Abstract
Lipocalins are a family of diverse low molecular weight proteins that act extracellularly. They use multiple recognition properties that include: 1) ligand binding to small hydrophobic molecules, 2) macromolecular complexation with other soluble macromolecules, and 3) binding to specific cell surface receptors to deliver cargo. Tear lipocalin (TLC) is a major protein in tears and has a large ligand binding cavity that allows the lipocalin to bind an extensive and diverse set of lipophilic molecules. TLC can also bind to macromolecules including the tear proteins lactoferin and lysozyme. The receptor to which TLC binds is termed tear lipocalin interacting membrane receptor (LIMR). LIMR appears to work by endocytosis. TLC has a variety of suggested functions in tears including regulation of tear viscosity, binding and release of lipids, endonuclease inactivation of viral DNA, binding of microbial siderophores (iron chelators used to deliver essential iron to bacteria), use as a biomarker for dry eye, and possession of anti-inflammatory activity. Additional research is warranted to determine the actual functions of TLC in tears and the presence of its receptor on the ocular surface.
Keywords: tear lipocalin, tears, lipocalin interacting membrane receptor, meibomian gland, lipids
I. Introduction
Tear lipocalin is a member of the lipocalin family and the calycin superfamily of proteins. These families are a diverse set of proteins that function as extracellular binding proteins. The proteins of these families are found in almost all phyla and have wide-spread functions. In spite of the research to date, the native cargo and the cellular functions of many of these proteins, including tear lipocalin, remains speculative. The present review will focus on tear lipocalin, its structure, function in tears, and place in the lipocalin familiy of proteins.
II. Lipocalin Family of Proteins
A. Structure
Lipocalins are a family of diverse, low molecular weight proteins (18–40 kDa) that function predominantly extracellularly. The family members include retinol-binding protein (RBP), oroseromucoid (α1-acid glycoprotein), retinoic acid binding protein, α-1 microglobulin, purpurin, apolipoprotein D, complement C8 γ, tear lipocalin (human tear prealbumin, Von Ebner’s gland protein), prostaglandin D synthase, human neutrophil lipocalin (neutrophil gelatinase-associated lipocalin), β-lactoglobulin, major urinary protein (MUP), oderant binding protein (OBP), glycodelin, and all important mammalian acroallergens (Table 1)1, 2. Lipocalins are varied proteins with a common structure and possess multiple molecular recognition properties (Figure 1). These recognition properties include: 1) ligand binding principally to small hydrophobic molecules, 2) macromolecular complexation with formation of covalent and non-covalent complexes with other soluble macromolecules, and 3) binding to specific cell surface receptors 3, 4.
Table 1.
The human lipocalins. Reprinted from Breustedt et al, 2006
| Lipocalin (abbreviation) | Synonyms | Tissue of primary synthesis | Amino acid residues | Oligomeric status | Glycosylation status | Disulfide bridges | Physiological ligands |
|---|---|---|---|---|---|---|---|
| α1-Acid glycoprotein (AGP) | orosomucoid (ORM), seromucoid α1 fraction, α1-S | liver | 183 | M | + | 2 | unknown |
| Apolipoprotein D (ApoD) | gross cystic disease fluid protein (GCDFP-24), apocrine secretion odour-binding protein (ASOB-2) | adrenals spleen placenta kidneys CNS | 169 | M/H | + | 2 | progesterone arachidonic acid |
| Complement factor 8 γ chain (C8γ) |
liver | 182 | H | − | 1 | unknown | |
| Glycodelin (GLY) | pregnancy protein 14 (PP14), human pregnancy-associated endometrial protein, α2-globulin (α2-PEG), chorionic α2-micro-microglobulin, progest-agen-associated endometrial protein (PAEP), endometrial protein (PAEP), α-uterine protein | endometrium fallopian tube ovary breast | 162 | D | + | 2 | unknown |
| α1-Microglobulin (α1m) | protein HC, α1-microglycoprotein | liver blood plasma kidneys | 183 | M/H | + | 1 | heme |
| Neutrophil gelatinase-associated lipocalin (NGAL) | human neutrophil lipocalin (HNL), 24p3, SIP24, uterocalin, α2-microglobulin-related protein, neu-related lipocalin (NRL) siderocalin, LCN2 | neutrophils | 178 | M/D/H | + | 1 | enterobactin |
| Odorant-binding protein | frog Bowman’s gland protein | olfactory epithelium | 155 | M | − | 1 | unknown |
| Lipocalin type prostaglandin D synthase (PGDS) | β-trace | CNS | 168 | M | + | 1 | prostaglandin D |
| Retinol-binding protein (RBP) | plasma retinol-binding protein, serum retinol-binding protein (sRBP) | liver | 183 | M/H | − | 3 | all-trans retinol |
| Tear lipocalin (Tlc) | protein migrating faster than albumin (PMFA), specific tear albumin (STP), von Ebner’s gland protein (VEGP), LVN1 | lacrimal glands | 158 | M/D | − | 1 | stearic acid palmitic acid cholesterol lauric acid triacylglycerols fatty alcohols |
Figure 1.
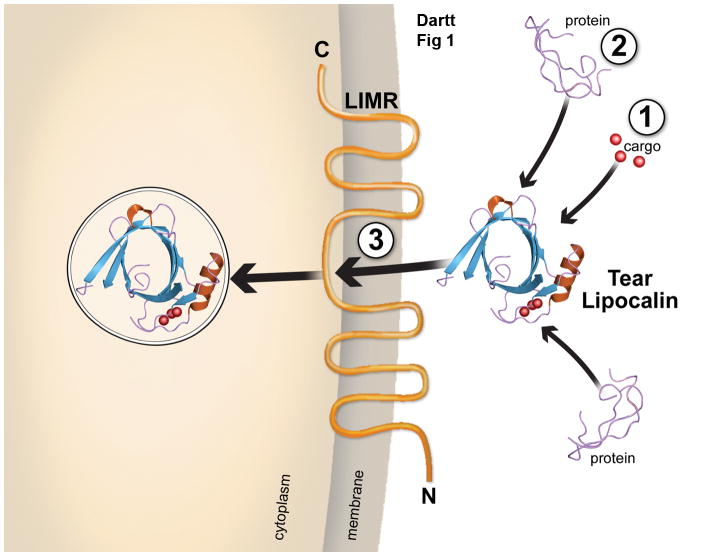
Schematic of tear lipocalin illustrating the three types of molecular recognition properties exhibited by the lipocalin family members. Lipocalins can bind to: 1) ligands illustrated here as cargo, 2) soluble macromolecules indicated here as protein strands, or 3) specific cell receptors indicated here as LIMR, the tear lipocalin receptor. Tear lipocalin once bound to its receptor is transported into the cell with its cargo by endocytosis.
The lipocalin family has low sequence similarity, but high enough to prove shared origin. Even though there is low sequence similarity there is high family unity at the 3D structural level so that lipocalins share one to three short conserved sequence motifs. As determined by high resolution X-ray crystallography all lipocalins contain a conserved ligand-binding pocket 1. Furthermore, most lipocalin genes are clustered on the same chromosome. The lipocalins that contain three characteristic conserved sequence motifs are termed kernel lipocalins. The lipocalins that share only one or two conserved sequence motifs are known as outlier lipocalins 5. The three conserved sequence motifs are all located in the ligand binding pocket known as the lipocalin fold. This fold is a highly symmetrical all-β strand structure dominated by a single eight-stranded antiparallel β-sheet closed back on itself to form a continuously hydrogen-bonded β-barrel (Figure 2) 5. The β-barrel encloses a ligand-binding site that contains an internal cavity and an external loop scaffold. The diversity of the pocket and scaffold allows a variety of ligands of different sizes, shapes and chemical characteristics to bind to specific types of lipocalins.
Figure 2.
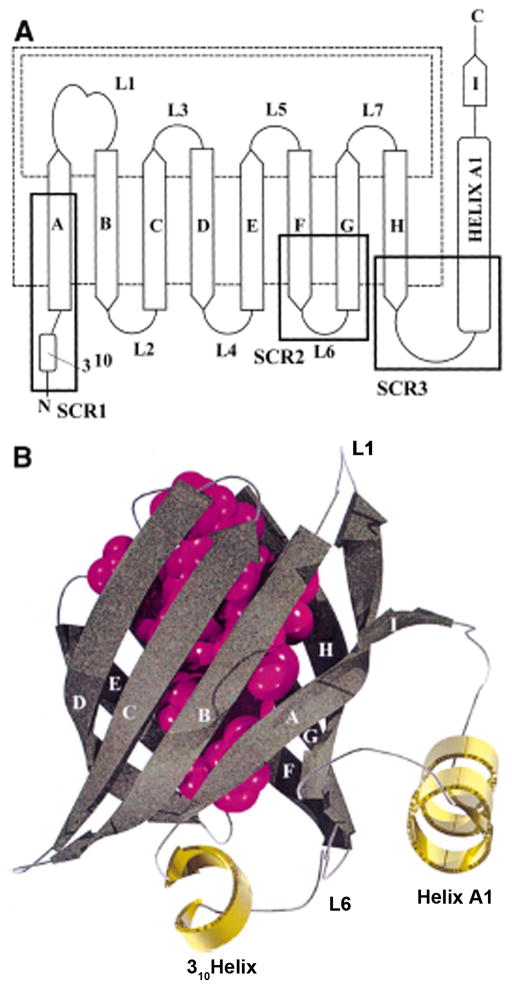
Structure of lipocalin fold. (A) An unwound view of the lipocalin fold. The nine b-strands of the antiparallel β-sheet are labeled A-I. The C-terminal α-helix A1 and N-terminal 310 are indicated. Dotted lines indicate hydrogen bond between the two strands. Connecting loops are shown as solid lines and labeled L1-L7. The opening of the internal ligand-binding site is here and is called the Open end of the molecule. The three amino structures and conserved regions (SCRS) of the fold are marked in heavy boxes. (B) A schematic of the lipocalin fold. The ligand binding site is depicted in purple depicted representing the overlay of ligands. β-strands are shown in smoothly curving arrow; a-helices as gold spirals; loops as silver cords. Reprinted from Flower et al 2000.
The lipocalin family is in fact part of a superfamily known as the calycins. This superfamily contains the fatty acid binding proteins, avidins, and metalloproteinase inhibitors in addition to lipocalin 5. All family members have a ligand binding cavity that for fatty acid-binding proteins binds fatty acids, for avidin binds the vitamin biotin, and the metalloproteinase inhibitors bind metalloproteinases. In fact some lipocalins function as proteinase inhibitors as exemplified by the metalloproteinase inhibitors. In addition to the β-barrel all calycins including the lipocalins have an argine or lysine that is able to form a number of potential hygrogen bonds with the short 310 helix that closes the end of the barrel using a conserved tryptophan 5.
Lipocalins have been found predominantly in eukaryotic organisms, mostly in vertebrates, although some have been identified in other phyla 5. Apart from vertebrates the largest number of lipocalins is found in the arthropoda. Lipocalins in arthropods include butterfly insecticyanin, grasshopper lazarillo, and lobster crustacyanin. Lipocalins are also present in plants, slime molds, Drosophila, and microbes.
On the basis of the presence of the conserved sequences, lipocalins have been divided into kernel and outliers. Kernel lipocalins have all three conserved sequences, whereas outlier lipocalins have one or two. Oderant-binding proteins and Von Ebner’s gland proteins have more similarity to the kernel lipocalins than do the other outlier lipocalins. Von Ebners’s gland proteins contain vomeronasal proteins, odor binding protein-II, the major dog allergen Can f 1, and tear lipocalin (TLC). Thus TLC is an outlier lipocalin, but has more similarity to the kernel lipocalins than many other outlier lipocalins.
Lipocalins were originally classified as transport proteins. The classic lipocalin the lipocalin RBP binds to its ligand all-trans-retinol. For the body to maintain a homeostatic level of retinol, retinol binds to RBP, which allows retinol to circulate without loss by oxidation 6. RBP is primarily synthesized in the liver, which is the main storage site of RBP 7. RBP is also produced in the kidney, testis, retinal pigmented epithelium, and choroid plexus. Retinol triggers the secretion of RBP into the extracellular space where it binds retinol. RBP also binds to transthyretin that prevents RBP from being filtered out of the blood in the glomerulus. The RBP transthyretin interaction allows RBP to bind to its receptor megalin and transfer retinol into a target cell where retinol binds cellular retinol binding protein. RBP is the prototypic lipocalin and illustrates the three types of interactions: 1) ligand binding (retinol), 2) binding with other macromolecules (transthyretin), and 3) binding with its receptor (megalin).
B. Functions
In the past years a wide variety of functions have been discovered for the lipocalins. One type of function is modulation of cell growth and metabolism by apolipoprotein D, quiescence-specific protein, purpurin, α1-microglobulin, and neutrophil gelatinase-associated lipocalin. Other lipocalins participate in the immune response (α1-microglobulin, glycodelin, and the γ chain of complement factor 8), smell reception (odorant-binding protein), tissue development (lazarillo), animal behavior (α2-urinary globulin, major urinary protein), and allergic reaction initiation as allergens (cockroach allergen, mouse urine allergen, rat allergen, dog dander allergen 1 and 2, horse allergen 1 and 2, major cow dander allergen) 1. Although functions have been identified for several lipocalins, many of their functions remain an enigma and, furthermore, the functions of many are unknown.
Functions of several of the individual lipocalins may shed light on the function of TLC. Lipocalin type prostaglandin D synthase (LPGDS) is a lipocalin that has two very different functions. When located intracellularly LPGDS catalyzes the isomerization of prostaglandin H2 8. Upon secretion into the extracellular space LPGDS functions as a lipocalin and can bind all-trans- or 9-cis-retinoic acid, although its endogenous ligands have yet to be identified. LPGDS is found in the central nervous system, male genital organs, and the heart. Secreted LPGDS is detected in cerebral spinal fluid, eye fluids, seminal plasma, and plasma. Knockout mice developed abnormalities in nociception and sleep. Transgenic mice displayed abnormalities in allergic reaction and adipogenesis. Similarly to LPGDS TLC could have different functions depending on the tissue in which it is located and its cellular location (intracellular or secreted).
A subset of lipocalins has been termed immunocalins. These include α1-microglobulin, glycodelin, the γ chain of complement factor 8, α1-acid glycoprotein, NGAL, TLC, and L-PGDS. All seven have protective immunoregulatory, anti-inflammatory, and antimicrobial effects 9. The immunocalins play a role in the acute phase response to infection and injury and are acute phase proteins (APP). In general they protect the body from excessive inflammation and function as part of the extended cytokine immune network. The immunocalins function by the three typical mechanisms of lipocalins: 1) the “lipocalin pocket” scavenges tissue toxic, small molecular weight lipophilic compounds released by microorganisms or inflammatory cells; 2) immunocalins bind plasma proteins regulating their function; and 3) immunocalins bind to cell surface receptors on inflammatory cells allowing immunocalins to directly regulate these cells (Figure 1). Evidence suggests that immunocalins can regulate natural killer (NK cells), neutrophils, monocytes, macrophages; platelet aggregation; adherence of neutrophils and monocytes to vascular endothelium; and B and T lymphocytes. As a member of the immunocalin subset, TLC could have protective immunoregulatory, anti-inflammatory, and antimicrobial effects in the tears and in relation to the ocular surface.
Lipocalins also appear to be markers of disease. The major lipocalins associated with disease are α1-acid glycoprotein, α1-microglobulin, apolipoprotein D, RBP, complement C8γ, TLC, prostaglandin D synthase, and human neutrophil lipocalin/neutrophil gelatinase-asscoated lipocalin (NGAL)10. The majority of the literature on lipocalins as biomarkers of disease is focused on NGAL and suggests that an elevation of urinary NGAL indicates kidney disease 11. A second disease marker is retinol-binding protein 4 that indicates coronary disease. Additionally, the eight lipocalins listed above are all associated with inflammation and most have been linked to inflammatory diseases such as coronary artery disease, rheumatoid arthritis, inflammatory bowel disease, Crohn’s disease, and diabetes. Several of the above listed lipocalins have been associated with cancer and cancer progression. TLC has been suggested to be a biomarker of dry eye.
C. Ligands
Lipocalins are characterized by their ability to bind small, principally hydrophobic ligands 1. Retinol is the typical lipocalin ligand. The number of ligands continues to grow including diverse molecules such as oderants, steroids, and pheromones. Ligands are lipocalin specific and too numerous to list here.
D. Receptors
Receptors for a number of lipocalins have been identified including α1-acid glycoprotein, α1-microglobulin, RBP, insecticyanin, glycodellin, β-lactoglobulin, TLC, and odorant-binding protein 4. Lipocalins bind their receptors by both carbohydrate-binding as well as protein-protein interactions. The former are inhibited by small molecule sugars, where as the latter are not. Different lipocalins can be bound by the same receptor, megalin. In contrast, several receptors can bind the same lipocalin. For example AGP is bound by both lectin (carbohydrate) and non-lectin (protein-protein interaction). Finally, lipocalin receptor interaction can activate signaling pathways or mediate endocytosis.
One well-studied lipocalin receptor is the receptor for RBP, STRA6 12. This receptor is not homologous to any other protein. It is a high-affinity receptor for plasma RBP and mediates cellular uptake of retinol (Vitamin A) from the retinol-RBP complex (retinol is the ligand for RBP). STRA6 has nine transmembrane domains with an extracellular N terminus domain and a long intracellular C terminus domain consistent with its being a membrane transport protein or channel. It has been hypothesized that after retinol loaded RBP binds to STRA6 the RBP ligand (retinol) could pass through the channel made by STRA6 entering the cell leaving RBP and STRA6 outside. Not surprisingly retinal pigmented epithelium is especially rich in STRA6 consistent with its role supplying the retinal photoreceptors with retinol 13.
E. Binding to Other Macromolecules
In addition to binding ligands and receptors, lipocalins can bind other proteins via protein-protein interactions. The best studied is the lipocalin RBP with the protein transthyretin. Retinol the major ligand of RBP is produced in the liver. In the liver RBP binds to retinol in the RBP’s binding cavity and then binds to transthyretin 7. RBP circulates in the plasma as this macromolecular complex keeps RBP from being filtered from the plasma by the renal glomerulus. HoloRBP (lipid loaded) binds more tightly to transthyretin than apoRBP (lipid free) and apoRBP is cleared from the circulation in the kidney. The stoichiometry of binding is 1RBP: 2 transthyretin. Thus the binding of RBP to transthyretin favors the retinol-loaded form of RBP. When this complex reaches the target tissue, RBP binds to its receptor releasing retinol into the cell. RBP remains attached to transthyretin.
NGAL is a second lipocalin that binds to a non-ligand, non-receptor protein. NGAL binds to the matrix metalloproteinase (MMP) 9 also known as gelatinase B 14. Binding of MMP9 to NGAL protects the activity of the former to digest the extracellular matrix. Extracellular matrix degradation is important in cancer metastatsis and tumor progression as well as in osteoarthritis 15. The NGAL/MMP9 complex in the urine can be predictive of metastatic cancer.
A third lipocalin macromolecular complex is based on the lipocalin Lazarillo. Lazarillo is a glycoprotein involved in axon growth and guidance in the grasshopper embryo 16. Lazarillo is a unique lipocalin in that it is anchored to the cell surface by GPI. Lazarillo oligomerizes in solution as well as when bound to GPI. Lazarillo binds to multiple ligands including heme, retinoic acid, and fatty acids. In addition, a function-blocking antibody mAB 10E6 which prevents binding of a protein to lazarillo prevents clustering in the cell membrane as well as perturbs its axon growth and pathfinding capabilities without altering ligand binding. The protein that binds to lazarillo has yet to be identified.
Two other examples of macromolecular binding to lipocalins are α1-microglobin binding to IgA and to fibronectin 1.
III. Tear Lipocalin Structure and Molecular Binding
A. Discovery and Tissue Distribution
TLC was first discovered in 1956 using non-denatured paper electrophoresis of human tears 17. TLC migrated faster than albumin so was first called protein migrating faster that albumin. As this protein was only found in tears it was thought to be tear specific and was thus named tear specific prealbumin or tear prealbumin 18. In 1992, tear prealbumin was cloned and identified as a lipocalin and renamed tear lipocalin (abbreviated TLC or LNC1) 19, 20. In 1993, TLC was also found in a salivary gland known as von Ebner’s gland where it was called von Ebner’s gland protein. TLC was subsequently identified in the nasal, mammary, sweat glandular, prostate, testicular, and tracheobronchial mucosae, as well as in the skin and corticotrophs of the pituitary gland 21, 22. Thus TLC can no longer be considered tear specific, although it is still named tear lipocalin. In tears TLC accounts for 15–33% of the total protein.
B. Molecular Weight and Isoforms
There are several isoforms of TLC due to the heterogeneity of the N terminus. The full-length isoform of TLC is 17.446 kDa. The most truncated form has a mass of 16.870 kDa 22. Three genes synthesize TLC: LCN1a, LCN1b, and LCN1c. LCN1a encodes TLC, LCN1b is not expressed, and LCN1c is a truncated pseudogene. Genetic polymorphism does not appear to be responsible for TLC heterogeneity, but proteolytic activity could account for the truncated isoforms.
C. Amino Acid Sequence Similarities
As presented in the section on the lipocalin family, TLC is an outlier rather than a kernel or classical lipocalin 22. When protein databases are analyzed of the twenty closest matches to TLC only two are kernel lipocalins (β-lactoglobulin and α1-macroglobulin). Accounting for this is the fact that TLC contains only two of the three kernel lipocalin motifs. The proteins to which TLC is similar are the outlier lipocalins rat and pig von Ebner’s gland protein, vomeral nasal proteins I and II, oderant binding protein II of rat, a protein of tammar wallaby named “late lactation protein”, two major allergens (Can f 1 from dog and Equ c 1 from horse), and a major protein of amphibian choroid plexus 22. All of these proteins are secreted from a variety of mucosa either epithelial or glandular except Equ c 1, which is an allergen in horse dandruff.
D. Ligand Binding and Structural Features
In biological samples a variety of ligands are found bound to TLC (Table 2). They include lipophilic compounds such as fatty acids, fatty alcohols, phospholipids, glycolipids, retinol, arachidonic acid, and cholesterol 23. TLC can also bind microbial siderophores (iron scavenging molecules), the antibiotic rifampin, and several synthetic fluorescent probes in vitro 24. TLC can also bind to lipid peroxidation products 21. The ability of TLC to bind such a large number of compounds from different chemical classes is unusual among lipocalins. This broad ligand specificity of TLC occurs both in vivo and in vitro. The reason for the ability of TLC to bind a wide range of ligands is found in its structure.
Table 2.
Ligands that bind TL: (A) extracted from native protein (B) binding in vitro. Reprinted from Redl, 2000
| Ligand | Kinetic parameters | Ref. | |
|---|---|---|---|
| (A) | Stearic acid | ND | [28] |
| Palmitic acid | ND | [28] | |
| Cholesterol | ND | [28] | |
| Lauric acid | ND | [28] | |
| Triacylglycerols | ND | [28] | |
| Fatty alcohols | ND | [28] | |
| (B) | Retinol | Kd ~ 0.6 μM | [8] |
| Oleic acid | ND | [46] | |
| 16-APa | Kd ~ 0.8 μM | [29] | |
| DAUDAb | Ki ~ 2.8 μM | [29] | |
| Cholesterol | Ki ~ 15.9 μM | [29] | |
| Lauric acid | Ki ~ 9.1 μM | [29] | |
| Palmitic acid | Ki ~ 3.2 μM | [29] | |
| Stearic acid | Ki ~ 1.3 μM | [29] | |
| Phosphatidylcholine | Ki ~ 1.2 μM | [29] |
16-AP, 16-(9-anthroyloxy)palmitic acid.
DAUDA, 11-(5-dimethylamino-1-naphthalenylsulphonyl)amino-undecanoic acid.
In spite of being an outlier lipocalin, TLC contains the classic structural feature of the lipocalin family, the eight β-strands that form a barrel and the C-terminal α-helical composition. However, the cavity inside the β-barrel is remarkably large, has a wide mouth, and an even wider base with a bifurcated shape 21. Four loops connect the β-strands 24 (indicated by 1–4 in Figure 3A). The size and shape of the cavity allows TLC to bind both slim fatty acids and bulkier compounds such as cholesterol and rifampin. The TLC cavity consists of β-strands that are elongated at the open end of the barrel. The amino acids Met39 and Phe99 protrude into the cavity and lead to a bifurcation at the bottom as well as to shielding the aromatic side chain of Trp17.
Figure 3.
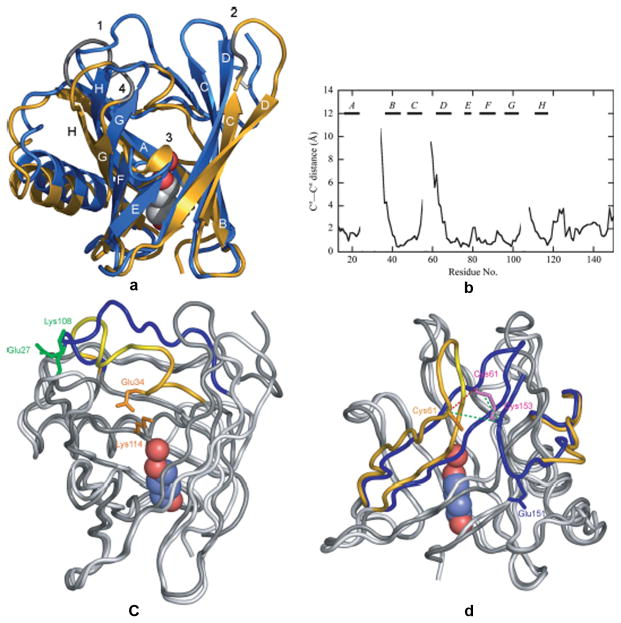
Comparison of the TLC structures obtained from two crystal forms. A. Superposition of TLC crystallized in space group P21 (chain A, blue) and is space group C2 (gold with modeled residues in grey). The bound 1,4-butanediol and hydrogen-bonded water are represented as spheres and the conserved disulfide bond is shown in white. B. Pairwise Ca-atom distances between the two different TLC crystal structures after superposition of the 58 conserved Cα positions of the β-barrel. C. Electrostatic interactions that may trigger the two alternative conformations of loop 1. In the P21 structure (dark grey) Glu27 and Lys108 (green) make an electrostatic contact that fixes the “open” conformation of loop 1 (blue). The conformation of this loop in C2 structure (light grey) is stabilized by interaction between Glu34 and Lys114 (orange). D. Flexibility of the region around the disulfide bridges. The disulfide bond between Cys61 and Cys153 is well ordered in the P21 structure (dark grey; loop 2 and the C-terminal peptide segment (blue). Only Cys61 was resolved in the C2 structure (light grey; loop 2 and the C-terminal (gold). Reprinted from Breustedt, et al 2009.
The structure of TLC, unlike many other lipocalins, can be determined when it is bound to ligands (holo-TLC) or with ligands absent (apo-TLC) 22. Substantial changes to the structure of TLC occur when a ligand binds. The changes upon ligand binding have been measured by X-ray structural analysis of crystals using non-endogenous ligands such as 1,4-butanediol. When ligand is bound the reversible formation of an additional β-sheet structure occurs in TLC making the molecule more rigid. The ligand pocket can adapt to different ligands via side chain rearrangements within TLC mainly involving Phe 99. However, ligand binding induces another large conformational change such that Met39 is pushed outward and with it the entire β-strand B (Figure 4). This changes the loop 1 that connects strands A and B and strands C and D as well as their connector loop 2. Loop 1 (AB in Figure 4) and loop 4 (GH in Figure 4) form the gate region in TLC and provide access for ligand binding 25. The ligand induced conformational change was observed in two different analytical methods 24, 26. Ligand induced conformational changes are not found in another outlier lipocalin, OBP.
Figure 4.
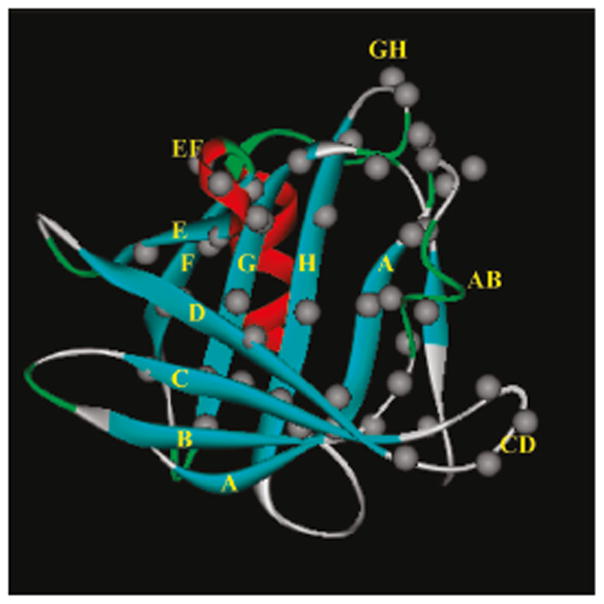
Positions of the residues that were sequentially substituted with Trp to explore the binding energy landscape of TLC. Grey circles show locations of the Cα atoms of the amino acid residues. The side chains of all β-strand residues are oriented inside the cavity. Single and double letters denote the identities of the β-strands and loops, respectively. The ribbon diagram (blue, β-strands; red, α-helix; green, turns, grey, loops) of TLC was generated from PDB 1XKI. Reprinted from Gasymov et al, 2009.
In TLC the ligand can be buried deeply within the β–barrel and laterally shifted to form direct contacts with Trp 17 and Tyr97 at the bottom of the cavity. The TLC cavity is larger than other lipocalins, but can extensively adapt to ligand binding by side-chain rearrangements especially involving Phe99. In addition, all ligands bind entirely in the interior of the β-barrel cavity in a relatively unrestricted binding site 27. Thus the loop region and adjoining parts of the β-barrel exhibit considerable flexibility allowing structural adaptation of TLC to various natural and artificial ligands that can differ vastly in shape and size. This mechanism for promiscuity in ligand binding has not been previously identified in the lipocalin family, but could be relevant to them.
The strength of ligand binding to TLC correlates with the length of the hydrocarbon tail for both alkyl amines and fatty acids 27. Thus TLC is able to bind most strongly the least soluble and longest lipids.
E. Tear Lipocalin Receptor
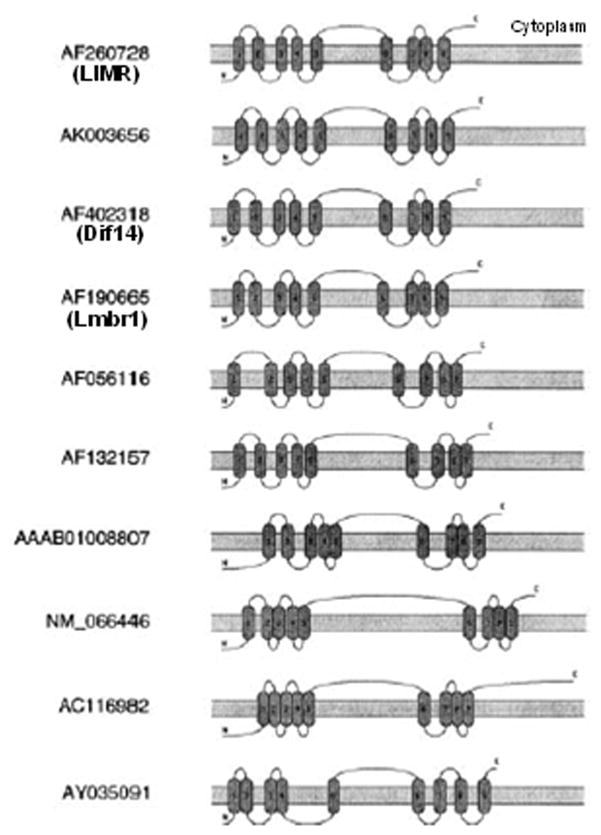
Once TLC has bound its ligand (goes from apo-TLC to holo-TLC) its F-G loop becomes more exposed and is available to interact with the TLC receptor 28. The TLC receptor was identified by Wojnar et al 29 using a phage display technique with a cDNA expression library with TLC as bait. Several clones of proteins that had not been previously characterized were identified in the screen. A protein of 487 amino acids and a molecular weight of 55 kDa was identified as the TLC receptor and was termed lipocalin interacting membrane receptor (LIMR) (Figure 5). A hydropathy analysis revealed that LIMR has nine putative transmembrane helices and indicating that LMR is a membrane receptor. Computer analysis suggested that the N-terminal domain is extracellular and that TLC interacts with (binds to) this domain. The central cytoplasmic loop of LIMR is quite large, whereas the other cytoplasmic loops are small. Immunohistochemical analysis demonstrated that LIMR localized in membranes. Binding assays showed that TLC binds specifically to LIMR, where as bovine serum albumin does not. Thus a specific receptor for TLC has been identified and TLC is one of the few lipocalins to have a specific receptor.
Figure 5.
Punative topography of LIMR orthologous proteins. The analysis was performed using TopPred 2 topology predicition program. AF260728 has been identified as H. sapiens LIMR; AF402318 as H. sapiens Dif14, and AF190665 as M. musculus Lmbr1. The rest are unknown. Reprinted from Wojnar, et al, 2003.
Lipocalins interact with their receptors by either binding to carbohydrate moieties on the receptors or protein-protein interactions. LIMR, which is specific to TLC, binds to LIMR by a protein-protein interaction.
It is the holo (ligand bound) form of TLC that binds to LIMR. There are several mechanisms by which the ligand (or cargo) could then enter the target cell 30. First, after TLC-LIMR binding TLC would release its ligand, which would then diffuse through the target cell membrane and interact with an intracellular fatty acid binding protein or other intracellular receptor. Second, TLC with its ligand could be internalized by receptor-mediated endocytosis. Third, the ligand would not be internalized, but the TLC-LIMR interaction would activate a signal to induce a physiologic response. Wojnar et al (2001) found TLC and LIMR expression in a cell line, NT2 cells 31. Using this cell line Wojnar et al found that LIMR is an endocytic receptor and mediates cellular uptake of TLC and its ligand 30. In fact there is a family of receptors that are similar in structure to LIMR and form a novel family of endocytic receptors. Wojnar et al speculate that receptor-mediated endocytosis would protect the cell from the TLC cargo, as TLC is so promiscuous in its ligand binding and some types of cargo could be cell toxic 30.
LIMR tissue expression was performed and indicated that LIMR was detected in testis, pituitary gland, adrenal gland, trachea, placenta, thymus, cerebellum, stomach, mammary gland, and spinal cord 31. Most of these tissues also express TLC. Of note, lacrimal gland was not tested; nor were cornea, conjunctiva, or lacrimal gland drainage duct used in the screen. It is possible that after TLC is secreted by the lacrimal gland into the tear film and binds lipophilic ligands, holo-TLC could then bind a membrane receptor in the cornea and conjunctiva or perhaps the nasolacrimal (tear drainage) duct. At the apical (mucosal) membranes in these epithelia, the holo-TLC ligand would enter the epithelial cells by endocytosis after TLC/LIMR interaction or would deliver its lipid cargo into the cells. Experiments testing this mechanism of action have not yet been published.
LIMR can be compared with another type of receptor, G protein-coupled receptors (GPCR), that are receptors for neurotransmitters, peptide hormones, and thousands of other compounds. GPCR have an N-terminal extracellular domain, seven transmembrane spanning domains, and an intracellular C-terminal domain. Similarly to GPCR, LIMR has an N-terminal extracellular domain and an intracellular C-terminal domain. LIMR have nine transmembrane spanning domains, two more than possessed by GPCR. GPCR do not use carbohydrate binding to interact with their ligands, but if the ligand is a peptide or protein use protein/protein interactions, as do LIMR. In both types of receptors the ligand binds to the extracellular N terminal domain. Thus GPCR and LIMR have many characteristics in common.
LIMR can also be compared to another lipocalin receptor megalin. Megalin has a single transmembrane domain compared to the nine transmembrane domains of LIMR. Megalin, however, has an extensive N terminal extracellular domain that contains 53 cysteine rich repeats characteristic on low density lipoprotein receptor family. Megalin interacts with several different lipocalins including TLC as well as the non-lipocalins albumin and insulin. Megalin, like LIMR, appears to function by endocytotic uptake of its ligand with its cargo, although RBP bound to megalin does not appear to enter the target cell.
Two other proteins bind LIMR. These proteins are: 1) the lipocalin β-lactoglobin, a major food allergen present in milk 32 and 2) uteroglobin a non-lipocalin that is anti-inflammatory and anti-chemotactic, but binds retinoids and interacts with lipophilic compounds similarly to lipocalins 33. After binding to LIMR, β-lactoglobin, like TLC, was endocytosed into the target cell. Thus uptake could function either to trigger the immunoreaction to β-lactoglobin or to prevent the allergic response through internalization of the allergen.
Thus TLC is one of the few lipocalins to have its own specific receptor. Determination of the cellular localization of LIMR on the ocular surface including the lacrimal gland and nasolacrimal duct will help to unravel the function of TLC in the eye.
F. Tear Lipocalin Binding to Macromolecules
TLC like other lipocalins has the potential to bind protein macromolecules in addition to its receptor. Redl et al 34 used phage display to identify proteins that interact with TLC. They identified thioredoxin. Binding of thioredoxin to TLC reduces the TLC by disulfide bond reduction and increases its affinity for retinoic acid. The increased affinity of TLC for its ligand could be a fast and efficient mechanism to increase the protective potential of the lipid scavenger in response to inflammation or chronic infection. A second protein was also detected, but not identified.
TLC is also known to interact with other proteins secreted by the lacrimal gland. The tear proteins lactoferrin and lysozyme are co-localized with TLC in the lacrimal gland secretory granules. Thus they are most likely secreted together and could either have similar functions or influence the activity of each other. As all three proteins have anti-microbial activity they could enhance the microbial activity of each other as suggested for the rabbit 35. One mechanism for this enhancement could be that TLC could bind the long chain fatty acids (C-18) secreted by the meibomian glands 23. These fatty acids are known to inhibit lysozyme. Thus TLC would enhance the activity of lysozyme. Lysozyme can also be inhibited by nucleic acids. Lactoferrin binds nucleic acids and thus could increase lysozyme activity 36.
In addition, TLC, lysozyme, and lactoferrin could directly interact with each other. Gasymov using purified proteins found that lysozyme and lactoferrin decrease the mobility at two sites on TLC consistent with direct interaction. In contrast, albumin did not interact with TLC 27. The evidence to data is suggestive of interaction of lactoferrin, lysozyme, and TLC by two different mechanisms. First, by one protein enhancing the environment of the other by binding negative regulators and the second mechanism is by a direct interaction of the three proteins, a known property of lipocalins.
IV. Function of Tear Lipocalin in Tears
A. Tear Viscosity
Lipids have an important function in tears, as the lipids account for the non-Newtonian behavior of tears 37, 38 that is tears have high viscosities at low shear rates. Tear viscosity drops sharply as sheer rate increases. Tears have to be viscous enough to protect and lubricate the surface, but not too viscous so that the high shear forces caused by the blink do not damage the ocular surface. At the sheer forces generated by the blink tears are not viscous. However at low sheer forces such as between blinks, tear viscosity is very high and tears remain on the ocular surface. If lipids are removed from tears, tears become non-Newtonian. In the manuscript by Gouveia and Tiffany the term whole tears appears to indicate aqueous/mucin tears with no components of the lipid layer, but from which lipids (such as those bound to TLC) have not been removed 38. Gouveia and Tiffany found that there are no free lipids in aqueous/mucin-containing tears. Instead the lipids are bound by TLC (holo-TLC) and this keeps the tears non-Newtonian in behavior 38. When the bound lipids are removed from the holo-TLC to form apo-TLC, the tears become Newtonian in behavior. Furthermore, combinations of the tear proteins secretory IgA, lysozyme and lactoferrin, but not any compound alone, can cause non-Newtonian tear behavior. Holo-TLC, but not apo-TLC, can interact with other tear proteins increasing tear viscosity. Thus the type of TLC, apo or holo, present in tears can affect tear viscosity. In turn the viscosity of tears can protect or damage the ocular surface.
B. Lipid Binding and Release
There are three possible ways that TLC could function based on its ability to bind a wide range of lipids. One, TLC could scavenge lipids from the cornea and conjunctiva. Second, TLC could scavenge lipids from the tear film, removing lipids that are potentially harmful to the cornea and conjunctiva. Third, TLC could deliver lipids to the cornea, conjunctiva, or epithelium lining the tear drainage duct. In the first two cases lipids bound to lipocalin (holo-TLC) wound be removed from the tears by the tear drainage system. In the third case TLC loaded with lipids (holo-TLC) would bind to its receptor and deliver lipids to the ocular surface or drainage duct epithelial cells.
The first potential function of TLC is scavenging lipids from the corneal surface. The hypothesis for this function is that lipids can contaminate the apical surface of the cornea especially in areas in which the membrane spanning mucin MUC 16 has been lost or shed and a dry spot has formed. This lipid contamination makes the cornea unwettable and eventually can result in dessication. This process is seen clinically by fluorescein staining of the cornea. In fact increased fluorescein staining is a diagnostic criteria for dry eye. TLC would function to bind the lipids and remove them from the cornea. The findings that support this function are that TLC: 1) is the third most abundant protein in tears with a concentration of 70 μM; 2) binds lipids that are normally insoluble in aqueous solution; 3) removes lipids from a variety of surfaces including glass, quartz, and Teflon; and 4) contains a capacious molecular mouth that permits relatively large lipids to enter its binding cavity. To demonstrate the removal of lipids from the cornea Gasymov et al used human tears, human corneas, and a fluorescent-labeled fatty acid and a fluorescent-labeled phospholipid both of which are insoluble in aqueous solutions 39. They found that in both fixed and fresh corneas tears removed the labeled fatty acid or phospholipid from the cornea by saturable kinetics indicative of enzyme binding. Tears depleted of TLC did not remove the two types of lipid, whereas tears depleted of TLC and then TLC was added back regained the ability to bind lipid. The lipid removed co-eluted with the TLC. Furthermore, TLC was the only component of tears to bind lipids. Thus TLC can extract insoluble lipids from the corneal surface and stabilize the removed lipids in an aqueous solution.
Glasgow et al measured the removal of fluorescent-labeled phospholipids from human corneas with the ocular surface pathology of bullous keratopathy and dry eye 40. Tears removed lipid from pathological corneas at the same rate as the normal cornea. Although a small number of corneas were used (6 in each group), lipid was not removed faster or to a greater level from the pathological compared to normal corneas. Furthermore both formalin-fixed and fresh corneas had the same rate of lipid removal. Thus TLC in tears can remove lipids from human cornea, but its role in vivo is not yet demonstrated as the native tear film was not present in any of the studies and in fact some of the corneas had been fixed in formalin. Nor was it demonstrated that TLC removal of lipids depended upon dry spot formation or that increased lipid removal occurred with increased dry spots. Finally it is not clear what happens to the lipids bound to the TLC, whether the lipids are transferred to the lipid layer or are removed by drainage of tears with TLC and adsorbed lipids (holo-TLC) into the nasolacrimal drainage duct. These questions were not addressed experimentally 39, 40.
The second potential function is that TLC could scavenge lipids from the tear film. That is TLC would bind lipids in the aqueous and mucin layers of the tear film or remove them from the cornea and transfer them to the lipid layer. TLC can be adsorbed to an air/buffer interface and can absorb to a phospholipid monolayer 41, 42. Mudgil determined if lipid loaded TLC (holoTLC) and lipid depleted TLC (apo-TLC) adsorbed to meibomian lipids and if holoTLC released its lipids. In these experiments bovine meibomian lipids were placed as a film in a Lagmuir trough 43. The sides of the trough were moved together increasing the pressure in the film with time and the pressure recorded. A fluorescent-tagged lipid was added to the film so the fluorescence could be measured. Apo-TLC or holo-TLC was then added to the fluorescent meibomian lipid film. Both apo- and holo-TLC adsorbed to the film, apo- more quickly than holo-TLC and both increased the surface pressure. If holo-TLC released its lipids after adsorbing into the meibomian film the surface pressure should increase and be higher than apo-TLC. The rationale for the delivery of the lipids from the holo-TLC is that the local acidic environment of the lipid-aqueous layer would lower the pH to allow relaxation of the holo-TLC and allow the bound lipids to be released from the binding pocket. Holo-TLC, however, did not release its lipids. Thus TLC does not appear to deliver lipids into the lipid layer of the tear film. Furthermore, apo-TLC once in the lipid layer underwent a conformational change, but holo-TLC did not. This suggests that holo-TLC remains in the aqueous layer, but apo-TLC does not. Interaction with both apo- and holo-TLC led to an increase stability of the meibomian lipid layer. Lysozyme caused a similar effect as TLC 43. These results also led Millar to propose a new structure to the tear film in which proteins and mucins adsorb to the lipid layer and the proteins can unfold increasing the stability of the tears. Holo-TLC would remain in the aqueous layer and could encourage protein aggregation increasing the viscosity of tears 38. Holo-TLC with its bound lipids would then be removed from the tear film by the nasolacrimal duct drainage system.
However, Millar 44 repeated the Langmuir trough experiments with human, rather than bovine, meibomian gland lipids, and obtained remarkably different results even though human TLC was used in both experiments 44. TLC adsorbed to human meibomian lipids, but its binding was much slower than that of lysozyme and lactoferrin. The appearance and hence the structure of meibomian gland lipids after addition of TLC was different in humans and cows. Furthermore the behaviors of lipidated, delipidated, and delipidated/relipidated TLC differed between humans and cows. In cows relipidation of delipidated TLC returned the pressure values back to those of lipidated TLC. In contrast in human meibomian gland lipids, this return did not occur. In spite of these differences, TLC was able to absorb to and penetrate human meibomian gland lipid films thereby stabilizing them. It was surprising that bovine and human meibomian gland lipids behaved so differently as they are relatively similar in composition. This finding suggests that small changes in meibomian lipids can have an effect on the behavior of these lipids. The Millar study used meibomian lipids pooled from six individuals 44. Study of individual samples is necessary to determine the extent of differences between individual meibomian gland samples and their effect on TLC behavior.
In the Millar study the lipids extracted from the human TLC, which unlike the meibomian lipids, were from one individual 44. When the removed lipids were analyzed they were found to be predominantly diacyglycerols. This is in contrast to Glasgow and others who found a broad array of lipids bound to TLC including fatty acids, cholesterol, and phospholipids. Thus it is controversial what types of lipids TLC actually binds in tears and whether this differs between individuals. This is an important controversy in need of resolution, as TLC is known to bind a broad array of lipids in vitro.
For the third potential function of TLC, delivering lipids to the cornea, Redl proposed that TLC carries retinol to the cornea on its way to the retina and retinal pigemented epithelium 19. Glasgow argued against this as TLC has not been detected in the cornea, retina, or retinal pigmented epithelium 23. Furthermore, a 20 kDa retinol-binding protein has been identified in lacrimal gland secretion and tears 45. The redundancy of nature is not consistent with the argument of Glasgow 41. More recently Saaren-Seppala et al 46 investigated the lipid transferring ability of TLC, that is the binding and releasing of lipids 42. This group used artificially created lipid monolayers that could be neutral, anionic, or cationic in charge. They found that TLC bound avidly to all three types of membranes. However, TLC did not transfer any neutral or polar lipids into the lipid vesicles. Thus TLC can bind lipids and membranes and remove lipids from membranes as well as different types of surfaces, but does not transfer lipids. This group and another did identify a protein in tears that does transfer lipids, a protein named phospholipid transfer protein (PLTP) 46, 47. Thus to date TLC does not appear to transfer lipids to membranes. However, the lipid monolayer did not contain the lipocalin receptor and this could completely alter the delivery of lipid.
To summarize the lipid binding and release properties of TLC, TLC can be absorbed into the lipid layer of the tear film, but does not release its lipid cargo into this layer. In addition, TLC can scavenge lipids from the cornea without its glycocalyx of MUC16. It appears that TLC does not release these lipids into the lipid layer of the tear film either, but rather is drained through the tear drainage system. To date TLC does not appear to release its lipids into the corneal or conjunctival epithelial cells, although the appropriate experiments have not been performed. Finally, it is not clear what type of lipids TLC actually binds in vivo.
C. Endonuclease Activity: A Mechanism for Inactivating Viral DNA in Tears
Nuclease activity nicks DNA thereby inactivating it. The LEDFXR sequence is responsible for the nuclease activity of a known endonuclease the Mg2+-dependent nuclease of the bacteria Serratia marcescens 48. TLC contains conserved amino acids for the LEDFXR sequence at Glu-128 and this is responsible for the nuclease activity of TLC that allows TLC to hydrolyze DNA. A mutation in Glu-28 depletes TLC of it nuclease activity. Yusifov et al studied human tears to determine if TLC functioned as an endonuclease in tears 49. They found two major endonculeases in tears, TLC and a 34 kDa protein. TLC accounted for 75% of the DNA catalytic activity in tears and thus is the major nuclease in tears.
TLC endonuclease activity plays a role in protecting the ocular surface from viruses and in clearing DNA in tears from shed epithelial cells 48, 49. DNA from a variety of viral sources has been identified in tears. This is viral DNA that escaped destruction by intracellular caspases. TLC endonuclease activity could destroy the viral DNA and prevent transfection of viruses to other cells or other patients. In fact the virus HIV can be cultured from blood, but not tears, of infected patients. The endonuclease activity of TLC is critical as the major human extracellular nucleases, DNase I and DNase X, are not present in tears. In addition, DNA inhibits the bactericidal activity of lysozyme. Destruction of DNA by TLC would protect the activity of lysozyme.
D. Antibacterial and Antifungal Activity
TLC has antimicrobial activity that is dependent on its ability to bind microbial siderophores 50. Siderophores are molecules found in microbes that are powerful iron chelators, because iron is an essential nutrient for micorganisms. However, iron is strongly bound to mammalian host proteins and microorganisms must compete for iron. Inhibition of the microbial iron acquisition process by scavenging microbial siderphores is a powerful defense against microbes and infection. TLC synthesized in bacteria was used for these in vitro biochemical studies. TLC bound all bacterial and fungal siderophores tested. The binding was specific as it replaced a known ligand of TLC. Although TLC binds a broad spectrum of siderophores, consistent with its general scavenger function, the affinity of binding was low in some cases. In addition to binding bacterial and fungal siderophores, TLC also inhibited the growth of the bacteria E. coli and blocked conidia formation of a specific fungus. The question then becomes, can TLC function in vivo in the tear film to kill bacteria and fungi. These experiments were not performed, but Fluckinger et al speculated that TLC might be a more efficient inhibitor of fungal growth than bacterial as TLC binds fungal siderophores with higher affinity than it binds bacterial siderophores 50. In this connection, TLC was one of six proteins changed in tears from patients with fungal infections and one of four proteins downregulated. Tear proteins were comprehensively analyzed by MALDI-TOF spectrophotometry ensuring that most proteins altered by infection were analyzed. Thus it is possible to conclude that a decrease in TLC could have at least partly contributed to the fungal infection.
A role for TLC as an antimicrobial is consistent with it being a member of the immunocalins, a lipocalin subfamily that is antiflammatory and antimicrobial. This subfamily was described in section I B.
E. Role in Dry Eye
Research on TLC and dry eye is limited. Two papers addresses TLC as a biomarker of dry eye and one on its potential as an autoantigen in Sjogren’s syndrome. In a study using complex spectroscopy methods to identify proteins in tears, Zhou et al found six proteins upregulated and four proteins downregulated in tears of patients with dry eye compared to tears of normal patients 51. The ten proteins that are potential biomarkers included proteins decreased in lacrimal gland secretion, increased by elevation of tear osmolarity, increased by ocular surface inflammation, and increased by unknown mechanisms. TLC along with lactoferrin and lysozyme was downregulated. This is consistent with the finding that lacrimal gland secretion is decreased in dry eye disease and the three proteins secreted by the regulated pathway are all decreased. The Zhou study included discovery, verification, and verification by ELISA arms. A similar study was performed by Caffrey et al 52, who analyzed TLC, lysozyme, and total tear protein in patients with Sjogren’s syndrome, non-Sjogren’s dry eye, and non-dry eye. In this study TLC and total protein were decreased in patients with Sjogren’s syndrome compared to those with non-Sjogren’s dry eye and non-dry eye patients. Thus studies to date indicate that a decrease in TLC is associated with dry eye syndrome and could in fact be a biomarker of dry eye.
The studies of Zhou et al and Caffrey did not determine if lipocalin plays a role in the pathogenesis of dry eye or Sjogren’s syndrome 51, 52. However, Navone et al found that TLC is a novel autoantigen target in Sjogren’s syndrome 53. This group analyzed blood from 40 patients with Sjogren’s syndrome, 40 patients with non-Sjogren’ autoimmune disease, and 40 normal individuals. In 73% of Sjogren’s syndrome patients and in none of the others, the IgG fraction recognized a peptide that had homology with Epstein Barr virus early antigen protein, alpha-fodrin (a known antigen in Sjogren’s syndrome), and TLC. Numerous antigens have been identified in the sera of patients with Sjogren’s syndrome. To explain this finding, Mircheff has suggested that chronic overstimulation of the lacrimal gland can induce errors in membrane trafficking so that internal proteins get mistakenly presented externally causing an autoimmune reaction 54. As TLC is a secretory protein present in high concentration it is not surprising that it is present in high levels in the different synthetic and endocytic compartments of the lacrimal gland cell and that TLC has a high probability of being mistakenly trafficked to the external environment of the lacrimal acinar cells. TLC appears to be one of the many autoantigens associated with Sjogren’s syndrome.
F. Anti-inflammatory Activity
The lipocalin superfamily has an amino acid motif QDVSGTWY, which contains the motif QNVNG 55. This latter motif is a well-conserved domain in the family 2 cystatins that is responsible for their ability to inhibit cysteine proteinases. This proteinase ability allows cystatins to inhibit bacterial and viral proteinases and control inflammation. Three domains have been identified in cysteinases that are crucial for their cysteine proteinase inhibitory activity. Three analogous domains are recognized in von Ebner’s protein and hence in TLC. In in vitro experiments, von Ebner’s protein inhibited the activity of papain, a cysteine proteinase. Recombinant protein had the same cysteine proteinase inhibitory properties as the native protein 31. The cysteine proteinase inhibitory activity of TLC combined with its nuclease activity gives TLC its anti-microbial, anti-inflammatory properties.
V. Conclusion
As a major protein constituent of tears, TLC has a diverse set of critical functions in maintaining the health of the ocular surface. These range from controlling the non-Newtonian viscosity of tears, to removing lipids from the cornea, to serving as a biomarker of dry eye. The basis for these far ranging functions is the promisicuity of the types of lipids bound by TLC in its large, bifurcated binding domain. Because of this variability the native lipid(s) bound by TLC have yet to be discovered and hence the major function of TLC in tears may still be unknown. In particular the presence of the TLC binding protein (LIMR) on the ocular surface has yet to be investigated and hence the role of TLC in removal or delivery of lipids to the tears or ocular surface is unknown.
Acknowledgments
The literature review and preparation of this article were supported by Johnson & Johnson Vision Care, Inc but under the control of Dr. Dartt.
References
- 1.Akerstrom B, Flower DR, Salier JP. Lipocalins: unity in diversity. Biochim Biophys Acta. 2000;1482(1–2):1–8. doi: 10.1016/s0167-4838(00)00137-0. [DOI] [PubMed] [Google Scholar]
- 2.Flower DR. The lipocalin protein family: a role in cell regulation. FEBS Lett. 1994;354(1):7–11. doi: 10.1016/0014-5793(94)01078-1. [DOI] [PubMed] [Google Scholar]
- 3.Flower DR. Multiple molecular recognition properties of the lipocalin protein family. J Mol Recognit. 1995;8(3):185–95. doi: 10.1002/jmr.300080304. [DOI] [PubMed] [Google Scholar]
- 4.Flower DR. Beyond the superfamily: the lipocalin receptors. Biochim Biophys Acta. 2000;1482(1–2):327–36. doi: 10.1016/s0167-4838(00)00169-2. [DOI] [PubMed] [Google Scholar]
- 5.Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000;1482(1–2):9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 6.Newcomer ME, Ong DE. Plasma retinol binding protein: structure and function of the prototypic lipocalin. Biochim Biophys Acta. 2000;1482(1–2):57–64. doi: 10.1016/s0167-4838(00)00150-3. [DOI] [PubMed] [Google Scholar]
- 7.Monaco HL. The transthyretin-retinol-binding protein complex. Biochim Biophys Acta. 2000;1482(1–2):65–72. doi: 10.1016/s0167-4838(00)00140-0. [DOI] [PubMed] [Google Scholar]
- 8.Urade Y, Hayaishi O. Biochemical, structural, genetic, physiological, and pathophysiological features of lipocalin-type prostaglandin D synthase. Biochim Biophys Acta. 2000;1482(1–2):259–71. doi: 10.1016/s0167-4838(00)00161-8. [DOI] [PubMed] [Google Scholar]
- 9.Logdberg L, Wester L. Immunocalins: a lipocalin subfamily that modulates immune and inflammatory responses. Biochim Biophys Acta. 2000;1482(1–2):284–97. doi: 10.1016/s0167-4838(00)00164-3. [DOI] [PubMed] [Google Scholar]
- 10.Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000;1482(1–2):298–307. doi: 10.1016/s0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 11.Rosner MH. Urinary biomarkers for the detection of renal injury. Adv Clin Chem. 2009;49:73–97. doi: 10.1016/s0065-2423(09)49004-8. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi R, Yu J, Wiita P, Ter-Stepanian M, Sun H. Mapping the membrane topology and extracellular ligand binding domains of the retinol binding protein receptor. Biochemistry. 2008;47(19):5387–95. doi: 10.1021/bi8002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf G. Identification of a membrane receptor for retinol-binding protein functioning in the cellular uptake of retinol. Nutr Rev. 2007;65(8 Pt 1):385–8. doi: 10.1301/nr.2007.aug.385-388. [DOI] [PubMed] [Google Scholar]
- 14.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276(40):37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 15.Gupta K, Shukla M, Cowland JB, Malemud CJ, Haqqi TM. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56(10):3326–35. doi: 10.1002/art.22879. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez D, Ortega-Cubero S, Akerstrom B, Herrera M, Bastiani MJ, Ganfornina MD. Molecular interactions of the neuronal GPI-anchored lipocalin Lazarillo. J Mol Recognit. 2008;21(5):313–23. doi: 10.1002/jmr.902. [DOI] [PubMed] [Google Scholar]
- 17.Erickson O. Stanford Med Bull. 1956;14:124–125. [Google Scholar]
- 18.Josephson AS, Weiner RS. Studies of the proteins of lacrimal secretions. J Immunol. 1968;100(5):1080–1. [PubMed] [Google Scholar]
- 19.Redl B, Holzfeind P, Lottspeich F. cDNA cloning and sequencing reveals human tear prealbumin to be a member of the lipophilic-ligand carrier protein superfamily. J Biol Chem. 1992;267(28):20282–7. [PubMed] [Google Scholar]
- 20.Lassagne H, Gachon AM. Cloning of a human lacrimal lipocalin secreted in tears. Exp Eye Res. 1993;56(5):605–9. doi: 10.1006/exer.1993.1075. [DOI] [PubMed] [Google Scholar]
- 21.Breustedt DA, Korndorfer IP, Redl B, Skerra A. The 1.8-A crystal structure of human tear lipocalin reveals an extended branched cavity with capacity for multiple ligands. J Biol Chem. 2005;280(1):484–93. doi: 10.1074/jbc.M410466200. [DOI] [PubMed] [Google Scholar]
- 22.Redl B. Human tear lipocalin. Biochim Biophys Acta. 2000;1482(1–2):241–8. doi: 10.1016/s0167-4838(00)00142-4. [DOI] [PubMed] [Google Scholar]
- 23.Glasgow BJ. Tissue expression of lipocalins in human lacrimal and von Ebner’s glands: colocalization with lysozyme. Graefes Arch Clin Exp Ophthalmol. 1995;233(8):513–22. doi: 10.1007/BF00183433. [DOI] [PubMed] [Google Scholar]
- 24.Breustedt DA, Chatwell L, Skerra A. A new crystal form of human tear lipocalin reveals high flexibility in the loop region and induced fit in the ligand cavity. Acta Crystallogr D Biol Crystallogr. 2009;65(Pt 10):1118–25. doi: 10.1107/S0907444909031011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasymov OK, Abduragimov AR, Glasgow BJ. Intracavitary ligand distribution in tear lipocalin by site-directed tryptophan fluorescence. Biochemistry. 2009;48(30):7219–28. doi: 10.1021/bi9005557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Structural changes in human tear lipocalins associated with lipid binding. Biochim Biophys Acta. 1998;1386(1):145–56. doi: 10.1016/s0167-4838(98)00092-2. [DOI] [PubMed] [Google Scholar]
- 27.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Interaction of tear lipocalin with lysozyme and lactoferrin. Biochem Biophys Res Commun. 1999;265(2):322–5. doi: 10.1006/bbrc.1999.1668. [DOI] [PubMed] [Google Scholar]
- 28.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Resolution of ligand positions by site-directed tryptophan fluorescence in tear lipocalin. Protein Sci. 2000;9(2):325–31. doi: 10.1110/ps.9.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojnar P, van’t Hof W, Merschak P, Lechner M, Redl B. The N-terminal part of recombinant human tear lipocalin/von Ebner’s gland protein confers cysteine proteinase inhibition depending on the presence of the entire cystatin-like sequence motifs. Biol Chem. 2001;382(10):1515–20. doi: 10.1515/BC.2001.186. [DOI] [PubMed] [Google Scholar]
- 30.Wojnar P, Lechner M, Redl B. Antisense down-regulation of lipocalin-interacting membrane receptor expression inhibits cellular internalization of lipocalin-1 in human NT2 cells. J Biol Chem. 2003;278(18):16209–15. doi: 10.1074/jbc.M210922200. [DOI] [PubMed] [Google Scholar]
- 31.Wojnar P, Lechner M, Merschak P, Redl B. Molecular cloning of a novel lipocalin-1 interacting human cell membrane receptor using phage display. J Biol Chem. 2001;276(23):20206–12. doi: 10.1074/jbc.M101762200. [DOI] [PubMed] [Google Scholar]
- 32.Fluckinger M, Merschak P, Hermann M, Haertle T, Redl B. Lipocalin-interacting-membrane-receptor (LIMR) mediates cellular internalization of beta-lactoglobulin. Biochim Biophys Acta. 2008;1778(1):342–7. doi: 10.1016/j.bbamem.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Kim SJ, Chowdhury B, Wang J, Lee YC, Tsai PC, Choi M, Mukherjee AB. Interaction of uteroglobin with lipocalin-1 receptor suppresses cancer cell motility and invasion. Gene. 2006;369:66–71. doi: 10.1016/j.gene.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 34.Redl B, Merschak P, Abt B, Wojnar P. Phage display reveals a novel interaction of human tear lipocalin and thioredoxin which is relevant for ligand binding. FEBS Lett. 1999;460(1):182–6. doi: 10.1016/s0014-5793(99)01331-9. [DOI] [PubMed] [Google Scholar]
- 35.Josephson AS, Wald A. Enhancement of lysozyme activity by anodal tear protein. Proc Soc Exp Biol Med. 1969;131(2):677–9. doi: 10.3181/00379727-131-33951. [DOI] [PubMed] [Google Scholar]
- 36.Laktionov PP, Rykova E, Krepkii DV, Bryksin AV, Vlassov VV. Interaction of oligonucleotides with barrier fluid proteins. Biochemistry (Mosc) 1997;62(6):613–8. [PubMed] [Google Scholar]
- 37.Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78(3):347–60. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Gouveia SM, Tiffany JM. Human tear viscosity: an interactive role for proteins and lipids. Biochim Biophys Acta. 2005;1753(2):155–63. doi: 10.1016/j.bbapap.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Gasymov OK, Abduragimov AR, Prasher P, Yusifov TN, Glasgow BJ. Tear lipocalin: evidence for a scavenging function to remove lipids from the human corneal surface. Invest Ophthalmol Vis Sci. 2005;46(10):3589–96. doi: 10.1167/iovs.05-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glasgow BJ, Gasymov OK, Abduragimov AR, Engle JJ, Casey RC. Tear Lipocalin Captures Exogenous Lipid from Abnormal Corneal Surfaces. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glasgow BJ, Marshall G, Gasymov OK, Abduragimov AR, Yusifov TN, Knobler CM. Tear lipocalins: potential lipid scavengers for the corneal surface. Invest Ophthalmol Vis Sci. 1999;40(13):3100–7. [PubMed] [Google Scholar]
- 42.Saaren-Seppala H, Jauhiainen M, Tervo TM, Redl B, Kinnunen PK, Holopainen JM. Interaction of purified tear lipocalin with lipid membranes. Invest Ophthalmol Vis Sci. 2005;46(10):3649–56. doi: 10.1167/iovs.05-0176. [DOI] [PubMed] [Google Scholar]
- 43.Mudgil P, Millar TJ. Adsorption of apo- and holo-tear lipocalin to a bovine Meibomian lipid film. Exp Eye Res. 2008;86(4):622–8. doi: 10.1016/j.exer.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2009;50(1):140–51. doi: 10.1167/iovs.08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SY, Ubels JL, Soprano DR. The lacrimal gland synthesizes retinol-binding protein. Exp Eye Res. 1992;55(1):163–71. doi: 10.1016/0014-4835(92)90104-z. [DOI] [PubMed] [Google Scholar]
- 46.Huuskonen J, Wohlfahrt G, Jauhiainen M, Ehnholm C, Teleman O, Olkkonen VM. Structure and phospholipid transfer activity of human PLTP: analysis by molecular modeling and site-directed mutagenesis. J Lipid Res. 1999;40(6):1123–30. [PubMed] [Google Scholar]
- 47.Nishida HI, Nishida T. Phospholipid transfer protein mediates transfer of not only phosphatidylcholine but also cholesterol from phosphatidylcholine-cholesterol vesicles to high density lipoproteins. J Biol Chem. 1997;272(11):6959–64. doi: 10.1074/jbc.272.11.6959. [DOI] [PubMed] [Google Scholar]
- 48.Yusifov TN, Abduragimov AR, Gasymov OK, Glasgow BJ. Endonuclease activity in lipocalins. Biochem J. 2000;347(Pt 3):815–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Yusifov TN, Abduragimov AR, Narsinh K, Gasymov OK, Glasgow BJ. Tear lipocalin is the major endonuclease in tears. Mol Vis. 2008;14:180–8. [PMC free article] [PubMed] [Google Scholar]
- 50.Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother. 2004;48(9):3367–72. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, Tong L, Liu S, Stern ME, Tan D. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8(11):4889–905. doi: 10.1021/pr900686s. [DOI] [PubMed] [Google Scholar]
- 52.Caffery B, Joyce E, Boone A, Slomovic A, Simpson T, Jones L, Senchyna M. Tear lipocalin and lysozyme in Sjogren and non-Sjogren dry eye. Optom Vis Sci. 2008;85(8):661–7. doi: 10.1097/OPX.0b013e318181ae4f. [DOI] [PubMed] [Google Scholar]
- 53.Navone R, Lunardi C, Gerli R, Tinazzi E, Peterlana D, Bason C, Corrocher R, Puccetti A. Identification of tear lipocalin as a novel autoantigen target in Sjogren’s syndrome. J Autoimmun. 2005;25(3):229–34. doi: 10.1016/j.jaut.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 54.Qian L, Xie J, Rose CM, Sou E, Zeng H, Hamm-Alvarez SF, Mircheff AK. Altered traffic to the lysosome in an ex vivo lacrimal acinar cell model for chronic muscarinic receptor stimulation. Exp Eye Res. 2004;79(5):665–75. doi: 10.1016/j.exer.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 55.van’t Hof W, Blankenvoorde MF, Veerman EC, Amerongen AV. The salivary lipocalin von Ebner’s gland protein is a cysteine proteinase inhibitor. J Biol Chem. 1997;272(3):1837–41. doi: 10.1074/jbc.272.3.1837. [DOI] [PubMed] [Google Scholar]