Abstract
Alzheimer’s is a neurodegenerative disease with a complex and progressive pathological phenotype characterized first by hypometabolism and impaired mitochondrial bioenergetics followed by pathological burden. The progressive and multifaceted degenerative phenotype of Alzheimer’s suggests that successful treatment strategies necessarily will be equally multi-faceted and disease stage specific. Traditional therapeutic strategies based on the pathological aspect of the disease have achieved success in preclinical models which has not translated into clinical therapeutic efficacy. Meanwhile, increasing evidence indicates an antecedent and potentially causal role of mitochondrial bioenergetic deficits and brain hypometabolism coupled with increased mitochondrial oxidative stress in AD pathogenesis. The essential role of mitochondrial bioenergetics and the unique trajectory of alterations in brain metabolic capacity enable a bioenergetic-centric strategy that targets disease-stage specific pattern of brain metabolism for disease prevention and treatment. A combination of nutraceutical and pharmaceutical intervention that enhances glucose-driven metabolic activity and potentiates mitochondrial bioenergetic function could prevent the antecedent decline in brain glucose metabolism, promote healthy aging and prevent AD. Alternatively, during the prodromal incipient phase of AD, sustained activation of ketogenic metabolic pathways coupled with supplement of the alternative fuel source, ketone bodies, could sustain mitochondrial bioenergetic function to prevent or delay further progression of the disease.
Keywords: Mitochondria, Bioenergetics, Alzheimer’s disease, Ketogenesis, Hypometabolism, Oxidative Stress
ALZHEIMER’S DISEASE – UNLIMITED COST BUT LIMITED REMEDY
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease and the leading cause of dementia among the aged population. Currently, the estimated AD patient population in the US reaches 5.4 million and is projected to at least double by the year 2050 [1]. The disease is symptomatically characterized by progressive memory deficits, cognitive impairments and personality change, which can be attributed to progressive synaptic dysfunction and the subsequent loss of neurons in vulnerable regions of the brain, including the neocortex, the limbic system and the subcortical regions [2]. From a histo-pathological view, AD is characterized by senile plaques and neurofibrillary tangles in the medial temporal lobe and cortical areas of the brain together with neurodegeneration and loss of synaptic functions [3]. According to the genetics of the disease, AD has been categorized into two major forms: familial AD (FAD) and sporadic AD (SAD) or late onset age-related AD (LOAD), with the latter being the leading cause of dementia, accounting for about 50~60% of all cases. FAD is an autosomal dominant disorder with the onset age before 65. The majority of FAD cases have been attributed to mutations in three genes, the amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2)[4]. In contrast, the complete etiology of SAD has yet to be fully elucidated, with age being identified as the greatest risk factor. The prevalence of AD increases exponentially with age in people aged 65 or older [3].
Financially, the cost of Medicare for AD and other types of dementia dwarf all other Medicare beneficiaries in the same age group by 9 fold. As of 2010, the estimated cost of AD related payments is $172 billion and the cost is projected to increase exponentially in the next 20 years with more baby boomers into senile age [1].
Despite unprecedented demand for AD medications, currently no treatment exists to prevent, modify, or halt disease progression. Available drugs approved by FDA only offer moderate and temporary symptom relief [5, 6]. Therapeutic development for AD, particularly SAD, has been largely impeded due to limited understanding of the disease etiology. The prevailing “amyloid cascade” hypothesis, which was first introduced almost 20 years ago and has been enriched over the past decade, emphasizes the neurotoxic characteristics of β-amyloid (Aβ) and proposes that the deposition of Aβ initiates a cascade of neurotoxic events, including the formation of neurofibrillary tangles (NFT), prolonged inflammatory responses, increased oxidative stress and mitochondrial dysfunction, which eventually lead to cell death and dementia [7–10]. While this “amyloid cascade” hypothesis proposes a unified etiopathogenic mechanism for both FAD and SAD, findings from both basic research and clinical observations indicate a far more complex mechanism for the etiopathogenesis of SAD. Further, rather than being the cause of the disease, recent studies indicate that in SAD both Aβ deposition and NFTs may serve as reactive products that arise from increased vulnerability to genetic and environmental risk factors as a function of aging [7, 11–13]. Moreover, candidates that directly target amyloid pathways, either through passive immunotherapy against Aβ bapineuzumab) [14] or via inhibition of pathways involved in Aβ generation (Tarenflurbil, Semagacestat, or Flurizan)[15], failed to achieve efficacy in recent clinical trials, indicating the therapeutic limitation of amyloid-specific strategies. On the other hand, increasing evidence suggest that Alzheimer’s disease, particularly SAD, is a multifaceted disease that could at least be partially attributed to decline in mitochondrial function and altered brain metabolic activity.
MITOCHONDRIAL DYSFUNCTION AND β-AMYLOID – A VICIOUS CYCLE
The fundamental role of mitochondria in cellular bioenergetics and survival has been well established [16–18]. The evidence for mitochondrial dysfunction as a pivotal factor in age-associated neurodegenerative diseases such as Alzheimer's and Parkinson's continues to mount [16, 19–22]. Perturbations in mitochondrial function have long been observed in samples derived from clinically confirmed AD patients, including altered mitochondrial morphology, compromised enzyme complexes in the Krebs cycle, and reduced cytochrome c oxidase (COX) activity [23–28]. Later, the “cybrid model” of AD, generated by transferring mtDNA from human AD patients into cell cultures that are devoid of endogenous mtDNA (ρ0 cells), exhibited symptoms that recapitulated previous findings in clinical AD specimens, including decreased mitochondrial mobility, increased oxidative stress, decreased COX activity, decreased mitochondrial membrane potential, and increased Aβ production, therefore provided further evidence for involvement of mitochondria and mtDNA in AD etiopathogenesis [29, 30]. Moreover, increasing evidence indicates that mitochondria are direct targets of Aβ. Aβ has been demonstrated to accumulate within mitochondria and interact with a mitochondrial protein, Aβ̃ binding-alcohol-dehydrogenase (ABAD), resulting in decreased COX activity and increased oxidative stress [31–33]. Further, the Aβ induced neurotoxicity requires functional mitochondrial respiratory chain [34] and is further exacerbated in synergy with mitochondrial dysfunction in similar AD cybrid models [35].
While the neurotoxic mechanisms of Aβ converge upon mitochondria, compromised mitochondrial function, particularly inhibition in mitochondrial bioenergetics and increase in oxidative stress, exacerbates the degenerative process by increasing Aβ generation thereby generating a vicious cycle in which excessive Aβ accumulation and -prolonged mitochondrial dysfunction synergize to activate a cascade of neurodegenerative pathways, including disturbed cytoskeleton network and lysosomal pathways [36–39].
MITOCHONDRIAL BIOENERGETIC DEFICITS IN AD – AN EARLY AND POTENTIALLY CAUSAL EVENT
Multiple levels of analysis and multiple experimental paradigms that range from genomic analyses in animal models and postmortem autopsy of human brains to in vitro cell model systems to brain imaging in humans, demonstrate that dysfunction in glucose metabolism, bioenergetics and mitochondrial function are consistent antecedents to development of Alzheimer pathology [13, 40–42]. The decline in brain glucose metabolism and mitochondrial function can appear decades prior to the onset of histopathologcial and/or clinical features and thus may serve as a biomarker of AD risk as well as therapeutic target. Studies using multiple preclinical in vitro and in vivo AD models demonstrated antecedent decline in mitochondrial function prior to the development of Alzheimer’s pathology, including decreased mitochondrial respiration, decreased metabolic enzyme expression and activity, decreased cerebral glucose metabolism, increased oxidative stress, and increased mitochondrial Aβ load and ABAD expression [40–45]. Further, the antecedent decline in mitochondrial function deteriorates with AD progression [31, 32]. Consistent with basic science findings, many clinical observations also report antecedent abnormality in cerebral glucose utilization decades prior to the onset of AD [46–52]. In addition to the distinct pattern of brain hypometabolism observed in clinical confirmed AD patients, multiple positron emission tomography (PET) studies demonstrated that the antecedent decline in glucose utilization, particularly in the hippocampal and entorhinal cortical regions, preceded the clinical diagnosis of AD by years and predicted the cognitive decline in normal aging [50] or the progression of patients from mild cognitive impairment (MCI) to AD [53] with high accuracy. Recent clinical studies revealed a significant overlap between brain regions that exhibited abnormal glucose metabolism and regions that are most vulnerable to development of AD pathology [54–56], providing further evidence of the association between disturbed glucose metabolism and AD pathogenesis.
ACTIVATION OF ALTERNATIVE FUEL SOURCE – COMPENSATION WITH DISEASE PROGRESSION
In parallel with the decline in glucose metabolism in AD, there is a generalized shift away from glucose-derived energy production, which is associated with a decrease in the expression of glycolytic enzymes coupled to a decrease in the activity of the pyruvate dehydrogenase (PDH) complex [24]. Alteration in brain metabolic profile in AD is further evidenced by concomitant activation of compensatory pathways that promote the usage of alternative substrates, such as ketone bodies, to compensate for the decline in glucose-driven ATP generation. We have previously reported that in the female 3xTgAD mouse model, pre-pathological decrease in PDH expression and mitochondrial bioenergetics were paralleled by increased expression of succinyl-CoA:3-ketoacid coenzyme A transferase (SCOT) at a young age (3 months), indicating early activation of ketogenic pathways to compensate for compromised PDH capacity, provide alternative sources of acetyl-CoA, and consequently maintain energy-conservation mechanisms required for ATP generation [57]. Consistently, previous clinical observations have also demonstrated the projectile of substrate switch along with AD progression. While there is a 100:0 ratio of glucose to other substrates utilization in young controls, there is a 2:1 ratio in incipient AD patients compared to a ratio of 29:1 in healthy elderly controls [58]. Cerebral utilization of ketone bodies requires supply from the peripheral ketogenic organ, the liver, or local synthesis of ketone bodies, potentially by astroglia via fatty acid oxidation [59]. Concomitant with the increase in ketone utilization in aging and AD brains, AD patients demonstrated various degrees of compromised brain white matter integrity [60–62]. In a preclinical AD rodent model, white matter degeneration was observed in the corpus callosum, fornix and hippocampus [63]. Lesions in white matter integrity suggests either hyper-activation of the fatty acid oxidation pathway for local astroglial ketone production or incompetency for lipid synthesis due to competition between consumption of ketones/ acetyle-CoA for bioenergetics and lipid synthesis [59].
BIOENERGETIC DEFICITS AND OXIDATIVE STRESS – WHEN IT RAINS IT POURS
Impairment of mitochondrial bioenergetics and oxidative phosphorylation (OXPHOS) is often closely associated with increased free radical production and the consequent oxidative damage. As the major source for cellular reactive oxygen species (ROS), mitochondria generate free radicals as the by-product during OXPHOS [64, 65]. On the other hand, oxidative damage to mitochondrial membranes and proteins is well documented to impair mitochondrial OXPHOS efficiency and result in increased electron leak as observed by increased hydrogen peroxide levels and higher oxidative stress [33, 66]. Key enzymes involved in mitochondrial bioenergetics, such as α-ketoglutarate dehydrogenase complex (αKGDHC) and the pyruvate dehydrogenase complex (PDHC), often are the targets of oxidative modifications, which lead to deceased enzyme activity and increased production of free radicals [67, 68].
Overproduction of reactive oxygen species and higher oxidative stress is characteristic of AD brains [13, 69]. In AD patients, significant increase in oxidatively modified molecules, such as lipid peroxide, 8-oxoguanine, and oxidized amino acid, have been identified in vulnerable brain regions [70, 71]. In preclinical AD animal models, increased generation of ROS, such as hydrogen peroxide, and elevated oxidative damage on cellular components have also been demonstrated to precede the development of AD pathology [40, 72–76]. In fact, increase in oxidative stress has been demonstrated to increase β-amyloid production in vitro and in vivo [77– 79].
CURRENT ALZHEIMER’S THERAPEUTICS – A MILESTONE YET TO BE ACHIEVED
Alzheimer’s disease is a complex disease with a prolonged trajectory of etiopathogenic changes in brain bioenergetics decades prior to the clinical onset of the disease. Therefore, targeting only the pathological aspect, particularly the β-amyloid pathway, is insufficient to achieve full efficacy to prevent, delay, or even reverse the disease progression.
Recently, Eli Lilly announced cessation of its phase III clinical trial of semagacestat, a γ-secretase inhibitor, as the drug candidate failed to achieve efficacy in slowing disease progression and was associated with worsening of clinical measurement of cognitive function [80]. Similarly, other Aβ targeting candidates, tarenflurbil, tramiprosate and flurizan, also failed in Phase II or phase III clinical trials [80–82], although these candidates have been demonstrated to lower or reduce Aβ production in preclinical and early phase clinical studies [80–82]. Further, the clinical potential of the γ-secretase inhibitors are often complicated by its off-target interferences on the Notch signaling pathway.
Another category of anti-Aβ therapeutics includes Aβ42 vaccines, monoclonal Aβ antibodies and polyclonal antibodies. These candidates act via an immunotherapeutic mechanism to promote the clearance of amyloid. Yet, the active immunization approach, such as the amyloid vaccine AN-1792 (Elan/Wyeth), was discontinued due to increased risk of severe meningoencephalitis in the patients despite the trend towards positive efficacy it achieved [83]. On the other hand, bapineuzumab (Elan/Wyeth), a passive immunization monoclonal antibody of Aβ, failed to achieve expected efficacy in a Phase II clinical trial, although it did exhibit some benefits in AD patients who did not carry the APOE4 genetic risk factor [83]. Other Aβ antibodies are currently under various stages of clinical trials and the efficacy is yet to be determined. Similarly, candidates that target Tau pathology as disease modifying therapeutics for AD are still in the early stage of development despite the potential benefits exhibited in preclinical animal studies.
Aside from the anti-Aβ/tau strategy, antioxidants have been proposed as potential therapeutics for AD. Analyses on specimens obtained from AD patients and various preclinical animal models clearly documented elevated oxidative damage on cellular components [40, 70–76]. The therapeutic potential of antioxidants is further supported by vast epidemiological analyses that demonstrated a positive correlation between the usage of antioxidant, particularly vitamin E and C, and cognitive function. Further early administration of antioxidants such as curcumin or vitamin E, has been demonstrated to suppress amyloidogenesis in several preclinical AD rodent models [84, 85]. However, multiple randomized clinical trials of high dosage of vitamin E usage failed to achieve significant efficacy [86], indicating the therapeutic limit of using exogenous antioxidants to scavenge oxidative insults rather than suppress the generation of oxidative insults.
THERAPEUTICS TARGETING MITOCHONDRIA AND BIOENERGETICS
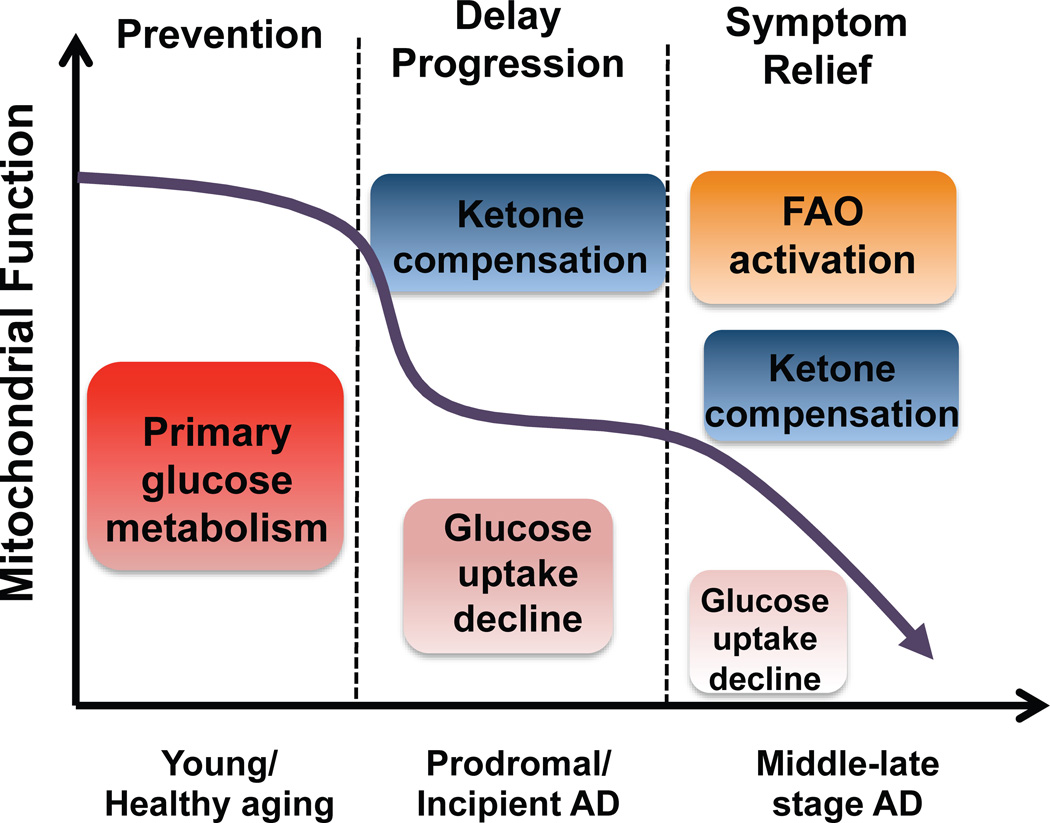
Alzheimer’s is a neurodegenerative disease with a complex and progressive pathological phenotype characterized first by hypometabolism and impaired mitochondrial bioenergetics followed by pathological burden. The progressive and multifaceted degenerative phenotype of Alzheimer’s suggests that successful treatment strategies need to be equally multi-faceted and stage specific. Increasing evidence indicates an antecedent and potentially causal role of mitochondrial bioenergetic deficits and brain hypometabolism coupled with increased mitochondrial oxidative stress in AD pathogenesis. Mitochondrial deficits have been demonstrated to activate a cassette of neurotoxic events that all contribute to synaptic dysfunction, pathology development and eventually neuronal loss and cognitive impairment [33, 66]. Further, deficits in mitochondrial bioenergetics and brain metabolism exhibit a stage-specific trajectory with disease progression, which was first evidenced by the decline in glucose uptake and utilization that takes place decades prior to AD onset, followed by parallel activation of pathways to use alternative fuel substrates, ketone bodies, to compensate for the decline in glucose metabolism [40, 57]. As disease progresses, exacerbated decline in glucose utilization and exhaustion of available ketone reservoir leads to further disturbance of mitochondrial function and activation of fatty acid oxidation (FAO) pathway that eventually results in white matter generation and neuronal death observed in AD [16, 60, 62, 87]. This unique trajectory of glucose-ketone-FAO progression of brain mitochondrial metabolic alteration provides an ideal therapeutic target that is both disease modifying and stage specific (Fig. 1).
Fig. (1). Trajectory of mitochondrial function, substrate utilization during AD progression and therapeutic strategy.
At young age or during healthy aging, brain metabolic activity is largely supported by glucose the primary fuel source whereas in prodromal and incipient AD the antecedent decline in glucose metabolism is paralleled by compensatory activation of ketogenic pathways, which later diminishes and progresses to local fatty acid oxidation leading to white matter degeneration observed with disease progression. The prevention strategy aims to enhance the glucose driven mitochondrial bioenergetics to promote healthy aging and prevent AD. Alternatively, in prodromal and incipient AD, sustained activation of ketogenesis provides prolonged supplement of the alternative fuel source, ketone bodies, and therefore sustains mitochondrial bioenergetic function and prevents/delays further progression of the disease. At the middle to late stage of AD, rather than modifying disease progression, treatments merely offer symptom relief.
Candidates that potentiate mitochondrial bioenergetics and enhance brain glucose metabolism are expected to prevent the antecedent decline in brain glucose metabolism, promote healthy aging and therefore prevent AD. Interestingly, many candidates within this category are naturally occurring herbals and small co-factors, which often are on the GRAS (generally considered as safe) list. R-α-lipoic acid, an important co-factor for key mitochondrial metabolic enzymes, including PDH, KGDH, and branched chain α-ketoacid dehydrogenase (BCKDH), has been demonstrated to upregulate mitochondrial bioenergetics, promote glucose metabolism, and suppress oxidative stress due to its potent antioxidant capacity [88]. Resveratrol, a redox active ingredient in grapes and wine, improves brain energy metabolism and reduces amyloid accumulation in preclinical animal models [89–92]. Both R-α-lipoic acid and resveratrol are currently under clinical trials for their efficacy in AD prevention and treatment [88, 93]. Other important regulators of mitochondrial metabolic activity include B-vitamins which are also co-factors of key metabolic/mitochondrial enzymes. These naturally occurring compounds often possess antioxidant properties. All together, these compounds exhibit potential to promote brain glucose utilization, potentiate brain metabolic activity, and simultaneously suppress oxidative damage with relatively low toxicity, which make them ideal candidates for development of nutraceutical cocktails to promote brain metabolism during healthy aging and therefore prevent AD.
While the preventive strategy focuses heavily on the enhancement of brain glucose metabolism, the shift towards an alternative fuel source, ketone bodies, observed in both preclinical AD models and in AD patients provides a second therapeutic window that targets the specific glucose – ketone transition stage to sustain brain metabolic activity and therefore prevent or delay further exacerbation in brain bioenergetic deficits. Ketone bodies are mainly synthesized in the liver and are well documented to serve as alternative energy substrates for the heart, muscle, and brain. Ketogenic pathways have been demonstrated to exist in astrocytes [94, 95]. Epidemiological analyses indicate a positive association between dietary intake of ketones/consumption of ketogenic diets and reduced risk for AD [59, 96]. The switch from glucose as the primary fuel to the alternative of ketone bodies in the AD brain was the basis for Accera to develop Ketasyn, which is converted to ketone bodies in the liver for subsequent use by the brain. This approach capitalizes on the brain’s relative inability to utilize glucose and its dependency on ketone bodies. Phase II clinical trial in Alzheimer's patients and in individuals suffering from age-associated memory impairment has been completed and both groups showed improvement in memory function using the ketone body alternative fuel source (http://www.accerapharma.com).
While increasing ketone body supply provides more substrate to the brain to utilize as an alternative fuel, the therapeutic efficacy could be limited due to a diminished brain capacity to utilize ketone bodies. To address the issue of deficits in the ketogenic metabolic pathway, our group investigated the efficacy of the ketogenic modulator, 2-deoxy-d-glucose (2-DG) to increase brain capacity to utilize ketone bodies as fuel. Results of these analyses demonstrated that dietary 2-DG intake induced ketogenesis, sustained mitochondrial bioenergetics, and reduced pathology in the triple transgenic Alzheimer’s (3xTgAD) mouse model [97]. Based on these clinical and preclinical findings, a combination of nutraceutical and pharmaceutical modulators that simultaneously enhance mitochondrial bioenergetics while sustaining availability and utilization of an alternative fuel substrate (ketone bodies), could prevent further decline in brain metabolism and to delay progression of AD.
SUMMARY
Alzheimer’s is a progressive and complex neurodegenerative disease that requires therapeutic development to target beyond histopathological aspects of the disease. Increasing evidence indicates an antecedent and causal role of mitochondrial bioenergetic deficits and brain hypometabolism coupled with oxidative stress that initiates multiple neurotoxic events in AD etiopathogenesis. The essential role of mitochondrial bioenergetics and the unique trajectory of alterations in brain metabolic profile provides the foundation upon which to construct a bioenergetic-centric strategy that targets disease-stage specific pattern of brain metabolism to prevent AD and/or delay the disease progression.
ACKNOWLEDGEMENTS
This study was supported by National Institute on Aging Grant 2R01AG032236 (to RDB) and Eileen L. Norris Foundation (to RDB).
REFERENCES
- 1.Association As. Alzheimer’s Disease Facts and Figures. 2011:12. [Google Scholar]
- 2.Fassbender K, Masters C, Beyreuther K. Alzheimer's disease: molecular concepts and therapeutic targets. Naturwissenschaften. 2001;88:261–267. doi: 10.1007/s001140100237. [DOI] [PubMed] [Google Scholar]
- 3.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Reiman EM, Alexander GE, et al. Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am J Psychiatry. 2007;164:916–921. doi: 10.1176/ajp.2007.164.6.916. [DOI] [PubMed] [Google Scholar]
- 5.Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Learning from failure. Nature reviews. Drug discovery. 2010;9:499. doi: 10.1038/nrd3222. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong RA. The pathogenesis of Alzheimer's disease: a reevaluation of the "amyloid cascade hypothesis". Int J Alzheimer's disease. 2011;2011:630865. doi: 10.4061/2011/630865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy J. Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimer's disease : JAD. 2006;9:151–153. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- 9.Sommer B. Alzheimer's disease and the amyloid cascade hypothesis: ten years on. Current opinion in pharmacology. 2002;2:87–92. doi: 10.1016/s1471-4892(01)00126-6. [DOI] [PubMed] [Google Scholar]
- 10.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 11.Simon AM, Frechilla D, del Rio J. Perspectives on the amyloid cascade hypothesis of Alzheimer's disease. Revista de neurologia. 2010;50:667–675. [PubMed] [Google Scholar]
- 12.Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer's disease. The international J biochemistry & cell biology. 2009;41:1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson GE, Shi Q. A mitocentric view of Alzheimer's disease suggests multi-faceted treatments. J Alzheimer's disease : JAD. 2010;20(Suppl 2):S591–S607. doi: 10.3233/JAD-2010-100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prins ND, Visser PJ, Scheltens P. Can novel therapeutics halt the amyloid cascade? Alzheimer's research & therapy. 2010;2:5. doi: 10.1186/alzrt28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imbimbo BP, Giardina GA. gamma-Secretase Inhibitors and Modulators for the Treatment of Alzheimer's Disease: Disappointments and Hopes. Current topics in medicinal chemistry. 2011 doi: 10.2174/156802611795860942. [DOI] [PubMed] [Google Scholar]
- 16.Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev. 2008;60:1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 18.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swerdlow RH, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218:308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosconi L, Mistur R, Switalski R, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreira PI, Zhu X, Wang X, et al. Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta. 2010;1802:212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreira PI, Cardoso SM, Santos MS, Oliveira CR. The key role of mitochondria in Alzheimer's disease. J Alzheimer's disease : JAD. 2006;9:101–110. doi: 10.3233/jad-2006-9202. [DOI] [PubMed] [Google Scholar]
- 23.Perry EK, Perry RH, Tomlinson BE, Blessed G, Gibson PH. Coenzyme A-acetylating enzymes in Alzheimer's disease: possible cholinergic 'compartment' of pyruvate dehydrogenase. Neuroscience letters. 1980;18:105–110. doi: 10.1016/0304-3940(80)90220-7. [DOI] [PubMed] [Google Scholar]
- 24.Blass JP. The mitochondrial spiral. An adequate cause of dementia in the Alzheimer's syndrome. Ann N Y Acad Sci. 2000;924:170–183. doi: 10.1111/j.1749-6632.2000.tb05576.x. [DOI] [PubMed] [Google Scholar]
- 25.Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Annals of neurology. 1983;13:72–78. doi: 10.1002/ana.410130116. [DOI] [PubMed] [Google Scholar]
- 26.Gibson GE, Sheu KF, Blass JP, et al. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Archives of neurology. 1988;45:836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- 27.Parker WD., Jr Cytochrome oxidase deficiency in Alzheimer's disease. Annals of the New York Academy of Sciences. 1991;640:59–64. doi: 10.1111/j.1749-6632.1991.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso SM, Proenca MT, Santos S, Santana I, Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer's disease platelets. Neurobiology of aging. 2004;25:105–110. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 29.Khan SM, Cassarino DS, Abramova NN, et al. Alzheimer's disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Annals of neurology. 2000;48:148–155. [PubMed] [Google Scholar]
- 30.Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J neuroscience research. 2007;85:3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- 31.Takuma K, Yao J, Huang J, et al. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- 32.Lustbader JW, Cirilli M, Lin C, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 33.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardoso SM, Santos S, Swerdlow RH, Oliveira CR. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:1439–1441. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- 35.Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer's disease cybrids enhances Abeta toxicity. J neurochemistry. 2004;89:1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 36.Silva DF, Esteves AR, Arduino DM, Oliveira CR, Cardoso SM. Amyloid-beta-Induced Mitochondrial Dysfunction Impairs the Autophagic Lysosomal Pathway in a Tubulin Dependent Pathway. J Alzheimer's disease : JAD. 2011 doi: 10.3233/JAD-2011-110423. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimer's disease : JAD. 2010;20(Suppl 2):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swerdlow RH, Khan SM. A"mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Medical hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 39.Swerdlow RH, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: an update. Experimental neurology. 2009;218:308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson RM, Kusne Y, Nowak LA, LaFerla FM, Reiman EM, Valla J. Regional cerebral glucose uptake in the 3xTG model of Alzheimer's disease highlights common regional vulnerability across AD mouse models. Brain research. 2010;1347:179–185. doi: 10.1016/j.brainres.2010.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauptmann S, Scherping I, Drose S, et al. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiology of aging. 2009;30:1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Chou JL, Shenoy DV, Thomas N, et al. Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer's disease. J proteomics. 2011;74:466–479. doi: 10.1016/j.jprot.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Diana FF, Silva Esteves AR, Oliveira CR, Cardoso SM. Mitochondria: The Common Upstream Driver of Abeta and Tau Pathology in Alzheimer s Disease. Current Alzheimer research. 2011 doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- 45.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishii K, Sasaki M, Kitagaki H, et al. Reduction of cerebellar glucose metabolism in advanced Alzheimer's disease. J Nucl Med. 1997;38:925–928. [PubMed] [Google Scholar]
- 47.Spulber G, Niskanen E, Macdonald S, et al. Whole brain atrophy rate predicts progression from MCI to Alzheimer's disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 48.de Leon MJ, Convit A, Wolf OT, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-ons et Alzheimer's dementia. Proceedings of the National Academy of Sciences of the USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosconi L, De Santi S, Li J, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiology of aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosconi L, Mistur R, Switalski R, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. European J nuclear medicine and molecular imaging. 2009;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbloom MH, Alkalay A, Agarwal N, et al. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011 doi: 10.1212/WNL.0b013e31821cccad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 54.Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vlassenko AG, Vaishnavi SN, Couture L, et al. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta ) deposition. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bero AW, Yan P, Roh JH, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nature neuroscience. 2011 doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bioenergetics and shift to ketogenic profile in brain during reproductive senescence. Biochim Biophys Acta. 2010;1800:1121–1126. doi: 10.1016/j.bbagen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3:1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- 59.Morris AA. Cerebral ketone body metabolism. J inherited metabolic disease. 2005;28:109–121. doi: 10.1007/s10545-005-5518-0. [DOI] [PubMed] [Google Scholar]
- 60.Kuczynski B, Targan E, Madison C, et al. White matter integrity and cortical metabolic associations in aging and dementia. Alzheimer's & dementia : the J the Alzheimer's Association. 2010;6:54–62. doi: 10.1016/j.jalz.2009.04.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agosta F, Pievani M, Sala S, et al. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology. 2011;258:853–863. doi: 10.1148/radiol.10101284. [DOI] [PubMed] [Google Scholar]
- 62.Carmichael O, Schwarz C, Drucker D, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Archives of neurology. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez-Lima F, Berndt JD, Valla JE, Games D, Reiman EM. Reduced corpus callosum, fornix and hippocampus in PDAPP transgenic mouse model of Alzheimer's disease. Neuroreport. 2001;12:2375–2379. doi: 10.1097/00001756-200108080-00018. [DOI] [PubMed] [Google Scholar]
- 64.Dumont M, Lin MT, Beal MF. Mitochondria and antioxidant targeted therapeutic strategies for Alzheimer's disease. J Alzheimer's disease : JAD. 2010;20(Suppl 2):S633–S643. doi: 10.3233/JAD-2010-100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 66.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 67.Park LC, Zhang H, Sheu KF, et al. Metabolic impairment induces oxidative stress, compromises inflammatory responses, and inactivates a key mitochondrial enzyme in microglia. J neurochemistry. 1999;72:1948–1958. doi: 10.1046/j.1471-4159.1999.0721948.x. [DOI] [PubMed] [Google Scholar]
- 68.Starkov AA, Fiskum G, Chinopoulos C, et al. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. The J neuroscience : the official J the Society for Neuroscience. 2004;24:7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atamna H, Frey WH., 2nd Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer's disease. Mitochondrion. 2007 doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Nunomura A, Chiba S, Lippa CF, et al. Neuronal RNA oxidation is a prominent feature of familial Alzheimer's disease. Neurobiology of disease. 2004;17:108–113. doi: 10.1016/j.nbd.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer's disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol. 2006;2006:31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta neuropathologica. 2009;118:151–166. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- 73.Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 74.Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer's disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 75.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. The J neuroscience : the official J the Society for Neuroscience. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rhein V, Song X, Wiesner A, et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J neuropathology and experimental neurology. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 78.Moreira PI, Santos MS, Seica R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer's disease and diabetes. J Neurol Sci. 2007;257:206–214. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 79.Li F, Calingasan NY, Yu F, et al. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J neurochemistry. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- 80.Extance A. Alzheimer's failure raises questions about disease-modifying strategies. Nat Rev Drug Discov. 2010;9:749–751. doi: 10.1038/nrd3288. [DOI] [PubMed] [Google Scholar]
- 81.Bateman RJ, Siemers ER, Mawuenyega KG, et al. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Annals neurology. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raybon JJ, Albright CF. In response to "a gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system". Annals of neurology. 2010;67:143–144. doi: 10.1002/ana.21929. author reply 144–5. [DOI] [PubMed] [Google Scholar]
- 83.Rafii MS, Aisen PS. Recent developments in Alzheimer's disease therapeutics. BMC medicine. 2009;7:7. doi: 10.1186/1741-7015-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. The J neuroscience : the official J the Society for Neuroscience. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conte V, Uryu K, Fujimoto S, et al. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J neurochemistry. 2004;90:758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- 86.Brewer GJ. Why vitamin E therapy fails for treatment of Alzheimer's disease. J Alzheimer's disease : JAD. 2010;19:27–30. doi: 10.3233/JAD-2010-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical "disconnection" in aging and Alzheimer's disease. Neurobiol Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 88.Packer L, Cadenas E. Lipoic acid: energy metabolism and redox regulation of transcription and cell signaling. J clinical biochemistry and nutrition. 2011;48:26–32. doi: 10.3164/jcbn.11-005FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vingtdeux V, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer's disease. BMC neuroscience. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochemistry international. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. The J biological chemistry. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 92.Li H, Yan Z, Zhu J, Yang J, He J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology. 2011;60:252–258. doi: 10.1016/j.neuropharm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Wollen KA. Alzheimer's disease: the pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Alternative medicine review : a J clinical therapeutic. 2010;15:223–244. [PubMed] [Google Scholar]
- 94.Auestad N, Korsak RA, Morrow JW, Edmond J. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem. 1991;56:1376–1386. doi: 10.1111/j.1471-4159.1991.tb11435.x. [DOI] [PubMed] [Google Scholar]
- 95.Guzman M, Blazquez C. Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot Essent Fatty Acids. 2004;70:287–292. doi: 10.1016/j.plefa.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 96.Henderson ST. Ketone bodies as a therapeutic for Alzheimer's disease. Neurotherapeutics : the J the American Society for Experimental NeuroTherapeutics. 2008;5:470–480. doi: 10.1016/j.nurt.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao Jia SC, Mao Zisu, Cadenas Enrique, Brinton Roberta Diaz. 2-Deoxy-D-Glucose Treatment Induces Ketogenesis, Sustains Mitochondrial Function, and Reduces Pathology in Female Mouse Model of Alzheimer's Disease. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0021788. [DOI] [PMC free article] [PubMed] [Google Scholar]