 635 STD clinic samples had identical non-reactive CMIA results, with an ARCHITECT assay S/CO median value and IQR of 0.14 [0.11–0.16] and 0.13 [0.11–0.15] respectively. In addition, from among these hospital/referral results, 167 (0.8%) were ARCHITECT non-reactive with an S/CO values between 0.5 and 0.99. The ARCHITECT assay S/CO median and [IQR] for 21,610 negative samples was very low 0.14 [0.11–0.16]; for 15-acute HIV-1 infections, 33 [2.1–36]; and for 413 established HIV infection, 794 [444–1,028] (Kruskal–Wallis, p < 0.0001). In the case of five indeterminate WB with reactive MS, the ARCHITECT assay S/CO values (55 [9–62]) overlapped with the S/CO for the 15 acute HIV-1 infections.
There was a log-linear relationship between the ARCHITECT assay S/CO value and HIV-1 RNA level in the acute infection group (Fig. 3) with ARCHITECT assay S/CO values of 0.5 and 1.0 representing 4.0 [95% CI, 3.96–4.17] and 4.38 [95% CI, 4.27–4.46] HIV-1 RNA log10 copies/mL, respectively.
Fig. 3.
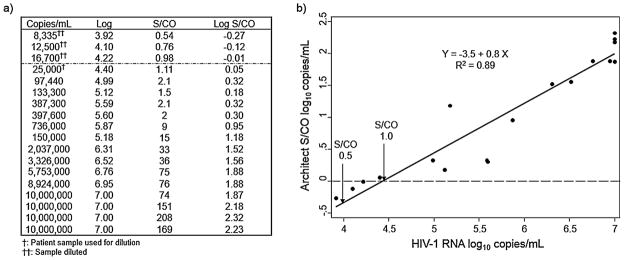
(a) Table showing 15 acute HIV-1 infection cases plus three extended dilutions from a patient sample with a signal-to-cutoff (S/CO) value = 1.11. (b) Figure showing the Log10 linear relationship between the HIV-1 RNA copies/mL of plasma (abscissa) and the ARCHITECT assay S/CO values (ordinate).
5. Discussion
The proposed 4th-generation-based HIV testing algorithm based on the ARCHITECT Ag/Ab Combo Assay, orthogonal Multispot confirmation of CMIA-reactive specimens and HIV NAAT as needed, identified all 413 WB-positive specimens, 15 primary (acute) HIV-1 infections in addition to one each of HIV-1 and HIV-2 infection with mixed Multispot rapid test results. Among nine WB-indeterminate specimens with HIV-1-reactive Multispot rapid test results, four were subsequently found to be seroconverting while five were considered to be indeterminate since we did not know the clinical diagnostic outcome. However, if we used the new MS rapid test report format approved by the FDA in 2013 (both HIV-1 spots must be reactive), three of the five WB-positive specimens were categorized as Multispot rapid test indeterminate with a single-spot gp41 recombinant peptide reactive, and the remaining two specimens were considered confirmed HIV-1 infection with both HIV-1 spots reactive.
Importantly, the 15 acute HIV-1 infections occurred from among 83 Multispot-non-reactive specimens which were, not surprisingly, also missed by the HIV-1 WB confirmation assay; and a single HIV-2 infection was identified from among four Multispot dually HIV-1/HIV-2 reactive specimens, which would have been erroneously classified as a HIV-1 infection by the WB assay alone. There were an additional four early HIV-1 infections with indeterminate WB results from among the 422 Multispot HIV-1 positive tests that were identified by subsequent follow-up serological and NAAT.
It is important to mention that without further supplemental or confirmatory WB and/or NAAT, the 4th-generation testing algorithm would have misidentified 68 of 83 (81.9%) Multispot-negative samples as possible acute HIV-1 infections. From this group, 14 specimens were WB-indeterminate and 54 were WB-negative; however, the ARCHITECT assay S/CO values were similarly low with median values of 2.5 and 1.8, respectively.
The CMIA technology used by the ARCHITECT Ag/Ab Combo assay is characterized by a three-log10 dynamic range of S/CO values, which contrasts with a one-log10 range for the other FDA- approved 4th-generation assay (Bio-Rad, Redmond, WA), earlier generation HIV-1/2 EIA platforms and a recently FDA-approved lateral flow HIV-1/2 antigen/antibody rapid test assay (Orgenics, Alere Determine HIV-1/2 Ag/Ab Combo assay). We reasoned that this difference in S/CO value dynamic range would allow the CMIA test to discriminate between true-negative samples, p24-antigen associated with acute infection, and IgM/IgG antibodies associated with established infection; as such, there was a significant association between the S/CO values and stage of HIV infection. There was also a strong log10 linear relationship (R2 = 0.89) between the ARCHITECT assay S/CO values and HIV-1 RNA levels in acute infection, which is related to the p24-antigen concentration, with an HIV-1 RNA log10 copies/mL of 4.44 [95% CI, 4.35–4.53] when the S/CO value = 1. This relationship agrees with the published ARCHITECT Combo assay performance data, which identifies a lower limited of detection (LLOD) between 4.5 and 4.7 HIV-1 RNA log10 copies/mL [16].
Given the public health importance of identifying early acute infection to limit onward transmission of HIV-1, we explored improving the sensitivity of the ARCHITECT assay to detect more acute infections by decreasing the S/CO ratio required to trigger confirmatory Multispot rapid testing and supplemental HIV-1 NAAT. Currently, there is no intermediate or “gray zone” for an S/CO value <1 defined by the manufacturer that would trigger additional testing. The ARCHITECT assay S/CO median and IQR from negative samples in a high-risk STD population was not significantly different from that for a low-risk hospital and referral population; thus, considering a S/CO value between ≥0.5 and <1.0 as indeterminate likely would not interfere with detecting true-negative samples but would define a LLOD of 4.0 [95% CI, 3.96–4.17] HIV-1 RNA log10 copies/mL. Our study suggests that there is potential for defining an intermediate or “gray zone” of S/CO values for identifying acute HIV-1 infections with a supplemental viral load testing to confirm the infection status but a more precise definition warrants further study. The 167 specimens with S/CO values between 0.5 and 0.99 were not available retrospectively for HIV-1 NAAT although such testing is now being done prospectively in our laboratory.
6. Conclusions
We showed that the Abbott 4th-generation assay with orthogonal supplemental Multispot HIV-1/2 confirmation and reserved use of HIV-1 WB, HIV-2 IB and HIV-1/2 NAAT for Multispot dual HIV-1/2 infection, and NAAT alone for Multispot-negative specimens, had a test performance suitable for detecting acute and established HIV infection without the current mandatory use of confirmatory WB providing that HIV-1 and HIV-2 NAAT (RNA and DNA) are available. The success of a 4th-generation testing algorithm to provide timely results without relying on presumptive diagnostic test reporting will depend upon the availability of FDA-approved rapid supplemental testing for HIV NAAT. The advent of HIV-1 RNA suppressive antiretroviral prophylaxis for pre- and post-exposures and to prevent mother-to-child transmission will require the availability of FDA-approved rapid HIV DNA tests. Moreover, similar rapid HIV DNA testing may also apply to HIV-1 vaccine recipients because of vaccine-induced seropositivity/reactivity (VISPR) for which WB is still an important discriminatory test until such time that a broader vaccine-induced response more completely mimics the WB gag-, pol- and env-related banding patterns that currently define serological confirmation for HIV-1 infection. The broad dynamic range of the Abbott S/CO ratio with further definition of an indeterminate “gray zone” value <1 may provide additional guidance for the use of NAAT to detect acute HIV-1 infection and prevent onward transmission.
Acknowledgments
Funding
This study was supported by the follow grants: ACTG Virology Specialty Laboratory (AI-38858), HVTN HIV Diagnostic Laboratory (AI-68618), and UW CFAR Clinical Retrovirology Core (AI-27757).
The authors would like to thanks all the Virology Specialty Laboratory staff for their contribution in the processing and diagnostic testing of specimens for HIV and special thanks to Public Health - Seattle and King County for providing the high-risk patient samples.
Footnotes
This study was presented at the 2012 HIV Diagnostics Conference, Atlanta, GA; December 12–14, 2012.
Competing interests
The authors declare no financial or other conflict of interest.
Ethical approval
University of Washington Human Subjects application #29860, titled “Laboratory Medicine Quality Assurance Project Research”.
Authorship
All authors have made substantial contributions to each of the following: (1) the concept and design of the study (EMR, JD, RWC), or acquisition of data (EMR, SH, JD, PS), or analysis and interpretation of data (EMR, SH, JD, PS, JS, RWC); (2) drafting of the article or revising it critically for important intellectual content (EMR, JD, PS, JS, RWC); (3) final approval of the version to be submitted (EMR, SH, JD, PS, JS, RWC).
References
- 1.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med. 2011;364:1943–54. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller WC, Rosenberg NE, Rutstein SE, Powers KA. Role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:277–82. doi: 10.1097/COH.0b013e32833a0d3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–68. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma ZM, Stone M, Piatak M, Jr, Schweighardt B, Haigwood NL, Montefiori D, et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol. 2009;83:3288–97. doi: 10.1128/JVI.02423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell-Yesufu OT, Gandhi RT. Update on human immunodeficiency virus (HIV)-2 infection. Clin Infect Dis. 2011;52:780–7. doi: 10.1093/cid/ciq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M, Gottlieb GS, Dragavon JA, Cherne SL, Kenney DL, Hawes SE, et al. Validation for clinical use of a novel HIV-2 plasma RNA viral load assay using the Abbott m2000 platform. J Clin Virol. 2012;55:128–33. doi: 10.1016/j.jcv.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasrullah M, Wesolowski LG, Meyer WA, 3rd, Owen SM, Masciotra S, Vorwald C, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2012;27:731–7. doi: 10.1097/QAD.0b013e32835bc535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm – United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2013;62:489–94. [PMC free article] [PubMed] [Google Scholar]
- 9.Branson BM, Stekler JD. Detection of acute HIV infection: we can’t close the window. J Infect Dis. 2012;205:521–4. doi: 10.1093/infdis/jir793. [DOI] [PubMed] [Google Scholar]
- 10.Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(Suppl 1):S17–22. doi: 10.1016/j.jcv.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Styer LM, Sullivan TJ, Parker MM. Evaluation of an alternative supplemental testing strategy for HIV diagnosis by retrospective analysis of clinical HIV testing data. J Clin Virol. 2011;52(Suppl 1):S35–40. doi: 10.1016/j.jcv.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Branson BM. The Future of HIV Testing. J Acquir Immune Defic Syndr. 2010;55:S102–5. doi: 10.1097/QAI.0b013e3181fbca44. [DOI] [PubMed] [Google Scholar]
- 13.Peters P, Westheimer E, Moss N, Gay C, Tsoi B, Pandori M, et al. HIV combination antigen/antibody testing detects a high proportion of acute HIV infections and improves HIV diagnostic yield—three regions of the United States. Conference on Retroviruses and Opportunistic Infections; 2013. [Google Scholar]
- 14.Stekler JD, Swenson PD, Coombs RW, Dragavon J, Thomas KK, Brennan CA, et al. HIV testing in a high-incidence population: is antibody testing alone good enough? Clin Infect Dis. 2009;49:444–53. doi: 10.1086/600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schupbach J, Tomasik Z, Knuchel M, Opravil M, Gunthard HF, Nadal D, et al. Optimized virus disruption improves detection of HIV-1 p24 in particles and uncovers a p24 reactivity in patients with undetectable HIV-1 RNA under long-term HAART. J Med Virol. 2006;78:1003–10. doi: 10.1002/jmv.20655. [DOI] [PubMed] [Google Scholar]
- 16.Brennan CA, Yamaguchi J, Vallari A, Swanson P, Hackett JR., Jr ARCHITECT((R)) HIV Ag/Ab Combo assay: Correlation of HIV-1 p24 antigen sensitivity and RNA viral load using genetically diverse virus isolates. J Clin Virol. 2013;57:169–72. doi: 10.1016/j.jcv.2013.01.017. [DOI] [PubMed] [Google Scholar]