Abstract
Background
Food reinforcement, the extent to which people are willing to work to earn a preferred snack food, and parental obesity are risk factors for weight gain, but there is no research comparing the predictive effects of these factors for adolescent weight gain.
Methods
130 non-obese adolescents (M age = 15.2 ± 1.0; M BMI = 20.7 ± 2.0; M zBMI = 0.16 ± 0.64) at differential risk for weight gain based on parental obesity completed baseline food and money reinforcement tasks, and provided zBMI data over 2-yr follow-up.
Results
The number of obese (BMI ≥ 30) parents (p = 0.007) and high food reinforcement (p = 0.046) were both significant independent predictors of greater zBMI increases, controlling for age, sex, parent education and minority status. Having no obese parents or being low or average in food reinforcement was associated with reductions in zBMI, but those high in food reinforcement showed larger zBMI increases (0.102) than having one obese parent (0.025) but less than having two obese parents (0.177).
Discussion
Food reinforcement and parental obesity independently predict future weight gain among adolescents. It might be fruitful for obesity prevention programs to target both high risk groups.
Keywords: Food reinforcement, adolescent, parental obesity, preventing weight gain
Adolescence may be one of the critical periods for weight gain (Dietz, 1994). Adolescence is associated with hormonal changes that favor adipose tissue increase in girls (Biro, 2008), and genetic and familial predispositions to excess weight gain may be expressed more strongly during adolescence than during preadolescent periods (Stice, Presnell, Shaw, & Rohde, 2005). For this reason, examining parental obesity as a risk factor for weight gain in adolescence is particularly relevant. Adolescent children of obese versus lean parents have shown a fourfold increase in risk for obesity onset (Epstein, et al., 2007; Magarey, Daniels, Boulton, & Cockington, 2003; Whitaker, Wright, Pepe, Seidel, & Dietz, 1997), but the effects of this risk factor may vary across development, as some studies have shown parental obesity exerts an influence on weight gain throughout development (Crossman, Sullivan, & Benin, 2006), while other studies have shown parental obesity is important only for young children (Epstein, et al., 2007; Whitaker, et al., 1997). Parental obesity may be a risk factor for adolescent weight gain due to genetic predisposition (Wardle, Carnell, Haworth, & Plomin, 2008; Warrington, et al.), or due to behavioral factors within families (Birch & Fisher, 1998; Crossman, et al., 2006; Francis, Lee, & Birch, 2003), such as the shared eating and activity environment, modeling, and support for healthy or unhealthy behaviors.
Another risk factor for weight gain is the motivation to eat, or food reinforcement. Food reinforcement is related to energy intake in the laboratory and natural environment assessed by dietary recalls and food frequency questionnaires (Epstein, Carr, Lin, Fletcher, & Roemmich, 2012) and cross sectional data show that obese children (Temple, Legierski, Giacomelli, Salvy, & Epstein, 2008) and adults (Epstein, et al., 2012; Epstein, et al., 2007) find highly palatable food more reinforcing and are more motivated to eat than leaner peers. In addition, prospective data shows the reinforcing value of food predicts future body fat gain in children (Hill, Saxton, Webber, Blundell, & Wardle, 2009) and weight gain in adults (Carr, Lin, Fletcher, & Epstein, 2014).
The primary aim of this study is to assess the independent effects of food reinforcement and parental obesity on weight gain for adolescents in the healthy weight range, and to quantify the degree of risk associated with food reinforcement in terms of the well- established risk of parental obesity. In addition, the relationship between parental obesity and child food reinforcement will be assessed. While there is no research on the relation between these risk factors, research suggests that food reinforcement is correlated in parents and their children (Epstein, Dearing, Temple, & Cavanaugh, 2008), which may be in part responsible for the relation between parental and adolescent offspring obesity status (Crossman, et al., 2006). If a parent became obese due to their excessive motivation to eat (Carr, et al., 2014), then parental obesity may be related to child food reinforcement. In addition, parental obesity may increase risk of weight gain for youth high in food reinforcement. Since obesity is related to greater consumption of high energy dense foods (Mendoza, Drewnowski, Cheadle, & Christakis, 2006; Mendoza, Drewnowski, & Christakis, 2007), healthy weight teens who are high in food reinforcement and who reside in homes with obese parents with access to high-fat/high-sugar foods may be a higher risk of weight gain.
Methods and Materials
Participants
Participants were 160 adolescent males and females (M age = 15.3 ± 1.07, zBMI = 0.2 ± 0.6, BMI = 20.8 ± 1.90); 4.1% Hispanic, 0.6% Native American, 0.6% Asian/Pacific Islanders, 76.5% European Americans, and 17.9% mixed racial heritage. Adolescents had to have an age-adjusted BMI >18 and < 25. Those who reported binge eating or compensatory behaviors in the past 3 months, any use of psychotropic medications or illicit drugs, head injury with a loss of consciousness, or current Axis I psychiatric disorder per Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria (American Psychiatric Association, 1994) were excluded. Informed consent was obtained from parents and assent from adolescents. For the present investigation, analyses were performed on the 130 participants (M age = 15.2 years ± 1.0; 70 females) who met the inclusion criteria, with complete food and monetary reinforcement, who were not obese (<95th BMI percentile) at baseline, and for whom we collected objective height and weight data at 2-year follow-up. Participants were asked to refrain from eating or drinking caffeinated beverages 2 hours prior to their assessment.
Recruitment occurred between June 2009 and June 2011. The study was approved by the Oregon Research Institute Institutional Review Board. Participants were paid $75 for completing the 2-hour baseline assessment/behavioral test, and $30 for completing each annual follow-up assessment. Characteristics of adolescents and parents as a function of the number of obese parents are shown in Table 1.
Table 1.
Characteristics of adolescents based on number of obese parents.
| Number of obese parents | ||||
|---|---|---|---|---|
|
| ||||
| Variable | 0 (N = 58) | 1 (N = 54) | 2 (N = 18) | p |
| Age (years) | 15.4 ± 1.1 | 15.0 ± 0.9 | 15.0 ± 1.1 | 0.043 |
| Tanner stage | 4.3 ± 0.8 | 4.3 ± 0.6 | 4.4 ± 0.7 | 0.643 |
| Baseline BMI | 20.7 ± 2.1 | 20.6 ± 1.8 | 21.1 ± 2.0 | 0.562 |
| Baseline zBMI | 0.09 ± 0.7 | 0.17 ± 0.6 | 0.36 ± 0.6 | 0.289 |
| Mother BMI | 24.2 ± 3.2 | 29.5 ± 6.5 | 39.4 ± 6.4 | < 0.0001 |
| Father BMI | 25.3 ± 2.3 | 31.6 ± 5.0 | 37.9 ± 5.8 | < 0.0001 |
| Sex (M/F) | 23/35 | 28/26 | 9/9 | 0.413 |
| Minority (Minority/Non-minority) | 11/47 | 11/43 | 5/13 | 0.725 |
| Highest parent education (years) | 15.8 ± 2.7 | 16.2 ± 2.7 | 14.7 ± 2.8 | 0.107 |
| Reinforcing value tasks | ||||
| Food reinforcement (log Pmax) | 3.01 ± 1.0 | 3.08 ± 1.1 | 3.04 ± 1.1 | 0.945 |
| Monetary reinforcement (log Pmax) | 4.04 ± 1.3 | 3.94 ± 1.6 | 3.97 ± 1.2 | 0.935 |
Note - Data are mean ± SD. P-values are based on ANOVA or Chi-Square tests comparing between groups based on the number of obese (BMI > 30) parents. N=53 for tanner staging for adolescents with one obese parent; N=57 for father BMI and money p max for adolescents with no obese parents.
Measures
Height and weight
Height was measured to the nearest millimeter using a portable direct reading stadiometer. Weight was assessed to the nearest 0.1 kg using digital scales with participants wearing light clothing without shoes or coats. Two measures of height and weight were obtained and averaged. Parental BMI values are based on parental self-report. Although self-report may underestimate actual BMI, this is a conservative method for identifying obese parents. On the basis of height and weight data, BMI was calculated according to the following formula: BMI=kg/m2. Parental obesity was defined as BMI greater than 30 (NHLBI Obesity Education Initiative Expert Panel, 1998), and risk was assessed in terms of the number of obese parents. Adolescent BMI values were standardized for age and sex, and converted to BMI percentiles and zBMI values for analyses (Kuczmarski, et al., 2002).
Pubertal development
Youth reported their current pubertal development using a series of sex-specific line drawings of youth at various stages of pubertal development (Bonat, Pathomvanich, Keil, Field, & Yanovski, 2002). Girls were given line drawings of the 5 stages of breast and female pubic hair development with appropriate written descriptions of each stage. Boys are given line drawings of boys showing the 5 stages of pubic hair development and appropriate written descriptions of each stage. The description of each stage were read to the participant and they indicate which best captures their present stage of pubertal development. Self-rated pubertal development correlates with Tanner ratings made by physicians (r = 0.86) (Bonat, et al., 2002).
Food reinforcement task
Behavioral choice methods, adapted from Epstein and colleagues (Epstein, et al., 2007), were used to assess the reinforcing value of favorite snack foods or money. Money was chosen as the alternative reinforcer since it is a generalized conditioned reinforcer, is a strong reinforcer for most people, and can be used to purchase a wide variety of alternatives to food. In the reinforcement task, participants worked toward a snack food reward of their choice (e.g., small standard snack size bags/servings of salted peanuts, potato chips, Reeses’ peanut butter cups, M&M’s, chocolate cookies, and gummy bears) and for money. Participants were first asked to perform a taste test of 1g of each food and rate the pleasantness and intensity of each taste and how much they crave each food on cross-modal visual analogue scales. Participants earned the snack food that they rated the most pleasant. The reinforcement task is similar to a slot machine with shapes that rotate on the screen and a point is earned each time the three shapes match in shape and color. Reinforcers are first earned on a variable ratio 4 (VR4) schedule, which means that on the average of every four responses, a point is earned. The progressive ratios double each time the participant earned five points. Participants were told that it would get progressively harder to earn points. A total of 10 points was worth 1 standard serving of the food. In the monetary reinforcement task, participants completed the same task to earn $1 each time schedule requirements were met. The monetary reinforcement task was implemented first for all participants. Participants were told to do the task as long as they liked. They received the food and monetary rewards as they earned them.
The dependent measure was the breakpoint, or Pmax (Bickel, Marsch, & Carroll, 2000), which is the maximum schedule (e.g., 4, 8, 16 32, etc.) at which subjects met response requirements for access to the food and non-food alternative. Since the distribution of food and money reinforcement values were skewed, the values were logged, and log Pmax values were used in all analyses. The food reinforcement paradigm has shown 2–7 day test-retest reliability (r = 0.80) (Epstein, et al., 2007). Individuals who work longer for snack foods consume more food ad libitum (Epstein, et al., 2007; Epstein, et al., 2004) and individuals who rate the snack foods as more hedonically pleasurable work longer for the snack foods (Goldfield & Legg, 2006). Obese participants also work longer to earn food relative to lean participants (Epstein, et al., 2007; Temple, et al., 2008). Additionally, the reinforcing value of food is related to energy intake in the laboratory (Epstein, et al., 2007; Temple, et al., 2008), and energy intake outside of the laboratory assessed using dietary records and food frequency questionnaires (Epstein, et al., 2012).
The reinforcement task has also been correlated with brain activation in fMRI paradigms (Stice & Yokum, In press). Reward-related activation (caudate; r = 0.30) in response to milkshake receipt correlated with how much participants worked for energy dense snacks. Activation in regions involved in attention (inferior parietal lobe; r = 0.42, anterior cingulate cortex; r = 0.32), visual processing (cuneus; r = 0.38), and reward (mid insula; r = 0.31) in response to monetary reward receipt or anticipated receipt correlated with how much participants worked for money (N = 162). These data suggest that both paradigms are related to individual differences in food and monetary reward sensitivity.
Analytic Plan
Differences in participant characteristics as a function of the number of obese parents were established using ANOVA for continuous variables and Chi-Square for categorical variables (Table 1). Zero-order correlations were run to assess parental obesity, and food and monetary reinforcement as predictors of zBMI change over two year follow-up. Multiple regression models assessed whether parental obesity, and food and monetary reinforcement were independent predictors of zBMI change, controlling for adolescent age, sex, parental education and minority status. The interaction of parental obesity x food and monetary reinforcement was tested. In addition, since girls start puberty earlier, and may be at greater risk for obesity during adolescence (Dietz, 1994), self-reported Tanner staging was included as a covariate in a preliminary multiple regression model, and the interaction of sex x parental obesity and sex x food reinforcement was tested.
Results
Subjects with complete reinforcement data (N = 157) who did not provide objective weight data at 2-year follow-up (n = 27) did not differ with respect to baseline zBMI (F1,155 = 0.00, p = 0.98), BMI (F1,155 = 0.97, p = 0.32) parental obesity (F1,155 = 0.03, p = 0.86), food reinforcement (F1,155 = 0.25, p = 0.62) and monetary reinforcement (F1,155 = 0.03, p = 0.86) compared to those with complete measured data.
Zero-order relationships among the predictors, covariates and outcome are shown in Table 2. Zero-order correlations showed the number of obese parents (r = 0.26, p = 0.003) and food reinforcement (r = 0.18 p = 0.035) significantly correlated with zBMI change (M = −0.02 ± 0.45), but monetary reinforcement was not related to zBMI change (r = 0.06, p = 0.53). Food reinforcement was significantly correlated with monetary reinforcement, and non-minority status was significantly correlated with greater years of parental education. In addition to the number of obese parents, paternal BMI also significantly correlated with zBMI change (r = 0.297, p = 0.001), as did average parental BMI (r = 0.196, p = 0.025), but maternal BMI was not significantly correlated with this outcome (r = 0.056, p = 0.529, data not shown in Table 2). We chose to use the number of obese parents as the predictor of zBMI change as it provides a summary of both parents’ obesity, and is a stronger predictor than average parental BMI.
Table 2.
Correlations among predictors, covariates and 2-year zBMI change
| zBMI | Age | Sex | zBMI | RRVfood | RRVmoney | # obese Parents | Minority | |
|---|---|---|---|---|---|---|---|---|
| Age | −0.099 | |||||||
| Sex | −0.122 | −0.110 | ||||||
| zBMI | −0.028 | −0.160 | 0.110 | |||||
| RRVfood | 0.182* | −0.028 | −0.044 | −0.065 | ||||
| RRVmoney | 0.055 | 0.081 | −0.038 | −0.137 | 0.515*** | |||
| # obese parents | 0.263** | −0.192* | −0.098 | 0.134 | 0.019 | −0.026 | ||
| Minority | −0.018 | −0.040 | 0.018 | 0.017 | 0.005 | −0.060 | 0.063 | |
| Highest parent ed | −0.010 | −0.026 | −0.058 | −0.061 | 0.012 | 0.025 | −0.085 | −0.179* |
Note.
p < 0.05,
p < 0.01,
p < 0.001, zBMI refers to baseline zBMI, RRVfood and RRVmoney refer to relative reinforcing value of food and money, respectively, # obese parents refers to number of parents with BMI ≥ 30, minority refers to non-Caucasian, and highest parent ed refers to the years of education for the most educated parent.
Multiple regression (Table 3) showed that parental obesity (β = 0.152, p = 0.007) and food reinforcement (β = 0.071, p = 0.046) were significant independent predictors of zBMI change over 2 year follow-up, controlling for age, sex, parental education and minority status, accounting for 11.3% of the variance. No interaction between food reinforcement and parental obesity was observed to predict zBMI gain (p = 0.50). Similarly, no interactions between sex and parental obesity (p = 0.65) or sex and food reinforcement (p = 0.51) were observed. Inclusion of self-reported Tanner stage slightly improved the effect of food reinforcement (0.046 to 0.037) but did not change the significance of parental obesity (0.007).
Table 3.
Multiple regression to predict 0–2 year zBMI change
| Effect | B coeff. | SE | t | p |
|---|---|---|---|---|
| Constant | 0.111 | 0.652 | 0.170 | 0.866 |
| RRVfood | 0.071 | 0.035 | 2.02 | 0.046 |
| # Obese parents | 0.152 | 0.056 | 2.73 | 0.007 |
| Age | −0.026 | 0.037 | 0.694 | 0.489 |
| Sex | −0.087 | 0.077 | 1.127 | 0.262 |
| Parental education | −0.001 | 0.014 | 0.064 | 0.949 |
| Minority status | −0.039 | 0.095 | 0.409 | 0.683 |
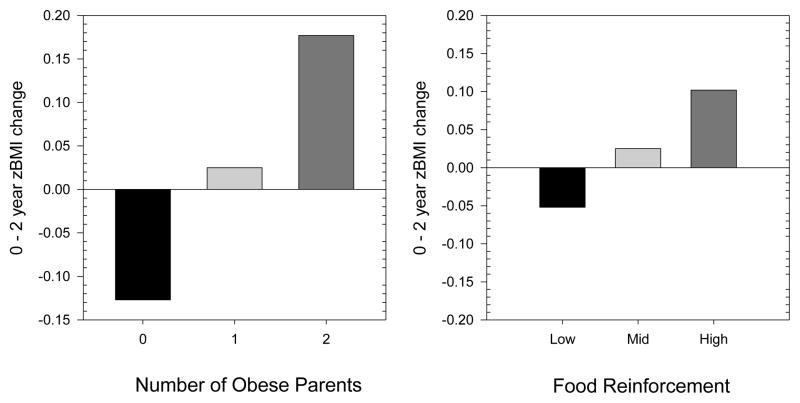
Based on the multiple regression model, Figure 1 shows the relation between the number of obese parents and those low, average and high in food reinforcement, and zBMI change.
Figure 1.
Two-year zBMI changes predicted by the number of obese parents (left graph), and levels of food reinforcement based on ± 1 SD from the mean (right graph), controlling for age, sex, parental education and minority status.
Food reinforcement values were based on ± 1 SD from the mean of food reinforcement scores. For those with no obese parents, or low or moderate in food reinforcement, the average non-obese adolescent showed a reduction in zBMI, with a reduction in −0.127 zBMI units if there were no obese parents or −0.052 zBMI units for being low in food reinforcement. Being high in food reinforcement was associated with greater zBMI increases than having one obese parent (0.102 vs 0.025 zBMI units), but less than having two obese parents (0.177).
Discussion
Both the number of obese parents and food reinforcement predicted relative weight gain over a 2-year follow-up among adolescents with an initially healthy weight. Being high in food reinforcement was associated with greater zBMI increases than having one obese parent, but less than having two obese parents. There were no interactions between the two predictors, and sex did not moderate the predictive relationships between the number of obese parents or food reinforcement and zBMI change. Parental weight is a known risk factor for child (Agras, Hammer, McNicholas, & Kraemer, 2004; Reilly, et al., 2005) and adolescent weight gain (Stice, et al., 2005). Food reinforcement has been shown to predict child body fat gain (Hill, et al., 2009) and adult weight gain (Carr, et al., 2014), but to our knowledge has not been studied in adolescents. Monetary reinforcement did not predict adolescent weight gain, showing that the risk is specifically due to the motivation to eat, and not a general sensitivity to different types of reinforcers. Parental weight and child food reinforcement are independent risk factors, and account for over 11% of the variance in predicting relative weight increase.
Parental obesity can influence increases in their children’s weight in many ways, including modeling unhealthy behaviors, providing a shared family environment that stimulates and supports unhealthy eating and a sedentary lifestyle (Birch & Fisher, 1998; Crossman, et al., 2006; Francis, et al., 2003), as well as similarities in behavioral phenotypes that are related to weight gain, such as elevated motivation to eat. While previous data suggest that parent and child food reinforcement are related (Epstein, et al., 2008), no measures of parental food reinforcement were collected in this study to test this hypothesis. Genetic data show that concordance of the number of Taq1A1 alleles, which has been related to food reinforcement (Epstein, et al., 2007; Epstein, et al., 2004), is a strong predictor of the concordance between parent and child weight loss in family-based behavioral programs (Epstein, Dearing, & Erbe, 2010). Research is needed on how concordance of behavioral phenotypes such as the motivation to eat may predict similarities in weight gain or weight loss across multiple generations.
The reinforcing value of food is an individual difference variable that has been shown to predict weight gain in multiple age groups (Carr, et al., 2014; Hill, et al., 2009), but perhaps one of the most interesting implications is that some people who find food to be very reinforcing do not show excess weight gain. Examination of the individual patterns of weight change shows that 42.4% of adolescents who had food reinforcement scores greater than 1 SD from the mean (14/33) did not show an increase in zBMI. Understanding what protects these children from weight gain may be of great interest in developing efficacious obesity prevention programs. While focusing prevention efforts on children and adolescents with obese parents and who are high in food reinforcement makes sense, understanding what protects children and adolescents from weight gain when they have risk factors may be a unique first step in developing effective prevention programs that go beyond the mantra of eat healthier and get more physical activity.
Identification of variables associated with relative weight gain may suggest that by manipulating the risk factor you can prevent relative weight gain in a randomized trial, as this is arguably the best way to differentiate a risk factor from a causal etiologic factor. The consistency between the relation between parental weight and child weight gain suggests modification of parental weight may be important to prevent child weight gain (Boutelle, Cafri, & Crow, 2012; Temple, Wrotniak, Paluch, Roemmich, & Epstein, 2006; Wrotniak, Epstein, Paluch, & Roemmich, 2004). Research suggests positive health behavior changes for non-obese children of parents who participate in family-based behavioral weight loss programs (Epstein, et al., 2001), as treating parental obesity may have spillover effects on other family members due to a shared environment, modeling by child of parental behaviors or parental reinforcement or support of child healthy behaviors.
Elevated food reinforcement is a consistent predictor of concurrent obesity (Epstein, et al., 2012; Epstein, et al., 2007; Temple, et al., 2008), prospective weight or body fat gain (Carr, et al., 2014; Hill, et al., 2009), but there is very little research on methods to change food reinforcement. It would be interesting to test incentive based ways to increase the reinforcing value of healthy foods (Cooke, et al., 2011) and ways to promote the substitution of healthy for less healthy foods (Goldfield & Epstein, 2002). In addition, since the reinforcing value of food depends on the alternatives available (Temple, et al., 2008), attempts to increase the reinforcing value of alternatives to food, or place constraints on access to very reinforcing foods that drive the motivation to eat may reduce consumption of less healthy foods and/or increase consumption of healthier foods (Goldfield & Epstein, 2002). An alternative to directly modifying food reinforcement is to improve self-regulation skills that compete with food reinforcement. Research suggests that delay discounting, a form of delay of gratification, interacts with food reinforcement to influence eating and weight change. Those individuals high in food reinforcement and unable to delay gratification consume the most food (Rollins, Dearing, & Epstein, 2010) were the least successful in a pediatric weight control program (Best, et al., 2012). The drive to eat and the inability to delay gratification is conceptualized as reinforcement pathology (Carr, Daniel, Lin, & Epstein, 2011; Epstein, Salvy, Carr, Dearing, & Bickel, 2010), and these studies suggest that enhancing self-control may reduce energy intake. One approach to reducing delay discounting (Daniel & Epstein, 2013; Daniel, Stanton, & Epstein, 2013; Lin & Epstein, 2014) and reducing energy intake (Daniel, et al., 2013) is episodic future thinking, which shifts focus from small immediate rewards to larger, but delayed and future rewards. Research is needed to assess whether episodic future thinking would work with individuals who are high in food reinforcement and who cannot delay immediate gratification.
It is important to consider the study limitations. First, while we supported theoretically relevant predictions of zBMI increase, these increases were relatively small, and may not lead to obesity over time. Second, the population of older adolescents who are not obese may be at less risk for becoming obese than someone who gained excess weight at an earlier age. Finally, the number of adolescents with two obese parents was quite small (N = 15), and as such may not provide an unbiased estimate of the influence of two obese parents on adolescent weight gain.
Collectively, these data support the study of food reinforcement as a risk factor for weight gain, and suggest that food reinforcement predicts body weight independent of parental obesity. In terms of relative risk, food reinforcement is associated with greater zBMI increases than having one obese parent, but those with two obese parents showed greater zBMI increases. The challenge is to move beyond demonstrating these risk factors to interventions that target those with obese parents and/or high food reinforcement to prevent weight gain.
Highlights.
We examined weight gain in adolescents by food reinforcement and parental obesity
High food reinforcement and parental obesity predicted weight gain
High food reinforcement associated with greater weight gain than one obese parent
Food reinforcement and parental obesity are risk factors for adolescent weight gain
Acknowledgments
This research was funded in part by grant R01 DK-080760 from the National Institute of Diabetes and Digestive Diseases and grant HD-044725 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. Study sponsors had no role in study design, collection, analysis and interpretation of data, writing of the report, or decision to submit the manuscript for publication.
Footnotes
Authors responsibilities were as follows: LHE developed the study concept in collaboration with ES and SY; ES and SY contributed to the study design; protocol implementation and data collection were performed by SY; LHE and DMF analyzed and interpreted the data; LHE drafted the manuscript, prepared the figure and tables; and all authors provided critical revisions and approved the final version of the manuscript for submission.
None of the authors declared had conflicts of interest with respect to their authorship or the publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. Journal of Pediatrics. 2004;145:20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, Perri MG, Schechtman KB, Epstein LH, Wilfley DE. Behavioral economic predictors of overweight children’s weight loss. Journal of Consulting and Clinical Psychology. 2012;80:1086–1096. doi: 10.1037/a0029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology (Berl) 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–549. [PubMed] [Google Scholar]
- Biro F. Normal growth and development. In: Slap G, editor. Adolescent Medicine: the requisites in pediatrics. Philadelphia, PA: Mosby, Inc; 2008. [Google Scholar]
- Bonat S, Pathomvanich A, Keil MF, Field AE, Yanovski JA. Self-assessment of pubertal stage in overweight children. Pediatrics. 2002;110:743–747. doi: 10.1542/peds.110.4.743. [DOI] [PubMed] [Google Scholar]
- Boutelle KN, Cafri G, Crow SJ. Parent predictors of child weight change in family based behavioral obesity treatment. Obesity. 2012;20:1539–1543. doi: 10.1038/oby.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KA, Daniel TO, Lin H, Epstein LH. Reinforcement pathology and obesity. Current Drug Abuse Reviews. 2011;4:190–196. doi: 10.2174/1874473711104030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KA, Lin H, Fletcher KD, Epstein LH. Food reinforcement, dietary disinhibition and weight gain in nonobese adults. Obesity (Silver Spring) 2014;22:254–259. doi: 10.1002/oby.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke LJ, Chambers LC, Anez EV, Croker HA, Boniface D, Yeomans MR, Wardle J. Eating for pleasure or profit: the effect of incentives on children’s enjoyment of vegetables. Psychological Science. 2011;22:190–196. doi: 10.1177/0956797610394662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman A, Sullivan DA, Benin M. The family environment and American adolescents’ risk of obesity as young adults. Social Science & Medicine. 2006;63:2255–2267. doi: 10.1016/j.socscimed.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Epstein LH. The future is now: Comparing the effect of episodic future thinking on impulsivity in lean and obese individuals. Appetite. 2013;71:120–125. doi: 10.1016/j.appet.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, Epstein LH. The future is now: Reducing impulsivity and energy intake using episodic future thinking. Psychological Science. 2013;24:2339–2342. doi: 10.1177/0956797613488780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. Critical periods in childhood for the development of obesity. American Journal of Clinical Nutrition. 1994;59:955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Carr KA, Lin H, Fletcher KD, Roemmich JN. Usual energy intake mediates the relationship between food reinforcement and BMI. Obesity (Silver Spring) 2012;20:1815–1819. doi: 10.1038/oby.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Erbe RW. Parent-child concordance of Taq1 A1 allele predicts similarity of parent-child weight loss in behavioral family-based treatment programs. Appetite. 2010;55:363–366. doi: 10.1016/j.appet.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Temple JL, Cavanaugh MD. Food reinforcement and impulsivity in overweight children and their parents. Eating Behaviors. 2008;9:319–327. doi: 10.1016/j.eatbeh.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Gordy CC, Raynor HA, Beddome M, Kilanowski CK, Paluch R. Increasing fruit and vegetable intake and decreasing fat and sugar intake in families at risk for childhood obesity. Obesity Research. 2001;9:171–178. doi: 10.1038/oby.2001.18. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiology and Behavior. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype and energy intake in obese and non-obese humans. Behavioral Neuroscience. 2007;121:877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LW, Jr, Jaroni JL, Saad FG, Crystal-Mansour S, Shields PG, Lerman C. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. American Journal of Clinical Nutrition. 2004;80:82–88. doi: 10.1093/ajcn/80.1.82. [DOI] [PubMed] [Google Scholar]
- Francis LA, Lee Y, Birch LL. Parental weight status and girls’ television viewing, snacking, and body mass indexes. Obesity Research. 2003;11:143–151. doi: 10.1038/oby.2003.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfield GS, Epstein LH. Can fruits and vegetables and activities substitute for snack foods? Health Psychology. 2002;21:299–303. [PubMed] [Google Scholar]
- Goldfield GS, Legg C. Dietary restraint, anxiety, and the relative reinforcing value of snack food in non-obese women. Eating Behaviors. 2006;7:323–332. doi: 10.1016/j.eatbeh.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7–10-y-old children. American Journal of Clinical Nutrition. 2009;90:276–281. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. Vital Health Statistics. Series 11. Vol. 246. Hyattsville, MD: National Center for Health Statistics; 2002. CDC growth charts for the United States: Methods and development; pp. 1–90. [PubMed] [Google Scholar]
- Lin H, Epstein LH. Living in the moment: effects of time perspective and emotional valence of episodic thinking on delay discounting. Behavioral Neuroscience. 2014;128:12–19. doi: 10.1037/a0035705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. International Journal of Obesity and Related Metabolic Disorders. 2003;27:505–513. doi: 10.1038/sj.ijo.0802251. [DOI] [PubMed] [Google Scholar]
- Mendoza JA, Drewnowski A, Cheadle A, Christakis DA. Dietary energy density is associated with selected predictors of obesity in US children. Journal of Nutrition. 2006;136:1318–1322. doi: 10.1093/jn/136.5.1318. [DOI] [PubMed] [Google Scholar]
- Mendoza JA, Drewnowski A, Christakis DA. Dietary energy density is associated with obesity and the metabolic syndrome in US adults. Diabetes Care. 2007;30:974–979. doi: 10.2337/dc06-2188. [DOI] [PubMed] [Google Scholar]
- NHLBI Obesity Education Initiative Expert Panel. Clinical Guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–The evidence report. Obesity Research. 1998;6(Supplement 2):51S–209S. [PubMed] [Google Scholar]
- Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, Steer C, Sherriff A. Early life risk factors for obesity in childhood: cohort study. British Medical Journal. 2005;330:1357–1359. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55:420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Presnell K, Shaw H, Rohde P. Psychological and behavioral risk factors for obesity onset in adolescent girls: a prospective study. Journal of Consulting and Clinical Psychology. 2005;73:195–202. doi: 10.1037/0022-006X.73.2.195. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S. Brain reward region responsivity of adolescents with and without parental substance use disorders. Psychology of Addictive Behaviors. doi: 10.1037/a0034460. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. American Journal of Clinical Nutrition. 2008;87:1121–1127. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Wrotniak BH, Paluch RA, Roemmich JN, Epstein LH. Relationship between sex of parent and child on weight loss and maintenance in a family-based obesity treatment program. International Journal of Obesity (Lond) 2006;30:1260–1264. doi: 10.1038/sj.ijo.0803256. [DOI] [PubMed] [Google Scholar]
- Wardle J, Carnell S, Haworth CMA, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. American Journal of Clinical Nutrition. 2008;87:398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- Warrington NM, Howe LD, Wu YY, Timpson NJ, Tilling K, Pennell CE, Newnham J, Davey-Smith G, Palmer LJ, Beilin LJ, Lye SJ, Lawlor DA, Briollais L. Association of a body mass index genetic risk score with growth throughout childhood and adolescence. PLoS One. 8:e79547. doi: 10.1371/journal.pone.0079547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. Parent weight change as a predictor of child weight change in family-based behavioral obesity treatment. Archives of Pediatric and Adolescent Medicine. 2004;158:342–347. doi: 10.1001/archpedi.158.4.342. [DOI] [PubMed] [Google Scholar]